Abstract
‘Valentine’ heart shape is a common qualifier used in veterinary radiology to describe a cardiac silhouette with focal enlargement at the level of the base of the heart in feline patients. Anecdotally, this sign has been thought to be related to biatrial enlargement and also to hypertrophic cardiomyopathy (HCM). However, to our knowledge, there has been no study performed to assess the association between cardiac chamber enlargement and cardiac disease with the ‘valentine’-shaped heart. The aim of this study was to verify the association between the ‘valentine’ heart shape observed in ventrodorsal thoracic radiographs and the presence of singular or combined cardiac chamber enlargement, and also the presence and type of cardiomyopathy (CM) in cats. A search of the database of the Small Animal Veterinary Hospital of the University of Florida for cats with a radiology report of thoracic radiographs that contained the words ‘valentine’ and ‘biatrial’, and echocardiography performed within 1 week, was undertaken; 41 cases met the inclusion criteria. Eighty-two percent of the cats of the study sample had some form of CM. The ‘valentine’ heart shape was associated with biatrial enlargement in 41% of the patients in our study sample that had some form of CM and just 8% of cases diagnosed with HCM, suggesting that the ‘valentine’ heart shape has a low association with HCM or biatrial enlargement; however, it should be considered a sign of feline CM.
Introduction
Radiographic evaluation of the thoracic cavity is recognized as an excellent screening test for thoracic disease, and thoracic radiography is often used to evaluate the cardiac silhouette using the classic Roentgen terminology. Increased cardiac size is considered reliable evidence of heart disease, and its degree may determine severity. 1 Objective and semiobjective measures of cardiac size have been described.1–4 Additionally, changes in shape can be suggestive of focal cardiac enlargements. Often, radiographic findings that suggest cardiac enlargement – focal or generalized – are non-specific with regard to the underlying etiology of heart disease. Additional findings, including changes in pulmonary or great vessel size, as well as fluid accumulations (pleural effusion, pericardial effusion, pulmonary edema), must be present in order to narrow the differential diagnosis list. However, ‘valentine’ shape has been commonly used in textbooks, and is perpetuated anecdotally as a feature of feline hypertrophic cardiomyopathy (HCM).5–12
Objective cardiac measurements, such as the vertebral heart scale of size, aid in the assessment of cardiomegaly. 2 Objective radiographic evaluation of left atrial (LA) enlargement using a modified vertebral heart scale in cats has been described, with a sensitivity of 0.28–0.36, a specificity of 0.95, a positive predictive value (PPV) of 0.88–0.90, and a negative predictive value of 0.5–0.53. 13 Commonly, evaluations of the cardiac silhouette are subjective, 2 but shape changes are largely responsible for determining specific chamber enlargements, and often the clock-face analogy is employed. 14 Most texts agree that the ‘valentine’ shape is caused by LA or a combination of LA and right atrial (RA) enlargement with a shift of the apex toward midline.5,6,8,9,14–16 However, some debate exists on exactly which atrium is enlarged in the ‘valentine’-shaped heart. Some texts suggest that LA enlargement is most likely the cause of most ‘valentine’-shaped hearts, especially in cats with HCM, 10 while others suggest this shape occurs as a result of biatrial enlargement. 15
In one study of 21 cats with subclinical HCM, 43% (6/14) with cardiomegaly had a ‘valentine’-shaped cardiac silhouette. 17 Only 33% (2/6) had evidence of atrial enlargement, and 100% (2/2) with atrial enlargement had only LA enlargement, as determined by a LA/aortic (LA/Ao) ratio >2. 17 Additional references describing the ‘valentine’ shape or the combination of chamber enlargements that lead to the formation of the ‘valentine’ shape were not found. Therefore, the purpose of this study was to evaluate the echocardiographic findings in cats with cardiac silhouettes that were described as ‘valentine’-shaped or as having biatrial enlargement by veterinary radiologists in order to determine the association between ‘valentine’ shape and chamber enlargement, as well as echocardiographic diagnosis. We hypothesized that the ‘valentine’ shape is not specifically associated with feline HCM or with specific chamber enlargement.
Materials and methods
Case selection
For the purposes of the study, a ‘valentine’-shaped heart was defined on the ventrodorsal projection as those cardiac silhouettes having a broadening of the cranial aspect of the cardiac silhouette with or without a rightward shift of the caudally oriented cardiac apex. Retrospectively, the radiology information system at the University of Florida College of Veterinary Medicine was searched for all radiographic reports containing the word ‘valentine’ or ‘biatrial’ from January 2006 to January 2013. Of the cases retrieved, only those cases described as having a ‘valentine’ heart shape or biatrial enlargement, and that had echocardiography performed within 7 days of thoracic radiography, were included in our study sample.
Echocardiography
Echocardiographic reports for these cases were reviewed for each patient. Cardiac chamber enlargement and clinical diagnosis were recorded. Determination of LA enlargement was based on measurements of the maximal LA and Ao diameter in a right parasternal short-axis view of heart base and, from these, the LA/Ao ratio was calculated as previously described. 18 An LA/Ao >1.4 was considered enlarged. Evaluation of RA size was subjective and based on comparison with the LA in a four-chamber view from the right parasternal or left apical window. If the LA measured normally based on the LA/Ao and the RA was similar in size or smaller than the LA, the RA was considered normal in size. If the LA measured normally and the RA appeared larger than the LA, the RA was considered enlarged. If the LA measured enlarged with an LA/Ao ratio >1.4 and the RA appeared considerably smaller than the LA, the RA was considered to be normal in size. If the LA measured enlarged and the RA appeared proportional to the LA, the RA was considered enlarged. Determination of left ventricular (LV) size was based on measurements of diastolic wall thickness and diastolic internal dimension (LVIDd) from two dimensional (2D) and M-mode measurements. If either exceeded 5.5 mm for wall thickness and 1.80 cm for LVIDd, the LV was considered enlarged. Similar to the criteria for RA size, the assessment of right ventricular (RV) size was subjective and based on comparison with the LV in a four-chamber view from the right parasternal or left apical window. The RV was considered normal in size if the RV wall thickness and chamber dimension in diastole were less than or equal to one half of the normal LV free wall thickness in diastole (LVFWd) and LVIDd based on the aforementioned criteria. If the RV wall thickness or chamber dimension in diastole exceeded one half of the normal LVFWd or LVIDd, the RV was considered enlarged.
A diagnosis of HCM was made if any area of the LV walls measured >6.0 mm on 2D or M-mode echocardiography, and systemic blood pressure and thyroid hormone level were normal. 19 In order to make the diagnosis of restrictive cardiomyopathy (RCM), several factors were assessed. Pulsed-wave Doppler of transmitral inflows was completed, the peak velocity of the early diastolic transmitral flow (E wave) and transmitral flow due to atrial contraction (A wave) were measured, and the E/A ratio was calculated. RCM was diagnosed when there was considerable LA, RA or biatrial enlargement with normal or near-normal LV wall measurements (<6.0 mm) and either the E/A was >2.0, consistent with restrictive physiology, or hyperechoic structures were observed at the endocardial surfaces or bridging the LV chamber.20,21 A diagnosis of unclassified cardiomyopathy (UCM) was made when there was considerable LA, RA or biatrial enlargement with normal or near-normal LV wall thickness and the E/A was not consistent with restrictive filling or could not be calculated owing to E and A wave summation.22,23
Data analysis
The associations between a diagnosis of CM (yes, no) and chamber enlargements were tested using the Fisher’s χ2 test. The associations between different diagnoses of CM and chamber enlargements were tested using a χ2 test. P values ⩽0.05 were considered significant.
Results
Forty-one cats met the inclusion criteria. The median age of the study cats was 11 years (interquartile range [IQR] 7–13). Median age was significantly higher in cats affected with cardiomyopathies (12 years; IQR 8–13) compared with cats without cardiomyopathies (6 years; IQR 1–10) (P = 0.03). Similarly, median age was significantly higher in cats affected with LA enlargement (7 years; IQR 1–10) compared with cats without LA enlargement (6 years; IQR 8–14) (P <0.01).
The range of echocardiographic diagnoses included normal (N; n = 3) (Figure 1), HCM (n = 13) (Figure 2), RCM (n = 7) (Figure 3), UCM (n = 14) (Figure 4) and other (O; n = 4: mitral dysplasia [n = 1]; endocardiosis [n = 1]; Gerbode ventriculoseptal defect [n = 1]; volume overload [n = 1]). There were no cases of dilated CM in the study population; therefore, discussion is limited to non-dilated forms of CM. Based on these results, the PPV for a ‘valentine’ heart shape for CM is 82%; for cardiac disease in general, the PPV of a ‘valentine’ heart shape is 93%. With regard to breed, the sample population included domestic shorthair (n = 22), domestic longhair (n = 8), Persian (n = 2), Siamese (n = 2), Sphynx (n = 2), Balinese (n = 1), Bengal (n = 1), Birman (n = 1), Burmese (n = 1) and Maine Coon (n = 1).
Figure 1.

Ventrodorsal projection of a 10-year-old domestic shorthair showing equivocal enlargement characterized by a rightward apical shift and a bulge in the region of the right atrium, as well as a ‘valentine’ shape. No abnormalities were noted on echocardiography
Figure 2.

Ventrodorsal projection of a 14-year-old domestic shorthair cat with hypertrophic cardiomyopathy showing a markedly enlarged cardiac silhouette and a ‘valentine’ shape. On echocardiography, enlargement of only the left atrium was documented
Figure 3.
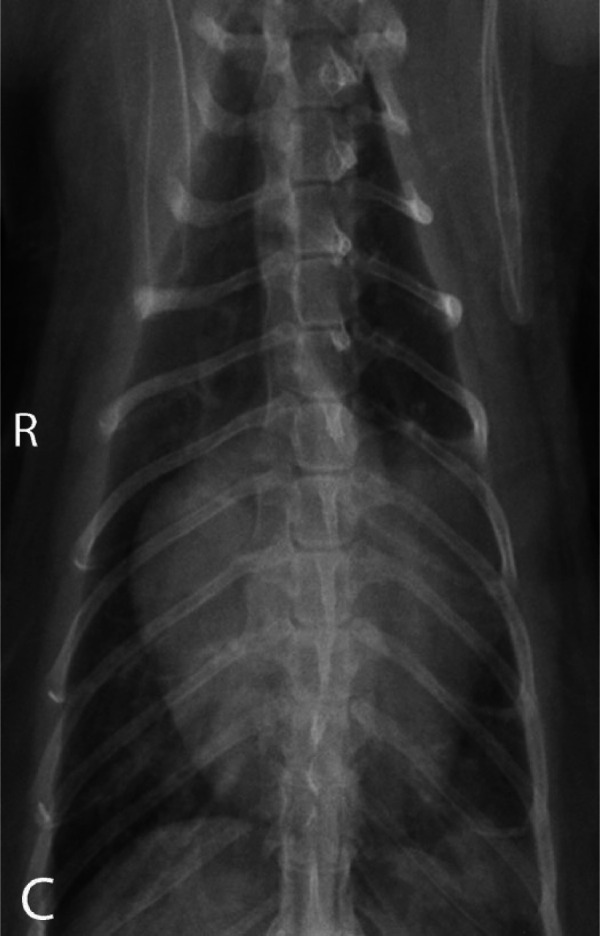
Ventrodorsal image of a 10-year-old domestic longhair cat with restrictive cardiomyopathy showing a markedly enlarged cardiac silhouette and a ‘valentine’ shape. On echocardiography, severe enlargement of only the left atrium was documented
Figure 4.

Ventrodorsal projection of a 14-year-old domestic shorthair cat with unclassified cardiomyopathy showing a markedly enlarged cardiac silhouette with a ‘valentine’ shape. On echocardiography, enlargement of the left atrium, left ventricle and right atrium was documented
The patterns of chamber enlargement are summarized in Table 1. Of the cats with a ‘valentine’-shaped heart, 34% (14/41) had LA and LV enlargement, 15% (6/41) had LA enlargement alone, 24% (10/41) had biatrial enlargement along with LV and/or RV enlargement, 7% (3/41) had LV enlargement alone, 5% (2/41) had biatrial enlargement, 5% (2/41) had biatrial and RV enlargement, and 3% (1/41) had only RA enlargement. No cats in the study population had RV enlargement alone or the combination of RA and RV enlargement. Cats with a ‘valentine’ heart shape with no cardiac chamber enlargements in the echocardiograms comprised 7% (3/41) of our study population.
Table 1.
Descriptive summary of the combination of chamber enlargement by type of cardiomyopathy
| Chamber enlargements | HCM | RCM | UCM | Others | Normal | Total |
|---|---|---|---|---|---|---|
| LA | – | 2 | 3 | 1 | – | 6 |
| RA | – | – | – | 1 | – | 1 |
| LV | 2 | – | – | 1 | – | 3 |
| RV | – | – | – | – | – | 0 |
| LA + RA | – | – | 2 | – | – | 2 |
| LA + LV | 10 | – | 3 | 1 | – | 14 |
| RA + RV | – | – | – | – | – | 0 |
| RA + LA + LV | 1 | 2 | 2 | – | – | 5 |
| RA + LA + RV | – | 1 | 1 | – | – | 2 |
| RA + LA + RV + LV | – | 2 | 3 | – | – | 5 |
| No chamber enlargement | – | – | – | – | 3 | 3 |
| Total | 13 | 7 | 14 | 4 | 3 | 41 |
HCM = hypertrophic cardiomyopathy; RCM = restrictive cardiomyopathy; UCM = unclassified cardiomyopathy; LA = left atrium; RA = right atrium; LV = left ventricle; RV = right ventricle
Associations between chamber enlargement and diagnosis are also summarized in Table 1.
In the study population of cats with a ‘valentine’-shaped heart, 83% (34/41) were diagnosed with some form of CM. Of cats with CM, 41% (14/34) were diagnosed with UCM, 38% (13/34) were diagnosed with HCM and 21% (7/34) were diagnosed with RCM. In cats with HCM, 85% (11/13) had a ‘valentine’ heart shape resulting from the combination of LA and LV enlargement. Combinations of chamber enlargement with concurrent biatrial enlargement resulted in a ‘valentine’ heart shape in only 8% (1/13) of cats with HCM.
The number of cats diagnosed with combinations of chamber enlargement and concurrent biatrial enlargement was significantly higher in cats with CM (14/34) compared with cats without CM (0/7) (P = 0.03). None of the cats with HCM with a ‘valentine’ heart shape had biatrial enlargement alone, and 8% (1/13) showed biatrial enlargement in combination with LV enlargement. Biatrial enlargement alone was not observed with RCM and occurred in combination with mild ventricular enlargement in 71% (5/7) of the cases. Biatrial enlargement alone was observed in 14% (2/14) of cats with UCM and occurred in combination with ventricular enlargement in 57% (8/14) of cases. This difference in the frequency of cats diagnosed with biatrial enlargement in combination with ventricular enlargement was significantly less for cats with HCM (1/13) compared with cats with other types of CM (13/21) (P = 0.05). In cats with diseases other than CM, none had evidence of biatrial enlargement concurrent with a ‘valentine’ heart shape. There were no cats with CM that had only RA enlargement. The one cat with RA enlargement was diagnosed with a Gerbode defect.
Discussion
In the study population, a ‘valentine’ heart shape was strongly associated with cardiac disease (38/41), yielding a false positive rate of 7%. The ‘valentine’ heart shape was also strongly correlated with non-dilated CM (34/41), yielding a false positive rate of 17%. The ‘valentine’ heart shape was most commonly seen in diseases causing LA enlargement or a combination of LA and LV enlargement (20/41). While the frequency of biatrial enlargement alone in cats with a ‘valentine’ heart shape was low (2/41), it is of interest to note that the combination of biatrial enlargement with LV and/or RV enlargement occurred in 34% of our study population.
UCM and HCM were the most common types of CM in the study sample (14 and 13/41 cases, respectively). This agrees, in part, with other studies in which HCM was the most common form of CM. 23 Based on the results of our study, it is not accurate to associate the finding of a ‘valentine’ heart shape with HCM, but rather with feline CM in general.
Biatrial enlargement in combination with other chamber enlargement was rarely seen in cases of HCM, and was more commonly seen in cases of RCM and UCM. Indeed, Ferasin et al 23 defined RCM in terms of having echocardiographic evidence of LA or biatrial enlargement, and UCM as potentially affecting both sides of the heart. It has also been stated that RCM is a subclassification of UCM, and not necessarily a separate entity; 24 therefore, similar radiographic features and patterns of atrial enlargement with UCM and RCM may not be unexpected. HCM is most commonly associated with left-sided enlargement, although biatrial enlargement has been reported. 25 In a retrospective study of feline idiopathic CM, 6.6% of cats with HCM had LA enlargement, and 6.6% of cats with HCM had RA enlargement, 23 suggesting that the atria could be equally affected in the study population. It should be noted that the percentage of cats with biatrial enlargement in that study was not reported.
It is important to note that three cases in our study sample were normal, suggesting that other factors may contribute to a ‘valentine’ shape. It has been shown that peri- or epicardial fat can affect assessment of cardiac size in obese cats. 3 While we did not specifically look at the effects of body condition or attempt to quantify the amount of pericardial fat in each of our patients, we speculate that a possible cause for our false positive result may be adiposity. Positioning and body conformation may also affect assessment of cardiac shape. Indeed, the presence of normal cats in our study sample reflects the subjective nature of the ‘valentine’ shape finding and factors other than cardiac disease that can affect cardiac shape.
Based on our findings, the recognition of a ‘valentine’ heart shape should not lead a clinician directly to a diagnosis of HCM or to the anatomic assumption of biatrial enlargement. However, the majority of patients in our study population did have some form of CM (83%; 34/41). Therefore, it may be reasonable to suspect that the anecdotally perpetuated observation that cats with HCM have a ‘valentine’ heart shape began when distinction between the different types of feline CM was less commonly performed. In addition, because the prevalence of HCM is higher than the sum of other types of CM, 23 it may be surmised that the majority of cats with the ‘valentine’ heart shape would likely have HCM rather than RCM or UCM in the population at large. However, it would perhaps be more accurate to state that the observation of a ‘valentine’ heart shape is most commonly seen in cases of CM. Finally, RA and biatrial enlargement have low prevalence. However, in cases of RCM, biatrial enlargement is more prevalent; therefore, the ‘valentine’ heart shape may be associated with biatrial enlargement in cases of RCM.
In our study population, cats with LA enlargement and with CM were older than those without LA enlargement or CM, with a median age of our CM population of 12 years compared with 6 years in the non-CM patients. This is not a surprising finding, and is in agreement with other studies that have found the median age of cats diagnosed with some form of CM to be 5.5 years with a range of 4 months to 16 years. 22
Our study is limited by a small study sample, particularly the low number of cats with RCM and cardiac abnormalities other than CM. We intentionally limited our study sample to those cats that had radiographs and echocardiograms in close temporal relation, which excluded some cats described as having a ‘valentine’ heart shape. In addition, patients with UCM may actually have had RCM or, potentially, end-stage HCM, but it is not always possible to clearly separate these types of CM. Finally, the assessment of right-sided cardiac chamber enlargement is subjective, and may affect our assessment of RA, biatrial and RV enlargement in our study population.
Conclusions
The ‘valentine’ heart shape was associated with LA enlargement in approximately 50% of our cases, and was associated with biatrial enlargement in approximately 34% of cases. In our study population, the ‘valentine’ heart shape was strongly associated with CM (83% of cases), but was not specific for HCM. The ‘valentine’ heart shape may occasionally be seen in cats without true cardiomegaly, and may rarely be seen in cats without atriomegaly.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 11 July 2014
References
- 1. Buchanan JW. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract 2000; 30: 379–393. [PubMed] [Google Scholar]
- 2. Litster AL, Buchanan JW. Vertebral scale system to measure heart size in radiographs of cats. J Am Vet Med Assoc 2000; 216: 210–214. [DOI] [PubMed] [Google Scholar]
- 3. Litster AL, Buchanan JW. Radiographic and echocardiographic measurement of the heart in obese cats. Vet Radiol Ultrasound 2000; 41: 320–325. [DOI] [PubMed] [Google Scholar]
- 4. Ghadiri A, Avizeh R, Rasekh A, et al. Radiographic measurement of vertebral heart size in healthy stray cats. J Feline Med Surg 2008; 10: 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tilley LP. Hypertrophic cardiomyopathy. In: Norsworthy GD, Grace SF, Crystal MA, et al. (eds). The feline patient. Ames: Wiley-Blackwell, 2010, pp 261–264. [Google Scholar]
- 6. Owens JM, Biery DN. Heart. In: Owens JM, Biery DN. (eds). Radiographic interpretation for the small animal clinician. Philadelphia: Williams & Wilkins, 1999, pp 185–216. [Google Scholar]
- 7. Fox P. Restrictive cardiomyopathy. In: Proceedings of the ACVIM Forum ACVIM, San Antonio, TX, May 23-26, 1996, p 235. [Google Scholar]
- 8. French A, Wotton P. The cardiovascular system. In: Chandler EA, Gaskell RM, Gaskell CJ. (eds). Feline medicine and therapeutics. 3rd ed. Oxford: Blackwell, 2008, pp 493–526. [Google Scholar]
- 9. Burk RL. Radiographic examination of the cardiopulmonary system. Vet Clin North Am Small Anim Pract 1983; 13: 241–258. [DOI] [PubMed] [Google Scholar]
- 10. Rishniw M. Radiography of feline cardiac disease. Vet Clin North Am Small Anim Pract 2000; 30: 395–425. [PubMed] [Google Scholar]
- 11. Hause WR. Management of acute illness in cats. Mod Vet Pract 1984; 65: 461–465. [PubMed] [Google Scholar]
- 12. Hamlin RL, Smetzer DL, Smith CR. Radiographic anatomy of the normal cat heart. J Am Vet Med Assoc 1963; 143: 957–961. [PubMed] [Google Scholar]
- 13. Schober KE, Maerz I, Ludewig E, et al. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2-dimensional echocardiography. J Vet Intern Med 2007; 21: 709–718. [DOI] [PubMed] [Google Scholar]
- 14. Bahr R. The heart and pulmonary vessels. In: Thrall D. (ed). Textbook of veterinary diagnostic radiology. 6th ed. St Louis, MO, Saunders, 2012, pp 585–607. [Google Scholar]
- 15. Ferasin L. Feline myocardial disease 2: diagnosis, prognosis and clinical management. J Feline Med Surg 2009; 11: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrtage M. Cardiovascular disorders. In: Schaer M. (ed). Clinical medicine of the dog and cat. 3rd ed. Barcelona: Manson Publishing, 2010, pp 141–186. [Google Scholar]
- 17. Taillefer M, Di Fruscia R. Benazepril and subclinical feline hypertrophic cardiomyopathy: a prospective, blinded, controlled study. Can Vet J 2006; 47: 437–445. [PMC free article] [PubMed] [Google Scholar]
- 18. Abbott JA, MacLean HN. Two-dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med 2006; 20: 111–119. [DOI] [PubMed] [Google Scholar]
- 19. Fox PR, Liu S-K, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy: an animal model of human disease. Circulation 1995; 92: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 20. Fox PR. Endomyocardial fibrosis and restrictive cardiomyopathy: pathologic and clinical features. J Vet Cardiol 2004; 6: 25–31. [DOI] [PubMed] [Google Scholar]
- 21. Boon JA. Acquired heart disease. In: Veterinary echocardiography. Ames: Wiley-Blackwell, 2011. [Google Scholar]
- 22. Ferasin L. Feline myocardial disease 1: classification, pathophysiology and clinical presentation. J Feline Med Surg 2009; 11: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kittleson MD. Feline myocardial disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. St Louis, MO: Elsevier, 2005, pp 1082–1104. [Google Scholar]
- 25. Kittleson M, Kienle R. Hypertrophic cardiomyopathy. In: Small animal cardiovascular medicine. St Louis, MO: Mosby, 1998, pp 347–362. [Google Scholar]


