Abstract
Cholangiohepatitis/cholangitis is second only to hepatic lipidosis as the most common liver disease in cats and is often associated with concurrent pancreatitis. Magnetic resonance imaging (MRI) and MR cholangiopancreatography (MRCP) have developed into an accurate, highly sensitive and specific imaging tool for the diagnosis of biliary and pancreatic duct disorders in humans. In this prospective case series, 10 cats with suspected cholangitis and/or pancreatitis were enrolled based on clinical history, physical examination and appropriate diagnostic test results. MRI and MRCP sequences with secretin stimulation of the cranial abdomen were performed, and sonography and laparoscopic biopsies for histologic diagnosis were obtained for comparison. MRI detected pancreatic abnormalities in cats suspected of pancreatitis, including T1 pre-contrast hypointense and T2 hyperintense pancreatic parenchyma and a dilated pancreatic duct. The MRI findings of the liver were non-specific. Nine of 10 cats had biliary abnormalities, including gall bladder wall thickening, gall bladder wall moderate contrast enhancement and/or gall bladder debris. Eight of 10 cats had histologic evidence of pancreatitis, as well as hepatitis or cholangitis, with one cat diagnosed with hepatic lymphoma. The advantages of MRI/MRCP over sonography of these cats included the striking pancreatic signal changes associated with pancreatitis and the ability to comprehensibly assess and measure the pancreas and hepatobiliary structures without operator dependence or interference from bowel gas. MRI/MRCP imaging of the feline abdomen may be beneficial in cases with equivocal ultrasound imaging findings.
Introduction
Cholangiohepatitis/cholangitis is second only to hepatic lipidosis as the most common liver disease in cats and is often associated with concurrent pancreatitis and inflammatory bowel disease — a condition referred to as ‘triaditis’. This is due to the unique feline ductal anatomy in which the common bile duct and pancreatic duct both open onto the major duodenal papilla, allowing for infection and inflammation to spread from one area, such as the biliary system, into the pancreas and duodenum. 1 In multiple retrospective and prospective studies evaluating feline cholangitis, concurrent pancreatitis was common and found in 39–65% of cats.2–4
In veterinary medicine, B-mode ultrasound imaging is used frequently in an attempt to diagnose this condition in cats, but has proven to be unsatisfactory in many patients. Sonography has low sensitivity and specificity to cholangitis and pancreatitis, and correlates poorly with histopathological diagnosis, although advanced stages of disease can be detected.3–6 Inflammatory liver conditions are also difficult to detect sonographically owing to their diffuse nature, but findings are non- specific and can range from normal, to hypo- or hyper-echoic parenchyma.7–9
Magnetic resonance imaging (MRI) that incorporates cholangiopancreatography (MRCP) sequences is an accurate, highly sensitive and specific imaging tool for the diagnosis of human biliary and pancreatic duct disorders.10–21 MRI is more sensitive to soft tissue pathology than ultrasonography because it allows detection of subtle shifts in cellular water and protons. Inflammatory processes of the hepatobiliary system and pancreas can therefore lead to visible signal differences and changes in contrast enhancement. MRI can detect changes associated with human pancreatitis including increased or decreased size of the entire gland, pancreatic edema and inflammation (based on parenchymal signal changes on T1- and T2-weighted images and abnormal contrast enhancement) and peri-pancreatic inflammation. 22
The addition of MRCP to anatomic MRI abdominal sequences provides a complete non-invasive anatomical map of the biliary tree and pancreatic duct without the use of ionizing radiation or intravenous contrast. 14 MRCP is based on the principle that slow moving fluids are hyperintense on heavily T2-weighted images, so bile and pancreatic fluids will be bright, resulting in increased duct to background contrast.12,13 The advantages of MRCP over sonography include decreased operator dependence and improved demonstration of biliary and pancreatic ducts. The combination of MRCP with conventional MR T1 and T2 imaging results in complete anatomic imaging of ductal, as well as extra-ductal, disease within the liver and pancreas.10,21
The feline pancreatic duct has been studied sonographically in normal cats, documenting normal ductal size, age-related changes and the response of the duct to secretin administration.6,23–25 Abnormal ductal values and morphology of structures in cats with pancreaticobiliary disease have not been established with either ultrasound or MRI, however.
MRI/MRCP of the normal feline cranial abdomen has been reported recently from a study that established the optimal imaging protocol for the cat, including scans obtained pre and post-secretin stimulation. 26 Feline MRI/MRCP provided high quality images of the liver, biliary ductal system, pancreas, and pancreatic duct. Visualization of the small normal feline pancreatic duct was feasible, although its conspicuity was further improved with secretin stimulation. Additionally, the use of multiple imaging planes in this MRI protocol resulted in excellent comprehensive views of hepatobiliary and pancreatic anatomy.
We hypothesized that signs of cholangiohepatitis-pancreatitis would be detected in a series of clinically affected cats similar to those reported for affected human patients. Our main aim was to document MRI/MRCP abnormalities visible in affected cats using the previously studied normal cats and human results as a standard of comparison. As sonography is commonly used in the cat to evaluate these clinical conditions, an ancillary aim was to compare the MRI/MRCP with sonographic results. Histopathology was obtained laparoscopically for further comparison. Our overall goal was to determine whether MRI/MRCP provided valuable observations in this group of affected cats. That was considered particularly important given the expense and need for anesthesia that is associated with veterinary MRI.
Materials and methods
Inclusion criteria
A prospective case series of 10 cats was conducted at Colorado State University from July 2011 to June 2012. Cats were enrolled if suspected by the clinical internist (DT) to have cholangiohepatitis-pancreatitis based on history, physical examination, biochemical and hematologic tests. The study protocol was approved by Institutional Animal Care and Use Committee (IACUC) and all participants gave informed consent. Cats included in the study were healthy enough to tolerate general anesthesia and had no additional previously diagnosed chronic illness. A minimum patient database was obtained of complete blood count, chemistry profile and feline pancreas- specific lipase (fPLI) assay (IDEXX Laboratories Inc).
Ultrasound and MRI/MRCP
A complete abdominal ultrasound examination was performed on the day of arrival by one of the participating radiologists (AM), with MRI/MRCP on the following day. Both imaging procedures included the use of secretin (ChiRhoStim; ChiRhoClin, established feline dose of 2 U/kg IV) to evaluate its effects on pancreatic duct dilation.25,26 The first three enrolled cats of the project received the secretin during the initial ultrasound study and also a second secretin injection during MRI/MRCP the next day.
However, the protocol was changed for the remaining seven cats. Those seven cats had a baseline ultrasound examination on the first day without secretin, and received only a single secretin injection during the MRI/MRCP examination. Immediately after MR/MRCP imaging, post-secretin ultrasonography of the pancreatic duct was performed. This protocol change was made owing to concerns in the first three cats that the secretin given during ultrasound could potentially lead to prolonged duct dilation and thereby affect the baseline ductal appearance on MRI/MRCP the next day. Owing to the change in protocol the post-secretin pancreatic ductal assessment for the first three cats was eliminated from the pool of data.
After the ultrasound examination, the cats were fasted overnight and, on the following day, were anesthetized for MRI/MRCP. The previously established feline MRI/MRCP protocol (GE Signa 1.5 T; GE Medical Systems) was utilized for these cats including T1 fast spin (FS) gradient echo (GRE) pre- and post-contrast in dorsal and transverse planes, T2 fat saturated in dorsal and transverse planes, MRCP in dorsal planes, and fast spoiled gradient recalled (fSPGR) in/out of phase sequences in transverse planes. 26 Secretin was given as described above after the first MRCP sequence identical to the previously established protocol, followed by additional post-secretin imaging sequences.
Laparoscopy
Following MRI/MRCP, the cats had laparoscopic evaluation of the liver, biliary tract and pancreas with subsequent biopsies of their liver and pancreas (DT). Three individual liver biopsies were obtained in three different liver lobes with one biopsy obtained in an affected area of the visible pancreas. These biopsies were obtained for histology and culture to confirm the involved disease processes.
Each tissue sample was fixed in 10% formalin for 24 h and embedded in paraffin with 5 μm thick sections cut and stained with standard hematoxylin and eosin.
Data analysis
The ultrasound images were reviewed by a board- certified radiologist (AM). The following parameters were assessed and compared to established normals for the cat: pancreatic thickness (measured in multiple locations in both limbs and the body using electronic calipers in the transverse plane), echogenicity (normal, hypo-echoic, hyperechoic or heterogeneous compared with the adjacent mesentery and liver), pancreatic duct diameter (pre- and post-secretin injection) and peri-pancreatic abnormalities (± free fluid, ± presence of hyperechoic mesentery). 24 Parameters to evaluate hepatobiliary structures included liver echogenicity (normal, hypo-echoic, hyperechoic, heterogeneous compared with the falciform fat), presence of focal or multifocal hepatic abnormalities, common bile duct diameter, gall bladder wall echogenicity (normal or hyperechoic) and thickness and the presence or absence of abnormal gall bladder contents (echogenic sediment).27,28
The MR images were reviewed by two board-certified veterinary radiologists (AM and SK) and a physician radiologist (TD) via consensus. This method was used to optimize detection of any potential abnormalities as MRI/MRCP is a new imaging application. Blinded interpretation was not used for this study because enrolled cats were known to be clinically affected. Pancreatic size was evaluated subjectively and was also measured in a dorsal to ventral orientation on the transverse T1 weighted sequence post-contrast at the level of the splenic vein (Figure 1). Pancreatic signal intensity was compared with liver on T1- and fat saturated T2-weighted images, and its pattern of contrast enhancement was evaluated as minimal, homogenous or heterogeneous. Additional criteria included intensity of the peri-pancreatic tissues (isointense, hyperintense relative to pancreas), and size of the pancreatic duct pre- and post-secretin. The size of the pancreatic duct and common bile duct were evaluated in fat saturated T2-weighted and MRCP images pre- and post-secretin injection and measured via electronic calipers.
Figure 1.
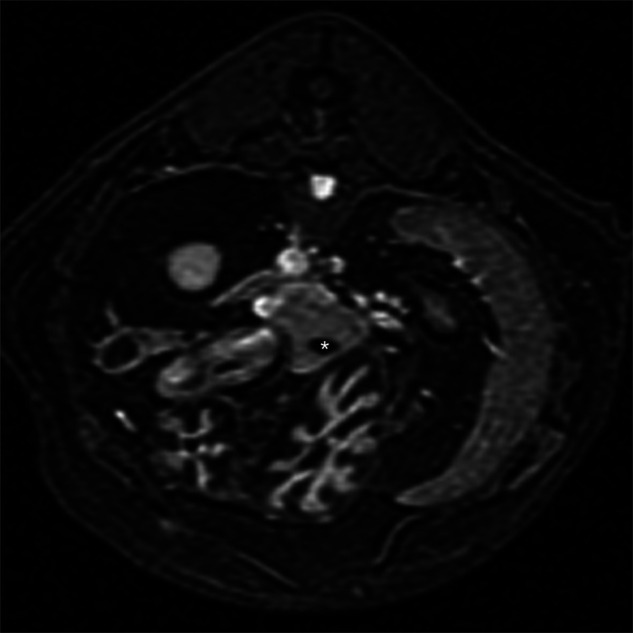
Tranverse plane T1 sequence post-intravenous contrast used for measuring the pancreatic thickness. The pancreas is uniformly and mildly contrast enhancing with well-defined margins. The hypoechoic pancreatic duct is noted in the ventral portion of the pancreatic body (asterisk)
The liver intensity was evaluated on T1 pre-contrast, fat saturated T2-weighted, fSGRE in/out of phase sequences (hypointense, hyperintense, heterogeneous) by comparing it with the spleen. Contrast enhancement (homogenous or heterogeneous) was evaluated on T1-weighted images. The liver was also assessed for focal abnormalities, such as hyper- or hypointense nodules or cysts and heterogeneity of the parenchyma. The biliary tract was evaluated by the presence of gall bladder wall thickness (on the post- contrast T1 transverse images), gall bladder wall enhancement (minimal, mild, moderate), presence and nature of gall bladder contents on T1 pre- and post-contrast and fat saturated T2-weighted images and diameter measurement of the common bile duct.
Mean, median and standard deviations were calculated on pancreatic size thickness, pancreatic duct size pre- and post-secretin and gall bladder wall thickness, for both imaging modalities. Median values were not calculated for small subsets of data as this was not feasible. Although this study was not performed to assess sensitivity or specificity of MRI for these conditions, the results were compared with histology and ultrasound findings to identify whether MRI contributed unique or valuable information.
Results
Signalment, clinical signs, biochemistry findings and fPLI assay
There were seven neutered male cats and three spayed female cats with an age range of 12–17 years (mean 13, median 12 years), nine of which were domestic shorthair cats and one was a domestic longhair cat. The cats presented for a variety of reasons, including inappetence, weight loss, lethargy, diarrhea, vomiting and abdominal pain. Seven cats had elevations in liver enzymes and total bilirubin, and three cats had normal liver enzymes. Five of the 10 cats had normal fPLI assays and the remaining five had elevated fPLI assays.
Ultrasound imaging results
A summary of pancreatic sonography is presented in Table 1. Nine of 10 cats had evidence of pancreatic enlargement in a portion of the pancreas (based on reported normal feline pancreatic thickness <1 cm). 24 There were areas of the pancreas that also measured within normal limits in each cat. Two of the 10 cats had anechoic pancreatic cystic structures. Half of the cats had pancreatic duct diameters that exceeded the normal reported value of up to 2.5 mm.23,29 After secretin, the pancreatic duct mildly dilated in 8/10 cats. The two remaining cats’ pancreatic ducts did not dilate further post-secretin; these cats had the largest pancreatic duct diameters and had pancreatic cysts. Three cats had evidence of peri-pancreatic inflammation.
Table 1.
Ultrasound pancreatic findings
| Pancreatic size – largest thickness measurement (in mm) | Echogenicity | Pancreatic duct size pre-secretin (mm)* | Pancreatic duct size post-secretin (mm) (excludes first three cats)† | Peri-pancreatic tissues |
|---|---|---|---|---|
| 12.2 mm (mean) ± 2.5 mm
(11 mm median) |
8 hypoechoic
1 heterogeneous 1 normal |
2.5 mm (mean) ± 0.9 mm
(1.4 mm median) |
5 dilated ducts
2.7 mm (mean) ± 0.7 mm 2 static ducts 3.6 mm (mean) ± 0.4 mm |
7 normal
2 hyperechoic 1 hyperechoic with free fluid |
Normal cat pancreatic duct measurements pre-secretin 0.77 mm (mean) ± 0.33 mm25 and 1.3 mm ± 0.04 mm (normal older cats) 29
Normal cat pancreatic duct measurements post-secretin 1.42 mm (mean) ± 0.40 mm25
Liver echogenicity was considered normal (3/10), hypoechoic (3/10), heterogeneous (1/10) and hyper-echoic (3/10). Two cats had hypoechoic and/or hyper-echoic liver nodules, and one cat had multiple biliary cystadenomas. The common bile duct was normal in eight cats (based on reported values of normal being <4 mm) and dilated in two cats (4.0–9.9 mm). 27 Five cats had no gall bladder debris and five cats had mild-to-moderate echogenic gall bladder debris. Four of 10 cats had a hyperechoic thickened gall bladder wall (1.8–2. 8 mm thick) compared with normal being ≤1 mm. 28
MRI/MRCP results
Nine of 10 cats had an abnormal pancreas based on MRI, including overall mild-to-moderate enlargement of the gland, as summarized in Table 2. The pancreas was also diffusely enlarged in nine cats, as viewed on the dorsal plane images compared with normal (Figure 2). One cat had a normally sized pancreas focally (6.9 mm) and generally.
Table 2.
Magnetic resonance pancreatic findings
| Pancreatic thickness (mm)* | T1 intensity | T2 intensity | Contrast enhancement | Pancreatic duct size pre-secretin (mm) (excludes first three affected cats)† | Pancreatic duct size post-secretin (mm) (excludes first three affected cats)‡ | Peri-pancreatic tissues |
|---|---|---|---|---|---|---|
| 13.0 mm (mean) ± 3.0 mm (14.6 mm median) | 9 hypointense
1 hyperintense (normal) |
9 hyperintense
1 isointense (normal) |
8 homogeneous
1 heterogeneous 1 minimal enhancement |
2.1 mm (mean) ± 0.9 mm (1.9 mm median) | 5 dilated ducts
2.8 mm (mean) ± 0.5 mm (2.8 mm median) 2 static ducts 3.8 mm (mean) ± 0.1 mm |
9 normal
1 hyperintense with fluid |
Pancreatic thickness normal cats 9.5 mm (mean) ± 1.2 mm26, †Pancreatic duct size pre-secretin 1.65 mm (mean) ± 0.05 mm26
Pancreatic duct size post-secretin 2.5 mm (mean) ± 0.01 mm26
Figure 2.
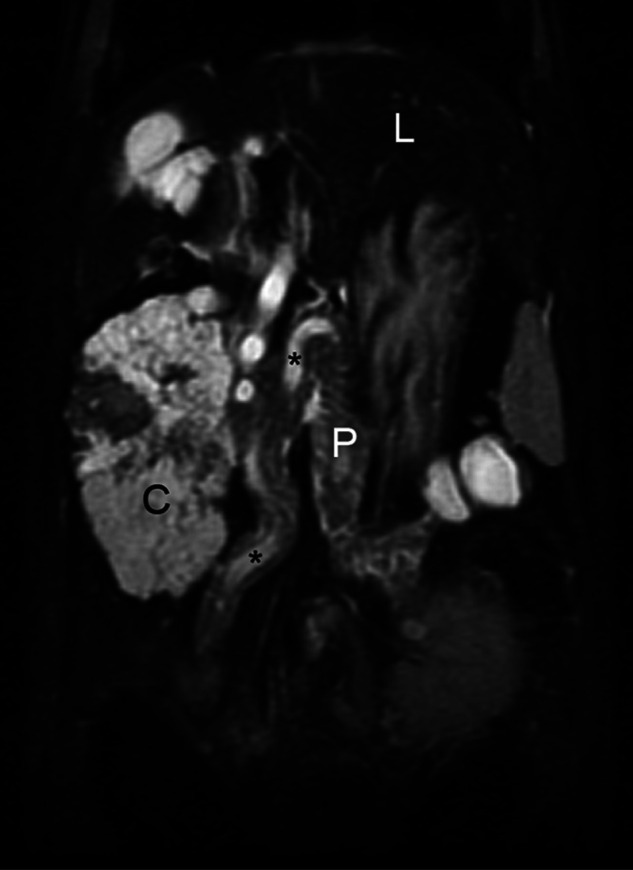
Dorsal plane fat-saturated T2 sequence which provides an excellent global image of the pancreas. Note the hyperintense pancreas (P) relative to the liver (L) with multiple interdigitating hyperintensities within its folds, which is indicative of edema. Hyperintense cysts are present in the left limb just caudal to the spleen. The markedly enlarged pancreatic duct is noted intermittently as it courses through the entire pancreas (*). A large hyperintense biliary cystadenoma is present adjacent to the right pancreatic limb (C)
Nine of 10 cats had abnormal signal intensity of the pancreatic parenchyma compared with the liver (Table 2 and Figure 3 a, c). Some of these changes were patchy and multifocal throughout the gland. In comparison, the normal cat pancreas was hyperintense on pre-contrast T1-weighted images and isointense/hypointense on fat saturated T2-weighted images compared with liver (Figure 3 b, d). 26 Most cats had similar pancreatic contrast enhancement when compared with normal cats in which the pancreas was mildly and homogenously contrast enhancing. 26 Two cats had cystic structures within the pancreas which were hyperintense on T2-weighted sequences and hypointense on T1-weighted sequences; these were the same cats in which cysts were identified sonographically. The one cat with the normally sized pancreas had normal pancreatic intensity and diffuse contrast enhancement. The pancreatic duct dimensions were greater than the range reported for normal cats both pre- and post-secretion (Table 2). Eight of 10 cats’ pancreatic ducts dilated after secretin stimulation. Two of the cats with the pancreatic cysts and the most dilated pancreatic ducts had no evidence of increased dilation after secretin administration. Many of the cats had a smoother and more uniform dilation of the pancreatic duct after receiving secretin (Figure 4).
Figure 3.
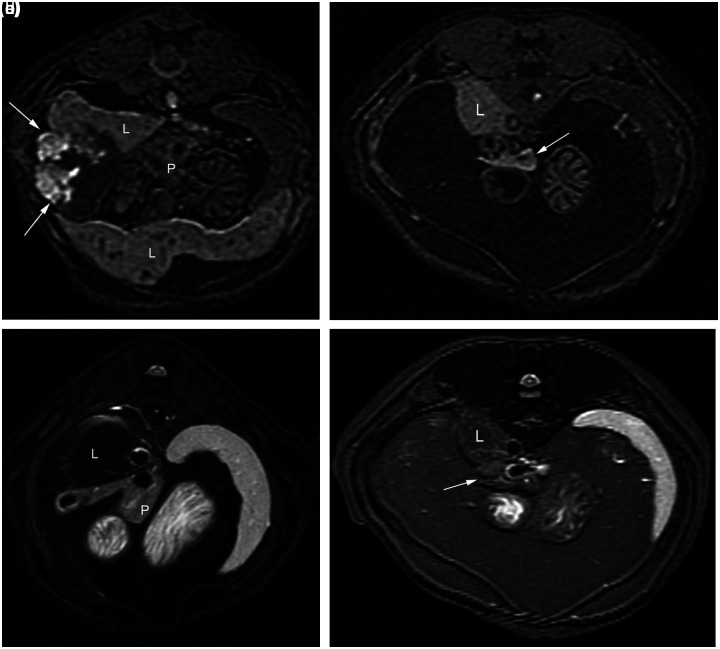
(a) Transverse plane T1 pre-contrast image. The pancreas (P) is hypointense relative to liver (L). Mixed intensity biliary cystadenoma is lateral to the pancreas (arrows). (b) Transverse plane T1 pre-contrast image of normal cat for comparison. Note the hyperintense pancreas (arrow) next to liver (L). (c) Transverse plane fat-saturated T2 image. The pancreas (P) is hyperintense relative to liver (L). (d) Transverse plane fat-saturated T2 image of normal cat for comparison. Note the hypointense pancreas (arrow) next to liver (L)
Figure 4.
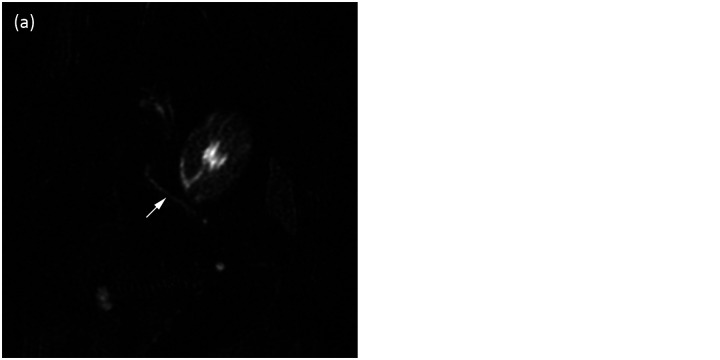
(a) Dorsal plane MRCP image pre-secretin. Note the pancreatic duct (arrow). (b) Dorsal plane MRCP image post-secretin. Note the increased dilation and improved conspicuity of the pancreatic duct after secretin stimulation (arrow)
Eight of 10 cats had a hypointense liver relative to the spleen on almost all imaging sequences similar to normal cats. 26 In normal cats the liver was hypointense relative to spleen on T1- and T2-weighted sequences, and retained similar intensity between in- and out-of-phase fGRE sequences. 26 Two of 10 cats had a heterogeneous liver parenchyma. Five of 10 cats had multifocal cysts or nodules in the liver. One cat had several large biliary cystadenomas, which were predominantly hyperintense on T2 sequences and hypointense on T1 sequences. Seven of 10 cats had gall bladder debris (Figure 5a). This debris was typically hyperintense on T1 and hypointense on T2 sequences. Two of five normal cats had gall bladder debris which had similar imaging characteristics. 26 Nine of 10 cats had a mildly thickened and moderately enhancing gall bladder wall [mean 2.2 mm ± 0.3 mm (median 1.9 mm)] (Figure 5b). In normal cats, the gall bladder wall was mildly contrast enhancing and was difficult to measure with electronic calipers owing to its small size, which required magnifying the image until pixelated [1.7 mm–0.5 mm (median 1.2 mm)]. 26 The common bile duct was normal in 8/10 cats and too small for measurement with electronic calipers. In two cats the common bile duct was dilated and tortuous measuring 4.4 mm and 5.6 mm. The common bile duct was not measured in normal cats owing to its small size. Only one cat had abnormal peri-pancreatic tissues.
Figure 5.
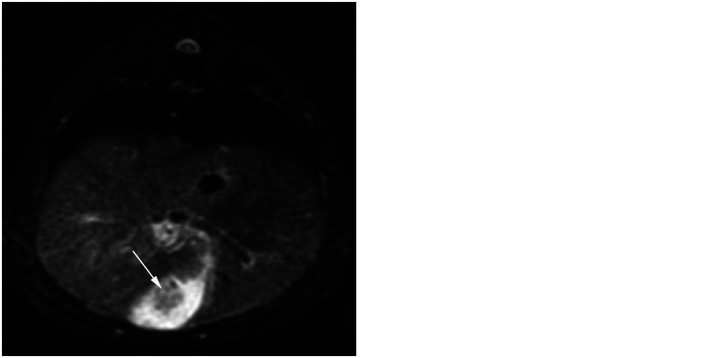
(a) Transverse plane fat-saturated T2 image. There are hypointense gall bladder intraluminal contents (arrow). The surrounding bile is hyperintense. (b) Transverse plane T1 post-contrast image. The gall bladder is thickened and uniformly contrast enhancing
Comparison of MRI/MRCP to sonographic results
In nine cats similar pancreatic abnormalities were identified by both modalities. In 1/10 cats, MRI demonstrated a dilated pancreatic duct which was considered normal on ultrasound. Peri-pancreatic regions were considered abnormal sonographically in two additional cats compared with MRI/MRCP. Sonographically, 7/10 cats had hepatic parenchymal abnormalities and 7/10 cats had biliary tract abnormalities (not all the same as those with liver parenchymal changes). Based on MRI/MRCP, fewer (2/10) cats had hepatic parenchymal changes. MRI demonstrated gall bladder wall changes more often than ultrasound with 9/10 cats having a mean thickness (mean 2.2 mm) greater than normal cats (mean 1.7 mm) and increased wall contrast enhancement compared with 4/10 cats with increased gall bladder wall thickness and hyperechogenicity sonographically. MRI and ultrasound positively identified gall bladder contents in 7/10 and 5/10 cats, respectively.
Laparoscopic findings
Eight of nine cats had an abnormal pancreas laparoscopically. Changes included pancreatic enlargement and edema, irregular to nodular texture and presence of multiple white superficial nodules. One of the cats had free abdominal fluid and evidence of multifocal peri-pancreatic saponification. The cat with a normal pancreas on ultrasound and MRI/MRCP also had a normal appearing pancreas on laparoscopy.
The liver was abnormal laparoscopically in 9/10 cats. Changes included diffuse and focal enlargement, mottled or ‘nutmeg’ appearance, tan discoloration with red reticular markings and the presence of multifocal pale pink or white nodules. One cat had a normal appearing liver. Seven of the cats’ biliary tracts were considered normal. The gall bladder was large with a distended cystic duct in two cats, which was not noted on either imaging modality. Another cat had a severely dilated and tortuous common bile duct which was identified on both ultrasound and MRI.
Laparoscopic hepatic biopsies were obtained from 9/10 cats and pancreatic biopsies obtained in 10/10 cats. Liver biopsies could not be obtained in one of the cats owing to technical difficulties; however, this cat had a grossly abnormal liver consistent with cholangitis.
Histology and culture results
Eight of the 10 biopsied cats had histologic evidence of pancreatitis characterized as either chronic (6/8), chronic active (1/8) or acute (1/8). Two of 10 had a normal pancreas histologically. One of those cats had an abnormal pancreas based on imaging and laparoscopy and the second cat had a normal pancreas based on imaging and laparoscopy.
All cats with liver biopsies had evidence of cholangitis or hepatitis histologically. One cat was initially diagnosed with lymphocytic plasmacytic hepatitis, but re-diagnosed with lymphoma based on special stains. There was no evidence of aerobic or anaerobic growth based on liver culture for any cats.
Discussion
The signalment, clinical history and biochemistry abnormalities in these cats correlated well with previous descriptions of cholangitis and pancreatitis.3,30–32 Five cats had a negative fPLI assay, with three of these cats having a false-negative test result based on histology. A large-scale prospective clinical study to determine the sensitivity and specificity of the fPLI assay has not been reported; however, the false-negative fPLI assay results occurred in cats with chronic pancreatitis and may indicate lack of enzyme activity and release in these patients.
Histologic pancreatic changes are not always confirmatory either and have been shown to be patchy and multifocal in nature in dogs with similar findings expected for cats. 33 Two cats had normal pancreatic histology. In the first cat, imaging and laparoscopic findings corroborated the normal histologic diagnosis. However, the second cat had an abnormal pancreas on imaging and laparoscopy, but had normal pancreatic histology. The second cat could have truly had a normal pancreas; however, the small biopsy samples obtained may have missed areas of inflammation. These issues highlight the potential value of imaging in patients with suspected pancreatitis.
The most salient advantage of MRI/MRCP in these cats was the pronounced signal intensity abnormalities associated with pancreatitis. Affected cats had a T1 pre-contrast hypointense and T2 hyperintense pancreas, as has been reported in humans.22,34 These signal changes have been attributed to fibrosis and edema.22,34 Other significant advantages included the ability to obtain images with excellent soft tissue anatomy in multiple imaging planes. MRI indicated pancreatic enlargement based on the single objective measure on transverse plane T1 images, but, also subjectively when evaluating the entire pancreas on the dorsal plane T1- and T2-weighted images. The dorsal plane MR images provided an entire view of the pancreas, pancreatic duct and hepatobiliary system without superimposition of bowel or lack of patient compliance that can occur with ultrasound, allowing complete evaluation of both limbs and body for parenchymal, pancreatic duct and peri-pancreatic changes. The dorsal plane images assisted in identifying focal changes that could be, and were, missed on ultrasound imaging, including the patchy nature of some intensity changes. This information was useful prior to laparoscopy in providing landmarks and could be useful for surgical planning. Further advantages of MRI/MRCP include its independence from operator skill and experience level.
It has been suggested that evidence of pancreatic duct dilation may be a better measure of pancreatic inflammation in the cat.6,35 Based on ultrasound and MRI findings, most of the cats with histologic evidence of pancreatitis had a dilated pre-secretin pancreatic duct compared with normal cats.23–26,29 Similar changes have been identified in humans, where pancreatic duct dilation and pancreatic duct changes on abdominal MRI/MRCP sequences are associated with chronic pancreatitis, due to fibrosis in the gland, which causes traction around the duct and subsequent dilation.22,34,36 Two of the cats with pancreatic cysts had the most severe pancreatic duct distention, which was likely secondary to ductal obstruction. Ductal dilation is not specific in cats with pancreatitis because age-related pancreatic duct dilation has also been identified in older cats.23,29 All of the cats in this study were over 10 years of age, so some pancreatic duct dilation may have been due to age-related changes in addition to the pancreatic inflammation.
Most of the cats’ pancreatic ducts dilated mildly after secretin stimulation, which was also found in normal cats and humans based on ultrasound and MRI.25,26,37,38 All cats in this study had more pronounced dilation of the pancreatic duct post-secretin stimulation compared with previously studied normal cats.25,26 It has been suggested that mild dilation post-secretin could indicate retention of ductal elasticity. 37 In contrast, the two cats with pancreatic cysts and the most severe pancreatic duct dilation did not respond to secretin stimulation, suggesting minimal duct elasticity. In normal cats, the pancreatic duct was more conspicuous and better visualized after secretin. In these abnormal cats that already had dilated prominent pancreatic ducts, the advantage of secretin was less evident, which has been noted in humans. 37 In humans, the advantage of secretin administration lies in the evaluation of ductal strictures, which may be missed without secretin.37,38 The meaning and interpretation of the feline post-secretin ductal changes relative to the detection and staging of pancreatitis needs more study.
Nine of 10 cats had a moderately enhancing and mildly thickened gall bladder wall. In humans, a contrast enhancing, thickened gall bladder wall can indicate inflammation secondary to cholangitis or cholecystitis. 39 The presence of gall bladder debris in the cat may be associated with cholangitis or cholestasis.40,41 Further investigation is needed to determine the association of gall bladder intraluminal debris and gall bladder wall enhancement with biliary disease. Cats with cholangitis or hepatitis diagnosed histologically had no specific hepatic parenchymal changes evident by either MRI or ultrasound examinations. Both modalities readily identified focal abnormalities, such as cysts and nodules. Histology is still necessary in diagnosing liver disorders.
Ultrasound imaging by an experienced sonographer also identified pancreatic abnormalities in all affected cats, including abnormal pancreatic echogenicity, size, and/or pancreatic duct dilation. However, many of the cats only had mild enlargement of sections of the pancreas and other sections measured within normal size limits. Pancreatic inflammation in cats can be chronic, which can make sonographic changes more subtle and challenging. 42 Furthermore, the operator dependence of sonography and interference by superimposed gas-filled bowel can limit its usefulness for some cats. The known lower sensitivity and specificity of ultrasound in diagnosing pancreatitis may be related to those, and other, factors.5,6
Limitations of this study include the small number of cats and the inherent bias of the reviewers who were intentionally not blinded. The main goal was to determine the MRI changes associated with pancreatitis and cholangiohepatitis. A large prospective study would be needed to determine the specificity, sensitivity and accuracy of MRI compared with ultrasonography. Biopsies were obtained laparoscopically to minimize invasiveness and morbidity. However, this technique had limitations due to the small number and size of the samples obtained. Surgical biopsies were not considered owing to higher potential morbidity and risk.
Conclusions
MRI/MRCP was useful in detecting abnormalities associated with histologically confirmed pancreatitis compared with normal cats. The MRI findings of a T1 pre-contrast hypointense and T2 hyperintense pancreatic parenchyma were associated with feline pancreatitis. Pancreatic ducts were dilated pre-secretin by both imaging modalities in these cats with pancreatitis. MRI/MRCP provided excellent comprehensive visualization of the pancreatic and hepatobiliary systems. MRI/MRCP was advantageous over sonography in visualizing the pancreatic duct, but the MRI findings for hepatitis/cholangitis were non-specific. Sonography remains the recommended first imaging modality for feline pancreatic and hepatobiliary disease owing to its availability, lower cost and lack of requirement of anesthesia. There are, however, certain advantages of MRI/MRCP that may, at times, justify the risk of general anesthesia and added expense of that imaging procedure, particularly in situations in which ultrasound results are equivocal or in which the pancreas was incompletely assessed sonographically owing to bowel gas or lack of patient compliance. Abdominal MRI examination should be considered as a potentially valuable second tier diagnostic imaging tool for pancreatitis.
Acknowledgments
The authors would like to thank Jeff Stewart for excellent MRI technical work.
Footnotes
Funding: The authors would like to thank the Colorado State University’s College Research Council for funding this study, and ChiRhoClin for donating the secretin used in the project
The authors do not have any potential conflicts of interest to declare.
Accepted: 5 October 2012
References
- 1. Smallwood JE. Digestive system. In: Hudson L, Hamilton W. (eds). Atlas of feline anatomy for veterinarians. Philadelphia: WB Saunders, 1993, pp 166–167. [Google Scholar]
- 2. Weiss DJ, Gagne P, Armstrong J. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis and nephritis in cats. J Am Vet Med Assoc 1996; 209: 1114–1116. [PubMed] [Google Scholar]
- 3. Callahan J, Haddad J, Brown D, et al. Feline cholangitis: a necropsy study of 44 cats (1986–2008). J Feline Med Surg 2011; 13: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swift NC, Marks SL, MacLachlan NJ, et al. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc 2000; 217: 37–42. [DOI] [PubMed] [Google Scholar]
- 5. Ferreri JA, Hardam E, Kimmel SE, et al. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996–2001). J Am Vet Med Assoc 2003; 223: 469–474. [DOI] [PubMed] [Google Scholar]
- 6. Saunders JM, Van Winkle TJ, Drobatz K, et al. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994–2001). J Am Vet Med Assoc 2001; 221: 1724–1730. [DOI] [PubMed] [Google Scholar]
- 7. Newell SM, Selcer BA, Girard E, et al. Correlations between ultrasonographic findings and specific hepatic disease in cats: 72 cases (1985–1997). J Am Vet Med Assoc 1998; 213: 94–98. [PubMed] [Google Scholar]
- 8. Biller DS, Kantrowitz B, Miyabayashi T. Ultrasonography of diffuse liver disease: a review. J Vet Intern Med 1992; 6: 71–76. [DOI] [PubMed] [Google Scholar]
- 9. Penninck D, Berry C. Liver imaging in the cat. Sem Vet Med and Surg 1997; 12: 10–21. [DOI] [PubMed] [Google Scholar]
- 10. Fulcher AS, Turner MA, Capps GW, et al. Half-Fourier RARE MR cholangiopancreatography: Experience in 300 subjects. Radiology 1998; 201: 21–32. [DOI] [PubMed] [Google Scholar]
- 11. Palmucci S, Mauro LA, LaScola S, et al. Magnetic resonance cholangiopancreatography and contrast-enhanced magnetic resonance cholangiopancreatography versus endoscopic ultrasonography in the diagnosis of extrahepatic biliary pathology. Radiol Med 2010; 115: 732–746. [DOI] [PubMed] [Google Scholar]
- 12. Becker CD, Grossholz M, Becker M, et al. Choledocholithiasis and bile duct stenosis: diagnostic accuracy of MR cholangiopancreatography. Radiology 1997; 205: 523–430. [DOI] [PubMed] [Google Scholar]
- 13. Viltellas KM, Keogan MT, Freed KS, et al. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics 2000; 20: 959–975. [DOI] [PubMed] [Google Scholar]
- 14. Lee MG, Lee JH, Kim MH, et al. Extrahepatic biliary diseases: 3D MR Cholangiopancreatography compared with endoscopic retrograde cholangiopancreatography. Radiology 1997; 202: 663–669. [DOI] [PubMed] [Google Scholar]
- 15. Maurea S, Caleo O, Mollica C, et al. Comparative diagnostic evaluation with MR cholangiopancreatography, ultrasonography, and CT in patients with pancreatobiliary disease. Radiol Med 2009; 114: 390–402. [DOI] [PubMed] [Google Scholar]
- 16. Fernandez-Esparrach G, Gines A, Sanchez M, et al. Comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the diagnosis of pancreatobiliary diseases: a prospective study. Am J Gastroenterol 2007; 102: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt S, Chevallier P, Novellas S, et al. Choledocholithiasis: repetitive thick-slab single-shot projection magnetic resonance cholangiopancreatography versus endoscopic ultrasonography. Eur Radiol 2007; 17: 241–250. [DOI] [PubMed] [Google Scholar]
- 18. Chan YL, Chan AC, Lam WW. Choledocholithiasis: comparison of MR cholangiopancreatography and endoscopic retrograde cholangiography. Radiology 1996; 200: 85–89. [DOI] [PubMed] [Google Scholar]
- 19. Guibaud L, Bret PM, Reinhold C, et al. Bile duct obstruction and choledocholithiasis: diagnosis with MR cholangiography. Radiology 1995; 197: 109–115. [DOI] [PubMed] [Google Scholar]
- 20. Takehara Y, Katsutoschi I, Tooyama N, et al. Breath-hold MR cholangiopancreatography with a long echo train fast spin echo sequence and a surface coil in chronic pancreatitis. Radiology 1994; 192: 3–78. [DOI] [PubMed] [Google Scholar]
- 21. Matos C, Thierry M, Deviere J, et al. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology 1997; 203: 435–441. [DOI] [PubMed] [Google Scholar]
- 22. Balci C, Bieneman B, Bilgin M, et al. Magnetic resonance imaging in pancreatitis. Top Magn Reson Imaging 2009; 20: 25–20. [DOI] [PubMed] [Google Scholar]
- 23. Larson MM, Panciera DL, Ward DL, et al. Age-related changes in the ultrasound appearance of the normal feline pancreas. Vet Radiol Ultrasound 2005; 46: 238–242. [DOI] [PubMed] [Google Scholar]
- 24. Etue SM, Penninck DG, Labato MA, et al. Ultrasonography of the normal feline pancreas and associated anatomic landmarks: a prospective study of 20 cats. Vet Radiol Ultrasound 2001; 42: 220–226. [DOI] [PubMed] [Google Scholar]
- 25. Baron ML, Hecht S, Matthews AR, et al. Ultrasonographic observation of secretin-induced pancreatic duct dilation in healthy cats. Vet Radiol Ultrasound 2010; 51: 86–89. [DOI] [PubMed] [Google Scholar]
- 26. Marolf A, Stewart J, Dunphy T, Kraft S. Hepatic and pancreaticobiliary MRI and MR cholangiopancreatography with and without secretin stimulation in normal cats. Vet Radiol Ultrasound 2011; 52: 415–421. [DOI] [PubMed] [Google Scholar]
- 27. Leveille R, Biller D, Shiroma J. Sonographic evaluation of the common bile duct in cats. J of Vet Int Med 1996; 10: 296–299. [DOI] [PubMed] [Google Scholar]
- 28. Hittmair KM, Vielgrader HD, Loupal G. Ultrasonographic evaluation of gallbladder wall thickness in cats. Vet Radiol Ultrasound 2001; 42: 149–155. [DOI] [PubMed] [Google Scholar]
- 29. Hecht S, Penninck DG, Mahony OM, et al. Relationship of pancreatic duct dilation to age and clinical findings in cats. Vet Radiol Ultrasound 2006; 47: 287–294. [DOI] [PubMed] [Google Scholar]
- 30. Gagne J, Armstrong P, Weiss D, et al. Clinical features in inflammatory liver disease in cats: 41 cases (1983–1993). J Am Vet Med Assoc 1999; 241: 513–516. [PubMed] [Google Scholar]
- 31. Zawie D, Garvey M. Feline hepatic disease. Vet Clin North Am Small Anim Pract 1984; 14: 1201–1230. [DOI] [PubMed] [Google Scholar]
- 32. Mansfield C, Jones B. Review of feline pancreatitis part two: clinical signs, diagnosis and treatment. J Feline Med Surg 2001; 3:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman S, Steiner M, Woosley K, et al. Histologic assessment and grading of the exocrine pancreas in the dog. J Vet Diagn Invest 2006; 18: 115–118. [DOI] [PubMed] [Google Scholar]
- 34. Balci C. MRI assessment of chronic pancreatitis. Diagn Interv Radiol 2011; 17: 249–254. [DOI] [PubMed] [Google Scholar]
- 35. Wall M, Biller D, Schoning P, et al. Pancreatitis in a cat demonstrating pancreatic duct dilatation ultrasonographically. J Am Anim Hosp Assoc 2001; 37: 49–53. [DOI] [PubMed] [Google Scholar]
- 36. Choueiri N, Balci C, Alkaade S, et al. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep 2010; 12: 114–120. [DOI] [PubMed] [Google Scholar]
- 37. Manfredi R, Costamagna G, Brizi M, et al. Severe chronic pancreatitis versus suspected pancreatic disease: dynamic MR cholangiopancreatography after secretin stimulation. Radiology 2000; 214: 849–855. [DOI] [PubMed] [Google Scholar]
- 38. Remer E, Baker M. Imaging of chronic pancreatitis. Radiol Clin North Am; 2002; 40: 1229–1242. [DOI] [PubMed] [Google Scholar]
- 39. Catalano O, Sahani D, Sanjeeva P, et al. MR imaging of the gallbladder: a pictorial essay. Radiographics 2008; 28: 1335–1355. [DOI] [PubMed] [Google Scholar]
- 40. Marolf A, Leach L, Gibbons D, et al. Ultrasonographic findings in feline cholangitis. J Am Anim Hosp Assoc 2012; 48: 36–42. [DOI] [PubMed] [Google Scholar]
- 41. Harran N, d’Anjou M, Dunn M, et al. Gallbladder sludge on ultrasound is predictive of increased liver enzymes and total bilirubin in cats. Can Vet J, 2011; 52: 999–1003. [PMC free article] [PubMed] [Google Scholar]
- 42. Williams D. The pancreas. In: Guilford WG, Center SA, Strombeck DR, Williams DA.(eds). Strombeck’s small animal gastroenterology. 3rd ed. Philadelphia: WB Saunders, 1996, pp 381–410. [Google Scholar]