Abstract
Practical relevance:
Aged pets comprise a significant proportion of the small animal veterinarian’s patient population; in the USA, for example, it was estimated that over 20% of pet cats were 11 years of age or older in 2011. Certain changes associated with aging are neither positive nor negative, but others are less desirable, associated with illness, changes in mobility or the development of unwanted behaviors. These changes can greatly affect the health and wellbeing of the cat and have a tremendous impact on the owner.
Clinical challenges:
Regular veterinary examinations are essential for evaluating the health of older patients and for providing owners with guidance regarding optimal care. With the exception of overt disease, however, it is difficult to definitively determine if a cat is displaying changes that are appropriate for age or if they reflect an abnormal process or condition.
Goals:
This is the first of two review articles in a Special Issue devoted to feline healthy aging. The goals of the project culminating in these publications included developing a working definition for healthy aging in feline patients and identifying clinical methods that can be used to accurately classify healthy aged cats. This first review provides a thorough, systems-based overview of common health-related changes observed in cats as they age.
Evidence base:
There is a paucity of research in feline aging. The authors have drawn on expert opinion and available data in both the cat and other species.
Introduction
Aging is defined as a complex set of biological processes resulting in the progressive reduction of an individual’s ability to maintain homeostasis when exposed to internal physiologic and external environmental stressors. 1 These changes lead to decreased vitality, increased vulnerability to disease and eventual death.
Some of the changes associated with aging may be thought of as favorable; for example, aged cats often are well behaved and have well-established habits. Other changes are neither positive nor negative, such as graying of the coat or a moderately reduced activity level. Less desirable changes are those associated with illness, changes in mobility or the development of unwanted behaviors. Collectively, deteriorative changes that may negatively affect an aging pet’s overall health and quality of life are referred to as senescence. 2
A variety of theories have been proposed to explain the phenomena of aging and senescence. 3 Several of these focus on genetic controls, such as the somatic mutation and gene regulation theories. Others examine the impact of temporal changes in homeostatic mechanisms of different body systems or the accumulation of the damaging products of cellular aging. For example, the oxidative stress theory postulates that aging is linked to energy expenditure and the cumulative cellular damage caused by free radicals from external sources as well as internal sources, such as mitochondrial respiration and immune cell reactions.4,5 While not mutually exclusive, neither of these theories solely explain all of the changes that are observed during aging. A more cogent view is that aging and senescence are multifaceted processes influenced by genetics and a myriad of internal and external environmental factors.

The rate at which an individual cat exhibits signs of aging may be influenced by breed, genetic make-up, exposure to injuries and disease, physical environment, stress and nutritional status.1,2,6 With good care, many cats live into their late teens and early 20s. A suggested classification for aging cats is to consider them as ‘mature’ or ‘middle-aged’ at 7–10 years, ‘senior’ at 11–14 years and ‘geriatric’ at 15+ years. 7 These classifications are based on epidemiological data; each cat should be evaluated as an individual.
Aged pets comprise a significant proportion of the small animal veterinarian’s patient population. Of the 74.1 million pet cats in the United States in 2011, it was estimated that 20.4% (15.1 million) were 11 years of age or older. 8 Naturally, many of these patients are cherished family members with owners who are committed to providing them with the best of care (Figure 1). Regular veterinary examinations are essential for evaluating the health of older patients, and for providing owners with guidance regarding optimal care including, when needed, nutritional, behavioral or medical interventions.
Figure 1.

Many of the animals within the veterinarian’s aged feline caseload are cherished family members with committed owners seeking to provide the best of care. © iStock/KatarzynaBialasiewicz
With the exception of overt disease, it is difficult to definitively determine if an aged cat is displaying age-related changes that are appropriate for age (ie, healthy aged cat) or if they reflect an abnormal process or condition. Effectively characterizing these aged cats as healthy or not requires a thorough, systems-based overview of common health-related changes observed in cats as they age. The objective of this review is to provide that overview.
General physical changes
Typical age-related physical effects in healthy cats, as noticed by most pet owners, manifest as changes in behavior, appearance and daily function (see box). These changes can happen in the absence of any disease process, as part of normal aging.
Cognitive and behavioral health
The classic signs of aging noted by cat owners are commonly behavioral and related to changes in the musculoskeletal system, sensory systems and cognitive function. Pet owners often characterize these changes as just part of the aging process and may not mention them to their veterinarian, yet identification of these signs by veterinary health care professionals may permit timely intervention that can improve quality of life for the patient. Changes in behavior that possibly are not related to systemic disease include changes in attitude, activity, appetite, sleep and cognitive ability, and are often the result of underlying neurodegenerative changes.
Over the past two decades, relatively robust evidence has been compiled for cognitive decline in senior dogs, and a growing body of evidence supports similar changes in the cat (see box on page 535).9–14 Like dogs, an aging cat may show cognitive and behavioral changes without any underlying or concurrent systemic disease.
Decline in cognitive ability in aged dogs is supported by neuropsychological testing.14–16 Only limited neuropsychological testing results are available for the aged cat. One such study evaluated the responses of cats, divided into three age groups (young adult, 3.0–3.8 years; old, 7.7–9.0 years; senior, 10.5–15 years), to a battery of cognitive tasks; 17 these were modeled on tasks and apparatus developed to assess cognitive function in dogs. The subjects showed significant age-dependent decline in learning in T-maze tasks, in both discrimination learning and reversal learning. Additionally, there were significant differences between adult and aged cats on a matching task. These data establish age-related differences in the ability of cats to learn certain cognitive tasks, which suggests that advancing age in cats is associated with a progressive decline in cognitive function. 17 Based on results from case studies, Landsberg et al suggest that these declines may begin at around 10 years of age, depending on the individual cat. 11
Skin and coat health
With advancing age, various changes occur in the skin and coat of cats (Figure 3). These include epidermal, dermal and follicular atrophy and hyperkeratosis, which may result in a thinning haircoat and focal alopecia. 18 Loss of melanocytes in the hair follicles and reduced activity of the enzyme tyrosinase result in the production of white hairs; however, paradoxically whiskers may turn black. Changes in sebum production, along with reduced self-grooming activity, can lead to scaly skin and cause the coat to become dry or oily and dull. 19 Decreased skin elasticity and brittle claws have also been noted in apparently healthy older cats. 7
Figure 3.
Reduced grooming activity is reflected by the clumped and spiked appearance of the coat in this older cat. Also note the black whisker (inset), commonly seen with aging in cats. Courtesy of Margie Scherk
In addition, the effects of solar exposure may be more evident in older cats. A positive correlation between age and the degree of photodamage was found in cats chronically exposed to natural solar radiation. In those cats, non-neoplastic photodamage was characterized by subepidermal edema and sclerosis, telangiectasia, squamatization of basal keratinocytes and increased epidermal thickness. 20 Such solar keratotic lesions may progress to squamous cell carcinoma, which is primarily a cancer of older cats, the median age of affected cats being 10–12 years. 21
In general, neoplastic skin changes are more likely to be seen with advancing age in cats. In addition to squamous cell carcinomas, the more common skin tumors observed in older cats include benign basal cell tumors, basal cell carcinomas and fibrosarcomas. 22
Weight and body condition
Maintenance of a healthy body weight and body composition throughout the life cycle is well accepted to reduce the risks associated with morbidity and mortality in all mammals. 23 Despite this, overweight, ranging from mild to severe (obesity), represents one of the most common health conditions in cats. 24 While obesity is technically defined as having excess body fat (>12% body fat), 25 in practical situations it is diagnosed as body weight that is >20% above ideal. 26
Weight gain is often unrecognized by the owner and under-reported by the veterinarian. Given the insidious nature of body composition changes, it is important that body composition is tracked throughout a cat’s life. Although body weight and fat:lean ratio are generally accepted to either plateau or increase throughout adulthood, 27 they are more likely to decrease in cats over 11 years of age.23,28–30 Older cats commonly lose weight and body condition. While mild changes are possibly normal in the older individual, several underlying medical conditions may contribute to weight loss and, where possible, any underlying cause should be determined. This is critically important in the light of the observations that every 100 g loss of weight increases the risk of death by 6.4%, every 100 g loss of lean body mass increases the risk of death by 20%, and every 100 g loss of body fat increases the risk of death by 40%. 29
Recent data suggest the importance of veterinarians (as opposed to trained or untrained veterinary support staff) in the evaluation of body composition in at-risk cats. 25 This was most evident in cats with a body condition score (BCS) >3.5 (scale of 1–5, where 1 = thin and 5 = obese) and suggested that veterinarians are more familiar than veterinary technicians and both trained and untrained animal care staff with body fat depots and body conformation differences through the life stages. If cats have proper nutritional, environmental and health care management, they will not experience weight gain or loss of lean body mass up through 11 years of age; however, minor increases in blood urea nitrogen (BUN) (Table 1) without concomitant changes in creatinine (data shown in Table 1 of the accompanying article) may suggest minor increases in protein breakdown. Changes in BUN and creatinine are non-specific, and are not always renal in nature. Slight changes in these parameters may be related to changes in diet or lean body mass. Cumulatively, these data suggest that practitioners should first seek to limit weight gain and maintain lean mass in cats throughout adulthood, and thereafter maintain both weight and lean mass in cats >11 years of age.
Table 1.
Association between blood urea nitrogen (mg/dl) and age in cats in a controlled animal facility*
| Gender | Age (years) | n | 2.5th percentile and CI | 97.5th percentile and CI | Reference interval |
|---|---|---|---|---|---|
| Male | 0–1 | 285 | 15.1 (14.0–16.0) | 29.0 (28.0–31.0) | 14.0–31.0 |
| 2–7 | 520 | 16.0 (15.0–16.1) | 29.4 (28.7–30.3) | 15.0–30.3 | |
| 8–11 | 268 | 16.0 (15.5–16.2) | 34.80 (32.0–39.0) | (15.5–39.0 | |
| 12+ | 182 | 17.0 (14.0–18.0) | 44.69 (39.0–57.0) | 14.0–57.0 | |
| Female | 0–1 | 335 | 15.2 (13.8–16.2) | 30.0 (29.0–33.0) | 13.8–33.0 |
| 2–7 | 1345 | 15.8 (15.0–16.0) | 30.0 (29.6–31.0) | 15.0–31.0 | |
| 8–11 | 617 | 15.7 (15.0–16.0) | 31.00 (29.0–33.0) | 15.0–33.0 | |
| 12+ | 557 | 17.2 (16.0–18.2) | 44.66 (42.9–47.9) | 16.0–47.9 |
Data were generated from results for 627 healthy cats, including 211 cats aged 7–10 years and 149 cats aged 11+ years, housed at the Iams Pet Health and Nutrition Center between 2002 and 2011
CI = confidence interval
Energy expenditure and intake are the variables contributing to energy balance. Many factors affect energy expenditure and intake, including age, body size, activity level, nutrient digestibility, neutering, health status and environmental conditions. These factors need to be considered for individual cats. The National Research Council 31 and the Association of American Feed Control Officials 32 base maintenance energy requirements (MER) and recommendations, respectively, for adult cats on data from unstressed, healthy adult laboratory animals that are of typical body weight and condition, undergoing modest amounts of exercise and housed in a thermoneutral environment to which they have been acclimated. These criteria may not adequately represent client-owned cats and/or cats >11 years of age. Indeed, Bermingham et al demonstrated that there is a significant effect of age and season on energy expenditure, but when standardized for lean body mass, energy expenditure, as evaluated in the summer, was not different between young and old cats. 33 However, when dietary energy intake required to maintain body weight is used to determine MER, daily energy requirements appear to increase in cats 10–12 years old and more dramatically in cats >12 years old. 34
Studies report conflicting findings on the relative food intake of senior cats. Peachey and Harper demonstrated that food and energy intake of senior cats was not different from that of young adults when fed a wet food. 35 However, the small number of cats (n = 6) in the study may not have been sufficient to detect numerically small, but physiologically significant, differences. In addition, there was a tendency for senior cats to consume less energy per meal than young cats, and this could contribute to an overall negative energy balance and weight loss over time. 35 Conversely, Taylor et al reported that senior cats (10–14 years) had significantly greater energy intakes compared with younger cats. 36 The Bermingham study found that older cats had a lower total metabolizable energy intake in the winter compared with the summer, but that energy requirements based on energy expenditure were similar between summer and winter in older cats. 33
The overall challenge in detecting any differences in energy intake is compounded by the small quantities of food consumed by most cats and the high energy density of most diets. While it takes quite a large change in body weight (at least 2 lb or 1 kg) to fall into a new feeding guideline amount (typically ± 1/4 to 1/3 cup) for most commercially labeled dry cat foods, a much smaller change in daily intake (<15 g or 1/8 cup) can result in significant increases or decreases in weight over time. Unlike humans and dogs, many cats do not have outdoor access; this may limit their ability to adequately compensate for energy intake excess or deficiency with concurrent changes in physical activity. 27 There is a great need for additional research to better elucidate the relationship between food intake and energy balance in senior cats.
Diet digestibility can also impact energy balance. Studies that have examined age as a variable have suggested either lower or similar energy digestibility in older cats compared with younger cats.35–37 When energy digestibility has been found to be lower, it is predominantly driven by a decrease in fat digestibility. 36 A reduction in digestibility of fat and energy may result in an energy imbalance and, in turn, loss of body and/or muscle condition. More frequent meals may improve digestibility and availability of nutrients. Recent research has suggested that increased feeding frequency and water intake increase physical activity, 38 which can help maintain lean body mass. Therefore, for management of senior cats consideration might be given to increased frequency of feeding and methods to encourage increased intake of fresh, high quality water.
Figure 4.

Methods to encourage water intake can promote physical activity and help maintain lean body mass in aged cats. Courtesy of University of Bristol, UK/International Cat Care
Musculoskeletal system health
The major changes in the musculoskeletal system that occur with age are a decrease in lean body mass (muscle, bone, skin, organs), deterioration of joint components and functional decline. These changes occur with normal aging and are distinct from changes in these systems that are induced by disease.
Sarcopenia is a syndrome seen during aging in the absence of disease.39,40 The European Working Group on Sarcopenia in Older People defines sarcopenia in humans as ‘a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse clinical outcomes, such as physical disability, poor quality of life, and death’. 40 The term frailty has also been used in relation to age-related deterioration in the musculoskeletal system. Frailty is a multisystem impairment associated with increased vulnerability to stressors and describes individuals that are at increased risk of adverse health outcomes. Frailty is related, but not synonymous, with comorbidity and disability. In the human literature, there is controversy regarding the exact definitions of sarcopenia and frailty. 41 Regardless, there appears to be increasing interest in understanding the mechanisms behind age-related deterioration of the musculoskeletal system, how to phenotype it and how to slow the process. 41
In contrast to humans, little work has been performed in this area in the cat, but most clinicians recognize a similar decline in musculoskeletal function with age. And, as discussed earlier, loss of lean body mass has been shown to be related to decreased survival in cats.29,30 A longitudinal analysis of cats suggested that absolute lean mass declines at a steady rate for many years prior to death, while absolute fat is maintained until around 3 years before death, from which time it increasingly declines. 30 Various studies have highlighted the high prevalence of degenerative joint disease (DJD) in cats (Figure 5), even those of a young age,42–44 and the fact that this is not necessarily associated with clinical signs. Thus, many cats may have radiographically detectable DJD, apparently without any associated clinical signs, and so may still present as healthy individuals. Additionally, cartilage deterioration occurs in cats before radiological features of DJD are seen. 45 This mirrors what is seen in aging humans. Again, the important aspect here is that DJD may be present in the healthy cat without causing any clinically evident signs.
Figure 5.
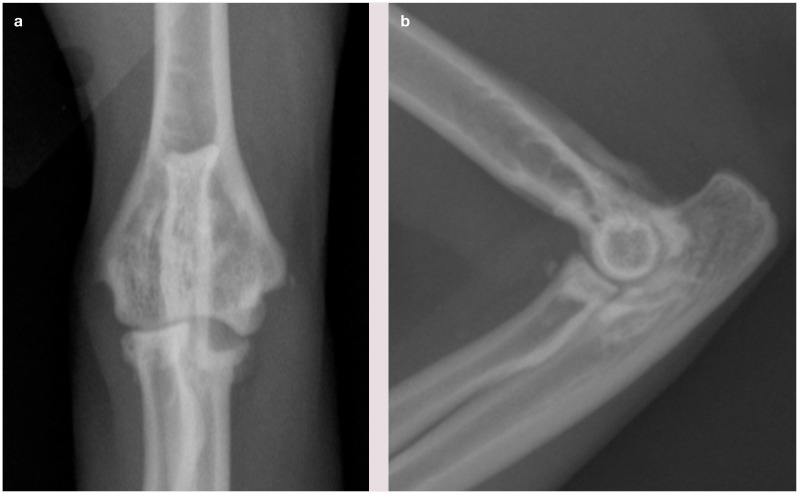
Craniocaudal (a) and lateral (b) view of the elbow of a cat showing changes associated with degenerative joint disease (DJD), including osteophytes and sclerosis, and also joint-associated mineralization and erosion of the medial coronoid area of the ulna (visible in a). This erosion is caused by osteochondral bodies within the joint, resulting in wear of the medial compartment, and is likely a manifestation of osteochondromatosis. It is unknown if this is a primary disease in the cat, or occurs secondarily to DJD. Courtesy of Duncan Lascelles
Clinically apparent, age-appropriate changes in the cat may include some muscle loss, decreased muscle strength, decreased agility and flexibility, DJD (not associated with pain) and decreased bone density and cortical thickness. Such age-related changes are most likely to be seen after 10 years of age.
Special senses health
Aging cats often experience a decline in their sense of smell, hearing and vision. This decline can result in changes in how the individual responds to stimuli, people and the environment.
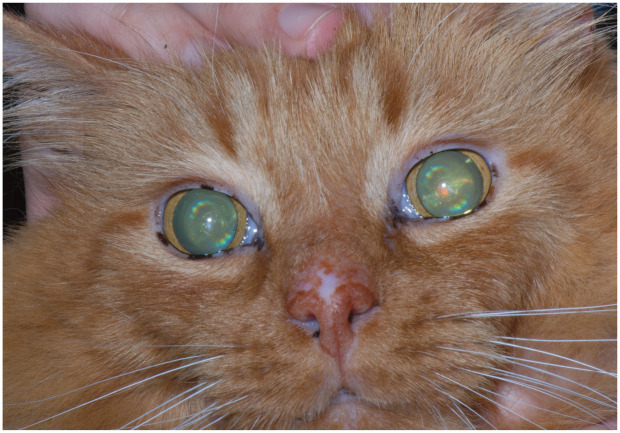
The most common visually apparent change in healthy aged cats is an increase in the density of the central portion of the lens as it ages. Known as lenticular (nuclear) sclerosis (Figure 6), this does not typically affect vision (except perhaps in low lighting) but is often noted by owners as a bluish haze in the lenticular tissue. 46 Commonly in an aged cat there will be changes in the iris tissue, resulting in atrophy and the appearance of scalloping along the iris edge. 47 Although likewise this does not appear to affect vision, these senile changes may progress with age and result in a slower pupillary response to changes in light. Benign deposition of melanin in the iris may also occur, and is difficult to distinguish from diffuse iris melanoma in the early stages. 48
Figure 6.
Lenticular sclerosis in an aging cat. Courtesy of David A Wilkie
Hearing may become impaired in the senior cat, especially at higher frequencies. It is speculated that this hearing loss may result in confusion, anxiety and increased vocalization in some senior cats. To the authors’ knowledge, there is no documented evidence of loss of hearing with age.
It is likely that olfaction diminishes with age in the cat, as it does in other species. The loss of smell may manifest most obviously as changes in eating habits; cats use smell not only to find their food, but are attracted to, and more likely to eat, certain foods based on their aroma. 49 Although changes in olfaction have been noted in dogs and humans with advancing age, no such information is available for the cat.
Oral and gingival health
The oral cavity of a healthy aged cat is defined by the absence of periodontal disease, tooth resorption, oropharyngeal inflammation, oral masses and fractured teeth.
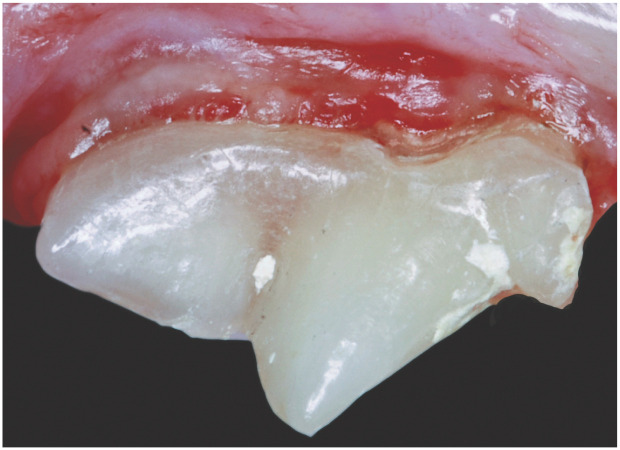
The appearance of individual teeth often changes as cats age. The outer enamel layer of the tooth is non-living and remains approximately the same thickness throughout life. Young cats have wide pulp chambers and thin dentin walls compared with older cats. As cats age, the dentin walls thicken, resulting in greater tooth density with a yellow, tan or ‘off-white’ appearance. Elderly cats occasionally develop sclerotic dentin and pulp chamber shrinkage, which may result in a glassy or transparent appearance to the teeth (Figure 7). 50
Figure 7.
Partially transparent dentin covering the maxillary fourth premolar of a 14-year-old domestic shorthair cat. Courtesy of Jan Bellows
Plaque biofilm attaches continuously on the crowns of feline teeth unless mechanically or chemically removed. Calcium and phosphorus in the saliva mineralize the plaque to produce calculus. It is common to find minimal plaque and calculus on healthy aged feline teeth. In some cats, the plaque irritates the gingiva, allowing pathogenic gram-negative bacteria (Porphyromonas species, Prevotella species, Peptostreptococcus species, Fusobacterium species) to survive subgingivally. By-products of these bacteria stimulate the host’s immune system to release cytokines and prostaglandins that weaken and destroy the tooth’s supporting tissues, resulting in oropharyngeal inflammation. The progression of periodontal disease depends on the complex regulatory interaction between bacteria and immune modulators of the host response. 51
Breed type is a significant risk factor for oral disease in cats. Clinically, the aged domestic shorthair cat may have a healthy mouth with little plaque and calculus accumulation, while brachycephalic cats (eg, Persian, Himalayan) may present with a greater accumulation of plaque and resulting oropharyngeal inflammation caused by tooth crowding.
According to the Banfield Pet Hospital State of Pet Health 2011 Report, 52 68% of cats over 3 years of age are affected by dental disease. Increasing age also positively correlates with the severity of periodontal disease as measured by the intensity of gingival inflammation, quantity of calculus deposited, and the degree of bone resorption and tooth mobility.
Gastrointestinal health
A number of progressive physiologic changes involving the gastrointestinal (GI) system are appreciated in aging humans. This is despite the tremendous physiologic reserve supporting food digestion, and nutrient absorption and metabolism, which minimizes the clinical effects of many age-related changes until they are considerable. Although relatively few feline studies have focused on anatomic or functional changes, similar effects of aging may occur in cats.
Appetite can be reduced in aging cats because of many factors, including common dental issues that can result in pain, altered taste or olfaction, or compromised ability to prehend, masticate or swallow (see also ‘oral and gingival health’ above). Other possible contributing factors, based on what is known in humans, are outlined in the box below.
Morphologic and functional esophageal studies have disclosed neuronal loss and reduced amplitude of peristaltic waves in geriatric humans.59–61 Even in apparently healthy geriatric humans, inverse correlations between age, esophageal sphincter pressures, and amplitude and velocity of peristaltic esophageal waves have been shown.59,61 It is anticipated that similar changes occur in geriatric cats. These changes decrease clearance of esophageal contents and predispose to the dysphagia and gastroesophageal reflux that may be provoked by non-digestive disorders.
Reduced nutrient digestibility in humans, and also confirmed in cats (see below), associated with aging may be the result of alterations in bile composition, reduced pancreatic enzyme availability, delayed gastric emptying or altered enteric transit. 61 Rapid transit times can lead to maldigestive malabsorption, while slower transit can promote bacterial overgrowth and stool dehydration and predispose to constipation. Numerous studies in humans and senescent rodents suggest that the number of enteric neurons declines in nearly all regions of the GI tract during ‘normal’ aging. These degenerative losses involve both the myenteric and submucosal plexus, are slowly progressive and correlate with altered function (motility, coordinated contractions). 62 A study in healthy young and senior (11.6 ± 1.4 years) female cats revealed a trend for slower GI transit in the senior cats, with far more variability among individuals than observed in the young cats. 63 Indeed, a large clinical study of apparently healthy geriatric and young humans showed that only colonic transit was significantly influenced by age. 64 These findings support the proclivity for chronic constipation that has long been recognized as clinically important in aging cats. Dehydration undoubtedly also plays a role in this problem.
Advanced age is known to alter physiologic control systems regulating thirst and satiety (Figure 8), and homeostatic mechanisms controlling hydration. Although geriatric humans may consume adequate fluids on a daily basis in controlled environments, they may experience decreased thirst sensation and consequently a reduced fluid intake when challenged by fluid deprivation, a hyperosmotic stimulus or exercise in warm/humid conditions. 65 Studies show that full restoration of fluid balance is slower compared with younger individuals. Similar responses may exist in aging cats, explaining their risk for constipation when challenged with dry coarse foods, limited exercise or illnesses leading to dehydration.
Figure 8.

Aging is recognized to alter physiologic control systems regulating satiety, and influences digestibility of energy and other nutrients. © iStock/Haykirdi
Studies suggest that aging can influence digestibility of energy and other nutrients in cats.35,37,66–68 Although results among studies are inconsistent, lower apparent digestibility values for protein, fat, carbohydrate and energy in older vs younger cats have been detected.66–68 Greatest inter-individual variability was also found in old cats (13.3 ± 0.6 years) compared with younger age groups. 67 Collectively, these studies suggest that age-related changes in digestion or absorption occur, but are variable among cats. The inter-individual variability is not surprising because differences between chronologic and physiologic aging are well recognized in other species and likely exist in cats.
Studies in multiple species have suggested age-related changes in enteric microbiota, and cats are no exception (see box below). The reported reduction in bifidobacteria by Jia et al 70 may confound the GI signs related to the changes in digestion and absorption and the propensity for constipation in older cats. This work indicates that future studies should investigate microbial changes at the family level, rather than genus or species level, in order to determine whether alterations in microbiota could also impact feline weight or metabolism.
Age-related change in hepatobiliary function has been detected in humans and some other animals, but not confirmed in cats. One change that has been documented in older cats, however, is accumulation of lipofuscin in hepatocytes. Lipofuscin, often called age pigment, is considered a hallmark of aging, with accumulation inversely related to longevity. 74 Pathophysiologic mechanisms associated with lipofuscin accumulation include age-related oxidative stress and reduced ability to eliminate reactive oxygen intermediates. It is not clear, however, whether this histologic finding correlates with an age-related, clinically significant change in hepatic function in older cats.
Cardiac health
Disorders of the cardiovascular system are among the most commonly encountered disease entities in aging cats.75,76 Similar to dogs and humans, normal vascular changes including increased calcium deposition within the aorta and peripheral arteries occur with advancing age, 77 although cats tend to develop fibrosis of the intima rather than atherosclerosis. Accordingly, there is appropriate concern for the stress that this might place on the aging feline heart.
Routine monitoring of systolic blood pressure (SBP) is recommended as part of the geriatric health screening process for feline patients over 8 years of age (Figure 9), even when underlying causes for hypertension (eg, chronic renal disease, hyperthyroidism) are not suspected. There is published evidence to show a trend towards higher SBP as feline patients age (see box below); systemic hypertension, however, does not appear to be a normal age-associated finding.
Figure 9.
Blood pressure measurement using headphones to eliminate Doppler noise and help reduce stress to the cat. Courtesy of Amara Estrada
Auscultatory findings suggestive of underlying cardiac disease in a feline patient include murmurs, gallop rhythms and arrhythmias. Multiple studies in apparently healthy cats and cats with cardiomyopathy have repeatedly shown, however, that cardiac auscultation is insensitive for detecting underlying cardiac disease. As these studies have revealed, heart murmurs are commonly auscultated in older feline patients on routine physical examination, despite there being no evidence of underlying heart disease on subsequent echocardiographic investigation.81–84 Many of these patients appear to develop a murmur as a result of stress and anxiety producing increased blood flow through the right ventricular outflow tract. Thus, while a murmur in an older cat should not be ignored, many of these patients have physiologic murmurs that are not caused by underlying cardiac pathology.
Auscultation of an arrhythmia in an older feline patient is not as common as a murmur, and, likewise, this finding does not necessarily indicate underlying heart disease. Previous studies of healthy feline patients have shown that cats over 6 years of age have extrasystolic rhythms (atrial premature and ventricular premature complexes) recorded and auscultated more commonly than younger cats.85,86 Results of 24 h Holter monitoring tests have confirmed that healthy older cats commonly have ventricular premature complexes.85,86
A gallop rhythm is a dysrhythmia that occurs when there is reduced compliance of the left ventricle during either early or late ventricular diastolic filling. Although gallop rhythms are auscultated in many cats with myocardial disease, it is also noteworthy that older feline patients (>10 years of age) may occasionally have a gallop rhythm that is related to an aging, stiff ventricle, in the absence of significant cardiovascular disease. Reduced ventricular compliance and delayed relaxation of the left ventricle is a frequent finding in human geriatric patients; 87 while no studies specifically looking at diastolic function in geriatric cats have been published, similar changes appear to occur commonly.
Therefore, a murmur, arrhythmia or gallop rhythm does not necessarily indicate underlying heart disease and may be an age-associated change. In cats with abnormal auscultatory findings, an echocardiogram is recommended to rule out an underlying morphologic problem. In the event of a normal echocardiogram, a gallop rhythm is still clinically relevant, as age-associated changes in diastolic filling (rather than heart disease) may make these patients more sensitive to volume overload during intravenous fluid administration. A recent study evaluated sleeping and resting respiratory rates in healthy cats and cats with subclinical heart disease and determined that cats with multiple or mean sleeping respiratory rate measurements >30 breaths/minute likely warrant additional evaluation. 88
The most common cardiac disease in cats over 6 years of age is hypertrophic cardiomyopathy (HCM). Because this is a disease that causes concentric hypertrophy, thoracic radiographs are not very helpful in assessing whether the auscultatory findings are caused by physiologic murmurs or underlying cardiac pathology. One of the newer methods of screening for heart disease in both human and veterinary medicine involves use of cardiac biomarkers, specifically N-terminal pro-brain natriuretic peptide (NT-proBNP). Increased NT-proBNP levels in older human patients are confounded by several aging-related physiologic changes, including reduction in body mass and kidney function, and anemia. 89 Thus, until the effect of aging on NT-proBNP in feline patients is specifically studied, interpretation of this test may be challenging in the geriatric cat population.
Echocardiography is considered the ‘gold standard’ for diagnosing and staging feline cardiac disease. Although there are many different phenotypes of HCM, one particular pattern of hypertrophy is often seen in feline patients >10 years of age and is felt to represent an age-associated change, as opposed to actual underlying HCM. Many geriatric cats (with or without auscultatory abnormalities) have a focal area of thickening at the level of the left ventricular outflow tract. When following these patients longitudinally, there does not appear to be progression of the echographic change. The authors believe that this small septal bulge is likely related to the change in the angulation of the aortic root (which also occurs in geriatric human patients), rather than disease. 90
Respiratory health
Although aging changes in the respiratory health of geriatric humans have been well characterized, less is known about respiratory changes in old cats. In older humans, age-related changes include a decline in pulmonary elasticity, respiratory muscle strength and chest wall compliance. 91 Alterations in immune function, in pulmonary beta-adrenoceptors and in airway reactivity associated with aging have also been described. 92
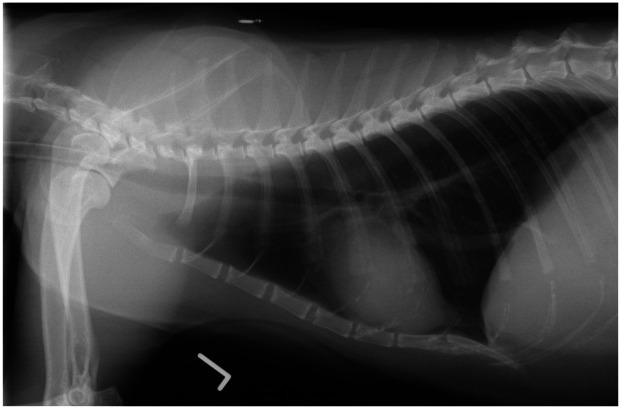
One study compared radiographic patterns and airway reactivity between groups of young (1–2 years) and senior (12–13 years) clinically healthy cats. 93 A significantly higher proportion of senior cats showed bronchointerstitial patterns on radiography when compared with the young cats. The presence of this pattern radiographically in older feline patients is thus believed to be a normal aging change (Figure 10). Additionally, airway reactivity appears to be decreased in older cats, as suggested by the finding that naturally occurring asthma is typically reported in young to middle-aged cats, and not in elderly cats. 94
Figure 10.
Left lateral thoracic radiograph of a 14-year-old domestic shorthair cat, showing a mild bronchointerstitial pattern. Courtesy of Langford Veterinary Services, University of Bristol, UK
Renal/urinary health
Aging is a commonly associated risk factor for the development of chronic kidney disease (CKD) in cats. The reported prevalence of CKD in older feline populations varies, ranging from 28% in cats >12 years 95 up to 80.9% in cats aged 15–20 years. 96 IRIS staging in a randomly selected population showed a CKD prevalence of 50% in cats up to 20 years of age, with 27.9% of affected cats in stage 1 disease, 65.1% in stage 2, 7.0% in stage 3 and 0% in stage 4. 96 This same study found a higher prevalence of CKD (68.8%) in a population of cats with DJD, with the majority of affected cats being in stage 2 (65.9%). In both the randomly selected and DJD populations, the prevalence of CKD was similar in cats up to 14.9 years, but it significantly increased after 15 years of age. In both populations, the most advanced stage seen (stage 3) was limited to cats ⩾10 years. The prevalence rates in this study were higher than previously reported, but this may partially be due to the inclusion of earlier stages of CKD than in previous reports. The underlying association between CKD and DJD is not known, but warrants further investigation to elucidate possible common immune and inflammatory mechanisms.
Among aged cats with CKD, tubulointerstitial fibrosis is the most prevalent pathology, with glomerular disease secondary to neoplasia or infectious disease seen less commonly.97–100 One study evaluating a population of cats with CKD found older cats (>7 years) to be over-represented (52.9%) compared with the total older cat hospital population (12.7%). 97 Morphologic diagnoses included chronic tubulointerstital nephritis (52.7%), renal lymphoma (16.2%), renal amyloidosis (9.5%), chronic pyelonephritis (9.5%), chronic glomerulonephritis (8.1%), polycystic renal disease (2.7%) and pyogranulomatous nephritis secondary to feline infectious peritonitis (1.3%). Infectious and familial causes were more commonly seen in younger cats, while non-specific causes were more commonly seen in the older population. Another study evaluating an older population of cats (>9 years) showed a correlation between interstitial inflammation and fibrosis scores, suggesting that these non-specific tubulointerstitial lesions could represent a slowly progressing inflammatory process resulting in eventual fibrosis. 98
While the inciting cause for non-specific tubular inflammation and fibrosis has yet to be identified, it has been suggested that these changes could represent a survival-driven adaptive process. 100 In an evaluation of 676 adult colony cats, cats that died of tubulo-interstitial renal disease (38% of the population) lived significantly longer (11.67 years) than those that died from other causes (10.0 years). Glomerular changes occurred less frequently (11% of the population), and cats with glomerular changes died at a younger age (9.96 years), compared with those without glomerular changes (12.23 years). Of the cats that died of non-renal causes, life span was greater when histological renal changes were present. Based on these findings, the investigators hypothesized that age-associated renal changes may be a defensive, life-preserving adaptation. Additional research in this area is needed to understand the association between renal changes and age, and the impact or association with other disease processes over time.
There is a significant increase in SBP with increasing age in cats with CKD. 101 When compared with cats with CKD, healthy controls had a lower risk (hazard ratio 0.2) of developing hypertension. 101 The investigators of this study defined hypertension in cats as SBP ⩾170 mmHg on two consecutive visits, or SBP ⩾160 mmHg with concurrent evidence of hypertensive retinopathy or choroidopathy on indirect fundoscopy. They found that in a population of cats that were normotensive at the time of diagnosis of CKD, 17% developed hypertension, while only 7% of the healthy controls developed hypertension over the follow-up period (3–40 months).
The concentrations of glycosaminoglycans and chondroitin sulfate in urine are negatively associated with aging in cats. 102 These polysaccharides are the products of connective tissue turnover, with the higher concentration of these substances in younger cats believed to be a reflection of their higher metabolic rate, tissue remodeling and glycosaminoglycan turnover when compared with older cats. Reduced urinary glycosaminoglycans concentrations are also observed in cats with lower urinary tract disease. 102 This reduction is believed to be driven by damage to the bladder surface, resulting in increased absorption and/or degradation of the endogenous urinary glycosaminoglycans. This is another area requiring further study to understand the significance of these changes in aging cats and cats with lower urinary tract disease.
Aging is additionally associated with a shift in the relative prevalence of struvite vs calcium oxalate uroliths in cats, with the majority of cats with struvite uroliths being under 7 years of age and the greatest risk of developing calcium oxalate uroliths being between 10 and 15 years of age.103,104 One study evaluated urine pH and relative supersaturation (RSS) in senior and younger adult cats fed the same diet. 105 Results showed that the older cats produced more acidic urine, lower struvite RSS and a higher calcium oxalate RSS when compared with the younger controls. This study demonstrates a change in both urine pH and composition as cats age, influencing the risk for specific urolith types. While the prevalence of feline calcium oxalate uroliths has been increasing over the past 20 years, the proportion of feline urethral plugs that comprise calcium oxalate has remained very low (1%), compared with struvite (92%). 104 In contrast, there has been an increase in the frequency of upper urinary tract uroliths, with 70% composed of calcium oxalate. 104 The association between upper urinary tract uroliths and kidney disease warrants further evaluation, because it is not clear if urolith formation is the cause or consequence of CKD.
Endocrine health
At the present time, there is very little information about normal age-associated changes of the endocrine system of cats. Unlike their canine counterparts, there are no published studies showing alterations in adrenal responsiveness. Pituitary, reproductive and thyroid hormones, renin, angiotensin and aldosterone concentrations, and pancreatic, hepatic, renal and GI hormone levels have likewise not been evaluated longitudinally in the healthy cat.
One paper, 106 as well as unpublished work carried out in a private clinical diagnostic laboratory (Central Lab for Veterinarians, Langley, Canada), establishing regional age-group reference intervals, showed that thyroxine (total T4) levels are low in kittens, are maintained at adult levels between 1 and 5 years of age, and increase mildly above 5 years of age (S Lester, 2013, personal communication). Studies are lacking to verify whether a gradual increase in total T4 levels is normal in cats >10 years of age; however, this may contribute to the common finding of weight loss in cats >12 years of age. 34
There are two studies evaluating age and glucose regulation in cats. When adjusted for body weight, there was no difference in insulin sensitivity between young (0.8–2.3 years) and mature (4.0–7.0 years) cats. 107 Body weight gain was more likely than feeding a dry-type diet to induce insulin resistance and dysfunctional secretion. 107 Similarly, the second study found that diet had little effect on metabolic parameters. 108 These data suggest that aging may predispose cats to insulin resistance, contributing to the risk of development of diabetes in older cats, especially if they are obese.
Hypertension is seen commonly in older cats. Age-related changes in blood pressure may be normal; however, as mentioned earlier, studies present contradictory results.77–80 There are numerous endocrine mechanisms implicated in the development of hypertension, many of which are related to disease (eg, hyperthyroidism, CKD, hyperaldosteronism). Because hypertension is associated with so many pathologies of the older cat, blood pressure measurement should be incorporated into the examination of all older cats.
Immune system health
The immune system comprises an interactive network of cells, proteins and signaling molecules designed to provide protection to the host from environmental pathogens, parasites, malignant cells, allergens and toxins. 109 It is divided into two interactive components: the innate and adaptive immune systems. 110 The innate component is non-specific and includes humoral elements such as lysozyme, complement system, phagocytic cells, and protective barriers such as the skin, GI tract and lung. The adaptive component is antigen specific and includes humoral immunity (B cell derived immunoglobulins IgG, IgA, IgM, IgD and IgE) and cell-mediated immunity (bone marrow derived macrophages and B cells, and thymus derived T cells).
Similar to that of humans and other species, the immune system of the cat is known to undergo multifactorial change with age, a process known as immunosenescence. 111 Although the body of literature describing immunosenescence in the cat is not as extensive as in many other species, there have been a number of feline-specific studies demonstrating age-related changes (see Table 2 and box on page 546).112–116
Table 2.
Immunosenescence and inflammaging in cats
| Reported age-related changes | Mean age or age range (years) ± SD |
|---|---|
| Lower total WBC, lymphocyte and eosinophil counts; lower T cells, B cells and NK cells (no difference in neutrophil, monocyte and basophil counts) 109 | 10–14 |
| Decreased mitogen response to Con A and PHA108,110,111 | 9.92 ± 0.89; 10–14; 10–14 |
| Decreased delayed-type hypersensitivity response to sRBC 108 | 9.92 ± 0.89 |
| Decreased antibody response to sRBC | No range |
| Reduced blood CD4+ T cells 110 | 10–14 |
| Reduced blood CD5+ T cells 111 | 10–14 |
| Increased serum immunoglobulins IgA and IgM 109 | 10–14 |
| Increased monocyte production of proinflammatory cytokine (IL-1β, IL-6, IL-12 p40) mRNA 112 | 8–10 |
Con A = concanavalin A; NK = natural killer; PHA = phytohemagglutinin; sRBC = sheep red blood cells; WBC = white blood cells
In addition to the changes associated with immunosenescence, there is a growing understanding of a second feature of the aging process known as inflammaging. This term has been used to describe changes in inflammatory mediators that may contribute to proinflammatory changes with age (see box on page 546).111,116
Key points
Considerable changes occur in virtually every body system of the cat with advancing age.
Some of these changes can greatly affect the health and wellbeing of the cat and have a tremendous impact on the owner, both from the standpoint of the pet–owner relationship and the cost of management.
An appreciation of normal aging in cats is critical from both a clinical and research perspective. In this regard, it is imperative to be able to distinguish between what would be considered ‘normal for age’ vs unhealthy aging changes.
Importantly, this distinction would impact clinical management of the aging cat in that the aggressiveness of the approach could be adjusted based on normal aging expectations.
Research efforts in cats could be focused on evaluating the effect of intervention on either a normal aging cat or disease processes.
This article has reviewed systems-based, age-related changes and considerations regarding health vs disease as an initial step toward defining healthy aging in the cat. Additional effort will be needed to define the clinical assessments required to determine healthy aging vs disease within each of the systems reviewed.
Acknowledgments
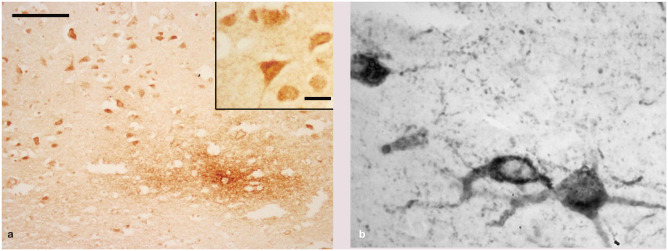
The authors are grateful to those colleagues who loaned images for this review. Professor Danièlle Gunn-Moore’s work on brain aging, from which Figure 2 is derived, was supported by Petsavers.
Figure 2.
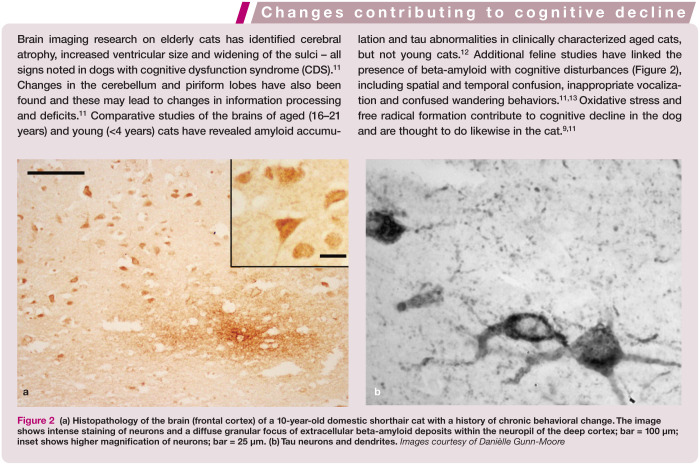
(a) Histopathology of the brain (frontal cortex) of a 10-year-old domestic shorthair cat with a history of chronic behavioral change. The image shows intense staining of neurons and a diffuse granular focus of extracellular beta-amyloid deposits within the neuropil of the deep cortex; bar = 100 μm; inset shows higher magnification of neurons; bar = 25 μm. (b) Tau neurons and dendrites. Images courtesy of Danièlle Gunn-Moore
Footnotes
Funding: This work was supported by Procter & Gamble Pet Care, Mason, Ohio, USA.
JB, SC, AHE, DFH, BDXL and MS all were paid for their contributions by Procter & Gamble Pet Care. LD, EAF, AL, SP and AKS have financial and personal interest in the Procter & Gamble Company; LD and EAF were employees of the company at the time of coauthoring this review. SP is an employee of Royal Canin, Mars Incorporated.
References
- 1. Goldston RT. Introduction and overview of geriatrics. In: Goldston RT, Hoskins JD. (eds). Geriatrics and gerontology of the dog and cat. Philadelphia: WB Saunders, 1995, pp 1–8. [Google Scholar]
- 2. Waters DJ. Aging well: how the science of aging informs the practice of wellness. Proceedings of the NAVC conference; 2008 Jan 19–23; Orlando, FL, USA, pp 4–7. [Google Scholar]
- 3. Sharma R. Theories of aging. In: Timiars PS. (ed). Physiological basis of aging and geriatrics. Boca Raton, FL: CRC Press, 1994, pp 37–46. [Google Scholar]
- 4. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300. [DOI] [PubMed] [Google Scholar]
- 5. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 1998; 78: 547–581. [DOI] [PubMed] [Google Scholar]
- 6. Pittari J, Rodan I, Beekman G, et al. American Association of Feline Practitioners: senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vogt AH, Rodan I, Brown S, et al. AAFP–AAHA: feline life stage guidelines. J Feline Med Surg 2010; 12: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Veterinary Medical Association (AVMA). Total pet ownership and pet population. In: US pet ownership & demographics sourcebook. Schaumburg, IL: AVMA, 2012, pp 25–34. [Google Scholar]
- 9. Gunn-Moore D, Moffat K, Christie LA, et al. Cognitive dysfunction and the neurobiology of ageing in cats. J Small Anim Pract 2007; 48: 546–553. [DOI] [PubMed] [Google Scholar]
- 10. Gunn-Moore D, McVee J, Bradshaw JM, et al. Ageing changes in cat brains demonstrated by beta amyloid and AT8-immunoreactive phosphorylated tau deposits. J Feline Med Surg 2006; 8: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landsberg GM, Denenberg S, Araujo JA. Cognitive dysfunction in cats: a syndrome we used to dismiss as old age. J Feline Med Surg 2010; 12: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Head E, Moffatt K, Das P, et al. Beta-amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol Aging 2005; 26: 749–763. [DOI] [PubMed] [Google Scholar]
- 13. Gunn-Moore DA. Cognitive dysfunction in cats: clinical assessment and management. Top Companion Anim Med 2011; 26: 17–24. [DOI] [PubMed] [Google Scholar]
- 14. Cummings BJ, Head E, Afagh AJ, et al. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem 1996; 66: 11–23. [DOI] [PubMed] [Google Scholar]
- 15. Cummings BJ, Satou T, Head E, et al. Diffuse plaques contain c-terminal AB42 and not AB40: evidence from cats and dogs. Neurobiol Aging 1996; 17: 653–659. [DOI] [PubMed] [Google Scholar]
- 16. Head E, Milgram NW, Cotman CW. Neuropathology and cognitive dysfunction in aging dogs. Symposium on brain aging and related behavioral changes in dogs; 2002; Orlando, FL, USA, 2002, pp 6–12. [Google Scholar]
- 17. Landsberg GM, Milgram NW, De Rivera C, et al. Age and cognitive function in the domestic cat. ACVB/AVSAB behavior symposium proceedings; 2011, pp 28–29. [Google Scholar]
- 18. Miller WH, Griffin CE, Campbell KL. Structure and function of the skin. In: Muller & Kirk’s small animal dermatology. 7th ed. St Louis, MO: Elsevier, 2013, p 49. [Google Scholar]
- 19. Case LP, Daristotle L, Hayek MG, et al. Canine and feline nutrition. 3rd ed. Maryland Heights, MO: Elsevier, 2011, p 265. [Google Scholar]
- 20. Almeida EMP, Caraça RA, Adam RL, et al. Photodamage in feline skin: clinical and histomorphometric analysis. Vet Pathol 2008; 45: 327–335. [DOI] [PubMed] [Google Scholar]
- 21. Murphy S. Cutaneous squamous cell carcinoma in the cat: current understanding and treatment approaches. J Feline Med Surg 2013; 15: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moriello KA, Klei TR, Stiller D, et al. Tumors of the skin in cats. Merck Manual. Kenilworth, NJ, USA. http://www.merckmanuals.com/pethealth/cat_disorders_and_diseases/skin_disorders_of_cats/tumors_of_the_skin_in_cats.html (2011, accessed May 20, 2015). [Google Scholar]
- 23. Scarlett JM, Donoghue S, Saidla J, et al. Overweight cats: prevalence and risk factors. Int J Obes Relat Metab Disord 1994; 18 Suppl 1: S22–S28. [PubMed] [Google Scholar]
- 24. Banfield Pet Hospital. State of Pet Health 2014. Report. https://www.banfield.com/Banfield/media/PDF/Downloads/soph/Banfield-State-of-Pet-Health-Report_2014.pdf (2014, accessed April 14, 2016).
- 25. Shoveller AK, DiGennari J, Lanman C, et al. Trained vs untrained evaluator assessment of body condition score as a predictor of percent body fat in adult cats. J Feline Med Surg 2014; 16: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brooks D, Churchill J, Fein K, et al. 2014 AAHA weight management guidelines for dogs and cats. J Am Anim Hosp Assoc 2014; 50: 1–11. [DOI] [PubMed] [Google Scholar]
- 27. Harper JE. Changing perspectives on aging and energy requirements: aging, body weight and body composition in humans, dogs and cats. J Nutr 1998; 128: 2627–2631. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong PJ, Lund EM. Changes in body composition and energy balance with aging. Vet Clin Nutr 1996; 3: 83–87. [Google Scholar]
- 29. Perez-Camargo G, Patil AR, Cupp CJ. Body composition changes in aging cats. Compend Contin Educ Pract Vet 2004; 26 Suppl 2A: 71. [Google Scholar]
- 30. Cupp CJ, Kerr WW, Jean-Philippe C, et al. The role of nutritional interventions in the longevity and maintenance of long-term health in aging cats. Int J Appl Res Vet Med 2008; 6: 69–81. [Google Scholar]
- 31. National Research Council. Nutrient requirements of dogs and cats. Washington, DC: The National Academies Press, 2006. [Google Scholar]
- 32. Association of American Feed Control Officials. AAFCO Manual. Minnesota: AAFCO, 2010. [Google Scholar]
- 33. Bermingham EN, Weidgraaf K, Hekman M, et al. Seasonal and age effects on energy requirements in domestic short-hair cats (Felis catus) in a temperate environment. J Anim Physiol Anim Nutr (Berl) 2013; 97: 522–530. [DOI] [PubMed] [Google Scholar]
- 34. Cupp C, Perez-Camargo G, Patil A, et al. Long-term food consumption and body weight changes in a controlled population of geriatric cats [abstract]. Compend Contin Educ Pract Vet 2004; 26 Suppl 2A: 60. [Google Scholar]
- 35. Peachey E, Harper EJ. Aging does not influence feeding behavior in cats. J Nutr 2002. Suppl 2; 132: 1735S–1739S. [DOI] [PubMed] [Google Scholar]
- 36. Taylor EJ, Adams C, Neville R. Some nutritional aspects of ageing in dogs and cats. Proc Nutr Soc 1995; 54: 645–656. [DOI] [PubMed] [Google Scholar]
- 37. Anantharaman-Barr HG, Gicquello P, Rabot R. The effect of age on the digestibility of macronutrients and energy in cats [abstract]. Proc Br Small Anim Vet Assoc 1991: 164. [Google Scholar]
- 38. Deng O, Iwazaki E, Suchy SA, et al. Effects of feeding frequency and dietary water content on voluntary physical activity in healthy adult cats. J Anim Sci 2014; 92: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 39. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int 2012; 23: 1839–1848. [DOI] [PubMed] [Google Scholar]
- 42. Freire M, Brown J, Robertson ID, et al. Meniscal mineralization in domestic cats. Vet Surg 2010; 39: 545–552. [DOI] [PubMed] [Google Scholar]
- 43. Lascelles BD, Henry JB, 3rd, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 44. Freire M, Meuten D, Lascelles D. Histopathology of articular cartilage and synovial membrane from elbow joints with and without degenerative joint disease in domestic cats. Vet Pathol 2014; 51: 968–978. [DOI] [PubMed] [Google Scholar]
- 45. Freire M, Robertson I, Bondell HD., et al. Radiographic evaluation of feline appendicular degenerative joint disease vs. macroscopic appearance of articular cartilage. Vet Radiol Ultrasound 2011; 52: 239–247. [DOI] [PubMed] [Google Scholar]
- 46. Lovelace KM. Age approximation. In: Norsworthy GD, Grace SF, Crystal MA, et al. (eds). The feline patient. 4th ed. Wiley-Blackwell, 2010, p 934. [Google Scholar]
- 47. Pot SA. Iris atrophy. In: Tilley LP, Smith FS. (eds). Blackwell’s five-minute veterinary consult: canine and feline. Wiley-Blackwell, 2011, p 714. [Google Scholar]
- 48. Lim CC, Maggs DJ. Ophthalmology. In: Little S. (ed). The cat: clinical medicine and management. Elsevier, 2012, pp 807–845. [Google Scholar]
- 49. Hullár I, Fekete S, Andrásofszky E, et al. Factors influencing the food preference of cats. J Anim Physiol Anim Nutr 2001; 85: 205–211. [DOI] [PubMed] [Google Scholar]
- 50. Kressin D. Oral examination of cats and dogs. Compend Contin Educ Vet 2009; 31: 72–85. [PubMed] [Google Scholar]
- 51. Bellows JE. Oral pathology. In: Bellow J. (ed). Feline dentistry. Blackwell, 2010, pp 101–103. [Google Scholar]
- 52. Verdon DR. Banfield releases major veterinary study showing spike in diabetes, dental disease and otitis externa. http://veterinarynews.dvm360.com/banfield-releases-major-veterinary-study-showing-spike-diabetes-dental-disease-and-otitis-externa (2011, accessed April 14, 2016).
- 53. Loo AT, Youngentob SL, Kent PF, et al. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int J Dev Neurosci 1996; 14: 881–900. [DOI] [PubMed] [Google Scholar]
- 54. Duffy VB. Variation in oral sensation: implications for diet and health. Curr Opin Gastroenterol 2007; 23: 171–177. [DOI] [PubMed] [Google Scholar]
- 55. Bhutto A, Morley JE. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care 2008; 11: 651–660. [DOI] [PubMed] [Google Scholar]
- 56. Groth K. Age-related changes in the gastrointestinal tract. Geriatr Nurs 1988; 9: 278–280. [DOI] [PubMed] [Google Scholar]
- 57. Miletic ID, Schiffman SS, Miletic VD, et al. Salivary IgA secretion rate in young and elderly persons. Physiol Behav 1996; 60: 243–248. [DOI] [PubMed] [Google Scholar]
- 58. Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. J Oral Rehabil 2007; 34: 711–723. [DOI] [PubMed] [Google Scholar]
- 59. Ferriolli E, Oliveira RB, Matsuda NM, et al. Aging, esophageal motility, and gastroesophageal reflux. J Am Geriatr Soc 1998; 46: 1534–1537. [DOI] [PubMed] [Google Scholar]
- 60. Shimamoto C, Hirata I, Hiraike Y, et al. Evaluation of gastric motor activity in the elderly by electrogastrography and the (13)C-acetate breath test. Gerontology 2002; 48: 381–386. [DOI] [PubMed] [Google Scholar]
- 61. Grassi M, Petraccia L, Mennuni G, et al. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr Hosp 2011; 26: 659–668. [DOI] [PubMed] [Google Scholar]
- 62. Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci 2007; 136: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peachey SE, Dawson JM, Harper EJ. Gastrointestinal transit times in young and old cats. Comp Biochem Physiol A Mol Integr Physiol 2000; 126: 85–90. [DOI] [PubMed] [Google Scholar]
- 64. Wade PR, Hornby PJ. Age-related neurodegenerative changes and how they affect the gut. Sci Aging Knowledge Environ 2005; 2005: 8. [DOI] [PubMed] [Google Scholar]
- 65. Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc 2001; 33: 1524–1532. [DOI] [PubMed] [Google Scholar]
- 66. Peachey SE, Dawson JM, Harper EJ. The effect of ageing on nutrient digestibility by cats fed beef tallow-, sunflower oil- or olive oil-enriched diets. Growth Dev Aging 1999; 63: 61–70. [PubMed] [Google Scholar]
- 67. Teshima E, Brunetto MA, Vasconcellos RS, et al. Nutrient digestibility, but not mineral absorption, is age-dependent in cats. J Anim Physiol Anim Nutr (Berl) 2010; 94: e251–e258. [DOI] [PubMed] [Google Scholar]
- 68. Patil A, Cupp C, Perez-Camargo G. Incidence of impaired nutrient digestibility in aging cats. Compend Contin Educ Pract Vet 2004; 26 Suppl 2A: 72. [Google Scholar]
- 69. Ritchie LE, Steiner JM, Suchodolski JS. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol 2008; 66: 590–598. [DOI] [PubMed] [Google Scholar]
- 70. Jia J, Frantz N, Khoo C, et al. Investigation of the faecal microbiota of geriatric cats. Lett Appl Microbiol 2011; 53: 288–293. [DOI] [PubMed] [Google Scholar]
- 71. Abecia L, Hoyles L, Khoo C, et al. Effects of a novel GOS on the faecal microbiota of inflammatory bowel disease cats during a randomized, double-blind, cross-over feeding study. Int J Probiotics Prebiotics 2010; 5: 61–68. [Google Scholar]
- 72. Patil AR, Czarnecki-Maulden GC, Dowling KE. Effect of advances in age on faecal microflora of cats. FASEB J 2000; 14: A488. [Google Scholar]
- 73. Bermingham EN, Young W, Kittelmann S, et al. Dietary format alters fecal bacterial populations in the domestic cat (Felis catus). Microbiologyopen 2013; 2: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 2002; 269: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 75. Hoskins JD, McCumin DM. Geriatric care in the late 1990s. Vet Clin North Am Small Anim Pract 1997; 27: 1273–1284. [DOI] [PubMed] [Google Scholar]
- 76. Epstein M, Kuehn NF, Landsberg G, et al. AAHA senior guidelines for dogs and cats. J Am Anim Hosp Assoc 2005; 41: 81–91. [DOI] [PubMed] [Google Scholar]
- 77. Bright JM, Mears E. Chronic heart disease and its management. Vet Clin North Am Small Anim Pract 1997; 27: 1305–1329. [DOI] [PubMed] [Google Scholar]
- 78. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998; 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 79. Sparkes AH, Caney SM, King MC, et al. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–318. [DOI] [PubMed] [Google Scholar]
- 80. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Drourr LT, Gordon SG, Roland RM, et al. Prevalence of heart murmurs and occult heart disease in apparently healthy adult cats. American College of Veterinary Internal Medicine Proceedings; 2010, p 159. [Google Scholar]
- 82. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Feline Med Surg 2011; 13: 266–271.21276739 [Google Scholar]
- 83. Côté E, Manning AM, Emerson D, et al. Assessment of the prevalence of heart murmurs in overtly healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 84. Nakamura RK, Rishniw M, King MK, et al. Prevalence of echocardiographic evidence of cardiac disease in apparently healthy cats with murmurs. J Vet Cardiol 2009; 11: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ware WA. Twenty-four-hour ambulatory electrocardiography in normal cats. J Feline Med Surg 2009; 11: 293–304. [DOI] [PubMed] [Google Scholar]
- 86. Hanås S, Tidholm A, Egenvall A, et al. Twenty-four hour Holter monitoring of unsedated healthy cats in the home environment. J Vet Intern Med 1999; 13: 175–180. [DOI] [PubMed] [Google Scholar]
- 87. Ruan Q, Nagueh SF. Effect of age on left ventricular systolic function in humans: a study of systolic isovolumic acceleration rate. Exp Physiol 2005; 90: 527–534. [DOI] [PubMed] [Google Scholar]
- 88. Ljungvall I, Rishniw M, Porciello F, et al. Sleeping and resting respiratory rates in healthy adult cats and cats with subclinical heart disease. J Feline Med Surg 2014; 16: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Krim SR, Vivo RP, de Lemos JA. B-type natriuretic peptides in acute coronary syndromes: implications in an aging population. Clin Cardiol 2012; 35: 682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moon ML, Keene BW, Lessard P, et al. Age related changes in the feline cardiac silhouette. Vet Radiol Ultrasound 1993; 34: 315–320. [Google Scholar]
- 91. Dyer C. The interaction of ageing and lung disease. Chron Respir Dis 2012; 9: 63–67. [DOI] [PubMed] [Google Scholar]
- 92. Yager EJ, Ahmed M, Lanzer K, et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med 2008; 205: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hirt RA, Dederichs D, Boehler A, et al. Relationship of age, sex, body weight, and hematologic and respiratory variables with airway reactivity in adult cats. Am J Vet Res 2003; 64: 26–31. [DOI] [PubMed] [Google Scholar]
- 94. Moise NS, Wiedenkeller D, Yeager AE, et al. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986). J Am Vet Med Assoc 1989; 194: 1467–1473. [PubMed] [Google Scholar]
- 95. Bartlett PC, Van Buren JW, Neterer M, et al. Disease surveillance and referral bias in the veterinary medical database. Preventive Vet Med 2010; 94: 264–271. [DOI] [PubMed] [Google Scholar]
- 96. Marino CL, Lascelles BDX, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 98. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2012; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 99. Reynolds BS, Lefebvre HP. Feline chronic kidney disease: pathophysiology and risk factors – what do we know? J Feline Med Surg 2013; 15 Suppl 1: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lawler DF, Evans RH, Chase K, et al. The aging feline kidney: a model mortality antagonist? J Feline Med Surg 2006; 8: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bijsmans ES, Jepson RE, Chang YM, et al. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med 2015; 29: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pereira DA, Aguiar JAK, Hagiwara MK, et al. Changes in cat urinary glycosaminoglycans with age and in feline urologic syndrome. Biochim Biophys Acta 2014; 1672: 1–11. [DOI] [PubMed] [Google Scholar]
- 103. Cannon AB, Westropp JL, Ruby AL, et al. Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004). J Am Vet Med Assoc 2007; 231: 570–578. [DOI] [PubMed] [Google Scholar]
- 104. Osborne CA, Lulich JP, Kruger JM, et al. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract 2008; 39: 183–197. [DOI] [PubMed] [Google Scholar]
- 105. Smith BHE, Moodie SJ, Wensley S, et al. Differences in urinary pH and relative supersaturation values between senior and young adult cats [abstract]. J Vet Intern Med 1997; 11: 127. [Google Scholar]
- 106. Thoday KL, Seth J, Elton RA. Radioimmunoassay of serum total thyroxine and triiodothyronine in healthy cats: assay methodology and effects of age, sex, breed, heredity and environment. J Small Anim Pract 1984; 25: 457–472. [Google Scholar]
- 107. Backus RC, Cave NJ, Ganjam VK, et al. Age and body weight effects on glucose and insulin tolerance in colony cats maintained since weaning on high dietary carbohydrate. J Anim Physiol Anim Nutr 2010; 94: e318–e328. [DOI] [PubMed] [Google Scholar]
- 108. Hoenig M, Jordan ET, Glushka J, et al. Effect of macronutrients, age, and obesity on 6- and 24-h postprandial glucose metabolism in cats. Am J Physiol Regul Integr Comp Physiol 2011; 301: R1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chandra RK. Basic immunology and its application to nutritional problems. In: Forse RA, Bell S, Blackburn GL, et al. (eds). Diet, nutrition and immunity. Boca Raton: CRC Press, 1994, pp 1–8. [Google Scholar]
- 110. Hayek MG, Massimino SP, Ceddia MA. Interactions of nutrition and the aging immune system of the dog and cat. Clinical and nutritional management of senior dogs and cats. Proceedings of the World Small Animal Veterinary Association world congress; 2002 Oct 2; Granada, Spain, 2002, pp 22–28. [Google Scholar]
- 111. Day MJ. Ageing, immunosenescence and inflammageing in the dog and cat. J Comp Pathol 2010; 124: S60–S69. [DOI] [PubMed] [Google Scholar]
- 112. Campbell DJ, Rawlings JM, Koelsch S, et al. Age-related differences in parameters of feline immune status. Vet Immunol Immunopathol 2004; 100: 73–80. [DOI] [PubMed] [Google Scholar]
- 113. Hayek MG, Massimino SP, Burr JR, et al. Dietary vitamin E improves immune function in cats. In: Reinhard GA, Carey DRP. (eds). Recent advances in canine and feline nutrition. Vol 3. Iams Company, 2000, pp 555–563. [Google Scholar]
- 114. Campbell DJ, Rawlings JM, Heaton PR, et al. Insulin-like growth factor-1 (IGF-1) and its association with lymphocyte homeostasis in the ageing cat. Mech Ageing Dev 2004; 125: 497–505. [DOI] [PubMed] [Google Scholar]
- 115. Campbell DJ, Heaton PR, Pritchard DI, et al. Assessment of ex vivo responses to T-cell mitogens and oxidative stress in lymphocytes from healthy adult and senior cats. J Nutr 2006; 136: 2084S–2086S. [DOI] [PubMed] [Google Scholar]
- 116. Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J Leukoc Biol 2010; 87: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kipar A, Baptiste K, Meli ML, et al. Age-related dynamics of constitutive cytokine transcription levels of feline monocytes. Exp Gerontol 2005; 40: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]