Abstract
Objectives
The aim of the study was to describe faecal consistency, prevalence and risk factors for diarrhoea and constipation in a rescue cat population.
Methods
Faecal samples in litter trays from a stratified random sample of cats in pens at 25 UK rehoming centres were scored for consistency in two discrete time periods, summer and winter. A six-point scale was used, with diarrhoea ⩽3, severe diarrhoea ⩽2 and constipation as 6. The effect on faecal consistency of age, number of cats per pen and season was investigated using multivariable hierarchical logistic regression with centre and pen as random effects. Intraclass correlation coefficients were used to estimate the effect of pen and centre.
Results
Overall, 11.9% (95% confidence interval [CI]:10.4–13.7) of cats had diarrhoea, 2.4% (95% CI 1.6–3.7) had severe diarrhoea and 5.6% (95% CI 4.2–7.5) were constipated. The prevalence of diarrhoea (median 11.0%, interquartile range [IQR] 5.0–14.5%) and constipation (median 4.2%, IQR 1.8–5.9) varied at the centre level. Diarrhoea was associated with being a kitten (odds ratio [OR] 2.54, 95% CI 1.45–4.46; P = 0.001) and being in a multi-cat pen (OR 1.24, 95% CI 1.04–1.48; P = 0.02) but not with season (OR 0.99, 95% CI 0.55–1.77; P = 0.96). Severe diarrhoea was associated with senior cats (OR 4.66, 95% CI 1.25–17.44; P = 0.02). Constipation was associated with increasing age (OR 1.01; 95% CI 1.00–1.01; P = 0.02) and winter (OR 0.43, 95% CI 0.21–0.89;P = 0.02). Both diarrhoea and constipation showed moderate correlation with pens within a centre.
Conclusions and relevance
From IQRs, we suggest acceptable levels for diarrhoea and constipation of 11% and 4%, respectively, targets of 5% and 2%, and intervention at 15% and 6%. Increasing age was associated with decreased risk of diarrhoea and increased risk of constipation. However, severe diarrhoea was associated with being a senior cat. Season (winter) was a risk factor for constipation; multi-cat pens were a risk factor for diarrhoea. Describing the prevalence and risk factors for diarrhoea and constipation in cats will assist their management in this population. Understanding and managing constipation may be more important than interventions to reduce severe diarrhoea.
Introduction
Faecal consistency is an important indicator of gut health. Scoring systems have been developed for humans and animals,1–6 but there are few published quantitative data on the distribution of faecal consistency scores in any population. Feline diarrhoea and constipation are cited as common clinical problems,7–9 but their prevalence in the general cat population is unknown. Surveillance data from referred populations indicates that diarrhoea accounts for 1.8% (95% confidence interval [CI] 1.6–2.0%), 10 3.2% (95% CI 2.6–3.8) 11 and 2.6% (95% CI 2.1–3.1) 12 of the reasons for veterinary consultations. There are no data describing the frequency of constipation.10,12
Rescue and re-homing shelters offer the opportunity to present population-based data on feline health. To date, this has focused on seroprevalence studies,13,14 the detection of infectious agents15–18 and feline behaviour.19,20 Here we report representative data on the distribution of faecal scores in cat populations in 25 rehoming centres or shelters in the UK, and the influence of age, season, and multi-cat pens on faecal consistency.
Materials and methods
Study population and design
The study population and design have been described in detail elsewhere, for investigation of the prevalence and genotypic diversity of rotavirus in UK cats. 18 Briefly, the population comprised cats held in the 25 UK rehoming or adoption centres run by the UK’s largest feline welfare charity, Cats Protection. These centres are widely distributed geographically (Figure 1), and vary in size and construction, accommodating between 12 and 202 cat accommodation units (pens). 18
Figure 1.
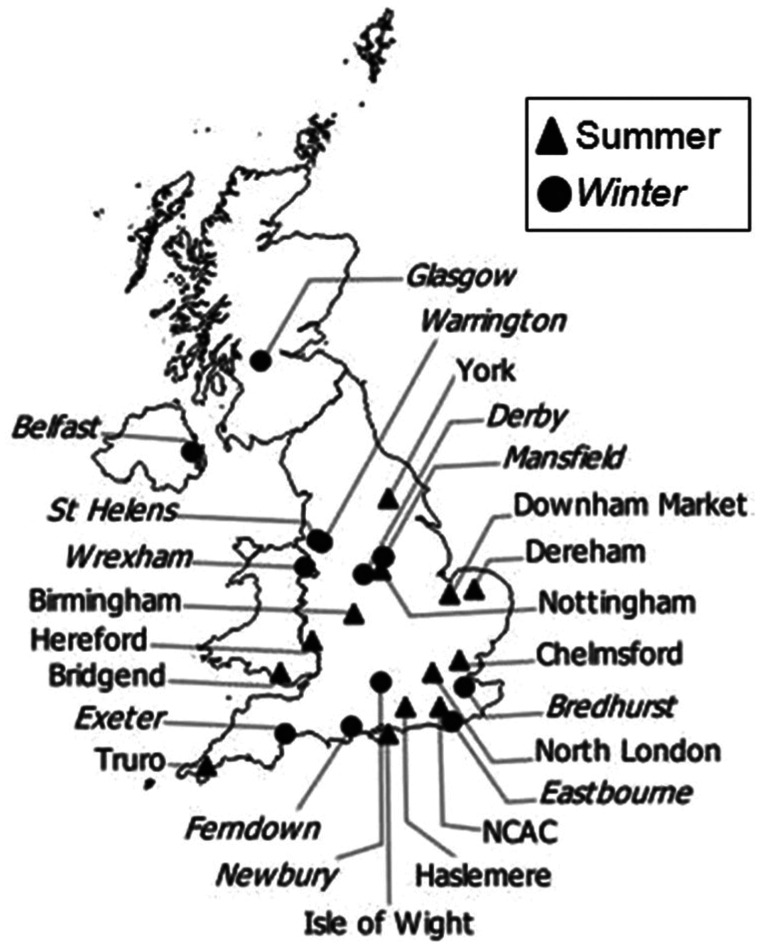
Distribution of Cats Protection adoption centres across the UK, indicating in which season faecal collection was performed. NCAC = National Cat Adoption Centre
Two cross-sectional studies were undertaken, in winter (3 February to 30 March) and summer (29 May to 17 August) 2012, to account for seasonal breeding and the consequent changes in demography. Centres were stratified by size (small, medium and large) and randomly allocated to the two collection periods (Figure 1). 18 This gave 12 centres for study in the winter and 13 for study in the summer.
The unit of sampling was the pen. Cats were housed individually unless they presented at the centre as a compatible pair, or as a related group (eg, a litter of kittens with queen). In a few centres, long-stay cats were kept together in a group of more than two. All pens containing at least one kitten and a random sample of those housing one or more adult cats were selected. Kittens were defined as cats aged <6 months. The sample size was calculated to allow one case of any faecal score to be detected at or above a prevalence of 2%, with 95% confidence and 1.3–1.9% precision.
Recording sheets were used to transcribe exposure data from a number of sources, including the centre admission records, pen data recording sheets, veterinary records, Cats Protection database (‘PAWS’: an outsourced software programme [Claws, https://www.advancednfp.com/downloads/product-brochures/claws-product-brochure.aspx] with Cats Protection internal data) and from observation of pen content.
Sample collection and processing
Faecal samples were collected from litter trays and, where necessary, the pen floor by two operators between 6.30 am and 12.00 pm (noon) on the first day of the study visit; where faeces were not present, cats were monitored for the duration of the visit. In dual occupancy or multi-cat pens (if cats were not directly observed to defaecate), when the number of faecal deposits was equal to the number of cats in the pen, deposits were randomly assigned to each cat. This was based on the premise that most healthy cats usually defaecate no more than once overnight. Kitten stools were differentiated from adult ones based on diameter/size. In multiple occupancy pens, when the number of faecal deposits exceeded the number of cats, each deposit was sampled and, where necessary, recorded as ‘pooled sample from more than one cat’. In single-cat pens with multiple deposits, all deposits were sampled and recorded as ‘pooled sample from an individual’. Faecal consistency was graded 1 (watery) to 6 (hard, dry) using the Cats Protection Faecal Scoring System (Figure 2), a modified version of the Bristol Stool Scale (Meyers Scale). 1
Figure 2.
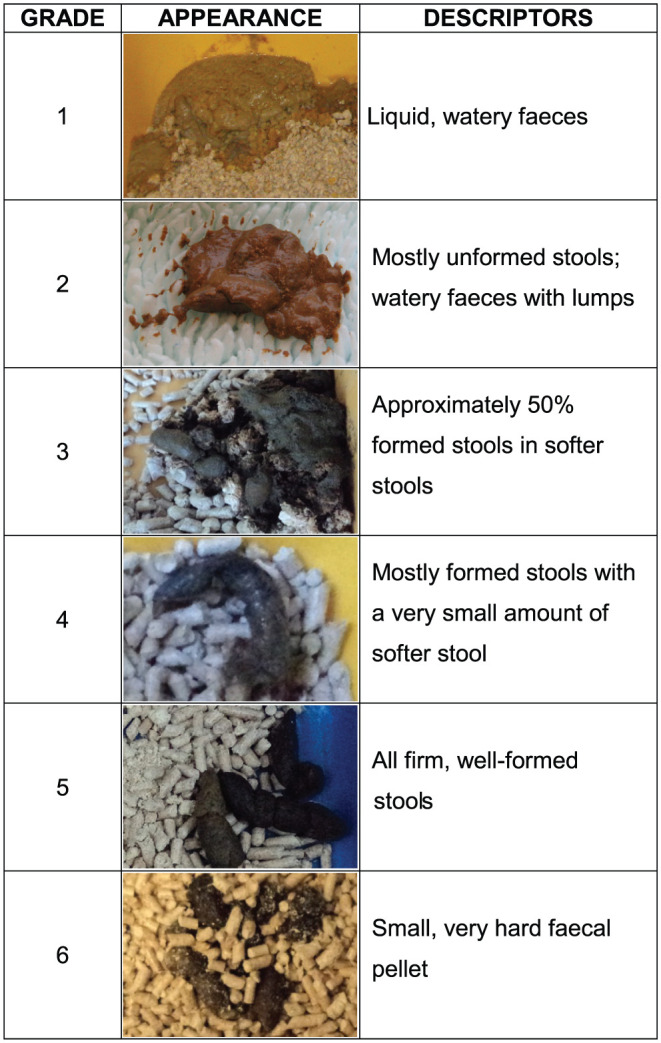
Cats Protection Faecal Scoring System. Copyright Cats Protection. Used with permission
Statistical analysis
Prevalence of individual faecal scores was estimated using the svy commands in STATA (StataCorp) to adjust for stratification by season and clustering by centre and pen. Sampling weights were adjusted for those cats that did not defaecate on the day of collection.
Two definitions of diarrhoea were used: a faecal score of ⩽3 (diarrhoea) and a faecal score of ⩽2 (severe diarrhoea). Constipation was defined as a faecal score of 6.
Using the melogit commands in STATA, hierarchical uni- and multivariable logistic regressions were used to examine associations between diarrhoea or constipation and centre, pen, age and season. Odds ratios (OR) and 95% CI were calculated. Intraclass correlation coefficients were estimated using the estaticc command. Age was modelled as a continuous and as two binary variables: kittens and senior cats. These were offered to separate multivariable models. Kittens were cats <6 months old and senior cats were ⩾11 years (132 months), as suggested by the American Association of Feline Practitioners–American Animal Hospital Association guidelines. 21
Ethical approval
This study was approved by the University of Liverpool Veterinary Research Ethics Committee (VREC20) and the Cats Protection ethical review committee.
Results
Population
In total, 1727 faecal samples were collected and scores were available for 97.6% (1686/1727) of them. Overall, 33.5% of the population were kittens (565/1686; 95% CI 28.2–39.2). The proportion of kittens was much greater in the summer than in winter months; 47.6% in summer (485/1019; 95% CI 42.2–53.1) and 12.0% (80/667; 95% CI 8.4–1.9) in winter (P <0.01). The proportion of senior cats was 7.7% overall (127/1657; 95% CI 6.0–9.7); 10.6% in winter (68/644; 95% CI 8.1–13.6) and 5.8% (59/1013; 95% CI 3.9–8.6) in summer.
Faecal scores
The distribution of faecal scores is shown in Table 1. The majority of samples were faecal score 5.
Table 1.
Overall distribution of faecal scores
| Faecal score | Frequency | Percent | 95% CI |
|---|---|---|---|
| 1 | 8 | 0.5 | 0.2–1.0 |
| 2 | 33 | 2.0 | 1.3–2.9 |
| 3 | 160 | 9.5 | 8.3–10.8 |
| 4 | 344 | 20.4 | 17.7–23.4 |
| 5 | 1046 | 62.0 | 58.8–65.2 |
| 6 | 95 | 5.6 | 4.2–7.5 |
| Total | 1686 | 100.0 |
CI = confidence interval
Prevalence of diarrhoea
When adjusted for stratification by season and centre, and clustering by pen, the estimated prevalence of diarrhoea ⩽3 in the overall cat population was 11.9% (95% CI 10.4–13.7) and that of diarrhoea ⩽2 was 2.4% (95% CI 1.6–3.7). When individual centres were considered, the prevalence of diarrhoea ⩽3 varied from 0–22.6% (Table 2). The median was 11.0% (interquartile range [IQR] 5.0–14.5%). There was a statistical difference in the prevalence of diarrhoea ⩽3 between centres (P = 0.03).
Table 2.
Prevalence of diarrhoea at individual rescue centres
| Centre ID | Summer (S) or winter (W) | Total cats | Diarrhoea ⩽3 |
||
|---|---|---|---|---|---|
| n | % | 95% CI | |||
| 18 | S | 8 | 0 | 0.0 | NE |
| 24 | W | 27 | 1 | 3.7 | 1.9–7.2 |
| 6 | S | 24 | 1 | 4.2 | 2.1–8.0 |
| 7 | W | 68 | 3 | 4.4 | 2.9–6.8 |
| 22 | W | 23 | 1 | 4.4 | 2.8–6.7 |
| 14 | W | 129 | 6 | 4.7 | 3.9–515 |
| 11 | S | 38 | 2 | 5.3 | 2.9–9.5 |
| 25 | S | 43 | 3 | 7.0 | 3.9–12.2 |
| 20 | W | 24 | 2 | 8.3 | 4.7–14.3 |
| 15 | W | 43 | 4 | 9.3 | 6.2–13.7 |
| 9 | W | 90 | 9 | 10.0 | 7.9–12.6 |
| 5 | W | 28 | 3 | 10.7 | 7.0–16.1 |
| 4 | S | 210 | 23 | 11.0 | 9.5–12.6 |
| 17 | S | 85 | 10 | 11.8 | 9.9–13.9 |
| 2 | S | 175 | 21 | 12.0 | 10.3–13.9 |
| 16 | W | 31 | 4 | 12.9 | 9.0–18.2 |
| 13 | W | 46 | 6 | 13.0 | 10.7–15.8 |
| 10 | S | 57 | 8 | 14.0 | 9.5–20.2 |
| 21 | W | 97 | 14 | 14.4 | 12.1–17.2 |
| 8 | S | 75 | 11 | 14.7 | 12.1–17.7 |
| 23 | W | 61 | 10 | 16.4 | 12.7–20.9 |
| 1 | S | 168 | 30 | 17.9 | 14.7–21.6 |
| 12 | S | 34 | 7 | 20.6 | 17.9–23.5 |
| 3 | S | 71 | 15 | 21.1 | 19.0–23.4 |
| 19 | S | 31 | 7 | 22.6 | 14.1–34.2 |
Data are presented in order of ascending prevalence
CI = confidence interval; NE = not estimated
Risk factors for diarrhoea
Diarrhoea ⩽3 was associated with age and number of cats per pen in univariable analysis. The odds of diarrhoea ⩽3 decreased with age, with an OR of 0.99 (95% CI 0.99–1.0; P = 0.04) for every month increase in age. This relationship was non-linear and the effect was more evident when the binary variable ‘kitten’ was used to compare cats aged <6 months with the rest of the population. The OR was 3.5 (95% CI 2.1–5.7; P <0.001). There was no association between diarrhoea ⩽3 and being a senior cat (OR 1.3, 95% CI 0.58–2.89; P = 0.53). The more cats per pen the greater the odds of having diarrhoea ⩽3. The OR was 1.42 (95% CI 1.20–1.68; P <0.001) for each additional cat. There was no association between the summer season and diarrhoea ⩽3 when compared with winter (OR 1.6, 95% CI 0.96–2.6; P = 0.07). When adjusted for confounding, diarrhoea ⩽3 was associated with being a kitten and the number of cats per pen, but not with season, being a senior cat or age modelled as a continuous variable (Table 3).
Table 3.
Risk factors for diarrhoea ⩽3 identified by multivariable analysis
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Fixed effects | |||
| Kitten | 2.54 | 1.45–4.46 | 0.001 |
| Cats per pen | 1.24 | 1.04–1.48 | 0.02 |
| Season (summer vs winter) | 0.99 | 0.55–1.77 | 0.96 |
| Random effects | |||
| Centre | 0.07 | 0.003–1.490 | |
| Centre >pen | 3.42 | 1.89–6.17 |
OR = odds ratio; CI = confidence interval
In contrast, diarrhoea ⩽2 was not associated with age (OR 1.0, 95% CI 0.99–1.01; P = 0.61), being a kitten (OR 1.8, 95% CI 0.69–4.49; P = 0.24), number of cats per pen (OR 1.24, 95% CI 0.92–1.67; P = 0.16) or season (OR 1.2, 95% CI 0.3–3.8; P = 0.87). However, it was associated with being a ‘senior’ cat (OR 3.95, 95% CI 1.15–13.65; P = 0.03). When adjusted for confounding, senior cats remained significant (Table 4).
Table 4.
Risk factors for diarrhoea ⩽2 identified by multivariable analysis
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Fixed effects | |||
| Senior cats | 4.66 | 1.25–17.44 | 0.02 |
| Cats per pen | 1.30 | 0.95–1.77 | 0.11 |
| Season (summer vs winter) | 0.92 | 0.21–3.98 | 0.91 |
| Random effects | |||
| Centre | 1.42 | 0.34–5.99 | |
| Centre >pen | 4.58 | 1.64–12.8 |
OR = odds ratio; CI = confidence interval
The effects of clustering of populations by centre and by pen within centre (Centre and Centre >pen in Tables 3 and 4) on the occurrence of diarrhoea was investigated using the residual intraclass correlation coefficients (ICC) obtained after developing the multilevel models for diarrhoea ⩽3 and diarrhoea ⩽2 (Table 5).
Table 5.
Residual intraclass correlation coefficients (ICC)
| Diarrhoea ⩽3 |
Diarrhoea ⩽2 |
|||||
|---|---|---|---|---|---|---|
| Level | ICC | SE | 95% CI | ICC | SE | 95% CI |
| Centre | 0.010 | 0.016 | 0.001–0.184 | 0.153 | 0.092 | 0.043–0.421 |
| Pen/centre | 0.515 | 0.074 | 0.372–0.655 | 0.646 | 0.110 | 0.415–0.824 |
CI = confidence interval
There was moderate correlation between diarrhoea and cats in the same pen within a centre (51.5% and 64.6% for diarrhoea ⩽3 and diarrhoea ⩽2, respectively) but poor correlation with being in the same centre (0.1% and 15.3%, respectively).
Prevalence of constipation
The prevalence of constipation was 5.6% (95% CI 4.2–7.5). The prevalence in individual centres varied from 0–19.7% (P <0.001) (Table 6). The median prevalence was 4.2% (IQR 1.8–5.9).
Table 6.
Prevalence of constipation in different centres
| Centre ID | Summer (S) or winter (W) | Total cats | Constipation |
||
|---|---|---|---|---|---|
| n | % | 95% CI | |||
| 3 | S | 71 | 0 | 0 | NE |
| 12 | S | 34 | 0 | 0 | NE |
| 13 | W | 46 | 0 | 0 | NE |
| 16 | W | 31 | 0 | 0 | NE |
| 18 | S | 8 | 0 | 0 | NE |
| 25 | S | 43 | 0 | 0 | NE |
| 10 | S | 57 | 1 | 1.8 | 0.6–4.9 |
| 17 | S | 85 | 2 | 2.4 | 1.6–3.4 |
| 19 | S | 31 | 1 | 3.2 | 1.3–7.7 |
| 4 | S | 210 | 7 | 3.3 | 2.7–4.1 |
| 5 | W | 28 | 1 | 3.6 | 2.0–6.4 |
| 8 | S | 75 | 3 | 4.0 | 3.0–5.4 |
| 6 | S | 24 | 1 | 4.2 | 2.1–8.0 |
| 20 | W | 24 | 1 | 4.2 | 1.9–9.1 |
| 9 | W | 90 | 4 | 4.4 | 3.1–6.4 |
| 1 | S | 168 | 8 | 4.8 | 3.6–6.4 |
| 11 | S | 38 | 2 | 5.3 | 3.2–8.6 |
| 2 | S | 175 | 10 | 5.7 | 4.8–6.8 |
| 7 | W | 68 | 4 | 5.9 | 4.1–8.5 |
| 21 | W | 97 | 6 | 6.2 | 4.7–8.1 |
| 24 | W | 27 | 2 | 7.4 | 4.5–12.0 |
| 15 | W | 43 | 5 | 11.6 | 8.9–15.4 |
| 22 | W | 23 | 3 | 13.0 | 10.2–16.6 |
| 14 | W | 129 | 22 | 17.1 | 15.3–18.9 |
| 23 | W | 61 | 12 | 19.7 | 15.7–24.4 |
Data are presented in ascending order of prevalence
CI = confidence interval; NE = not estimated
Risk factors for constipation
In univariable analysis, constipation was associated with age and season but not with the number of cats per pen. The odds increased with age (OR 1.01, 95% CI 1.00–1.01; P = 0.005) for every monthly increase in age. Kittens were at a reduced risk of constipation (OR 0.24; 95% CI 0.11–0.55; P = 0.001).
The mean age of constipated cats was 63.1 months (95% CI 53.1–73.0) compared with 40 months (95% CI 35.4–46.34) for cats with a faecal score <6 (P <0.001). Based on these data, a binary variable was created using the average of 50 months of age as a cut-off; the odds of older cats being constipated was 2.12 (95% CI 1.11–4.05; P = 0.02). However, senior cats (>11 years), showed no increase in odds of being constipated (OR 1.14, 95% CI 0.40–3.29; P = 0.81) when compared with the rest of the population.
Summer months were associated with a reduced risk of constipation (OR 0.36, 95% CI 0.17–0.78; P = 0.01). There was no association between constipation and the number of cats per pen (OR 0.86, 95% CI 0.67–1.12; P = 0.26).
When adjusted for confounding, both season and age remained in the multivariable model. This was seen consistently when age was represented as a continuous variable, as kittens <6 months (OR 0.26, 95% CI 0.11–0.64; P = 0.003) or cats >50 months (OR 1.86; 95% CI 1.0–3.47; P = 0.05). The data for age, season and cats per pen are shown in Table 7.
Table 7.
Risk factors for constipation identified by multivariable analysis
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Fixed effects | |||
| Age | 1.01 | 1.00–1.01 | 0.02 |
| Season (summer vs winter) | 0.43 | 0.21–0.89 | 0.02 |
| Cats per pen | 0.94 | 0.75–1.18 | 0.59 |
| Random effects | |||
| Centre | 0.19 | 0.02–1.63 | |
| Centre >pen | 3.31 | 1.13–9.67 |
OR = odds ratio; CI = confidence interval
The residual ICC indicated that constipation was moderately correlated with being in the same pen within a centre (ICC 0.515, 95% CI 0.276–0.748) but poorly correlated with being in the same centre (ICC 0.028, 95% CI 0.003–0.204).
Discussion
It is often stated that diarrhoea is common in cats housed in animal shelters, 22 but there are few representative data on the distribution of feline faecal scores in rescue catteries. In this cross-sectional study of 1727 cats in 25 rescue catteries in the UK, the overall prevalence of diarrhoea was 11.9% (95% CI 10.2–13.7). Severe diarrhoea, classed as ⩽grade 2, was only observed in 2.4% (95% CI 1.6–3.7) of cats. This is considerably less than the overall estimates of 28.6% (95% CI 22.9–34.6) and 53.6% (95% CI 47.0–60.1) reported by Bybee et al. 22 These authors used a seven-point faecal score, where 1/7 described hard dry faeces and 7/7 watery diarrhoea. However, diarrhoea was defined as score ⩾4, which, by descriptors, incorporated an equivalent to our ‘normal’ score of 4/6 (Figure 2 and Table 1). Conversely, the prevalence from our study was more than that recorded in a study of Campylobacter species in a rescue cattery, where only one of the 58 cats sampled (1.7%, 95% CI 0.09–8.20) was reported to have diarrhoea, and no faecal scoring system was used. 17
Surprisingly, the prevalence of constipation (5.6%, 95% CI 4.2–7.5) was more than double that of severe diarrhoea (2.4%, 95% CI 1.6–3.7), suggesting that, in numerical terms, constipation may be a greater problem in this population than severe diarrhoea. Constipation is defined as infrequent or difficult evacuation of dry, hard faeces. 23 In this study, voiding dry, hard-grade faeces was used as a measure of constipation; neither frequency nor ease of defaecation was recorded. In humans, faecal consistency is correlated with gut transit time and constipation,1,24 and it seems reasonable to assume that this is also the case in cats.
Three potential risk factors for diarrhoea and constipation were examined: age, number of cats per pen and season. The influence of the individual centre and individual pens within a centre was also examined using ICCs. The use of hierarchical logistic regression models is a valuable way of identifying the variance associated with each hierarchical level.25–27
Being a kitten (OR 2.54, 95% CI 1.45–4.46; P = 0.001) and living in a multi-cat pen (OR 1.24, 95% CI 1.04–1.48; P = 0.02) were associated with diarrhoea. There was also a moderate correlation with being in the same pen within a centre (ICC 0.515, 95% CI 0.372–0.655). Faecal consistency may be influenced by dietary components, infectious and non-infectious diseases, dehydration, stress and environment. The increased risk of diarrhoea in multi-cat pens in this study suggests either that transmissible agents may have contributed to diarrhoea or that some other effect, for example stress from higher stocking densities, influenced faecal consistency. Unacquainted cats were not penned together. This effect was independent of age and so applied to adult cats, as well as kittens. The only infectious agent investigated in this study was rotavirus, and this was not associated with diarrhoea. 18 However, the samples are stored at −80ºC and are available for collaborative analysis.
Diarrhoea is a common clinical sign in the young of all species and the association with being a kitten was not surprising. To our knowledge, the OR of 2.54 estimated here is the first quantitative estimate of the increased risk of diarrhoea in kittens. More surprising was that severe diarrhoea (diarrhoea ⩽2) was associated with being a senior cat. Observation of a bimodal age distribution of severe diarrhoea gave us reason to investigate this. Senior cats have been classified as those >11 years of age. 21 When this definition was used, these cats were at increased risk of having severe diarrhoea (OR 4.66; 95% CI 1.25–17.44; P = 0.02). There was no effect of number of cats per pen or season. The reason for this association with senior cats is unclear; it may represent increased susceptibility to gastrointestinal infection, or a chronic, non-infectious gastrointestinal disease, which may have influenced relinquishment to the shelter, increased time to rehoming or return of the cat to the centre. Interestingly, the ICC of 0.646 (95% CI 0.415–0.824) indicated a moderate correlation between severe diarrhoea and clustering of the population at the pen level (ie, cats being in the same pen within a centre), not with clustering at the centre level (ie, cats being in the same centre) (ICC 0.153; 95% CI 0.043–0.421).
The ICCs suggested that if diarrhoea is attributed to an infectious agent, the management routines and hygiene standards within the centres are effective in confining infection to individual pens rather than promoting spread throughout the centre. The partitioning of the residual variance to ‘pen within a centre’ rather than the centre itself provides the evidence for this. This was also suggested as a reason for the scarcity of transmission events in a longitudinal study of calicivirus in a smaller UK shelter cat population. 28
Constipation was associated with age, but in contrast to diarrhoea the risk increased with increasing age. The odds increased by 1.01 for every monthly increase in age. This effect of age has also been reported in humans. 29 The absence of an association with the number of cats per pen argues against an infectious component of constipation, or a stocking density effect. However, there was an association with season. Interestingly, the risk of constipation decreased in the summer months when dehydration might be expected to be more common (OR 0.43; 95% CI 0.21–0.89). Increased physical activity has been associated with a decreased risk of constipation in humans,30,31 and it is possible that the design of cat accommodation in these centres, indoor heated areas and outdoor exercise areas means that cats are more active in the summer months. It is also possible that the location of drinking bowls and litter trays in the outdoor compartments of the cat pens discourages cats from drinking or defaecating during the colder months. Like rats, 32 goats 33 and cattle, 34 cats may also prefer warm water.
To our knowledge, this is the first report of the prevalence of constipation in cat shelters. This needs further investigation to identify additional risk factors. It is possible that these cats were exhibiting faecal retention due to stress associated with a recent move into the shelter. This effect of stress has been reported in elderly humans.35,36 Surprisingly few of the studies that have investigated stress in cats introduced to a shelter have investigated defaecatory behaviour.19,37–39
In addition to their role in identifying risk factors, population-based surveys have been used in benchmarking and setting health targets. In this study, the prevalence of diarrhoea and constipation in different centres showed wide variation, from 0–22.6% and from 0–19.7%, respectively. While the presence of zero prevalence in some centres demonstrates that this is achievable, lower, median and upper quartile values may provide more realistic benchmarks or targets. This study suggests that target levels for diarrhoea could be set at 5%, with 11% as acceptable, and 15% as a level requiring intervention or investigation; for constipation, target levels of 2% (optimal) and 4% (acceptable), with intervention above 6%. However, while the use of targets and benchmarking in health is common,40–42 their value is controversial.43–45 Contention relates to the selection of targets, 46 methods of measurement, 47 uncertainty around target interpretation,48,49 and the ‘gaming’ and ‘effort substitution’ human behaviours that they invoke.44–46 Although this study provides representative cross-sectional prevalences of diarrhoea and constipation in individual centres housing this population, the ICC indicates that centres accounted for very little of the variance within the data. This suggests that using measures of diarrhoea or constipation as indicators of performance of individual centres or targeting interventions at the centre level rather than the pen or cat level may have little impact.
Conclusions
This study identifies constipation as a more prevalent problem than severe diarrhoea in rescue catteries. It sets normal prevalence targets for constipation and diarrhoea and suggests levels at which interventions should occur. Quantitative estimates of the effect of age, number of cats per pen and season, and the influence of hierarchical clustering by centre and pens within centres are provided. The evidence suggests that current hygiene protocols and centre management appear to prevent pen-to-pen spread of infectious agents. Understanding the risk factors for diarrhoea and constipation in shelter cat populations will further facilitate improvements in feeding and management.
Acknowledgments
We thank the Cats Protection and the cooperation of their staff and cats with faecal sample and data collection. Additionally, the assistance of Anna Edwards, Harriet Campbell, Anneka Summan and Sabrina Knight with data entry is gratefully acknowledged.
Footnotes
Allison C German held a lectureship supported by Cats Protection.
Funding: This work was supported by a Royal College of Veterinary Surgeons Charitable Trust Blue Sky Award in Virology (BSR11 1428) and internal funding from the Institute of Infection and Global Health, University of Liverpool.
Accepted: 11 September 2015
References
- 1. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 2. Moxham G. The Waltham faeces scoring system – a tool for veterinarians and pet owners: how does your pet rate? Waltham Focus 2001; 11: 24–25. [Google Scholar]
- 3. Whelan K, Judd PA, Taylor MA. Assessment of fecal output in patients receiving enteral tube feeding: validation of a novel chart. Eur J Clin Nutr 2004; 58: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 4. Sorensen MT, Vestergaard EM, Jensen SK, et al. Performance and diarrhoea in piglets following weaning at seven weeks of age: challenge with E coli O 149 and effect of dietary factors. Livest Sci 2009; 123: 314–321. [Google Scholar]
- 5. Bellosa ML, Nydam DV, Liotta JL, et al. A comparison of fecal percent dry matter and number of Cryptosporidium parvumoocysts shed to observational fecal consistency scoring in dairy calves. J Parasitol 2011; 97: 349–351. [DOI] [PubMed] [Google Scholar]
- 6. Laflamme DP, Xu H, Long GM. Effect of diets differing in fat content on chronic diarrhea in cats. J Vet Intern Med 2011; 25: 230–235. [DOI] [PubMed] [Google Scholar]
- 7. Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 6th ed. St Louis, MO: Elsevier, 2005, pp 1332–1378. [Google Scholar]
- 8. Washabau RJ, Holt DE. Diseases of the large intestine. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 6th ed. St Louis, MO: Elsevier, 2005, pp 1378–1408. [Google Scholar]
- 9. Washabau RJ, Hasler AH. Constipation, obstipation, and megacolon. In: August JR. (ed). Consultations in feline internal medicine. 3rd ed. Philadelphia, PA: WB Saunders, 1996, pp 104–112. [Google Scholar]
- 10. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999; 214: 1336–1341. [PubMed] [Google Scholar]
- 11. Jones PH, Dawson S, Gaskell RM, et al. Surveillance of diarrhoea in small animal practice through the Small Animal Veterinary Surveillance Network (SAVSNET). Vet J 2014; 201: 412–418. [DOI] [PubMed] [Google Scholar]
- 12. O’Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary-care veterinary practices in England. Vet J 2014; 202: 286–291. [DOI] [PubMed] [Google Scholar]
- 13. Case JB, Chomel B, Nicholson W, et al. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J Feline Med Surg 2006; 8: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiGangi BA, Levy JK, Griffin B, et al. Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. J Am Vet Med Assoc 2012; 241: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 15. Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg 2005; 7: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen NC, Sato R, Foley JE, et al. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J Feline Med Surg 2004; 6: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acke E, Whyte P, Jones BR, et al. Prevalence of thermophilic Campylobacter species in cats and dogs in two animal shelters in Ireland. Vet Rec 2006; 158: 51–54. [DOI] [PubMed] [Google Scholar]
- 18. German AC, Iturriza-Gómara M, Dove W, et al. Molecular epidemiology of rotavirus in cats in the United kingdom. J Clin Microbiol 2015; 53: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCobb EC, Patronek GJ, Marder A, et al. Assessment of stress levels among cats in four animal shelters. J Am Vet Med Assoc 2005; 226: 548–555. [DOI] [PubMed] [Google Scholar]
- 20. Gouveia K, Magalhaes A, de Sousa L. The behaviour of domestic cats in a shelter: residence time, density and sex ratio. Appl Anim Behav Sci 2011; 130: 53–59. [Google Scholar]
- 21. Vogt AH, Rodan I, Brown M, et al. AAFP-AAHA: feline life stage guidelines. J Am Anim Hosp Assoc 2010; 46: 70–85. [DOI] [PubMed] [Google Scholar]
- 22. Bybee SN, Scorza AV, Lappin MR. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med 2011; 25: 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones BD. Constipation, tenesmus, dyschezia, and faecal incontinence. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine, 5th ed. Philadelphia, PA: Saunders, 2000, pp 129–135. [Google Scholar]
- 24. Russo M, Martinelli M, Sciorio E, et al. Stool consistency, not frequency, correlates with total gastrointestinal transit time in children. J Pediatr 2013; 162: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 25. Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. Am J Epidemiol 1999; 149: 876–883. [DOI] [PubMed] [Google Scholar]
- 26. Hepworth PJ, Nefedov AV, Muchnik IB, et al. Early warning indicators for hock burn in broiler flocks. Avian Pathol 2010; 39: 405–409. [DOI] [PubMed] [Google Scholar]
- 27. Hepworth PJ, Nefedov AV, Muchnik IB, et al. Hock burn: an indicator of broiler flock health. Vet Rec 2011; 168: 303–305. [DOI] [PubMed] [Google Scholar]
- 28. Coyne KP, Edwards D, Radford AD, et al. Longitudinal molecular epidemiological analysis of feline calicivirus infection in an animal shelter: a model for investigating calicivirus transmission within high-density, high-turnover populations. J Clin Microbiol 2007; 45: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt FM, Santos VL. Prevalence of constipation in the general adult population: an integrative review. J Wound Ostomy Continence Nurs 2014; 41: 70–76. [DOI] [PubMed] [Google Scholar]
- 30. Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003; 98: 1790–1796. [DOI] [PubMed] [Google Scholar]
- 31. Donald IP, Smith RG, Cruikshank JG, et al. A study of constipation in the elderly living at home. Gerontology 1985; 31: 112–118. [DOI] [PubMed] [Google Scholar]
- 32. Kapatos G, Gold RM. Tongue cooling during drinking: a regulator of water intake in rats. Science 1972; 176: 685–686. [DOI] [PubMed] [Google Scholar]
- 33. Olsson K, Hydbring E. The preference for warm drinking water induces hyperhydration in heat-stressed lactating goats. Acta Physiol Scand 1996; 157: 109–1014. [DOI] [PubMed] [Google Scholar]
- 34. Wilks DL, Coppock CE, Lanham JK, et al. Responses of lactating Holstein cows to chilled drinking water in high ambient temperatures. J Dairy Sci 1990; 73: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 35. Whitehead WE, Drinkwater D, Cheskin LJ, et al. Constipation in the elderly living at home. Definition, prevalence, and relationship to lifestyle and health status. J Am Geriatr Soc 1989; 37: 423–429. [DOI] [PubMed] [Google Scholar]
- 36. Talley NJ, Fleming KC, Evans JM, et al. Constipation in an elderly community: a study of prevalence and potential risk factors. Am J Gastroenterol 1996; 91: 19–25. [PubMed] [Google Scholar]
- 37. Kessler MR, Turner DC. Stress and adaptations of cats (Felis silvestris catus) housed singly, in pairs and in groups in boarding catteries. Anim Welf 1997; 6: 243–254. [Google Scholar]
- 38. Ottway DS, Hawkins DM. Cat housing in rescue shelters: a welfare comparison between communal and discrete-unit housing. Anim Welf 2003; 12: 173–189. [Google Scholar]
- 39. Dybdall K, Strasser R, Katz T. Behavioral differences between owner surrender and stray domestic cats after entering an animal shelter. Appl Anim Behav Sci 2007; 104: 85–94. [Google Scholar]
- 40. van Herten LM, Gunning-Schepers LJ. Targets as a tool in health policy. Part I: lessons learned. Health Policy 2000; 53: 1–11. [DOI] [PubMed] [Google Scholar]
- 41. van Herten LM, Gunning-Schepers LJ. Targets as a tool in health policy. Part II: guidelines for application. Health Policy 2000; 53: 13–23. [DOI] [PubMed] [Google Scholar]
- 42. Soares S, Green DM, Turnbull JF, et al. A baseline method for benchmarking mortality losses in Atlantic salmon (Salmo salar) production. Aquaculture 2011; 314: 7–12. [Google Scholar]
- 43. Paddock SM, Adams JL, Hoces de la Guardia F. Better-than-average and worse-than-average hospitals may not significantly differ from average hospitals: an analysis of Medicare Hospital Compare ratings. BMJ Qual Saf 2015; 24: 128–134. [DOI] [PubMed] [Google Scholar]
- 44. Bird SM, Cox D, Farewell VT, et al. Performance indicators: good, bad and ugly. J Roy Stat Soc A 2005; 168: 1–27. [Google Scholar]
- 45. Kelman S, Freidman JN. Performance improvement and performance dysfunction: an empirical examination of impacts of the emergency room wait-time target in the English National Health Service. J Publ Adm Res Theor 2009; 19: 917–946. [Google Scholar]
- 46. Bevan G, Hood C. What’s measured is what matters: targets and gaming in the English public health care system. Public Admin 2006; 84: 517–538. [Google Scholar]
- 47. Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 48. Paddock SM, Louis TA. Percentile-based empirical distribution function estimates for performance evaluation of healthcare providers. J R Stat Soc Ser C Appl Stat 2011; 60: 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldstein H, Spiegelhalter DJ. League tables and their limitations: statistical issues in comparisons of institutional performance (with discussion). J R Stat Soc Ser A 1996; 159: 385–443. [Google Scholar]


