Abstract
Hodgkin’s-like lymphoma is a slow growing neoplasm, usually affecting the lymph nodes of the head and neck, which has been sporadically described in veterinary patients. This report describes the clinical and histopathological features in a 9-year-old male neutered Siamese cat that presented with a 6 week history of mid-dorsocranial swelling. Immunohistochemistry demonstrated positive staining for CD79a, paired box protein and B lymphocyte antigen-36, with variable, weak-to-moderate cytoplasmic staining for human leukocyte antigen-DR and CD18, and negative staining for antimacrophage antibody. The diagnosis based on incisional biopsy was Hodgkin’s-like lymphoma; however, no evidence of neoplasia was found following wide surgical excision. This case report demonstrates two unreported items of note: the novel extranodal site of Hodgkin’s-like lymphoma in a cat and tumour regression following initial biopsy. It is hypothesised that the surgical trauma of biopsying the lesion or the introduction of foreign material may have caused the regression of the neoplastic cells through induction of an anti-tumour immune or inflammatory response.
Case Report
A 9-year-old, 5 kg, male neutered, indoor and outdoor Siamese cat presented with a 6 week history of progressive swelling of the mid-dorsal cranium that was not preceded by trauma and was not associated with systemic clinical signs. The mass (10 mm wide × 20 mm long) was smooth, minimally mobile and not painful on palpation. No superficial pathology was noted and it was deemed to be located within the cutaneous tissue. Clinical examination was otherwise unremarkable, with no lymphadenopathy palpated. Feline leukaemia virus and feline immunodeficiency virus serology was negative. Three days after initial presentation, two wedge biopsies were taken through a single skin incision from the centre of the lesion under general anaesthesia and under standard sterile conditions. A lateral skull radiograph taken at the time of biopsy demonstrated a small soft tissue-density cutaneous mass in the area corresponding to the skin lesion with no associated periosteal reaction. The defect created was closed routinely in two layers using 2 metric chromic catgut (Catgut Chrom; SMI) subcutaneously and 2 metric polyamide sheathed multifilament (Supramid White; SMI) in the skin. Findings on histopathological examination were consistent with Hodgkin’s-like lymphoma. Ten days after initial presentation, further investigations continued to rule out concurrent disease, paraneoplastic syndromes and metastatic spread. The cat remained otherwise healthy. Complete blood count was within normal limits with normal cell morphology on smear examination. Biochemistry showed mild non-specific findings with creatine kinase moderately elevated at 287 µl/l (range 0–152 µl/l), this was likely due to muscle damage from the biopsy or from the intramuscular injection as part of the premedication protocol. Radiographs of the thorax and abdomen were unremarkable, as was the abdominal ultrasound. The mass was excised surgically 25 days after initial presentation with wide margins (20 mm co-laterally, 25 mm cranially and caudally, and one fascial plane deep); the defect was subsequently closed using a single-pedicle advancement flap (Figures 1 and 2). Recovery was uneventful, with sutures removed 21 days postsurgery, extended from the usual 10–14 days owing to mild overlapping of the wound edges. The cat continued to be clinically well; the owner was last contacted 8 months postsurgery.
Figure 1.
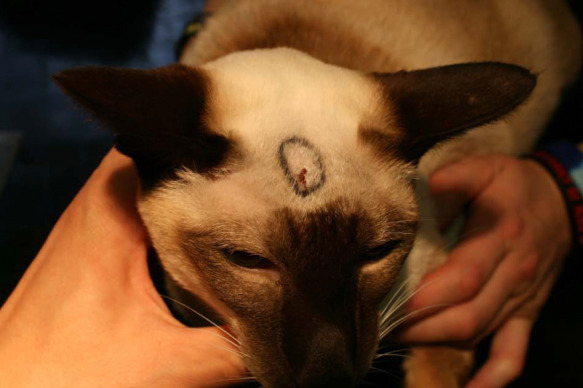
Presurgical resection with the mass outlined for surgical planning. The mass measured approximately 20 mm × 10 mm
Figure 2.
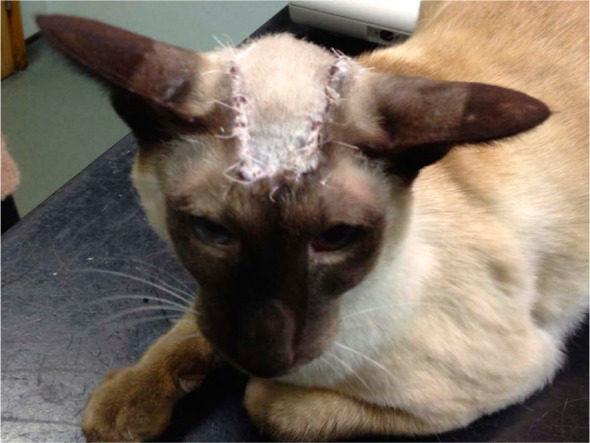
Picture taken prior to suture removal 21 days postsurgical resection
The initial incisional biopsies consisted of connective tissue heavily infiltrated by heterogeneous populations of cells. There were multiple areas of pyogranulomatous inflammation and necrosis. There were also areas where the tissue was infiltrated by a population of large neoplastic round cells, which formed variably dense sheets supported by a fine fibrovascular stroma (Figure 3). These cells had indistinct borders and scant-to-moderate amounts of pale, vacuolated cytoplasm. Nuclei were large, rounded-to-irregular-to-lobed, and they contained vesicular chromatin and often-prominent magenta nucleoli. There was moderate anisokaryosis and anisocytosis in this population. Mitotic figures were up to five per high power fields. These cells were mixed with moderate numbers of small lymphocytes, which were fairly evenly scattered between the neoplastic cells as well as fewer macrophages. Special stains (periodic acid–Schiff and Ziehl–Neelsen) were negative, excluding an inflammatory origin of this lesion. Immunohistochemical stains were used to further diagnose the neoplastic cells, including CD79a (B lymphocyte marker), CD18 (a panleukocyte marker) and human leukocyte antigen (HLA)-DR (expressed by all cells involved in antigen presentation). The majority of the neoplastic cells demonstrated moderate-to-strong cytoplasmic staining for CD79a (Figure 4). A proportion of these cells also demonstrated moderate staining for HLA-DR and CD18. The atypical cells were negative for CD3 (T-lymphocyte marker), but this antibody did mark moderate numbers of reactive small T lymphocytes scattered between the neoplastic cells. Following these findings, additional stains were performed to further characterise the atypical cells. The atypical cells did not stain for antimacrophage antibody (MAC 387), refuting a histiocytic origin of these cells; however, they did stain positive for paired box protein (PAX-5; Figure 5) and BLA-36 (Figure 6), which are specific B lymphocyte markers.
Figure 3.
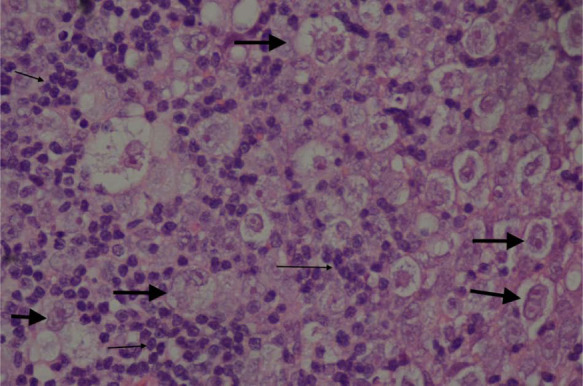
Haematoxylin and eosin section at high power (x 400) demonstrating the neoplastic cells (arrowed) from the initial wedge biopsy. Larger arrows indicate neoplastic cells. Smaller arrows indicate clusters of small reactive lymphocytes
Figure 4.
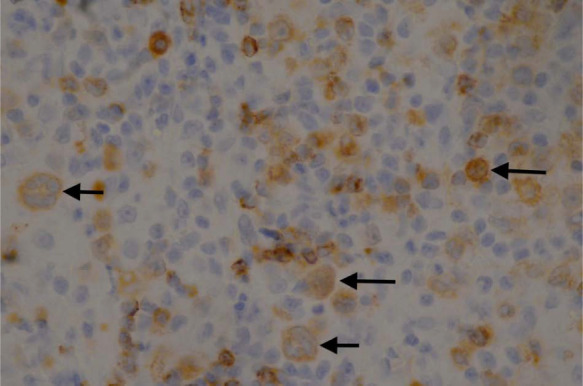
Immunohistochemistry demonstrating the atypical cells (arrowed) staining positive for CD79a (B-cell marker) from the initial wedge biopsy
Figure 5.
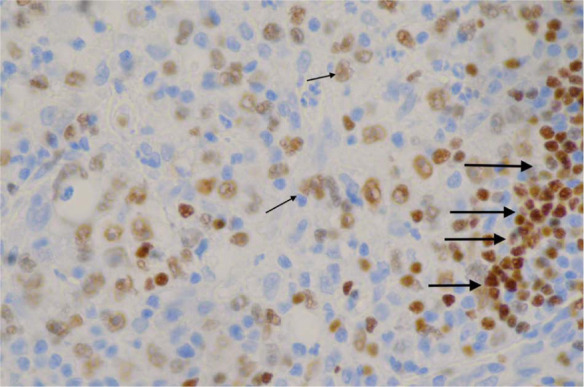
Immunohistochemistry demonstrating the atypical cells (arrowed) staining positive for paired box protein (B-cell marker) from the initial wedge biopsy. Small arrows indicate positively staining neoplastic cell nuclei. Larger arrows highlight a cluster of reactive small B lymphocytes
Figure 6.
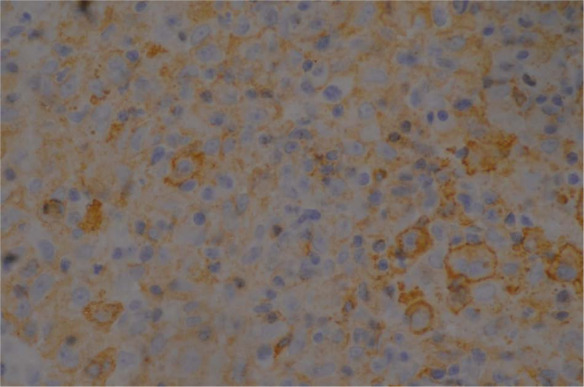
Immunohistochemistry demonstrating the atypical cells staining positive for B lymphocyte antigen (B-cell marker) from the initial wedge biopsy
Histopathology of the postsurgical specimen revealed a single aggregate of plant material embedded in the subcutis, surrounded by accumulations of epithelioid and multinucleated macrophages. In addition, there were aggregates of suture material, surrounded bylarge numbers of epithelioid and multinucleated macrophages, and moderate numbers of lymphocytes, plasma cells and scattered neutrophils within the subcutis and extending the underlying skeletal muscle. There was mild fibrosis around this focus. No neoplastic cells were found. The histological appearance in this specimen was compatible with a diagnosis of granulomatous inflammation with intralesional suture and plant material.
The morphological features and immunohistochemical staining pattern of the tumour in the initial biopsy specimens were compatible with a diagnosis of Hodgkin’s-like lymphoma. Similar lesions in cats have also been diagnosed as T-cell-rich B-cell lymphoma, 1 but there is no consensus on the nomenclature of the tumours in the veterinary literature. In humans, differentiating between these tumour types is not straightforward and misdiagnosis has been previously noted in up to 26% of cases.2,3 Differentiation is important in humans as these tumours have a significantly different prognosis. Five forms of Hodgkin’s lymphoma are described in humans, and identification of Reed–Sternberg (RS) cells is requisite for confirming diagnosis. Classic RS cells are found in all forms, but are rare and difficult to find in the lymphocyte-predominant subtype where the so-called lymphohistiocytic variant of the RS cells predominates. The neoplastic cells found in the present case most closely resemble this variant of the RS cells, which are also described in the lymphocyte-predominant subtype of a series of Hodgkin’s-like lymphoma in cats. 4 That study also describes positive CD79a immunohistochemical staining of the lymphohistiocytic variant of RS cells, along with positive CD3 staining of a population of reactive T lymphocytes in the background of the lymphocyte predominate subtype of this tumour. A very similar staining pattern was demonstrated in our case.
Hodgkin’s-like lymphoma is a slow-growing neoplasm that predominantly affects one or a chain of lymph nodes and spreads contiguously. 5 It is usually found in the head or neck, and appears to be a less aggressive form of lymphoma. 4 It has been previously reported in both domestic and non-domestic species.1,6 –18 There have been several cases in humans that presented as a solitary subcutaneous mass,19–22 but this is very rare, affecting approximately 0.5–3.4% of patients with Hodgkin’s lymphoma.23,24 Several extranodal lymphoma cases have been reported in domestic animals. In 31 cases of equine lymphoma, 11 had Hodgkin’s-like lymphoma, with eight of these having subcutaneous lesions and seven of those horses having only subcutaneous lesions. 9 A separate horse had a subcutaneous Hodgkin’s-like lymphoma mass that regressed following removal of an ovarian granulosa theca cell tumour. 7 A dog was reported to have an orbital Hodgkin’s-like lymphoma mass. 6 A cat with a conjunctival Hodgkin’s-like lymphoma has also been reported, although this cat had concurrent lymphadenopathy for an unknown reason. 8 Hodgkin’s-like lymphoma appears to have a better prognosis than non-Hodgkin’s lymphoma, whereby surgical resection of affected lymph nodes has been reported to be curative in both humans and animals.15,25
The phenomenon of cancer regression in humans is well known; it was first reported in the literature in 1899 with many subsequent reports since then. 26–32 Hodgkin’s lymphoma is no different, with 14 cases of regression reported.21,33–36 Papac reports that cutaneous and pulmonary locations seem to be predilection sites for regression, and postulates that the micro-environment plays a role in regression. 31 In animals, regression of Hodgkin’s-like lymphoma has been reported once, in a horse, with a subcutaneous mass regressing following removal of an ovarian granulosa theca cell tumour, although the reason for this is unclear. 7 It is interesting to note the theory put forward by Franklin of ‘the more common the tumour, the less likely the regression will be’, 37 suggesting a possible immune tolerance in common neoplasms. The exact mechanisms of tumour regression are not fully understood, although the role of immunoglobulins, interferons, infection, enzymes and many other factors have been discussed.27,28,31
The cause of tumour regression in this case is uncertain, although two distinct hypotheses are proposed. The organic plant material may have been present prior to neoplastic transformation, meaning that it may have contributed to neoplastic change. Alternatively, it may have been inoculated peri- or postbiopsy, in which case it may have had an influence on the destruction of the tumour by stimulating an immune response. Or, trauma caused by the biopsy method itself may have stimulated an immune response responsible for initiating tumour regression. Either way, it is hypothesised that the tumour regression was due to the inflammatory response and the immune reaction caused by the presence of the foreign body or the surgical trauma itself. Regression following surgical trauma has been reported in humans,27,28,31 with the hypotheses of increased immunogenicity to tumour growth,29,37 and possible compromise of tumour blood supply and subsequent necrosis. 38 An additional hypothesis would be that the three elements – inoculated plant material, surgical trauma during biopsy and spontaneous regression of the neoplasm – could be entirely unrelated and therefore completely coincidental. A tumour-free margin postincisional biopsy was not apparent in the sections examined histologically, and during the initial surgery the mass grossly appeared to extend beyond the biopsy incisions, with the appearance being diffusely homogenous. Therefore, it is unlikely that all the neoplastic tissue was removed surgically at that time.
Conclusions
This report illustrates a novel presentation of Hodgkin’s-like lymphoma in the cat and offers an alternative differential for a mass located on the head. This case highlights the importance of histopathology (postbiopsy and after full resection). Further work needs to be undertaken to investigate both Hodgkin’s-like lymphoma, including its classification, and the phenomenon of tumour regression in animals.
Acknowledgments
We thank Maggie Costello MVB, DVR, DipECVDI, MRCVS, for her contribution and specialist diagnostic imaging of this case.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
Accepted: 2 June 2014
References
- 1. Day MJ, Silkstona MA, Luck VM, et al. T-cell-rich B-cell lymphoma in the cat. J Comp Pathol 1999; 120: 155–167. [DOI] [PubMed] [Google Scholar]
- 2. Chittal SM, Brousset P, Voigt JJ, et al. Large B-cell lymphoma rich in T-cells and simulating Hodgkin’s disease. Histopathology 1991; 19: 211–220. [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez J, Pug WC, Cabanillas F. T-cell-rich B-cell lymphoma. Blood 1993; 82: 1586–1589. [PubMed] [Google Scholar]
- 4. Walton RM, Hendrick MJ. Feline Hodgkin’s-like Lymphoma: 20 Cases (1992–1999). Vet Pathol 2001; 38: 504–511. [DOI] [PubMed] [Google Scholar]
- 5. Jose BO, Koerner P, Spanos WJ, Jr, et al. Hodgkin’s lymphoma in adults – clinical features. J Ky Med Assoc 2005; 103: 15–17. [PubMed] [Google Scholar]
- 6. Aquino SM, Hamor RE, Valli VE, et al. Progression of an orbital T-cell rich B-cell lymphoma to a B-cell lymphoma in a dog. Vet Pathol 2000; 37: 465–469. [DOI] [PubMed] [Google Scholar]
- 7. Henson KL, Alleman AR, Cutler TJ, et al. Regression of subcutaneous lymphoma following removal of an ovarian granulosa theca cell tumor in a horse. J Am Vet Med Assoc 1998; 212: 1419–1422. [PubMed] [Google Scholar]
- 8. Holt E, Goldschmidt MH, Skorupski K. Extranodal conjunctival Hodgkin’s-like lymphoma in a cat. Am J Ophthalmol 2006; 9: 141–144. [DOI] [PubMed] [Google Scholar]
- 9. Kelley LC, Mahaffey EA. Equine malignant lymphomas: morphologic and immunohistochemical classification. Vet Pathol 1998; 35: 241–252. [DOI] [PubMed] [Google Scholar]
- 10. Maeda H, Ozaki K, Honaga S, et al. Hodgkin’s-like lymphoma in a dog. Zentralbl Veterinarmed A 1993; 40: 200–204. [DOI] [PubMed] [Google Scholar]
- 11. Majeed SK, Gopinath C. Hodgkin’s disease-like lesion in a rat. J Comp Pathol 1985; 95: 123–126. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama H, Nakanaga K, Ogihara S, et al. Three cases of feline lymphosarcoma with formation of multinucleated giant cells. Nippon Ika Daigaku Zasshi 1984; 46: 225–228. [DOI] [PubMed] [Google Scholar]
- 13. Roperto F, Damiano S, Galati P. Hodgkin’s disease in a cat. Zentralbl Veterinarmed A 1983; 30: 182–188. [DOI] [PubMed] [Google Scholar]
- 14. Smith DA, Barker IK. Four cases of Hodgkin’s disease in striped skunks (Mephitis mephitis). Vet Pathol 1983; 20: 223–229. [DOI] [PubMed] [Google Scholar]
- 15. Steele KE, Saunder GK, Coleman GD. T-cell-rich B-cell lymphoma in a cat. Vet Pathol 1997; 34: 47–49. [DOI] [PubMed] [Google Scholar]
- 16. Tanimoto T, Ohtsuki Y. T-cell-rich B-cell lymphoma in a pig. Vet Pathol 1998; 35: 147–149. [DOI] [PubMed] [Google Scholar]
- 17. Wells GAH. Hodgkin’s disease-like lesions in the dog. J Pathol 1974; 112: 5–10. [DOI] [PubMed] [Google Scholar]
- 18. Yonezawa M, Nakamine H, Tanaka T, et al. Hodgkin’s disease in a killer whale (Orcinus orca). J Comp Pathol 1989; 100: 203–207. [DOI] [PubMed] [Google Scholar]
- 19. Mukesh M, Shuttleworth D, Murray P. Primary cutaneous Hodgkin’s lymphoma. Clin Exp Dermatol 2009; 34: 673–675. [DOI] [PubMed] [Google Scholar]
- 20. Sioutos N, Kerl H, Murphy SB, et al. Primary cutaneous Hodgkin’s disease. Unique clinical, morphological and immunophenotypic findings. Am J Dermatopathol 1994; 16: 2–8. [DOI] [PubMed] [Google Scholar]
- 21. Szur L, Harrison CV, Levene GM, et al. Primary cutaneous Hodgkin’s disease. Lancet 1970; 1: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 22. Van der Meiren L. Three cases of Hodgkin’s disease with predominant cutaneous localization. Br J Dermatol 1948; 60: 181–185. [DOI] [PubMed] [Google Scholar]
- 23. Smith J, Butler J. Skin involvement in Hodgkin’s disease. Cancer 1980; 45: 354–361. [DOI] [PubMed] [Google Scholar]
- 24. White R, Patterson J. Cutaneous involvement in Hodgkin’s disease. Cancer 1985; 55: 1136–1145. [DOI] [PubMed] [Google Scholar]
- 25. Aster J, Kumar V. The hematopoietic and lymphoid systems. In: Cotran RS, Kumar V, Collins T. (eds) Pathologic basis of disease. 6th ed. Philadelphia, PA: WB Saunders, 1999, pp 644–695. [Google Scholar]
- 26. Bennett WH. Some peculiarities in the behaviour of certain malignant and innocent growths. Lancet 1899; 1: 3–7. [Google Scholar]
- 27. Challis GB, Stam HJ. The spontaneous regression of cancer. Acta Oncol 1989; 29: 545–550. [DOI] [PubMed] [Google Scholar]
- 28. Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol 1981; 17: 201–209. [DOI] [PubMed] [Google Scholar]
- 29. Cole WH. Spontaneous regression of cancer: the metabolic triumph of the host? Ann N Y Acad Sci 1974; 230: 111–141. [DOI] [PubMed] [Google Scholar]
- 30. Everson TC. Spontaneous regression of cancer. Ann N Y Acad Sci 1964; 114: 721–735. [PubMed] [Google Scholar]
- 31. Papac RJ. Spontaneous regression of cancer. Cancer Treat Rev 1996; 22: 395–423. [DOI] [PubMed] [Google Scholar]
- 32. Rohdenburg GL. Fluctuations in the growth energy of malignant tumours in man, with especial reference to spontaneous regression. J Cancer Res 1918; 3: 192–221. [Google Scholar]
- 33. Mangel J, Bart D, MacEachren J, et al. Lymphoproliferative disease. Am J Hematol 2003; 8: 191–196. [Google Scholar]
- 34. Taqi AM, Abdurrahman MB, Yakubu AM, et al. Regression of Hodgkin’s disease after measles. Lancet 1981; 16: 1112. [DOI] [PubMed] [Google Scholar]
- 35. Williams MV. Spontaneous regression of cutaneous Hodgkin’s disease. BMJ 1980; 280: 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zygiert Z. Hodgkin’s disease: remissions after measles. Lancet 1971; 20: 593. [DOI] [PubMed] [Google Scholar]
- 37. Franklin CI. Spontaneous regression in cancer. In: Stoll BA. (ed). Prolonged arrest of cancer. Toronto: JB Wiley, 1982, pp 103–116. [Google Scholar]
- 38. Parbhoo S. Necrosis in cancer tissue. In: Stoll BA. (ed). Prolonged arrest of cancer. Toronto: JB Wiley, 1982, pp 243–280. [Google Scholar]


