Abstract
Borrelia burgdorferi, the spirochete that causes Lyme disease, binds decorin, a collagen-associated extracellular matrix proteoglycan found in the skin (the site of entry for the spirochete) and in many other tissues. Two borrelial adhesins that recognize this proteoglycan, decorin binding proteins A and B (DbpA and DbpB, respectively), have recently been identified. Infection of mice by low-dose B. burgdorferi challenge elicited antibodies against DbpA and DbpB that were sustained at high levels, suggesting that these antigens are expressed in vivo. Scanning immunoelectron microscopy showed that DbpA was surface accessible on intact borreliae. Passive administration of DbpA antiserum protected mice from infection following challenge with heterologous B. burgdorferi sensu stricto isolates, even when serum administration was delayed for up to 4 days after challenge. DbpA is the first antigen target identified that is capable of mediating immune resolution of early, localized B. burgdorferi infections. DbpA immunization also protected mice from B. burgdorferi challenge; DbpB immunization was much less effective. DbpA antiserum inhibited in vitro growth of many B. burgdorferi sensu lato isolates of diverse geographic, phylogenetic, and clinical origins. In combination, these findings support a role for DbpA in the immunoprophylaxis of Lyme disease and suggest that DbpA vaccines have the potential to eliminate early-stage B. burgdorferi infections.
Lyme disease (53), or Lyme borreliosis, is caused by a group of related tick-borne spirochetes classified as Borrelia burgdorferi sensu lato (including B. burgdorferi sensu stricto, B. afzelii, and B. garinii). Although much progress has been made in the characterization of the organism, spirochetal factors responsible for infectivity, immune evasion, and disease pathogenesis remain largely obscure. The most-studied B. burgdorferi membrane protein is outer surface protein A (OspA), a lipoprotein antigen expressed by borreliae in resting ticks and the most abundant protein expressed in vitro by most B. burgdorferi sensu lato isolates (2, 32). Experimental OspA vaccines have demonstrated efficacy in several animal models (11, 19, 24, 33), and OspA vaccines for human use are the subjects of current clinical evaluations (35, 36, 57).
During tick engorgment, OspA expression by borreliae diminishes (15) while expression of other proteins, exemplified by OspC, increases (51). By the time of B. burgdorferi transmission to hosts, spirochetes in the tick salivary glands express little or no OspA. This diminished expression of OspA appears to explain the weak early immune responses to this antigen in experimental or natural infection following tick-borne infection (26, 34, 47, 49) or following inoculation with minimal infectious doses of cultured spirochetes (3, 47). OspA antibodies are sometimes detected in later stages of infection (34, 50), implying that this antigen may be reexpressed by at least some spirochetes during infection. For these reasons OspA-specific antibodies are ineffective in eliminating infection when they are administered after infection is established by syringe inoculation (18, 48) or tick bite (15). Additionally, OspA immunity is circumvented by challenges of in vivo-adapted borreliae in the form of transplants of skin from infected donors into OspA-immunized mice (7). OspA immunization has been shown to mediate killing of spirochetes directly in the midgut of feeding ticks and therefore has a primary mode of action at the vector stage (15, 24). To be efficacious, OspA vaccines must elicit protective levels of antibody that must be maintained throughout periods of tick exposure in order to block spirochete transmission from the vector.
Vaccines against pathogens other than Borrelia are often based on in vivo-expressed antigens that boost anamnestic responses upon infection, potentiate the action of immune effector cells and complement, and inhibit key virulence mechanisms. Many laboratories have examined the vaccine potential of B. burgdorferi antigens that are immunogenic during infection and therefore presumably expressed in vivo. OspC is expressed during infection (37) and elicits protective immunity in rodents (27, 40, 42). However, OspC-immunized mice appear not to be protected against challenge with heterologous B. burgdorferi isolates (41), and in some cases OspC immunization failed to protect mice against challenge with even homologous B. burgdorferi isolates (6, 13). OspE and OspF are immunogenic during infection, but OspF elicits only partial protection against tick-borne or low-dose (102 borreliae) intradermal challenge and OspE is ineffective as a protective immunogen (38). Other immunogenic in vivo-expressed antigens that have been evaluated as targets for protection, including 41-kDa flagellin, P30, P39, P55, P83, IpLA-7, the OspE homolog P21, and the OspF homolog pG, have thus far failed to show efficacy in the prevention of infection (14, 14a, 17, 20, 27, 42, 59, 60). Identification of in vivo-expressed B. burgdorferi antigens that are capable of eliciting broadly protective immune responses has remained elusive.
In mammalian hosts, B. burgdorferi infection is initiated when the tick vector deposits the spirochetes into the dermis during feeding. At both initial and later stages of infection, B. burgdorferi is commonly found in association with collagen fibers in the extracellular matrix (5, 58). Recent findings (29) suggest that colonization of these collagenous tissues may be mediated by spirochetal surface adhesins binding specifically to the collagen-associated proteoglycan decorin (12). These adhesins were expressed at low-to-moderate copy numbers on cultured borreliae (29). Genes for two adhesins of B. burgdorferi recognizing decorin, lipoproteins with apparent masses of 18 to 20 kDa called decorin binding proteins A and B (DbpA and DbpB, respectively), have been partially characterized (28). In the current study, we examined the immunogenicity of DbpA and DbpB during infection, examined their accessibility to antibodies and efficacy as potential vaccine candidates, and evaluated the serological conservation of these proteins.
(This work was presented, in part, at the 14th annual meeting on Modern Approaches to the Control of Infectious Diseases, 9 to 13 September 1996, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y., and in a preliminary report [10a] resulting from that meeting.)
MATERIALS AND METHODS
B. burgdorferi isolates.
Isolates of B. burgdorferi sensu lato were donated by the laboratories of A. Barbour, S. Barthold, R. Johnson, J. Leong, and S. Norris or from the Rocky Mountain Laboratories Microscopy Branch collection. The biologic and geographic origins of these isolates are listed in Table 1. The molecular typing method described by Postic et al. (39) was used to confirm the phylogenetic designation of each of these isolates. Spirochetes were propagated in tightly closed containers at 34°C in modified Barbour-Stoenner-Kelly (BSKII) medium (1) overlaid with a 5% O2–5% CO2–90% N2 gas mixture. Cell densities of these cultures were determined by dark-field microscopy at a magnification of ×400. Batches of BSKII were qualified for spirochete propagation and infection testing by confirming that they supported the growth of 1 to 5 cells of isolate B31. Infectivity of the B. burgdorferi sensu lato isolates for mice was assessed as described below. The following isolates used for immunologic protection studies were determined to have the median infectious dose (ID50) values indicated: B31, 6 × 101; Sh-2-82, 6 × 102; N40, 3 × 102; 297, 3 × 103; 25015, 6 × 101; HB19, 3 × 103; and CA-3-87, 3 × 101.
TABLE 1.
Evaluation of diverse B. burgdorferi sensu lato isolates for growth inhibition by rabbit preDbpA297 and OspAB31 antisera, for immunoblot reactivity, and for decorin binding activity
| Borrelia isolate | Source and origina | Decorin binding activityd | DbpA antiserum
|
OspA antiserum
|
||
|---|---|---|---|---|---|---|
| Growth inhibition end point | Immunoblot reactivityd | Growth inhibition end point | Immunoblot reactivityd | |||
| B. burgdorferi sensu stricto | ||||||
| B31 | I. scapularis, New York | +b | 5,120 | ++ | 51,200 | ++ |
| 297 | CSF, New York | + | 5,120 | ++ | 51,200 | ++ |
| Sh-2-82 | I. scapularis, New York | ++ | 5,120 | ++ | 51,200 | ++ |
| N40 | I. scapularis, New York | ++b | 12,800 | ++ | 51,200 | ++ |
| JD1 | I. scapularis, Massachusetts | ++b | 800 | ++ | 1,600 | ++ |
| HB19 | Blood, United States | ± | <50 | ± | 100 | ++ |
| 3028 | Human pus, Texas | +b | 50c | ++ | 1,600 | ++ |
| G39/40 | I. scapularis, Connecticut | ± | 100 | ± | 3,200 | ++ |
| LP4 | Skin (EM), Connecticut | +b | 800 | ++ | <50 | ++ |
| LP5 | Skin (EM), Connecticut | +b | 800 | ++ | <50 | ++ |
| LP7 | Skin (EM), Connecticut | +b | 400 | ++ | <50 | ++ |
| NCH-1 | Skin, United States | +b | 50c | ++ | 100 | ++ |
| ZS7 | I. ricinus, Germany | ++ | <50 | + | 400 | ++ |
| H11 | Blood, Italy | + | 200 | ++ | 400 | ++ |
| CA-3-87 | I. pacificus, California | ± | <50 | − | 1,600 | ++ |
| FRED | Human, Missouri | − | 1,600 | + | 3,200 | ++ |
| HBNC | Blood, California | ± | 3,200 | ± | 3,200 | ++ |
| B. afzelii | ||||||
| PGau | Skin (ACA), Germany | ++ | 50c | ++ | 50 | ++ |
| ACA I | Skin (ACA), Sweden | + | <50 | ± | <50 | ++ |
| M7 | I. persulcatus, China | ± | 1,600 | + | <50 | ++ |
| IPF | I. persulcatus, Japan | − | 1,600 | ± | 200 | ++ |
| BO23 | Skin, Germany | ++ | 50c | ++ | <50 | + |
| ECM-1 | Skin (EM), Sweden | ++ | 50c | ++ | 100 | + |
| B. garinii | ||||||
| PBr | CSF, Germany | ++ | 12,800 | + | <50 | ++ |
| PBi | CSF, Germany | ++ | 800 | − | <50 | ++ |
| B4 91 | Skin, Norway | ++ | <100 | − | <50 | ++ |
| G2.22 | CSF, Germany | ++ | <50 | − | <50 | ++ |
| Ip90 | I. persulcatus, Russia | + | <50 | ± | <50 | ++ |
| IP89 | I. persulcatus, Russia | ++ | <50 | − | <50 | + |
| 2226 | I. persulcatus, China | + | 200 | ± | <50 | ++ |
| Fuji P1 | I. persulcatus, Japan | ++ | 100 | ++ | <50 | ++ |
| 20047 | I. ricinus, France | ++ | 50c | ± | <50 | ++ |
| B. japonica HO14 | I. ovatus, Japan | + | <50 | ± | 50 | + |
| Group 25015 25015 | I. scapularis, United States | − | 10 | ± | 6,400 | ++ |
| B. andersonii 21038 | I. dentatus, United States | ++ | 1,600 | + | <50 | ++ |
Isolates were derived from patients with Lyme disease or Ixodes tick species. CSF, cerebrospinal fluid; EM, erythema migrans; ACA, acrodermatitis chronicum atrophicans.
Two decorin binding bands at 18 to 20 kDa.
Partial reduction in the number of cells at lowest dilution tested.
Reactivities were scored on a scale from “−” to “++,” with “−” representing background reactivity comparable to background obtained with nonimmune serum or immune serum against an irrelevant antigen and “++” representing the strongest specific signal observed. For decorin blotting, “−” represented reactivity comparable to background obtained in the absence of digoxigenin-conjugated decorin.
Expression and purification of recombinant immunogens.
Several recombinant forms of DbpA, schematically represented in Fig. 1, were expressed, purified, and used for immunologic analyses and immunizations. Molecular cloning was accomplished by standard methods (46). A library (a gift of Robin Isaacs) of approximately 2-kb Sau3A I fragments of total genomic DNA from a low-passage culture of B. burgdorferi 297 was constructed in Lambda Zap II (Stratagene Cloning Systems, La Jolla, Calif.), and plaques were plated and blotted onto nitrocellulose membranes by standard methods (46). Phage was recovered from plaques binding digoxigenin-conjugated decorin (29), and B. burgdorferi DNA inserts were excised as clones in the phagemid vector pBluescript II (pBsII) SK(−) according to the manufacturer’s protocol. One of these clones, recombinant plasmid pBG26 (ATCC 69791), harbored a 2.5-kb B. burgdorferi DNA insert containing two 561-bp open reading frames (28). Both of these two genes expressed products with decorin binding activity and were subsequently named dbpA (GenBank accession no. U75866) and dbpB (GenBank accession no. U75867). The nucleotide sequences of dbpA and dbpB shared 50% identity. Single homologs of the dbpA and dbpB genes have been identified recently in the whole genome sequence of B. burgdorferi B31, residing on the linear plasmid lp54, which also contains the ospAB operon (25a). The B31 homologs share 94 and 100% similarity, respectively, with the original dbpA and dbpB genes from B. burgdorferi 297. Recombinant clone pBG29 was constructed from pBG26 by HindIII partial digestion, with deletion of the 5′ half of the dbpB gene and retaining full-length dbpA and expression of its product. DbpA297 was purified from Escherichia coli JM101/pBG29 by affinity chromatography on decorin-conjugated Sepharose. Decorin purified (12) from fetal bovine skin, a kind gift of L. C. Rosenberg (Montefiore Medical Center, New York, N.Y.), was covalently linked to CNBr-activated Sepharose 4B (Pharmacia, Uppsala, Sweden) according to the manufacturer’s instructions. A 1-liter culture of JM101/pBG29 in LB medium (46) plus 100 μg of ampicillin per ml was shaken until an A600 value of 0.6 to 0.8 was reached, isopropylthiogalactoside (IPTG) was added to 0.2 mM, and shaking was continued for 2 to 3 h. Cells were suspended in 10 ml of phosphate-buffered saline (PBS) and lysed in a French pressure cell, and the soluble fraction was collected following centrifugation at 200,000 × g. The supernatant fluid was passed through a 0.45-μm-pore-size filter, and 5 ml of the filtrate was passed through a 2-ml decorin-Sepharose column that bound the DbpA. After the column was washed with 20 ml of PBS, DbpA was eluted from the column with 1 M NaCl and recovered at >90% homogeneity as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). DbpA remained soluble after dialysis against PBS. Amino-terminal sequencing showed that this soluble DbpA retained the leader peptide and was the precursor form (pre-DbpA297) of the mature lipoprotein.
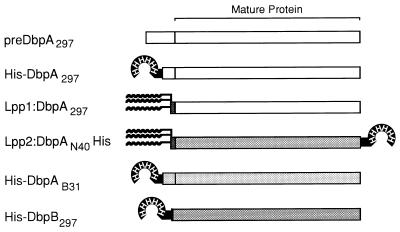
FIG. 1.
Diagrammatic representation of the recombinant Dbp immunogens evaluated for vaccine efficacy. All proteins included the complete DbpA or DbpB coding sequence following Cys at the site of the presumed posttranslational processing. Lpp1:DbpA297 is a fusion of this mature DbpA protein sequence to a modified lpp leader peptide. Fusions of DbpA and DbpB to a (His)6 tag were made within, or in place of, their respective leader peptides. Lpp2:DbpAN40His has both a N-terminal lpp leader and a C-terminal (His)6 fusion.
Posttranslational modification may influence the immunogenicity of some antigens such as OspA (16, 33, 54, 57). Because posttranslational processing of pre-DbpA297 expressed by JM101/pBG29 appeared to be inefficient, we constructed new vectors, pT7Lpp1 and pT7Lpp2, capable of expressing proteins as fusions with leader peptides derived from the abundant E. coli lipoprotein Lpp in order to increase posttranslational processing efficiency. Others had previously shown that a similar Lpp leader fusion strategy was effective for OspA (30). To construct pT7Lpp1 the NdeI-EcoRV fragment of pET-30b (Novagen, Inc., Madison, Wis.) was replaced with a synthetic DNA fragment created by annealing the oligonucleotides 5′-TATGAAAGCTACTAAACTGGTACTGGGCGCGGTAATCCTGGGTTCTACTCTGCTGGCAGCATGCGATCAGAT-3′ and 5′-ATCTGATCGCATGCTGCCAGCAGAGTAGAACCCAGGATTACCGCGCCCAGTACCAGTTTAGTAGCTTTCA-3′ encoding MKATKLVLGAVILGSTLLAACDQ, a modified version of the 5′ end of the lpp gene (GenBank accession no. V00302). A second vector, pT7Lpp2, was created in a similar manner with the oligonucleotides 5′-TATGAAAGCTACTAAACTGGTACTGGGCGCGGTAATCCTGGGTTCTACTCTGCTGGCAGGTTGCTCCTCGAT-3′ and 5′-ATCGAGGAGCAACCTGCCAGCAGAGTAGAACCCAGGATTACCGCGCCCA GTACCAGTTTAGTAGCTTTCA-3′, encoding the exact sequence of the amino-terminal end of Lpp, MKATKLVLGAVILGSTLLAGCSS. DNA encoding the entire sequence (28) of the mature DbpA297 protein (GenBank accession no. U75866), after the cysteine at the site of posttranslational modification, was amplified from B. burgdorferi 297 template DNA by PCR with oligonucleotide primers 5′-CCGGATCCCGGACTAACAGGAGCAACAAAAATC-3′ (the added BamHI site is underlined) and 5′-TGGTCTAAGCTTTTGAGTTGCATATAAAAATGG-3′ (the added HindIII site is underlined). After digestion of PCR products and vector with BamHI and HindIII, the dbpA gene fragment was ligated into pT7Lpp1 yielding pMSH24. This plasmid expresses chimeric lipoprotein Lpp1:DbpA297 that differs from the natural sequence by the vector-added residues DQISDP between the amino-terminal C and the natural G at DbpA297 position +2 following processing of the leader peptide. E. coli BL21(DE3)/pLysS (55) was transformed with pT7Lpp1 (negative control for protein expression) and pMSH24.
BL21(DE3)/pLysS/pMSH24 was grown at 37°C in LB plus 50 μg of kanamycin per ml and 20 μg of chloramphenicol per ml to mid-exponential phase, protein expression was induced by the addition of IPTG to 1.0 mM, and after an additional 2 h of growth, the cells were harvested by centrifugation. The cell paste was suspended in cold PBS at ∼0.25 g (wet weight) of Triton X-114 per ml (10% [vol/vol] in PBS) was added to 2%, and the cells were lysed by sonication in an ice bath. After gentle overnight agitation at 4°C, insoluble material was removed by centrifugation at 100,000 × g, and the supernatant was decanted. Cloud point extraction (9) of the Triton X-114 supernatant enriched for the Lpp1:DbpA297 in the detergent phase. This detergent phase was diluted 20-fold with 20 mM NaH2PO4 buffer (PB), pH 7.4, containing 1% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1 propane-sulfonate (CHAPS) and loaded onto a DEAE Sepharose Fast Flow (FF) column. The flowthrough containing Lpp1:DbpA297 was adjusted to pH 4.0 and applied to an SP Sepharose FF column. After the mixture was washed with column buffer (PB [pH 4.0]–1% CHAPS), application of a NaCl step gradient in column buffer resulted in elution of bound Lpp1:DbpA297 from the column at the 0.5 M NaCl step. Lpp1:DbpA297-containing fractions were adjusted to neutral pH and concentrated in a Prodicon (Spectrum Medical Industries, Inc., Houston, Tex.) vacuum concentrator against PBS–0.1% CHAPS. Lpp1:DbpA297 was recovered at >95% purity.
DbpAN40 was expressed from pT7Lpp2 as a chimeric lipoprotein with the addition of a vector-encoded carboxy-terminal extension VDKLAAALEHHHHHH to facilitate purification by immobilized metal-affinity chromatography. DNA encoding the entire sequence (44) of the mature DbpAN40 protein after the cysteine at the site of posttranslational modification was amplified from B. burgdorferi N40 template DNA by PCR with oligonucleotide primers 5′-CCGGATCCCGGATTAAAAGGAGAAACAAA-3′ (the added BamHI site is underlined) and 5′-CTGTCTAAGCTTAGTCGACGTTATTTTTGCATTTTTC-3′ (the added HindIII and SalI sites are underlined), digested with BamHI and SalI, and ligated into the comparable sites of pT7Lpp2 to yield plasmid pWCR129. A Triton X-114 extract of BL21(DE3)/pLysS/pWCR129 was made as described above and applied to a Ni2+-charged ToyoPearl AF-Chelate-650M (TosoHaas, Montgomeryville, Pa.) column equilibrated with PBS–1.0% CHAPS. Lpp2:DbpAN40His was eluted from the column with a 0 to 200 mM gradient of l-histidine in PBS-CHAPS. After concentration, Lpp2:DbpAN40His purity was estimated at ∼90%.
DbpA297, DbpAB31, and DbpB297 were expressed in E. coli M15/pREP4 from the vector pQE30 (Qiagen, Inc., Santa Clarita, Calif.) as amino-terminal fusions with the vector-encoded peptide MRGSHHHHHHGS and purified essentially according to Qiagen protocols. Cloning by PCR amplification, expression, and purification of His-DbpA297 and His-DbpB297 (28) will be described elsewhere. The gene fragment encoding DbpAB31 was PCR amplified with the same primers used for construction of His-DbpA297. The first 10 codons of dbpA and all 20 codons of the dbpB leader peptide were deleted in these constructions.
For expression of lipoprotein OspA, plasmid pSO3 was constructed by subcloning the NcoI-BclI fragment from pMV251 (54), containing the full-length B. burgdorferi B31 ospA gene, into the NcoI and BamHI sites of pET-3d (55). After growth in LB plus 100 μg of ampicillin per ml and 20 μg of chloramphenicol per ml, lysis, and fractionation of BL21(DE3)/pLysS/pSO3 through the cloud point extraction step were carried out essentially as for BL21(DE3)/pLysS/pMSH24 described above. The detergent phase containing lipoprotein OspAB31 was diluted approximately twofold to a final concentration of 50 mM citrate–10 mM EDTA–15 mM CHAPS (pH 4.2), and applied to an SP Sepharose FF column. Elution of the column with a linear pH gradient to pH 5.7 allowed recovery of lipoprotein OspAB31 at ∼90% homogeneity.
Negative-control immunogens for vaccination studies of mice were prepared by making extracts of each recombinant E. coli host that were comparable to those used for chromatography of the Dbp recombinant proteins expressed in these host strains. These were soluble fractions from lysates of JM101/pBsII and M15/pREP4 and a detergent-phase extract of BL21(DE3)/pLysS/pT7Lpp1.
Recombinant P39 protein was produced to evaluate seroconversion of mice following B. burgdorferi inoculation or challenge. Oligonucleotide primers 5′-ATGGATCCGAGTGGTAAAGGTAGTCTTGGGAGC-3′ (the added BamHI site is underlined) and 5′-AGAGAAGCTTAGTCGACAATAAATTCTTTAAGAAACTTCTC-3′ (the added HindIII and SalI sites are underlined) were designed based on the sequence (GenBank accession no. L24194) of the B. burgdorferi Sh-2-82 bmpA gene encoding P39 and used to amplify the entire mature region of the P39 protein from B. burgdorferi B31 template DNA by PCR. After digestion with BamHI and HindIII, the P39B31 gene fragment was cloned into the same two sites of plasmid pGMal-c, a derivative of the maltose binding protein (MBP) fusion protein expression vector pMal-c (New England Biolabs, Beverly, Mass.) with a replacement of the multiple cloning site from pMV261 (54). After transformation of the resulting plasmid, pNKP2, into E. coli DH5α, MBP-P39B31 fusion protein was expressed and purified essentially according to the manufacturer’s protocols. As a negative protein control reagent, MBP was purified from DH5α/pGMAL-c in a similar manner.
Antibody reagents.
Antisera were raised against recombinant preDbpA297 and His-DbpB297 in New Zealand White rabbits. Antisera were also generated in rabbits against recombinant B. burgdorferi B31 OspA lipoprotein purified from the E. coli clone containing plasmid pOA1 (16) and against recombinant pneumococcal surface protein A (PspA) purified from the E. coli clone containing plasmid pJY4306 (62). The predicted sequences of the lipoprotein OspA’s expressed by plasmids pOA1 and pSO3 are identical, but their products used for mouse and rabbit immunizations, respectively, were purified by different chromatographic procedures. Each animal received a primary subcutaneous (s.c.) immunization of 200 μg of protein emulsified with complete Freund’s adjuvant (CFA), followed by two or three booster injections of 100 μg with incomplete Freund’s adjuvant (IFA) at 3- to 4-week intervals. Titers of rabbit sera against their homologous recombinant borrelial antigens were 128,000 to 256,000 by enzyme-linked immunosorbent assay (ELISA). Rabbit anti-His-DbpB297 reacted weakly with pre-DbpA297 (titer of 4,000), but reactivity of rabbit anti-preDbpA297 with His-DbpB297 was at background levels. These proteins were previously observed to have minimal serologic cross-reactivity (28) consistent with their limited sequence homology (40% amino acid identity and 56% similarity). Monoclonal antibody (MAb) H5332 (2) against OspA, an immunoglobulin G1 (IgG1), was a gift of Alan Barbour. The DbpA-specific MAb 7D2B.3G6, an IgG2b, was obtained from a cloned hybridoma of splenic B cells from preDbpA-immunized BALB/cByJ (BALB; The Jackson Laboratory, Bar Harbor, Maine) mice fused with P3X63Ag8U.1 myeloma cells by standard methods (61).
Antisera against Lpp1:DbpA297 and OspAB31 were also obtained, prior to challenge, from C3H/HeJ (C3H; Jackson Laboratory) mice immunized (see below) for use in protection experiments (Table 2, experiment D, groups 2 and 3) and used for scanning immunoelectron microscopy and evaluation of passive immunization.
TABLE 2.
Protection of mice by active immunization with different forms of DbpA compared with that by immunization with OspA
| Expt (mouse strain/challenge isolate) | Mouse group | Immunogena | No. of positive cultures/total no. tested
|
P39 IgGb | No. of mice infected/total no. | ||
|---|---|---|---|---|---|---|---|
| Bladder | Ear | Joint | |||||
| A (C3H/297) | 1 | His-DbpA297 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | Lpp1:DbpA297 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 3 | OspAB31 | 0/5 | 0/5 | 0/5 | − | 0/5 | |
| 4 | PBS | 5/5 | 5/5 | 4/5 | + | 5/5 | |
| B (C3H/N40) | 1 | Lpp2:DbpAN40His | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | OspAB31 | 5/5 | 0/5 | 0/5 | + | 5/5 | |
| 3 | E. coli BL21 (det.) | 4/4 | 4/4 | 1/4 | + | 4/4c | |
| 4 | None | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| Cd (BALB/B31) | 1 | Pre-DbpA297 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | OspAB31 | 0/5 | 0/5 | 0/5 | − | 0/5 | |
| 3 | E. coli JM101 (sol.) | 5/5 | 5/5 | 5/5 | ND | 5/5 | |
| 4 | None | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| (C3H/B31) | 5 | Pre-DbpA297 | 5/5 | 0/5 | 0/5 | 5/5 | 5/5 |
| 6 | OspAB31 | 0/5 | 0/5 | 0/5 | − | 0/5 | |
| 7 | E. coli JM101 (sol.) | 5/5 | 5/5 | 5/5 | ND | 5/5 | |
| 8 | None | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| D (C3H/B31) | 1 | Pre-DbpA297 | 5/5 | 0/5 | 0/5 | 5/5 | 5/5 |
| 2 | Lpp1:DbpA297 | 5/5 | 0/5 | 0/5 | 5/5 | 5/5 | |
| 3 | OspAB31 | 0/5 | 0/5 | 0/5 | − | 0/5 | |
| 4 | E. coli JM101 (sol.) | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| 5 | E. coli BL21 (det.) | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| 6 | None | 5/5 | 5/5 | 5/5 | + | 5/5 | |
| Ed (C3H/B31) | 1 | Pre-DbpA297 | 3/5 | 0/5 | 0/5 | 3/5 | 3/5 |
| 2 | His-DbpA297 | 1/5 | 0/5 | 1/5 | 1/5 | 1/5 | |
| 3 | His-DbpAB31 | 1/4 | 0/4 | 0/4 | 0/4 | 1/4c | |
| 4 | OspAB31 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 5 | E. coli M15 (sol.) | 5/5 | 5/5 | 5/5 | ND | 5/5 | |
| 6 | None | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | |
Immunogen doses were 20 μg for DbpA, 5 μg for OspA, and 5 μg for E. coli soluble (sol.) and detergent (det.) extracts, except 20 μg in experiment C.
Presence of IgG to P39 at the time of sacrifice was determined for most groups of mice (number positive/number tested). When sera were assayed as pools, the presence or absence of P39 IgG was scored as + or −, respectively. ND, not determined.
Data were not recorded for one mouse in groups B3 and E3 that died prior to challenge.
Data for experiments C and E are reproduced from Vaccines ’97 (10a) with permission of the publisher.
Scanning immunoelectron microscopy.
In vitro-passage-5 B. burgdorferi B31 was collected from 0.5 ml of a mid-log-phase culture by centrifugation for 5 min at 1,000 × g. Sedimented cells were gently resuspended in 0.2 ml of BSKII containing 12 μl of immune or preimmune mouse serum and incubated for 30 min at room temperature with mixing. Spirochetes were then collected by centrifugation and washed twice in BSKII. The bacteria were labeled by incubation in 0.2 ml of BSKII containing 16 μl of goat anti-mouse IgG plus IgM conjugated to 30-nm-diameter gold particles (Ted Pella, Inc., Redding, Calif.). Labeled spirochetes were collected and gently washed twice in Tyrode’s buffer and allowed to settle onto 0.1% poly-1-lysine-coated coverslips. After 10 min, the buffer was replaced with fixative containing 2.5% glutaraldehyde in 0.2 M sodium cacodylate (pH 7.2). Following fixation, the coverslips were washed twice in cacodylate and postfixed in 1% osmium tetroxide in cacodylate. After being washed in water and dehydrated in ethanol, the samples were critical-point dried from ethanol through carbon dioxide, lightly sputter coated with chromium, and observed with a Hitachi S4500 field emission scanning electron microscope equipped with an ultralow-voltage backscattered electron detector (GW Electronics, Norcross, Ga.).
Immunization of mice and challenge with B. burgdorferi.
For passive immunizations, undiluted rabbit antiserum, or antiserum diluted in PBS, was administered intraperitoneally (i.p.) to 6- to 8-week-old female C3H mice within 1 h prior to challenge (day 0) or at various times afterward. For active immunizations, C3H or BALB mice were injected i.p. at week 0 with 20 μg of DbpA or DbpB, 5 μg of OspA, 20 or 5 μg of soluble or detergent extract of E. coli or PBS emulsified with CFA, given a similar booster immunization in IFA at week 4, and challenged at week 6. B. burgdorferi spirochetes were diluted in BSKII from exponentially growing cultures, and mice were injected s.c. at the base of the tail with 0.1 ml of these dilutions (typically 104 borreliae unless noted otherwise). Isolates used for challenge were passaged fewer than six times in vitro. To assess infection, mice were sacrificed at 14 to 17 days postchallenge, and specimens derived from ears, urinary bladders, and tibiotarsal joints were placed in BSKII–1.4% gelatin–13 μg of amphotericin B per ml–1.5 μg of phosphomycin per ml–15 μg of rifampin per ml, and borrelial outgrowth at 2 or 3 weeks was assessed by dark-field microscopy. Mice were scored as infected when any of the three tissues were culture positive. In some instances, seroconversion for protein P39 reactivity was also used to confirm infections (see below). Median effective dilutions (ED50) of passively administered antisera and ID50 of borreliae were assessed in groups of three C3H mice per dose and were calculated by a standard method (43). Immune serum was also obtained from C3H mice persistently infected with B31.
In vitro growth inhibition assay.
A microwell antibody titration assay (45) was used to evaluate the growth inhibition properties of antisera for various isolates of B. burgdorferi sensu lato. Briefly, 105 spirochetes in 100 μl of BSKII were added to serial twofold dilutions of antisera in 100 μl of BSKII in 96-well plates, and the plates were covered and incubated at 34°C in a 5% O2–5% CO2–90% N2 gas mixture for 72 h prior to quantification of borrelia growth by dark-field microscopy. The highest serum dilution showing growth inhibition of >90% reduction in the number of cells and motility was designated as the end point growth inhibition titer. Since, in most cases, antisera diluted less than 1:100 gave only partial growth inhibition, a titer of ≥100 was defined as positive inhibition.
Immunoblotting and decorin blotting.
In a single-well format, samples (2 μg) of Lpp1:DbpA297, OspAB31, or MBP-P39B31 were boiled in sample buffer containing 1% SDS–2.5% 2-mercaptoethanol before SDS-PAGE through 3% stacking and 12.5% acrylamide resolving gels. Gels were electroblotted onto nitrocellulose membranes, and lanes were probed with 1:50 dilutions of immune sera from infected mice or 1:2,000 dilutions of purified MAbs, followed by the appropriate secondary antibody-enzyme conjugate. Membranes used for DbpA and OspA comparative immunogenicity studies were stained with Ponceau S to confirm that equivalent amounts of purified protein had transferred following electroblotting. Immunoblot signals were detected by fluorography with ECL chemiluminescence reagents (Amersham Corp., Arlington Heights, Ill.).
Assay of decorin binding activity was performed essentially as described by Guo et al. (29). Briefly, spirochetes were harvested from culture by centrifugation, washed once with 10 mM HEPES (pH 7.4)–20 mM NaCl, and suspended to 1 × 107 to 5 × 107 cells/ml in SDS–2-mercaptoethanol sample buffer. Samples of spirochetes were subjected to SDS-PAGE as described above, electroblotted to nitrocellulose, and probed with digoxigenin-conjugated decorin (29) followed by antidigoxigenin secondary antibody-enzyme conjugate (Genius System; Boehringer Mannheim Corp., Indianapolis, Ind.). Antidigoxigenin signals were detected by fluorography following incubation of the filters with Lumi-Phos 530 (Boehringer Mannheim).
Reactivities were scored on a scale from “−” to “++,” with “−” representing background reactivity comparable to background obtained with nonimmune serum or immune serum against an irrelevant antigen and “++” representing the strongest specific signal observed. For decorin blotting, “−” represented reactivity comparable to background obtained in the absence of digoxigenin-conjugated decorin.
ELISA.
Wells of 96-well microtiter plates (Immulon 2; Dynatech, Chantilly, Va.) were coated with antigen by incubating 50 μl of a 1-μg/ml antigen solution in 0.1 M sodium carbonate buffer at pH 9.6. After unbound antigen was decanted, additional binding sites were blocked by incubating 200 μl of 3% nonfat milk in wash buffer (PBS–0.2% Tween 20 [pH 7.4]). After washing, duplicate serial twofold dilutions of sera in PBS–Tween 20–1% fetal bovine serum were incubated for 1 h and removed, and the wells were washed three times. The wells were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG, as appropriate. After three washes, bound antibodies were detected with H2O2 and 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) (ABTS; Kirkegaard & Perry, Gaithersburg, Md.), and A405 was quantified with a Molecular Devices Corp. (Menlo Park, Ca.) Vmax plate reader. IgG levels that were less than or equal to twice the background level in serum samples from naive mice or rabbits were assigned the minimum titer of 1:100 or 1:500, respectively.
RESULTS
DbpA is surface accessible.
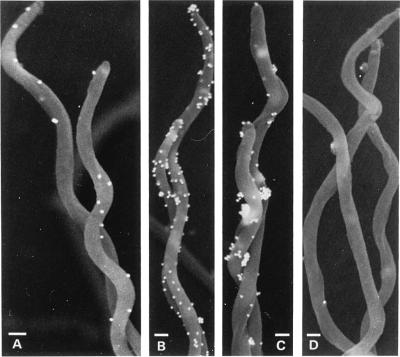
Determination of the subcellular location of B. burgdorferi proteins is complicated by the chemical and mechanical fragility of the spirochetal outer membrane (13). We used scanning immunoelectron microscopy to evaluate the location of DbpA and OspA on in vitro-grown unfixed B. burgdorferi sensu stricto isolate B31 and to confirm the integrity of the antibody-labeled cells. Antibodies against both DbpA and OspA bound to the surface of intact borreliae (Fig. 2). Anti-DbpA labeling was uniformly scattered along the spirochete surface (Fig. 2A). Occasionally spirochetes (fewer than 1 in 10) were observed to label heavily with anti-DbpA, suggesting variability in the expression or the accessibility of this protein among cells in this population (Fig. 2B). Anti-OspA labeling was typically more dense and tended to occur in aggregates, possibly reflecting antibody cross-linking of these target proteins (Fig. 2C). The heavier labeling of OspA was consistent with analyses by SDS-PAGE indicating that in vitro OspA levels were 5- to 10-fold-higher than DbpA (29) (data not shown). Periplasmic endoflagella, which are normally contained within the spirochetal outer membrane, did not protrude from these antibody-labeled cells, leading us to tentatively conclude that these membranes were intact and that DbpA and OspA were surface exposed. Endoflagellar filaments were observed protruding from other cells that were partially disrupted (data not shown). These observations do not preclude the possibility of additional quantities of these lipoproteins at a subsurface location, as suggested by others (13).
FIG. 2.
Detection of surface-directed antibody labeling by scanning immunoelectron microscopy. B. burgdorferi B31 was incubated in BSKII plus mouse anti-DbpA (A and B), mouse anti-OspA (C), or normal mouse serum (D) prior to labeling of bound antibodies with goat anti-mouse IgG plus IgM-conjugated colloidal gold particles. Typical electron micrographs for these samples are shown except for panel B, which represents spirochetes with atypically heavy anti-DbpA labeling. Bars, 0.2 μm.
Infection elicits strong antibody responses against DbpA and DbpB but not OspA.
Dermal inoculation with low numbers of B. burgdorferi organisms near the ID50 failed to elicit antibody responses to OspA but did elicit antibodies to other proteins early in the infection (3, 47). It has been proposed that such inoculations approximate the number of borreliae delivered by tick bites (47). We examined the kinetics and level of the antibody response of C3H mice to DbpA following s.c. inoculation with 102, 103, or 104 B31 spirochetes. Isolate B31 (uncloned) was chosen because it was one of the most infectious isolates and could be consistently cultured from multiple tissues (including bladder, ear, and joint) of infected mice. Three of 10 mice inoculated with 102 B31 became infected, and all mice were infected at the higher doses. Sera from infected mice within each dose group were pooled and analyzed for immune responses to specific borrelial proteins. Antibodies reactive with DbpA were detectable by immunoblotting within 2 weeks of infection with 102 borreliae, while OspA-specific antibodies remained at background levels within the first 8 weeks after infection (Fig. 3). Sera from culture-negative mice in the 102 dose group did not react with DbpA or OspA (data not shown), indicating that the amount of these proteins in 102 borreliae was subimmunogenic and suggesting that DbpA immune responses were against antigens synthesized in vivo during spirochete multiplication. At inoculum doses of 103 and 104 spirochetes, early IgG responses to both DbpA and OspA were observed, but anti-DbpA responses were strongest. This was particularly evident at 8 weeks, when anti-OspA reactivity waned and responses to DbpA increased (lanes 9 and 12). Transient anti-DbpA IgM was detectable at all inoculum doses, but anti-OspA IgM responses were much weaker relative to background (lane 3) and were detectable only at inoculum doses of 103 and 104 spirochetes (lanes 8 and 11).
FIG. 3.
Early antibody responses to DbpA and OspA elicited by low-dose challenge. Multilane immunoblot showing antibodies reactive with DbpA and OspA at weeks 2, 4, and 8 postchallenge (lanes 4 to 12) in sera pooled from mice infected by challenge with 102, 103, or 104 B. burgdorferi (B.b.) B31 spirochetes. Lanes: 1, anti-DbpA MAb, 1.0 μg/ml; 2, anti-OspA MAb, 0.2 μg/ml; 3, normal mouse serum. Samples for SDS-PAGE were 2 μg of purified Lpp1:DbpA297 or OspAB31.
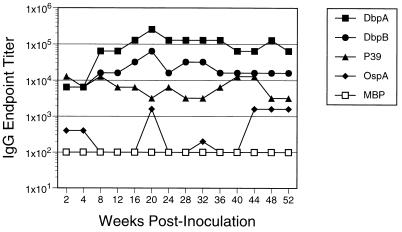
We extended serologic analysis to include antibody reactivity to DbpB and the lipoprotein P39 (BmpA), as well as DbpA and OspA, using an ELISA and serum pools from mice with culture-verified infection collected at intervals after inoculation with 102 B31 spirochetes. Others have previously shown that mice elicited antibodies to P39 when inoculated with live borreliae by syringe or tick bite but not with killed borreliae (52). At nearly every time point, the relative reactivity of antibodies against DbpA was greater than that against DbpB, which in turn was greater than antibody reactivity against P39 (Fig. 4). Antibody reactivity against OspA was near background (i.e., MBP) during early infection but rose somewhat at later times.
FIG. 4.
Relative levels of IgG antibodies to DbpA, DbpB, and other selected borrelial antigens during chronic infection of mice. Sera were collected for a 1-year period from mice infected by challenge with 102 B31. Sera were pooled at each time point, and end point titers of IgG specific for each of four recombinant borrelial antigens were determined by ELISA. E. coli MBP, the fusion partner for recombinant P39, was used as a negative control protein.
Passive immunization of mice with DbpA and DbpB antisera.
As we initially lacked an isolate of B. burgdorferi 297 that was infectious for mice, isolate B31 was chosen for our first protection studies since it was found to be sensitive to killing in vitro by both preDbpA and OspA antisera (Table 1). Additionally, we sought to determine if antiserum against DbpA would passively protect mice against challenge with a heterologous B. burgdorferi sensu stricto isolate. Rabbit antiserum against pre-DbpA297 and antiserum against OspAB31 were passively administered at the time of challenge with 104 B. burgdorferi B31 spirochetes. Undiluted, both antisera protected mice from infection (Table 3; data not shown). The ELISA IgG titers of the rabbit pre-DbpA297 and OspAB31 antisera against their homologous recombinant antigens were comparable (∼256,000). When 0.1 ml of antiserum was given at the time of challenge, the ED50 values of the DbpA and OspA sera were comparable (1:11.2 versus 1:7.5). A second administration of OspA serum on day 2 had no effect, whereas a second administration of DbpA antiserum was much more effective than the single treatment on day 0 (Table 3). It is noteworthy that the higher ED50 for the anti-DbpA297 serum, relative to the anti-OspAB31 serum, was obtained with the B31 isolate, which was heterologous to the DbpA297 antigen. Passive immunization of C3H mice with rabbit His-DbpB297 antiserum (ELISA IgG titer of 128,000) showed only partial protection against challenge with 104 B. burgdorferi B31 spirochetes (two mice protected of five) and no protection against challenge with 104 B. burgdorferi N40 spirochetes (no mice protected of five). Passive immunization against DbpB was not evaluated further in this study.
TABLE 3.
Relative potencies of rabbit pre-DbpA297 and OspAB31 antisera for passive protection of C3H mice from challenge with B. burgdorferi B31
| Antiserum (0.1-ml dose) | Day(s) of antiserum administration | No. of mice infected for serum dilution of:
|
ED50 | |||
|---|---|---|---|---|---|---|
| None (undiluted) | 1:5 | 1:25 | 1:125 | |||
| Pre-DbpA297 | 0 | 0/3 | 1/3 | 2/3 | 3/3 | 1:11.2 |
| 0, 2 | 0/3 | 0/3 | 0/3 | 2/3 | 1:83.1 | |
| OspAB31 | 0 | 0/3 | 1/3 | 3/3 | NDa | 1:7.5 |
| 0, 2 | 0/3 | 1/3 | 3/3 | ND | 1:7.5 | |
ND, not determined.
Elimination of infection by postinoculation administration of DbpA, but not OspA, antisera.
Passive protection results suggested that DbpA may be accessible to antibodies for longer than OspA after challenge and that these relative changes in protein expression or accessibility happen in periods as short as 2 days. The possibility that DbpA is a target for protective antibodies after OspA is no longer accessible was addressed further by administering antisera against both of these antigens to mice at increasing intervals after challenge (Table 4). Cohorts of 30 or 36 C3H mice were inoculated with 104 B. burgdorferi B31 spirochetes, and then groups of three mice each received antisera starting at 0, 2, 4, 5, 6, 7, or 10 days later. In two separate experiments, passive transfer of rabbit pre-DbpA297 antiserum completely protected mice from infection with B. burgdorferi B31, even when serum administration was delayed as long as 4 days but not 5 or more days postinoculation. OspAB31 antisera were protective when administered at the time of challenge, but not at later times, similar to observations of others (48). Our observations showed that DbpA, unlike OspA, was a target for antibody-mediated elimination of borreliae during the early stage of infection and provided further evidence that the relative in vivo levels or accessibility of DbpA and OspA change during this period.
TABLE 4.
Postchallenge administration of rabbit pre-DbpA297 antiserum aborts infection of B. burgdorferi B31
| Expt no.a | Antiserum | No. of mice infected on day
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 5 | 6 | 7 | 10 | ||
| 1 | Pre-DbpA297 | 0/3 | 0/3 | 0/3 | NDb | ND | 3/3 | 3/3 |
| OspAB31 | 0/3 | 3/3 | 3/3 | |||||
| PspAc | 3/3 | |||||||
| None | 3/3 | |||||||
| 2 | Pre-DbpA297 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| OspAB31 | 0/3 | 3/3 | 3/3 | |||||
| PspA | 3/3 | |||||||
| None | 3/3 | |||||||
In experiment 1, mice were given a single passive administration of rabbit antiserum (0.1 ml i.p.) at the time of challenge (day 0) or on one of several intervals afterward. On day 17, mice were sacrificed and evaluated for infection. Data are reproduced from Vaccines ’97 (10a) with permission of the publisher. In experiment 2, serum was given in two 0.05-ml doses 48 h apart, starting at the interval indicated.
ND, not determined.
PspA, pneumococcal surface protein A, irrelevant immunogen.
Active immunization of mice with DbpA protects against infection.
C3H and BALB mice were actively hyperimmunized with three forms of recombinant DbpA297 differing only at their amino termini, one form of DbpAB31, and one form of DbpAN40 and then challenged with both homologous and heterologous B. burgdorferi sensu stricto isolates (Table 2). Immunizations with DbpA or OspA preparations elicited high titers of serum IgG (ELISA end point titers were >105). C3H mice immunized with either His-DbpA297 or Lpp1:DbpA297 were protected from challenge with the homologous 297 isolate as judged by culture and P39 seroconversion (experiment A). C3H mice immunized with Lpp2:DbpAN40His were protected against N40 homologous challenge (experiment B). However, OspAB31 did not confer protection against the heterologous N40 challenge (experiment B). BALB mice immunized with pre-DbpA297 were protected from challenge with the heterologous B31 isolate (experiment C), but protection of C3H mice from B31 challenge by immunization with the various forms of DbpA was less complete (experiments C, D, and E). Although protection of C3H mice against B31 challenge was somewhat higher in one experiment with His-tagged versions of DbpA (experiment E), more-extensive studies are required to determine whether this form of the immunogen elicits a more potent immune response in mice. Preliminary experiments comparing immunizations with 5 or 20 μg His-DbpA indicated that fewer mice were protected by the lower DbpA dose (2 of 5 protected at 5 μg versus 4 of 5 at 20 μg). Using Freund’s adjuvants, we did not observe any substantial difference in immunogenicities between the acylated Lpp1:DbpA297 and nonacylated pre-DbpA297 and His-DbpA297. Such differences may be more apparent with other adjuvants that are clinically relevant, as has been observed for OspA (2, 16, 33).
In almost every DbpA-immunized mouse where infection (positive bladder culture and P39 IgG) was observed, infection of ear and joint tissues was below detectable levels (Table 2). Unlike borreliae cultured from naive or sham-immunized mice, spirochetes recovered from bladders of the DbpA-immunized C3H mice were elongated and morphologically irregular, had reduced motility, and became nonviable before exceeding 106 cells/ml. We were unable to propagate sufficient quantities of these spirochetes to permit further physiological or genetic analyses.
As was the case with passive immunization against DbpB, only partial protection against challenge with 104 B. burgdorferi B31 spirochetes was achieved by active immunization of mice with His-DbpB297. Three of five BALB mice were protected, but all five C3H mice immunized with His-DbpB297 were infected at multiple sites.
Passive immunization of mice against diverse B. burgdorferi sensu stricto isolates.
We next compared the abilities of rabbit DbpA and OspA antisera to protect C3H mice against challenge with five B. burgdorferi sensu stricto isolates and isolate 25015, which is classified as phylogenetically distinct by some typing methods (10, 39), to assess the conservation of protective epitope(s) on these antigens (Table 5). Antiserum against pre-DbpA297 protected C3H mice against challenge with the three genetically heterologous B. burgdorferi sensu stricto isolates B31, Sh-2-82, and N40. Significantly, DbpAN40 shares only 67% amino acid sequence identity (75% similarity) with DbpA297 (44), demonstrating broad reactivity of rabbit DbpA antiserum. Pre-DbpA297 antiserum gave broader protection than anti-OspAB31 serum against these isolates (Table 5). Isolates 25015, HB19, and CA-3-87 were resistant to both passively administered pre-DbpA297 and OspAB31 antisera. With respect to OspA heterogeneity, the lack of cross-protection between OspA of B. burgdorferi sensu lato isolate 25015 and OspA from a B. burgdorferi sensu stricto isolate (N40) has previously been observed by others (21).
TABLE 5.
Comparison of pre-DbpA297 and OspAB31 antiserum for protection of mice against challenge with heterologous B. burgdorferi isolates
| Expt no. and B. burgdorferi sensu lato challenge isolatea | No. of mice infectedb
|
||
|---|---|---|---|
| DbpA antiserum | OspA antiserum | No serum | |
| 1 | |||
| B31 | 0/5 | 0/5 | 5/5 |
| Sh-2-82 | 0/5 | 1/5 | 5/5 |
| N40 | 0/5 | 5/5 | 5/5 |
| 2 | |||
| B31 | 0/5 | 0/5 | 5/5 |
| 25015 | 5/5 | 5/5 | 5/5 |
| 3 | |||
| B31 | 0/5 | 0/5 | 5/5 |
| HB19 | 5/5 | 5/5 | 5/5 |
| CA-3-87 | 5/5 | 5/5 | 5/5 |
Challenge dose with HB19 was 105 borreliae; the challenge dose with other isolates was 104.
Rabbit pre-DbpA297 or OspAB31 antiserum (0.05 ml) was administered 1 h before challenge (day 0) and again on day 2 postchallenge. Data are reproduced from Vaccines ’97 (10a) with permission of the publisher.
Conservation of DbpA epitope(s) binding growth-inhibitory antibodies.
We next examined a large panel of B. burgdorferi sensu lato isolates with diverse geographic and biologic origins and representing six of the major phylogenetic groups (10, 39, 53) using a microwell-based growth inhibition assay (45) and by immunoblotting to evaluate the in vitro expression and serologic conservation of DbpA and OspA among these isolates (Table 1). To supplement immunoblotting, we also used a decorin blot assay (29) to evaluate expression of DbpA and DbpB. Sensitivities of these isolates to growth inhibition by rabbit pre-DbpA297 or OspAB31 antisera were assessed in parallel. Growth of 19 of the 35 B. burgdorferi sensu lato isolates (54%) was inhibited (defined as growth inhibitory titer of ≥100) by pre-DbpA297 antiserum. This growth-inhibitory effect did not require addition of serum complement, similar to observations by others using this assay (45). Most isolates of B. burgdorferi sensu stricto (12 of 17 [71%]) and nearly half of the isolates of B. afzelii (2 of 6) and B. garinii (4 of 9) were inhibited by pre-DbpA297 antiserum (range of inhibitory titers, 100 to 12,800). In some cases resistance to DbpA antiserum correlated with weak or negative reactions by immunoblotting and decorin blotting (HB19, CA-3-87, and 25015); these isolates apparently expressed little or no DbpA in vitro although PCR analysis indicated that each isolate contained a dbpA gene homolog (44). Some B. garinii isolates were inhibited weakly, or not at all, by pre-DbpA297 antiserum but were positive by decorin blotting (PBi, B4 91, G2.22, and IP89). Isolate PBi was inhibited by pre-DbpA297 antiserum but was negative by immunoblotting. For a few isolates (FRED and IPF), decorin binding activity appeared to be lost after SDS-PAGE, yet the isolates remained immunoreactive. Possibly differences in DbpA in vitro expression levels, in epitope structure, and in antibody accessibility, all contributed to differences in anti-DbpA inhibitory end point titers among these isolates. About half of the isolates (17 of 35 [49%]) were inhibited by OspAB31 antiserum (range of inhibitory titers, 100 to 51,000) even though all 35 isolates were positive for OspA expression by immunoblotting. The OspAB31 antiserum had negligible inhibitory activity against the nine B. garinii isolates. Thus, DbpA epitopes capable of binding growth-inhibitory antibodies appeared to be as conserved as, if not more conserved than, those of OspA. Most of the isolates (26 of 35 [74%]) were inhibited by at least one of the two antisera, suggesting that combinations of these two antigens might provide broader vaccine coverage than either one alone.
Rabbit His-DbpB297 antiserum was also tested for in vitro growth inhibition activity. This antiserum had negligible effect on the in vitro growth of B. burgdorferi B31, Sh-2-82, and N40 (data not shown); therefore, this analysis was not expanded further. However, all three isolates were positive for DbpB expression by immunoblotting (data not shown), demonstrating that they expressed DbpB in vitro.
DISCUSSION
Many proteins of B. burgdorferi, including Osps A-F, 41-kDa flagellin, IpLA-7, P21, P30, P35, P37, P39, P55, P83, pG, and lp6.6, have been evaluated as targets for protection in animal models of Lyme borreliosis (14–17, 19–24, 27, 33, 36, 38, 40–42, 48, 54, 59, 60). Among these, only antibodies to OspA, OspB, and OspC have been shown to prevent infection completely. Combined administration of antisera against two in vivo-expressed proteins, P35 and P37, 1 day after homologous challenge also provided protection in a recent study (22) but only against 102, but not 104, spirochetes. The duration of P35/P37 target accessibility beyond day 1, or the level of serologic conservation of these antigens, was not reported. We show here that DbpA is accessible to antibodies capable of aborting infection up to 4 days after inoculation and is serologically cross-reactive among many borrelial isolates. In contrast, antibodies to OspA were ineffective at aborting established infection, as previously reported by others (48).
DbpA and DbpB are relatively minor proteins of cultured B. burgdorferi compared to OspA (28, 29). The antigenic stimulus provided to mice by a minimal infectious dose (102) of B. burgdorferi B31 was insufficient to elicit immune responses to these proteins in mice that failed to become infected, but persistent and high titers of DbpA and DbpB antibodies, and not OspA antibodies, were observed in culture-positive mice. These observations provide evidence that DbpA and DbpB are expressed in vivo during infection.
DbpA antibodies bound specifically to the surface of intact cultured spirochetes (Fig. 2) and were able directly to inhibit the growth of B. burgdorferi both in vivo and in vitro (Tables 1, 2, and 5). Similar observations were also made for OspA antibodies. However, the experiments evaluating the postchallenge protective efficacies of these antibodies were able to distinguish major differences in the expression level or surface accessibility of OspA, relative to that of DbpA, during the first 4 days after dermal inoculation of spirochetes (Tables 3 and 4). This decrease in expression or accessibility of OspA can be taken as evidence that the spirochetes are undergoing a host adaptation process at this stage of the infection. Methods capable of directly demonstrating the in vivo surface accessibility of B. burgdorferi outer membrane components are not currently available. The observation that B. burgdorferi remains sensitive to the growth-inhibitory effects of DbpA antiserum after a 4-day period of host adaptation provides evidence that DbpA, and not OspA, is surface accessible in vivo during this stage of infection. This is the first report demonstrating evidence of in vivo surface accessibility of a defined target in this manner. In order for decorin adherence (29) to play a role in the virulence of B. burgdorferi, decorin binding proteins would presumably require in vivo surface exposure.
Others have shown that passive transfer of immune sera from persistently infected mice to naive mice will eliminate spirochetes in newly infected mice at 4 days postinfection but will not eliminate infection when immune serum is administered at 12 days postinfection (4). Antigenic targets for this effect have not been identified, but DbpA may be such a target. In the present study, spirochetes remained sensitive to the effects of DbpA antiserum through the first 4 days of infection in mice but became resistant to DbpA antiserum administrations at later times. Several mechanisms for this resistance are possible: (i) DbpA expression becomes down-regulated during or after infection; (ii) DbpA becomes inaccessible on the spirochete to antibodies; or (iii) spirochetes are sequestered in cells or tissues inaccessible to antibodies. A model suggesting that surface-exposed lipoproteins may be globally down-regulated in vivo by spirochetes or differentially localized during infection as part of the host adaptation process has been recently proposed (13). Our observations are consistent with this model but suggest that this may happen in a stage-specific or tissue-specific manner and that changes in lipoproteins occur in a programmed, rather than global, fashion since OspA and DbpA lipoproteins appeared to lose antibody-mediated vulnerability at different times during early infection (Table 4). In C3H mice the dissemination of B. burgdorferi from the site of dermal inoculation by syringe begins about 3 to 4 days postinoculation (8). This coincides with the time at which DbpA antibodies were no longer effective at aborting infection in the present studies. For instance, decorin adherence may contribute to establishing localized infection in collagen-rich connective tissues yet may be dispensable during spirochete dissemination. B. burgdorferi shows tropism for the skin of infected animals (5, 8), and cutaneous manifestations of human Lyme borreliosis are seen in both early and late disease (53). Immune response-mediated disease resolution has been observed in the immunocompetent mouse model of Lyme disease (3, 4) and has been interpreted to explain intermittent remissions during human Lyme disease. Anti-DbpA responses may contribute to these effects and to the control of persistent infection. The sustained anti-DbpA, as well as DbpB and P39, IgG levels in persistently infected mice may be a result of an immune stimulus by spirochetes when they periodically exit a putative immunity-privileged niche and ultimately become eliminated by the host’s immune response.
Active immunization of C3H mice with DbpA conferred complete, or nearly complete, protection against challenge with 104 spirochetes of the isolate homologous to the immunogen. Immunization with DbpA conferred complete protection against infection following challenge with a heterologous B. burgdorferi sensu stricto isolate in BALB mice and in some C3H mice and appeared to limit dissemination in the remaining C3H mice. Spirochetes were recovered only from bladders, and not from ears or joints, of the partially protected DbpA-immunized mice, and these spirochetes were highly moribund. Although C3H mice hyperimmunized with OspAB31 were protected from dermal challenge with 104 spirochetes of the homologous B31 isolate, OspA immunity can be overcome by challenge doses greater than 105 (23) (data not shown). The spirochetes recovered from OspA-immunized mice in the study by Fikrig et al. (23) were shown to be ospA escape mutants and remained viable and infectious, unlike the spirochetes recovered from DbpA-immunized mice. Future experiments will evaluate the disease status of DbpA-immunized mice harboring reduced numbers of spirochetes at 2 weeks postchallenge and will determine whether they eventually eliminate their infections. It is not known at this time whether the mice partially protected from infection also had reduced severity of disease or duration of symptoms.
Passive immunization with rabbit antiserum against DbpA297 appeared to elicit more-complete and broader protection of C3H mice than active immunization. This response included protection against isolate N40 expressing DbpA having only 67% amino acid sequence identity (75% similarity) with the DbpA297 immunogen (44). Additionally, we found that two passive immunizations of as little as 4 μl of the rabbit pre-DbpA297 antiserum completely protected C3H mice from challenge with 104 B. burgdorferi B31 spirochetes (Table 3), but two passive immunizations of the equivalent of 4 μl of BALB or C3H mouse pre-DbpA297 antiserum (with even higher DbpA IgG ELISA titers) did not protect naive mice from a similar challenge (data not shown). Differences in the potencies of the various sera may be due to differences in the responses of mice, which demonstrate a persistent B. burgdorferi infection (5, 8), and rabbits, which can clear a B. burgdorferi infection (25), to protective versus nonprotective epitopes or the relative avidities of the antibodies contained in the sera. We do not yet know how broad heterologous protection by DbpA antibodies may be in nonmurine species, but our in vitro results summarized in Table 1 provide encouragement.
By immunoblotting, and by blotting with tagged decorin, isolates HB19, CA-3-87, and 25015 were found to express little or no DbpA in vitro (Table 1), indicating that these borreliae had few DbpA targets at the time of challenge. Interestingly, sera from naive mice infected with HB19 or CA-3-87 reacted with purified Lpp1:DbpA297 by immunoblotting (data not shown), suggesting that expression of dbpA by these isolates was up-regulated in vivo after infection began, possibly at the time when the passively administered DbpA297 antiserum had diminished to levels below which protection may have otherwise been provided.
The high levels of antibodies to DbpB elicited during persistent infection of C3H mice suggested that, like DbpA, DbpB is also expressed in vivo (Fig. 4). However, the results of protection and in vitro growth inhibition studies suggest that DbpB is less vulnerable to antibodies or is expressed at lower levels than DbpA in vivo, or both. The DbpB sequences of the isolates examined in this study share >98% identity with DbpB297 (44), suggesting that serologic variation is not an explanation for their lack of sensitivity to DbpB297 antibodies. As we observed that rabbit anti-His-DbpB297 was able to protect mice partially against a challenge with 104 borreliae, it appears that accessibility of DbpB to antibodies may differ in vivo and in vitro. In this regard, others have shown that antibodies from OspC-immunized mice that were protected against infection had very weak in vitro growth-inhibitory activity against the same borrelial isolate used for challenge (41, 42). These preliminary experiments suggested that DbpB is a less effective target for protection than DbpA, but additional studies are required to confirm this possibility.
Like the initial reports showing protective immunity targeting OspA (19, 48) and OspC (40), we have utilized cultured B. burgdorferi to demonstrate the protective effect of anti-DbpA immunity. We have also shown that cross-protection among heterologous DbpAs is feasible. The next and most critical step in the evaluation of DbpA vaccines will be to assess whether DbpA immunity will also confer protection against the tick-borne route of infection as was eventually shown for OspA and OspC (24, 27). The protective effects of an anti-DbpA immune response may extend beyond the transmission-blocking effect of an OspA immune response, which is apparently effective only against the spirochete’s arthropod stage in the tick midgut. If so, immune responses against DbpA may surpass the efficacy of those elicited by OspA-based vaccines by acting in concert with immune effector functions in vivo. Furthermore, mixtures of DbpA and OspA may be synergistic for efficacy. A multistage, multiantigen strategy is under consideration for vaccines against Plasmodium falciparum malaria, another arthropod-borne pathogen with developmental regulation of antigen expression (31, 56). This strategy may also be effective for protection against both infection and disease caused by B. burgdorferi.
ACKNOWLEDGMENTS
We thank Alan Barbour and coworkers for advice on culture and manipulation of borrelia and Stephen Barthold for sharing important information on the mouse model for Lyme disease, including unpublished data. We thank Scott Koenig for thoughtful discussions throughout this study and for review of the manuscript. We also thank Lorne Erdile and Patrick McVerry of Connaught Laboratories, Inc., for purified OspA and PspA used for rabbit antiserum. We gratefully acknowledge Will Roberts, David Wood, Shawn Offutt, Debra Couchenour, Wendy White, and Kannaki Senthil for their technical assistance.
This study was supported in part by NIH grant AI39865 to M.S.H.
REFERENCES
- 1.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold S W, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Invest. 1996;74:57–67. [PubMed] [Google Scholar]
- 5.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt M A, Riley B S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casjens S, Delange M, Levy III H L, Rosa P, Huang W M. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J Bacteriol. 1995;177:2769–2780. doi: 10.1128/jb.177.10.2769-2780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Cassatt D R, Patel N K, Hanson M S, Guo B P, Höök M. Protection burgdorferi infection by antibodies to decorin-binding protein. In: Brown F, Burton D, Doherty P, Mekalanos J, Norby E, editors. Vaccines ’97. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 191–195. [Google Scholar]
- 11.Chang Y F, Appel M J G, Jacobson R H, Shin S J, Harpending P, Straubinger R, Patrican L A, Mohammed H, Summers B A. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect Immun. 1995;63:3543–3549. doi: 10.1128/iai.63.9.3543-3549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H U, Johnson T L, Pal S, Tang L H, Rosenberg L, Neame P J. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-sepharose chromatography. J Biol Chem. 1989;264:2876–2884. [PubMed] [Google Scholar]
- 13.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, Barthold S W, Stocker Giles S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Invest. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Das S, Shraga D, Gannon C, Lam T T, Feng S, Brunet L R, Telford S R, Barthold S W, Flavell R A, Fikrig E. Characterization of a 30-kDa Borrelia burgdorferi substrate-binding protein homologue. Res Microbiol. 1996;147:739–751. doi: 10.1016/s0923-2508(97)85121-2. [DOI] [PubMed] [Google Scholar]
- 15.deSilva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdile L F, Brandt M A, Warakomski D J, Westrack G J, Sadziene A, Barbour A G, Mays J P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng S, Barthold S W, Telford III S R, Fikrig E. P55, an immunogenic but nonprotective 55-kilodalton Borrelia burgdorferi protein in murine Lyme disease. Infect Immun. 1996;64:363–365. doi: 10.1128/iai.64.1.363-365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fikrig E, Barthold S W, Flavell R A. OspA vaccination of mice with established Borrelia burgdorferi infection alters disease but not infection. Infect Immun. 1993;61:2553–2557. doi: 10.1128/iai.61.6.2553-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 20.Fikrig E, Barthold S W, Marcantonio N, Deponte K, Kantor F S, Flavell R A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fikrig E, Barthold S W, Persing D H, Sun X, Kantor F S, Flavell R A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992;148:2256–2260. [PubMed] [Google Scholar]
- 22.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 23.Fikrig E, Tao H, Barthold S W, Flavell R A. Selection of variant Borrelia burgdorferi isolates from mice immunized with outer surface protein A or B. Infect Immun. 1995;63:1658–1662. doi: 10.1128/iai.63.5.1658-1662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley D M, Gayek R J, Skare J T, Wagar E A, Champion C I, Blanco D R, Lovett M A, Miller J N. Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J Clin Invest. 1995;96:965–975. doi: 10.1172/JCI118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Frazer C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M S, Van Vugt R, Palmer N A, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 26.Gern L, Schiable U E, Simon M M. Mode of infection of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis. 1993;167:971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- 27.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J B. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Höök. 1996. Unpublished data.
- 29.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson L, Noppa L, Nilsson A K, Stromqvist M, Bergström S. Expression of truncated and full-length forms of the Lyme disease Borrelia outer surface protein A in Escherichia coli. Protein Expr Purif. 1995;6:15–24. doi: 10.1006/prep.1995.1003. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman S L, Sacci J B., Jr . Rationale and approaches to constructing preerythrocytic malaria vaccines. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1997. pp. 787–803. [DOI] [PubMed] [Google Scholar]
- 32.Howe T R, Mayer L W, Barbour A G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985;227:645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- 33.Johnson B J B, Sviat S L, Happ C M, Dunn J J, Frantz J C, Mayer L W, Piesman J. Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by mAb LA-2. Vaccine. 1995;13:1086–1094. doi: 10.1016/0264-410x(95)00035-y. [DOI] [PubMed] [Google Scholar]
- 34.Kalish R A, Leong J M, Steere A C. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–2235. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller D, Koster F T, Marks D H, Hosbach P, Erdile L F, Mays J P. Safety and immunogenicity of a recombinant outer surface protein A Lyme vaccine. JAMA. 1994;271:1764–1768. [PubMed] [Google Scholar]
- 36.Meurice F, Parenti D, Fu D, Krause D S. Specific issues in the design and implementation of an efficacy trial for a Lyme disease vaccine. Clin Infect Dis. 1997;25:S71–S75. doi: 10.1086/516167. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen T P, Lam T T, Barthold S W, Telford III S R, Flavell R A, Fikrig E. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect Immun. 1994;62:2079–2084. doi: 10.1128/iai.62.5.2079-2084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 40.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 41.Probert W S, Crawford M, Cadiz R B, LeFebvre R B. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 42.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 44.Roberts, W. C., R. Lathigra, and M. S. Hanson. 1996. Unpublished data.
- 45.Sadziene A, Thompson P A, Barbour A G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47.Schiable U E, Gern L, Wallich R, Kramer M D, Prester M, Simon M M. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993;36:219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 48.Schiable U E, Kramer M D, Eichman K, Modelell M, Museteanu C, Simon M M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (SCID) mice. Proc Natl Acad Sci USA. 1990;87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schutzer S E, Coyle P K, Dunn J J, Luft B J, Brunner M. Early and specific antibody response to OspA in Lyme disease. J Clin Invest. 1994;94:454–457. doi: 10.1172/JCI117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schutzer S E, Coyle P K, Krupp L B, Deng Z, Belman A L, Dattwyler R, Luft B J. Simultaneous expression of Borrelia OspA and OspC and IgM response in cerebrospinal fluid in early neurologic Lyme disease. J Clin Invest. 1997;100:763–767. doi: 10.1172/JCI119589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson W J, Burgdorfer W, Schrumpf M E, Karstens R H, Schwann T G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991;29:236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steere A C. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci USA. 1994;91:2378–2383. doi: 10.1073/pnas.91.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stover C K, Bansal G P, Hanson M S, Burlein J E, Palaszynski S R, Young J F, Koenig S, Young D B, Sadziene A, Barbour A G. Protective immunity elicited by recombinant Bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med. 1993;178:197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 56.Tine J A, Lanar D E, Smith D M, Wellde B T, Schultheiss P, Ware L A, Kauffman E B, Wirtz R A, DeTaisne C, Hui G S, Chang S P, Church P, Hollingdale M R, Kaslow D C, Hoffman S, Guito K P, Ballou W R, Sadoff J C, Paoletti E. NYVAC-pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996;64:3833–3844. doi: 10.1128/iai.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Hoecke C, Comberbach M, De Grave D, Desmons P, Fu D, Hauser P, Lebacq E, Lobet Y, Voet P. Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) lyme vaccines in healthy adults. Vaccine. 1996;14:1620–1626. doi: 10.1016/s0264-410x(96)00146-6. [DOI] [PubMed] [Google Scholar]
- 58.Van Mierlo P, Jacob W, Dockx P. Erythema chronicum migrans: an electron-microscopic study. Dermatology. 1993;186:306–310. doi: 10.1159/000247384. [DOI] [PubMed] [Google Scholar]
- 59.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallich R, Simon M M, Hofmann H, Moter S E, Schiable U E, Kramer M D. Molecular and immunological characterization of a novel polymorphic lipoprotein of Borrelia burgdorferi. Infect Immun. 1993;61:4158–4166. doi: 10.1128/iai.61.10.4158-4166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama W M. Production of monoclonal antibodies. In: Coligan J E, Kruisbeek A M, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1991. pp. 2.5.1–2.5.17. [Google Scholar]
- 62.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the PspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]