Abstract
Infected necrotizing pancreatitis (INP) is associated with an increased risk of organ failure and mortality. Its early recognition and timely initiation of antibiotic therapy can save patients’ lives. We systematically searched three databases on 27 October 2022. In the eligible studies, the presence of infection in necrotizing pancreatitis was confirmed via a reference test, which involved either the identification of gas within the necrotic collection through computed tomography imaging or the examination of collected samples, which yielded positive results in Gram staining or culture. Laboratory biomarkers compared between sterile necrotizing pancreatitis and INP were used as the index test, and our outcome measures included sensitivity, specificity, the receiver operating characteristic (ROC) curve and area under the ROC curve (AUC). Within the first 72 hours (h) after admission, the AUC of C-reactive protein (CRP) was 0.69 (confidence interval (CI): 0.62–0.76), for procalcitonin (PCT), it was 0.69 (CI: 0.60–0.78), and for white blood cell count, it was 0.61 (CI: 0.47–0.75). After the first 72 h, the pooled AUC of CRP showed an elevated level of 0.88 (CI: 0.75–1.00), and for PCT, it was 0.86 (CI: 0.60–1.11). The predictive value of CRP and PCT for infection is poor within 72 h after hospital admission but seems good after the first 72 h. Based on these results, infection is likely in case of persistently high CRP and PCT, and antibiotic initiation may be recommended.
Keywords: scoring system, antibiotic therapy, sepsis, necrosis, infection
1. Introduction
Acute pancreatitis (AP) ranks among the most prevalent gastroenterological conditions, affecting many patients worldwide [1]. While interstitial edematous pancreatitis is the most common form, pancreatic necrosis occurs in approximately 5–10% of cases [2].
Pancreatic necrosis arises from compromised pancreatic perfusion, emphasizing the pivotal role of pancreatic ischemia and microcirculatory disturbances in acute pancreatitis development. This injury, whether primary or secondary to non-vascular causes, manifests early in acute pancreatitis and correlates with severity progression. Conversely, enhancing blood flow not only averts acute pancreatitis but also expedites recovery. Therefore, it is recommended to provide aggressive hydration to all patients, especially during the first 12–24 hours (h), unless restricted by cardiovascular or renal comorbidities [2,3,4].
AP is an inflammatory condition, thus exhibiting systemic manifestations of inflammation, including fever, tachycardia, hypotension, elevated white blood cell count (WBC), and increased levels of C-reactive protein (CRP) [5,6,7]. These characteristics do not differentiate between inflammation and infection, leading to an excessive utilization of antibiotics across the entire range of disease severity without distinguishing between the two [8].
Nonetheless, approximately 30% of individuals diagnosed with acute necrotizing pancreatitis (ANP) will experience debris infection due to the migration of intestinal microbial flora, resulting in infected necrotizing pancreatitis (INP). The presence of sepsis exacerbates the complexity of the condition, leading to alarmingly high mortality rates, as high as 40% [9,10].
Diagnosing and treating infected necrosis remains challenging. Antimicrobial therapy is most appropriate when there is a culture-proven infection in pancreatic necrosis or a strong suspicion of infection indicated by factors such as gas in the collection, bacteremia, sepsis, or clinical deterioration. Prophylactic antibiotics for preventing the infection of sterile necrosis are not recommended. Initiating enteral feeding early in patients with pancreatic necrosis is recommended to reduce the risk of infection. This preventive measure enhances the integrity of the mucosal barrier and reduces the likelihood of bacterial translocation in the gastrointestinal tract. The drainage and/or debridement of pancreatic necrosis is warranted in patients with infected necrosis. It is recommended to avoid pancreatic debridement in the early acute period (first 2 weeks) due to its association with increased morbidity and mortality; optimal debridement is ideally delayed for 4 weeks [11,12].
While several scoring systems have been developed to predict the severity of AP, there is currently a lack of scoring systems specifically designed to predict the presence of infection [13,14]. Identifying infection early is crucial for timely interventions and appropriate management, especially in high-risk patients who may benefit from early antibiotic therapy. To address this gap in the literature, we conducted a meta-analysis on laboratory markers to investigate their potential for predicting the presence of infection in AP. Our meta-analysis aimed to identify early predictors for INP.
2. Methods
2.1. Protocol
The meta-analysis was reported following the Preferred Reporting Items for Systematic Review (PRISMA) statement (Supplementary Document S1), and we followed the Cochrane Handbook [15,16]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42022370672.
2.2. Information Sources and Search Strategy
A systematic search was conducted in Medline (via Pubmed), Embase, and The Cochrane Central Register of Controlled Trials (CENTRAL) from inception to 27 October 2022 (Supplementary Document S2).
2.3. Eligibility Criteria
We used the PIRD (patient; index test; reference test; diagnosis of interest) framework to formulate our research question. We included randomized controlled trials, prospective and retrospective observational cohort studies with the following criteria: (1) the study was conducted in the adult population with necrotizing pancreatitis, and the diagnosis of AP adhered to the ‘two out of three’ criteria outlined in the International Association of Pancreatology and American Pancreatic Association guidelines, which included: (a) upper abdominal pain, (b) serum amylase or lipase levels elevated to at least three times the upper limit of normal, and (c) the presence of characteristic findings on pancreatic imaging; (2) the reference test confirmed infection by computed tomography imaging with the presence of gas in the necrotic collection or by examination of the sample acquired by an intervention using Gram staining or culture; (3) the index test was using any laboratory biomarker that was compared between patients suffering from sterile necrotizing pancreatitis (SNP) or INP; (4) and data on the sensitivity, specificity, receiver operating characteristic (ROC) curve or area under the ROC curve (AUC) to predict pancreatic infection, differentiating SNP from INP [2,17].
We excluded animal or in vitro studies, case reports, case series, and abstracts. There was no limitation on the publication language or date.
2.4. Selection Process
The publications were processed by the EndNote software (version 20.4.1.16297). After excluding duplicates, two reviewers (DT and ML) independently assessed the titles and abstracts. Later, the full text of relevant studies was obtained and independently evaluated. Cohen’s kappa was used to assess the level of agreement among the review authors. Disagreements between the two authors were resolved by a third author (AM). To identify additional eligible studies, we also investigated the references of relevant reviews or included articles. Also, CitationChaser was used for both backward and forward citation chasing of the included studies [18].
2.5. Data Collection Process and Data Items
The eligible articles were reviewed by two independent reviewers who extracted the following data using a pre-designed data table: first author, study location, study design, study duration, demographic information about the study population, number of cases of IPN, type of reference test, timing of laboratory test measurements, type of laboratory tests, and outcomes (laboratory biomarkers’ sensitivity, specificity, AUC, true positive, false positive, false negative, true negative, cut-off value).
2.6. Study Risk of Bias Assessment
Two authors (D.T. and M.L.) independently assessed the risk of bias in the included studies using the Quality Assessment of Diagnostic Accuracy Study-2 (QUADAS-2) Tool [19]. Each item was evaluated as having a low, unclear, or high risk of bias. Disagreements were resolved by a third reviewer (A.M.).
2.7. Certainty of Evidence
To assess the certainty of evidence, we employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology [20]. The GRADEpro tool was used to generate a table.
2.8. Synthesis Methods
Statistical analyses were carried out using the R statistical software (version 4.1.2.) and the R script of the online tool described by Freeman [21]. For all statistical analyses, a p-value of less than 0.05 was considered significant.
We collected the AUC values and the confidence intervals (CIs) of the different diagnostic scores from the studies. From the CIs, we estimated the standard deviations of the AUC values, and we applied classical inverse-variance random-effects meta-analysis with the restricted maximum likelihood estimator to gain pooled AUC estimates with 95% CI. A test’s discrimination ability is considered excellent when its AUC falls between 0.90 and 1.00, while AUC values in the ranges of 0.80 to 0.90, 0.70 to 0.80, 0.60 to 0.70, and 0.50 to 0.60 signify good, fair, poor, and failed discrimination, respectively [22]. As only a few studies contributed to the meta-analyses, the Hartung–Knapp adjustment was applied. The I2 measure, its confidence interval and the Cochrane Q test were used to examine heterogeneity. Heterogeneity levels were categorized as low, moderate, and high when I2 values were 25%, 50%, and 75%, respectively. We also performed subgroup analysis according to the time horizons. We did not assume that the standard deviations of the random effects were the same in the subgroups.
In the case of the short-term diagnostic performances of PCT and CRP, we collected from a sufficient number of studies the total number of patients with and without infected necrosis, sensitivity, and specificity values, in most cases, along with the corresponding thresholds. From these data, we calculated two-by-two contingency tables for each threshold containing the true positive, false positive, false negative, and true negative values. Since the thresholds differed across studies, we fitted the summary ROC (SROC) curve using the non-Bayesian version of the approach [23]. For clarity, we note that Harbor et al. show that the employed method is mathematically equivalent to the bivariate model [24,25,26]. We plotted on an ROC plot the resulting SROC curves and the study-level estimates with their confidence intervals. The SROC curve shows the trade-off between sensitivity and specificity as the employed threshold varies. Publication bias analysis was not possible, since the number of involved studies was less than 10.
3. Results
3.1. Search and Selection
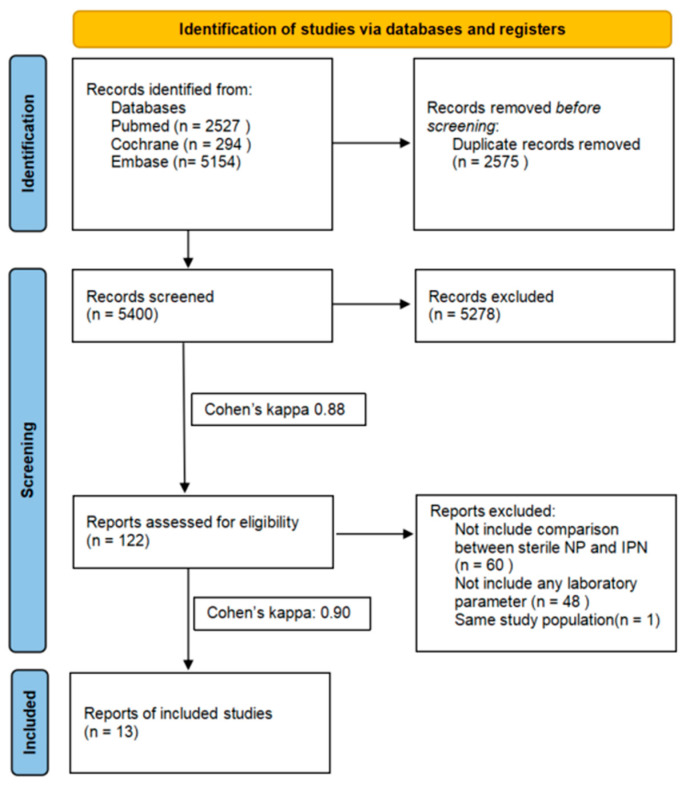
Altogether, 7975 articles were identified through our systematic search strategy. After the duplicate removal, 5400 titles and abstracts were screened, leaving 122 studies for full-text review. We excluded 60 studies because they did not compare SNP and IPN. Most commonly, they included a comparison between severe AP and IPN. Another 48 articles were not eligible for the analysis because they did not contain any data for laboratory parameters (Supplementary Document S3). One study had to be excluded because of overlapping populations [27]. Using citation chasing, we discovered no new articles relevant to our research topic. Finally, eight [28,29,30,31,32,33,34,35] of the thirteen studies included in our analysis were deemed eligible for meta-analysis, while the remaining five [36,37,38,39,40] were considered for systematic review. The study selection process is shown in Figure 1.
Figure 1.
PRISMA 2020 flowchart representing the study selection process.
3.2. Study Characteristics
All studies included in our analysis were observational studies with four being retrospective and nine being prospective in design (Table 1). The quantitative synthesis involved 758 patients diagnosed with necrotizing acute pancreatitis, of whom 324 were identified as having INP.
Table 1.
Basic characteristics of the included studies.
| Publication Data | Study Design |
Demography | Reference Test | Index Tests | Assessed Outcomes | Time Points for Laboratory Parameter Measurements | The Time Interval between Onset of Abdominal Pain and the Measurements of Laboratory Parameters | ||
|---|---|---|---|---|---|---|---|---|---|
| Country | Population | Age (Years) | |||||||
| Block et al. † (1987) [37] | cross-sectional (cohort-type accuracy study); prospective | Germany | 161 | N/A | surgery | Alb; Ca; HCT; WBC | Se, Sp | within 48 h | N/A |
| Brand et al. (2014) [36] | cross-sectional (cohort-type accuracy study); retrospective | Germany | 99 | 52 a (18–84) b | FNA | Alb, Ca, CRP, WBC | AUC; ROC; Se, Sp | within 36 h (most within 24 h) |
N/A |
| Chen et al. (2017) [29] | cross-sectional (cohort-type accuracy study); retrospective | China | 215 | 42.2 c (11.6) d | CECT, US- or CT- guided FNA, invasive therapeutic procedures | BUN; Cr; CRP; D-dim; HCT; PCT; PLT; WBC | AUC; ROC; Se; Sp | within 48 h | <48 h before hospital admission |
| Dambrauskas et al. (2007) [35] | cross-sectional (cohort-type accuracy study); prospective | Lithuania | 52 | 51.15 c | CECT, FNA | CRP, WBC | AUC; NPV; PPV; ROC; Se; Sp | every fourth day until discharge | measurement of laboratory parameters occurred between days 21 and 40 after the onset of the disease in the subgroup analysis |
| Mándi et al. † (2000) [38] | cross-sectional (cohort-type accuracy study); prospective | Hungary | 20 | 45.5 c (18.2) d (20–63) b |
CECT, US-guided FNA | IL-6; sICAM-1; PCT | NPV; PPV; Se; Sp | within 48 h, blood samples daily | N/A |
| Müller et al. (1999) [32] | cross-sectional (cohort-type accuracy study); prospective | Switzerland | 35 | 56.3 c (27–87) b |
CECT, US- or CT-guided FNA |
CRP; GCSF; PCT | AUC; ROC; Se; Sp | 1–14 days daily and thereafter every third day | from day 0 until day 14 after the onset of the symptoms |
| Rau et al. (2000) [34] | cross-sectional (cohort-type accuracy study); prospective |
Germany | 61 | (14–87) b | CECT, US-guided FNA |
CRP, PCT | AUC; ROC; Se; Sp | in 24 h intervals over 14 day | abdominal pain less than 120 h before hospital admission |
| Riché et al. (2003) [31] | cross-sectional (cohort-type accuracy study); prospective | France | 48 | (24–91) b | CECT, CT-guided FNA, surgical drainage | CRP; IL-6; PCT; TNF-alpha | AUC, ROC | within 72 h daily |
N/A |
| Rotar et al. (2022) [33] | cross-sectional (cohort-type accuracy study); prospective | Ukraine | 151 | (18–80) b | CECT, therapeutic intervention, | PCT | AUC; ROC; Se; Sp | 72 h before intervention | after the 4th weeks in case of 41 patients, before the 4th week in case of 74 patients |
| Rotar et al. † (2019) [39] | cross-sectional (cohort-type accuracy study); prospective | Ukraine | 70 | (18–80) b | CECT, therapeutic intervention | Presepsin | AUC; ROC; Se; Sp | 72 h | N/A |
| Ueda et al. † (2007) [40] | cross-sectional (cohort-type accuracy study); retrospective | Japan | 75 | 52 c (2) d | CECT, blood culture, US-guided FNA |
LDH, Lymphocyte count | AUC; ROC | within 72 h | within 72 h |
| Wiese et al. (2022) [28] | cross-sectional (cohort-type accuracy study); retrospective | Germany | 89 | 57.67 c | CECT, PC drainage, EUS-guided FNA | Alb; BUN; Ca; Crea; CRP; HCT; IL-6; PCT | AUC; ROC; Se; Sp | within 48 h | N/A |
| Zheng et al. (2011) [30] | cross-sectional (cohort-type accuracy study); prospective | China | 30 | 55.5 c | CECT, US- or CT-guided FNA |
CRP; IL-6, PCT; sTREM-1; TNF-alpha; WBC | AUC; NPV; PPV; ROC Se; Sp | 72 h | N/A |
a = median; b = range; c = mean; d = standard deviation; N/A = not applicable; Alb = albumin; AUC = area under the ROC curve; BUN = blood urea nitrogen; Ca = calcium; CECT = contrast-enhanced computed tomography; Crea = creatinine; CRP = c-reactive protein; D-dim = D-dimer; EUS = endoscopic ultrasound; FNA = fine needle aspiration; GCSF = granulocyte colony-stimulating factor; HCT = hematocrit; h = hours; IL-6 = interleukin-6; LDH = lactate dehydrogenase; NPV = negative predictive value; PCT = procalcitonin; PC = percutaneous; PLT = platelet; PPV = positive predictive value; ROC = receiver operating characteristic; Se = sensitivity; sICAM-1 = soluble intercelluar adhesion molecule-1; Sp = specificity; sTREM-1 = soluble triggering receptor expressed on myeloid cells; TNF-alpha = tumor necrosis factor-alpha; US = ultrasound; WBC = white blood cell; † = study included only in a systematic review.
Nine articles were published before the 2012 Atlanta classification, in which peripancreatic necrosis was added as necrotizing pancreatitis [2].
INP was identified through computed tomography imaging with the presence of gas in the necrotic collection or examination of samples obtained during an intervention or fine-needle aspiration with Gram stain, culture, or both. Interventions were defined as percutaneous or endoscopic drainage or percutaneous, endoscopic, or surgical necrosectomy.
CRP and PCT were the most frequently described predictors. However, many laboratory parameters such as albumin, hematocrit (HCT), blood urea nitrogen (BUN), creatinine, lymphocyte count, lactate dehydrogenase (LDH), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), soluble intercellular adhesion molecules (sICAM-1), soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), presepsin, and granulocyte colony-stimulating factor (G-CSF) were investigated in one study only. Due to limited comparable studies, we were unable to conduct a quantitative analysis of these factors.
3.3. Within 72 h after Admission CRP, PCT, and WBC Levels Alone Have Poor Predictive Value in ANP
A subgroup analysis was performed based on the time of the index test. Timing of the index test was within the first 72 h after admission in five articles [28,29,30,31,36].
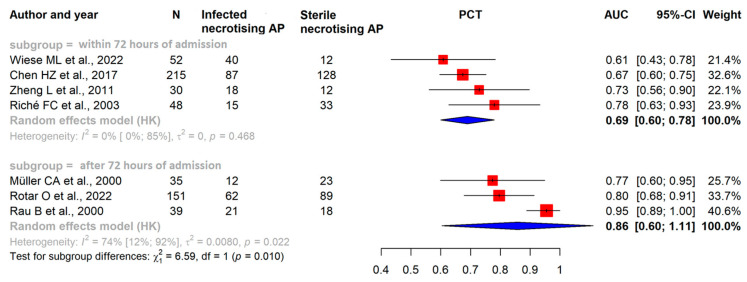
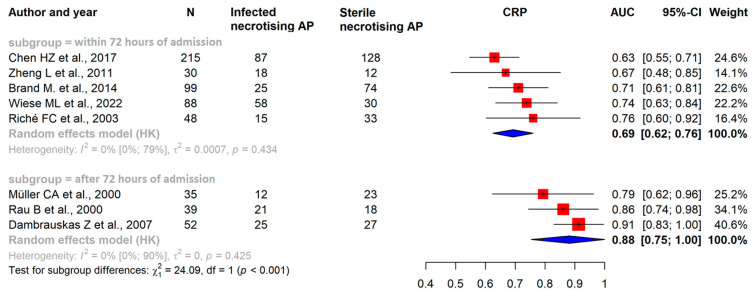
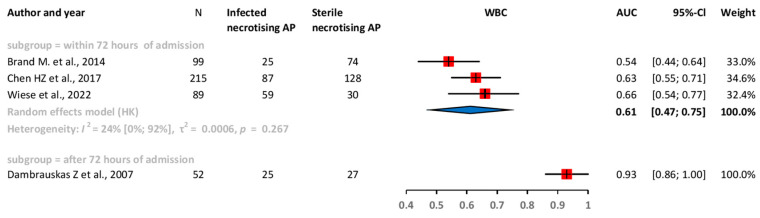
Our results confirmed that within the first 72 h after admission, the pooled AUC of CRP was 0.69 (CI: 0.62–0.76), for PCT, it was 0.69 (CI: 0.60–0.78), and for white blood cell count (WBC), it was 0.61 (CI: 0.47–0.75) (Figure 2, Figure 3 and Figure 4).
Figure 2.
Forest plots representing the AUC of PCT within the initial 72 h of admission and 72 h after admission, examining a two-week period of the disease: AP: acute pancreatitis; AUC: area under the ROC curve; CI: confidence interval; h: hours; PCT: procalcitonin [28,29,30,31,32,33,34].
Figure 3.
Forest plots representing the AUC of CRP within the initial 72 h of admission and 72 h after admission, examining a two-week period of the disease. AP: acute pancreatitis; AUC: area under the ROC curve; CI: confidence interval; CRP: C-reactive protein, h: hours [29,30,31,32,33,34,35].
Figure 4.
Forest plots representing the AUC of WBC within 72 h of admission. AP: acute pancreatitis; AUC: area under the ROC curve; CI: confidence interval; h: hours; WBC: white blood cell count [28,29,35,36].
3.4. After the First 72 h of Admission CRP and PCT Levels Have Good Predictive Value in ANP
After the first 72 h of admission, in studies investigating a minimum two-week period of the disease, the pooled AUC of CRP showed an elevated level of 0.88 (CI: 0.75–1.00); for PCT, it was 0.86 (CI: 0.60–1.11), which shows that it had a good predictive value (Figure 2 and Figure 3) [22].
3.5. Risk of Bias Assessment
The QUADAS-2 scores indicate that the included studies were moderately high quality (Supplementary Table S1).
3.6. Certainty of Evidence
The certainty of evidence derived from our analysis was graded as low. This is primarily due to imprecision in the data, as evidenced by wide confidence intervals. The imprecision suggests a certain degree of uncertainty in the estimates, which could impact the overall certainty of the findings (Supplementary Table S2).
3.7. Publication Bias and Heterogeneity
Funnel plot analyses could not be appropriately performed due to the low number of studies. No significant heterogeneity was observed among the subgroups.
4. Discussion
Our meta-analysis found that the effectiveness of CRP and PCT in predicting infection in necrotizing pancreatitis can vary depending on the stage of the disease. Initially, within the first three days of admission, CRP, PCT, and WBC had low predictive accuracy for infection, aligning with previous findings by Párniczky et al. [8,22]. However, after the initial three days of admission, investigating at least two weeks of the period of the disease, CRP and PCT demonstrated good predictive accuracy for infection [23]. Repeated measurements and monitoring these biomarkers over time may be essential to achieve more reliable results.
PCT, the inactive 116-amino-acid propeptide of calcitonin, demonstrates significant elevation within 2 to 4 h in severe systemic inflammation or bacterial infections while showing a minimal increase in response to viral infections. In contrast, the elevation of CRP and the WBC count occurs gradually and reaches its maximum level approximately 36 h after exposure to endotoxin. CRP is an acute-phase protein and needs more than 72 h to reach its highest level. Previous publications have shown that it can predict the severity of acute pancreatitis, and pancreatic necrosis is strongly associated with a CRP level exceeding 150 mg/L within the initial 72 h period [5,41,42]. PCT production can be triggered by microbial antigens such as endotoxin and the immune response by stimulating cytokines like IL-1b, TNF-alfa, and IL-6 [43,44]. PCT temporarily increases for 12–24 h after surgery but returns to baseline within 48 h if no infection is present. CRP and WBCs may stay elevated longer post-surgery, regardless of infection [45]. The PROCAP randomized trial was conducted at a single center, where a PCT algorithm (threshold: 1 ng/mL) was employed to steer antibiotic therapy. The findings indicate a noteworthy decline in the likelihood of antibiotic prescription when employing the PCT algorithm. This reduction effectively curtailed the inappropriate usage of antibiotics without resulting in a substantial rise in infections or prolongation of hospitalization [46]. The trial results highlight the importance of utilizing biomarkers like PCT in clinical practice to optimize patient care and improve outcomes in INP. Generally, the threshold for antibiotic administration is “encouraged” for values exceeding 0.5 ng/mL but “strongly recommended” for values surpassing 1.0 ng/mL [43]. Another study identified the inability to decrease PCT levels to less than 60% of the baseline value after seven days of intervention as a prognostic indicator for mortality [47].
In a prior systematic review, PCT was the most accurate indicator of IPN [48]. Chen et al. conducted a study that demonstrated the potential of PCT as a reliable predictor of infection within the first 48 h. Their findings may be attributed to the fact that their tertiary center specializes in treating critically ill patients more prone to developing infections [29]. The included studies did not have a follow-up period, which might restrict the assessment of long-term outcomes or the detection of delayed complications. In the statistical analysis of CRP and PCT, pooling sensitivity and specificity was unattainable due to variations in threshold values. This was further compounded by the restriction imposed by the limited number of eligible studies, preventing their aggregation.
Systemic inflammation is thought to play a pivotal role in the pathophysiology of organ dysfunction, with cytokines acting as key mediators in regulating the inflammatory response. IL-6 is a proinflammatory cytokine produced by various cell types in response to tissue damage and stimuli such as TNF-alpha and IL-1β. IL-6 acts by inducing the production of acute-phase proteins in hepatocytes, including CRP. IL-6 demonstrates significantly elevated serum levels in cases of necrotizing pancreatitis and severe acute pancreatitis on the day of admission, making it an excellent marker for early severity stratification and predicting remote organ failure [31,49]. IL-6 levels were significantly elevated in SNP and IPN [38].
TNF-alpha and sICAM were explored as potential markers for IPN, but they did not demonstrate satisfactory predictive value [31,38]. One study examined the predictive value of G-CSF, building upon previous findings, suggesting a potential association between low G-CSF levels and increased infection risk. However, the study’s results revealed that the G-CSF concentration was slightly elevated in cases of INP and did not serve as a useful marker [32,50].
STREM-1 and presepsin (soluble CD14-ST) were investigated individually in separate studies, and the results showed AUC values of 0.792 and 0.956 [30,33]. In recent studies, presepsin has emerged as a promising early biomarker for various infections and a valuable tool in identifying sepsis and determining severity and prognosis [51].
Hypoalbuminemia has been identified as a predictive factor for IPN in a study [28]. Additionally, it is a recognized dose-dependent risk factor for organ failure, local complications, and malnutrition in acute pancreatitis [52].
Elevated serum LDH levels, indicating cellular injury, have demonstrated significant associations with severe acute pancreatitis and are recognized as a sensitive biomarker for pancreatic necrosis; nevertheless, their ability to predict IPN yielded an AUC of 0.77 [40,53]. However, when LDH levels were combined with lymphocyte levels in the late phase of AP, the AUC significantly increased to 0.94 [40].
HCT, BUN, and creatinine are indicators of organ perfusion and volume status and have been identified as predictors of the severity of AP and mortality [54]. HCT has limited predictive value for IPN, but creatinine and BUN have shown associations with IPN and have been incorporated into scoring systems [28,29].
Chen et al. examined the combined diagnostic accuracy of PCT, CRP, HCT, and BUN within the first 48 h of admission [29]. Furthermore, Wiese et al. developed a prediction model that included creatinine, CRP, albumin, and alcoholic etiology. These models demonstrated better ROC curves and AUC values compared to individual laboratory parameters alone [28]. After conducting a meta-analysis, it was found that patients with severe or necrotizing pancreatitis had a higher risk of developing IPN if they experienced over 50% necrosis of the pancreas, delayed enteral nutrition, and required invasive mechanical ventilation [55]. This highlights the potential benefit of a comprehensive approach in predicting infected necrosis [56,57].
4.1. Strengths and Limitations
Regarding the strengths of our study, we adhered to our pre-registered protocol, ensuring methodological rigor and transparency throughout the study. In contrast to the prior systematic review, we conducted a subgroup analysis based on the timing of the laboratory parameter measurements [48].
The main limitations of our study were the low number of eligible studies included and the observed variations in study design and patient populations. Additionally, the enrolled patients varied in terms of onset and severity of the disease, and this may impact the generalizability and applicability of the findings to a broader population. Furthermore, certain studies were of low quality. Another limitation is that due to the varying thresholds used in the studies and their unavailability in some cases, we cannot provide a recommended value for suspecting infection.
4.2. Implication for Practice and Research
The immediate application of scientific results is of great significance [56,57]. CRP and PCT as biomarkers may serve as valuable tools to facilitate appropriate antibiotic therapy in the late phase of AP. Future research with larger sample sizes and longer follow-up durations is needed to validate further and refine the use of CRP, PCT, creatinine, BUN, presepsin, and albumin in predicting IPN. Furthermore, developing a scoring system that combines multiple biomarkers and clinical parameters, such as imaging findings and patient demographics, may enhance the accuracy of predicting IPN.
5. Conclusions
The predictive value of CRP and PCT for infection is poor within 72 h after hospital admission. However, after the first three days of admission, the predictive value of CRP and PCT appears to be high enough for clinical use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25021273/s1.
Author Contributions
D.T.: conceptualization, project administration, methodology, formal analysis, writing—original draft; E.S.: conceptualization, methodology, visualization, writing—review and editing; M.L.: conceptualization, writing—review and editing; T.K.: conceptualization, data curation, formal analysis, writing—review and editing; M.V.: conceptualization, data curation, writing—review and editing; B.T.: conceptualization, writing—review and editing; N.F.: conceptualization, writing—review and editing; B.E.: conceptualization, data curation, writing—review and editing; P.H.: conceptualization, funding acquisition, supervision, writing—review and editing; A.M.: conceptualization; supervision; funding acquisition, writing—original draft. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct, or interpretation of our study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The research was supported by the Hungarian Ministry of Innovation and Technology, National Research, Development and Innovation Fund (TKP2021-EGA-23 to PH), Translational Neuroscience National Laboratory program (RRF-2.3.1-21-2022-00011 to PH), project grants (K131996 and K147265 to PH, FK131864 to AM), the Translational Medicine Foundation, the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to AM), and by the ÚNKP–22–5 and ÚNKP-22-3 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (to AM and to BT- ÚNKP-23-3-II-PTE-1996). The funders had no effect on the concept, data collection, analysis, or writing of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Peery A.F., Dellon E.S., Lund J., Crockett S.D., McGowan C.E., Bulsiewicz W.J., Gangarosa L.M., Thiny M.T., Stizenberg K., Morgan D.R., et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., Tsiotos G.G., Vege S.S., Acute Pancreatitis Classification Working Group Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Konarska-Bajda K., Ceranowicz P., Cieszkowski J., Ginter G., Stempniewicz A., Gałązka K., Kuśnierz-Cabala B., Dumnicka P., Bonior J., Warzecha Z. Administration of Warfarin Inhibits the Development of Cerulein-Induced Edematous Acute Pancreatitis in Rats. Biomolecules. 2023;13:948. doi: 10.3390/biom13060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenner S., Baillie J., DeWitt J., Vege S.S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Off. J. Am. Coll. Gastroenterol.|ACG. 2013;108:1400–1415. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 5.Farkas N., Hanák L., Mikó A., Bajor J., Sarlós P., Czimmer J., Vincze Á., Gódi S., Pécsi D., Varjú P., et al. A Multicenter, International Cohort Analysis of 1435 Cases to Support Clinical Trial Design in Acute Pancreatitis. Front. Physiol. 2019;10:1092. doi: 10.3389/fphys.2019.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czapári D., Váradi A., Farkas N., Nyári G., Márta K., Váncsa S., Nagy R., Teutsch B., Bunduc S., Erőss B., et al. Detailed Characteristics of Post-discharge Mortality in Acute Pancreatitis. Gastroenterology. 2023;165:682–695. doi: 10.1053/j.gastro.2023.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Kiss S., Pintér J., Molontay R., Nagy M., Farkas N., Sipos Z., Fehérvári P., Pecze L., Földi M., Vincze Á., et al. Early prediction of acute necrotizing pancreatitis by artificial intelligence: A prospective cohort-analysis of 2387 cases. Sci. Rep. 2022;12:7827. doi: 10.1038/s41598-022-11517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Párniczky A., Lantos T., Tóth E.M., Szakács Z., Gódi S., Hágendorn R., Illés D., Koncz B., Márta K., Mikó A., et al. Antibiotic therapy in acute pancreatitis: From global overuse to evidence based recommendations. Pancreatology. 2019;19:488–499. doi: 10.1016/j.pan.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Werge M., Novovic S., Schmidt P.N., Gluud L.L. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:698–707. doi: 10.1016/j.pan.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Pavlidis E.T., Pavlidis T.E. Management of infected acute necrotizing pancreatitis. World J. Clin. Cases. 2023;11:482–486. doi: 10.12998/wjcc.v11.i2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron T.H., DiMaio C.J., Wang A.Y., Morgan K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67–75.e61. doi: 10.1053/j.gastro.2019.07.064. [DOI] [PubMed] [Google Scholar]
- 12.De Lucia S.S., Candelli M., Polito G., Maresca R., Mezza T., Schepis T., Pellegrino A., Verme L.Z.D., Nicoletti A., Franceschi F., et al. Nutrition in Acute Pancreatitis: From the Old Paradigm to the New Evidence. Nutrients. 2023;15:1939. doi: 10.3390/nu15081939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A.H., Singh Griwan M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol. Rep. 2018;6:127–131. doi: 10.1093/gastro/gox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva-Vaz P., Abrantes A.M., Castelo-Branco M., Gouveia A., Botelho M.F., Tralhão J.G. Multifactorial Scores and Biomarkers of Prognosis of Acute Pancreatitis: Applications to Research and Practice. Int. J. Mol. Sci. 2020;21:338. doi: 10.3390/ijms21010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumpston M., Li T., Page M., Chandler J., Welch V., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Working Group IAP/APA Acute Pancreatitis Guidelines IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13((Suppl. 2)):e1–e15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 18.Haddaway N.R., Grainger M.J., Gray C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods. 2022;13:533–545. doi: 10.1002/jrsm.1563. [DOI] [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M., QUADAS-2 Group Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Schünemann H.J., Oxman A.D., Brozek J., Glasziou P., Jaeschke R., Vist G.E., Williams J.W., Jr., Kunz R., Craig J., Montori V.M., et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman S.C., Kerby C.R., Patel A., Cooper N.J., Quinn T., Sutton A.J. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med. Res. Methodol. 2019;19:81. doi: 10.1186/s12874-019-0724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F., He H. Assessing the Accuracy of Diagnostic Tests. Shanghai Arch. Psychiatry. 2018;30:207–212. doi: 10.11919/j.issn.1002-0829.218052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutter C.M., Gatsonis C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001;20:2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 24.Chu H., Cole S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006;59:1331–1332. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Harbord R.M., Deeks J.J., Egger M., Whiting P., Sterne J.A.C. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8:239–251. doi: 10.1093/biostatistics/kxl004. [DOI] [PubMed] [Google Scholar]
- 27.Rau B., Steinbach G., Gansauge F., Mayer J.M., Grünert A., Beger H.G. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832–840. doi: 10.1136/gut.41.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiese M.L., Urban S., von Rheinbaben S., Frost F., Sendler M., Weiss F.U., Bülow R., Kromrey M.-L., Tran Q.T., Lerch M.M., et al. Identification of early predictors for infected necrosis in acute pancreatitis. BMC Gastroenterol. 2022;22:405. doi: 10.1186/s12876-022-02490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H.-Z., Ji L., Li L., Wang G., Bai X.-W., Cheng C.-D., Sun B. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine. 2017;96:e7487. doi: 10.1097/MD.0000000000007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z., Liu Y., Dong Y.-H., Zhan X.-B., Du Y.-Q., Gao J., Gong Y.-F., Li Z.-S. Soluble triggering receptor expressed on myeloid cells in severe acute pancreatitis: A biological marker of infected necrosis. Intensive Care Med. 2012;38:69–75. doi: 10.1007/s00134-011-2369-z. [DOI] [PubMed] [Google Scholar]
- 31.Riché F.C., Cholley B.P., Laisné M.-J.C., Vicaut E., Panis Y.H., Lajeunie E.J., Boudiaf M., Valleur P.D. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257–262. doi: 10.1067/msy.2003.70. [DOI] [PubMed] [Google Scholar]
- 32.Muller C., Uhl W., Printzen G., Gloor B., Bischofberger H., Tcholakov O., Buchler M. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut. 2000;46:233–238. doi: 10.1136/gut.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotar O., Khomiak I., Khristich T., Hontsariuk D., Ferfetska K., Temerivsjka T., Kropyva V. Procalcitonin is an effective tool for the diagnosis of generalized forms of infectious complications in patients with acute necrotizing pancreatitis. Med. Stud./Stud. Med. 2022;38:1–5. doi: 10.5114/ms.2022.115141. [DOI] [Google Scholar]
- 34.Rau B., Steinbach G., Baumgart K., Gansauge F., Grünert A., Beger H.G. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med. 2000;26((Suppl. S2)):S159–S164. doi: 10.1007/s001340051136. [DOI] [PubMed] [Google Scholar]
- 35.Dambrauskas Z., Pundzius J., Barauskas G. Predicting development of infected necrosis in acute necrotizing pancreatitis. Medicina. 2006;42:441–449. [PubMed] [Google Scholar]
- 36.Brand M., Götz A., Zeman F., Behrens G., Leitzmann M., Brünnler T., Hamer O.W., Stroszczynski C., Heiss P. Acute necrotizing pancreatitis: Laboratory, clinical, and imaging findings as predictors of patient outcome. AJR Am. J. Roentgenol. 2014;202:1215–1231. doi: 10.2214/AJR.13.10936. [DOI] [PubMed] [Google Scholar]
- 37.Block S., Büchler M., Bittner R., Beger H.G. Sepsis indicators in acute pancreatitis. Pancreas. 1987;2:499–505. doi: 10.1097/00006676-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Mándi Y., Farkas G., Takács T., Boda K., Lonovics J. Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of infected necrosis in acute pancreatitis. Int. J. Pancreatol. 2000;28:41–49. doi: 10.1385/IJGC:28:1:41. [DOI] [PubMed] [Google Scholar]
- 39.Rotar O., Khomiak I., Nazarchuck M., Rotar V. Utility of Presepsin for Diagnosis of Infected Acute Necrotizing Pancreatitis. JOP J. Pancreas. 2019;20:67–71. [Google Scholar]
- 40.Ueda T., Takeyama Y., Yasuda T., Shinzeki M., Sawa H., Nakajima T., Matsumoto I., Ajiki T., Fujino Y., Kuroda Y. Lactate dehydrogenase-to-lymphocyte ratio for the prediction of infection in acute necrotizing pancreatitis. Pancreas. 2007;35:378–380. doi: 10.1097/01.mpa.0000297827.05678.9e. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso F.S., Ricardo L.B., Oliveira A.M., Canena J.M., Horta D.V., Papoila A.L., Deus J.R. C-reactive protein prognostic accuracy in acute pancreatitis: Timing of measurement and cutoff points. Eur. J. Gastroenterol. Hepatol. 2013;25:784–789. doi: 10.1097/MEG.0b013e32835fd3f0. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Chen Z., Li L., Lai T., Peng H., Gui L., He W. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front. Cell Infect. Microbiol. 2022;12:933221. doi: 10.3389/fcimb.2022.933221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J. Intern. Med. 2013;28:285–291. doi: 10.3904/kjim.2013.28.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert D.N. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J. Clin. Microbiol. 2010;48:2325–2329. doi: 10.1128/JCM.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins R.R., Lemonovich T.L. Serum procalcitonin in the diagnosis and management of intra-abdominal infections. Expert. Rev. Anti-Infect. Ther. 2012;10:197–205. doi: 10.1586/eri.11.164. [DOI] [PubMed] [Google Scholar]
- 46.Siriwardena A.K., Jegatheeswaran S., Mason J.M., Baltatzis M., Sheen A.J., A O’Reilly D., Jamdar S., Deshpande R., Carino N.D.L., Satyadas T., et al. A procalcitonin-based algorithm to guide antibiotic use in patients with acute pancreatitis (PROCAP): A single-centre, patient-blinded, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2022;7:913–921. doi: 10.1016/S2468-1253(22)00212-6. [DOI] [PubMed] [Google Scholar]
- 47.Samanta J., Dhar J., Birda C.L., Gupta P., Yadav T.D., Gupta V., Sinha S.K., Kochhar R. Dynamics of Serum Procalcitonin Can Predict Outcome in Patients of Infected Pancreatic Necrosis: A Prospective Analysis. Dig. Dis. Sci. 2023;68:2080–2089. doi: 10.1007/s10620-022-07758-4. [DOI] [PubMed] [Google Scholar]
- 48.Yang C.J., Chen J., Phillips A.R., Windsor J.A., Petrov M.S. Predictors of severe and critical acute pancreatitis: A systematic review. Dig. Liver Dis. 2014;46:446–451. doi: 10.1016/j.dld.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 49.Sathyanarayan G., Garg P.K., Prasad H., Tandon R.K. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2007;22:550–554. doi: 10.1111/j.1440-1746.2006.04752.x. [DOI] [PubMed] [Google Scholar]
- 50.Rao R., Prinz R.A., Kazantsev G.B., Hecht D., Gattuso P., Jacobs H.K., Djuricin G., Castelli M. Effects of granulocyte colony-stimulating factor in severe pancreatitis. Surgery. 1996;119:657–663. doi: 10.1016/S0039-6060(96)80190-5. [DOI] [PubMed] [Google Scholar]
- 51.Xiao H.-L., Wang G.-X., Wang Y., Tan Z.-M., Zhou J., Yu H., Xie M.-R., Li C.-S. Dynamic blood presepsin levels are associated with severity and outcome of acute pancreatitis: A prospective cohort study. World J. Gastroenterol. 2022;28:5203–5216. doi: 10.3748/wjg.v28.i35.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ocskay K., Vinkó Z., Németh D., Szabó L., Bajor J., Gódi S., Sarlós P., Czakó L., Izbéki F., Hamvas J., et al. Hypoalbuminemia affects one third of acute pancreatitis patients and is independently associated with severity and mortality. Sci. Rep. 2021;11:24158. doi: 10.1038/s41598-021-03449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng Y.B., Zhan X.B., Guo X.R., Zhang H.G., Chen Y., Cai Q.C., Li Z.S. Risk factors for pancreatic infection in patients with severe acute pancreatitis: An analysis of 163 cases. J. Dig. Dis. 2014;15:377–385. doi: 10.1111/1751-2980.12150. [DOI] [PubMed] [Google Scholar]
- 54.He W., Zhu Y., Zhu Y., Jin Q., Xu H., Xion Z., Yu M., Xia L., Liu P., Lu N. Comparison of multifactor scoring systems and single serum markers for the early prediction of the severity of acute pancreatitis. J. Gastroenterol. Hepatol. 2017;32:1895–1901. doi: 10.1111/jgh.13803. [DOI] [PubMed] [Google Scholar]
- 55.Tran A., Fernando S.M., Rochwerg B., Inaba K., Bertens K.A., Engels P.T., Balaa F.K., Kubelik D., Matar M., Lenet T.I., et al. Prognostic factors associated with development of infected necrosis in patients with acute necrotizing or severe pancreatitis-A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2022;92:940–948. doi: 10.1097/TA.0000000000003502. [DOI] [PubMed] [Google Scholar]
- 56.Hegyi P., Erőss B., Izbéki F., Párniczky A., Szentesi A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021;27:1317–1319. doi: 10.1038/s41591-021-01458-8. [DOI] [PubMed] [Google Scholar]
- 57.Hegyi P., Petersen O.H., Holgate S., Erőss B., Garami A., Szakács Z., Dobszai D., Balaskó M., Kemény L., Peng S., et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020;9:1532. doi: 10.3390/jcm9051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.