Abstract
Practical relevance:
Feline chronic kidney disease (CKD) is frequently encountered by veterinarians. Timely diagnosis and staging may facilitate the initiation of adequate therapy and improve the prognosis for patients.
Clinical challenges:
Feline CKD is diagnosed based on the presence of compatible clinical signs and renal azotaemia, which implies that urinalysis (particularly urine specific gravity) is mandatory to confirm the diagnosis. Although the diagnosis of advanced feline CKD and associated complications is usually straightforward, based on complete blood and urine examination, all routine blood and urine tests have their limitations in detecting early CKD. Therefore, diagnosing early or non-azotaemic CKD is much more challenging. Although determination of glomerular filtration rate (GFR) would be ideal to identify early kidney dysfunction, practical limitations hamper its routine use in clinical practice.
Patient group:
CKD is typically a disease of aged cats, but may affect cats of all ages. Conclusive breed and sex predispositions for feline CKD are not reported.
Audience:
This review is directed at practising veterinarians and provides an overview of the required diagnostic tests, the classification system established by the International Renal Interest Society, and the importance of and possible techniques for early detection of CKD.
Evidence base:
Staging of cats with CKD is essential as it directs management and provides a prognostic guide. Given that diagnosis at early disease stages is associated with more prolonged survival times, simple, inexpensive and accurate methods for early CKD diagnosis are needed. Techniques currently under investigation include limited sampling strategies to estimate GFR, clearance marker cut-off concentrations to identify cats with low GFR, new indirect GFR markers and urinary biomarkers.
Minimum database for diagnosis
Feline chronic kidney disease (CKD) is diagnosed based on the presence of combined renal azotaemia and poorly concentrated urine (urine specific gravity [USG] ?1.035), with compatible historical and/or physical examination findings.1,2 If the urinary bladder is palpable, urine can be easily collected by cystocentesis (Figure 1).
Figure 1.
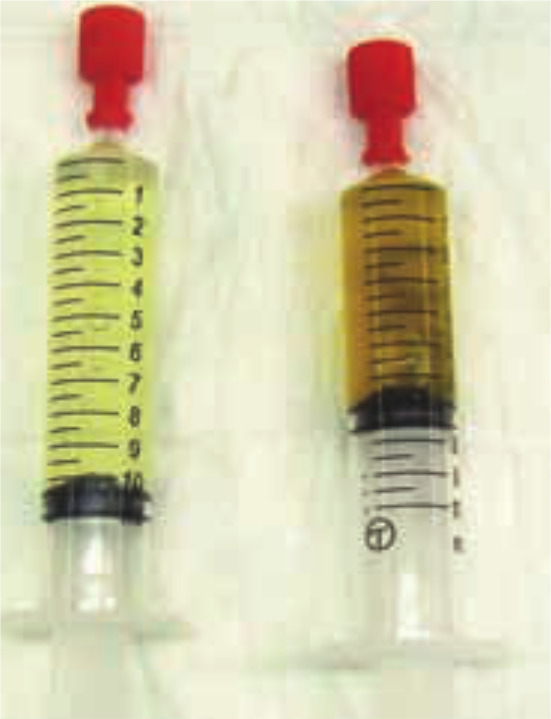
Urine samples taken by cystocentesis from (left) a cat with IRIS stage 3 CKD and (right) a healthy geriatric cat. The cat with CKD had poorly concentrated urine (USG 1.014); the healthy cat concentrated urine (USG 1.045). In the absence of bilirubinuria, the difference in urinary concentration is macroscopically visible

Signalment
The signalment may be helpful for diagnosing feline CKD. Veterinarians should have an increased index of suspicion for CKD in senior and geriatric cats. Although CKD typically is a disease of older cats and some breeds have been overrepresented in some studies, CKD may affect cats of all ages and conclusive breed and sex predispositions for feline CKD are not reported.3–6
History
Cats with CKD are presented at various stages of illness. Some are incidentally diagnosed during health screening; some demonstrate mild clinical signs; while others suffer from end-stage CKD with severe signs such as emaciation or dehydration.3,4 The illness duration prior to admission is highly variable. Although feline CKD is typically chronic, an acute history of illness is not uncommon. 3
Clinical signs
The most common clinical signs are non-specific and include inappetence, polyuria, polydipsia, weight loss, lethargy, halitosis and vomiting.3–5,7 Signs associated with nephrotic syndrome or hypertension are uncommon in cats with CKD.3,4,7 Physical examination reveals thin body condition, dehydration, periodontal disease, unkempt hair coat, abnormal findings on kidney palpation (small, irregular or enlarged kidneys) and pale mucous membranes (Figure 2).3,4
Figure 2.
Cat presenting with severe clinical signs due to acute-on-chronic kidney disease. Thin body condition (body condition score 3/9) and unkempt hair coat with scaling are visible (a,b). The cat also had pale mucous membranes and was salivating because of uraemic stomatitis and nausea (c). After stabilisation with infusion, supportive therapy (nasoesophageal tube feeding, antiemetics, antacids, analgesics) and antibiotics, the cat was discharged. Follow-up 2 weeks later revealed IRIS stage 3 CKD
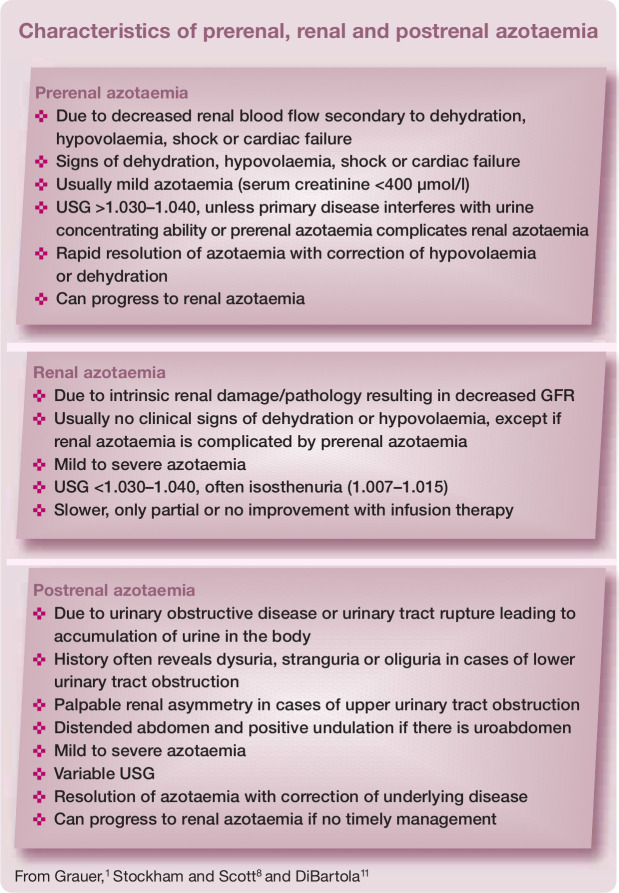
Serum creatinine
Renal azotaemia is defined as increased serum creatinine and urea concentrations due to intrinsic renal pathology (see box on the right). 8 Creatinine is more reliable than urea as an indirect marker for glomerular filtration rate (GFR) because creatinine is less influenced by extrarenal factors (eg, intestinal protein content, liver function) and undergoes glomerular filtration without tubular reabsorption. The daily production rate of creatinine depends on muscle mass, which may be of clinical importance in geriatric cats with age-related muscle wasting or when muscle mass gradually declines during CKD progression.9–11
The (modified) Jaffé and enzymatic assays are frequently used commercial creatinine assays. Both methods correlate well with a reference method. However, the Jaffé assay may overestimate low concentrations and underestimate high concentrations of feline serum creatinine. The enzymatic assay only slightly overestimates feline creatinine and appears to be the preferred method. 12 Although the (modified) Jaffé assay is being replaced gradually by enzymatic assays, many diagnostic laboratories still use this assay to measure serum creatinine concentrations. Reference intervals (RIs) for serum creatinine can differ widely between laboratories, which may lead to misclassification of samples as normal or abnormal.13,14 Given that assays and RIs often differ between laboratories, clinicians are encouraged to consistently determine serum creatinine in a single laboratory with good quality control. Development of age-dependent RIs for laboratory parameters, especially serum creatinine, would improve health screening in aged cats.9,15
Urine specific gravity
In veterinary medicine, USG is routinely measured by refractometry. Human hand-held optical refractometers can overestimate feline USG. 16 However, these errors are not clinically relevant and are unlikely to change clinical decision making. 17 Veterinary refractometers with a separate feline USG scale avoid these errors. 16 Alternatively, a conversion calculation is available (feline USG = [0.846 x SG of human refractometer] + 0.154), a formula that dates from 1956.16,17
Most cats with CKD have isosthenuric urine (USG 1.007–1.015),3,4 but this finding is less consistent compared with dogs. 18 Some cats, with spontaneous as well as with experimentally induced CKD, can retain their urine concentrating ability despite being azotaemic, particularly in the early stages of disease.3,4,19 With disease progression, USG usually gradually declines.4,20
Proteinuria – is it renal?
Although severe proteinuria is uncommon, low level proteinuria (urinary protein:creatinine ratio [UPC] <1) commonly affects feline CKD patients and is an important prognostic factor and therapeutic target.7,21–23 Therefore, quantification and longitudinal monitoring of proteinuria is very important in all cats with CKD.
Proteinuria may be prerenal, renal or postrenal in origin and persistent renal proteinuria indicates CKD. A step-wise diagnostic approach (see box on page 17) must be followed to eliminate prerenal (eg, haemoglobinuria, myoglobinuria, Bence-Jones proteinuria), postrenal urinary (eg, urolithiasis, cystitis, ureteritis, bladder or urethral neoplasia) and postrenal extraurinary proteinuria (eg, genital tract inflammation). 24
Routine tests for proteinuria
Several methods exist to evaluate whether cats with CKD are proteinuric. However, the UPC, which provides an index of total urinary protein loss and correlates closely with the gold standard of 24 h urinary protein excretion, is the only reliable method to determine the clinical implications.24,25 Because the UPC can vary depending on the methodology, monitoring of UPC requires that the same laboratory assay is consistently used. 26
In practice, dipstick tests are often used as a measure of urinary protein. Urine dipstick tests primarily measure albumin; are easy, rapid, in-house tests; and provide semiquantitative assessment of proteinuria. Unfortunately, urine dipsticks only reliably detect severe feline proteinuria. In cats with low level proteinuria, false-positive and false-negative results are common.27,28 A positive dipstick test can be confirmed by the more sensitive and specific semiquantitative sulfosalicylic acid (SSA) turbidity test, which has a lower detection limit (5 mg/dl) compared with dipstick tests (30 mg/dl). Dipstick and SSA results must be interpreted in the light of USG, because positive results in concentrated urine reflect less severe protein loss than in dilute urine.9,29 If a false-negative dipstick result is suspected, the SSA test or a species-specific microalbuminuria test (see below) can be employed.29,30
Measurement of UPC is recommended in all animals with positive semiquantitative proteinuria tests.9,24,30
(Micro)albuminuria
Microalbuminuria is defined as the presence of a small amount (1–30 mg/dl) of albumin in the urine, below the limit of detection of urinary dipstick tests.30,31 Microalbuminuria may also remain undetected by UPC determination. 28 Higher urinary albumin concentrations (>30 mg/dl) are termed (overt) albuminuria and are usually detected using urine dipstick tests or UPC.30,31 Persistent renal (micro)albuminuria may be indicative of renal disease;24,31 however, (micro)albuminuria has been observed in healthy cats and in cats with a wide variety of non-renal diseases (eg, infectious, inflammatory, endocrine, neoplastic and urinary tract disease).31–35 It is currently unknown whether microalbuminuria is a negative prognostic factor in cats, as it is in humans.31,34
(Micro)albuminuria can be measured with the urinary albumin:creatinine ratio (UAC) by quantifying urinary albumin with an enzyme-linked immunosorbent assay (ELISA) using a feline-specific albumin antibody. 31 Until now, an apparent benefit of UAC measurement over UPC has not been found.21,36,37 Alternatively, feline microalbuminuria can be detected with a commercial in-house semi-quantitative ELISA-based dipstick test (ERD-Health Screen, Heska Corporation, Fort Collins, Colorado, USA).31,32,38 Although most cats with a negative microalbuminuria dipstick test have a UPC <0.4,32,38,39 a negative microalbuminuria dipstick result does not preclude elevated UPC.32,35 A positive microalbuminuria test is very likely in feline CKD patients with a ?2+ urine dipstick result, positive SSA test or ?trace dipstick result combined with a positive SSA test. In any of these scenarios, quantification of UPC or UAC is warranted. Conversely, microalbuminuria is unlikely in CKD patients with UPC <0.2. 39
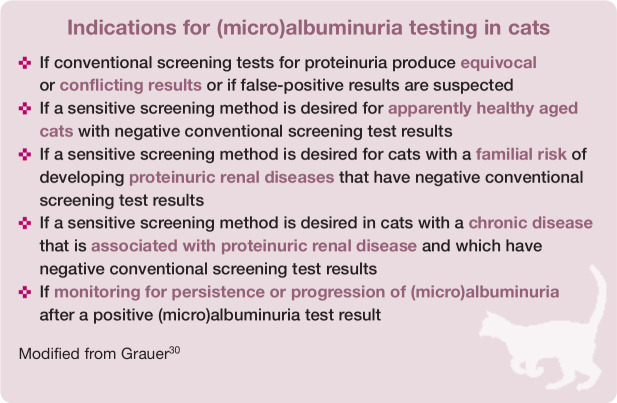
Routine evaluation for the presence of (micro)albuminuria in cats is not warranted for a number of reasons: principally, because (micro)albuminuria occurs with various diseases, UAC measurement is not widely commercially available, UAC lacks benefit over UPC, semiquantitative test interpretation might be difficult and negative microalbuminuria tests do not rule out proteinuria.9,27 However, there are some indications for (micro)albuminuria assessment, particularly in cats at risk of renal disease without overt proteinuria (see box below).9,30 It is important to remember that (micro)albuminuria is not necessarily associated with CKD and diagnostic tests to define the underlying cause are recommended.31,33
Additional diagnostic tests
Blood and urine examination
Additional blood and urine parameters need to be monitored carefully in cats with CKD, mainly to facilitate early recognition and treatment of complications.
Anaemia of renal disease Because 30–65% of cats with CKD develop anaemia during their disease course, and chronic anaemia is associated with diminished quality of life, timely diagnosis of anaemia of renal disease is important.3–6,40 The anaemia of CKD, which is usually non-regenerative, worsens with increasing severity of renal disease.4,7,20 Anaemia at diagnosis might be associated with more rapid disease progression and shorter survival, although this has not consistently been found.22,23,41
Renal secondary hyperparathyroidism and hyperphosphataemia A common complication of feline CKD, renal secondary hyperparathyroidism (RHPTH) is more common and more severe with higher degrees of renal dysfunction. 42 Higher phosphate concentration at diagnosis has been associated with shorter survival times and disease progression.6,23,41 Thus, phosphate and (preferably ionised) calcium concentrations should be assessed in cats with CKD, although these are relatively insensitive tests for detecting RHPTH. Measurement of parathyroid hormone (PTH) would provide more information regarding parathyroid status, but PTH assays are expensive, not widely available and correct sample handling is difficult.42,43 Both PTH and fibroblast growth factor 23 (FGF-23), a phosphaturic hormone secreted in response to hyperphosphataemia, progressively increase with more advanced CKD and may be increased before azotaemia develops.43–45 A recent study also found that FGF-23 was higher in hyperphosphataemic azotaemic cats compared with normophosphataemic azotaemic cats of the same IRIS stage. 44 Further studies looking at the potential value of FGF-23 for early diagnosis of RHPTH are warranted. 45
Approximately 15% of cats with mild CKD (IRIS stage 2) and up to 100% of cats with end-stage CKD have hyperphosphataemia.3,5,7,42 Hyperphosphataemia might be overlooked if the phosphate RI is based on healthy young cats, again indicating the need for age-dependent RIs. 15 Total and ionised calcium concentrations in cats with CKD vary from increased, to normal to decreased.20,42,46 Ionised calcium concentrations tend to decline with increasing severity of CKD.20,42 Because total calcium poorly predicts ionised calcium concentration, particularly in CKD patients, measurement of total calcium is of little value and ionised calcium determination is required to assess calcium status in CKD cats.42,46
Hypokalaemia Between 15% and 30% of cats with CKD, especially those at IRIS stages 2 and 3, are hypokalaemic. Hypokalaemia is less common in cats with more severe CKD, and cats with end-stage CKD (IRIS stage 4) may develop hyperkalaemia (12–22%) due to reduced potassium excretion.3–5,7,20 Monitoring of potassium concentrations is recommended for cats at all stages of CKD.
Metabolic acidosis Frequently, metabolic acidosis, characterised by decreased pH, low bicarbonate concentration, increased anion gap and/or decreased to normal chloride concentration, occurs in feline CKD patients, especially in cats with advanced disease.3,5,20 Cats with mild to moderate CKD usually maintain their acid–base balance. 20 Determination of acid–base status and appropriate treatment of acid–base disturbances is recommended in cats with advanced CKD (IRIS stages 3 and 4). Blood gas testing in cats with CKD can be performed on venous samples drawn into syringes that are coated with 1:1000 heparin or into specialised blood gas syringes containing pelleted heparin. 47 The amount of heparin in the syringe must be minimised by an evacuation technique to avoid pre-analytical errors due to dilution of the sample. 48 After sampling, the syringe must immediately be made airtight and sample analysis must be conducted within 15 mins on a bench-top analyser that has been specifically developed or validated to perform blood gas analysis in veterinary patients. 47
Bacterial UTIs Urine bacterial culture is recommended as routine at diagnosis and during follow-up of CKD, because bacterial urinary tract infections (UTIs) commonly affect cats with CKD, independent of the severity of CKD. Many cats with CKD and UTI do not show lower urinary tract signs and/or urinary sediment abnormalities (occult UTI). Therefore, a UTI may be overlooked based on history, physical examination and routine urinalysis.49–52
Blood pressure measurement
Hypertension frequently complicates CKD (20–65% of cases)53–55 and renal dysfunction is the most common underlying cause of feline hypertension (31.9–87% of cases).56–59 In humans, hypertension is considered to be both a cause and a consequence of CKD and a contributor to progressive CKD. 60 Similarly, and regardless of the underlying cause of hypertension, azotaemia is observed in many hypertensive cats.56,58,59,61 Idiopathic hypertension is diagnosed in 13–20% of hypertensive cats; however, it is uncertain whether these non-azotaemic and non-hyperthyroid cats have primary hypertension or hypertension secondary to early, subclinical, non-azotaemic CKD.57–59,62
Although an association between hypertension and progressive kidney disease is generally presumed, it remains uncertain whether feline systemic hypertension might cause CKD and what role it plays in CKD progression. 62 Nevertheless, blood pressure should be measured in all cats with kidney disease and renal function should be assessed in all hypertensive cats.58,61,63
Techniques for blood pressure measurement are reviewed in detail elsewhere.63,64 Because it is inexpensive, easy and accurate, the Doppler ultrasonic technique (Figure 3) is the most suitable method for indirect systolic blood pressure (SBP) measurement in practice. Oscillometric techniques are less accurate in cats, but may be advantageous in patients that prefer minimal restraint.63,65 Hypertension is considered and further diagnostic tests are advised if SBP, measured with a Doppler device, exceeds 160 mmHg on repeated occasions or on a single occasion with clinical manifestations of hypertension.63,64,66
Figure 3.
Systolic blood pressure measurement in cats using the Doppler ultrasonic technique; in a sitting position (a), in sternal recumbency (b) and in lateral recumbency (c). Points of note are to restrain the cat gently in a comfortable position and to hold the cuff at the level of the heart base. In cats, the authors always use headphones to avoid stress hypertension due to the sounds of the Doppler machine and to improve audibility of the Doppler sounds
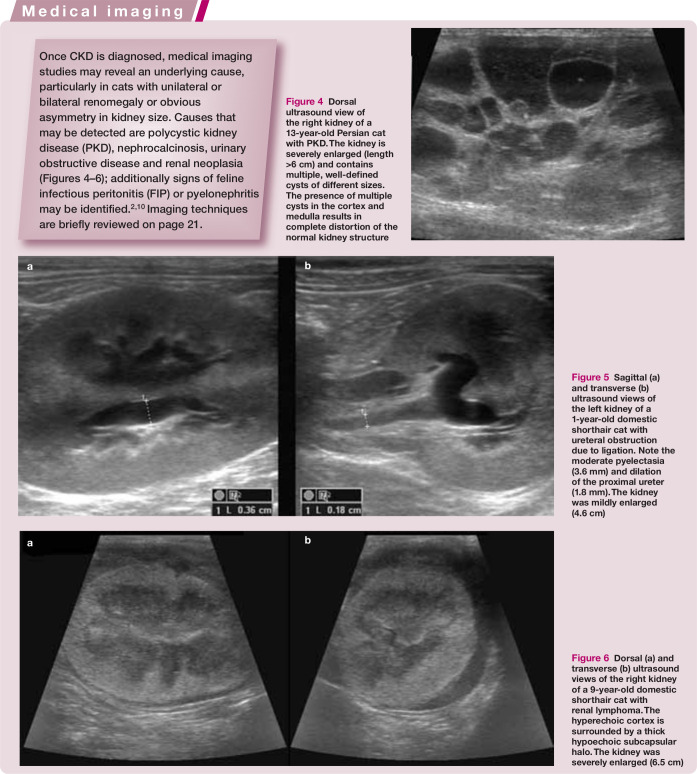
Figure 4.
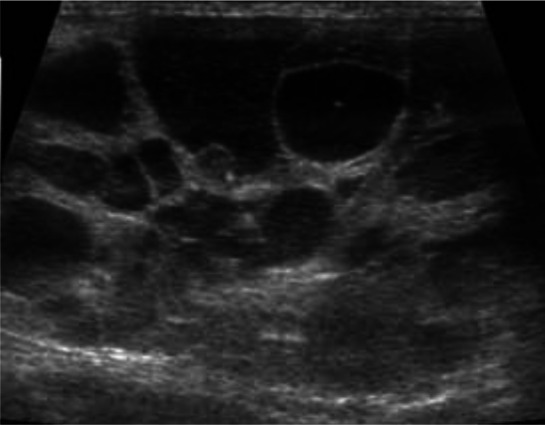
Dorsal ultrasound view of the right kidney of a 13-year-old Persian cat with PKD. The kidney is severely enlarged (length >6 cm) and contains multiple, well-defined cysts of different sizes. The presence of multiple cysts in the cortex and medulla results in complete distortion of the normal kidney structureMedical imaging
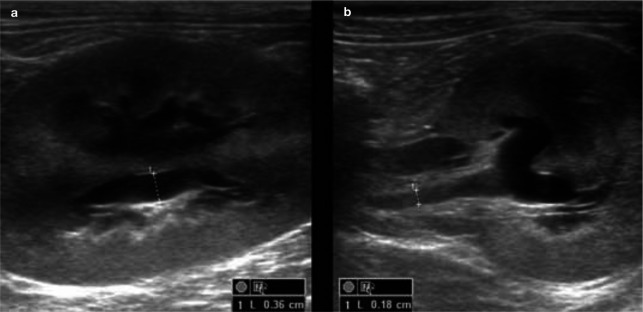
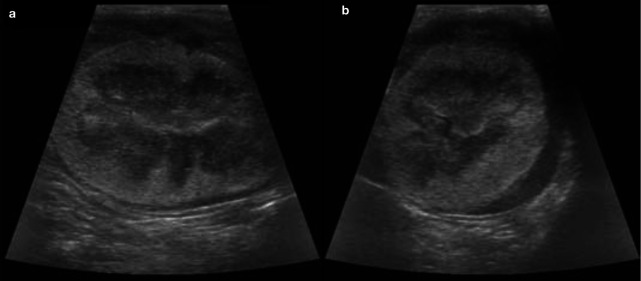
Figure 5.
Sagittal (a) and transverse (b) ultrasound views of the left kidney of a 1-year-old domestic shorthair cat with ureteral obstruction due to ligation. Note the moderate pyelectasia (3.6 mm) and dilation of the proximal ureter (1.8 mm). The kidney was mildly enlarged (4.6 cm)
Figure 6.
Dorsal (a) and transverse (b) ultrasound views of the right kidney of a 9-year-old domestic shorthair cat with renal lymphoma. The hyperechoic cortex is surrounded by a thick hypoechoic subcapsular halo. The kidney was severely enlarged (6.5 cm)
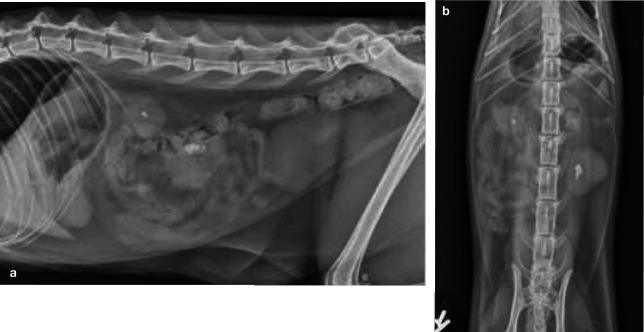
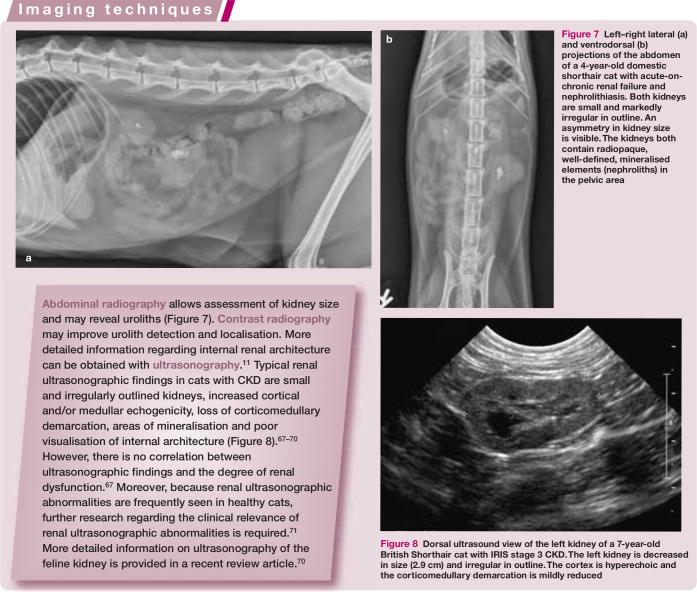
Figure 7.
Left–right lateral (a) and ventrodorsal (b) projections of the abdomen of a 4-year-old domestic shorthair cat with acute-on-chronic renal failure and nephrolithiasis. Both kidneys are small and markedly irregular in outline. An asymmetry in kidney size is visible. The kidneys both contain radiopaque, well-defined, mineralised elements (nephroliths) in the pelvic area
Figure 8.
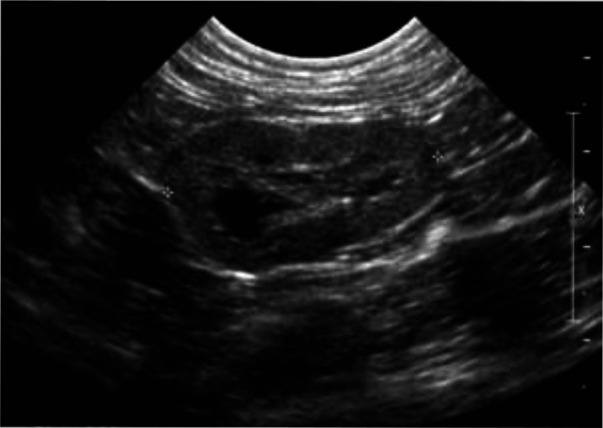
Dorsal ultrasound view of the left kidney of a 7-year-old British Shorthair cat with IRIS stage 3 CKD. The left kidney is decreased in size (2.9 cm) and irregular in outline. The cortex is hyperechoic and the corticomedullary demarcation is mildly reduced
Uncontrolled hypertension leads to end-organ damage at the level of the kidneys, heart, brain and eyes. The majority of hypertensive cats have ocular or cardiac abnormalities on physical examination.54,56–58,61,64
Glomerular filtration rate
Determination of GFR is considered to be the gold standard method for evaluation of kidney function. 72 For research purposes, plasma clearance of an intravenously administered marker, commonly iohexol or creatinine, is frequently used in cats to estimate GFR. Unfortunately, iohexol assays and injectable creatinine are not commercially available. Inulin and radioisotopes have also been used as clearance markers, but inulin assays are technically challenging and not widely available and radioisotopes require specialised equipment and carry the risk of radiation exposure.11,73 Such multi-sample techniques for GFR estimation are additionally labour-intensive, time-consuming and may be stressful or painful for the patient, which limits their practical use in cats.
However, GFR determination might be valuable for cats with doubtful renal function (eg, unexplained weight loss or polyuria/ polydipsia; IRIS stage 1 CKD patients; idiopathic hypertension; or azotaemia, poorly concentrated urine or pathological renal proteinuria as a single laboratory abnormality). GFR determination is also recommended to guide dosage adjustment for potentially toxic drugs that primarily undergo renal excretion.9,10
Several limited sampling strategies have been described to estimate feline GFR based on a reduced number of blood samples. Unfortunately, in most of the studies to date no or only few renally impaired cats were evaluated and none of the methods have been sufficiently validated in cats with CKD to be used in practice.74–82 The authors’ group is currently working on limited sampling strategies in cats with a wide range of GFR. 83
Renal biopsies
Kidney histology in cats with CKD often cannot reveal the underlying cause. However, some primary causes such as renal lymphoma, amyloidosis and FIP can usually be identified on renal biopsies, regardless of the disease stage. 3 Renal biopsy should be considered when knowledge of morphological alterations in renal structure will substantially influence patient management; for example, in cats with renal lymphoma, amyloidosis or glomerulonephritis. However, this is not true for the majority of cats that suffer from chronic generalised tubulointerstitial nephritis, glomerulosclerosis, tubular necrosis or PKD, or for cats with significant azotaemia or end-stage CKD regardless of the underlying cause.5,10
Maximum information will be obtained by evaluating kidney biopsies with light, electron and immunofluorescent microscopy, which is particularly recommended in patients with persistent severe proteinuria (UPC ?2) without severe azotaemia (IRIS stages 1 to early 3). Potential underlying diseases leading to proteinuria should be ruled out before taking kidney biopsies.29,84
Screening for early CKD
Routine health screening of aged cats is important for early detection of chronic conditions, such as CKD.15,87,88 Survival rates for cats with CKD are significantly associated with azotaemia and proteinuria, and cats diagnosed early in the disease course live longer than cats diagnosed with more severe azotaemia.6,21 So, an even better prognosis might be expected for cats diagnosed in the non-azotaemic disease stage (IRIS stage 1) because timely therapeutic intervention might prevent or delay disease progression and complications.9,89 Similarly, in humans, many adverse events of CKD such as progressive deterioration of kidney function, complications of decreased kidney function, and cardiovascular disease can be prevented or delayed by early detection and treatment.90–94
Unfortunately, diagnosis of early feline CKD is challenging. Over two-thirds of functional renal mass must be lost before kidneys lose their urine concentrating ability and over three-quarters must be lost before azotaemia develops. Thus, serum creatinine and urea concentrations and USG are often within RIs in cats with early CKD, particularly because some cats may maintain their urine concentrating ability.8,11,72
Practical, inexpensive and accurate methods to detect early feline CKD are urgently needed. Until these are available, veterinarians should improve owner awareness of early signs of CKD. Poor body condition, weight loss and polyuria/polydipsia are not always recognised by cat owners.15,95,96 Regular nutritional assessments (diet history, body weight, body and muscle condition score) may improve early detection of chronic diseases.97–99
Minimum laboratory database
The minimum laboratory database for CKD screening consists of serum creatinine, USG and proteinuria or (micro)albuminuria measurements.15,87,88 If a blood pressure device is not available, fundoscopy may be used to investigate for the presence of hypertension. 58
Physical and laboratory parameters should be compared with values obtained at previous health screenings to detect clinically relevant changes. Increasing serum creatinine concentrations, even within the RI, may indicate early kidney dysfunction, particularly in cats with weight loss or muscle wasting or USG consistently below 1.035.9,89,95 However, many factors influence USG and daily USG fluctuations can be seen in healthy animals. Thus, low USG without other indicators of CKD does not necessarily suggest kidney dysfunction.8,9,71
In a study of healthy non-azotaemic geriatric cats, plasma creatinine concentration combined with UPC was predictive of the development of azotaemia, indicating that high–normal creatinine concentrations and/or UPC values consistent with borderline or overt proteinuria might indicate early kidney dysfunction. 37
More advanced tests
More advanced tests to evaluate kidney function might be considered in cats returning doubtful routine blood and urine test results. As discussed earlier, GFR estimation would be ideal but has important practical limitations. Some of these limitations might be avoided by limited sampling strategies, but research is ongoing. Because, in daily practice, detection of early renal dysfunction is more important than knowing the exact GFR value, the authors’ group recently developed cut-off concentrations for creatinine, exo-iohexol and endo-iohexol at three time points after intravenous injection of iohexol and creatinine. These cut-off points identified cats with low GFR with high sensitivity and specificity. 103
Studies evaluating the value of serum cystatin C (sCysC) as an indirect marker of GFR in cats are also ongoing. Cystatin C, a low molecular weight protein produced at a constant rate by all nucleated cells, meets the criteria required for endogenous GFR markers. 104 Serum CysC is superior to serum creatinine for the detection of renal dysfunction in humans 104 and also has some advantages over serum creatinine in dogs. 105 Cats with CKD have higher sCysC concentrations compared with healthy cats,106,107 but evidence showing advantages of sCysC over serum creatinine in the detection of early feline CKD is currently not available.
Another pathway for identifying kidney disease involves the use of urinary biomarkers for tubular or glomerular damage.108,109 Retinol binding protein (RBP), N-acetyl-β-glucosaminidase (NAG) activity, urinary cystatin C (uCysC), transforming growth factor-β1 (TGF-β1), interleukin-8 (IL-8) and (micro)albuminuria (see above) are promising candidate urinary biomarkers for cats.31,32,36,107,109–114 Low molecular weight proteins (NAG, uCysC, RBP) and tubular enzymes (NAG) are not present in the urine of healthy animals, whereas patients with CKD might have detectable urinary concentrations secondary to tubulointerstitial damage or inflammation. Tubulointerstitial inflammation or fibrosis might also result in overexpression and increased urinary concentrations of inflammatory cytokines (TGF-β1, IL-8).108,109
In humans, careful selection of biomarkers allows detection of site-specific changes (glomerular versus tubular). 108 Whether the same is true in cats, and whether these urinary biomarkers have benefit over routine parameters in the detection of early feline CKD, is currently unknown.
Footnotes
Funding: The preparation of this article was supported by an educational grant from Boehringer Ingelheim.
The authors do not have any potential conflicts of interest to declare.
Key Points
Feline CKD is typically diagnosed based on compatible clinical signs, azotaemia and poorly concentrated urine (USG ≤1.035). The diagnosis of advanced feline CKD and associated complications is usually straightforward based on complete blood (urea, creatinine, hematocrit, electrolytes, acid–base status) and urine (USG, UPC, sediment, bacterial culture) examination. Medical imaging may identify specific underlying causes for CKD.
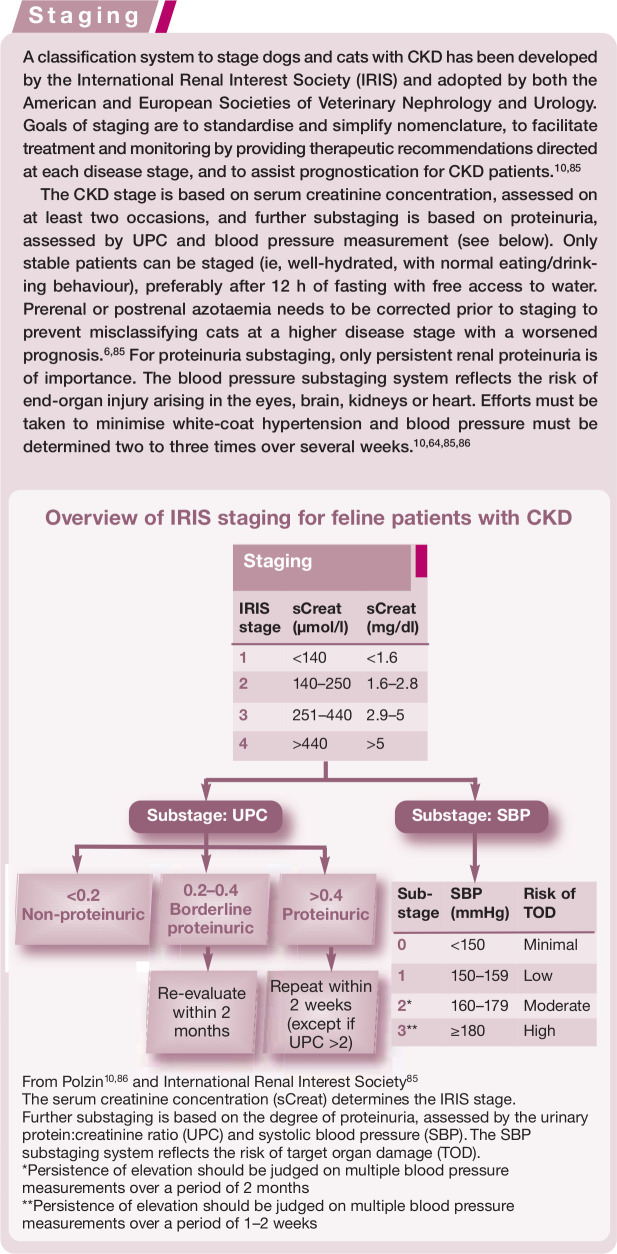
To facilitate communication, treatment, monitoring and prognostication, all CKD patients must be classified according to the IRIS system, using creatinine for staging and proteinuria and SBP for substaging.
In contrast, early or non-azotaemic CKD is more challenging to diagnose. Because early detection might improve prognosis, research regarding feline CKD should focus on the development of simple, inexpensive and accurate methods for early disease diagnosis. Possible future techniques are limited sampling strategies to estimate GFR, methods to identify cats with low GFR, new indirect GFR markers and urinary biomarkers.
References
- 1. Grauer GF. Urinary tract disorders. In: Nelson RW, Couto CG. (eds). Small animal internal medicine. 2nd ed. St Louis, Missouri: Mosby, 1998, pp 571–670. [Google Scholar]
- 2. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42: 669–692. [DOI] [PubMed] [Google Scholar]
- 3. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 9: 1196–1202. [PubMed] [Google Scholar]
- 4. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 5. Lulich JP, Osborne CA, O’Brien TD, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet 1992; 14: 127–152. [Google Scholar]
- 6. Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 7. King JN, Gunn-Moore DA, Tasker S, Gleadhill A, Strehlau G; Benazepril in Renal insufficiency in Cats Study Group. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006; 20: 1054–1064. [DOI] [PubMed] [Google Scholar]
- 8. Stockham SL, Scott MA. Urinary system. In: Stockham SL, Scott MA. (eds). Fundamentals of veterinary clinical pathology. 2nd ed. Oxford: Blackwell Publishing, 2008, pp 415–494. [Google Scholar]
- 9. Lees GE. Early diagnosis of renal disease and renal failure. Vet Clin North Am Small Anim Pract 2004; 34: 867–885. [DOI] [PubMed] [Google Scholar]
- 10. Polzin DJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 1990–2021. [Google Scholar]
- 11. DiBartola SP. Clinical approach and laboratory evaluation of renal disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 1955–1969. [Google Scholar]
- 12. Le Garreres A, Laroute V, De La Farge F, Boudet KG, Lefebvre HP. Disposition of plasma creatinine in non-azotaemic and moderately azotaemic cats. J Feline Med Surg 2007; 9: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boozer L, Cartier L, Heldon S, Mathur S, Brown S. Lack of utility of laboratory ‘normal’ ranges for serum creatinine concentration for the diagnosis of feline chronic renal insufficiency [abstract]. J Vet Intern Med 2002; 16: 354. [Google Scholar]
- 14. Ulleberg T, Robben J, Nordahl KM, Ulleberg T, Heiene R. Plasma creatinine in dogs: intra- and inter-laboratory variation in 10 European veterinary laboratories. Acta Vet Scand 2011; 53: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paepe D, Verjans G, Duchateau L, Piron K, Ghys L, Daminet S. Routine health screening. Findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George JW. The usefulness and limitations of hand-held refractometers in veterinary laboratory medicine: an historical and technical review. Vet Clin Pathol 2001; 30: 201–210. [DOI] [PubMed] [Google Scholar]
- 17. Bennett AD, McKnight GE, Dodkin SJ, Simpson KE, Schwartz AM, Gunn-Moore DA. Comparison of digital and optical hand-held refractometers for the measurement of feline urine specific gravity. J Feline Med Surg 2011; 13: 152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finco DR. Evaluation of renal functions. In: Osborne CA, Finco DR. (eds). Canine and feline nephrology and urology. 1st ed. Baltimore: Williams and Wilkins, 1995, pp 216–229. [Google Scholar]
- 19. Ross LA, Finco DR. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. Am J Vet Res 1981; 42: 1704–1710. [PubMed] [Google Scholar]
- 20. Elliott J, Syme HM, Reubens E, Markwell PJ. Assessment of acid-base status of cats with naturally occurring chronic renal failure. J Small Anim Pract 2003; 44: 65–70. [DOI] [PubMed] [Google Scholar]
- 21. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 22. Kuwahara Y, Ohba Y, Kitoh K, Kuwahara N, Kitagawa H. Association of laboratory data and death within one month in cats with chronic renal failure. J Small Anim Pract 2006; 47: 446–450. [DOI] [PubMed] [Google Scholar]
- 23. King JN, Tasker S, Gunn-Moore DA, Strehlau G; Benazepril in Renal insufficiency in Cats Study Group. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–916. [PubMed] [Google Scholar]
- 24. Lees GE, Brown SA, Elliott J, Grauer GF, Vaden SL. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 25. Adams LG, Polzin DJ, Osborne CA, O’Brien TD. Correlation of urine protein/creatinine ratio and twenty-four-hour urinary protein excretion in normal cats and cats with surgically induced chronic renal failure. J Vet Intern Med 1992; 6: 36–40. [DOI] [PubMed] [Google Scholar]
- 26. Fernandes P, Kahn M, Yang V, Weilbacher A. Comparison of methods used for determining urine protein-to-creatinine ratio in dogs and cats [abstract]. J Vet Intern Med 2005; 19: 431. [Google Scholar]
- 27. Syme H. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg 2009; 11: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyon SD, Sanderson MW, Vaden SL, Lappin MR, Jensen WA, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein-to-creatinine ratio, and species-specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc 2010; 236: 874–879. [DOI] [PubMed] [Google Scholar]
- 29. Segev G. Proteinuria. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 168–171. [Google Scholar]
- 30. Grauer GF. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet Clin North Am Small Anim Pract 2007; 37: 283–295. [DOI] [PubMed] [Google Scholar]
- 31. Langston C. Microalbuminuria in cats. J Am Anim Hosp Assoc 2004; 40: 251–254. [DOI] [PubMed] [Google Scholar]
- 32. Mardell EJ, Sparkes AH. Evaluation of a commercial in-house test kit for the semi-quantitative assessment of microalbuminuria in cats. J Feline Med Surg 2006; 8: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whittemore JC, Miyoshi Z, Jensen WA, Radecki SV, Lappin MR. Association of microalbuminuria and the urine albumin-to-creatinine ratio with systemic disease in cats. J Am Vet Med Assoc 2007; 230: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 34. Vaden SL, Turman CA, Harris TL, Marks SL. The prevalence of albuminuria in dogs and cats in an ICU or recovering from anesthesia. J Vet Emerg Crit Care 2010; 20: 479–487. [DOI] [PubMed] [Google Scholar]
- 35. Al-Ghazlat SA, Langston CE, Greco DS, Reine NJ, May SN, Schofer FS. The prevalence of microalbuminuria and proteinuria in cats with diabetes mellitus. Top Companion Anim Med 2011; 26: 154–157. [DOI] [PubMed] [Google Scholar]
- 36. Syme HM, Elliott J. Comparison of urinary albumin excretion normalized by creatinine concentration or urine specific gravity [abstract]. J Vet Intern Med 2005; 19: 466. [Google Scholar]
- 37. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–813. [DOI] [PubMed] [Google Scholar]
- 38. Syme HM, Elliott J. Semi-quantitative evaluation of protein in feline urine [abstract]. J Vet Intern Med 2005; 19: 432. [Google Scholar]
- 39. Hanzlicek AS, Roof CJ, Sanderson MW, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein-to-creatinine ratio and a feline-specific immunoassay for detection of albuminuria in cats with chronic kidney disease. J Feline Med Surg 2012; 14: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chalhoub S, Langston C, Eatroff A. Anemia of renal disease. What it is, what to do and what’s new. J Feline Med Surg 2011; 13: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 42. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108–116. [DOI] [PubMed] [Google Scholar]
- 43. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentrations in geriatric cats with various degrees of renal function. J Am Vet Med Assoc 2012; 241: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 44. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013; 27: 234–241. [DOI] [PubMed] [Google Scholar]
- 45. Finch NC, Geddes RF, Syme HM, Elliott J. Fibroblast growth factor 23 (FGF-23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013; 27: 227–233. [DOI] [PubMed] [Google Scholar]
- 46. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010; 74: 209–213. [PMC free article] [PubMed] [Google Scholar]
- 47. Kerl ME. Acid-base, oximetry, and blood gas emergencies. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 467–471. [Google Scholar]
- 48. Hopper K, Rezende ML, Haskins SC. Assessment of the effect of dilution of blood samples with sodium heparin on blood gas, electrolyte and lactate measurements in dogs. Am J Vet Res 2005; 66: 656–660. [DOI] [PubMed] [Google Scholar]
- 49. Mayer-Roenne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Feline Med Surg 2007; 9: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bailiff NL, Westropp JL, Nelson RW, Sykes JE, Owens SD, Kass PH. Evaluation of urine specific gravity and urine sediment as risk factors for urinary tract infections in cats. Vet Clin Pathol 2008; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 51. Martinez-Ruzafa I, Kruger JM, Miller RA, Swenson CL, Bolin CA, Kaneene JB. Clinical features and risk factors for development of urinary tract infections in cats. J Feline Med Surg 2012; 14: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White JD, Stevenson M, Malik R, Snow D, Norris JM. Urinary tract infections in cats with chronic kidney disease. J Feline Med Surg 2013; 15: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stiles J, Polzin DJ, Bistner SI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994; 30: 564–572. [Google Scholar]
- 54. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799–1804. [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi DL, Peterson ME, Graves TK, Lesser M, Nichols CE. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–62. [DOI] [PubMed] [Google Scholar]
- 56. Littman MP. Spontaneous hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 57. Elliott J, Barber PJ, Syme HM, Rawlings JM, Markwell PJ. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122–129. [DOI] [PubMed] [Google Scholar]
- 58. Maggio F, DeFrancesco TC, Atkins CE, Pizzirani S, Gilger BC, Davidson MG. Ocular lesions associated with hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 59. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–409. [DOI] [PubMed] [Google Scholar]
- 60. Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens 2011; Article ID: 132405. DOI: 10.4061/2011/132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chetboul V, Lefebvre HP, Pinhas C, Clerc B, Boussouf M, Pouchelon JL. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 62. Syme H. Hypertension in small animal kidney disease. Vet Clin North Am Small Anim Pract 2011; 41: 63–89. [DOI] [PubMed] [Google Scholar]
- 63. Stepien RL. Feline systemic hypertension. Diagnosis and management. J Feline Med Surg 2011; 13: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown S, Atkins C, Bagley R, Carr A, Cowgill L, Davidson M, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 65. Stepien RL. Pathophysiology of systemic hypertension and blood pressure assessment. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 577–582. [Google Scholar]
- 66. Lin CH, Yan CJ, Lien YH, Huang HP. Systolic blood pressure of clinically normal and conscious cats determined by an indirect Doppler method in a clinical setting. J Vet Med Sci 2006; 68: 827–832. [DOI] [PubMed] [Google Scholar]
- 67. Grooters AM, Biller DS. Ultrasonographic findings in renal disease. In: Bonagura JD. (ed). Kirk’s current veterinary therapy. 12th ed. Philadelphia, Pennsylvania: WB Saunders, 1995, pp 933–936. [Google Scholar]
- 68. Widmer WR, Biller DS, Adams LG. Ultrasonography of the urinary tract in small animals. J Am Vet Med Assoc 2004; 225: 46–54. [DOI] [PubMed] [Google Scholar]
- 69. d’Anjou MA. Kidneys and ureters. In: Penninck MA, d’Anjou MA. (eds). Atlas of small animal ultrasonography. 1st ed. Ames, Iowa: Blackwell Publishing, 2008, pp 339–364. [Google Scholar]
- 70. Debruyn K, Haers H, Combes A, Paepe D, Peremans K, Vanderperren K, et al. Ultrasonography of the feline kidney. Technique, anatomy and changes associated with disease. J Feline Med Surg 2012; 14: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paepe D, Bavegems V, Combes A, Saunders JH, Daminet S. Prospective evaluation of healthy ragdoll cats for chronic kidney disease by routine laboratory parameters and ultrasonography. J Feline Med Surg. Epub ahead of print 14 February 2013. DOI: 10.1177/1098612X13477415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Braun JP, Lefebvre HP. Kidney function and damage. In: Kaneko JJH, Harvey JW, Bruss ML. (eds). Clinical biochemistry of domestic animals. 6th ed. London: Elsevier; 2008: 485–528. [Google Scholar]
- 73. Von Hendy-Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J 2011; 188: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barthez PY, Chew DJ, DiBartola SP. Effect of sample number and time on determination of plasma clearance of technetium Tc 99m pentetate and orthoiodohippurate sodium I131 in dogs and cats. Am J Vet Res 2000; 61: 280–285. [DOI] [PubMed] [Google Scholar]
- 75. Barthez PY, Chew DJ, DiBartola SP. Simplified methods for estimation of 99mTc-pentetate and 131I-orthoiodohippurate plasma clearance in dogs and cats. J Vet Intern Med 2001; 15: 200–208. [DOI] [PubMed] [Google Scholar]
- 76. Goy-Thollot I, Besse S, Garnier F, Marignan M, Barthez PY. Simplified methods for estimation of plasma clearance of iohexol in dogs and cats. J Vet Intern Med 2006; 20: 52–56. [DOI] [PubMed] [Google Scholar]
- 77. Vandermeulen E, van Hoek I, De Sadeleer C, Piepsz A, Ham HR, Bosmans T, et al. A single sample method for evaluating 51chromium-ethylene diaminic tetraacetic acid clearance in normal and hyperthyroid cats. J Vet Intern Med 2008; 22: 266–272. [DOI] [PubMed] [Google Scholar]
- 78. Heiene R, Reynolds BS, Bexfield NH, Larsen S, Gerritsen RJ. Estimation of glomerular filtration rate via 2- and 4- sample plasma clearance of iohexol and creatinine in clinically normal cats. Am J Vet Res 2009; 70: 176–185. [DOI] [PubMed] [Google Scholar]
- 79. Miyagawa Y, Takemura N, Hirose H. Evaluation of a single sampling method for estimation of plasma iohexol clearance in dogs and cats with various kidney functions. J Vet Med Sci 2010; 72: 271–278. [DOI] [PubMed] [Google Scholar]
- 80. Vandermeulen E, De Sadeleer C, Piepsz A, Ham HR, Dobbeleir AA, Vermeire ST, et al. Determination of optimal sampling times for a two blood sample clearance method using 51Cr-EDTA in cats. J Feline Med Surg 2010; 12: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Katayama R, Saito J, Katayama M, Yamagishi N, Yamashita T, Kato M, et al. Simplified procedure for the estimation of glomerular filtration rate following intravenous administration of iodixanol in cats. Am J Vet Res 2012; 73: 1344–1349. [DOI] [PubMed] [Google Scholar]
- 82. Katayama M, Saito J, Katayama R, Yamagishi N, Murayama I, Miyano A, et al. A single-blood-sample method using inulin for estimating feline glomerular filtration rate. J Vet Intern Med 2013; 27: 17–21. [DOI] [PubMed] [Google Scholar]
- 83. Paepe D, Lefebvre HP, Concordet D, van Hoek I, Smets P, et al. Limited sampling strategies to estimate glomerular filtration rate in cats with low, normal and high glomerular filtration rates [abstract]. J Vet Intern Med 2010; 24: 679–680. [Google Scholar]
- 84. Vaden SL. Glomerular diseases. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, Missouri: Elsevier Saunders, 2010, pp 2021–2036. [Google Scholar]
- 85. International Renal Interest Society. IRIS staging of CKD. http://www.iris-kidney.com/pdf/IRIS2009_Staging_CKD.pdf (2009, accessed February 18, 2013).
- 86. Polzin DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 15–30. [DOI] [PubMed] [Google Scholar]
- 87. FAB (Feline Advisory Bureau). WellCat for life. A guide to engaging your clients in a lifelong partnership. In: WellCat veterinary handbook. 1st ed. Tisbury, Wiltshire, UK: International Cat Care, 2008, pp 1–30. [Google Scholar]
- 88. Vogt AH, Rodan I, Brown M, Brown S, Buffington CAT, Forman MJL, et al. AAFP–AAHA feline life stage guidelines. J Feline Med Surg 2010; 12: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grauer GF. Early detection of renal damage and disease in dogs and cats. Vet Clin North Am Small Anim Pract 2005; 35: 581–596. [DOI] [PubMed] [Google Scholar]
- 90. Maschio G, Alberti D, Janin G, Locatelli F, Mann JFE, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 1996; 334: 939–945. [DOI] [PubMed] [Google Scholar]
- 91. Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: renoprotective benefits of renin-angiotensin system inhibition. Ann Intern Med 2002; 136: 604–615. [DOI] [PubMed] [Google Scholar]
- 92. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139: 137–147. [DOI] [PubMed] [Google Scholar]
- 93. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 94. Levin A, Stevens PE. Early detection of CKD: the benefits, limitations and effects on prognosis. Nat Rev Nephrol 2011; 7: 446–457. [DOI] [PubMed] [Google Scholar]
- 95. Pittari J, Rodan I, Beekman G, Gunn-Moore D, Polzin D, Taboada J, et al. American Association of Feline Practitioners senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bartlett PC, Van Buren JW, Bartlett AD, Zhou C. Case control study of risk factors associated with feline and canine chronic kidney disease. Vet Med Int 2010; DOI: 10.4061/2010/957570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hughes KL, Slater MR, Geller S, Burkholder WJ, Fitzgerald C. Diet and lifestyle variables as risk factors for chronic renal failure in pet cats. Prev Vet Med 2002; 55: 1–15. [DOI] [PubMed] [Google Scholar]
- 98. WSAVA Nutritional Assessment Guidelines Task Force. WSAVA nutritional assessment guidelines. J Feline Med Surg 2011; 13: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Freeman LM. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med 2012; 26: 3–17. [DOI] [PubMed] [Google Scholar]
- 100. Avila A, Reche Júnior A, Kogika MM, Daniel AGT, Merlo A, Junior CAG, et al. Occurrence of chronic kidney disease in cats naturally infected with immunodeficiency virus [abstract]. J Vet Intern Med 2010; 24: 760–761. [Google Scholar]
- 101. White JD, Malik R, Norris JM, Malikides N. Association between naturally occurring chronic kidney disease and feline immunodeficiency virus infection status in cats. J Am Vet Med Assoc 2010; 236: 424–429. [DOI] [PubMed] [Google Scholar]
- 102. Baxter KJ, Levy JK, Edinboro CH, Vaden SL, Tompkins MB. Renal disease in cats infected with feline immunodeficiency virus. J Vet Intern Med 2012; 26: 238–243. [DOI] [PubMed] [Google Scholar]
- 103. Paepe D, Lefebvre HP, Concordet D, van Hoek I, Croubels S, Daminet S. Development of cut-off exo-iohexol, creatinine, endo-iohexol concentrations at three time points after intravenous iohexol and creatinine injection to predict low glomerular filtration rate in cats. Proceedings of the ACVIM Forum 2013, June 12–15, Seattle, Washington, USA, p 506. [Google Scholar]
- 104. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002; 40: 221–226. [DOI] [PubMed] [Google Scholar]
- 105. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc 2008; 44: 131–138. [DOI] [PubMed] [Google Scholar]
- 106. Poświatowska-Kaszczyszyn I. Usefulness of serum cystatin C measurement for assessing renal function in cats. Bull Vet Inst Pulawy 2012; 56: 235–239. [Google Scholar]
- 107. Ghys LFE, Meyer E, Paepe D, Delanghe J, Daminet S. Analytical validation of the particle-enhanced nephelometer for cystatin C measurement in feline serum and urine. Vet Clin Pathol. In Press 2013. [DOI] [PubMed] [Google Scholar]
- 108. Price RG. Early markers of nephrotoxicity. Comp Clin Path 2002; 11: 2–7. [Google Scholar]
- 109. De Loor J, Daminet S, Pascale S, Maddens B, Meyer E. Urinary biomarkers for acute kidney injury in dogs. J Vet Intern Med. In Press 2013. [DOI] [PubMed] [Google Scholar]
- 110. Arata S, Ohmi A, Mizukoshi F, Baba K, Ohno K, Setoguchi S, et al. Urinary transforming growth factor-β1 in feline chronic renal failure. J Vet Med Sci 2005; 67: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 111. van Hoek I, Daminet S, Notebaert S, Janssens I, Meyer E. Immunoassay of urinary retinol binding protein as a putative renal marker in cats. J Immunol Methods 2008; 329: 208–213. [DOI] [PubMed] [Google Scholar]
- 112. van Hoek I, Meyer E, Duchateau L, Peremans K, Smets P, Daminet S. Retinol-binding protein in serum and urine of hyperthyroid cats before and after treatment with radioiodine. J Vet Intern Med 2009; 23: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 113. Jepson RE, Vallance C, Syme HM, Elliott J. Assessment of urinary N-acetyl-β-D-glucosaminidase activity in geriatric cats with variable plasma creatinine concentrations with and without azotemia. Am J Vet Res 2010; 71: 241–247. [DOI] [PubMed] [Google Scholar]
- 114. Habenicht LM, Webb TL, Clauss LA, Dow SW, Quimby JM. Urinary cytokine levels in apparently healthy cats and cats with chronic kidney disease. J Feline Med Surg 2013; 15: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]