Abstract
Tularemia is a disease caused by the facultative intracellular bacterium Francisella tularensis. Here we demonstrate that during the first weeks of infection, a significant increase in levels of Vγ9Vδ2 cells occurred in peripheral blood: in 13 patients analyzed 7 to 18 days after the onset of disease, these lymphocytes represented, on average, 30.5% of CD3+ cells and nearly 100% of γδ+ T cells. By contrast, after vaccination with the live vaccine strain (LVS) of F. tularensis, only a minor increase occurred. Eleven days after vaccination, γδ T cells represented an average of 6.7% and Vγ9Vδ2 cells represented an average of 5.3% of T cells, as in control subjects. Since derivatives of nonpeptidic pyrophosphorylated molecules, referred to as phosphoantigens, are powerful stimuli for Vγ9Vδ2 cells, this observation prompted an investigation of phosphoantigens in F. tularensis strains. The F. tularensis phosphoantigens triggered in vitro a proliferative response of human Vγ9Vδ2 peripheral blood leukocytes as well as a cytotoxic response and tumor necrosis factor release from a Vγ9Vδ2 T-cell clone. Quantitatively similar phosphoantigenic activity was detected in acellular extracts from two clinical isolates (FSC171 and Schu) and from LVS. Taken together, the chemical nature of the stimulus from the clinical isolates and the significant increase in levels of Vγ9Vδ2 cells in peripheral blood of tularemia patients indicate that phosphoantigens produced by virulent strains of F. tularensis trigger in vivo expansion of γδ T cells in tularemia.
Francisella tularensis is a gram-negative, facultative intracellular bacterium causing tularemia in small rodents and in humans exposed to infected animals or insects. In humans, tularemia is most often manifested as an ulceroglandular disease (26). This form is characterized by an infected wound at the site of a mosquito or tick bite and subsequent swelling of local lymph nodes. There is virtually no mortality when the disease is caused by Francisella tularensis subsp. palaearctica, and antibiotic treatment instituted early, e.g., tetracycline, is usually effective (26). Francisella tularensis subsp. tularensis strains are considerably more virulent for humans and other mammals. As with other intracellular bacteria, host protection against F. tularensis is dependent on cell-mediated immunity (reviewed in reference 26). Protection ensues after natural infection or after vaccination with the F. tularensis live vaccine strain (LVS). This strain was originally derived by nonspecific attenuation from a Russian clinical isolate of F. tularensis subsp. palaearctica. It was subsequently introduced in the United States, and its protective efficacy has been well documented (5).
Considering the cell-mediated nature of host protection against F. tularensis, it is necessary to delineate the specificity of the human T-cell response to the parasite, in terms of both cell subsets and nominal antigens, to understand the physiopathology of tularemia and to design effective vaccines. It has been demonstrated that immunity in vaccinated and naturally infected individuals is long lasting and specific. Recent studies have documented that CD4 and CD8 αβ T cells with a wide range of specificities are present in the peripheral blood of immune individuals (19, 21). Both subsets respond to antigenic stimulation with gamma interferon production (21). A few commonly recognized antigens, such as a 17-kDa lipoprotein, have been identified (18, 20).
Besides the activation of αβ T lymphocytes, a strong increase in levels of γδ T cells in the peripheral blood of a tularemia patient has been reported (22). There was a predominant expression of T-cell receptors (TCR) comprising Vγ9 and Vδ2 regions among the reactive T cells, suggesting the occurrence of superantigenic stimulation (22). However, it has not been established if this is a general occurrence during the disease, and the antigens or superantigens responsible for this in vivo γδ T-cell expansion are unknown. Specific antigens for Vγ9Vδ2 T cells have been identified in Mycobacterium tuberculosis and Plasmodium falciparum, pathogens causing prominent activation of this subset (2, 7, 8, 14, 24). The human Vγ9Vδ2 response to M. tuberculosis is due to four distinct stimulating molecules (7). These antigens are structurally related nonpeptidic phosphoesters having a common substituted pyrophosphoester moiety, either alone in TUBag1 and TUBag2 (7) or as a 5′-phosphate substituent of UMP or dTMP (7, 24) in TUBag4 (7) and of UMP in TUBag3 (14). Their common pyrophosphoester moiety constitutes a structural determinant essential to the antigenic property (16, 25). Chemical syntheses have revealed that diverse small alkyl-pyrophosphoesters are actually able to function as antigens for γδ T cells (4, 24, 25). Together, these studies have proven that human Vγ9Vδ2 T cells recognize in a TCR-dependent fashion (3) a new set of compounds that have been collectively designated phosphoantigens (11).
The striking similarity between γδ T-cell responses in tuberculosis and the recently reported onset of Vγ9Vδ2 T cells in a tularemia patient (22) prompted us to search for the presence of a possible phosphoantigen(s) in F. tularensis. Here we show that phosphorylated compounds in cell extracts from a vaccine strain and two virulent strains of F. tularensis are able to activate in vitro responses of human γδ peripheral blood leukocytes (PBL) and that the expansion of Vγ9Vδ2 cells in PBL is a general phenomenon in tularemia patients but not in vaccinees.
MATERIALS AND METHODS
Bacterial strains.
F. tularensis LVS was supplied by the U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Md. It was stored at −70°C and cultivated on modified Thayer-Martin agar containing GC medium base (15). Strain FSC171 was isolated from a wound sample from a patient with typical ulceroglandular, serologically confirmed tularemia. Subsequent biochemical and genetic analyses revealed that it belonged to F. tularensis subsp. palaearctica. The highly virulent Schu strain is a prototypic representative of F. tularensis subsp. tularensis.
Patients and vaccinees.
The vaccinees included in the study were healthy volunteers. All gave informed consent, and the study was approved by the ethical committee at the University of Umeå. Individuals were examined by a physician before being enrolled in the study. Vaccination was performed by scarification following the instructions of the manufacturer (Salk Institute, Swiftwater, Pa.). Approximately 7.0 × 107 bacteria in 0.1 ml of saline were administered to each individual. The vaccinees were monitored by blood sampling until 4 months after vaccination. No adverse reactions were recorded. All showed serological conversion within 3 weeks of vaccination.
Through general practitioners at various locations in northern Sweden and Finland, blood samples were obtained from patients with suspected ulceroglandular tularemia. All cases were subsequently confirmed as tularemia by serology. For all patients, onset occurred in August 1996. Most individuals were administered tetracycline at the first visit to the general practitioner, usually 7 to 12 days after the onset of symptoms. According to existing medical records, the instituted treatment was effective.
F. tularensis cultures.
An overnight culture (200 ml) of F. tularensis bacteria was diluted in a total volume of 2.0 liters of Chamberlain medium (6), grown to an optical density at 540 nm of 0.7, and harvested by centrifugation. Fifty milliliters of chloroform-methanol (1:1, vol/vol) was added to the cell pellet and allowed to stand overnight to kill bacteria.
FE preparations.
The F. tularensis cell suspension was dried, resuspended in chloroform-water, and decanted for partitioning. The water-soluble fraction was concentrated and used for stimulation of the γδ T-cell clone G12 as previously described (2). However, this fraction still comprised many glycolipidic components hindering the proliferative bioassays (see below), so it was further separated on a Sep-Pak C18 cartridge (Waters-Millipore, Boston, Mass.) by flash chromatography. Two fractions were obtained after elution with water and methanol. The fractions were dried and reconstituted at 100 mg/ml in water. The biological activity was recovered in the fraction eluted with water. This fraction, referred to as Francisella extract (FE), was used for further analysis.
Phosphatase treatments.
Ten microliters of FE was added to 3 μl (2 U) of calf intestinal alkaline phosphatase (Boehringer, Meslan, France), 3 μl (0.2 U) of Crotalus adamanteus venom nucleotide pyrophosphatase (Sigma, St. Quentin, France), and 1 μl of dephosphorylation buffer (Boehringer) and incubated for 30 min at 37°C before titration of the bioactivity. Control assays were performed without phosphatase treatment in order to quantify the spontaneous degradation of FE during the use of the above-described protocol.
In vitro polyclonal γδ T-cell expansions and γδ T-cell clone cytotoxic responses.
T-cell expansion and T-cell clone cytotoxic response assays were performed according to previously described procedures (2).
MAbs and flow cytometry.
Flow cytofluorometric analysis was carried out on 50 μl of whole blood from each individual. Surface phenotyping was carried out by two-color analysis on cell suspensions following the protocol of Becton-Dickinson (Sunnyvale, Calif.). Briefly, cells were incubated with 10 μl of normal mouse serum at a dilution of 1:500 (DAKO, Glostrup, Denmark) for 15 min at room temperature to block nonspecific binding of monoclonal antibodies (MAbs). Ten microliters of mouse anti-human CD3 MAb (Leu-4, clone SK7) directly conjugated to PerCP (Becton-Dickinson) or 5 μl of pan-αβ (immunoglobulin G2b [IgG2b], BMA031), pan-γδ (IgG1, 5.A6.E9 or IMMU 510), Vγ9 (IgG1, 7A5 or IMMU 360), or Vδ2 (IgG1, 15D or IMMU 389) fluorescein isothiocyanate (FITC)-conjugated MAb (purchased from Serotec, Oxford, England, or Immunotech, Marseille, France) was added and incubated for 25 min in the dark at room temperature. After incubation, 2 ml of fluorescence-activated cell sorter lysing solution (Becton-Dickinson) was added to each tube for lysis of erythrocytes. After 10 min of incubation, the cells were washed twice with cell wash solution (Becton-Dickinson) and finally resuspended in 500 μl of FACSflow solution (Becton-Dickinson). Data for 10,000 events from each sample were acquired with a FACSsort flow cytometer (Becton-Dickinson). For analysis, lymphocytes were gated according to their morphological parameters and analyzed with Cell Quest software (Becton-Dickinson). Results were expressed as percentage of lymphocytes or as percentage of CD3+ cells staining positively for a given label.
TNF production assay.
Supernatants were assayed for tumor necrosis factor (TNF) by use of a previously described cytotoxic assay against WEHI164 clone 13 cells (13). Briefly, supernatants of activated T-cell clone G12 were tested undiluted and diluted 1/5 in triplicate by the following procedure. Fifty microliters of sample was added to 50 μl of actinomycin D (2 μg/ml in phosphate-buffered saline; Sigma)-treated WEHI cells (6 × 105 cells/ml) in flat-bottom 96-well plates and incubated for 18 h at 37°C. After incubation, 50 μl of tetrazolium salt (2.5 mg/ml in phosphate-buffered saline; Sigma) was added to each well and incubated for 3 h. Formazan crystals were solubilized with 100 μl of lysis buffer (1 volume of N,N-dimethyl formamide, 2 volumes of 30% sodium dodecyl sulfate [pH 4.7]), and the optical density at 570 nm was read. Serial dilutions of recombinant TNF (Boehringer) were included in each experiment to provide a reference for quantification.
Statistical analysis.
The Mann-Whitney U test was used for statistical analysis.
RESULTS
Vγ9Vδ2 response during tularemia infection in humans.
As a first step, we attempted to ascertain whether the recently described expansion of Vγ9Vδ2 TCR-expressing cells in the peripheral blood of a tularemia patient is a common feature of tularemia. Therefore, the percentages of this T-cell subset in PBL were compared between a group of age-matched healthy donors and a group of recently diagnosed tularemia patients. One sample from each of 13 patients was obtained 7 to 21 days after the onset of disease (Table 1). For two patients sampling was performed before the institution of antibiotic treatment, but in all other cases samples were drawn 2 to 5 days after the start of antibiotic treatment.
TABLE 1.
T-cell subsets in peripheral blood of tularemia-infected persons
| Subjects (n) | Age of subjects (yr) (mean ± SD) | Mean ± SD % of CD3+ cells
|
||
|---|---|---|---|---|
| Pan-αβ | Pan-γδ | γ9 | ||
| Controlsa (14) | 38.0 ± 10.8 | 90.9 ± 8.8 | 6.5 ± 4.3 | 4.9 ± 3.6c |
| Patientsb (13) | 36.3 ± 18.8 | 66.4 ± 12.6 | 30.5 ± 8.8 | 30.5 ± 8.9c |
Healthy individuals with no known exposure to F. tularensis or M. tuberculosis.
Samples were obtained 7 to 21 days after the onset of tularemia.
The total and relative numbers of δ2 cells were determined for the patients and the controls. The total numbers were 95 to 108% the numbers of γ9 cells.
As demonstrated in Table 1, an unambiguous increase in the numbers of Vγ9 cells was observed in the patients: on average, these cells represented 30.5% of CD3+ cells and 24.5% of all lymphocytes, compared to 4.9 and 3.4%, respectively, in healthy controls (P < 0.001). Representative CD3 and Vγ9 staining results for PBL from a patient and a control are shown in Fig. 1. Although not directly proven by two-color immunofluorescence staining, the high correlation between the percentages of Vγ9 and Vδ2 cells in PBL strongly suggested that the observed increase in γδ cells was almost exclusively due to cells coexpressing Vγ9 and Vδ2. In both groups, the proportion of γδ cells expressing a TCR other than Vγ9Vδ2 was constantly below 2% (data not shown), indicating a minor, if any, involvement of such cells in the observed responses.
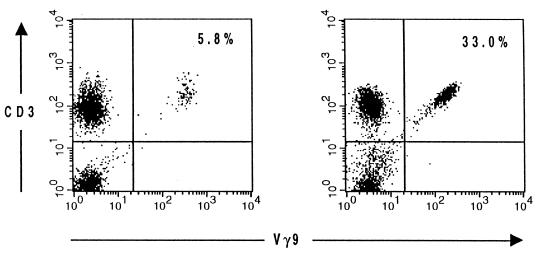
FIG. 1.
Flow cytometric analysis of lymphocytes in the peripheral blood of a control individual (left) and a patient 12 days after the onset of tularemia (right). Lymphocytes were gated according to their morphological parameters. The percentages of gated cells staining positively for CD3 and Vγ9 were determined.
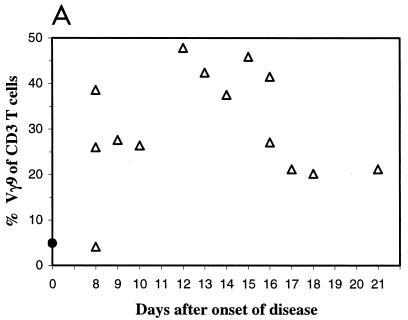
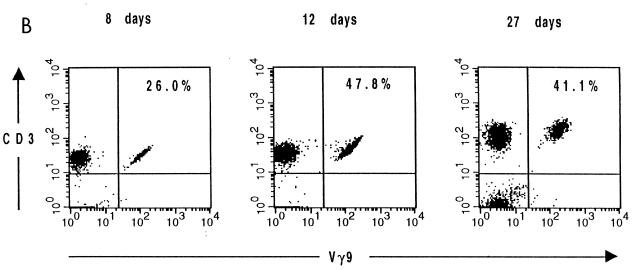
Moreover, this analysis showed that the number of Vγ9Vδ2 cells peaked approximately 12 to 16 days after the onset of disease (Fig. 2A). For example, in one individual Vγ9 cells comprised 26.0, 47.8, and 41.1% of CD3+ PBL in blood samples drawn at days 8, 12, and 27, respectively, after the onset of infection (Fig. 2B).
FIG. 2.
Evolution of the Vγ9 T-cell subset during tularemia infection. (A) Percentage of Vγ9 T cells in peripheral blood at various times following the onset of tularemia (triangles). The filled dot indicates the average number of Vγ9 T cells in a control group denying exposure to F. tularensis. (B) Representative flow cytometric analysis of successive peripheral blood samples from one patient (at 8, 12, and 27 days after onset). The numbers represent the percentages of Vγ9+ cells among CD3+ cells.
F. tularensis cell extract triggers in vitro selective expansion of human Vγ9Vδ2 T cells.
To determine the origin of the Vγ9Vδ2 T-cell expansion during tularemia (22), we probed for the presence of stimuli for γδ T lymphocytes in cell extracts of F. tularensis. To this end, a clinical isolate of F. tularensis (FSC171) was grown in vitro, bacteria were killed by overnight incubation with a chloroform-methanol mixture, and the preparations were subjected to filtration. The F. tularensis cell extract (filtrate) was partitioned between water and chloroform, and the aqueous phase was collected, concentrated, and tested for its ability to activate human Vγ9Vδ2 T lymphocytes. When adding various dilutions of the F. tularensis cell extract to an in vitro culture of human PBL, we repeatedly observed nonspecific suppression of lymphoproliferative responses at low but not at high dilutions. These effects were usually due to microbial glycolipids that nonspecifically interfered with responsive T cells and potentially hindered the detection of weak T-cell stimulation. Therefore, we separated the readily hydrophilic material from the remaining lipidic molecules on C18 cartridges using elution with water and methanol. While the latter eluate appeared devoid of activity, the water fraction, containing the most polar components from F. tularensis, was found stimulatory for human γδ cells. A representative in vitro amplification experiment with PBL obtained from two healthy donors and cultured for 2 weeks with either the F. tularensis cell extract or, as a control, mycobacterial TUBag3 or synthetic isopentenylpyrophosphate (IPP) phosphoantigen is presented in Table 2. In unstimulated wells, 3 to 7% of PBL were γδ cells; the latter represented about 80% (donor A) and 60% (donor B) of PBL stimulated with control phosphoantigens. At the end of culturing with the F. tularensis cell extract, γδ cells reached up to 80% (donor A) and 37% (donor B) of PBL. As expected, most of these γδ cells expressed Vγ9Vδ2 (Table 2), indicating unambiguously that a powerful polyclonal activator of human γδ cells was present in the F. tularensis cell extract.
TABLE 2.
In vitro expansion of Vγ9Vδ2 lymphocytesa
| Donor | Antigen | Concn | % of positive cells
|
||||
|---|---|---|---|---|---|---|---|
| —b | Pan-β | Pan-δ | Vγ9 | Vδ2 | |||
| A | None | 0 | 28 | 3 | 2 | 2 | |
| TUBag3 | 1/200 | 0 | 8 | 82 | 82 | 81 | |
| 1/1,000 | 0 | 11 | 80 | 77 | 77 | ||
| 1/5,000 | 0 | 21 | 61 | 59 | 60 | ||
| IPP | 30 μg/ml | 0 | 8 | 85 | 83 | 84 | |
| 10 μg/ml | 0 | 12 | 74 | 73 | 75 | ||
| 3 μg/ml | 1 | 11 | 72 | 71 | 71 | ||
| FSC171 | 1/30 | 0 | 6 | 83 | 83 | 82 | |
| 1/150 | 0 | 4 | 70 | 68 | 69 | ||
| 1/750 | 0 | 4 | 60 | 60 | 60 | ||
| 1/3,750 | 0 | 8 | 40 | 39 | 40 | ||
| B | None | 0 | 39 | 7 | 5 | 4 | |
| TUBag3 | 1/200 | 0 | 10 | 69 | 70 | 54 | |
| 1/1,000 | 0 | 16 | 56 | 52 | 54 | ||
| 1/5,000 | 1 | 23 | 34 | 34 | 27 | ||
| IPP | 30 μg/ml | 0 | 11 | 69 | 69 | ND | |
| 10 μg/ml | 1 | 15 | 61 | 63 | 63 | ||
| 3 μg/ml | 1 | 19 | 42 | 39 | 37 | ||
| FSC171 | 1/30 | 0 | 35 | 37 | 33 | 33 | |
| 1/150 | 0 | 18 | 31 | 26 | 28 | ||
| 1/750 | 1 | 31 | 12 | 8 | 9 | ||
| 1/3,750 | 0 | 35 | 20 | 11 | 9 | ||
PBL were cocultured with antigens (purified TUBag3, IPP, or extract from FSC171) at different concentrations for 15 days and phenotyped by flow cytometry with MAbs specific for pan-β, pan-δ, Vγ9, and Vδ2 cells. ND, not determined.
—, irrelevant IgG1 MAb.
The F. tularensis stimulus for Vγ9Vδ2 T cells has a phosphoantigenic nature.
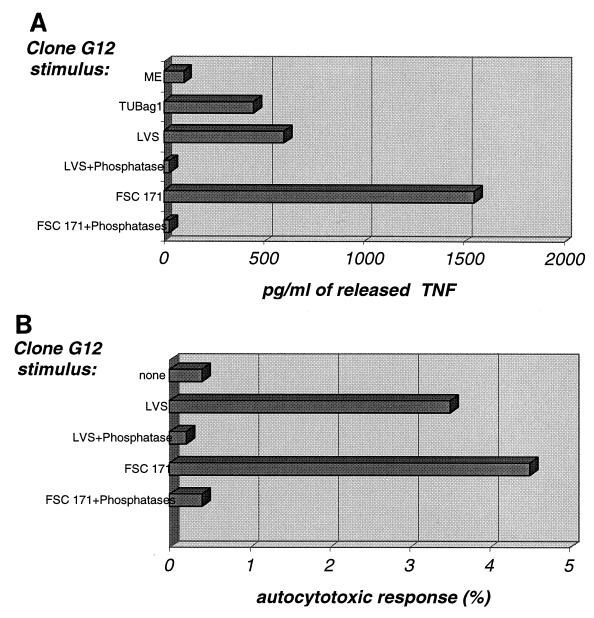
Since the above-described results demonstrated the presence of a specific stimulus for Vγ9Vδ2 T cells in F. tularensis cell extracts, we examined whether it had a phosphoantigenic nature. We previously (2, 13) showed that mycobacterial and malaria phosphoantigens triggered major histocompatibility complex-unrestricted lysis of a broad set of target cells by Vγ9Vδ2 clones, including the release of TNF and the induction of effector cell autocytotoxicity. Additionally, these stimuli were characteristically inactivated upon treatment with phosphatases. Using the phosphoantigen-reactive γδ T-cell clone G12 and cell extracts from two F. tularensis strains, namely, the previously described clinical isolate FSC171 and F. tularensis LVS, we studied the induction of TNF release (Fig. 3A) and of autocytotoxicity (Fig. 3B) by each extract before and after phosphatase treatment. As indicated by the results shown in Fig. 3, both F. tularensis cell extracts triggered responses that were totally abrogated by phosphatase treatment. Hence, these extracts stimulated γδ T cells through ligands corresponding to the previously defined phosphoantigens.
FIG. 3.
Phosphatases degrade the F. tularensis stimulus for the Vγ9Vδ2 clone G12. (A) TNF production by a Vγ9Vδ2 T-cell clone (G12) was estimated after 3 h of incubation in medium supplemented with crude mycobacterial extract (ME) (1 mg/ml), TUBag1 (phosphoantigen from M. tuberculosis; 0.1 μM), or F. tularensis cell extract (200 μg/ml) from the virulent strain (FSC171) or the vaccine strain (LVS) before and after treatment with calf intestinal alkaline phosphatase (1 U/ml) plus nucleotide pyrophosphatase (0.1 U/ml). Spontaneous release was subtracted to yield the data presented. (B) Vγ9Vδ2 T-cell clone (G12) autocytotoxicity was estimated after incubation in medium supplemented with F. tularensis cell extract (1,000 μg/ml) from the virulent strain (FSC171) or the vaccine strain (LVS) before and after treatment for 30 min at 37°C with calf intestinal alkaline phosphatase plus nucleotide pyrophosphatase as in panel A. Results are expressed as percent 51Cr release, calculated by dividing experimental minus spontaneous release by maximal release (i.e., from cells incubated with detergent).
Similar phosphoantigenic loads of virulent strains and F. tularensis LVS.
Prior studies of the phosphoantigenic stimuli of mycobacteria provided evidence of lower amounts in vaccine strain BCG than in virulent mycobacterial strains (8). We wondered whether a quantitative difference in phosphoantigenic loads between F. tularensis strains also existed. Thus, aqueous extracts of F. tularensis LVS and two virulent isolates (FSC171 and Schu) were prepared similarly and titrated comparatively together with a Mycobacterium fortuitum-derived extract. The titration was based on two distinct but sensitive in vitro measurements of Vγ9Vδ2 T-cell responses: the induction of TNF release by the Vγ9Vδ2 clone G12 (Fig. 4) and the expansion of Vγ9Vδ2 T cells in cultures of PBL from two healthy donors (a representative experiment is shown in Table 3). In both assays, all extracts triggered essentially the same levels of activation of Vγ9Vδ2 cells over a wide range of concentrations. We noticed, however, the presence of an inhibitory effect at the highest concentration of the LVS extract, 3 mg/ml, possibly due to interfering cell wall inhibitory glycolipids. Hence, the tentative phosphoantigens of F. tularensis strains both quantitatively and qualitatively closely resemble previously described mycobacterial phosphoantigenic extracts.
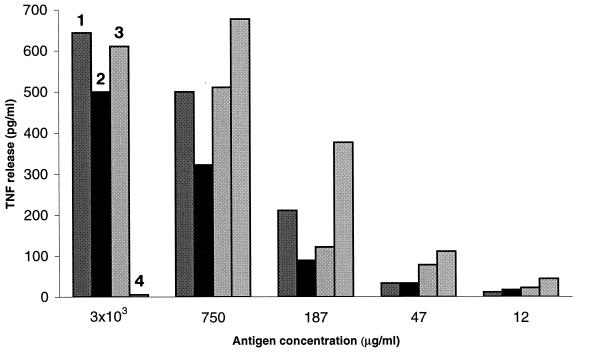
FIG. 4.
Comparative titration of phosphoantigenic loads in extracts from F. tularensis LVS and virulent strains. Comparison of TNF release by the G12 clone incubated with various concentrations of bacterial extracts (numbered bars): virulent strains of F. tularensis, FSC171 (bars 2) and Schu (bars 3), F. tularensis LVS (bars 4), and M. fortuitum extract (bars 1), all of which were used at a maximum concentration of 3 × 103 μg/ml. TNF release of 1 pg/ml resulted from culture medium alone.
TABLE 3.
Percentage of Vδ2-positive cells among CD3 cells cocultured with different bacterial extractsa
| Extract | % of Vδ2-positive cells in two donors at an antigen concn (μg/ml) of:
|
||||
|---|---|---|---|---|---|
| 3 × 103 | 750 | 187 | 47 | 12 | |
| M. fortuitum | 24.9 | 10.4 | 3.3 | 3.4 | 5.6 |
| FSC171 | 16.1 | 7.4 | 3.3 | 5 | 5.6 |
| LVS | ND | 14.6 | 3.9 | 4.3 | 3.6 |
| None (medium) | 3.4 | ||||
Fresh PBL from a healthy donor were incubated with decreasing antigen concentrations. The amplification of Vγ9Vδ2 cells was estimated by fluorescence-activated cell sorter analysis with a dual-stain (CD3-phycoerythrin and Vδ2-FITC) assay after 15 days of coculturing. ND, not done (no cells were found in wells containing 3 × 103 mg of LVS per ml). All of these cocultures were used for other donors and yielded the same results.
Minor increase in Vγ9Vδ2 cell levels after vaccination with F. tularensis LVS.
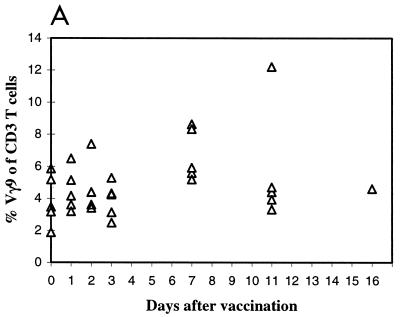
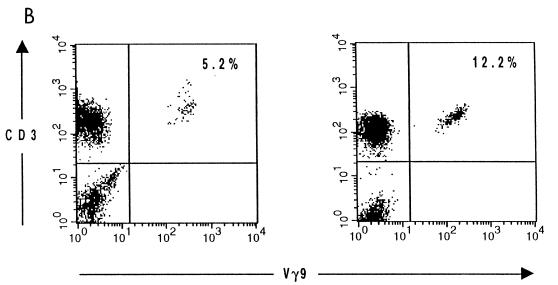
We wondered whether the phosphoantigenic stimuli present in the vaccine strain were as effective as those from the virulent strain in stimulating Vγ9Vδ2 T-cell expansion in peripheral blood after vaccination. Therefore, an analysis of γδ PBL was performed for a group of LVS-vaccinated volunteers from whom blood samples were drawn several times during the first 2 weeks, 5 to 6 weeks (five individuals), and 4 months after vaccination. Peak values in vaccinees were only slightly higher than prevaccination values, with means of 6.8 and 4.5%, respectively. The highest percentages were observed at day 8 (Fig. 5A), slightly earlier than in infected patients. Individual variations were noted: for instance, in one individual the percentage of Vγ9 cells increased from 5.2 to 12.2% (Fig. 5B). However, the average numbers of γδ and Vγ9 cells following vaccination did not differ significantly from those recorded in the controls (Table 1) or prior to vaccination (Table 4) (P > 0.10). So, the prominent increase in Vγ9Vδ2 PBL levels observed in tularemia patients was not seen in vaccinees.
FIG. 5.
Evolution of the Vγ9Vδ2 T-cell subset during vaccination with F. tularensis LVS. (A) Percentage of Vγ9Vδ2 T cells in peripheral blood at various times following vaccination with F. tularensis LVS (triangles). (B) Flow cytometric analysis of γδ T lymphocytes in the peripheral blood of a vaccinee (a total of seven individuals were vaccinated). Analysis was performed before (left) and 11 days after (right) vaccination. Lymphocytes were gated according to their morphological parameters. The percentages of CD3 cells staining positively for Vγ9 are indicated in the upper right quadrants.
TABLE 4.
T-cell subsets in peripheral blood of seven vaccinated healthy volunteers
| Day of samplinga | Age (yr) (mean ± SD) | Mean ± SD % of CD3+ cells
|
||
|---|---|---|---|---|
| Pan-αβ | Pan-γδ | γ9 | ||
| 0 | 37.0 ± 6.9 | 94.9 ± 3.8 | 4.5 ± 1.6 | 3.9 ± 1.6b |
| 11 | 91.6 ± 6.6 | 6.8 ± 3.7 | 5.3 ± 3.1b | |
Number of days between vaccination and sampling.
Determined for only five individuals.
DISCUSSION
A previous report described a massive expansion of Vγ9Vδ2 T cells in the peripheral blood of a Japanese patient with tularemia (22). Since this observation suggested a possible link between this expansion and the pathology of tularemia, an extended study of this phenomenon was warranted. The study presented here involved 13 tularemia patients from whom blood samples were obtained between days 7 and 21 of infection. The results unambiguously confirmed that the massive increase in the levels of Vγ9Vδ2 cells is a general characteristic of tularemia. Indeed, the average percentage of Vγ9 cells increased more than sixfold. When we monitored the kinetics of this onset in one tularemia patient, the percentage of Vγ9Vδ2 T cells was already elevated at day 8 (26%), peaked at day 12 (47%), and remained elevated for a long period of time (41% at 1 month). So, the observed increase in the levels of Vγ9Vδ2 T cells during tularemia is also a persistent cellular event. The persistence of this increase is being monitored in an ongoing study. Some individuals included in the present study still showed increased numbers of Vγ9Vδ2 T cells 1 year postinfection (12a).
The kinetics of the Vγ9Vδ2 increase could not be exactly determined, as most patients did not seek medical care before the second week of infection. However, as in vitro lymphocyte stimulation normally can be demonstrated 4 to 7 days after the onset of disease (23), the expansion of Vγ9Vδ2 cells probably occurs at a time when specific cell-mediated immunity is already present. In line with this idea, it has been demonstrated that Vγ9Vδ2 expansion occurs only after exposure to phosphoantigens in the presence of interleukin 2 (IL-2) or cytokines that signal through the IL-2 receptor (10). It is possible that the observed expansion in tularemia patients occurred only after IL-2-producing αβ T cells had begun to proliferate.
The lack of γδ T-cell expansion in vaccinees was in marked contrast to the prominent expansion observed in tularemia patients and was surprising, since no clear differences in the content of phosphoantigens could be detected in extracts from the vaccine strain and the two virulent strains, one of which, Schu, represents the highly virulent F. tularensis subsp. tularensis and the other of which, FSC171, represents the less virulent F. tularensis subsp. palaearctica. Analogously, despite the fact that virulent strains of M. tuberculosis contain large amounts of phosphoantigens (8), no unequivocal evidence has proved an expansion of Vγ9Vδ2 cells in peripheral blood or in granulomatous lesions of tuberculosis patients. Some studies have concluded that an increase occurs in some patients (1, 12), but in other studies no increase has been observed (17, 27, 29). This discrepancy between two phosphoantigen-containing pathogens may be inherent in bacteriological differences. While M. tuberculosis is a slow-growing gram-positive bacterium causing a chronic infection, tularemia is an acute disease manifested by rapid multiplication of gram-negative bacteria. Thus, it is possible that the stimulus for Vγ9Vδ2 cells is more intense during tularemia due to vigorous replication of bacteria, together with endotoxin-triggered cytokines. There are also clear differences between virulent strains of F. tularensis and the vaccine strain with regard to in vivo multiplication. In all experimental models, virulent strains replicate much faster than does the vaccine strain (9). Thus, it is likely that the stimulus for γδ T-cell expansion resulting from tularemia vaccination, as well as tuberculosis, is weaker than that encountered during tularemia due to differences in the rates of replication of the phosphoantigen-containing bacteria.
In vitro expansion of Vγ9Vδ2 cells after stimulation with extracts from mycobacteria, malaria parasites, viruses, tumor cells, and various gram-negative and gram-positive bacteria has been described (11). The stimuli of M. tuberculosis and P. falciparum have been biochemically characterized and collectively are referred to as phosphoantigens (11). Although the F. tularensis antigens have not been characterized in this study, they most likely resemble the characterized phosphoantigens, as all of these antigens induce a self-cytotoxic response of Vγ9Vδ2 cells and specifically induce in vitro expansion and TNF release by a T-cell clone also seen with M. tuberculosis- and P. falciparum-derived extracts. Moreover, all of these functions are critically dependent on the presence of phosphate in the stimulating ligands.
A common feature of pathogens stimulating Vγ9Vδ2 cells is their intracellular location. Mycobacteria are well known as invaders of monocytes and macrophages, plasmodium schizonts proliferate in erythrocytes, and F. tularensis is found as a facultative parasite of macrophages. Thus, the ability of human γδ cells to recognize these agents could constitute a general strategy for surveillance of parasitized cells.
It has been demonstrated that γδ cells can secrete an array of cytokines important for antimicrobial functions and display a broadly unrestricted cytotoxic response (11, 28). Thus, it is conceivable that expansion of the cell subset during an infection contributes to protection. The vaccine strain of F. tularensis affords good, albeit not complete, protection (5), whereas natural infection confers virtually complete protection (26). In view of this fact, the present observation that vaccination, in contrast to natural infection, resulted in only weak or no expansion of γδ T cells is of interest. An important question for future studies will be to determine whether this relative lack of γδ T-cell expansion is related to the incomplete protection observed after vaccination.
ACKNOWLEDGMENTS
We thank Pekka Ruuska, Kalle Antti, and Mats Ericsson for providing patient samples.
This research was supported by grants from the Medical Faculty, Umeå University, and the Medical Research Council (grant 9485 to A.S.) and by institutional grants from INSERM (to J.J.F. and M.B.), WHO-Global Programme for Vaccination, and la Région Midi-Pyrénées (to J.J.F.).
Yannick Poquet and Michal Kroca contributed equally to this work.
REFERENCES
- 1.Balbi B, Valle M T, Oddera S, Giunti D, Manca F, Rossi G A, Allegra L. T-lymphocytes with gamma delta+ V delta2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–1690. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 2.Behr C, Poupot R, Peyrat M-A, Poquet Y, Constant P, Dubois P, Bonneville M, Fournié J-J. Plasmodium falciparum stimuli for human γδ T cells are related to the phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–2896. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski J F, Morita C T, Tanaka Y, Bloom B R, Brenner M B, Band H. Vgamma 2 Vdelta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 4.Burk M R, Mori L, De Libero G. Human Vγ9-Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 5.Burke D S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain R E. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl Microbiol. 1965;13:232–235. doi: 10.1128/am.13.2.232-235.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournié J J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 8.Constant P, Poquet Y, Peyrat M-A, Davodeau F, Bonneville M, Fournié J-J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human γδ T cells. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eigelsbach H, Saslaw S, Tulis J, Hornick R. Tularemia: the monkey as a model for man. In: Vagtborg H, editor. Use of nonhuman primates in drug evaluation. A symposium. San Antonio, Tex: Southwestern Foundation of Research and Education; 1968. pp. 230–248. [Google Scholar]
- 10.Elloso M M, Van der Heyde H C, Troutt A, Manning D D, Weidanz W P. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol. 1996;157:2096–2102. [PubMed] [Google Scholar]
- 11.Fournié J J, Bonneville M. Stimulation of γδ T cells by phosphoantigens. Res Immunol. 1996;147:338–347. doi: 10.1016/0923-2494(96)89648-9. [DOI] [PubMed] [Google Scholar]
- 12.Ito M, Kojiro N, Ikeda T, Ito T, Funada J, Kokubu T. Increased proportions of peripheral blood gamma delta T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–197. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 12a.Kroca, M., et al. Unpublished data.
- 13.Lang F, Peyrat M A, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournié J J, Bonneville M. Early activation of human Vγ9Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 14.Poquet Y, Constant P, Halary F, Peyrat M A, Gilleron M, Davodeau F, Bonneville M, Fournié J J. A novel nucleotide-containing antigen for human blood γδ T lymphocytes. Eur J Immunol. 1996;26:2344–2349. doi: 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- 15.Sandström G, Tärnvik A, Wolf-Watz H. Antigen from Francisella tularensis: nonidentity between determinants participating in cell-mediated and humoral reactions. Infect Immun. 1984;45:101–106. doi: 10.1128/iai.45.1.101-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoel B, Sprenger S, Kaufmann S H E. Phosphate is essential for stimulation of Vgamma 9Vdelta 2 T lymphocytes by mycobacterial low molecular weight ligand. Eur J Immunol. 1994;24:1886–1892. doi: 10.1002/eji.1830240826. [DOI] [PubMed] [Google Scholar]
- 17.Shijubo N, Nakanishi F, Hirasawa M, Sigehara K, Sasaki H, Asakawa M, Suzuki A. Phenotypic analysis in peripheral blood lymphocytes of patients with pulmonary tuberculosis. Kekkaku. 1992;67:581–585. [PubMed] [Google Scholar]
- 18.Sjöstedt A, Sandström G, Tärnvik A. Nucleotide sequence and T cell epitopes of a membrane protein of Francisella tularensis. J Immunol. 1990;145:311–317. [PubMed] [Google Scholar]
- 19.Sjöstedt A, Sandström G, Tärnvik A. Several membrane polypeptides of the live vaccine strain Francisella tularensis LVS stimulate T cells from naturally infected individuals. J Clin Microbiol. 1990;28:43–48. doi: 10.1128/jcm.28.1.43-48.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjöstedt A, Tärnvik A, Sandström G. The T-cell-stimulating 17-kilodalton protein of Francisella tularensis LVS is a lipoprotein. Infect Immun. 1991;59:3163–3168. doi: 10.1128/iai.59.9.3163-3168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöstedt A, Sandström G, Eriksson M, Tärnvik A. Various membrane proteins of Francisella tularensis induce IFN-γ production in both CD4+ and CD8+ T cells of primed humans. Immunology. 1992;76:584–592. [PMC free article] [PubMed] [Google Scholar]
- 22.Sumida T, Maeda T, Takahashi H, Yoshida S, Yonaha F, Sakamoto A, Tomioka H, Koike T, Yoshida S. Predominant expansion of Vγ9/Vδ2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syrjälä H, Herva E, Ilonen J, Saukkonen K, Salminen A. A whole-blood lymphocyte stimulation test for the diagnosis of tularemia. J Infect Dis. 1984;150:912–915. doi: 10.1093/infdis/150.6.912. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Morita C T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin R L, Brenner M B, Bloom B R, Morita C T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 27.Tazi A, Fajac I, Soler P, Valeyre D, Battesti J P, Hance A J. Gamma/delta T-lymphocytes are not increased in number in granulomatous lesions of patients with tuberculosis or sarcoidosis. Am Rev Respir Dis. 1991;144:1373–1375. doi: 10.1164/ajrccm/144.6.1373. [DOI] [PubMed] [Google Scholar]
- 28.Tsukaguchi K, Balaji K, Boom W H. CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. Similarities and differences in antigen recognition, cytotoxic effector function and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 29.Ueta C, Tsuyuguchi I, Kawasumi H, Takashima T, Toba H, Kishimoto S. Increase of γ/δ T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–5441. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]