Abstract
Background
Twin‐twin transfusion syndrome, a condition affecting monochorionic twin pregnancies, is associated with a high risk of perinatal mortality and morbidity. A number of treatments have been introduced to treat the condition but it is unclear which intervention improves maternal and fetal outcome.
Objectives
The objective of this review was to evaluate the impact of treatment modalities in twin‐twin transfusion syndrome.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2013).
Selection criteria
Randomised and quasi‐randomised studies of amnioreduction versus laser coagulation, septostomy versus laser coagulation or septostomy versus amnioreduction.
Data collection and analysis
Two review authors independently assessed eligibility and extracted data. We contacted study authors for additional information.
Main results
Three studies (253 women and 506 babies) were included. All three trials were judged to be of moderate quality. One study compared amnioreduction with septostomy (71 women), whilst the other two studies compared amnioreduction with endoscopic laser coagulation (182 women). Not all trials provided outcome data that could be included in all meta‐analyses.
Amnioreduction compared with laser coagulation Although there was no difference in overall death between amnioreduction and laser coagulation (average risk ratio (RR) 0.87; 95% confidence interval (CI) 0.55 to 1.38 adjusted for clustering, two trials) or death of at least one infant per pregnancy (RR 0.91; 95% CI 0.75 to 1.09, two trials), or death of both infants per pregnancy (average RR 0.76; 95% 0.27 to 2.10, two trials), more babies were alive without neurological abnormality at the age of six years in the laser group than in the amnioreduction groups (RR 1.57; 95% CI 1.05 to 2.34 adjusted for clustering, one trial). There were no significant differences in the babies alive at six years with major neurological abnormality treated by laser coagulation or amnioreduction (RR 0.97; 95% CI 0.34 to 2.77 adjusted for clustering, one trial). Outcomes for death in this 2013 update are different from the previous 2008 update, where improvements in perinatal death and death of both infants per pregnancy were shown in the laser intervention arm. The NIHCD trial included in this update exerts an opposite direction of effects to the Eurofetus study, which was previously the only included laser study, hence the difference in outcome.
Amnioreduction compared with septostomy
There are no differences in overall death (RR 0.83; 95% CI 0.47 to 1.47, adjusted for clustering, one trial), death of at least one infant per pregnancy (RR 0.80; 95% CI 0.48 to 1.35, one trial), or death of both infants per pregnancy (RR 0.90; 95% CI 0.37 to 2.22, one trial) or gestational age at birth (RR 1.20; 95% CI ‐0.81 to 3.21, one trial) between amnioreduction and septostomy.
Authors' conclusions
Endoscopic laser coagulation of anastomotic vessels should continue to be considered in the treatment of all stages of twin‐twin transfusion syndrome to improve neurodevelopmental outcomes.
Further research targeted towards assessing the effect of treatment on milder (Quintero stage 1 and 2) and more severe (Quintero stage 4) forms of twin‐twin transfusion syndrome is required. Studies should aim to assess long‐term outcomes of survivors.
Keywords: Female; Humans; Pregnancy; Amniocentesis; Amniocentesis/methods; Amnion; Amnion/surgery; Fetofetal Transfusion; Fetofetal Transfusion/mortality; Fetofetal Transfusion/therapy; Laser Coagulation; Perinatal Mortality; Pregnancy Reduction, Multifetal; Pregnancy Reduction, Multifetal/methods; Punctures; Randomized Controlled Trials as Topic
Plain language summary
Interventions for the treatment of twin‐twin transfusion syndrome
Limited evidence suggests the best way to improve survival without neurological impairment in children with twin‐to‐twin transfusion syndrome is to perform laser treatment to the placenta.
Identical twins occur in about one in 320 pregnancies. Sometimes identical twins share the same placenta and blood flow, and the proportion of blood shared between the twins is usually equal. Twin‐to‐twin transfusion syndrome happens when the blood flow is uneven and passes from one twin (the donor) to the other (the recipient). This can happen when the placenta has deep artery‐to‐vein connections. The donor twin usually has very little amniotic fluid, and frequently does not grow well and is very small. The recipient twin has excessive amniotic fluid, and often has a distended bladder and other medical problems. The risk of death for both twins is high, around 80% if there is no treatment. There is also risk of physical or neurological damage to both twins if they survive. Various options for treatment exist. These include (1) the repeated removal of excessive amniotic fluid (amnioreduction); (2) laser treatment of the abnormal vessels in the placenta (endoscopic laser surgery); (3) puncture of the membrane between the twins (septostomy); and (4) the selective ending of one twin's life (selective feticide). The review found three trials, involving 253 women and 506 babies. There were no studies on laser treatment versus puncturing the membrane, nor on selective feticide. The evidence showed that laser treatment was associated with more babies being alive without a neurological abnormality when compared to removing the excess amniotic fluid. However, where there is insufficient expertise to perform laser surgery or when the pregnancy is beyond 26 weeks, amnioreduction remains the treatment of choice. Further research is needed on the best treatment for mild and very severe forms of the problem.
Background
Description of the condition
Twin‐twin transfusion syndrome (TTTS) is a condition that affects identical twin pregnancies. The diagnosis requires the ultrasound demonstration of excessive fluid (hydramnios) around one twin (the recipient) and little or no fluid (oligohydramnios) around the other twin (the donor) with the separating membrane completely covering this fetus. Both twins should be structurally normal. The recipient twin is usually appropriately grown for gestational age, has a large distended bladder and may, if severely compromised, show tricuspid regurgitation or hydrops fetalis. The donor twin on the other hand, is frequently severely growth restricted with abnormal umbilical artery Doppler waveforms.
The condition is now graded by the Quintero staging system (Quintero 1996) as follows.
Stage 1: abnormal amniotic fluid levels along with bladder filling in the donor.
Stage 2: collapsed bladder in the donor.
Stage 3: abnormal Doppler flow in the umbilical artery or ductus venosus of either twin.
Stage 4: hydrops in either twin.
Stage 5: intrauterine death of either twin.
These clinical features are associated with the death of one or both fetuses in more than 80% of untreated pregnancies, particularly if problems develop before 28 weeks' gestation (Saunders 1991; Urig 1990). The risks are those of spontaneous miscarriage, preterm prelabour rupture of membranes, preterm labour and growth retardation. Although twin‐twin transfusion is usually a gradual process, it can happen suddenly with the death of one twin, usually the recipient. This can lead to the death of the co‐twin or neurological handicap in the survivor (Fusi 1990; Van Heteren 1998).
Identical twin gestations occur in one in 320 pregnancies. Depending on when the embryos implant, the placenta will be dichorionic diamniotic, monochorionic diamniotic or monochorionic monoamniotic, a description of the pattern of membranes. Seventy‐five per cent of identical twin pregnancies are monochorionic, diamniotic. Vascular connections, called anastomoses, are common in monochorionic placentas. These can be superficial artery‐to‐artery or vein‐to‐vein connections or deep artery‐to‐vein anastomoses. Balanced blood flow across these connections occurs in most monochorionic twins. Unbalanced transfusion from the donor to the recipient can occur in those placentas that have deep artery‐to‐vein connections, causing TTTS (Denbow 1998). This is associated with a high risk of death and damage to twins and accounts for 15% to 17% of the overall perinatal mortality in twins (Steinberg 1990). The poor outcome of untreated TTTS leads to the introduction of a number of treatments, namely:
repeated serial amnioreduction;
endoscopic laser ablation of vascular anastomoses;
amniotic septostomy;
selective feticide.
Description of the intervention
Serial amnioreduction, the repeated removal of excessive amniotic fluid by amniocenteses, is the most established method of treatment. It was introduced initially as a treatment for hydramnios in the recipient sac, to try to prevent preterm labour or prelabour rupture of membranes, or both, but may have beneficial effects on the disease condition (Montan 1985; Schneider 1985). Colour‐flow Doppler waveforms of the uterine artery, a test of wellbeing of the fetus, have shown improvement following amnioreduction (Bower 1995). Other authors have reported a reduction in the rate of fluid accumulation following serial amnioreduction and therefore a lengthening of the interval between reductions (Schneider 1985; Urig 1990). They postulate that hydramnios compresses the placenta and increases the rate of transfusion to the recipient twin and that relieving this pressure with amnioreduction reverses this phenomenon (Urig 1990). On the other hand, the increase in fetoplacental blood volume with increasing gestation may reduce the effects of anastomoses therefore, showing an improvement in the condition (Saunders 1991). Whatever the actual physiology behind the procedure, amnioreduction appears to prolong pregnancy and thereby improves fetal survival. The survival rate following serial amnioreduction has been quoted at 37% to 60% (Saunders 1991; Trespidi 1997; Urig 1990) and the risk of neurological damage at 17% to 33%. Some papers have quoted very high survival rates at around 79% (Elliot 1991; Mahony 1990; Reisner 1993) but the results are debatable because non‐identical twin pregnancies and pregnancies with fetuses with structural abnormality were not excluded (see definition of TTTS). The cohort studies included mild cases of TTTS, and this could be the explanation for the better outcomes. Serial amnioreduction does not require special equipment and can be performed by most obstetricians specialised in fetal medicine. Procedure‐related complications occur with serial amnioreduction in the order of around 10% (Mahony 1990; Saunders 1991). This is mostly fetal death within 48 hours of the procedure or spontaneous abortion. Abruptio placentae has been reported (Mahony 1990; Reisner 1993).
Laser coagulation of the superficial blood vessels that cross the separating membrane of the placenta has been advocated as another method of managing TTTS (De Lia 1990). Theoretically, it has the advantage of solving the underlying mechanism that causes unbalanced twin‐twin transfusion. Controversy surrounds this theory because it has been shown that in TTTS, the anastomoses are more likely to be deep rather than superficial (Bajoria 1995; Machin 1996). The coagulation of superficial vessels therefore should not have any effect on the pathophysiology of TTTS but might indeed be cutting off some normal placental vessels with inherent fetal risks (De Lia 1995). Published series have however shown a 55% to 73% survival rate with a 4.2% neurological handicap rate (De Lia 1995; Ville 1995; Ville 1998). These authors argue that irrespective of the depth of the individual anastomoses, their afferent and efferent branches are superficial and can be seen on the placental surface. Systematic coagulation of all these vessels should include the branches of these anastomoses and currently this remains the only method that can prevent transfusion between the placentas. Endoscopic laser ablation requires fetoscopic skills and currently can only be performed in a small number of centres. It is a far more invasive procedure than amnioreduction. Maternal morbidity may be much higher than with amniocentesis‐related procedures and bleeding from the placental vessels is consistently reported (De Lia 1995; Hecher 1999). Further interventions such as amnioreduction are required in around 20% of cases.
The deliberate creation of a puncture in the inter twin membrane, 'septostomy', has been described by Saade et al (Saade 1998). They hypothesised that the improvement in amniotic fluid dynamics in some TTTS pregnancies may be due to an inadvertent puncturing of the inter twin membrane. Survival rates as high as 83% were achieved but no figures for neurological outcome are available (Saade 1998). The mechanism is thought to be an equalisation of the pressure in the two sacs, thus relieving the pressure on the placenta (Garry 1998). Septostomy may be a relatively safe procedure which does not require special equipment. The major risk associated with amniotic septostomy is cord entanglement, as the procedure is effectively creating a monoamniotic pregnancy within a single sac (Gilbert 1991).
Selective feticide, the deliberate ending of one twin's life, has been reported as a therapeutic option. In the event of the death of one twin, approximately 50% of surviving twins will experience mortality or neurological handicap (Van Heteren 1998). Feticide, using a technique which does not affect the circulation of the surviving twin, may prevent neurological injury to the survivor. Techniques such as fetoscopic cord ligation and ultrasound guided vascular embolisation have been described (Crombleholme 1996; Deprest 1998; Donner 1997; Quintero 1996). These procedures are performed through the skin (percutaneous) for uterine access. The number of women treated by this method is very small, as the procedure is usually only performed where the demise of the co‐twin is certain. The numbers are therefore too small to provide valid neurological outcomes for the surviving twin in these pregnancies and the survival rate by definition can at best be only 50%. Selective feticide has relative risks associated with the technique used.
Maternal digoxin therapy, used in conjunction with either serial amnioreduction or laser thermocoagulation, has been anecdotally reported (Arabin 1998; De Lia 1985; Roman 1995). Fetal heart and blood dynamics, however, suggest that it should not work and it has been abandoned. Indomethacin for reduction of hydramnios in the recipient sac has been described (Jones 1993) but the results were not encouraging and the authors concluded that it does not prevent perinatal mortality in TTTS. Indomethacin can have potentially adverse renal effects on the donor twin.
Why it is important to do this review
This review will assess which of the above treatments improves fetal, childhood and maternal outcomes.
Objectives
To evaluate the impact of treatment modalities in twin‐twin transfusion syndrome (TTTS).
Methods
Criteria for considering studies for this review
Types of studies
Due to an anticipated lack of randomised controlled trials, we considered both quasi‐randomised and randomised studies of one treatment versus another. Comparisons such as amnioreduction versus laser coagulation, septostomy versus laser coagulation or septostomy versus amnioreduction were suitable for inclusion.
Types of participants
Women with a twin pregnancy where TTTS has been diagnosed on ultrasound by:
confirmation of monochorionicity;
oligohydramnios in one sac and hydramnios in the other;
normal anatomy of both fetuses.
Types of interventions
We reviewed any intervention performed as a therapy for TTTS with a view to improving maternal symptoms, fetal, neonatal and childhood outcome and prolonging pregnancy.
Types of outcome measures
Primary outcomes
(1) Perinatal outcomes
Overall death (stillbirths neonatal and post‐neonatal).
Neurodevelopmental delay (as defined by trialists).
Secondary outcomes
(1) Procedure‐related details
Number of invasive interventions per pregnancy.
(2) Perinatal outcomes
Death of at least one infant per pregnancy.
Death of both infants per pregnancy.
Preterm labour within 48 hours of procedure.
Preterm prelabour rupture of membranes within 48 hours of procedure.
Gestational age at birth.
Use of mechanical ventilation.
Need for blood transfusion within 48 hours of delivery.
Weight or head circumference discordance at birth.
Intraventricular haemorrhage: grades III/IV.
Seizures within 28 days of delivery or anticonvulsant therapy.
Length of stay on neonatal intensive care unit.
(3) Maternal outcomes
Maternal death
Admission to intensive care unit for procedure‐related reasons.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 May 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
Weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
See Appendix 1, for search methods used in previous versions of this review.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeRoberts 2008a.
For this 2013 update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation; more than 20% missing data);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods, if appropriate in future updates.
Unit of analysis issues
Cluster‐randomised trials
We did not include cluster‐randomised trials in this review. In future updates, if high‐quality cluster‐randomised trials are identified, we will consider including them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook Section 16.3.4 using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not included as they are inappropriate to the question.
Other unit of analysis issues
The analysis in this review involves multiple pregnancies, therefore, wherever possible, analyses were adjusted for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004). Treating babies from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than babies from different pregnancies, will overestimate the sample size and give confidence intervals that are too narrow. Each woman can be considered a cluster in a multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of relative risk and adjustment of confidence intervals. Usually this will mean that the confidence intervals get wider. Although this may make little difference to the conclusion of a trial, it avoids misleading results in those trials where the difference may be substantial. We have used the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are more than 10 studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it further.
Data synthesis
We used the number randomised as the denominator, for outcomes that applied to women. The number of babies randomised was the denominator for outcomes applying to fetuses or babies. This included neonatal outcomes; we did not use the number of live births as the denominator for neonatal outcomes in the main analyses as this is a non‐randomised comparison, with consequent risk of bias.
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
It was not possible to carry out planned subgroup analysis because all trials included only one of the subgroups. In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analysis.
1. Percutaneous versus open procedures. We will use primary outcomes in subgroup analysis.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
When appropriate, in future updates, we will carry out sensitivity analysis to explore the effect of trial quality based on concealment of allocation, by excluding studies with unclear allocation concealment.
Results
Description of studies
A total of six trials were identified. We have included three studies (Eurofetus 2004; Moise 2005; NIHCD 2007), excluded two studies (Soothill 2000; Sutcliffe 2000), and one study is ongoing (Slaghekke 2008).
Included studies
Three studies were identified for inclusion (Eurofetus 2004; Moise 2005; NIHCD 2007) with data available for 253 women (seeCharacteristics of included studies table).
One study compares serial amnioinfusion with septostomy (Moise 2005), whilst the other two studies compare laser photocoagulation with serial amnioinfusion (Eurofetus 2004; NIHCD 2007). Both the septostomy trial and the NIHCD 2007 were conducted in the USA, whilst Eurofetus was conducted in Europe.
Two trials allowed cross‐over to the other arm (Eurofetus 2004; Moise 2005). The septostomy trial (Moise 2005) allowed cross‐over to the amnioreduction arm if two consecutive septostomies failed to resolve oligohydramnios in the donor sac or if the recipient sac continued to have polyhydramnios. Laser ablation of the anastomotic vessels and umbilical cord occlusion were used in cases where there was progression of TTTS. The endoscopic laser surgery trial (Eurofetus 2004) performed amnioreduction in the laser arm after 26 weeks' gestation as the trial protocol did not allow laser coagulation after that gestation. Six women in the amnioreduction arm in this trial underwent laser treatment for progression of TTTS after repeated amnioreductions. NIHCD 2007 was by nature a cross‐over study in that it only included those who were deemed to have failed amnioreduction therapy.
Most of the fetuses were classified as Quintero stage 2 or 3 in the endoscopic laser surgery trial (Eurofetus 2004), Quintero stage 1 to 3 in the septostomy trial (Moise 2005) and Quintero stage 2 to 4 in the NIHCD 2007 trial.
Excluded studies
For details of the two excluded studies, see the table of Characteristics of excluded studies.
Risk of bias in included studies
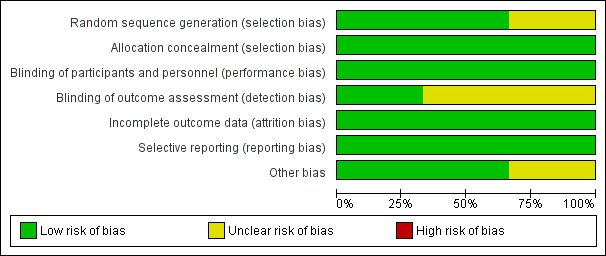
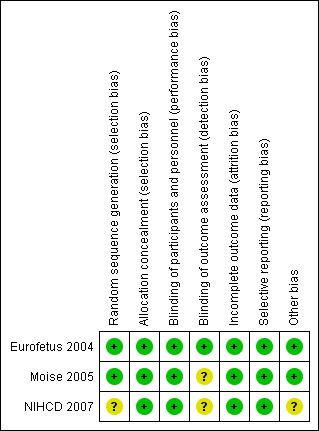
See Figure 1; Figure 2 for a summary of 'Risk of bias' assessments in included studies. All three trials were judged to be of moderate quality
1.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods of randomisation used in the included studies are summarised in the Characteristics of included studies table. Two studies (Eurofetus 2004; Moise 2005) used computer‐generated central randomisation sequences in order to maintain adequate allocation concealment. These studies were assessed as low risk of bias for selection bias. In one study (NIHCD 2007), the method of sequence generation was not reported. Allocation was reported as being centralised via faxed case reports and so this study was assessed as low risk of bias for allocation concealment.
Blinding
Performance bias is unlikely to have occurred in the studies included in this review. Blinding of outcome assessment was attempted in one trial (Eurofetus 2004).
Incomplete outcome data
All included studies (Eurofetus 2004; Moise 2005; NIHCD 2007) compared two treatment arms (253 pregnancies and 506 fetuses). There was evidence available to suggest that sample‐size calculations had been performed prospectively. Intention‐to‐treat analysis was mentioned. One trial (Moise 2005) lost two women to follow‐up in the septostomy arm and another trial (NIHCD 2007) lost two women, one from each arm of the trial. All trials were assessed as low risk of bias for completeness of follow‐up.
Selective reporting
All expected prespecified outcomes were reported in the trial reports and all trials are assessed as low risk of bias for selective reporting.
Other potential sources of bias
All three trials were stopped early on the basis of interim analyses (Eurofetus 2004; Moise 2005; NIHCD 2007). Two trials were assessed as being at low risk of bias (Eurofetus 2004; Moise 2005) and one was assessed as being at unclear risk of bias (NIHCD 2007). In the septostomy trial (Moise 2005), the recruitment rate to the trial was slower than predicted and it was felt that the primary end point would not be achieved. The included endoscopic laser surgery trial (Eurofetus 2004) was stopped after the second interim analysis showed a higher rate of survival of at least one twin in the laser arm. The NIHCD 2007 trial was stopped at the request of the investigators after recruiting only a quarter of the sample size (42 pregnancies) in five years. The study authors cited the unwillingness of referring centres to refer to units where laser photocoagulation was only available within the trial and a statistical trend in adverse outcomes affecting the recipient twin in one treatment arm. The results cannot be conclusive, particularly if many eligible participants were not referred. All trials quoted the O'Brien‐Fleming rule for stopping.
Effects of interventions
The results of this review are derived from three studies: a single study for the comparison of septostomy versus amnioreduction (Moise 2005); and two studies for the comparison of endoscopic laser surgery versus amnioreduction (Eurofetus 2004; NIHCD 2007).
Two included studies presented analyses that were adjusted for clustering but did not present estimates of the intracluster correlation coefficient (ICC) (Eurofetus 2004; Moise 2005). Eurofetus 2004 contained enough information to deduce the values of ICC that were used for adjustments for two outcomes; all deaths (0.29) and need for blood transfusion within 48 hours. The analysis of mechanical ventilation was not adjusted for clustering because the ICC was negative.
For other outcomes, we used the most appropriate ICC for the adjustment; the value derived from all deaths (0.29) for outcomes that involved death, and an ICC of 0.29 for survival with and without major neurological complications at six years.
Moise 2005 did not present any estimates of the ICC but the report contained sufficient information to calculate an ICC estimate for fetal death (0.47). This was used to adjust the analyses of overall death.
It was not possible to derive an ICC estimate for any outcome in NIHCD 2007.
Septostomy versus amnioreduction (one trial, 71 pregnancies, 142 fetuses)
Primary outcomes
No significant differences were found in overall death (risk ratio (RR) 0.83; 95% confidence interval (CI) 0.47 to 1.47, adjusted for clustering) Analysis 1.1, between amnioreduction and septostomy.
1.1. Analysis.
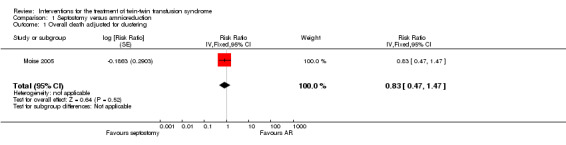
Comparison 1 Septostomy versus amnioreduction, Outcome 1 Overall death adjusted for clustering.
Neurodevelopmental delay was not reported in this study.
Secondary outcomes
No significant differences were found in death of at least one infant per pregnancy (RR 0.80; 95% CI 0.48 to 1.35) Analysis 1.2, or death of both infants per pregnancy (RR 0.90; 95% CI 0.37 to 2.22) Analysis 1.3, or mean gestational age at delivery in weeks (mean difference (MD) 1.20; 95% CI ‐0.81 to 3.21) Analysis 1.4 between amnioreduction and septostomy.
1.2. Analysis.
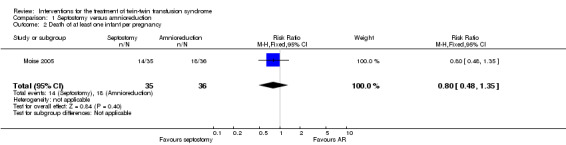
Comparison 1 Septostomy versus amnioreduction, Outcome 2 Death of at least one infant per pregnancy.
1.3. Analysis.
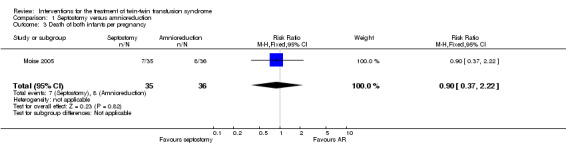
Comparison 1 Septostomy versus amnioreduction, Outcome 3 Death of both infants per pregnancy.
1.4. Analysis.
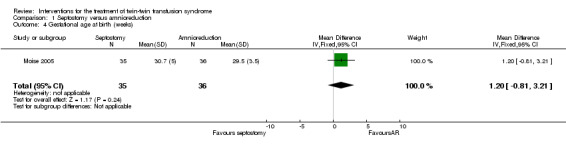
Comparison 1 Septostomy versus amnioreduction, Outcome 4 Gestational age at birth (weeks).
No other secondary outcomes were reported in this study.
Endoscopic laser surgery versus amnioreduction (two trials, 182 pregnancies, 364 fetuses)
Primary outcomes
No significant differences were found for overall death (average RR 0.87; 95% CI 0.55 to 1.38 adjusted for clustering) Analysis 2.1. Substantial heterogeneity was apparent in Analysis 2.1, and so a random‐effects analysis was performed (Heterogeneity: Tau² = 0.08; Chi² = 3.13, df = 1 (P = 0.08); I² = 68% Analysis 2.1). It should be noted that these two trials appear to have opposite directions of effect.
2.1. Analysis.
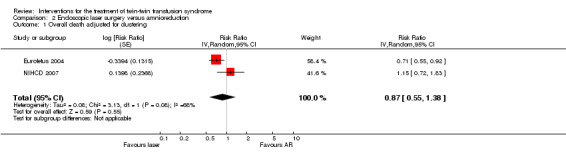
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 1 Overall death adjusted for clustering.
For the outcome neurodevelopmental delay, more babies were alive without neurological abnormality at the age of six years in the laser group than the amnioreduction groups (RR 1.57; 95% CI 1.05 to 2.34 adjusted for clustering), Analysis 2.2. There was no significant difference in the babies alive with major neurological abnormality treated by laser coagulation or amnioreduction at six years (RR 0.97; 95% CI 0.34 to 2.77 adjusted for clustering), Analysis 2.3.
2.2. Analysis.

Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 2 Alive without neurological complications at 6 years adjusted for clustering.
2.3. Analysis.
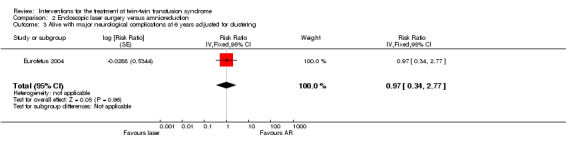
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 3 Alive with major neurological complications at 6 years adjusted for clustering.
Secondary outcomes
No significant differences were found for death of at least one infant per pregnancy (RR 0.91; 95% CI 0.75 to 1.09) Analysis 2.4, or death of both infants per pregnancy (average RR 0.76; 95% CI 0.27 to 2.10) Analysis 2.5, between laser surgery and amnioreduction. Substantial heterogeneity was apparent in Analysis 2.5 and so a random‐effects analysis was performed (Heterogeneity: Tau² = 0.41; Chi² = 3.69, df = 1 (P = 0.05); I² = 73% Analysis 2.5). It should be noted that these two trials appear to have opposite directions of effect.
2.4. Analysis.
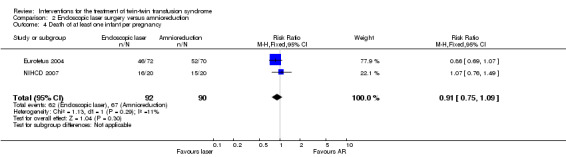
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 4 Death of at least one infant per pregnancy.
2.5. Analysis.
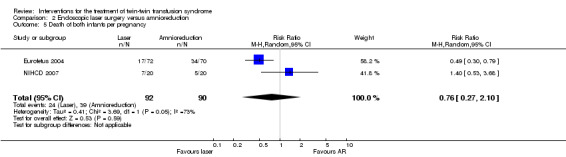
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 5 Death of both infants per pregnancy.
There were no differences between groups for preterm labour within 48 hours of procedure (RR 1.94; 95% CI 0.18 to 20.96) Analysis 2.6; rupture of membranes within 48 hours of procedure (RR 1.35; 95% CI 0.71 to 2.54) Analysis 2.7; need for mechanical ventilation (RR 0.79; 95% CI 0.44 to 1.43) Analysis 2.8; need for blood transfusion within 48 hours of delivery (RR 0.45; 95% CI 0.15 to 1.31) Analysis 2.9; or intraventricular haemorrhage grade III/IV (RR 0.24; 95% CI 0.05 to 1.12) Analysis 2.10.
2.6. Analysis.
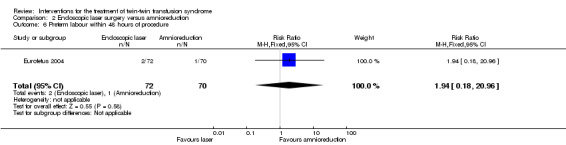
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 6 Preterm labour within 48 hours of procedure.
2.7. Analysis.
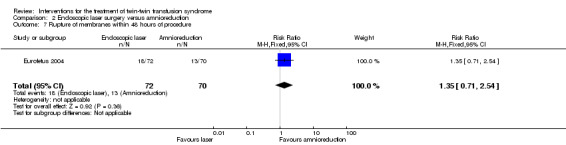
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 7 Rupture of membranes within 48 hours of procedure.
2.8. Analysis.
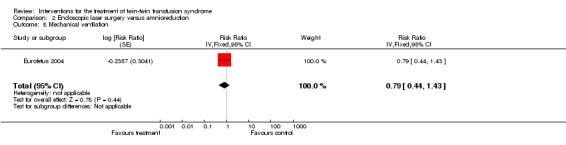
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 8 Mechanical ventilation.
2.9. Analysis.
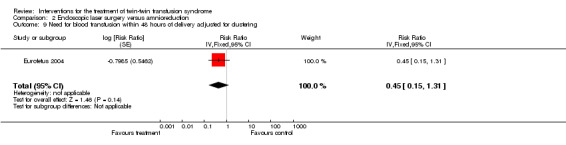
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 9 Need for blood transfusion within 48 hours of delivery adjusted for clustering.
2.10. Analysis.
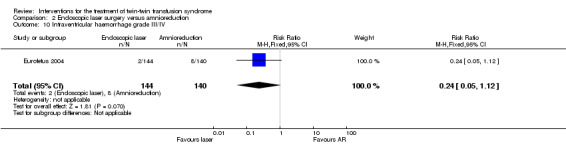
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 10 Intraventricular haemorrhage grade III/IV.
There were no reported maternal deaths or admissions to the intensive care unit (ICU) for procedure‐related reasons in either group (Analysis 2.11; Analysis 2.12).
2.11. Analysis.
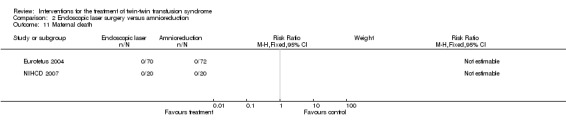
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 11 Maternal death.
2.12. Analysis.
Comparison 2 Endoscopic laser surgery versus amnioreduction, Outcome 12 Admission to intensive care unit for procedure‐related reasons.
No data were available for the other outcome measures for this comparison.
Discussion
Summary of main results
This updated review includes three studies (involving 253 women and 506 babies). The results of this review cannot be conclusive as the included trials are small and there are only meta‐analyses for four outcomes in the review at the moment. The findings would however, support the use of endoscopic laser coagulation in the treatment of twin‐twin transfusion syndrome (TTTS) to improve neurodevelopmental outcomes in the child. This update does not show any apparent difference in overall death or any secondary death outcomes between endoscopic laser coagulation and amnioreduction. It should be noted that the two trials analysed appear to have opposite directions of effect on these outcomes. There does not appear to be a difference in perinatal outcomes between amnioreduction and septostomy.
Overall completeness and applicability of evidence
The comparisons for the primary outcome represent the best available current evidence on interventions for twin‐twin transfusion syndrome. The ongoing Solomon study (Slaghekke 2008) aims to recruit 274 participants to a trial comparing two different methods of endoscopic laser coagulation. The composite primary outcome includes perinatal death, the recurrence of TTTS and another condition called twin anaemia‐polycythaemia sequence (TAPS). The Solomon study will have two‐year neurodevelopmental outcomes as well as data on mortality. Many factors affect the outcome in TTTS, one of which is the Quintero stage of the disease when the intervention is performed.
The trials included in this review enrolled fetuses with largely Quintero stage 2 to 3 disease. There were only two fetuses enrolled with Quintero stage 4 disease in Eurofetus 2004, three in NIHCD 2007 and two in the septostomy trial (Moise 2005), so the numbers are too small to reach any conclusions in this group of fetuses. There is insufficient evidence for this review to comment on either the primary or secondary outcomes by Quintero stage. The authors of Eurofetus 2004 suggest that staging should not influence the choice of treatment. This is supported by the findings of Quintero et al in a cohort study, where the relationship between perinatal mortality and stage was shown to be less apparent in pregnancies treated with laser photocoagulation than amnioreduction (Quintero 2003). The authors of NIHCD 2007 suggest that the presence of TTTS cardiomyopathy has a significant impact on recipient twin mortality.
Only one trial (Eurofetus 2004) analysed developmental outcomes at age two and beyond. The data for survival with and without neurological complications at six years have now been added to the review for this 2013 update. The six‐year follow‐up included 256 of the 284 infants recruited to the trial, and consistent with the overall trial results, more children had died by six years in the amnioreduction group (73/120, 60.8%) than the laser group (63/136, 46.3%). More babies were alive without neurological abnormality at the age of six years in the laser group (60/132) than in the amnioreduction group (33/114); but the proportion of children alive at six years with neurological abnormality was very similar in the two groups (laser 9/136 (6.6%); amnioreduction 8/114 (7.0%). It is usual for Cochrane reviews to exclude trials with an attrition rate greater than 20%. However, these data have been included as the attrition is secondary to death not loss to follow‐up. Neither the septostomy trial (Moise 2005) nor NIHCD 2007 has plans to follow up survivors.
Quality of the evidence
The analyses in this review have been adjusted for clustering for those outcomes in which it was possible to do so. This reduces the risk of bias due to non‐independence between twins. Confidence intervals become wider with cluster analysis suggesting that the true effect lies over a wider upper and lower value, i.e. that the true sample size is smaller than we think because of the inclusion of multiple pregnancies, making the estimate of effect less precise than by unadjusted analysis. The results of the adjusted analysis do not alter the conclusions in this review but may do if more pregnancies are added in the future.
All three trials included in this review were stopped early. All trials can be criticised for stopping early. This is because chance can lead to surprisingly large differences early in a trial, which disappear or reverse over time (Grant 2004). The process for stopping trials due to internal reasons is well recognised and has statistical methodology (Brocklehurst 2000). Two included studies were stopped by independent analysts: 1) an industrial partner, not involved in the study design or analysis of the data, in the case of the laser coagulation study (Eurofetus 2004) and 2) a Data Monitoring Safety (DMS) Officer in the septostomy trial (Moise 2005).
There was no formal Data Monitoring Committee mentioned for Eurofetus 2004. There was a higher rate of survival of at least one twin in the laser arm with a P value of 0.002, so the trial was stopped according to the O'Brien‐Fleming rule, with adjustment for multiple evaluations of the data (the rule states that at the second analysis the P value should be less than .015 in order to consider early termination). However, whatever method is used, the estimate of treatment effect will still be biased if a trial is stopped early.
The septostomy trial (Moise 2005) was stopped by the DMS Officer when an interim analysis showed no difference in outcome suggesting that the primary endpoint would not be reached. Data monitoring committees would also take into account other considerations, such as meta‐analyses of data from comparable trials, other existing evidence external to the trial and the nature of the condition and its alternative treatments (Doll 2001). It is unclear whether these were considered in either trial. These trials remain, however, the best evidence for the treatment of TTTS currently available.
The NIH‐sponsored trial (NIHCD 2007) was stopped at the request of the investigators after recruiting only a quarter of the sample size (42 of 146 pregnancies required) in five years. The authors cited the unwillingness of referring centres to refer to units where laser photocoagulation was only available within the trial. The authors also state that the Trial Oversight Committee noted a statistical trend in adverse outcome affecting the recipient twin in one treatment arm and made a recommendation to the Data Monitoring Committee to stop the trial with which the latter agreed. The results cannot be conclusive, particularly if many eligible participants were not referred and had laser coagulation performed elsewhere.
Potential biases in the review process
We are aware that the review process itself may introduce bias. We took various steps to reduce bias; at least two review authors independently carried out data extraction and assessed risk of bias. If study methods or results were unclear, we attempted to contact trial authors and several authors provided additional data or clarified study methods.
Authors' conclusions
Implications for practice.
Endoscopic laser coagulation of anastomotic vessels should continue to be considered in the treatment of all stages of TTTS to improve neurodevelopmental outcomes in the child.
When compared to amnioreduction, treatment with laser coagulation does not appear to increase or reduce the risk of overall death (stillbirth, neonatal and post‐neonatal) in this condition but it appears to result in more children being alive without neurological abnormality.
Amnioreduction can be retained as a treatment option for those situations where the expertise for laser coagulation is not available, pending transfer to a unit where such treatment can be obtained or when the condition is diagnosed after 26 weeks of pregnancy.
Implications for research.
Randomised evaluation of interventions such as septostomy, serial amniocentesis and placental laser ablation with regard to their respective effect on very mild forms of TTTS (Quintero stage 1) and more severe forms (Quintero stage 4) are required.
Future studies should include an economic analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2013 | New search has been performed | Search updated ‐ five trial reports identified. These included two additional reports for Eurofetus 2004 and two additional reports for NIHCD 2007. One trial is ongoing (Slaghekke 2008). Methods updated. Outcomes re‐defined. This updated review now contains three included studies (involving 253 women), two excluded studies and one ongoing study. |
| 31 May 2013 | New citation required and conclusions have changed | The NIHCD 2007 trial (which was previously awaiting assessment) has now been included. There are now long‐term data included for one already included study (Eurofetus 2004). At six‐years follow‐up more babies were alive without neurological abnormality in the laser group than in the amnioreduction group. Overall death and death of both infants per pregnancy are now not significantly different between laser and amnioreduction groups. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 10 April 2008 | New search has been performed | Search updated in January 2008. No new trials identified. We changed the statistical method from odds ratio to risk ratio for outcomes 1.4 and 2.5 in keeping with the statistical methods for all other outcomes. |
| 11 February 2008 | Amended | Converted to new review format. |
| 22 October 2007 | New citation required and conclusions have changed | Substantive amendment |
| 22 October 2007 | New search has been performed | The original review did not contain any data from completed trials. We updated the search in February 2007 and added the data from two trials now published (Eurofetus 2004; Moise 2005). We updated the search in October 2007 just before submission for publication and identified a published report for the NIHCD 2007a ongoing study. The outcomes from this trial have not been included in this review but the study is awaiting assessment pending further communication with authors. The results of the review cannot be conclusive as the included trials are small and no meta‐analysis is available at the moment. The findings would, however, support the use of endoscopic laser coagulation in the treatment of twin‐twin transfusion syndrome to improve perinatal outcome. There does not appear to be a difference in perinatal outcome between amnioreduction and septostomy. |
Acknowledgements
Acknowledgements to Kenneth Moise (Moise 2005), Professor Yves Ville and Michel Boulvain (Eurofetus 2004) for additional data supplied.
We would like to thank Leanne Jones, Research Associate, Cochrane Pregnancy and Childbirth Group for her support in preparing the 2013 update.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods used in previous versions of the review
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2007, Issue 4) using the following search strategy:
We did not apply any language restrictions.
TWIN‐TWIN
(TWIN near TRANSFUSION)
SEPTOSTOMY
((LASER next COAGULATION) and TWIN‐TWIN)
(FETO‐FETAL next TRANSFUSION)
(FOETO‐FOETAL next TRANSFUSION)
AMNIOREDUCTION
AMNIODRAINAGE
((LASER next ABLATION) and TWIN‐TWIN)
((((((((#1 or #2) or #3) or #4) or #5) or #6) or #7) or #8) or #9)
We also searched the following conference proceedings in February 2007: British Maternal and Fetal Medicine Society; Annual Clinical Meeting of the Society for Maternal and Fetal Medicine; International Society for Ultrasound in Obstetrics & Gynaecology and World Congress of Obstetrics and Gynaecology.
We made personal contact with experts or institutions active in the area.
We did not apply any language restrictions.
Data and analyses
Comparison 1. Septostomy versus amnioreduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall death adjusted for clustering | 1 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.47, 1.47] | |
| 2 Death of at least one infant per pregnancy | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.48, 1.35] |
| 3 Death of both infants per pregnancy | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.37, 2.22] |
| 4 Gestational age at birth (weeks) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.81, 3.21] |
Comparison 2. Endoscopic laser surgery versus amnioreduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall death adjusted for clustering | 2 | Risk Ratio (Random, 95% CI) | 0.87 [0.55, 1.38] | |
| 2 Alive without neurological complications at 6 years adjusted for clustering | 1 | Risk Ratio (Fixed, 95% CI) | 1.57 [1.05, 2.34] | |
| 3 Alive with major neurological complications at 6 years adjusted for clustering | 1 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.34, 2.77] | |
| 4 Death of at least one infant per pregnancy | 2 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 5 Death of both infants per pregnancy | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.27, 2.10] |
| 6 Preterm labour within 48 hours of procedure | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.18, 20.96] |
| 7 Rupture of membranes within 48 hours of procedure | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.71, 2.54] |
| 8 Mechanical ventilation | 1 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.44, 1.43] | |
| 9 Need for blood transfusion within 48 hours of delivery adjusted for clustering | 1 | Risk Ratio (Fixed, 95% CI) | 0.45 [0.15, 1.31] | |
| 10 Intraventricular haemorrhage grade III/IV | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.12] |
| 11 Maternal death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Admission to intensive care unit for procedure‐related reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eurofetus 2004.
| Methods | Type of study: randomised controlled trial. Method of treatment allocation: computer‐generated random sequence with randomisation allocated by a web‐based system. Stratification: none stated. Sample size calculation: yes. Intention‐to‐treat analyses: yes. Losses to follow‐up: no. Funding: Biology, Medicine and Development contract, European Commission, Flemish Government, French Direction de la Recherche Clinique and the 5th framework Program of the European Commission. | |
| Participants | Location: 6 countries, 122 women from France, 13 from Belgium, 3 from the Netherlands, 2 from Switzerland, 1 from Italy and 1 from the United States. Timeframe: January 1999 to March 2002. Inclusion criteria: women presenting between 15 and 26 weeks with polyuric polyhydramnios in the recipient twin, with a vertical pool of 8 cm at or before 20 weeks or 10 cm after 20 weeks with a distended fetal bladder. Oliguric oligohydramnios in the donor twin with a deepest pool measuring 2 cm. Exclusion criteria: fetal death, major fetal anomaly, ruptured membranes, maternal condition requiring delivery, and any previous invasive therapy for the syndrome. Total recruited: 142 women and 284 fetuses in both arms. | |
| Interventions | Fetoscopic laser coagulation of vessels crossing the membranes. Amniotic fluid was drained through the cannula until the deepest pool was 5‐6 cm on ultrasonography. Amnioreduction of the polyhydramniotic sac under local analgesia, with an 18‐gauge needle and either syringe aspiration or wall suction. Amniotic fluid was drained until the deepest pool was 5‐6 cm. Amnioreduction was repeated whenever polyhydramnios recurred. Prophylactic tocolytics and antibiotics administered prophylactically. Women kept in hospital for 24‐48 hours after the procedure, then seen weekly for ultrasound follow‐up. | |
| Outcomes | Primary outcomes: perinatal survival of at least 1 twin, survival of at least 1 twin to 7‐12 months and neurologic complications at 7‐12 months of age. Other outcomes: maternal complications (placental abruption, intra‐abdominal haemorrhage, or leakage of amniotic fluid with peritoneal irritation, chorioamnionitis, amniotic fluid embolus) and fetal complications. | |
| Notes | Interim analyses: 2 planned (after the inclusion of 72 and 144 women) to evaluate the rates of survival of at least one twin to discharge from NICU ‐ an end point considered more clinically relevant than survival at 28 days. First interim analysis did not reveal any differences between the groups. The second showed a significantly higher rate of survival of at least one twin to discharge from NICU, so the trial was stopped according to the O'Brien‐Fleming stopping rule for discontinuing enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence with randomisation allocated by a web‐based system. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random sequence with randomisation allocated by a web‐based system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "For practical reasons the treating perinatologist was not blinded to therapy", however, the main survival outcomes are not likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcomes were assigned by one neonatologist who had no knowledge of the assigned treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses: yes. Losses to follow‐up: no. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | This trial was stopped after the second interim analysis showed a higher rate of survival of at least one twin in the laser arm. The trial quoted the O'Brien‐Fleming rule for stopping. |
Moise 2005.
| Methods | Type of study: randomised controlled trial. Method of treatment allocation: computer‐generated randomisation with block size of 10 using a web‐based system. Stratification: none used. Sample size calculation: yes. Intention‐to‐treat analysis: yes. Losses to follow‐up: 2 women (3%) were lost to follow‐up in the septostomy arm. Funding: none mentioned. | |
| Participants | Location: University of North Carolina. Timeframe: September 1997 to July 2002. Inclusion criteria: women with monochorionic twin gestations less than 24 weeks, polyhydramnios in one amniotic cavity (deepest vertical pool > 8 cm at < 20 weeks, > 10 cm at 20‐22 weeks and > 12 cm after 22 weeks) and oligohydramnios in the second amniotic cavity (deepest vertical pool < 2 cm). Exclusion criteria: fetal structural abnormalities, premature contractions associated with cervical change, premature rupture of membranes, suspected chorioamnionitis, or other indications for delivery. Total recruited: 73 women and 146 fetuses in both arms. | |
| Interventions | Purposeful perforation of the inter twin membrane under ultrasound guidance with a 22‐gauge needle, from the donor sac into the recipient twin's amniotic cavity. Repeat septostomy, with or without amnioreduction was performed if re‐accumulation of the amniotic fluid in the donor twin's amniotic cavity did not occur. Cross‐over to amnioreduction arm was allowed if oligohydramnios had not resolved in the donor twin's sac or if the deepest vertical pool in the recipient twin's sac had increased by 30% over baseline value. Salvage amnioreduction at the time of septostomy was performed if maternal symptoms were present. Amnioreduction of the recipient amniotic sac using a 18‐gauge needle, connected either to wall suction or a syringe attached to extension tubing. Fluid was removed until the deepest pool was less than or equal to 6 cm or 5 L was removed. Amnioreduction was repeated if there was excessive uterine activity, maternal respiratory compromise or polyhydramnios recurred. |
|
| Outcomes | Primary outcomes: at least 1 infant surviving until hospital discharge. | |
| Notes | Interim analysis: planned at the midway point using O'Brien‐Fleming stopping rule for discontinuing enrolment. The trial was stopped by the Data Safety Monitoring Officer after the interim analysis because of slower than projected enrolment and almost identical perinatal mortality in either arm of the trial making it unlikely that the primary end point might be achieved. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation with block size of 10 using a web‐based system. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation with block size of 10 using a web‐based system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible, the main survival outcomes are not likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis: yes. Losses to follow‐up: 2 women (3%) were lost to follow‐up in the septostomy arm. |
| Selective reporting (reporting bias) | Low risk | All expected pre‐specified outcomes reported. |
| Other bias | Low risk | This trial was stopped early on the basis of interim analysis. The recruitment rate to the trial was slower than predicted and it was felt that the primary end point would not be achieved. This trial quoted the O'Brien‐Fleming rule for stopping. |
NIHCD 2007.
| Methods | Prospective, randomised, multicentre trial. | |
| Participants | Location: multicentre trial across 11 centres in USA. Inclusion criteria: with monochorionic diamniotic twin pregnancy less than 22 weeks' gestation with oligohydramnios in the donor twin (deepest vertical pool no more than 2 cm) and polyhydramnios (deepest vertical pool of > 8 cm) with or without Doppler or echocardiographic changes in the recipient twin. Decompressed bladder in donor unless Doppler velocimetry changes and/or echocardiographic changes already present. Exclusion criteria: randomisation after 24 weeks, cervical length < 2 cm, presence of cervical cerclage, uterine anomaly, refusal to accept randomisation, inability to pursue prenatal care at an approved centre coordinated by one of the participating institutions, inability to pursue postnatal evaluation at a NIHCD Neonatal Research Network Institution. Number required: 150 women. | |
| Interventions | Amnioreduction, n = 20 women. Selective fetal laser photocoagulation, n = 20 women. | |
| Outcomes | Primary outcomes: survival of at least 1 twin at 30 days after birth and no treatment failure. Secondary outcomes: survival times of each twin in utero or after birth, gestational age at delivery, placental insufficiency, echocardiographic evidence of cardiac compromise, evidence of brain injury preceding birth by magnetic resonance imaging, postnatal co‐morbidity. | |
| Notes | This trial was stopped early after recruiting only a quarter of the sample size (42 pregnancies) in five years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centralised via faxed case report forms and stratified by cluster and gestational age group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible, the main survival outcomes are not likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses: yes. Losses to follow‐up: 43 of 58 eligible cases consented to randomisation, 1 withdrew, 42(21.4% of original screened) were randomised, 1 dropped out of each arm leaving 20 in the AR arm and 20 in the SFLP arm. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | The trial was stopped early after 42 participants were randomised (only a quarter of sample size), at the request of the investigators and the Trial Oversight Committee. Evaluation of all adverse events detected a statistical trend in adverse outcome affecting the recipient twin in 1 treatment arm. |
AR: amnioreduction NICU: neonatal intensive care unit SFLP: selective fetal laser photocoagulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Soothill 2000 | Observational study of twins without therapeutic comparison groups. |
| Sutcliffe 2000 | Study not completed due to difficulty with recruitment. Observational data with no therapeutic comparison groups available. |
Characteristics of ongoing studies [ordered by study ID]
Slaghekke 2008.
| Trial name or title | Solomon study. |
| Methods | Multicentre, parallel group, randomised controlled trial. |
| Participants | All twin‐to‐twin transfusion syndrome pregnancies eligible for laser surgery up to 26 weeks' gestation. Exclusion criteria: triplet pregnancies; language problems for informed consent. |
| Interventions | 'Solomon laser‐technique', in which the entire vascular equator is coagulated compared to the 'selective laser‐technique' in which only the identifiable vascular anastomoses are coagulated. |
| Outcomes | Primary outcomes: Prevalence of TAPS; recurrence of TTTS. Secondary outcomes: Residual anastomoses on placental injection; perinatal mortality; neonatal morbidity. Follow‐up of TTTS survivors will be 2 years. |
| Starting date | 15 March 2008. |
| Contact information | Dr F Slaghekke, Leiden University Medical Center, Department of Obstetrics and Fetal Medicine. |
| Notes | Planned closing date: 15 March 2010. |
TAPS: twin anaemia‐polycythaemia sequence TTTS: twin‐twin transfusion syndrome
Differences between protocol and review
Methods updated. Primary and secondary outcomes were re‐defined for the 2014 update. The number of perinatal and neonatal mortality outcomes were reduced for the 2014 update (deleted the following: stillbirth; fetal survival per pregnancy; perinatal death; early or late neonatal death). A number of other outcomes were deleted from the review (first intervention to delivery time; need for a combination of therapies; type of anaesthesia; fetal haemoglobin discordance at birth; ventriculomegaly; intraventricular haemorrhage: any grade; cystic periventricular leukomalacia; admitted to neonatal intensive care unit; tocolysis; amniotic fluid embolism; placental abruption; chorioamnionitis; intraperitoneal bleeding; relief of symptoms; maternal satisfaction with procedure; termination of pregnancy (not a prespecified outcome).
Contributions of authors
This 2014 review update was prepared by D Roberts. S Gates provided statistical advice and commented on the revised draft. JP Neilson and M Kilby commented on drafts.
Sources of support
Internal sources
The University of Liverpool, UK.
Liverpool Women's NHS Foundation Trust, UK.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS:10/4001/02
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Eurofetus 2004 {published data only}
- Ortqvist L, Bussieres L, Staraci S, Fermanian C, Ville Y. Long‐term neurodevelopmental outcome in twin‐to‐twin transfusion syndrome (TTTS) in the Eurofoetus trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S118. [Google Scholar]
- Ortqvist L, Chevret S, Bussieres L, Staraci S, Huard F, Ville Y. Long‐term neurodevelopmental outcome in twin‐to‐twin transfusion syndrome in the Eurofetus trial. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S3. [Google Scholar]
- Salomon LJ, Ortqvist L, Aegerter P, Bussieres L, Staracci S, Stirnemann JJ, et al. Long‐term developmental follow‐up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin‐to‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 2010;203(5):444.e1‐444.e7. [DOI] [PubMed] [Google Scholar]
- Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin‐to‐twin transfusion syndrome. New England Journal of Medicine 2004;351(2):136‐44. [DOI] [PubMed] [Google Scholar]
- Senat MV, Deprest J, Boulvain M, Ville Y. Fetoscopic laser surgery versus serial amniodrainage in the management of severe twin‐to‐twin transfusion syndrome at midgestation. American Journal of Obstetrics and Gynecology 2003;189(6):S56. [Google Scholar]
- Ville Y. Treatment of TTTS. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(Suppl 1):35. [Google Scholar]
- Ville Y, Eurofetus Group. Treatment of twin‐to‐twin‐transfusion syndrome. The end of a long‐standing misunderstanding. Ultrasound in Obstetrics & Gynecology 2003;22(Suppl 1):64. [Google Scholar]
Moise 2005 {published and unpublished data}
- Fisk N. Twin‐twin transfusion syndrome: a multicentre randomised trial for the evaluation of septostomy versus serial amnioreduction for therapy. National Research Register 2000; Vol. Issue 1.
- Moise KJ Jr, Dorman K, Lamvu G, Saade GR, Fisk NM, Dickinson JE, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 1):701‐7. [DOI] [PubMed] [Google Scholar]
- Saade G, Moise K, Dorman K, Fisk N, Dickinson JE, Wilson RD. A randomized trial of septostomy versus amnioreduction in the treatment of twin oligohydramnios polyhydramnios sequence (TOPS). American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S54. [Google Scholar]
NIHCD 2007 {published and unpublished data}
- Crombleholme T. Amnioreduction versus selective fetoscopic laser photocoagulation for the treatment of severe twin‐twin transfusion syndrome. www.clinicaltrials.gov (accessed 12 April 2006).
- Crombleholme T, Shera D, Porter F, Lee H, Chyu J, Silver RK, et al. NIH sponsored prospecive randomized clinical trial of amnioreduction vs selective fetoscopic laser photocoagulation for twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombleholme TM, Shera D, Lee H, D'Alton M, Porter F, Chyu J, et al. A prospective randomized multicenter trial of amnioreduction vs. selective fetoscopic laser photocoagulation for the treatment of severe twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 2007;197(4):396.e1‐396.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks FI, Carr SR, O'Brien BM, Muratore CS. Power and interpretation of a randomized study on the treatment of severe twin‐to‐twin transfusion syndrome.[Comment]. American Journal of Obstetrics and Gynecology 2008;198(5):607; author reply 607‐8. [DOI] [PubMed] [Google Scholar]
- Quintero RA, Chmait R. The Con trial. American Journal of Obstetrics and Gynecology 2009;200(3):e14‐e15. [DOI] [PubMed] [Google Scholar]
- Silver R. Twin‐twin transfusion trial. www.enh.org (accessed 14 June 2005).
References to studies excluded from this review
Soothill 2000 {published data only (unpublished sought but not used)}
- Soothill PW. Twin to twin transfusion in dichorionic pregnancy. National Research Register 2000; Vol. Issue 1.
Sutcliffe 2000 {published data only (unpublished sought but not used)}
- Sutcliffe AG. A comparative study of amniotic drainage and laser cryotherapy for twin‐twin transfusion syndrome. National Research Register 2000; Vol. Issue 1.
References to ongoing studies
Slaghekke 2008 {published data only}
- Slaghekke F. Fetoscopic laser coagulation of the entire vascular equator for the treatment of twin‐to‐twin transfusion syndrome: the "Solomon study". http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1245 (accessed 7 January 2013).
Additional references
Arabin 1998
- Arabin B, Laurini RN, Eyck J, Nicolaides KH. Treatment of twin‐twin transfusion syndrome by laser and digoxin. Biophysical and angiographic evaluation. Fetal Diagnosis and Therapy 1998;13(3):141‐6. [DOI] [PubMed] [Google Scholar]
Bajoria 1995
- Bajoria R, Wigglesworth J, Fisk NM. Angioarchitecture of arterio‐arterial anastomoses in vivo with the feto‐fetal transfusion syndrome. American Journal of Obstetrics and Gynecology 1995;172:856‐63. [DOI] [PubMed] [Google Scholar]
Bower 1995
- Bower SJ, Flack NJ, Sepulveda W. Uterine artery blood flow response to correction of amniotic fluid volume. American Journal of Obstetrics and Gynecology 1995;173:502‐7. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2000
- Brocklehurst P, Elbourne D, Alfirevic Z. Role of external evidence in monitoring clinical trials: experience from a perinatal trial. BMJ 2000;320:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crombleholme 1996
- Crombleholme TM, Robertson F, Marx G, Yarnell R, D'Alton ME. Fetoscopic cord ligation to prevent neurological injury in monozygous twins. Lancet 1996;348:191. [DOI] [PubMed] [Google Scholar]
De Lia 1985
- Lia JE, Emery MG, Sheafor SA, Jennison TA. Twin transfusion syndrome: successful in utero treatment with digoxin. International Journal of Gynecology & Obstetrics 1985;23(3):197‐201. [DOI] [PubMed] [Google Scholar]
De Lia 1990
- Lia JE, Cruikshank DP, Keye W. Fetoscopic neodynium: YAG laser occlusion of placental vessels in severe twin‐twin transfusion syndrome. Obstetrics & Gynecology 1990;75:1046‐53. [PubMed] [Google Scholar]
De Lia 1995
- Lia JE, Kuhlmann RS, Harstad TW, Cruikshank DP. Fetoscopic laser ablation of placental vessels in severe previable twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 1995;172:1202‐8. [DOI] [PubMed] [Google Scholar]
Denbow 1998
- Denbow ML, Fisk NM. The consequences of monochorionic placentation. Baillieres Clinical Obstetrics and Gynaecology 1998;12(1):37‐51. [DOI] [PubMed] [Google Scholar]
Deprest 1998
- Deprest JA, Ballaer PP, Evrard VA, Peers KH, Spitz B, Steegers EA, et al. Experience with fetoscopic cord ligation. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;81(2):157‐64. [DOI] [PubMed] [Google Scholar]
Doll 2001
- Doll R. The role of data monitoring committees. In: Duley L, Farrell B editor(s). Clinical Trials. London: BMJ Publishing Group, 2001:97‐104. [Google Scholar]
Donner 1997
- Donner C, Shahabi S, Thomas D, Noel JC, Kirkpatrick C, Rysselberghe MV, et al. Selective feticide by embolization in twin‐twin transfusion syndrome. A report of two cases. Journal of Reproductive Medicine 1997;42(11):747‐50. [PubMed] [Google Scholar]
Elliot 1991
- Elliot JP, Urig MA, Clewell WH. Aggressive therapeutic amniocentesis for treatment of twin‐twin transfusion syndrome. Obstetrics & Gynecology 1991;77:537‐44. [PubMed] [Google Scholar]
Fusi 1990
- Fusi L, Gordon H. Multiple pregnancy complicated by single intrauterine death:problems and outcome with conservative management. British Journal of Obstetrics and Gynaecology 1990;97:511‐6. [DOI] [PubMed] [Google Scholar]
Garry 1998
- Garry D, Lysikiewicz A, Mays J, Canterino J, Tejani N. Intra‐amniotic pressure reduction in twin‐twin transfusion syndrome. Journal of Perinatology 1998;18(4):284‐6. [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should randomised trial including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111:213‐9. [DOI] [PubMed] [Google Scholar]
Gilbert 1991
- Gilbert WM, Davis SE, Kaplan C, Pretorius D, Merritt TA, Benirschke K. Morbidity associated with prenatal disruption of the dividing membrane in twin gestations. Obstetrics & Gynecology 1991;78(4):623‐30. [PubMed] [Google Scholar]
Grant 2004
- Grant A. Stopping clinical trials early. BMJ 2004;329:525‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hecher 1999
- Hecher K, Plath H, Bregenzer T, Hansmann M, Hackeloer BJ. Endoscopic surgery versus serial amniocenteses in the treatment of severe twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 1999;180(3):717‐24. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 1993
- Jones JM, Sbarra AJ, Dilillo L, Cetrulo CL, D'Alton ME. Indomethacin in severe twin‐twin transfusion syndrome. American Journal of Perinatology 1993;10:24‐6. [DOI] [PubMed] [Google Scholar]
Machin 1996
- Machin G, Still K, Lalani T. Correlations of placental vascular anatomy and clinical outcomes in 69 monochorionic twin pregnancies. American Journal of Medical Genetics 1996;61:229‐36. [DOI] [PubMed] [Google Scholar]
Mahony 1990
- Mahony BS, Petty CN, Nyberg DA, Luthy DA, Hickok DE, Hirsch JH. The "stuck twin" phenomenon: ultrasonographic findings, pregnancy outcome, and management with serial amniocentesis. American Journal of Obstetrics and Gynecology 1990;163:1513‐22. [DOI] [PubMed] [Google Scholar]
Montan 1985
- Montan S, Jorgensen C, Sjoberg N. Amniocentesis in the treatment of acute polyhydramnios in twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 1985;64:537‐9. [DOI] [PubMed] [Google Scholar]
Quintero 1996
- Quintero RA, Romero R, Reich H, Goncalves L, Johnson MP, Carreno C, et al. In utero percutaneous umbilical cord ligation in the management of complicated monochorionic multiple gestations. Ultrasound in Obstetrics & Gynecology 1996;8(1):16‐22. [DOI] [PubMed] [Google Scholar]
Quintero 2003
- Quintero RA, Dickinson JE, Morales WJ, Bornick PW, Bermudez C, Cincotta R, et al. Stage‐based treatment of twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 2003;188:1333‐40. [DOI] [PubMed] [Google Scholar]
Reisner 1993
- Reisner DP, Mahony BS, Petty CN, Nyberg DA, Porter TF, Zingheim RN, et al. Stuck twin syndrome: outcome in thirty seven cases. American Journal of Obstetrics and Gynecology 1993;169:991‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roman 1995
- Roman JD, Hare AA. Digoxin and decompression amniocentesis for treatment of feto‐fetal transfusion. British Journal of Obstetrics and Gynaecology 1995;102:421‐3. [DOI] [PubMed] [Google Scholar]
Saade 1998
- Saade GR, Belfort MA, Berry DL, Bui TH, Montgomery LD, Johnson A, et al. Amniotic septostomy for the treatment of twin oligohydramnios‐polyhydramnios sequence. Fetal Diagnosis and Therapy 1998;13(2):86‐93. [DOI] [PubMed] [Google Scholar]
Saunders 1991
- Saunders NJ, Snijders RJM, Nicolaides KH. Therapeutic amniocentesis in twin‐twin transfusion syndrome appearing in the second trimester of pregnancy. American Journal of Obstetrics and Gynecology 1991;166(3):820‐4. [DOI] [PubMed] [Google Scholar]
Schneider 1985
- Schneider KTM, Vetter K, Huch R. Acute polyhydramnios complicating twin pregnancies. Acta Geneticae Medicae et Gemellologiae 1985;34:179‐84. [DOI] [PubMed] [Google Scholar]
Steinberg 1990
- Steinberg LH, Hurley VA, Desnedt E, Besicher NA. Acute polyhydramnios in twin pregnancies. Australian and New Zealand Journal of Obstetrics and Gynaecology 1990;30:196‐200. [DOI] [PubMed] [Google Scholar]
Trespidi 1997
- Trespidi L, Boschetto C, Caravelli E, Villa L, Kusterman A, Nicolini U. Serial amniocenteses in the management of twin‐twin transfusion syndrome: when is it valuable?. Fetal Diagnosis and Therapy 1997;12:15‐20. [PubMed] [Google Scholar]
Urig 1990
- Urig MA, Clewell WH, Elliot JP. Twin‐twin transfusion syndrome. American Journal of Obstetrics and Gynecology 1990;163:1522‐6. [DOI] [PubMed] [Google Scholar]
Van Heteren 1998
- Heteren CF, Nijhuis JG, Semmekrot BA, Mulders LG, Berg PP. Risk for surviving twin after fetal death of co‐twin in twin‐twin transfusion syndrome. Obstetrics & Gynecology 1998;92(2):215‐9. [DOI] [PubMed] [Google Scholar]
Ville 1995
- Ville Y, Hyett J, Hecher K, Nicolaides K. Preliminary experience with endoscopic laser surgery for severe twin‐twin transfusion syndrome. New England Journal of Medicine 1995;332:224‐7. [DOI] [PubMed] [Google Scholar]
Ville 1998
- Ville Y, Hecher K, Gagnon A, Sebire N, Hyett J, Nicolaides K. Endoscopic laser coagulation in the management of severe twin‐twin transfusion syndrome. British Journal of Obstetrics and Gynaecology 1998;105:446‐53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Roberts 2001
- Roberts D, Neilson JP, Weindling AM. Interventions for the treatment of twin‐twin transfusion syndrome. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002073] [DOI] [PubMed] [Google Scholar]
Roberts 2008a
- Roberts D, Neilson JP, Kilby M, Gates S. Interventions for the treatment of twin‐twin transfusion syndrome. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002073.pub2] [DOI] [PubMed] [Google Scholar]
Roberts 2008b
- Roberts D, Gates S, Kilby M, Neilson JP. Interventions for twin‐twin transfusion syndrome: a Cochrane review. Ultrasound in Obstetrics & Gynecology 2008;31(6):701‐11. [DOI] [PubMed] [Google Scholar]


