Abstract
Alzheimer’s Disease (AD) is the most common neurodegenerative disease which manifests with progressive cognitive impairment, leading to dementia. Considering the noninvasive collection of saliva, we designed the systematic review to answer the question “Are salivary biomarkers reliable for the diagnosis of Alzheimer’s Disease?” Following the inclusion and exclusion criteria, 30 studies were included in this systematic review (according to the PRISMA statement guidelines). Potential biomarkers include mainly proteins, metabolites and even miRNAs. Based on meta-analysis, in AD patients, salivary levels of beta-amyloid42 and p-tau levels were significantly increased, and t-tau and lactoferrin were decreased at borderline statistical significance. However, according to pooled AUC, lactoferrin and beta-amyloid42 showed a significant predictive value for salivary-based AD diagnosis. In conclusion, potential markers such as beta-amyloid42, tau and lactoferrin can be detected in the saliva of AD patients, which could reliably support the early diagnosis of this neurodegenerative disease.
Keywords: neurodegenerative diseases, Alzheimer’s Disease, saliva, biomarkers, beta-amyloid, tau, lactoferrin
1. Introduction
Alzheimer’s Disease (AD) is the most common neurodegenerative disease and the leading cause of dementia [1]. Recent estimates suggest that around 50 million people suffer from dementia; however, the prognosis indicates that this number may reach 150 million by 2050 [2]. AD evolves via a progressive sequence from an asymptomatic, preclinical phase, followed by mild cognitive impairment (MCI) or mild behavioural impairment (MBI), up to AD dementia [3]. Usually, patients affected by AD develop progressive problems with episodic memory, apathy, neuropsychiatric, or mood alterations, leading to disturbances in daily living activities [4,5]. Despite several years of research, curative treatment is not available so far; therefore, the primary objective is preventing and alleviating AD risk factors [6].
The major histological hallmarks of AD include β-amyloid (Aβ) senile plaques and phosphorylated tau (p-tau) forming neurofibrillary tangles (NFTs) [7]. Furthermore, AD pathophysiology focuses on structural alterations in the synapse, synaptic damage or loss. A significant synaptic loss combined with general neuronal damage causes brain atrophy, which precedes the hallmarks mentioned above [8]. Moreover, growing evidence suggests oxidative stress, neuroinflammation, and mitochondrial dysfunction as other mechanisms of AD pathophysiology [9,10]. According to a 2018-released research framework, the diagnosis of AD should not be based on clinical symptoms but on biological biomarkers [11]. Currently, valid diagnostic tools for AD include both CSF biomarkers (Aβ42 and Tau) and imaging methods (MRI and PET) for the study of brain atrophy and metabolism or accumulation of pathogenic substances [12].
Saliva is an easily accessible body fluid with regular alterations in composition under different pathophysiological conditions [13]. The secretion and composition of saliva might be affected by various diseases, including gastrointestinal, thyroid, oncological, autoimmune, cardiovascular, neurological and other disorders [14,15,16,17,18,19,20,21]. Also, AD may influence these qualitative and quantitative salivary parameters. In addition, there seems to be a relationship between the human brain and saliva, which occurs via six different pathways communicating brain molecules with the saliva and vice versa. The oral–brain axis contains possible routes, such as the cranial nerves, the intranasal pathway, the lymphatic pathway, the sublingual route, the peripheral bloodstream, or the gut–brain axis with the vagus nerve [22].
Considering the beneficial aspects of saliva collection and its diagnostic values, in this systematic review, we sought to determine the quality of salivary biomarkers in AD diagnosis. In our review, we did not limit the selection of compounds based on biochemical nature but only salivary origin. Therefore, the design of this systematic review was based on the following question: “Are salivary biomarkers reliable for the diagnosis of Alzheimer’s Disease?”
2. Materials and Methods
2.1. Search Strategy and Data Extraction
Our systematic review was conducted based on the records published from 1 January 2008 to 30 September 2023, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [23], using the databases PubMed, Scopus and Web of Science. The search queries included the following:
-
-
For PubMed: saliva* AND (marker* OR biomarker* OR enzyme* OR metabolite* OR hormon*) AND (Parkinson* OR Alzheimer*);
-
-
For Scopus: TITLE-ABS-KEY (saliva* AND (marker* OR biomarker* OR enzyme* OR metabolite* OR hormon*) AND (parkinson* OR alzheimer*));
-
-
For Web of Science: TS = (saliva* AND (marker* OR biomarker* OR enzyme* OR metabolite* OR hormon*) AND (Parkinson* OR Alzheimer*)).
Retrieved search results were filtered by publication date after 1 January 2008. The search strategy deliberately included two major neurodegenerative diseases in connection with the planned publication of two separate papers.
Records were screened by the title, abstract and full text by two independent investigators. Studies included in this review matched all the predefined criteria according to PI(E)COS (“Population”, “Intervention”/“Exposure”, “Comparison”, “Outcomes” and “Study design”), as presented in Table 1. A detailed search flowchart is shown in the “Results” section. The study protocol was registered in the International prospective register of systematic reviews PROSPERO (CRD42023477115).
Table 1.
Inclusion and exclusion criteria according to the PI(E)COS.
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients aged 0–99 years, both genders; Sample size: 15 patients or more | Sample size: below 15 patients or controls |
| Intervention/Exposure | Alzheimer’s Disease | Other diseases, e.g., MCI |
| Comparison | Non-demented control group | Lack of non-demented control group |
| Outcomes | Alterations in salivary markers level | Alterations in other markers level (e.g., serum), microbiota |
| Study Design | Case control, cohort, and cross-sectional studies | Literature reviews, case reports, expert opinion, letters to the editor, conference reports |
| Published after 1 January 2008 | Not published in English |
The results of the meta-analysis were presented in forest plots using the MedCalc Statistical Software, version 22.014 (MedCalc Software Ltd., Ostend, Belgium). The meta-analysis was performed for the most often biomarkers in saliva from patients with AD. The standardised mean differences and pooled AUC were calculated.
2.2. Quality Assessment and Critical Appraisal for the Systematic Review of Included Studies
The risk of bias in each individual study was assessed according to the “Study Quality Assessment Tool” issued by the National Heart, Lung, and Blood Institute within the National Institute of Health [24]. These questionnaires were answered by two independent investigators, and any disagreements were resolved by discussion between them.
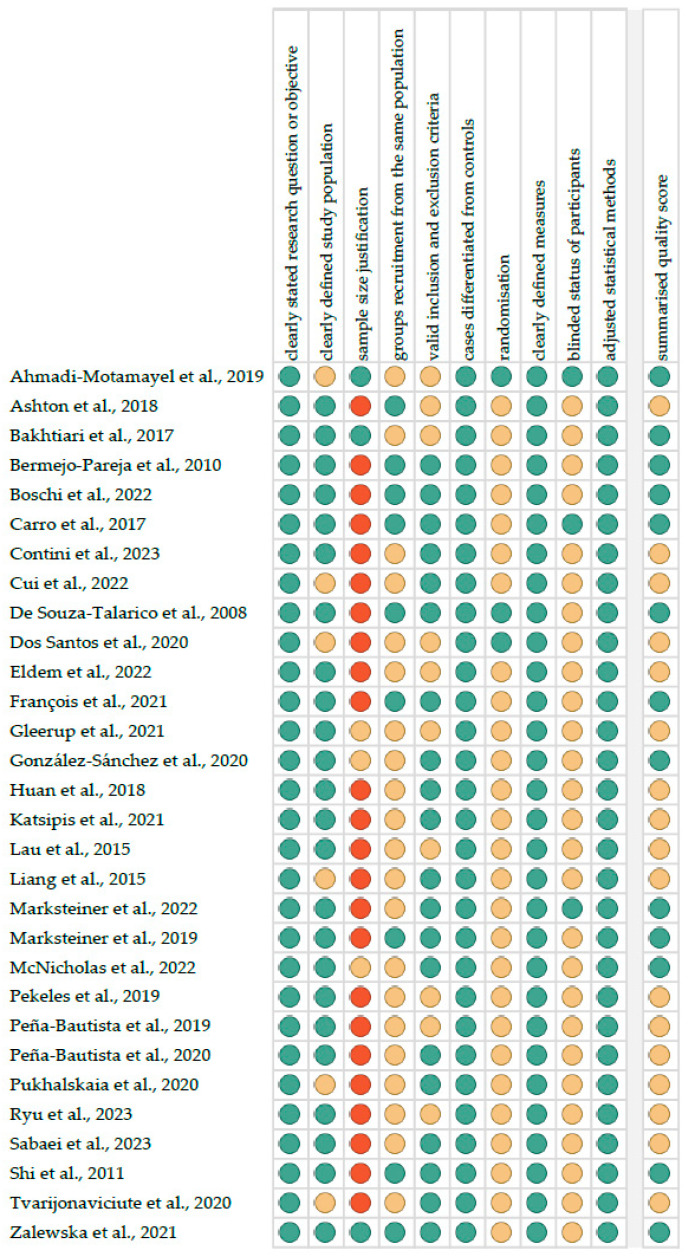
Figure 1 reports the summarised quality assessment. The most frequently encountered risks of bias were the absence of data regarding sample size justification, randomisation and blinding (each for twenty-seven studies). Critical appraisal was summarised by adding up the points for each criterion of potential risk (points: 1—low, 0.5—unspecified, 0—high). Thirteen studies (43.3%) were classified as having “good” quality (≥80% total score), and seventeen (56.7%) were classified as having “intermediate” quality (≥60% total score).
Figure 1.
Quality assessment, including the main potential risk of bias (risk level: green—low, yellow—unspecified, red—high; quality score: green—good, yellow—intermediate, red—poor) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
All of the included studies had the third or fourth level of evidence (case-control studies), according to the five-graded scale used for classification by the Oxford Centre for Evidence-Based Medicine levels for diagnosis [25].
3. Results
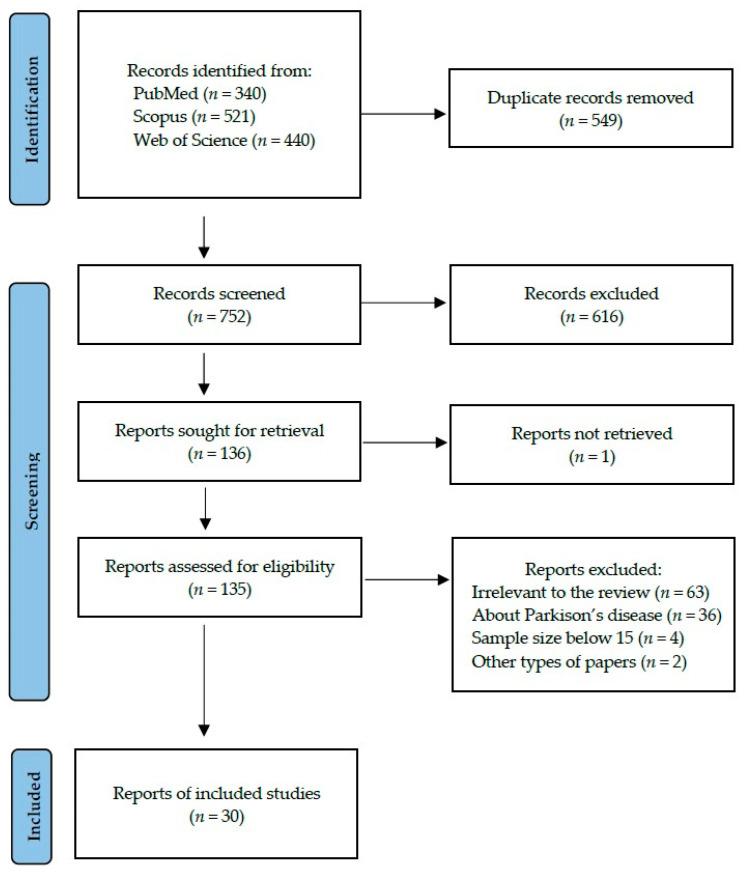
Following the search criteria presented in the Section 2, our systematic review included thirty studies, demonstrating data collected in seventeen different countries from a total of 1371 participants diagnosed with Alzheimer’s Disease. Figure 2 shows the detailed selection strategy of the searched records.
Figure 2.
PRISMA flow diagram presenting search strategy.
In Table 2, we presented data collected from each eligible study included in the present systematic review, which included its general characteristics, such as year of publication, setting and involved participants, as well as the detailed characteristics considering types of saliva, methods of collection, centrifugation, storing and laboratory analysis, and potential salivary biomarkers for AD. Most of the studies came from Europe, which was followed by Asia. The most commonly studied material was unstimulated saliva. Very different conditions of centrifugation and storage were reported by researchers. Among the diagnostic methods, ELISA prevailed. Proteins and metabolites were the most often determined potential biomarkers. Information on inclusion and exclusion criteria of study participants and their smoking status can be found in Table S1.
Table 2.
The characteristics of included studies.
| Author, Year | Setting | Study Group (F/M), Age | Control Group (F/M), Age | Type of Saliva and Method of Collection | Centrifugation and Storing | Method of Marker Determination | Salivary Biomarkers |
|---|---|---|---|---|---|---|---|
| Ashton et al., 2018 [26] | UK | AD: 53 (30/23), 81.4 ± 6.6; MCI: 68 (35/33), 79.8 ± 6.4 | 160 (94/66), 78.0 ± 6.7 | unstimulated saliva collected into preweighed sterile plastic 30 mL containers at 30 s intervals for 2 min or until 2 mL achieved, overnight fasting | centrifuged at 500× g for 10 min at 4 °C, immediately stored at −80 °C | Human Total Tau assay on an HD-1 Simoa instrument | t-tau (ns) |
| Bermejo-Pareja et al., 2010 [27] | Spain | AD: 70 (49/21), 77.20 (60–91); PD: 51 (25/26); 72.96 (60–93) | 56 (39/17), 74.35 (64–85) | approx. 1 mL of saliva collected at least 4 h after eating or drinking (at around 1 a.m.) in sterile plastic containers previously treated with 2% sodium azide solution | centrifuged at 1500 rpm for 5 min, immediately frozen at −80 °C until used | ELISA | Aβ42, Aβ40 (ns) |
| Boschi et al., 2022 [28] | Italy | AD: 18 (10/8), 72.13 ± 5.45 | 18 (11/7), 65.67 ± 12.02 | 10 mL of whole, unstimulated saliva collected in a 15 mL polypropylene Falcon tube, all patients fasting for at least 8 h | immediately placed on ice, precleared by a low spin at 600× g for 10 min at 4 °C, stored at −80 °C | ELISA | Aβ42 |
| Cui et al., 2022 [29] | China | AD: 30 (NR), NR | 30 (NR), NR | unstimulated/stimulated whole/sublingual/submandibular/parotid saliva collected in pre-chilled polypropylene tubes on ice between 9 and 9:30 a.m. using a Salivette, 5 mL in total | centrifuged at −20 °C, transferred to the laboratory on regular basis | ELISA | Aβ42, Aβ40 (ns), p-tau (ns), t-tau (ns) |
| Dos Santos et al., 2020 [30] | Brazil | AD: 60 (NR), NR | 60 (NR), NR | collected using Salivette, placed for 3 min in the mouth without chewing | stored in a cooler at −20 °C, centrifuged at 3000 rpm for 15 min | ELISA | Aβ42, t-tau |
| Katsipis et al., 2021 [31] | Greece | AD: 20 (9/11), 75 ± 5.5; MCI: 20 (12/8), 75 ± 7.2 | 20 (11/9), 79 ± 4.7 | unstimulated saliva collected in the morning, by passive drooling | centrifuged at 13,500 rpm for 15 min, stored at −80 °C | ELISA, Dot Blot, Western Blot | GFAP, p-tau, IL-1β, IL-6, TNF-α, caspase-8, COX-2, Aβ42 |
| Lau et al., 2015 [32] | Korea | AD: 20 (12/8), 72.5 ± 7.68; PD: 20 (11/9), 73 ± 8.07 | 20 (15/5), 66.1 ± 7.79 | 3 mL unstimulated saliva collected after at least 4 h of fasting | centrifuged at 1000× g for 15 min, stored at −80 °C | ELISA, EG-ISFET | Aβ42 (not detected), p-tau (ns), t-tau (ns), trehalose (undefined) |
| Marksteiner et al., 2022 [33] | Austria | AD: 44 (25/19), 79 ± 1; MCI: 45 (25/20), 74 ± 1 | 27 (14/13), 71 ± 1 | approx. 1.5 mL of saliva collected in the early morning using Salivette kept 2 min in the mouth | centrifuged at 3000× g 5 min, stored at −80 °C | HPLC-EC, robotic-automated enzymatic Lumipulse assay | norepinephrine, p-tau (ns), t-tau, Aβ40 and Aβ42 (not detected in cases) |
| Pekeles et al., 2019 [34] | Canada | AD: 46 (22/24), 80 (9); MCI: 55 (32/23), 78 (14) | 47 (32/15), 73 (6) | 4–5 mL of unstimulated saliva collected in the morning by spitting into a sterile 50 mL polypropylene tube | put on ice, then in 100 °C water bath for 20 min, centrifuged at 5000 rpm or 10,000 rpm for 10 min at 4 °C, stored at −80 °C | Western Blot, tau antibodies | p-tau (T181 (ns), S396, S404, S400, T403, T404) |
| Sabaei et al., 2023 [35] | Iran | AD: 24 (10/14), 73.5 ± 9.8; PD: 24 (10/14), 61.2 ± 8.7 | 22 (13/9), 64.1 ± 9.2 | dental cotton roll placed on the oral side of the cheek to, moist rolls located inside the salivary collector tubes | centrifuged at 1500 rpm for 5 min, stored at −80 °C | ELISA | Aβ 1–42, p-tau, α-synuclein |
| Shi et al., 2011 [36] | USA | AD: 21 (11/10), 68.8 (52–85) | 38 (19/19), 69.0 (40–88) | collected by placing a dental cotton roll between the cheek and gum of the mouth for at least 1 min, then spun inside a Salivette | stored at −70 °C | Luminex assays, IP/MS | t-tau, p-tau, Aβ42 (not detected) |
| Tvarijonaviciute et al., 2020 [37] | Spain | AD: 69 (NR), NR | 83 (NR), NR | up to 0.5 mL of unstimulated whole saliva collected passively by drooling into a propylene tube, between 9 and 12 a.m. | centrifuged at 3000× g for 10 min at 4 °C, stored at −80 °C | MILLIPLEX MAP, automated spectrophotometric method | FRAP (ns), ADA (ns), ChE (ns), Hp (ns), Aβ42, Aβ40 (ns), t-tau (ns), p-tau (ns), CRP (ns), PEDF (ns), SAP (ns), MIP-4 (ns), CC4, α1 antitrypsin (ns) |
| Zalewska et al., 2021 [38] | Poland | AD: 25 (15/10), 81.19 ± 6.77 | 25 (15/10), 82.1 ± 6.67 | stimulated whole saliva collected after drinking a glass of water and a 5 min conversation, saliva taken with a pipette after spraying of 100 μL of citric acid on the tip of the tongue every 30 s for 10 min | placed on ice, centrifuged at 5000× g for 20 min at 4 °C within 30 min from collection, stored at −84 °C | colorimetric, spectrofluorimetric, spectrophotometric methods, thioflavin T fluorescence, ELISA | Aβ, lactoferrin, IL-1β, SOD, CAT, GPx, UA (ns), GSH, TAC (ns), TOS, AGE, AOPP, MDA, NO, peroxynitrite, nitrotyrosine |
| Carro et al., 2017 [39] | Spain | AD: 80 (49/31), 76.2 ± 5.33; MCI: 44 (25/19), 75.16 ± 5.13; PD: 59 (32/27), 69.5 ± 8.6 | 91 (59/32), 73.7 ± 6.88 | 0.5 mL of unstimulated whole saliva collected into sterile plastic containers precoated with 2% sodium azide solution | immediately placed on ice, precleared by a low spin at 600× g for 10 min at 4 °C, stored at −80 °C | ELISA | lactoferrin |
| Gleerup et al., 2021 [40] | Denmark | AD: 71 (41/30), 72.1 ± 7.3; MCI: 56 (27/29), 70.4 ± 8.2 | 20 (8/12), 65.7 ± 10.1 | 1–3 mL of unstimulated whole saliva, collected between 9:15 and 10:15 a.m., or around noon, in a 15 mL polypropylene falcon tube | placed on ice, centrifuged at 2000 rpm for 10 min at 4 °C, stored at −80 °C | ELISA | lactoferrin (ns) |
| González-Sánchez et al., 2020 [41] | Spain | AD-PET+: 25 (12/13), 67.2 ± 9.2, MCI-PET+: 21 (8/13), 68.8 ± 7.5 | Control-PET-: 48 (33/15), 66.9 ± 5.9; control-PET+: 4 (2/2), 75.9 ± 3.6 | 0.5 mL of unstimulated whole saliva collected into sterile plastic containers precoated with 2% sodium azide solution | immediately placed on ice, precleared by a low spin at 600× g for 10 min at 4 °C, stored at −80 °C | ELISA | lactoferrin |
| Ahmadi-Motamayel et al., 2019 [42] | Iran | AD: 30 (NR), 56–85 | 30 (NR), 57–85 | unstimulated, whole saliva collected in falcon tubes within 5–10 min, from 8 to 10 a.m. | centrifuged at 3000 rpm for 10 min, stored at −80 °C | spectrophotometric assay using the Ellman colorimetric method | AChE, PChE |
| Bakhtiari et al., 2017 [43] | Iran | AD: 15 (6/9), 78.4 (64–90) | 15 (8/7), 71 (61–85) | 2 mL of whole, unstimulated saliva samples collected by spitting into 15 mL Falcon tubes, between 9 and 12 a.m. | immediately placed on ice and stored at −70 °C, centrifuged at 3000 rpm for 10 min | Ellman colorimetric method | AChE (ns) |
| De Souza-Talarico et al., 2008 [44] | Brazil | AD: 40 (27/13), 80.1 ± 6.0 | 40 (35/5), 72.2 ± 6.3 | drawn off with pipette and transferred to a sterile tube, collected within 2 h of waking in the next day after cognitive evaluation | centrifuged at 2200 rpm for 15 min at 4 °C, immediately placed on chipped ice, stored at −20 °C | radioimmunoassay | cortisol |
| Peña-Bautista et al., 2019 [45] | Spain | mild AD: 50 (29/21), 70 (68, 74); MCI-AD: 47 (30/17), 71 (69, 74) | 41 (16/25), 66 (62, 69) | according to previously described procedures, collected between 8 and 10 a.m. | stored at −80 °C, thawed on ice, homogenized and centrifuged at 3500× g for 10 min at 4 °C | UPLC-MS/MS | cortisol (ns) |
| Contini et al., 2023 [46] | Italy | AD: 35 (23/12), 80 ± 6; PD: 36 (11/15), 72 ± 7 | 36 (18/18), 78 ± 6 | unstimulated whole saliva collected between 9 and 12 a.m., with a soft plastic aspirator for less than 1 min, transferred to a plastic tube cooled on ice | centrifuged 20,000× g for 15 min at 4 °C, stored at −80 °C or immediately analysed | RP-HPLC-LR-ESI-MS | proteomics |
| Eldem et al., 2022 [47] | Switzerland | AD: 17 (9/8), 72 ± 1.36; MCI: 21 (16/5), 73 ± 1.51 | 19 (13/6), 64 ± 2.63 | 2 mL of whole unstimulated saliva collected between 9 and 11 a.m. | stored at –20 °C | LC-MS/MS, Western Blot, FASP | proteomics, t-tau (ns), transthyretin |
| François et al., 2021 [48] | Australia | AD: 20 (8/12), 78.0; MCI: 20 (11/9), 77.8 | 40 (19/21), 75.3 | collected using RNAProSAL | stored at −80 °C | GC-MS, LC-MS | proteomics, metabolomics |
| McNicholas et al., 2022 [49] | Australia | AD: 16 (6/10), 79 ± 6; MCI: 15 (8/7), 76 ± 6 | 29 (14/15), 74 ± 8 | collected using RNAProSAL | stored at −80 °C | ELISA | cystatin-C, IL-1 receptor antagonist, stratifin, haptoglobin, matrix metalloproteinase 9 |
| Pukhalskaia et al., 2020 [50] | Russia | AD: 64 (NR), elderly 63.0 ± 2.4, senile 82.0 ± 2.3 | 58 (NR), NR | collected between 10 and 12 a.m. | NR | ELISA | SIRT1, SIRT3, SIRT5 (ns), SIRT6 |
| Huan et al., 2018 [51] | Canada | AD: 22 (16/6), 77.09 ± 11.20; MCI: 25 (15/10), 70.40 ± 3.38 | 35 (22/13), 69.94 ± 3.80; 10 (5/5), 71.40 ± 2.84 | whole saliva collected using Oragene DNA Self-Collection Kit OG-500, sample placed inside the kit, shaken | stored at −80 °C | LC-MS | metabolomics |
| Liang et al., 2015 [52] | China | AD: 256 (NR), NR | 218 (NR), NR | collected between 9 and 11 a.m. | centrifuged at 10,000 rpm for 20 min at 4 °C, stored at −80 °C | FUPLC-MS | metabolomics |
| Marksteiner et al., 2019 [53] | Austria | AD: 25 (17/8), 80.4 ± 7.2; MCI: 25 (16/9), 75.9 ± 8.8 | 25 (16/9), 74.8 ± 4.4 | 1–2 mL of saliva collected in the early morning by spitting into a 15 mL sterile falcon tube for 2 min | stored at −80 °C until analysis, centrifuged at 14,000× g for 5 min | FIA-MS/MS, using the AbsoluteIDQ p150 Kit | metabolomics |
| Peña-Bautista et al., 2020 [54] | Spain | mild AD: 14 (NR), NR; MCI-AD: 17 (NR), NR | 12 (NR), NR | 1–2 mL of whole-mouth saliva collected by spitting into sterile bottles between 10 and 12 a.m. | stored at −80 °C, centrifuged at 1200× g for 5 min at 4 °C | UPLC-MS/MS | aspartic acid (ns), glutamic acid (ns), glutamine, GABA (ns), creatine, taurine, N-acetyl aspartate (ns), myoinositol, acetylcholine |
| Ryu et al., 2023 [55] | South Korea | AD: 27 (12/15), 72.59 ± 6.90 | 13 (11/2), 75.46 ± 6.58 | collected by oral swabs, transferred to sterilised tubes | centrifuged at 12,000× g for 10 min at 4 °C, stored at −70 °C | qPCR | miRNA-485-3p |
Legend: Aβ, beta-amyloid; AD, Alzheimer’s Disease; ADA, adenosine deaminase; AGE, advanced glycation end products; AOPP, advanced oxidation protein products; CC4, complement C4; CAT, catalase; ChE, cholinesterase; COX-2, cyclooxygenase 2; CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assay; FRAP, ferric-reducing ability of plasma; GABA, gamma-aminobutyric acid; GFAP, glial fibrillar acidic protein; GSH, glutathione; GPx, glutathione peroxidase; Hp, haptoglobin; IL, interleukin; IP/MS, immunoprecipitation/mass spectrometry identification; LC-MS/MS, liquid chromatography/mass spectroscopy; LF, lactoferrin; MCI, mild cognitive impairment; MDA, malondialdehyde; MIP-4, macrophage inflammatory protein-4; NO, nitric oxide; NR, not reported; ns, not significant; OSI, oxidative stress index; PChE, pseudocholinesterase; PEDF, pigment epithelium-derived protein; PGs, prostaglandins; p-tau, phosphorylated tau; qPCR, quantitative real-time polymerase chain reaction; SAP, salivary amyloid A; SIRT, sirtuin; SOD, superoxide dismutase; TAC, mean total antioxidant capacity; t-tau, total tau; TNF-α, tumour necrosis factor-alpha; TOS, total oxidant status; UA, uric acid; UPLC-MS/MS, ultra-performance liquid chromatography coupled to tandem mass spectrometry; UK, the United Kingdom; USA, the United States of America.
Additionally, we showed the predictive parameters for most discriminant potential AD markers from the included studies in Table 3. Since not all studies reported these data and only two salivary markers were repeatable with AUC values, a meta-analysis was performed only for them. For beta-amyloid, the pooled AUC was 0.803 (SE ± 0.056), and for lactoferrin, it was 0.896 (SE ± 0.067). Both markers showed significant predictive value for salivary-based AD diagnosis (for random effects, p-value < 0.001).
Table 3.
Reported predictive parameters of most discriminant potential biomarkers for Alzheimer’s Disease (vs. healthy subjects) from included studies.
| Study | Most Discriminant Markers | AUC | −95% CI | +95% CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Boschi et al., 2022 [28] | Aβ42 | 0.806 | - | - | 84 | 68 |
| Carro et al., 2017 [39] | lactoferrin | 1 | 1 | 1 | 100 | 100 |
| Cui et al., 2022 [29] | Aβ42 | 0.8483 | - | - | - | - |
| p-tau, t-tau, Aβ40, Aβ42 combined together | 0.9211 | - | - | - | - | |
| González-Sánchez et al., 2020 [41] | lactoferrin | 0.93 | 0.876 | 0.989 | 87.1 | 92.9 |
| Huan et al., 2018 [51] | methylguanosine, choline-cytidine, histidinyl-phenylalanine | 0.997 | 0.997 | 1.000 | 98.5 | 96.6 |
| phenylalanyl-proline, phenylalanylphenylalanine, urocanic acid | 0.831 | 0.770 | 0.888 | 82.2 | 73.6 | |
| Katsipis et al., 2021 [31] | GFAP (ELISA) | 1.000 | 1.000 | 1.000 | 75 | 100 |
| GFAP (Dot Blot) | 1.000 | 1.000 | 1.000 | 85 | 75 | |
| Liang et al., 2015 [52] | sphinganine-1-phosphate | 0.998 | - | - | 99.4 | 98.2 |
| ornithine | 0.927 | - | - | 81.9 | 90.7 | |
| phenyllactic acid | 0.831 | - | - | 79.5 | 84.3 | |
| inosine | 0.740 | - | - | 66.8 | 77.0 | |
| 3-dehydrocarnitine | 0.669 | - | - | 57.4 | 84.2 | |
| hypoxanthine | 0.674 | - | - | 53.7 | 73.9 | |
| Peña-Bautista et al., 2020 [54] | acetylcholine | 0.660 | 0.492 | 0.828 | - | - |
| glutamine | 0.777 | 0.619 | 0.935 | - | - | |
| creatine | 0.331 | 0.167 | 0.494 | - | - | |
| myoinositol | 0.261 | 0.113 | 0.408 | - | - | |
| myoinositol, glutamine, creatine, acetylcholine combined together | 0.806 | 0.674 | 0.939 | 61 | 92 | |
| Ryu et al., 2023 [55] | miRNA-485-3p | 0.895 | 0.796 | 0.994 | 74.1 | 92.3 |
| Sabaei et al., 2023 [35] | Aβ1–42 | 0.81 | - | - | 62.5 | 91.0 |
| p-tau | 0.78 | - | - | 91.7 | 63.6 | |
| α-synuclein | 0.71 | - | - | 66.7 | 68.2 | |
| Zalewska et al., 2021 [38] | CAT | 0.918 | 0.827 | 1.000 | 82.6 | 84.0 |
| GPx | 0.741 | 0.584 | 0.897 | 73.9 | 72.0 | |
| GSH | 0.684 | 0.526 | 0.842 | 72.7 | 72.0 | |
| IL-1β | 0.853 | 0.742 | 0.964 | 84.0 | 84.0 | |
| Aβ | 0.949 | 0.894 | 1.000 | 86.4 | 84.0 | |
| lactoferrin | 0.690 | 0.537 | 0.842 | 64.0 | 64.0 | |
| SOD | 0.777 | 0.629 | 0.926 | 69.6 | 68.0 | |
| TOS | 0.920 | 0.814 | 1.000 | 91.3 | 92.0 | |
| AGE | 0.936 | 0.851 | 1.000 | 87.0 | 88.0 | |
| AOPP | 0.680 | 0.531 | 0.829 | 56.0 | 56.0 | |
| MDA | 0.688 | 0.508 | 0.868 | 66.7 | 68.0 | |
| NO | 0.672 | 0.523 | 0.821 | 56.0 | 56.0 | |
| nitrotyrosine | 0.702 | 0.541 | 0.863 | 63.6 | 64.0 | |
| peroxynitrite | 0.816 | 0.693 | 0.940 | 63.6 | 79.2 | |
| OSI | 0.936 | 0.847 | 1.000 | 90.0 | 92.0 |
Legend: Aβ, beta-amyloid; AGE, advanced glycation end products; AOPP, advanced oxidation protein products; AUC, area under curve; CAT, catalase; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GFAP, glial fibrillar acidic protein; GPx, glutathione peroxidase; GSH, glutathione; IL-1β, interleukin 1 beta; MDA, malondialdehyde; NO, nitric oxide; OSI, oxidative stress index; p-tau, phosphorylated tau; SOD, superoxide dismutase; TOS, mean total oxidant status; t-tau, total tau.
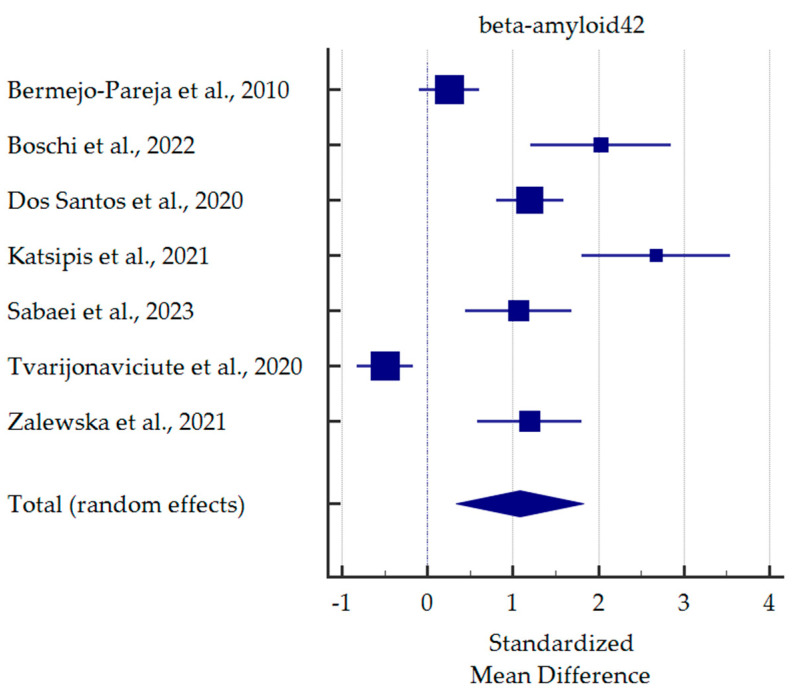
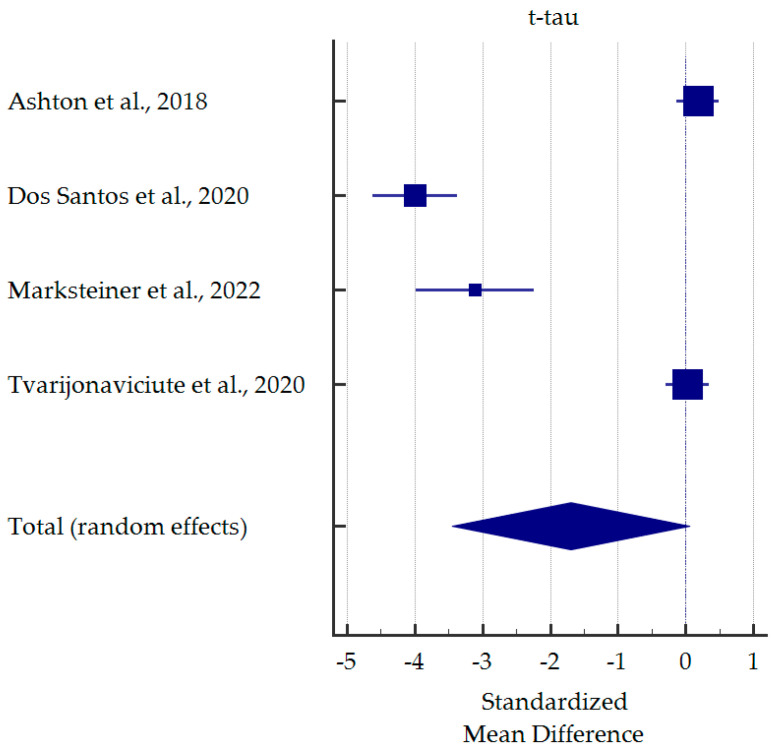
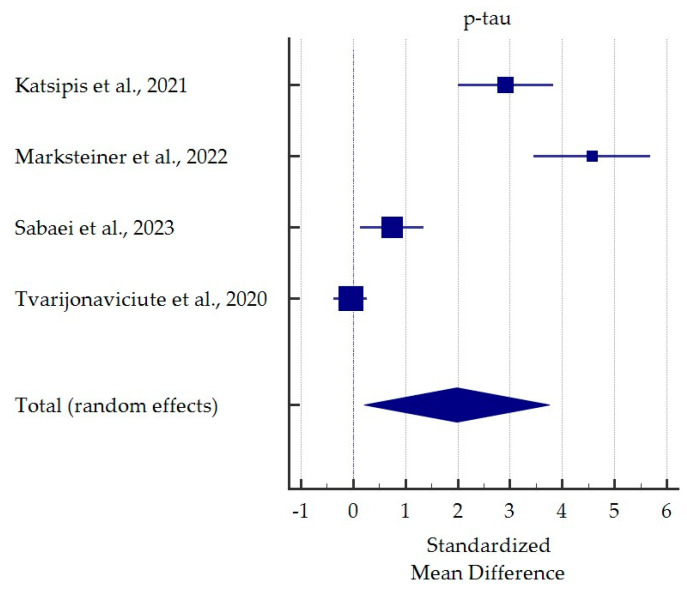
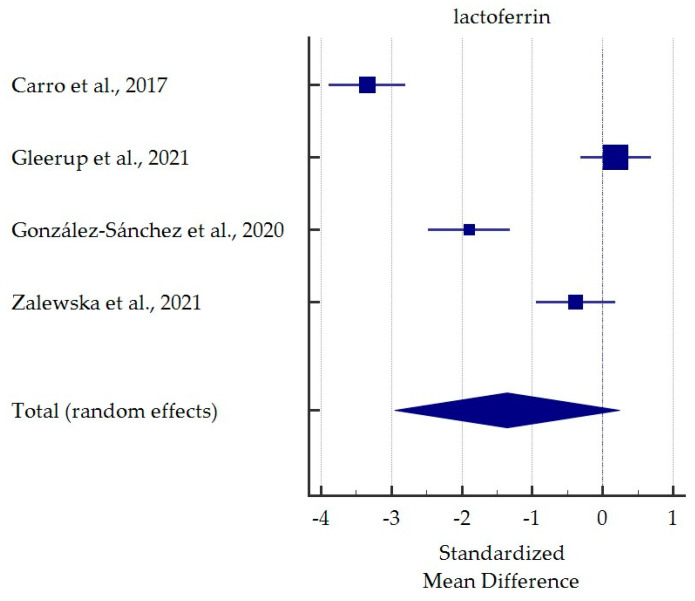
A meta-analysis of differences in saliva levels between AD patients and healthy subjects was performed for the most commonly reported markers (Figure 3, Figure 4, Figure 5 and Figure 6). Both beta-amyloid42 and p-tau levels were significantly higher in the saliva of AD patients. In contrast, salivary levels of t-tau and lactoferrin were lowered in patients with AD at borderline statistical significance. Detailed standardised mean differences are presented in Table 4.
Figure 3.
Standardised mean difference of beta-amyloid42 levels in saliva from patients with Alzheimer’s Disease compared to healthy subjects [27,28,30,31,35,37,38].
Figure 4.
Standardised mean difference of total tau levels in saliva from patients with Alzheimer’s Disease compared to healthy subjects [26,30,33,37].
Figure 5.
Standardised mean difference of phosphorylated tau levels in saliva from patients with Alzheimer’s Disease compared to healthy subjects [31,33,35,37].
Figure 6.
Standardised mean difference of lactoferrin levels in saliva from patients with Alzheimer’s Disease compared to healthy subjects [38,39,40,41].
Table 4.
Detailed results for meta-analysis comparing salivary levels of the most often potential markers for Alzheimer’s Disease vs. healthy subjects.
| Study | SMD | 95% CI | p-Value | Weight |
|---|---|---|---|---|
| Beta-amyloid42 | ||||
| Bermejo-Pareja et al., 2010 [27] | 0.254 | −0.100 to 0.609 | 15.11 | |
| Boschi et al., 2022 [28] | 2.025 | 1.204 to 2.846 | 13.29 | |
| Dos Santos et al., 2020 [30] | 1.198 | 0.807 to 1.588 | 15.01 | |
| Katsipis et al., 2021 [31] | 2.676 | 1.804 to 3.548 | 13.02 | |
| Sabaei et al., 2023 [35] | 1.064 | 0.438 to 1.690 | 14.16 | |
| Tvarijonaviciute et al., 2020 [37] | −0.497 | −0.822 to −0.172 | 15.19 | |
| Zalewska et al., 2021 [38] | 1.192 | 0.584 to 1.801 | 14.22 | |
| Total (random effects) | 1.080 | 0.334 to 1.827 | 0.005 | |
| t-tau | ||||
| Ashton et al., 2018 [26] | 0.179 | −0.132 to 0.491 | 25.47 | |
| Dos Santos et al., 2020 [30] | −4.000 | −4.625 to −3.375 | 24.88 | |
| Marksteiner et al., 2022 [33] | −3.110 | −3.986 to −2.233 | 24.20 | |
| Tvarijonaviciute et al., 2020 [37] | 0.022 | −0.299 to 0.342 | 25.46 | |
| Total (random effects) | −1.696 | −3.453 to 0.060 | 0.058 | |
| p-tau | ||||
| Katsipis et al., 2021 [31] | 2.916 | 2.005 to 3.827 | 24.63 | |
| Marksteiner et al., 2022 [33] | 4.569 | 3.445 to 5.693 | 23.85 | |
| Sabaei et al., 2023 [35] | 0.741 | 0.136 to 1.346 | 25.50 | |
| Tvarijonaviciute et al., 2020 [37] | −0.053 | −0.374 to 0.267 | 26.02 | |
| Total (random effects) | 1.983 | 0.209 to 3.757 | 0.029 | |
| Lactoferrin | ||||
| Carro et al., 2017 [39] | −3.346 | −3.889 to −2.803 | 25.00 | |
| Gleerup et al., 2021 [40] | 0.189 | −0.310 to 0.689 | 25.12 | |
| González-Sánchez et al., 2020 [41] | −1.897 | −2.475 to −1.318 | 24.92 | |
| Zalewska et al., 2021 [38] | −0.382 | −0.947 to 0.183 | 24.97 | |
| Total (random effects) | −1.357 | −2.953 to 0.239 | 0.095 | |
4. Discussion
4.1. β-amyloid
β-amyloid (Aβ) is a protein produced mainly in neuronal endosomes via amyloid precursor protein (APP) hydrolysis with β- and γ-secretases. In normal conditions, Aβ release is regulated by synaptic activity, which is, in turn, influenced by Aβ. Interestingly, Aβ may play an immunoprotective role [56]. Nevertheless, the accumulation of aggregated Aβ fibrils leads to the creation of Aβ plaques, which is a pathological phenomenon characteristic of AD [57].
In 2010, Bermejo-Pareja et al. [27] measured levels of Aβ40 and Aβ42 in the saliva of AD patients. Apart from healthy controls, 70 patients were enrolled, which were divided into three groups: the mild, moderate, and severe stages of AD (29, 24, and 17 patients, respectively). The results show that salivary levels of Aβ42 were significantly increased in patients in the mild AD stage. Moreover, a similar tendency was observed in moderate and severe stages although with a high standard deviation. Additionally, the authors observed a correlation between salivary Aβ42 concentration and sex. On the other hand, no significant differences were found in Aβ40 levels between AD patients and the control group.
Ten years later, another research focused on salivary Aβ42 levels. In this case, 60 healthy subjects and 60 patients with a probable diagnosis of AD were recruited and selected by geriatricians. There was no distinction between disease stages. Aβ42 levels in saliva were higher in AD patients but not significantly compared to healthy subjects [30].
In a study by Cui et al. [29], salivary Aβ40 and Aβ42 levels were assessed in a smaller sample (30 patients). Similarly, Aβ40 levels did not differ significantly between controls and patients, and Aβ42 levels were significantly increased. The performed ROC analysis revealed no significant predictive value for salivary Aβ40 and Aβ42 and their ratio.
On the other hand, Katsipis et al. [31] measured Aβ42 levels in the saliva of 60 participants (20 AD patients). Again, the results indicated that salivary Aβ42 levels significantly increased in AD patients compared to healthy individuals and MCI patients.
Consistent results were obtained by Boschi et al. [28] in a group of 100 participants (18 AD subjects, 18 controls, 64 patients with dementia other than AD). Salivary Aβ42 levels were significantly elevated in patients affected by AD compared to non-demented controls. No considerable correlations between gender or MMSE score and salivary Aβ42 level were observed. Interestingly, salivary Aβ42 concentrations were significantly and negatively associated with CSF Aβ42 levels in all diagnostic groups except for the AD group. Nevertheless, the ROC analysis revealed satisfactory performance of salivary Aβ42 in AD diagnosis (AUC 0.806, specificity 68%, sensitivity 84%, with a cut-off value of 92.5 pg/mL).
Furthermore, Sabaei et al. [35] investigated salivary Aβ1–42 levels in the study of 70 participants, including 24 patients with mild AD. Similarly, salivary Aβ1–42 levels were significantly higher in AD patients in comparison with healthy controls with a slightly lower difference after age adjustment. In addition, the ROC analysis confirmed the satisfactory performance of this potential biomarker with both the cut-off point equal to 60.3 pg/mL (AUC 0.81, specificity 91%, sensitivity 62.5%) and 15.5 pg/mL (AUC 0.77, specificity 59.1%, sensitivity 91.7%).
In turn, Tvarijonaviciute et al. [37] concluded that salivary Aβ42 levels are decreased in AD based on the sample of 69 patients. Analysis of the univariate logistic regression models revealed that individuals with decreased Aβ42 levels in saliva were more likely to be in the AD group. Moreover, no significant association between disease stage and salivary Aβ42 level was observed.
Another method, fluorescence of Aβ combined with the addition of Thioflavin T, was used to analyse Aβ by Zalewska et al. [38]. This research consisted of 25 controls and 25 AD patients. Concentrations of salivary Aβ were significantly higher in AD patients compared to non-demented controls (AUC 0.949, sensitivity 86.36%, specificity 84.00%).
In summary, in most studies, AD patients had elevated levels of beta-amyloid, which was statistically significant in our meta-analysis. However, in three studies, salivary Aβ40 and Aβ42 were not detected in AD patients [32,33,36]. Lau et al. [32] and Shi et al. [36] did not disclose Aβ42 levels employing the ELISA method and highly sensitive Luminex assays, respectively. Marksteiner et al. [33] used automated Lumipulse enzymatic light-emitting technology (Fujirebio G600II) and did not detect levels of both Aβ40 and Aβ42 in AD patients.
4.2. Tau
Tau belongs to the microtubule-associated protein group responsible for stabilising neuronal microtubules. In pathological conditions, tau may be hyperphosphorylated, which results in aggregation and neuronal toxicity [58]. Tau hyperphosphorylation and aggregation are connected with impaired both long- and short-term synaptic plasticity, which is a phenomenon observed in AD [59,60]. Tau protein has 85 phosphorylation sites, and in normal conditions, only 10 are phosphorylated, which is significantly less than the 55 in AD [61].
In 2011, Shi et al. [36] investigated both Aβ42 (previously mentioned) and tau levels in saliva. In comparing AD patients and controls, a non-significant decrease in t-tau concentrations in patients was observed; however, no difference was found after standardisation by total salivary protein levels. On the other hand, both absolute and standardised p-tau levels tended to increase in AD patients, but this was also insignificant. Nevertheless, a significant increase in the p-tau/t-tau ratio was observed in patients affected by AD.
Similarly, four years later, another study did not succeed in measuring salivary Aβ42 levels, but both p-tau and t-tau concentrations were detected. No significant differences between controls and patients with AD were found, although salivary p-tau tended to increase in the latter group. Moreover, none of these three biomarkers reflected the disease progression [32].
Interesting results were obtained by Ashton et al. [26] in a bigger sample of 53 AD patients using the Sioma platform. In contrast to the study mentioned above [36], salivary t-tau concentration tended to increase in the patients’ group compared to healthy subjects, although not significantly. In addition, the authors noticed a non-significant tendency in elevated t-tau levels associated with poorer cognitive abilities.
In a previously mentioned research study by Cui et al. [29], salivary p-tau and t-tau levels were also analysed. The Spearman rank analysis of both proteins’ salivary concentrations revealed no significant relationship. However, the p-tau/t-tau ratio increased significantly, which was consistent with a study by Shi et al. [36]. The ROC analysis showed no significant predictive value for t-tau and p-tau nor their ratio. Nevertheless, when p-tau, t-tau, Aβ40, and Aβ42 were combined, the ROC analysis revealed excellent diagnostic relevance (AUC 0.921).
On the other hand, Dos Santos et al. [30] noticed a statistically significant change in salivary t-tau levels in AD patients compared to healthy individuals. The median salivary t-tau of subjects without AD was significantly higher than that of AD patients. Conflicting results were obtained by Eldem et al. [47] in their proteomic study. In a group of 57 participants, 17 AD and 21 MCI patients were enrolled. T-tau levels were analysed using Western blot, and no significant differences between diagnostic groups were observed. Katsipis et al. [31] investigated p-tau levels in saliva. In this study, p-tau concentrations were significantly elevated in comparison not only to healthy controls but also to MCI patients.
Interestingly, although Marksteiner et al. [33] did not detect salivary Aβ40 and Aβ42 levels, the authors collected results about tau levels in saliva. T-tau levels significantly decreased in AD patients, especially in females. P-tau levels were significantly increased in MCI patients; slightly lower and not significant elevation in p-tau concentrations was observed in AD patients. Moreover, no statistically significant differences were found in the p-tau/t-tau ratio.
In 2019, Pekeles et al. [34] investigated the salivary p-tau/t-tau ratios among AD patients, MCI patients, and healthy controls, considering various phosphorylation sites. Interestingly, no significant differences were observed regarding one of the most extensively studied tau sites, T181. In contrast, analysis of both S396, S404, and a combination of S400, T403, and T404 sites showed significantly elevated levels of the p-tau/t-tau ratio in AD patients compared to the control group. S396 was most significantly increased and had better specificity than S404; however, it had worse sensitivity (S396 sensitivity 73%, specificity 50%, S404 sensitivity 83%, specificity 30%).
In one of the most recent studies included in this review, Sabaei et al. [35] also investigated salivary p-tau concentrations. Once again, significant elevations of p-tau levels were observed in the AD group compared to healthy subjects both regardless of the age-confounding variable and after adjusting the age variable. Moreover, the ROC curve analysis revealed satisfactory performance of this biomarker (AUC 0.78, specificity 63.6%, sensitivity 91.7%).
Finally, Tvarijonaviciute et al. [37] analysed salivary p-tau and t-tau in patients suffering from AD and non-demented individuals. No significant changes were observed. P-tau tended to decrease slightly in patients compared to controls. On the other hand, t-tau reached similar values in both groups.
4.3. Lactoferrin
Lactoferrin (LF) is a crucial protein that plays an important role in maintaining human health [62]. Antifungal, antibacterial, antiviral, anti-carcinogenic, anti-inflammatory, and iron-binding properties enhance its relevancy in biological processes [63]. LF may have neuroprotective effects in neurodegenerative diseases, such as AD. Several mechanisms in which LF likely alleviates cognitive impairment, Aβ accumulation, and neurodegeneration were reviewed in another paper [64].
In a study by Carro et al. [39], 116 AD patients were recruited. Also, patients affected by MCI, Parkinson’s Disease (PD), and healthy controls were enrolled. Salivary LF levels were significantly lower in AD and MCI patients than in healthy controls (4.78 ± 1.11 vs. 10.24 ± 1.96 µg/mL). Moreover, a statistically significant negative correlation was found between AD and MCI severity and LF level in saliva. The analogical association was observed regarding the MMSE score. In addition, salivary LF was significantly correlated with CSF t-tau and Aβ42. The performed ROC analysis, which included the MCI/AD group and healthy controls, reached 100% specificity and sensitivity with a cut-off value of 7.43 µg/mL.
Consistent results were obtained by González-Sánchez et al. [41] three years later. Significantly decreased salivary LF levels were observed in MCI patients with positive amyloid-PET scans and AD patients in comparison with cognitively normal individuals. No significant differences were observed between these two experimental groups. Similarly, such differences were not found between MCI patients with negative amyloid-PET scans and controls. Additionally, no significant correlation with disease stage was noticed. Nevertheless, salivary LF performance in differentiation between AD/MCI amyloid-PET positive patients and controls, visualised via the ROC curve analysis with a cut-off value of 5.63 µg/mL, showed satisfactory results (AUC 0.952, sensitivity 86.96%, specificity 91.67%).
In a study from 2021, Zalewska et al. [38] confirmed previously mentioned results. Indeed, in a smaller sample, LF levels, measured in stimulated whole saliva and analysed in µg/mg protein unit, significantly decreased in patients suffering from AD compared to non-demented controls. In this case, AUC was 0.6896. Again, no considerable relationships were observed between LF concentrations and disease stages. Opposite findings were presented in research by Gleerup et al. [40] from the same year. In a cohort of 222 participants, 71 AD patients were included. Surprisingly, no statistically significant differences between diagnostic groups were observed. Moreover, salivary LF tended to increase in AD patients compared to healthy controls. Standardisation by the total protein concentration in saliva did not reveal considerable results. The authors suggested that the inconsistency with previous studies may have appeared due to the inclusion of more heterogeneous and milder cases, which might have contributed to LF variations in their research.
4.4. Acetylcholinesterase, Pseudocholinesterase, Cholinesterase
Acetylcholinesterase (AChE) is an enzyme belonging to the serine hydrolases class, which is responsible for hydrolysing acetylcholine into choline and acetic acid and, therefore, finishing the action of this neurotransmitter [65]. AChE expression is performed in several forms, including homomeric and hetero-oligomeric states. This process can be observed in various tissues: peripheral and central nervous system neurons, skeletal muscles, and endocrine or exocrine glands [66]. AChE is considered a key target for the pharmacological treatment of AD, which is focused on the inhibitors of the hydrolysis of the neurotransmitter acetylcholine [67]. Additionally, higher AChE activity has been observed in several diseases, such as lung cancer, glaucoma, ALS, Hirschsprung’s disease, pesticide poisoning, neurotoxicity, or essential hypertension [68,69,70].
Ahmadi-Motamayel et al. [42] investigated AChE activity in patients with AD and non-demented controls. Moreover, the authors measured the activity of pseudocholinesterase (PChE), which is a sister enzyme of AChE hydrolysing exogenous choline-based esters [42,71,72]. In a group of AD patients, salivary AChE and PChE activities were significantly elevated compared to healthy subjects. Furthermore, the increase in activity was higher in males than females, but this difference was insignificant.
Another research analysed AChE activity in the sample of 15 AD patients and 15 healthy controls. Surprisingly, AChE activity was lower in the AD group compared to controls; however, the difference was not significant. Neither age nor disease duration were clearly associated with AChE activity. Moreover, in contrast to the previous study, enzyme activity was generally lower in males than in females. It is noteworthy that all patients were on therapy with memantine, which is a neurological drug that does not inhibit AChE [43]. Discrepancies between these two studies [42,43] are difficult to explain; however, unclear methods of diagnosis establishment, memantine therapy, and differences in the number of study participants might have influenced the results.
On the other hand, Tvarijonaviciute et al. [37] investigated salivary levels of cholinesterase. AD patients tended to have elevated levels of this enzyme compared to the control group; however, the results were not statistically significant.
4.5. Cortisol
Cortisol is the leading glucocorticoid hormone secreted by the adrenal cortex, fluctuating during the day [73,74]. Elevated cortisol level is associated with worse prognosis and the rapid progress of cognitive impairment in patients suffering from AD in the early stages or even the preclinical phase of the disease. Cortisol may contribute to the pathophysiology of AD by increasing both tau and Aβ pathologies as well as oxidative stress [75].
In 2008, De Souza-Talarico et al. [44] investigated salivary cortisol levels in mild AD patients (40 cases) and cognitively normal subjects (also 40 participants). Using a radioimmunoassay kit, AD patients presented significantly elevated salivary cortisol concentrations compared to controls. Slightly different times at sample collection between groups did not affect the results significantly. Interestingly, no significant correlation was observed between cortisol levels and working memory tests; however, AD patients with higher cortisol levels tended to have worse scores on one of the tests.
Different results were presented in another study published eleven years later. Peña-Bautista et al. [45] classified 97 participants into the AD group, consisting of both mild AD and MCI patients, who had positive neuroimaging and CSF biomarkers results. No significant association between AD and cortisol concentration in saliva was observed. Nevertheless, salivary cortisol levels in the AD group were increased compared to non-AD controls.
4.6. Biomarkers Related to Inflammation, Oxidative Stress or Redox Imbalance
Inflammation is clearly associated with AD pathology. Damage via various inflammatory mechanisms cumulates over years of disease progression and might considerably exacerbate pathogenic processes in this disorder [76]. Several factors participating in neuroinflammation concerning AD have been described, including cytokines, chemokines, caspases, complement system, and others [77].
Returning to research by Tvarijonaviciute et al. [37], several inflammation-related substances were also investigated. Salivary levels of haptoglobin, adenosine deaminase, and the ferric-reducing ability of plasma were decreased, whereas macrophage inflammatory protein-4, α1-antitrypsin, complement C4, and pigment epithelium-derived protein levels were increased in AD patients compared to controls. Nevertheless, only complement C4 alterations were considered significant.
On the other hand, Katsipis et al. [31] analysed salivary concentrations of glial fibrillary acidic protein (GFAP), interleukin-1β (IL-1β), IL-6, TNF-α, COX-2, and caspase-8. Interestingly, all these compounds presented significant changes between diagnostic groups. Levels of GFAP, COX-2, and caspase-8 were decreased, while IL-1β, IL-6, and TNF-α increased in patients affected by AD compared to MCI patients or healthy controls. The ROC analysis for distinguishing diagnostic groups revealed satisfactory results: between AD patients and healthy controls, AUC reached 0.998 or 1.000 (dot blot and ELISA methods, respectively), and between AD and MCI patients, AUC was 0.805 or 0.865 (dot blot and ELISA methods, respectively). Furthermore, a significant negative correlation between GFAP levels and COX-2, caspase-8, Aβ42, and p-tau concentrations was found. Analogically, a significant positive association was noted in regard to TNF-α, IL-1β, and IL-6 levels as well as the MMSE score.
Another study by Zalewska et al. [38] focused on several biomarkers related to inflammation, oxidative stress, or redox imbalance. Only stimulated saliva was used in this study. The ROC analysis indicated that salivary catalase, glutathione, glutathione peroxidase, the mean total antioxidant capacity/mean total oxidant status ratio (OSI), advanced glycation end products (AGEs), and IL-1β could be used to distinguish between AD patients and healthy controls clearly. The activity of salivary superoxide dismutase, glutathione peroxidase, and catalase as well as glutathione concentrations were significantly lower in the AD group compared to controls. In turn, NO, advanced oxidation protein products, AGEs, malondialdehyde, peroxynitrite, IL-1β, and nitrotyrosine concentrations, mean total oxidant status, and OSI were considerably increased in the same pattern. Moreover, a statistically significant association between the reduced activity of salivary peroxidase or superoxide dismutase and time elapsed from diagnosis of AD was observed.
On the other hand, McNicholas et al. [49] investigated the salivary levels of five inflammatory biomarkers (cystatin-C, IL-1 receptor antagonist, stratifin, haptoglobin, and matrix metalloproteinase 9) in a group of 16 AD, 15 MCI patients, and 29 non-demented controls. In general, cystatin-C, IL-1 receptor antagonist, and stratifin showed lower abundance in MCI and AD groups, whereas concentrations of haptoglobin and matrix metalloproteinase 9 were elevated. The results indicated that the levels of four of these biomarkers (without haptoglobin), adjusted for total salivary protein, were significantly altered in the AD group compared to healthy subjects, whereas only the absolute levels of haptoglobin and matrix metalloproteinase 9 were significantly changed in this comparison. Interestingly, in the MCI group, the absolute levels of all five biomarkers were significantly different compared to cognitively normal participants; however, after adjusting for total protein concentration, this significance dropped. Nevertheless, a panel consisting of the base model (only age, gender and APOEε4 allele status), cystatin-C, and IL-1 receptor antagonist (both adjusted for total protein concentration) showed excellent performance in distinguishing between AD patients and healthy controls (AUC 0.97). When matrix metalloproteinase 9 (adjusted for total protein concentration) and total protein concentration were added to this panel, it proved similar results in discriminating between either MCI or AD patients and non-demented individuals (AUC 0.97).
4.7. Amino Acids and Derivatives
Amino acids play an essential role in providing communication between neurons. These compounds can contribute to neurotransmission, acting as neurotransmitters, precursors, or neuromodulators [78]. Amino acids derivatives form an interesting group with a broad correlation spectrum, including obesity or neurological diseases [78,79,80,81]. Evidence shows that patients suffering from AD have impaired neurotransmission, which might be a result of a previously described accumulation of pathological compounds [82,83].
Interestingly, Peña-Bautista et al. [54] measured salivary levels of several amino acids and derivatives. Participants were divided into healthy controls (12 individuals) and the AD group, which consisted of patients with MCI due to AD and mild or moderate dementia due to AD (17 and 14 participants, respectively). Salivary acetylcholine levels were significantly higher in patients with mild AD than in controls, whereas creatine and myoinositol presented significantly lower concentrations in the AD group. Moreover, salivary levels of myoinositol, acetylcholine, glutamine, and creatine were significantly correlated with neuropsychological scales. In addition, myoinositol was considerably associated with CSF Aβ level. The performed ROC analysis revealed relatively satisfactory accuracy of glutamine and acetylcholine (AUC 0.777 and 0.660, respectively). Nevertheless, a multivariate analysis with combinations of previously mentioned biomarkers indicated that a set of all these compounds (myoinositol, glutamine, creatine, acetylcholine) showed the best performance and might be used to distinguish between AD patients and healthy subjects (AUC 0.806, sensitivity 61%, specificity 92%). In this study, only glutamine presented significant differences between genders.
In more recent research by Marksteiner et al. [33], apart from previously described tau and Aβ, norepinephrine concentrations were also investigated. The performed HPLC-EC method analysis revealed a significant decrease in salivary norepinephrine levels in AD patients compared with healthy controls.
4.8. miRNAs and Sirtuins
MicroRNAs (miRNAs) form a group of small endogenous non-coding RNA that regulates target gene expression [55,84]. Ryu et al. [55] investigated miRNA-485-3p concentrations in salivary exosome-enriched extracellular vesicles (EE-EV) of 27 AD patients and 13 healthy controls. The results revealed that miRNA-485-3p concentrations in salivary EE-EV from AD patients were significantly elevated compared to the control group. The ROC analysis regarding differentiating between AD and healthy individuals showed good performance of this biomarker: AUC 0.895. Moreover, statistically significant associations were observed between miRNA-485-3p concentrations in salivary EE-EV and MMSE or Aβ PET results with a stronger association with the latter ones (AUC 0.754 and 0.922, respectively).
Sirtuins (SIRT) belong to the histone deacetylases group and regulate processes like cell metabolism or gene expression via epigenetic mechanisms. Moreover, these enzymes might have neuroprotective effects [50,85]. Pukhalskaia et al. [50] enrolled 58 healthy participants and 64 AD patients in the initial or moderate stage of the disease. The results showed that SIRT1, SIRT3, and SIRT6 levels were significantly lower in the AD group compared to controls, while SIRT5 did not differ significantly. Among these biomarkers, SIRT1 and SIRT6 changed most considerably between diagnostic groups. Except for SIRT5, the rest of the mentioned SIRT significantly decreased along with patients’ age, while only SIRT1 and SIRT6 were significantly lower in older healthy subjects.
4.9. Trehalose
Trehalose is a natural disaccharide which exhibits neuroprotective effects via several potential ways, including an induction of autophagy or modulation of inflammatory responses [86]. Lau et al. [32] used an improved extended gate ion-sensitive field-effect transistor (EG-ISFET) to measure salivary trehalose levels in patients suffering from AD or PD and healthy controls. The findings showed that salivary trehalose levels were higher in AD patients compared to other diagnostic groups. Furthermore, the authors stated that using the EG-ISFET method, salivary trehalose levels of the AD group could be clearly distinguished from other diagnostic groups.
4.10. Metabolomics and Proteomics Panel Studies
Metabolomics, which analyses and profiles metabolites in biofluids, aids in the understanding of interactions between molecules and provides insights into mechanisms underlying diseases [87,88]. Similarly, proteomics evaluates both the structures and functions of proteins to better understand their characteristics in the organism [89]. In recent years, omics research has rapidly evolved and is predicted to develop even further [90].
In 2018, Huan et al. [51] developed a salivary diagnostic model of AD based on a metabolomic approach. A total sample of 109 participants (35 cognitively healthy, 25 MCI, and 22 AD patients) was divided into two phases: discovery (to determine the most significant metabolites that differentiate paired groups) and validation (to provisionally validate selected significant metabolites detected in the discovery phase). Using top-ranked but putatively identified biomarkers, a three-element panel to distinguish between AD and healthy controls was designed and consisted of methylguanosine, choline-cytidine, and histidinyl-phenylalanine. A similar panel for discriminating between AD and MCI groups included amino-dihydroxybenzene, glucosylgalactosyl hydroxylysine—H2O, and aminobutyric acid + H2. The performed ROC analysis revealed excellent results (overall AUC 0.997, sensitivity 98.52%, specificity 96.55%, and AUC 0.993, sensitivity 100%, specificity 97.70%, respectively). Analogically, using positively identified biomarkers, the designed panels included the following: phenylalanyl-proline, phenylalanylphenylalanine, urocanic acid (AD versus controls), and alanyl-phenylalanine with phenylalanyl-proline (AD versus MCI) (AUC 0.831, sensitivity 82.22%, specificity 73.56%, and AUC 0.843, sensitivity 81.90%, specificity 72.41%, respectively).
One year later, Marksteiner et al. [53] used targeted metabolomics to study salivary metabolomic changes in AD, MCI patients, and cognitively normal individuals; each group consisted of 25 participants. The results showed decreased salivary acyl-alkyl-phosphatidyl cholines (PCae) concentrations in AD and MCI groups compared to the control group. However, only alterations in PCae C34:1-2, PCae C36:1-2-3, PCaeCC38:1–3, and PCae C40:2-3 reached significant differences when comparing AD patients and healthy subjects. It is noteworthy that the significance was especially high when all these compounds were combined. Moreover, decreased salivary levels of PCae C36:1-2-3 significantly distinguish MCI patients from controls.
Another study investigated the metabolomic and proteomic parameters of saliva collected from 80 participants (20 AD, 20 MCI patients, and 40 cognitively normal controls). Statistical analysis revealed that 79 metabolites and 346 proteins were significantly altered in a comparison between AD and control groups. Interestingly, in the MCI group, 374 proteins and only six metabolites were considered significant compared to controls. All metabolites whose levels differed significantly between the MCI/AD and control groups (L-fucose, L-tyrosine, L-ornithine, L-aspartate, rhamnose, and serotonin) were upregulated (fold change > 2.0) [48].
Interestingly, another proteomic study, described earlier in the tau section, identified transthyretin as a potential biomarker of AD. Proteomic analysis showed a significant decrease in salivary transthyretin in AD patients, which was additionally confirmed by Western blot. The results revealed a 0.5-fold reduction in both MCI and AD groups compared to the cognitively normal subjects [47]. Transthyretin is considered a highly amyloidogenic protein that is responsible for creating amyloid deposits in the nerves, heart, arterioles, or ligaments [91]. In contrast, this protein is also believed to be a neuroprotective factor in AD due to its interaction with Aβ and decrease in Aβ aggregation [92].
On the other hand, Liang et al. [52] performed metabolomic screening on the sample of 256 AD patients and 218 cognitively normal controls to determine salivary biomarkers of early AD. The results indicated that six biomarkers significantly differed between diagnostic groups: inosine and 3-dehydrocarnitine were downregulated, while sphinganine-1-phosphate, ornithine, hypoxanthine, and phenyllactic acid were upregulated in the AD group compared with controls. Furthermore, the ROC analysis revealed that sphinganine-1-phosphate, ornithine, and phenyllactic acid seem most promising as salivary biomarkers of AD (AUC 0.998, sensitivity 99.4%, specificity 98.2%; AUC, 0.927 sensitivity 81.9%, specificity 90.7%; AUC 0.831, sensitivity 79.5%, specificity 84.3%, respectively); whereas inosine, 3-dehydrocarnitine, and hypoxanthine proved worse performance (AUC 0.740, sensitivity 66.8%, specificity 77.0%; AUC 0.669, sensitivity 57.4%, specificity 84.2%; AUC 0.674, sensitivity 53.7%, specificity 73.9%, respectively).
In a recent study, Contini et al. [46] enrolled 36 patients affected by PD, 36 healthy controls, and 35 AD patients (13 in moderate and 22 in mild disease stage). Using a proteomic approach, significant differences between various compounds in diagnostic groups were observed. AD patients had significantly higher abundances of thymosin β4, α-defensins—1, 2, 3, and sum of α-defensins, histatin 1 mono- and non-phosphorylated, statherin 2P, des 1-9 and des 1-13, P-C peptide, cystatin A, B-SSG, total cystatin B monomer, cystatin B S-S dimer, total cystatin B, S100A8-SNO, sum of S100A8-A8SNO, S100A9s, sum of S100A9s, and total S100A9 (s + l) compared to controls, and there were analogically significantly lower abundances of PRP1 0P. Moreover, 24 biomarkers were determined to distinguish between patients suffering from AD and PD—respectively, higher expression of α-defensins—1, 2, and sum, Hst1, Hst1 0P, Hst5, Hst6, statherin 2P, 1P, desD1, des1-9, des1-10 and des1-13, PRP1 1P, PRP3 2P, PRP3 1P, P-C peptide, cystatin SN and S100A9sox, and lower expression of SLPI, PB des1-5 and des1-12, SV1 and cystatin SA.
4.11. PD-Related Biomarkers in AD
One of the primary hallmarks of PD is α-synuclein [93]. Interestingly, in a previously mentioned study, Sabaei et al. [35] observed significantly decreased salivary total α-synuclein levels in AD patients compared to healthy controls either without age-confounding variables or after adjusting for age. Nevertheless, the ROC analysis with a cut-off point equal to 9.4 pg/mL did not prove the high reliability of this biomarker in AD diagnosis (AUC 0.71, sensitivity 66.7%, specificity 68.2%).
On the other hand, heme oxygenase-1 (HO-1) is associated with both AD and PD, since HO-1 dysregulation is linked with neuroinflammation presented in both disorders [94]. In a study by Galindez et al. [95], patients suffering from both diseases were included along with patients affected by other neurological disorders and healthy controls. Importantly, AD patients were combined together with MCI patients in one group. The results indicated that this group had significantly higher salivary HO-1 levels than healthy controls. After combining AD, MCI, and PD patients in one group (neurodegenerative) and non-neurodegenerative individuals in another, the ROC analysis revealed satisfactory results in distinguishing between neurodegenerative and non-neurodegenerative subjects (AUC 0.86, sensitivity 79%, specificity 80%).
4.12. Study Limitations
The limitations of the study include the heterogeneity of the included studies in terms of diagnostic methods and inclusion and exclusion criteria for participants (e.g., demographic characteristics, diagnosis criteria). Only some researchers conducted and reported the results of ROC analysis to assess the predictive reliability of potential salivary markers. Moreover, the diversity of the biomarkers studied made it difficult to compare their usefulness. In general, we meta-analysed repeated markers, but the others that appeared in individual studies were also discussed.
5. Conclusions
In conclusion, some potential biomarkers such as beta-amyloid42, t-tau, p-tau and lactoferrin could be detected in the saliva of patients with Alzheimer’s Disease. Therefore, these protein molecules could reliably support the early diagnosis of neurodegenerative diseases. However, further research is necessary to confirm these findings and to search for the predictive ability of other substances.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25021168/s1.
Author Contributions
Conceptualisation, K.N. and W.O.; methodology, K.N.; formal analysis, K.N., W.O. and J.J.; investigation and data curation, K.N. and W.O.; writing—original draft preparation, K.N. and W.O.; writing—review and editing, K.N. and J.J.; visualisation, K.N.; supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and Cellular Mechanisms Underlying the Pathogenesis of Alzheimer’s Disease. Mol. Neurodegener. 2020;15:40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X.-X., Tian Y., Wang Z.-T., Ma Y.-H., Tan L., Yu J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021;8:313–321. doi: 10.14283/jpad.2021.15. [DOI] [PubMed] [Google Scholar]
- 3.Porsteinsson A.P., Isaacson R.S., Knox S., Sabbagh M.N., Rubino I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021;8:371–386. doi: 10.14283/jpad.2021.23. [DOI] [PubMed] [Google Scholar]
- 4.Lane C.A., Hardy J., Schott J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Martinez L., Calfío C., Farias G.A., Vilches C., Prieto R., Maccioni R.B. New Frontiers in the Prevention, Diagnosis, and Treatment of Alzheimer’s Disease. J. Alzheimers Dis. 2021;82:S51–S63. doi: 10.3233/JAD-201059. [DOI] [PubMed] [Google Scholar]
- 6.Passeri E., Elkhoury K., Morsink M., Broersen K., Linder M., Tamayol A., Malaplate C., Yen F.T., Arab-Tehrany E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022;23:13954. doi: 10.3390/ijms232213954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahaman Y.A.R., Embaye K.S., Huang F., Li L., Zhu F., Wang J.-Z., Liu R., Feng J., Wang X. Biomarkers Used in Alzheimer’s Disease Diagnosis, Treatment, and Prevention. Ageing Res. Rev. 2022;74:101544. doi: 10.1016/j.arr.2021.101544. [DOI] [PubMed] [Google Scholar]
- 8.Peng L., Bestard-Lorigados I., Song W. The Synapse as a Treatment Avenue for Alzheimer’s Disease. Mol. Psychiatry. 2022;27:2940–2949. doi: 10.1038/s41380-022-01565-z. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z., Zhong C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song T., Song X., Zhu C., Patrick R., Skurla M., Santangelo I., Green M., Harper D., Ren B., Forester B.P., et al. Mitochondrial Dysfunction, Oxidative Stress, Neuroinflammation, and Metabolic Alterations in the Progression of Alzheimer’s Disease: A Meta-Analysis of in Vivo Magnetic Resonance Spectroscopy Studies. Ageing Res. Rev. 2021;72:101503. doi: 10.1016/j.arr.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klyucherev T.O., Olszewski P., Shalimova A.A., Chubarev V.N., Tarasov V.V., Attwood M.M., Syvänen S., Schiöth H.B. Advances in the Development of New Biomarkers for Alzheimer’s Disease. Transl. Neurodegener. 2022;11:25. doi: 10.1186/s40035-022-00296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Huang D., Cai Y., Cao Z., Liu Z., Zhang S., Zhao L., Wang X., Wang Y., Huang F., et al. Saliva Diagnostics: Emerging Techniques and Biomarkers for Salivaomics in Cancer Detection. Expert Rev. Mol. Diagn. 2022;22:1077–1097. doi: 10.1080/14737159.2022.2167556. [DOI] [PubMed] [Google Scholar]
- 14.Aragón F., Zea-Sevilla M.A., Montero J., Sancho P., Corral R., Tejedor C., Frades-Payo B., Paredes-Gallardo V., Albaladejo A. Oral Health in Alzheimer’s Disease: A Multicenter Case-Control Study. Clin. Oral Investig. 2018;22:3061–3070. doi: 10.1007/s00784-018-2396-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C.-Z., Cheng X.-Q., Li J.-Y., Zhang P., Yi P., Xu X., Zhou X.-D. Saliva in the Diagnosis of Diseases. Int. J. Oral Sci. 2016;8:133–137. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijakowski K., Zdrojewski J., Nowak M., Gruszczyński D., Knoll F., Surdacka A. Salivary Metabolomics for Systemic Cancer Diagnosis: A Systematic Review. Metabolites. 2022;13:28. doi: 10.3390/metabo13010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijakowski K., Surdacka A. Salivary Biomarkers for Diagnosis of Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2020;21:7477. doi: 10.3390/ijms21207477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortarzewska M., Nijakowski K., Kolasińska J., Gruszczyński D., Ruchała M.A., Lehmann A., Surdacka A. Salivary Alterations in Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Environ. Res. Public Health. 2023;20:4849. doi: 10.3390/ijerph20064849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijakowski K., Surdacki M., Sobieszczańska M. Salivary Melatonin Changes in Oncological Patients: A Systematic Review. Metabolites. 2022;12:439. doi: 10.3390/metabo12050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijakowski K., Jankowski J., Gruszczyński D., Surdacka A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2023;24:12078. doi: 10.3390/ijms241512078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekalarska-Dębek M., Dąbrowska M., Surdacka A. Biomarkers of Alzheimer’s Disease in Saliva—A Literature Review. Dent. Forum. 2017;45:83–87. [Google Scholar]
- 22.Zürcher C., Humpel C. Saliva: A Challenging Human Fluid to Diagnose Brain Disorders with a Focus on Alzheimer’s Disease. Neural Regen. Res. 2023;18:2606–2610. doi: 10.4103/1673-5374.373675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page M.J., McKenzie J., Bossuyt P., Boutron I., Hoffmann T., Mulrow C.D., Shamseer L., Tetzlaff J., Akl E., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Study Quality Assessment Tools|NHLBI, NIH. [(accessed on 22 August 2020)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 25.OCEBM Levels of Evidence. [(accessed on 22 August 2020)]. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/
- 26.Ashton N.J., Ide M., Zetterberg H., Blennow K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019;8:83–94. doi: 10.1007/s40120-019-00168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bermejo-Pareja F., Antequera D., Vargas T., Molina J.A., Carro E. Saliva Levels of Abeta1-42 as Potential Biomarker of Alzheimer’s Disease: A Pilot Study. BMC Neurol. 2010;10:108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boschi S., Roveta F., Grassini A., Marcinnò A., Cermelli A., Ferrandes F., Rainero I., Rubino E. Aβ42 as a Biomarker of Alzheimer’s Disease: Is Saliva a Viable Alternative to Cerebrospinal Fluid? Brain Sci. 2022;12:1729. doi: 10.3390/brainsci12121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y., Zhang H., Zhu J., Liao Z., Wang S., Liu W. Investigation of Whole and Glandular Saliva as a Biomarker for Alzheimer’s Disease Diagnosis. Brain Sci. 2022;12:595. doi: 10.3390/brainsci12050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dos Santos G.A.A., Olave E., Pardi P.C. Salivary Biomarkers in Alzheimer´s Disease; [Biomarcadores Salivales En La Enfermedad de Alzheimer] Int. J. Morphol. 2020;38:230–234. doi: 10.4067/S0717-95022020000100230. [DOI] [Google Scholar]
- 31.Katsipis G., Tzekaki E.E., Tsolaki M., Pantazaki A.A. Salivary GFAP as a Potential Biomarker for Diagnosis of Mild Cognitive Impairment and Alzheimer’s Disease and Its Correlation with Neuroinflammation and Apoptosis. J. Neuroimmunol. 2021;361:577744. doi: 10.1016/j.jneuroim.2021.577744. [DOI] [PubMed] [Google Scholar]
- 32.Lau H.-C., Lee I.-K., Ko P.-W., Lee H.-W., Huh J.-S., Cho W.-J., Lim J.-O. Non-Invasive Screening for Alzheimer’s Disease by Sensing Salivary Sugar Using Drosophila Cells Expressing Gustatory Receptor (Gr5a) Immobilized on an Extended Gate Ion-Sensitive Field-Effect Transistor (EG-ISFET) Biosensor. PLoS ONE. 2015;10:e0117810. doi: 10.1371/journal.pone.0117810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marksteiner J., Defrancesco M., Humpel C. Saliva Tau and Phospho-Tau-181 Measured by Lumipulse in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2022;14:1014305. doi: 10.3389/fnagi.2022.1014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pekeles H., Qureshi H.Y., Paudel H.K., Schipper H.M., Gornistky M., Chertkow H. Development and Validation of a Salivary Tau Biomarker in Alzheimer’s Disease. Alzheimers Dement. 2019;11:53–60. doi: 10.1016/j.dadm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabaei M., Rahimian S., Haj Mohamad Ebrahim Ketabforoush A., Rasoolijazi H., Zamani B., Hajiakhoundi F., Soleimani M., Shahidi G., Faramarzi M. Salivary Levels of Disease-Related Biomarkers in the Early Stages of Parkinson’s and Alzheimer’s Disease: A Cross-Sectional Study. IBRO Neurosci. Rep. 2023;14:285–292. doi: 10.1016/j.ibneur.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi M., Sui Y.-T., Peskind E.R., Li G., Hwang H., Devic I., Ginghina C., Edgar J.S., Pan C., Goodlett D.R., et al. Salivary Tau Species Are Potential Biomarkers of Alzheimer Disease. J. Alzheimers Dis. 2011;27:299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tvarijonaviciute A., Zamora C., Ceron J.J., Bravo-Cantero A.F., Pardo-Marin L., Valverde S., Lopez-Jornet P. Salivary Biomarkers in Alzheimer’s Disease. Clin. Oral Investig. 2020;24:3437–3444. doi: 10.1007/s00784-020-03214-7. [DOI] [PubMed] [Google Scholar]
- 38.Zalewska A., Klimiuk A., Zięba S., Wnorowska O., Rusak M., Waszkiewicz N., Szarmach I., Dzierżanowski K., Maciejczyk M. Salivary Gland Dysfunction and Salivary Redox Imbalance in Patients with Alzheimer’s Disease. Sci. Rep. 2021;11:23904. doi: 10.1038/s41598-021-03456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carro E., Bartolomé F., Bermejo-Pareja F., Villarejo-Galende A., Molina J.A., Ortiz P., Calero M., Rabano A., Cantero J.L., Orive G. Early Diagnosis of Mild Cognitive Impairment and Alzheimer’s Disease Based on Salivary Lactoferrin. Alzheimers Dement. 2017;8:131–138. doi: 10.1016/j.dadm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleerup H.S., Jensen C.S., Høgh P., Hasselbalch S.G., Simonsen A.H. Lactoferrin in Cerebrospinal Fluid and Saliva Is Not a Diagnostic Biomarker for Alzheimer’s Disease in a Mixed Memory Clinic Population. EBioMedicine. 2021;67:103361. doi: 10.1016/j.ebiom.2021.103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-Sánchez M., Bartolome F., Antequera D., Puertas-Martín V., González P., Gómez-Grande A., Llamas-Velasco S., Herrero-San Martín A., Pérez-Martínez D., Villarejo-Galende A., et al. Decreased Salivary Lactoferrin Levels Are Specific to Alzheimer’s Disease. EBioMedicine. 2020;57:102834. doi: 10.1016/j.ebiom.2020.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmadi-Motamayel F., Goodarzi M.T., Tarazi S., Vahabian M. Evaluation of Salivary Acetylcholinesterase and Pseudocholinesterase in Patients with Alzheimer’s Disease: A Case-Control Study. Spec. Care Dent. 2019;39:39–44. doi: 10.1111/scd.12342. [DOI] [PubMed] [Google Scholar]
- 43.Bakhtiari S., Moghadam N.B., Ehsani M., Mortazavi H., Sabour S., Bakhshi M. Can Salivary Acetylcholinesterase Be a Diagnostic Biomarker for Alzheimer? J. Clin. Diagn. Res. 2017;11:ZC58–ZC60. doi: 10.7860/JCDR/2017/21715.9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Souza-Talarico J.N., Caramelli P., Nitrini R., Chaves E.C. Effect of Cortisol Levels on Working Memory Performance in Elderly Subjects with Alzheimer’s Disease. Arq. De Neuro-Psiquiatr. 2008;66:619–624. doi: 10.1590/s0004-282x2008000500003. [DOI] [PubMed] [Google Scholar]
- 45.Peña-Bautista C., Baquero M., Ferrer I., Hervás D., Vento M., García-Blanco A., Cháfer-Pericás C. Neuropsychological Assessment and Cortisol Levels in Biofluids from Early Alzheimer’s Disease Patients. Exp. Gerontol. 2019;123:10–16. doi: 10.1016/j.exger.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Contini C., Fadda L., Lai G., Masala C., Olianas A., Castagnola M., Messana I., Iavarone F., Bizzarro A., Masullo C., et al. A Top-down Proteomic Approach Reveals a Salivary Protein Profile Able to Classify Parkinson’s Disease with Respect to Alzheimer’s Disease Patients and to Healthy Controls. Proteomics. 2023:e2300202. doi: 10.1002/pmic.202300202. [DOI] [PubMed] [Google Scholar]
- 47.Eldem E., Barve A., Sallin O., Foucras S., Annoni J.-M., Schmid A.W., Alberi Auber L. Salivary Proteomics Identifies Transthyretin as a Biomarker of Early Dementia Conversion. J. Alzheimers Dis. Rep. 2022;6:31–41. doi: 10.3233/ADR-210056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.François M., Karpe A., Liu J.-W., Beale D., Hor M., Hecker J., Faunt J., Maddison J., Johns S., Doecke J., et al. Salivaomics as a Potential Tool for Predicting Alzheimer’s Disease During the Early Stages of Neurodegeneration. J. Alzheimers Dis. 2021;82:1301–1313. doi: 10.3233/JAD-210283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNicholas K., François M., Liu J.-W., Doecke J.D., Hecker J., Faunt J., Maddison J., Johns S., Pukala T.L., Rush R.A., et al. Salivary Inflammatory Biomarkers Are Predictive of Mild Cognitive Impairment and Alzheimer’s Disease in a Feasibility Study. Front. Aging Neurosci. 2022;14:1019296. doi: 10.3389/fnagi.2022.1019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pukhalskaia A.E., Dyatlova A.S., Linkova N.S., Kozlov K.L., Kvetnaia T.V., Koroleva M.V., Kvetnoy I.M. Sirtuins as Possible Predictors of Aging and Alzheimer’s Disease Development: Verification in the Hippocampus and Saliva. Bull Exp. Biol. Med. 2020;169:821–824. doi: 10.1007/s10517-020-04986-4. [DOI] [PubMed] [Google Scholar]
- 51.Huan T., Tran T., Zheng J., Sapkota S., MacDonald S.W., Camicioli R., Dixon R.A., Li L. Metabolomics Analyses of Saliva Detect Novel Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2018;65:1401–1416. doi: 10.3233/JAD-180711. [DOI] [PubMed] [Google Scholar]
- 52.Liang Q., Liu H., Zhang T., Jiang Y., Xing H., Zhang A.-H. Metabolomics-Based Screening of Salivary Biomarkers for Early Diagnosis of Alzheimer’s Disease. RSC Adv. 2015;5:96074–96079. doi: 10.1039/C5RA19094K. [DOI] [Google Scholar]
- 53.Marksteiner J., Oberacher H., Humpel C. Acyl-Alkyl-Phosphatidlycholines Are Decreased in Saliva of Patients with Alzheimer’s Disease as Identified by Targeted Metabolomics. J. Alzheimers Dis. 2019;68:583–589. doi: 10.3233/JAD-181278. [DOI] [PubMed] [Google Scholar]
- 54.Peña-Bautista C., Torres-Cuevas I., Baquero M., Ferrer I., García L., Vento M., Cháfer-Pericás C. Early Neurotransmission Impairment in Non-Invasive Alzheimer Disease Detection. Sci. Rep. 2020;10:16396. doi: 10.1038/s41598-020-73362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryu I.S., Kim D.H., Ro J.-Y., Park B.-G., Kim S.H., Im J.-Y., Lee J.-Y., Yoon S.J., Kang H., Iwatsubo T., et al. The microRNA-485-3p Concentration in Salivary Exosome-Enriched Extracellular Vesicles Is Related to Amyloid β Deposition in the Brain of Patients with Alzheimer’s Disease. Clin. Biochem. 2023;118:110603. doi: 10.1016/j.clinbiochem.2023.110603. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y.-R., Liu R.-T. The Toxicity and Polymorphism of β-Amyloid Oligomers. Int. J. Mol. Sci. 2020;21:4477. doi: 10.3390/ijms21124477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouras G.K., Olsson T.T., Hansson O. β-Amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics. 2015;12:3–11. doi: 10.1007/s13311-014-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avila J., Lucas J.J., Perez M., Hernandez F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004;84:361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 59.Peineau S., Rabiant K., Pierrefiche O., Potier B. Synaptic Plasticity Modulation by Circulating Peptides and Metaplasticity: Involvement in Alzheimer’s Disease. Pharmacol. Res. 2018;130:385–401. doi: 10.1016/j.phrs.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Naseri N.N., Wang H., Guo J., Sharma M., Luo W. The Complexity of Tau in Alzheimer’s Disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duquette A., Pernègre C., Veilleux Carpentier A., Leclerc N. Similarities and Differences in the Pattern of Tau Hyperphosphorylation in Physiological and Pathological Conditions: Impacts on the Elaboration of Therapies to Prevent Tau Pathology. Front. Neurol. 2020;11:607680. doi: 10.3389/fneur.2020.607680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Lu C., Zhang J. Lactoferrin and Its Detection Methods: A Review. Nutrients. 2021;13:2492. doi: 10.3390/nu13082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang B., Timilsena Y.P., Blanch E., Adhikari B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019;59:580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- 64.Yong S.J., Veerakumarasivam A., Lim W.L., Chew J. Neuroprotective Effects of Lactoferrin in Alzheimer’s and Parkinson’s Diseases: A Narrative Review. ACS Chem. Neurosci. 2023 doi: 10.1021/acschemneuro.2c00679. [DOI] [PubMed] [Google Scholar]
- 65.Thapa S., Lv M., Xu H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini Rev. Med. Chem. 2017;17:1665–1676. doi: 10.2174/1389557517666170120153930. [DOI] [PubMed] [Google Scholar]
- 66.Rotundo R.L. Biogenesis, Assembly and Trafficking of Acetylcholinesterase. J. Neurochem. 2017;142((Suppl. S2)):52–58. doi: 10.1111/jnc.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxena M., Dubey R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019;19:264–275. doi: 10.2174/1568026619666190128125912. [DOI] [PubMed] [Google Scholar]
- 68.Lionetto M.G., Caricato R., Calisi A., Giordano M.E., Schettino T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. Biomed Res. Int. 2013;2013:321213. doi: 10.1155/2013/321213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saldanha C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules. 2017;22:1499. doi: 10.3390/molecules22091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xi H.-J., Wu R.-P., Liu J.-J., Zhang L.-J., Li Z.-S. Role of Acetylcholinesterase in Lung Cancer. Thorac. Cancer. 2015;6:390–398. doi: 10.1111/1759-7714.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robles A., Michael M., McCallum R. Pseudocholinesterase Deficiency: What the Proceduralist Needs to Know. Am. J. Med. Sci. 2019;357:263–267. doi: 10.1016/j.amjms.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Delacour H., Dedome E., Courcelle S., Hary B., Ceppa F. Butyrylcholinesterase Deficiency. Ann. Biol. Clin. 2016;74:279–285. doi: 10.1684/abc.2016.1141. [DOI] [PubMed] [Google Scholar]
- 73.Lee D.Y., Kim E., Choi M.H. Technical and Clinical Aspects of Cortisol as a Biochemical Marker of Chronic Stress. BMB Rep. 2015;48:209–216. doi: 10.5483/BMBRep.2015.48.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adam E.K., Quinn M.E., Tavernier R., McQuillan M.T., Dahlke K.A., Gilbert K.E. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouanes S., Popp J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019;11:43. doi: 10.3389/fnagi.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., et al. Inflammation and Alzheimer’s Disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalangin R., Kim A., Campbell R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020;21:6197. doi: 10.3390/ijms21176197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seckler J.M., Lewis S.J. Advances in D-Amino Acids in Neurological Research. Int. J. Mol. Sci. 2020;21:7325. doi: 10.3390/ijms21197325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pashaei S., Yarani R., Mohammadi P., Emami Aleagha M.S. The Potential Roles of Amino Acids and Their Major Derivatives in the Management of Multiple Sclerosis. Amino Acids. 2022;54:841–858. doi: 10.1007/s00726-022-03162-4. [DOI] [PubMed] [Google Scholar]
- 81.Chen J.J., Thiyagarajah M., Song J., Chen C., Herrmann N., Gallagher D., Rapoport M.J., Black S.E., Ramirez J., Andreazza A.C., et al. Altered Central and Blood Glutathione in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis. Alzheimers Res. Ther. 2022;14:23. doi: 10.1186/s13195-022-00961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy J., Tsui K.C., Ng J., Fung M.-L., Lim L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:6841. doi: 10.3390/ijms22136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paula-Lima A.C., Brito-Moreira J., Ferreira S.T. Deregulation of Excitatory Neurotransmission Underlying Synapse Failure in Alzheimer’s Disease. J. Neurochem. 2013;126:191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 84.Ho P.T.B., Clark I.M., Le L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022;23:7167. doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carafa V., Rotili D., Forgione M., Cuomo F., Serretiello E., Hailu G.S., Jarho E., Lahtela-Kakkonen M., Mai A., Altucci L. Sirtuin Functions and Modulation: From Chemistry to the Clinic. Clin. Epigenetics. 2016;8:61. doi: 10.1186/s13148-016-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khalifeh M., Read M.I., Barreto G.E., Sahebkar A. Trehalose against Alzheimer’s Disease: Insights into a Potential Therapy. Bioessays. 2020;42:e1900195. doi: 10.1002/bies.201900195. [DOI] [PubMed] [Google Scholar]
- 87.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: Beyond Biomarkers and towards Mechanisms. Nat. Rev. Mol. Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muthubharathi B.C., Gowripriya T., Balamurugan K. Metabolomics: Small Molecules That Matter More. Mol. Omics. 2021;17:210–229. doi: 10.1039/d0mo00176g. [DOI] [PubMed] [Google Scholar]
- 89.Al-Amrani S., Al-Jabri Z., Al-Zaabi A., Alshekaili J., Al-Khabori M. Proteomics: Concepts and Applications in Human Medicine. World J. Biol. Chem. 2021;12:57–69. doi: 10.4331/wjbc.v12.i5.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai X., Shen L. Advances and Trends in Omics Technology Development. Front. Med. 2022;9:911861. doi: 10.3389/fmed.2022.911861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ueda M. Transthyretin: Its Function and Amyloid Formation. Neurochem. Int. 2022;155:105313. doi: 10.1016/j.neuint.2022.105313. [DOI] [PubMed] [Google Scholar]
- 92.Gião T., Saavedra J., Cotrina E., Quintana J., Llop J., Arsequell G., Cardoso I. Undiscovered Roles for Transthyretin: From a Transporter Protein to a New Therapeutic Target for Alzheimer’s Disease. Int. J. Mol. Sci. 2020;21:2075. doi: 10.3390/ijms21062075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha E.M., De Miranda B., Sanders L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018;109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Wu Y.-H., Hsieh H.-L. Roles of Heme Oxygenase-1 in Neuroinflammation and Brain Disorders. Antioxidants. 2022;11:923. doi: 10.3390/antiox11050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galindez J.M., Juwara L., Cressatti M., Gornitsky M., Velly A.M., Schipper H.M. Salivary Heme Oxygenase-1: A Potential Biomarker for Central Neurodegeneration. J. Cent. Nerv. Syst. Dis. 2021;13:11795735211029114. doi: 10.1177/11795735211029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author.