Abstract
Obesity is a risk factor in the development of several respiratory diseases. Lung volumes tend to be decreased, especially expiratory reserve volume, increasing expiratory flow limitation during tidal breathing. Barometric whole-body plethysmography is a non-invasive pulmonary function test that allows a dynamic study of breathing patterns. The objective of this study was to compare pulmonary function variables between obese and non-obese cats through the use of barometric whole-body plethysmography. Nine normal-weight and six obese cats were placed in the plethysmograph chamber, and different respiratory variables were measured. There was a significant decrease in tidal volume per kilogram (P = 0.003), minute volume per kilogram (P = 0.001) and peak inspiratory and expiratory flows per kilogram (P = 0.001) in obese cats compared with non-obese cats. Obesity failed to demonstrate a significant increase in bronchoconstriction index variable enhanced pause (Penh), as previously reported in humans and dogs. The results show that feline obesity impairs pulmonary function in cats, although a significant increase in bronchoconstriction indexes was not observed. Non-invasive barometric whole-body plethysmography can help characterise mechanical dysfunction of the airways in obese cats.
Introduction
Obesity is a very common health problem in domestic cats. Obesity is defined as a pathological condition in which an excessive amount of adipose tissue is deposited, which can be either due to cellular hypertrophy (enlargement of cell size) or hyperplasia (increase in the number of cells). In humans, hypertrophy of adipocytes causes alteration in the production of adipokines, leading to chronic low-grade inflammation. Adipose tissue secretes several substances, including steroid hormones, growth factors and cytokines, eicosanoids, and so on.1–4 In contrast, hyperplasia has been shown to exert a protective effect against obesity-related disorders, in particular insulin resistance. 5
Obesity is recognised as an important risk factor for the development of several respiratory dysfunctions. Obesity impacts on respiratory physiology producing alterations in respiratory mechanics, reducing respiratory system compliance, muscle strength, maximal inspiratory and expiratory pressures, maximum voluntary ventilation and lung volumes (forced vital capacity, expiratory reserve volume, forced residual capacity and total lung capacity). As a consequence, the work and oxygen cost of breathing are usually increased. 6
It has been described that feline obesity prevalence varies between 19% (New Zealand) 7 and 52% (UK). 8 Epidemiological studies have identified several risk factors for the development of obesity, which vary according to local feeding and housing practices across the world.9,10
In human medicine, pulmonary function tests (PFTs) are widely used to characterise lung function 11 as well as response to therapy objectively. PFTs have been used in dogs and cats. However, these tests can be challenging, as most PFTs for people require voluntary maximal respiratory effort, which is not possible in animals.12–18 Barometric whole-body plethysmography (BWBP) is a non-invasive PFT that allows a dynamic study of breathing in dogs and cats.19–22
To the authors’ knowledge, there are no previous studies describing how obesity affects lung mechanics and function in cats. Therefore, the aims of this study were to evaluate pulmonary function variables in obese and non-obese cats by using BWBP, and to compare the results between both groups.
Materials and methods
Procedure
Cats included in the present study were recruited from the Hospital Veterinari Molins (Barcelona, Spain) from January 2011 to December 2012. All patients were client-owned cats, and none of them had a previous history of upper airway, cardiac or endocrine diseases. Additional inclusion criteria were having been dewormed within the 3 months prior to the study, no exposure to passive smoke inhalation, and negative results for feline leukaemia virus, feline immunodeficiency virus and heartworm. The animals studied presented normal cardiopulmonary auscultation and physical examination except for the weight status. Radiological evaluation of the thorax was normal in all cases. The body condition score (BCS) was determined using a nine-point scale. 23 Cats were considered to be of normal weight when presenting a BCS of 4/9 and 5/9, and were considered obese when the BCS was 7/9, 8/9 and 9/9.
The study was approved by the ethical committee of Veterinary Medicine Service of Las Palmas de Gran Canaria University (Spain), and it was carried out in accordance with the current European legislation on animal protection. The owners provided informed consent for their cats’ enrolment.
Animals
Nine normal-weight cats were included in this study (three males and six females), ranging from 2–12.5 years of age with a mean age of 6.08 years. Breed distribution was: European domestic shorthair (n = 7), Persian (n = 1) and Siamese (n = 1). All were indoor and sexually intact cats. Three (33.3%) presented a BCS of 4/9; the other six cats (66.6%) had a BCS of 5/9. Body weight (BW) ranged from 3–4 kg (mean ± SD: 3.44 ± 0.46 kg).
Six obese cats were included in the present study. Obesity was chronic (>12 months). Four were males and two were females, ranging from 4.2–14 years of age with a mean age of 9.05 years. Five cats were European domestic shorthairs and one was Siamese. All cats were indoor and sexually intact. Four cats (66.6%) had a BCS of 8/9; the other two (33.3%) presented a BCS of 9/9. BW ranged from 6.5–9.2 kg (mean ± SD: 7.30 ± 1.04 kg).
Barometric whole-body plethysmography
BWBP was performed by placing the cats in a transparent Plexiglass chamber (Unrestrained WBP plethysmograph PLY4219; Buxco Europe), with dimensions of 25 cm (h) × 51 cm (l) × 30 cm (w) (Figure 1). The chamber was ventilated with a continuous bias air flow (Buxco BFL0250) at 10 l/min. A MAX2275 card was used, which incorporated a calibration signal as well as the external gain. The process of calibration assigns appropriate units to the signals coming from the transducers, and should be conducted after the computer and electronics have been turned on and before recording data. Gain will fine-tune the sensitivity of the signal. The plethysmograph was connected to a preamplifier, and commercial software was used for analysis (Biosystem XA v2.10.1; Buxco Europe). The box pressure signal from the plethysmograph was calibrated before each recording by injecting 50 ml of air into the chamber with a calibrated syringe. The temperature and humidity of the room where the procedure was carried out were held constant at 20–23ºC and 60–65% humidity. Cats were acclimatised to the chamber for 5 mins, after which data recordings were taken over a period of 12 mins. The values were averaged.
Figure 1.
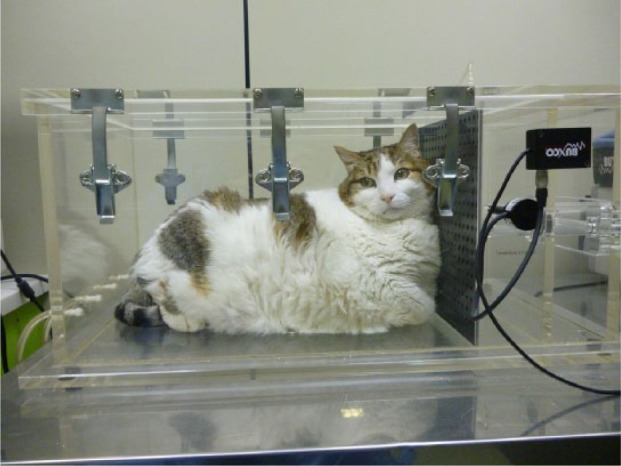
Obese cat placed in the barometric whole-body plethysmography chamber. The cat is very tolerant and seems to be very comfortable during testing
Pressure signals were recorded to obtain values in order to study the following pulmonary function variables: respiratory rate (RR [rpm]), tidal volume (TV [ml/kg]), minute volume (MV [ml/kg]), inspiratory (Ti [s]) and expiratory (Te [s]) intervals, Ti/Te ratio, bronchoconstriction index enhanced pause (Penh) and peak inspiratory and expiratory flows (PIF and PEF [ml/s]).
Waveforms with artefacts from sniffing or movement were automatically eliminated using the software if TV was <3 ml, Ti <0.1 s, Te >10 s or when there was a difference in inspiratory and expiratory volume of >60%. Cats were continuously monitored for signs of stress.
In order to standardise TV, MV, PIF and PEF according to BW, these parameters were divided by BW and called TV/BW, MV/BW, PIF/BW and PEF/BW, respectively.
Statistical study
Data are expressed as mean ± SD. Comparisons were performed by the non-parametric Mann-Whitney U-test and Fisher’s exact test. A P <0.05 was considered statistically significant. The statistical software SPSS v19.0 was used for this statistical study.
Results
Statistically significant differences were observed in TV/BW (P = 0.003), MV/BW (P = 0.001), PIF/BW (P = 0.001) and PEF/BW (P = 0.001) comparing obese and non-obese cats. There were no significant differences for RR, Te and Ti intervals, Ti/Te ratio and Penh between both groups of cats. The results are summarised in Table 1. There were no statistically significant differences in all lung function variables in gender distribution between groups (P = 0.315) or in age distribution between both groups (P = 0.125).
Table 1.
Means and SD of analysed barometric whole-body plethysmography variables and intergroup P values between obese and non-obese cats
| Cats | RR (rpm) | TV/BW (ml/kg) | MV/BW (ml/kg) | Ti (s) | Te (s) | Ti/Te | Penh | PIF/BW (ml/s kg) | PEF/BW (ml/s kg) |
|---|---|---|---|---|---|---|---|---|---|
| Non-obese (n = 9) | 59.074 ± 19.337 | 10.378 ± 3.931 | 508.586 ± 92.016 | 0.498 ± 0.122 | 0.784 ± 0.330 | 0.681 ± 0.143 | 0.389 ± 0.127 | 31.46 ± 5.396 | 21.616 ± 4.210 |
| Obese (n = 6) | 47.026 ± 25.57 | 4.775 ± 1.309 | 192.061 ± 47.718 | 0.592 ± 0.193 | 1.022 ± 0.482 | 0.607 ± 0.086 | 0.460 ± 0.082 | 12.495 ± 2.978 | 7.504 ± 1.963 |
| P = 0.195 | P = 0.003 | P = 0.001 | P = 0.239 | P = 0.409 | P = 0.239 | P = 0.346 | P = 0.001 | P = 0.001 |
RR = respiratory rate; Ti and Te = inspiratory and expiratory times; TV/BW = tidal volume per kilogram; MV/BW = minute ventilation per kilogram; PIF/BW = peak inspiratory flow per kilogram; PEF/BW = peak expiratory flow per kilogram; Penh = enhanced pause
Statistically significant differences (P <0.05) are shown in bold
Discussion
It has been observed that obesity reduces pulmonary and total chest compliance 24 and has effects on respiratory function due to an excess of abdominal and thoracic fat, which increase intra-abdominal pressure. Obesity is characterised by a restrictive pattern of lung function, associated with reductions in vital capacity, functional residual capacity and total lung capacity.6,24 This fact could increase the stiffness of the respiratory system and reduce lung volumes.
Obese people breathe at low lung volumes; their expiratory flow is very close to the maximal limit. The difficulty in the expiratory flow leads to incomplete expirations resulting in dynamic hyperinflation. In this setting, inspiratory muscles have to work at shorter length, increasing the sense of respiratory effort.6,25
TV is the volume of air displaced during breathing at rest and results from the MV divided by the RR. In this study, TV is estimated between 10 and 20 ml of inspired air per kilogram of BW in normal-weight cats. Other authors have evidenced similar values in healthy cats.21,26 Although the TV value obtained in the present study is higher than those reported by other authors, 27 these differences could be due to the fact that the animals from our study were made up of a heterogeneous group of client-owned cats comprising several breeds, ages and feeding patterns, while the other cited studies were designed using laboratory cats kept under experimental conditions, with identical feeding and handling procedures.
In the present study, TV/BW is decreased in obese cats compared with normal-weight cats. It is important to emphasise that TV results were indexed to BW in order to avoid the influence of the patient size. This ensures that the results are not influenced by the size of the animal (ie, small vs large cats) and only the body condition (obesity) is being evaluated. This result is in agreement with previous studies, both in dogs 28 and humans, 29 although this decrease had not been observed in all obese individuals. It is possible that this result may be due to the fact that the adipose tissue accumulated in the thorax reduced pulmonary and total chest compliances.
By contrast, the results of the present study do not support the increase in RR observed in other studies performed in dogs to compensate for the reduced TV. 28 Cats included in the study did not show signs of stress during data recordings, but this must be considered with caution, since normal-weight and obese cats in the Plexiglass chamber might show a different RR compared with cats outside the Plexiglass chamber of BWBP. More studies should be performed to confirm this assertion.
MV is the result of TV multiplied by RR. Our results evidence that MV is significantly decreased following TV reduction because of the absence of increase in RR. An increase in MV in obese women associated with both a reduced Te and a tendency to increase breathing frequency 30 has been described. There are different mechanisms by which excessive body fat might influence ventilatory function. These include mechanical effects on the diaphragm and on the chest wall. 31 The ventilatory deficits in the case of obesity could be due to compromised muscle strength or efficiency as a consequence of reduced chest wall and lung compliances. 32 It has been observed in humans that excess body fat alters respiratory control by blunting the central hypoxic ventilatory drive. 33 Similar mechanisms could explain this finding in obese cats.
With respect to Ti and Te, the results are in agreement with some studies.13,34 In healthy cats, there is a relationship between the Te and the Ti of approximately 1.0 ± 0.15 s, the Te being higher since inhalation is an active mechanism generated by the contraction of the thoracic muscles and diaphragm, whereas the exhalation is a passive mechanism generated by the elastic forces of the lung and the pressure exerted by the abdominal structures over the diaphragm.14,35 In the cats in our study, statistically significant differences were not detected in Te and Ti between obese and non-obese cats. This could be explained by the fact that the majority of the obese cats presented slight or moderate clinical signs associated with obesity.
Limitations in expiratory flow that lead to incomplete expirations resulting in dynamic hyperinflation 25 have been described in obese humans. Other studies show a significant decrease in Ti without changes in Te or mean inspiratory flow. 36 Due to a decrease in total respiratory compliance, it is possible to find an increase in expiratory flow rate. 29
Both PIF and PEF values are significantly reduced in obese cats. Obesity is a risk factor for dyspnoea but not for airflow obstruction. 37 In dogs, obesity seems to cause airflow limitation during the expiratory phase of breathing, but this has only been evidenced during hyperpnoea. This suggests that flow limitation is dynamic and likely to occur in the distal (rather than proximal) portions of the airways. Further studies are warranted to localise the flow-limited segment and to understand whether obesity is linked to exercise intolerance via airway dysfunction in dogs. 17 This expiratory limitation could be explained similarly in obese cats.
The Penh variable assesses the degree of resistance to the air flow in the airways. Penh value seems to be a valid indicator of bronchoconstriction. 38 Age-related variations in reference values of BWBP have been reported for Penh and TV. 19 Despite the fact that other reports are in disagreement, 39 we have included the results of the statistical study comparing gender and age between both groups of animals demonstrating that there are no significant differences in lung function variables between obese and non-obese cats. In humans, there seems to be a relationship between obesity and bronchoreactivity. However, obesity is not associated with airway obstruction or airway hyperreactivity. 25 Moreover, in humans, differences between genders have been described. 30
An inflammatory element has also been proposed as responsible for some of the respiratory alterations in overweight and obese individuals. 40 Fat tissue produces a plethora of inflammatory mediators such as leptin, low levels of adiponectin, C-reactive protein, interleukin-6 and tumour necrosis factor-α, that can potentially increase airway responsiveness.28,41,42 These inflammatory mediators should be studied in obese cats in order to evaluate their role in respiratory alterations observed in obese cats.
Conclusions
This study provides evidence that obesity in cats impairs BWBP measurement by decreasing TV/BW, MV/BW, PIF/BW and PEF/BW. This could reflect some grade of impairment in pulmonary function. Nevertheless, the study fails to demonstrate a significant increase in the Penh baseline value in obese cats, as previously reported in humans and dogs. It has been demonstrated that non-invasive BWBP can help to characterise mechanical dysfunction of the airways in obese cats.
However, due to the limited number of animals that were enrolled in this study, individual variability in breathing pattern should be taken into consideration. In the authors’ opinion, further studies with a larger number of patients to obtain correlations between the pulmonary function variables of obese cats are necessary. Likewise, a correlation between the respiratory findings and pulmonary histopathological studies are necessary, since this could be helpful in defining the nature of the lesions responsible for lung mechanics alterations. Also, it would be interesting to carry out further studies investigating the usefulness of BWBP as a short- and long-term therapeutic monitoring approach.
Footnotes
Preliminary results of this study were presented at the 23rd ECVIM-CA Congress, Liverpool, UK, September 2013
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Funding: The study has received no financial support from any public or private institution.
Accepted: 29 July 2014
References
- 1. German AJ, Ryan VH, German AC, et al. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 2010; 185: 4–9. [DOI] [PubMed] [Google Scholar]
- 2. Sideleva O, Suratt BT, Black KE, et al. Obesity and asthma. An inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 2012; 186: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van de Velde H, Janssens GPJ, de Rooster H, et al. The cat as a model for human obesity: insights into depot-specific inflammation associated with feline obesity. Br J Nutr 2013; 110: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 4. Van den Borst B, Schols AMWJ, de Theije C, et al. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J Appl Physiol 2013; 114: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 5. Hoenig M. The cat as a model for human obesity and diabetes. J Diabetes Sci Technol 2012; 6: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis 2008; 5: 233–242. [DOI] [PubMed] [Google Scholar]
- 7. Cave NJ, Allan FJ, Schokkenbroek SL, et al. A cross-sectional study to compare changes in the prevalence and risk factors for feline obesity between 1993 and 2007 in New Zealand. Prev Vet Med 2012; 107: 121–133. [DOI] [PubMed] [Google Scholar]
- 8. Courcier EA, O’Higgins R, Mellor DJ, et al. Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg 2010; 12: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courcier EA, Mellor DJ, Pendlebury E, et al. An investigation into the epidemiology of feline obesity in Great Britain: results of a cross-sectional study of 47 companion animal practises. Vet Rec 2012; 171: 560. [DOI] [PubMed] [Google Scholar]
- 10. Colliard L, Paragon BM, Lemuet B, et al. Prevalence and risk factors of obesity in an urban population of healthy cats. J Feline Med Surg 2009; 11: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Numa AH, Newth CJ. Anatomic dead space in infants and children. J Appl Physiol 1996; 80: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 12. McKiernan BC, Dye JA, Rozanski EA. Tidal breathing flow volume loops in healthy and bronchitic cats. J Vet Intern Med 1993; 7: 388–393. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman AM, Dhupa N, Cimetti L. Airway reactivity measured by barometric whole-body plethysmography in healthy cats. Am J Vet Res 1999; 60: 1487–1492. [PubMed] [Google Scholar]
- 14. Dye JA, Costa DL. Pulmonary mechanics. In: King LG. (ed). Textbook of respiratory disease in dogs and cats. St Louis, MO: Elsevier Saunders, 2004, pp 157–175. [Google Scholar]
- 15. Kirschvink N, Leemans J, Delvaux F, et al. Bronchodilators in bronchoscopy-induced airflow limitation in allergen-sensitized cats. J Vet Intern Med 2005; 19: 161–167. [DOI] [PubMed] [Google Scholar]
- 16. Kirschvink N, Leemans J, Delvaux F, et al. Functional, inflammatory and morphological characterization of a cat model of allergic airway inflammation. Vet J 2007; 174: 541–553. [DOI] [PubMed] [Google Scholar]
- 17. Bach JF, Rozansky EA, Bedenice D, et al. Association of expiratory airway dysfunction with marked obesity in healthy adult dogs. Am J Vet Res 2007; 68: 670–675. [DOI] [PubMed] [Google Scholar]
- 18. Leemans J, Kirschvink N, Bernaerts F, et al. A pilot study comparing the antispasmodic effects of inhaled salmeterol, salbutamol and ipratropium bromide using different aerosol devices on muscarinic bronchoconstriction in healthy cats. Vet J 2009; 180: 236–245. [DOI] [PubMed] [Google Scholar]
- 19. Kirschvink N, Leemans J, Delvaux F, et al. Non-invasive assessment of growth, gender and time of day related changes of respiratory pattern in healthy cats by use of barometric whole body plethysmography. Vet J 2006; 172: 446–454. [DOI] [PubMed] [Google Scholar]
- 20. Hirt RA, Leinker S, Mosing M, et al. Comparison of barometric whole-body plethysmography and its derived parameter enhanced pause (Penh) with conventional respiratory mechanics in healthy Beagle dogs. Vet J 2008; 176: 232–239. [DOI] [PubMed] [Google Scholar]
- 21. García-Guasch L, Caro-Vadillo A, Manubens-Grau J, et al. Evaluation of pulmonary function variables by using plethysmography in cats with respiratory disease associated to Dirofilaria immitis. Vet Parasitol 2012; 187: 254–258. [DOI] [PubMed] [Google Scholar]
- 22. García-Guasch L, Caro-Vadillo A, Manubens-Grau J, et al. Is Wolbachia participating in the bronchial reactivity of cats with heartworm associated respiratory disease? Vet Parasitol 2013; 196: 130–135. [DOI] [PubMed] [Google Scholar]
- 23. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–17. [Google Scholar]
- 24. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006; 13: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farah CS, Salome CM. Asthma and obesity: a known association but unknown mechanism. Respirology 2012; 17: 412–421. [DOI] [PubMed] [Google Scholar]
- 26. Johnson LR. Bronchial disease. In: August JR. (ed). Consultations in feline internal medicine. 5th ed. St Louis, MO: Elsevier Saunders, 2006, pp 361–367. [Google Scholar]
- 27. Hirt RA, Dederichs D, Boehler A, et al. Relationship of age, sex, body weight, and hematologic and respiratory variables with airway reactivity in adult cats. Am J Vet Res 2003; 64: 26–31. [DOI] [PubMed] [Google Scholar]
- 28. Manens J, Bolognin M, Bernaerts F, et al. Effects of obesity on lung function and airway reactivity in healthy dogs. Vet J 2012; 193: 217–221. [DOI] [PubMed] [Google Scholar]
- 29. Littleton SW. Impact of obesity on respiratory function. Respirology 2012; 17: 43–49. [DOI] [PubMed] [Google Scholar]
- 30. de Melo Barcelar J, Aliverti A, de Barros Melo TLL, et al. Chest wall regional volumes in obese women. Respir Physiol Neurobiol 2013; 189: 167–173. [DOI] [PubMed] [Google Scholar]
- 31. Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest 1997; 111: 891–898. [DOI] [PubMed] [Google Scholar]
- 32. Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. Can Med Assoc J 2006; 174: 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev 2004; 5: 145–151. [DOI] [PubMed] [Google Scholar]
- 34. Kirschvink N, Leemans J, Delvaux F, et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis. J Feline Med Surg 2006; 8: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lumb AB. Pulmonary ventilation. In: Lumb AB. (ed). Nunn’s applied respiratory physiology. 6th ed. Philadelphia: Elsevier/Butterworth Heinemann, 2005, pp 76–91. [Google Scholar]
- 36. Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol 1983; 55: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 37. Sin DD, Jones RL, Man SFP. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med 2002; 162: 1477–1481. [DOI] [PubMed] [Google Scholar]
- 38. Hamelmann E, Schwarze J, Takeda K, et al. Non-invasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997; 156: 766–775. [DOI] [PubMed] [Google Scholar]
- 39. García-Guasch L, Caro-Vadillo A, Laporta M, et al. Barometric whole-body plethysmography reference values in different age-range healthy cats. Proceedings of the 18th Annual ECVIM Congress; 2008 Sept 4–6; Ghent, Belgium. [Google Scholar]
- 40. Sebastian JC. Respiratory physiology and pulmonary complications in obesity. Best Pract Res Clin Endocrinol Metab 2013; 27: 157–161. [DOI] [PubMed] [Google Scholar]
- 41. Jensen ME, Collins CE, Gibson PG, et al. The obesity phenotype in children with asthma. Paediatr Respir Rev 2011; 12: 152–159. [DOI] [PubMed] [Google Scholar]
- 42. Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology 2012; 17: 402–411. [DOI] [PubMed] [Google Scholar]


