Abstract
The anatomical progression of chlamydial infection was studied in different areas of the placenta, using a mouse model and two inoculation times: early pregnancy (day 7, group A) and midpregnancy (day 11, group B). The first population cells affected were decidual cells and neutrophils located just at the limits of the maternal and fetal placenta. The following invaded area was the layer of giant cells. Complete colonization of the maternal placenta occurred after day 15 of pregnancy independently of the inoculation time, the metrial gland being the last area to be invaded; numerous granulated metrial gland (GMG) cells were infected. Finally, chlamydial inclusions were observed in labyrinthine trophoblastic cells from day 18 of pregnancy onward. Since no fetal damage was observed, it seems that an indirect mechanism involving the lysis of GMG cells and neutrophil infiltration of the decidua and metrial gland may be the pathogenic mechanism that leads to abortion.
Chlamydia psittaci serotype 1 is an obligate intracellular bacterium which can colonize very different types of placenta (ruminant, porcine, human, and murine), causing abortion during the last third of the gestation period. The disease is especially important in small ruminants, the natural hosts of the bacterium, because of the economic losses it causes. However, the bacteria also present a potential zoonotic risk to pregnant women, since several cases of human abortion followed by severe complications have been reported after exposure to goats or sheep in abattoirs or during lambing (11, 13, 28).
Chlamydiae show a unique growth cycle (17): infection begins with adhesion of an infectious elementary body (EB) to a susceptible cell, followed by phagocytosis within a phagosome. The EB is transformed into an active metabolic form, the reticulate body (RB), which divides by binary fission. The cycle ends with reorganization of RBs into EBs and the release of EBs, causing infection in nearby cells.
Previously we developed a model of chlamydial infection in pregnant mice (7, 21), in which intraperitoneal or intravenous inoculation with 105 to 106 PFU of C. psittaci serotype 1 caused abortion, usually by day 19 or 20 of pregnancy in nearly all of the pregnant mice inoculated. These late-term abortions appeared similar to those observed in cases of natural or experimentally induced abortion in small ruminants. In fact, this model has already been used to control the efficacy of commercial vaccines (20) and antigens suitable for vaccine development (10) or to measure the virulence of different strains (7, 21). In a preliminary study (23), we showed that C. psittaci infect the granulated metrial gland (GMG) cells, a lymphoid cell population, related to NK cells (18, 24), that are located during pregnancy mainly in the metrial gland and in the decidua basalis. These cells are the major lymphoid population associated with pregnancy. Their function is not clearly understood but, like their morphology, seems to vary during pregnancy. A putative defensive function against intracellular pathogens has been proposed (29), since these cells contain granules with cytotoxic molecules (perforins and serine esterases). Whatever the case may be, their functionality and number seem to decrease from day 15 of pregnancy onward (8, 9). Recently, several studies using different placental pathogens have been performed to show how the inoculation time influences the outcome of pregnancy (1, 2, 5, 16). However, there has been no description of the different areas of the placenta invaded by pathogens according to the time of infection, nor has it been observed whether these pathogens are able to invade GMG cells.
The aim of this work was to study which of the placental areas and cell populations are invaded by C. psittaci in an attempt to clarify the pathological mechanisms that cause abortion and to see whether there was any relationship with the time that inoculation was carried out.
MATERIALS AND METHODS
Microorganism.
The AB7 strain (21) of C. psittaci serotype 1 used in this study was isolated from an ovine abortion and was propagated in chicken embryo yolk sac in our laboratory. Chlamydiae were titered by a modification of the plaque-forming technique (3) on McCoy cells and stored at −80°C until use.
Mice.
Adult (56- to 60-day-old) OF1 mice (outbred) were obtained from the animal facility of the Institut de la Recherche Agronomique, Nouzilly, France. Virgin females were mated with males of the same strain. The presence of a vaginal plug was designated day 0 of pregnancy. The mice were then placed in individual cages and weighed daily.
Experimental design.
To evaluate the role of the time of inoculation in the development of chlamydial infection, the mice were divided into two groups: group A, consisting of mice inoculated at day 7 of pregnancy (early pregnancy), and group B, inoculated at day 11 of pregnancy (midpregnancy). Inoculation was carried out intraperitoneally with 5 × 105 PFU of C. psittaci in 0.2 ml of 0.1 M phosphate-buffered saline (PBS). Mice were killed at days 3, 5, 7, 9, 11, 13, 15, 17, and 21 postinfection (p.i.), four or more mice being sacrificed each time. Samples from the liver, spleen, and different areas of placenta were processed after necropsy for both light and electron microscopy. The control group consisted of 10 pregnant but uninoculated mice.
Immunohistochemistry.
Samples were collected and stained by the avidin-biotin-peroxidase complex (ABC) and immunogold techniques as previously described (23). Briefly, fetoplacental units from the mice were fixed in 10% formaldehyde in PBS, dehydrated, and embedded in paraffin wax at 56°C for light microscopy. To visualize chlamydial antigen on paraffin sections (5 μm), immunohistochemical staining was carried out with a biotinylated mouse monoclonal anti-chlamydial lipopolysaccharide (LPS) antibody (22) (dilution, 1:25), and the ABC according to the instructions of the manufacturer (Vector Laboratories, Burlingame, Calif.). A positive reaction was demonstrated by the precipitation of diaminobenzidine tetrahydrochloride. Sections were subsequently stained with hematoxylin or with the periodic acid-Schiff technique.
For electron microscopy, fetoplacental units were rapidly excised and immersed in a mixture of 2% paraformaldehyde and 1% glutaraldehyde in 0.1 M PBS. The metrial gland, decidua basalis, and labyrinth were cut into small pieces and fixed in the same fixative for 24 h at 4°C. After fixation, the tissues were dehydrated in ethanol and embedded in LR-White resin, soft grade (London Resin Company, Basingstoke, England). Thick sections (1 μm) were cut, stained with toluidine blue, and examined with the light microscope to locate the infected cells. Ultrathin sections (80 nm) were then cut and immunostained with the biotinylated anti-chlamydial LPS monoclonal antibody described above (dilution, 1:25) as previously described (22). Briefly, sections were collected on nickel grids and incubated for 1 h at 37°C with the primary biotinylated anti-LPS antibody. After several washes in PBS, the sections were incubated for 30 min at 37°C with streptavidin-gold (10 nm) (Sigma, Madrid, Spain), then washed in PBS, and counterstained in aqueous uranyl acetate and lead citrate.
Control sections were reacted by the ABC or immunogold technique. These control sections either were treated with nonimmune mouse serum instead of the primary antibody or were sections of tissue from noninfected mice. The control sections were all negative.
Statistical analysis.
The mean and standard deviation were calculated for each group of animals. Nonparametric tests (Mann-Whitney U test) were used to compare differences between groups A and B. Results were considered significant when P was <0.05.
RESULTS
Pregnancy outcome.
All the pregnant mice inoculated, if not previously sacrificed, aborted at days 19 to 20 of pregnancy (12 to 13 days p.i. for group A; 8 to 9 days p.i. for group B), while the noninoculated control group had an average litter of 12 live baby mice at days 21 to 22 of pregnancy.
Early stages of the infection.
In our model, which involved intraperitoneal inoculation, the placenta was not the primary target organ of C. psittaci, since positive immunoreaction was not detected until day 5 p.i. in the mice of group A and was detected only in some mice of group B at day 3 p.i. However, the spleen and liver of mice in both groups showed moderate immunoreaction at day 3 p.i. (data not shown). The first placental area to be colonized by C. psittaci was the decidua basalis, at the very limit of the giant cells layer (day 3 in group B; day 5 in group A), where chlamydial inclusion in decidual cells and immunolabelled neutrophils could be observed, always near maternal vessels.
Progression of the infection.
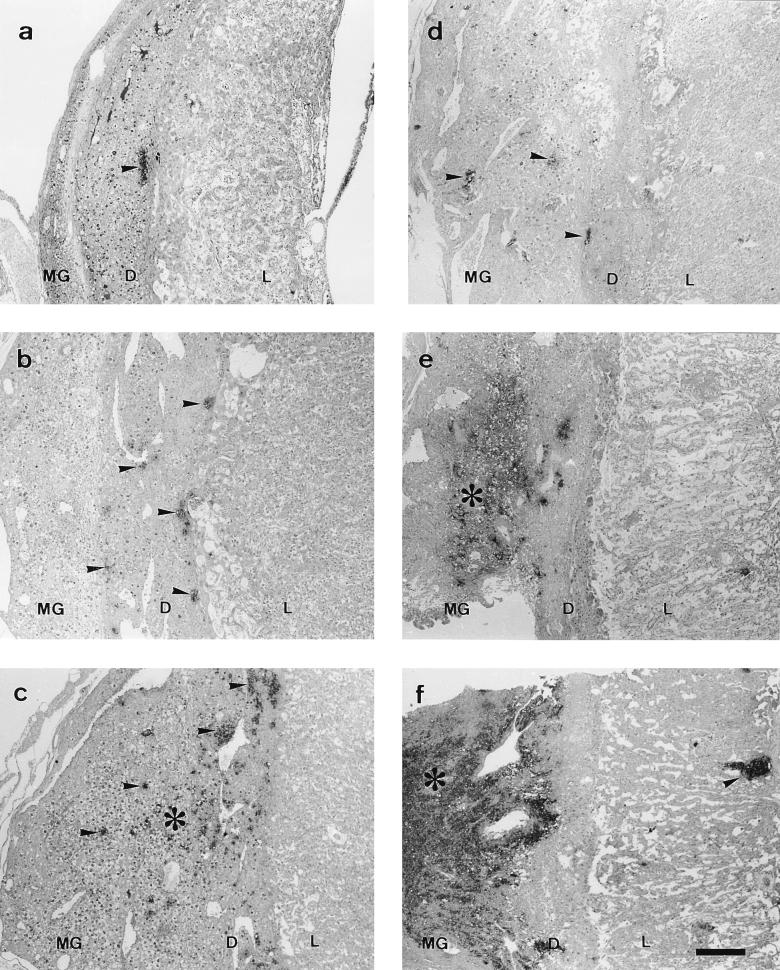
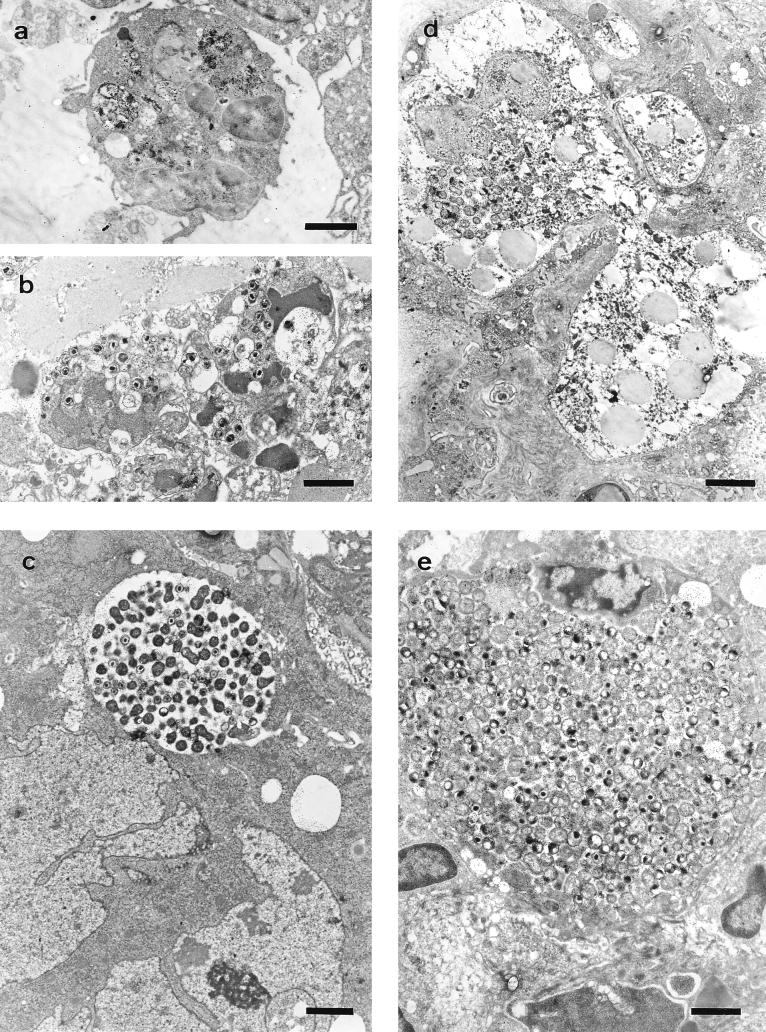
Progression of the infection depended on the day of inoculation (Tables 1 and 2). In group A it was quite slow: at day 5 p.i. (Fig. 1a), chlamydial antigen could be seen in the decidua basalis and decidua parietalis, decidual cells and neutrophils being the cell populations affected. Electron microscopy showed the decidual cells to have classical chlamydial inclusions, while the neutrophils continued to change as infection progressed in both groups. During the first stages of infection, the neutrophils contained immunolabelled remains that suggested a successful phagocytic function (Fig. 2a), while in more advanced stages they showed condensation of the nuclear chromatin and numerous EBs (Fig. 2b), reflecting a breakdown in the control of the infection. No sign of active chlamydial reproduction was observed in neutrophils (typical inclusions with RBs and EBs).
TABLE 1.
Distribution and intensity of the immunoreaction to C. psittaci antigen in the uterus, maternal placenta, fetal placenta, and fetus of mice inoculated on day 7 of pregnancy (group A)
| Tissue | Immunoreaction (mean ± SD of all mice in group)a at indicated day p.i.
|
||||||
|---|---|---|---|---|---|---|---|
| 3 (4b) | 5 (5) | 7 (4) | 9 (6) | 11 (4) | 13 (4) | 15c (5) | |
| Uterus | |||||||
| Endometrium | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium | 0 | 0 | 0 | 0.3 ± 0.5* | 0.5 ± 0.5 | 0.7 ± 0.5 | 0.8 ± 0.4* |
| Lumen | 0 | 0 | 0 | 0* | 0* | 2 ± 0d* | 2.2 ± 0.4* |
| Maternal placenta | |||||||
| Metrial gland | 0 | 0* | 0* | 0.5 ± 0.5* | 2.2 ± 0.5 | 2.5 ± 0.7 | NP |
| Decidua basalis | 0* | 1.2 ± 0.4 | 1.5 ± 0.5* | 2.1 ± 0.7 | 2.7 ± 0.5 | 3 ± 0 | NP |
| Decidua parietalis | 0 | 0.8 ± 0.4 | 1.2 ± 0.5 | 2 ± 0.6 | 2 ± 0.7 | 2 ± 0 | NP |
| Decidua capsularis | 0 | 0* | 0.7 ± 0.5 | 1.1 ± 0.4 | 1 ± 0 | 1 ± 0 | NP |
| Fetal placenta | |||||||
| Giant-cell layer | 0 | 0* | 0.7 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.7 | NP |
| Labyrinth | 0 | 0 | 0* | 0* | 0.7 ± 0.5 | 1 ± 0 | NP |
| Sites of previous placental attachment | NP | NP | NP | NP | NP | 3 ± 0d* | 2.2 ± 0.4* |
| Fetus | 0 | 0 | 0 | 0 | 0 | 0 | NP |
0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive; NP, tissue not present; ∗, significant differences (P < 0.05) with regard to group B at the same day postinfection.
Number of mice used.
All mice had aborted at day 15 p.i.
Values only for aborted mice in group.
TABLE 2.
Distribution and intensity of the immunoreaction to C. psittaci antigen in the uterus, maternal placenta, fetal placenta, and fetus of mice inoculated on day 11 of pregnancy (group B)
| Tissue | Immunoreaction (mean ± SD of all mice in group)a at indicated day p.i.
|
||||||
|---|---|---|---|---|---|---|---|
| 3 (5b) | 5 (5) | 7 (6) | 9 (4) | 11c (6) | 13 (5) | 15 (5) | |
| Uterus | |||||||
| Endometrium | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium | 0 | 0 | 0 | 1 ± 0* | 0.8 ± 0.4 | 0.8 ± 0.4 | 0* |
| Lumen | 0 | 0 | 0 | 2 ± 0d* | 2 ± 0.6* | 1.2 ± 0.5* | 0* |
| Maternal placenta | |||||||
| Metrial gland | 0 | 1.2 ± 0.4* | 2 ± 0.6* | 3 ± 0* | NP | NP | NP |
| Decidua basalis | 0.6 ± 0.5* | 1.4 ± 0.5 | 2.3 ± 0.5* | 2.5 ± 0.5 | NP | NP | NP |
| Decidua parietalis | 0 | 0.8 ± 0.4 | 1.5 ± 0.6 | 1.5 ± 0.7 | NP | NP | NP |
| Decidua capsularis | 0 | 0.8 ± 0.4* | 0.8 ± 0.4 | 1 ± 0 | NP | NP | NP |
| Fetal placenta | |||||||
| Giant-cell layer | 0 | 0.6 ± 0.5* | 0.8 ± 0.4 | 1 ± 0 | NP | NP | NP |
| Labyrinth | 0 | 0 | 0.6 ± 0.5* | 1 ± 0* | NP | NP | NP |
| Sites of previous placental attachment | NP | NP | NP | 2.5 ± 0.7d | 2.6 ± 0.5 | 2 ± 0.7* | 0.8 ± 0.4* |
| Fetus | 0 | 0 | 0 | 0 | NP | NP | NP |
0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive; NP, tissue not present; ∗, significant differences (P < 0.05) with regard to group A at the same day postinfection.
Number of mice used.
All mice had aborted at day 11 p.i.
Values only for aborted mice in group.
FIG. 1.
Paraffin wax sections immunostained by the ABC method showing the distribution of positive immunoreaction (arrowheads and asterisks) in the placental areas. Notice that the anatomical progression of infection was increased in group B with regard to group A. (a to c) Group A at 5 days p.i. (small focus in the decidua basalis next to giant-cell layer), 7 days p.i. (small foci disseminated in the decidua basalis), and 9 days p.i. (immunoreaction foci spread through the metrial gland), respectively. (d to f) Group B at 5 days p.i. (small foci disseminated in the decidua basalis and metrial gland), 7 days p.i. (foci forming extensive immunoreaction areas in the decidua basalis and metrial gland), and 9 days p.i. (very extensive immunoreaction area occupying decidua basalis and metrial gland [asterisk] and immunoreaction foci spread through the labyrinth [arrowhead]), respectively. Bar, 266.6 μm for all photographies. MG, metrial gland; D, decidua basalis; L, labyrinth.
FIG. 2.
Electron photomicrographs of the different cell populations affected by chlamydial infection labelled by the immunogold technique. (a) Neutrophil with remains of immunolabelled material within phagolysosomes, day 14 of pregnancy (7 p.i.). Bar, 1.66 μm. (b) Neutrophils with degenerative changes and chromatin condensation containing numerous EBs, day 18 of pregnancy (11 p.i.). Bar, 1.66 μm. (c) Giant cell with a chlamydial inclusion, day 14 of pregnancy (7 p.i.). Bar, 1.98 μm. (d) GMG cell with a young chlamydial inclusion, day 16 of pregnancy (9 p.i.). Bar, 2.64 μm. (e) Decidual cell with a very large chlamydial inclusion, day 18 of pregnancy (11 p.i.). Bar, 1.51 μm.
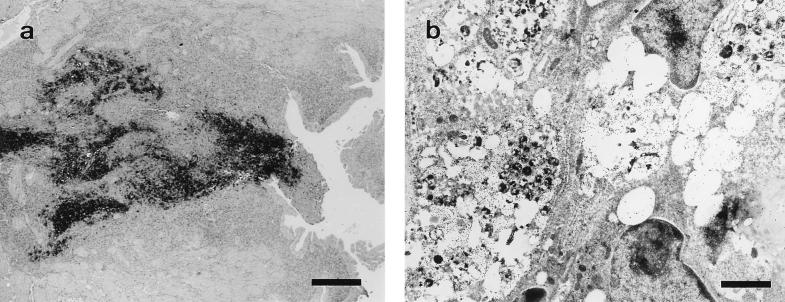
By day 7 p.i. (Fig. 1b), the decidua capsularis had also been colonized and very large inclusions appeared in the giant-cell layer (Fig. 2c), although no immunoreaction was found in the metrial gland. At day 9 p.i. (Fig. 1c), weak immunoreaction was observed in the metrial gland whereas the decidua basalis showed moderate reaction, some GMG cells showing chlamydial inclusions in both areas (Fig. 2d). At day 11 p.i., the immunoreaction was moderate in the metrial gland and strong in the decidua basalis, with many GMG cells infected. There were very large inclusions in the decidual cells (Fig. 2e) and a high degree of neutrophil infiltration of both areas. In the labyrinth, some inclusions were observed in trophoblast cells and also immunolabelled neutrophils in small foci. By day 13 p.i., some mice of group A had aborted. These mice showed a strong immunoreaction in the sites of previous attachment (Fig. 3a) and also in the uterus lumen, this positive immunoreaction remaining until day 17 p.i. Immunoreaction was not detected at day 21 p.i. Electron microscopy (Fig. 3b) showed that the immunoreaction was localized in the debris of necrotic cells, in degenerated neutrophils, and in extracellular groups of EBs. The decidua basalis and metrial gland of the mice that had not aborted showed an image similar to that at day 11 p.i., with substantial infiltration of neutrophils.
FIG. 3.
Site of previous attachment at day 2 postabortion. (a) Paraffin wax section immunostained by the ABC technique. Bar, 266.6 μm. (b) Electron photomicrograph labelled by the immunogold technique, showing groups of EBs, amorphous immunolabelled material, and cells with significant degenerative changes. Bar, 1.66 μm.
In group B, the anatomical progression of the infection was noticeably faster (Table 2). By day 5 p.i. (Fig. 1d), the metrial gland already showed immunoreaction and several GMG cells both in the decidua basalis and metrial gland were infected. The giant-cell layer was also invaded. At day 7 p.i. (Fig. 1e), the metrial gland and decidua basalis showed a strong immunoreaction, with substantial neutrophilic infiltration as well as numerous decidual and GMG cells with chlamydial inclusions. The infection stage was the equivalent to that at day 11 for group A; there were not significant differences in the immunoreaction (P < 0.05). Likewise, day 9 p.i. (Fig. 1f) was the equivalent of day 13 in group A, both in aborted and in nonaborted mice. The positive immunoreaction in the site of previous placental attachment remained in this group until day 15 p.i. Immunoreaction was not detected at day 17 or 21 p.i.
There was no sign of immunoreaction in any fetus or of histopathological lesions in either group A or group B.
DISCUSSION
The first placental area to be invaded by C. psittaci was the boundary between the maternal and fetal placenta, which was previously identified as the first center of infection in the placenta for different intracellular bacteria such as Coxiella burnetii (4), Listeria monocytogenes (19), and Brucella abortus (26). This border area between the maternal and fetal placenta is a suitable place for intracellular pathogen settlement since there are very few or no maternal macrophages or T cells to facilitate the survival of the fetal trophoblast (19). Neutrophils were the only defensive cell population which reached the decidua to any great extent as late as 13 p.i., by which time in the liver and spleen of the same animals they partially had been replaced by macrophages beginning on day 5 p.i. (data not shown). However, the neutrophils were unable to control chlamydial replication, while their massive accumulation, degeneration, and lysis could cause significant necrosis in the decidua. Neutrophil infiltration and extensive necrosis of the maternal-fetal junctions is a characteristic event of chlamydial infection of ruminant placenta (6), and similar lesions have been observed in several cases of gestational chlamydiosis in human beings, involving acute inflammatory cells in the intervillous spaces together with inflammation of the decidual bed (13, 28).
Extensive neutrophil infiltration has been observed in murine placental infection with other intracellular pathogens such as Coxiella burnetii (4), L. monocytogenes (19), and B. abortus (26).
A comparison of the rate at which infection developed in both groups indicated a delay of 4 days in group A; the metrial gland was not susceptible to chlamydia infection until day 15 of pregnancy regardless of the time of inoculation, this moment of pregnancy coinciding with the time at which the GMG cells start to lose their functionality (8). This fact suggests that the ability of chlamydiae to infect these cells and to completely colonize the maternal placenta depends on the functional state of the GMG cells. It has been suggested that the composition and increased granularity of the mature GMG cells accompanied by cell death may be a part of a mechanism to facilitate separation of the placenta from the uterine wall at parturition (8, 25), while the chlamydia-induced lysis of GMG cells and the release of their granules could contribute to a premature placental separation at abortion. In sheep, despite the differences in the type of placenta and local immune response in relation to mouse, it is well known that endometrial tissues contain cells which are morphologically and functionally analogous to the GMG cells of pregnant rodent uterus although they are γδ TCR+ CD8+ (12). However, it is not known whether these cells develop NK activity. During pregnancy, this large granulated lymphocyte subpopulation increases in the lumimal epithelium in the interplacentomal endometrium and may represent up to 10% of the cells of this tissue (15). In pregnant human uterus, large granulated lymphocytes have been identified as a subset of NK cells in the decidua (14) and seem to be related to GMG cells. The role of these cell populations in chlamydial infection of ruminant or human placenta has not been determined.
In our experiments there was no sign of immunoreaction in any fetus or of any histopathological findings, which suggests that chlamydiae do not directly damage the fetus. However, Rodolakis et al. (21) isolated chlamydiae from the fetus by the plaque-forming method, although in very low numbers (103- to 105-fold less than in placenta). This apparent disagreement could be due to the poor sensitivity of the immunohistochemical technique, since it may be able to detect easily chlamydial inclusions but not scarce free EBs. In our model, chlamydiae probably reached the fetus from day 18 of pregnancy, when the labyrinth was invaded, although they did not have time to cause lesions since abortion occurred on days 19 to 20. In sheep, chlamydiae also reach the fetus during the last third of pregnancy, causing focal necrosis in the liver and other organs (6). However, in this case the time elapsing between fetal infection and abortion is greater (about 30 days). In chlamydial human abortion, on the other hand, although bacteria were isolated from both fetal and placental tissue, histopathological changes were observed only in the placenta (28). The murine model therefore is quite similar to human chlamydial abortion.
A very similar occurrence has been observed with Coxiella burnetii, where hepatic lesions have been found in fetus from naturally infected sheep (27); however, Baumgärtner and Bachmann in an extensive immunohistochemical study (4) found no positive immunoreaction or lesions in the fetuses of experimentally infected pregnant mice but found both lesions and positive immunoreaction in surviving 9-day-old offspring, which suggests that fetal infection occurred just before or after birth.
In conclusion, our findings suggest that there is no important damage to the fetus prior to abortion, although two occurrences that could represent an indirect form of damage to the fetus took place in the decidua basalis: lysis of GMG cells with the subsequent release of granules containing cytotoxic proteins and pronounced infiltration of this area by neutrophils. Both of these occurrences, together with the direct destruction of the decidual cells by chlamydiae, could lead to a malfunctioning of the maternal placenta and a premature breaking of the decidua basalis, coinciding with the late-term abortion induced by chlamydiae.
ACKNOWLEDGMENTS
This work was supported in part by Comisión Interministerial de Ciencia y Tecnología (CICYT) grant AGF97-0459. A. J. Buendía was the recipient of a predoctoral grant from the Universidad de Murcia, Murcia, Spain.
We thank Ian J. Stewart for helpful suggestions at the outset of this study and A. Souriau for help in the biotinylation of the antichlamydial monoclonal antibody.
REFERENCES
- 1.Abzug M J, Rotbart H A, Magliato S A, Levin M J. Evolution of the placental barrier to fetal infection by murine enteroviruses. J Infect Dis. 1991;163:1336–1341. doi: 10.1093/infdis/163.6.1336. [DOI] [PubMed] [Google Scholar]
- 2.Awan A R, Baxi M, Field H J. EHV-1-induced abortion in mice and its relationship to stage of gestation. Res Vet Sci. 1995;59:139–145. doi: 10.1016/0034-5288(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 3.Banks J, Eddie B, Schachter J, Meyer K F. Plaque formation by Chlamydia in L cells. Infect Immun. 1970;1:259–262. doi: 10.1128/iai.1.3.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgärtner W, Bachmann S. Histological and immunocytochemical characterization of Coxiella burnetii-associated lesions in the murine uterus and placenta. Infect Immun. 1992;60:5232–5241. doi: 10.1128/iai.60.12.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M B, Steiner D A. Experimental genital mycoplasmosis: time of infection influences pregnancy outcome. Infect Immun. 1996;64:2315–2321. doi: 10.1128/iai.64.6.2315-2321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton D, Barlow R M, Finlayson J, Anderson I E, Mackellar A. Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J Comp Pathol. 1990;102:221–237. [PubMed] [Google Scholar]
- 7.Buzoni-Gatel D, Rodolakis A. A mouse model to compare virulence of abortive and intestinal ovine strains of Chlamydia psittaci: influence of the route of inoculation. Ann Microbiol. 1983;134:91–99. doi: 10.1016/0769-2609(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 8.Croy B A. Granulated metrial gland cells: hypothesis concerning possible functions during murine gestation. J Reprod Immunol. 1994;27:85–94. doi: 10.1016/0165-0378(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 9.Delgado S R, McBey B A, Yamashiro S, Fujita J, Kiso Y, Croy B A. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukocyte Biol. 1996;59:262–269. [PubMed] [Google Scholar]
- 10.De Sa C, Souriau A, Bernard F, Salinas J, Rodolakis A. An oligomer of the major outer membrane protein of Chlamydia psittaci is recognized by monoclonal antibodies which protect mice from abortion. Infect Immun. 1995;63:4912–4916. doi: 10.1128/iai.63.12.4912-4916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadley K M, Carrington D, Frew C E, Gibson A A, Hislop W S. Ovine chlamydiosis in an abattoir worker. J Infect. 1992;25:105–109. doi: 10.1016/0163-4453(92)92254-g. [DOI] [PubMed] [Google Scholar]
- 12.Hansen P J, Lee W-J. Immunological aspects of pregnancy: concepts and speculations using the sheep as a model. Anim Reprod Sci. 1996;42:483–493. [Google Scholar]
- 13.Jorgensen D M. Gestational psittacosis in a Montana sheep rancher. Emerging Infect Dis. 1997;3:191–194. doi: 10.3201/eid0302.970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King A, Wooding P, Gardner L, Loke Y W. Expression of perforin, granzyme A and TIA-1 by human uterine CD56+ NK cells implies they are activates and capable of effector functions. Hum Reprod. 1993;8:2061–2067. doi: 10.1093/oxfordjournals.humrep.a137982. [DOI] [PubMed] [Google Scholar]
- 15.Lee C S, Meeusen E, Gogolin-Ewens K, Brandon M R. Quantitative and qualitative changes in the intraepithelial lymphocyte population in the uterus of nonpregnant and pregnant sheep. Am J Reprod Immunol. 1992;28:90–96. doi: 10.1111/j.1600-0897.1992.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 16.Long M T, Bazler T V. Fetal loss in BALB/c mice infected with Neospora caninum. J Parasitol. 1996;82:608–611. [PubMed] [Google Scholar]
- 17.Moulder J W, Hatch T P, Kuo C-C, Schachter J, Storz J. Genus I. Chlamydia, Jones, Rake and Stearns 1945. In: Krieg N J, editor. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 729–739. [Google Scholar]
- 18.Parr E L, Young L H, Parr M B, Young J D-E. Granulated metrial gland cells of pregnant mouse uterus are natural killer-like cells that contain perforin and serine esterases. J Immunol. 1990;145:2365–2372. [PubMed] [Google Scholar]
- 19.Redline R W, Lu C Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–3955. [PubMed] [Google Scholar]
- 20.Rodolakis A, Gestin L, Bertin A. Méthode de contrôle des vaccins contre la chlamydiose abortive ovine utilisant la souris gestante. Ann Rech Vet. 1981;12:371–377. [PubMed] [Google Scholar]
- 21.Rodolakis A, Bernard F, Lantier F. Mouse models for evaluation of virulence of Chlamydia psittaci isolated from ruminants. Res Vet Sci. 1989;46:34–39. [PubMed] [Google Scholar]
- 22.Salinas J, Sánchez J, Buendía A J, Souriau A, Rodolakis A, Bernabé A, Cuello F. The LPS localization might explain the lack of protection of LPS-specific antibodies in abortion-causing Chlamydia psittaci infections. Res Microbiol. 1994;145:611–620. doi: 10.1016/0923-2508(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez J, Buendía A J, Salinas J, Bernabé A, Rodolakis A, Stewart I J. Murine granulated metrial gland cells are susceptible to Chlamydia psittaci infection in vivo. Infect Immun. 1996;64:3897–3900. doi: 10.1128/iai.64.9.3897-3900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart I J. Granulated metrial gland cells: pregnancy specific leukocytes? J Leukocyte Biol. 1991;50:198–207. doi: 10.1002/jlb.50.2.198. [DOI] [PubMed] [Google Scholar]
- 25.Straatsburg I H, Gossrau R. Enzyme histochemistry of the regressing rat decidua and metrial gland. Acta Histochem. 1993;94:202–219. doi: 10.1016/S0065-1281(11)80376-4. [DOI] [PubMed] [Google Scholar]
- 26.Tobias L, Cordes D O, Schurig G G. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol. 1993;30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 27.Van Moll P, Baumgärtner W, Eskens U, Hänichen T. Immunocytochemical demonstration of Coxiella burnetii antigen in the fetal placenta of naturally infected sheep and cattle. J Comp Pathol. 1993;109:295–301. doi: 10.1016/s0021-9975(08)80254-x. [DOI] [PubMed] [Google Scholar]
- 28.Wong S Y, Gray E S, Buxton D, Finlayson J, Johnson F W A. Acute placentitis and spontaneous abortion caused by Chlamydia psittaci: a histological and ultrastructural study. J Clin Pathol. 1985;38:707–711. doi: 10.1136/jcp.38.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng L M, Joag S V, Parr M B, Parr E L, Young J D-E. Perforin-expressing granulated metrial gland cells in murine deciduoma. J Exp Med. 1991;174:1221–1227. doi: 10.1084/jem.174.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]