ABSTRACT
While bone tissue is known for its inherent regenerative abilities, various pathological conditions and trauma can disrupt its meticulously regulated processes of bone formation and resorption. Bone tissue engineering aims to replicate the extracellular matrix of bone tissue as well as the sophisticated biochemical mechanisms crucial for effective regeneration. Traditionally, the field has relied on external agents like growth factors and pharmaceuticals to modulate these processes. Although efficacious in certain scenarios, this strategy is compromised by limitations such as safety issues and the transient nature of the compound release and half-life. Conversely, bioactive elements such as zinc (Zn), magnesium (Mg) and silicon (Si), have garnered increasing interest for their therapeutic benefits, superior stability, and reduced biotic risks. Moreover, these elements are often incorporated into biomaterials that function as multifaceted bioactive components, facilitating bone regeneration via release on-demand. By elucidating the mechanistic roles and therapeutic efficacy of the bioactive elements, this review aims to establish bioactive elements as a robust and clinically viable strategy for advanced bone regeneration.
Keywords: bioactive elements, biomaterials, bone organoid, bone regeneration, controllable release
Introduction
Bone is a complex and hierarchical organ, the remodelling of which depends on specific macro- and micro-environments. The primary function of bone tissue is to provide mechanical stability to the body and protect major organs. Additionally, bone tissue exhibits high metabolic turnover, aiding in maintaining ionic balance within the body.1-3 Diseases or traumatic injuries can impair bone function, making it crucial to restore lost functionality swiftly and efficiently. Autografts and allografts remain the benchmark in tissue engineering, yet their clinical utility is curtailed by supply constraints and the risk of disease transmission.4-6 In light of this, there is an urgent need to develop alternative bone substitutes through bone tissue engineering, which could offer functionalities similar to natural grafts while avoiding associated issues.
For successful bone regeneration, it is vital that bone substitutes mimic the highly ordered steps of bone regeneration to a maximum extent. Bone repair is a dynamic biological process evolving over time, mainly involving post-operative bleeding, clot formation, inflammatory response, angiogenesis, and new bone formation.7 The regulation at each stage impacts subsequent biological events and ultimately determines the pace and quality of bone regeneration. The primary focus has been on integrating growth factors into bone tissue scaffolds or implants.8-10 Growth factors like insulin-like growth factors can activate cellular signalling cascades in stem cells surrounding bone lesions, thereby inducing proactive repair, including the angiogenesis process crucial for tissue regeneration.11, 12 However, the use of these growth factors presents significant drawbacks, including instability, immunogenicity, and high costs.13-15 Recently, many small molecules such as peptides have attracted researchers’ attention. Yet, controlling their proper release in the defect area and their low half-life once implanted in the defect area are major barriers to their clinical translation.16-18
In contrast, employing bioactive elements is an appealing option, given their known therapeutic effects, higher stability, and lower risks compared to using biomolecules. The therapeutic elements released from biomaterials can regulate tissue regeneration steps in a manner akin to bioactive molecules. In recent years, researchers have been exploring the role of multiple bioactive elements in bone regeneration. Evidence suggests that elements, such as calcium (Ca),19 cobalt (Co),20 copper (Cu),21 fluoride (F),22 lithium (Li),23 magnesium (Mg),24 silicon (Si),25 silver (Ag),26 strontium (Sr),27 zinc (Zn),28 can induce osteoprogenitor cell differentiation through growth factor signalling pathways or stimulate other processes supporting bone tissue growth.
Overall, this review meticulously delineates the impact of bioactive elements during various stages of bone regeneration, elucidating their mechanisms in osteoimmunomodulation, orchestrating neuroregulation, stimulating angiogenesis, and promoting osteogenesis (Figure 1). The comprehensive analysis of these bioactive ions not only furnishes a robust theoretical scaffold for ensuing basic research but also unveils novel therapeutic vistas and design principles for clinicians and materials scientists. Especially in the milieu of confronting the limitations inherent to natural grafts and growth factors, the exploration of bioactive elements emerges as a cost-effective, low-risk, and viable avenue for bone regeneration.
Figure 1. The role of bioactive elements in bone regeneration. Created with BioRender.com.
The Process of Bone Regeneration
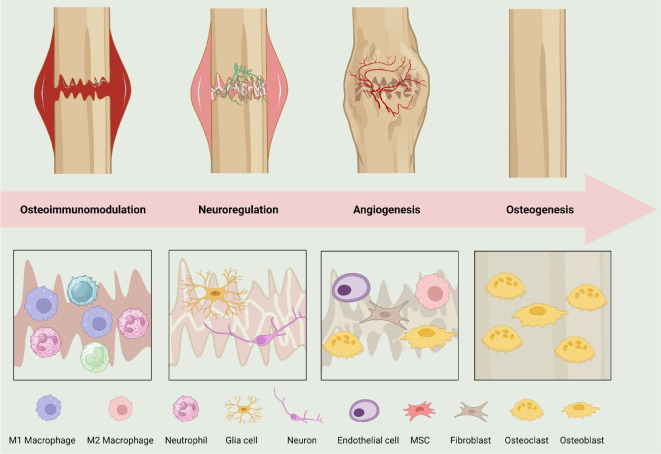
Bone regeneration unfolds through a meticulously orchestrated cascade of biological processes: osteoimmunomodulation, neuroregulation, angiogenesis, and bone formation (Figure 2). Initially, osteoimmunomodulation, mediated by macrophage polarisation, establishes a favourable inflammatory landscape.29 Concurrently, neuroregulation or skeletal interoception, steers physiological responses essential for bone healing.28 Advancing, angiogenesis, directed by endothelial progenitor and mature cells, forms new vasculature, delivering crucial resources to the regenerative callus.30 Lastly, osteogenesis, led by cellular entities like osteoblasts and mesenchymal stem cells (MSCs), governs the synthesis and remodelling of bone tissue.31
Figure 2. The process of bone regeneration. Created with BioRender.com. MSC: mesenchymal stem cell.
Osteoimmunomodulation
Osteoimmunomodulation represents a pivotal nexus where the realms of immunology and bone biology converge, under the auspices of bone biomaterials, to orchestrate a harmonious response between bone formation and resorption, thereby augmenting the bone repair capacity.19, 32-34 The crucible of this interplay lies in the role of immune cells, notably macrophages, whose interactions with bone cells and biomaterials significantly shape the trajectory and efficacy of bone repair endeavors.35-37 Macrophages, serving as the quintessential effector cells in immune responses to implants, are indispensable for promoting osteogenic functionality. The tapestry of immune-bone interplay is further enriched by the polarisation dynamics of macrophages into M1 and M2 subtypes, mediating pro-inflammatory and anti-inflammatory responses respectively, which are integral for bone repair across diverse stages.38 While M2 macrophages are emblematic for promoting bone tissue regeneration, the spotlight is gradually shifting towards unraveling the critical role of M1 macrophages in osteoimmunomodulation, particularly during the early inflammatory stages where they enhance the recruitment and commitment of angiogenic and osteogenic precursors.39 Recent scholarly endeavors have also unveiled the central role of exosomes, secreted by macrophages, in mediating osteoimmunomodulation. These exosomes, upon internalisation by pivotal cells engaged in de novo bone formation such as endothelial cells and osteoblasts, significantly intervene in osseointegration, thereby opening new frontiers in understanding and leveraging macrophage-mediated immune modulation for enhanced bone repair.40 In summation, osteoimmunomodulation, through the lens of macrophage-mediated interactions, unveils a complex yet rich array of mechanisms and interactions that not only deepen the comprehension of bone repair processes but also propel the development of novel therapeutic strategies and bone biomaterials.
Neuroregulation
Neuroregulation, a crucial mechanism orchestrated by the nervous system, governs the physiological and biochemical responses across various systems, organs, and cells in the body.41 This regulation is facilitated through the release of chemical substances, impacting an array of biological processes. In the realm of bone repair, neuroregulation, either known as skeletal interoception, emerges as a pivotal player in fostering the regeneration of bone tissues. It holds a significant sway in maintaining the equilibrium of bone mass. Moreover, an assortment of neural factors, including neurotrophic growth factors, neuropeptides, and prostaglandin E2 (PGE2), are known to exercise a substantial influence over the growth, regeneration, and repair processes of bone tissues.42 Neuroregulation manoeuvres the balance of bone remodelling through diverse mechanisms, such as the activation of TrkA signalling pathway in osteoblastic cells, modulation of Wnt signalling pathway, and regulation of PGE2 production and secretion, thereby propelling the process of bone rebuilding. The promising horizon of skeletal interoception in bone repair is further bolstered by the supplementary use of PGE2, which, by modulating the proliferation and differentiation of stem cells, and promoting bone regeneration, augments the processes of bone repair and regeneration.43-46 Hence, the domain of neuroregulation not only unveils a profound understanding of the intricate interplay between neural and bone tissues but also heralds a promising avenue for advancements in bone repair methodologies, thereby contributing significantly to the broader spectrum of regenerative medicine.
Angiogenesis
Angiogenesis, the formation of new blood vessels from pre-existing vessels, is a cornerstone of bone repair, serving as the conduit for essential resources to the regenerative callus, a temporary tissue formed during bone healing. This process is orchestrated by endothelial progenitor cells and mature endothelial cells through recruitment, proliferation, differentiation, and reconstruction, facilitating the sprouting of microvessels from existing blood vessels.47, 48 The newly formed vasculature is indispensable for delivering oxygen and nutrients to the metabolically active regenerating callus and facilitating the migration of inflammatory cells, as well as cartilage and bone precursor cells to the injury site, thereby providing a conducive environment for bone regeneration. The vitality of angiogenesis is underscored during fracture healing where the creation of new blood vessels is pivotal for supplying the requisite oxygen and nutrients to the evolving bone tissue. A sluggish or partial vascularisation process could hamper the supply of these essential resources to the bone defect area, which may lead to cell death, thus, emphasizing the need for prompt and robust angiogenesis during the bone regeneration. Notably, the angiogenic process is driven by various growth factors such as fibroblast growth factor, platelet-derived growth factor, and transforming growth factor-β (TGF-β), which invoke the proliferation, migration, differentiation, and vascularisation of endothelial cells and/ or endothelial progenitor cells.49, 50 The intricate biochemical and physical interplay between angiogenesis and bone repair processes elucidates a complex yet harmonious orchestration of events that are crucial for effective bone regeneration.
Osteogenesis
Osteogenesis is the pivotal process of bone tissue formation and remodelling, orchestrated chiefly by several cellular entities including osteoblasts, bone marrow-derived mesenchymal stem cells (BMSCs), osteoclasts, and osteocytes.51 Osteoblasts are the primary architects of bone formation. They migrate to the site of bone repair, especially at the implant-bone tissue interface during implant osseointegration, and govern the synthesis, secretion, and mineralisation of the extracellular matrix (ECM). Their activity is markedly enhanced by the expression of growth factors such as bone morphogenetic protein-2 (BMP-2) and TGF-β, which facilitate the formation and mineralisation of bone tissue.52, 53 BMSCs, residing within the bone structure, BMSCs hold the potential to differentiate into osteoblasts, a transformation triggered by signals such as the release of TGF-β1 from neighboring osteoclasts. This differentiation is a critical step towards the formation of new bone tissue, setting the stage for further maturation and mineralisation processes.54, 55 Osteoclasts are principally involved in bone resorption, a process vital for the subsequent bone formation by osteoblasts. The resorption pits created by osteoclasts serve as the sites for new bone formation, where osteoblasts deposit the new bone material. Moreover, the resorption process also releases factors that, in turn, activate osteoblast proliferation, maturation, and differentiation, establishing a coordinated homeostatic mechanism integral for bone remodelling.56 Osteocytes, originating from osteoblasts that become entrapped within the mineralized matrix, osteocytes play a crucial role in bone maintenance and remodelling. They are instrumental in attracting osteoclasts to implant sites, thereby facilitating the process of bone remodelling. In the process of bone regeneration, the orchestrated interplay of these cellular entities is indispensable.57, 58 Through a deeper understanding of the cellular and molecular mechanisms underlying osteogenesis, novel therapeutic strategies and biomaterials can be developed, potentially advancing orthopedic and dental applications.
The Role of Bioactive Elements in Bone Regeneration
The role of bioactive elements in osteoimmunomodulation
Ag
Chen et al.59 developed a titanium dioxide (TiO2) nanotube loaded with Ag nanoparticles (Ag@TiO2-NTs) that releases ultra-low amounts of Ag ions to improve immunoregulation and enhance bone repair. The study demonstrated that Ag@TiO2-NTs, releasing ultra-low-dose Ag ions, effectively induced M2 macrophage polarisation and promoted a favourable osteoimmune environment by modulating phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), glucose transporter 1, and autophagy in vitro. In vivo, Ag@TiO2-NTs enhanced bone formation, reduced inflammation, and promoted osteoimmune microenvironment compared to TiO2-NTs and polished Ti surfaces.
Boron
Boron (B) has been demonstrated to amplify pro-inflammatory conditions by facilitating lymphocyte proliferation and enhancing nitric oxide and pro-inflammatory cytokine secretion in lipopolysaccharide-activated macrophages from B-exposed mice.60 The precise molecular pathways involved are yet to be elucidated. Contrarily, B ions emanating from a calcium silicate layer on titanium surfaces have been found to downregulate pro-inflammatory cytokines and upregulate anti-inflammatory ones, potentially via attenuating the activation of the myeloid differentiation primary response 88-nuclear factor-κB (NF-κB) pathway.61
Ca
Ca, an essential mineral, plays a pivotal role in various physiological processes, including bone mineralisation, muscle contraction, and signal transduction in human body. Bioceramic degradation generated Ca ions, which triggered the Wnt/β-catenin signalling pathway through calcium-sensing receptor (CaSR).62 Blocking CaSR activity decreased the inflow of macrophage-promoting Ca ions, hindered Wnt/β-catenin signalling and the production of M2-like macrophages, and attenuated the MSC mineralisation promoted by the supernatants when they were treated with a CaSR antagonist. It implies that the Ca ions in bioceramics are dependent on CaSR-mediated Wnt/β-catenin activation for macrophage M2 polarisation and novo bone production. The Wnt/β-catenin pathway has been shown to be an important mediator of M2 polarisation in macrophages. During phage M2 polarisation, β-catenin is activated and transported to the nucleus, where it binds to the transcription factor T-cell factor/lymphoid enhancer factor family, triggering transcription of downstream target genes. As a major inhibitor of the Wnt/β-catenin signal axis, glycogen synthase kinase (GSK)3β can enhance the ubiquitination and degradation of β-catenin, diminish its protein stability, and limit its nuclear entrance in this pathway. By increasing the phosphorylation of downstream GSK3, phosphorylated AKT suppresses its function. It’s worth mentioning that Ca can play a role in Wnt/β-catenin signalling by boosting the phosphorylation of GSK3.19 Extracellular Ca influx may positively control the Wnt/β-catenin signalling pathway by increasing GSK3 phosphorylation, because GSK3 is a negative regulator of Wnt/β-catenin. As a result, increased intracellular Ca stimulated the PI3K/AKT signalling pathway and caused AKT phosphorylation, which was followed by decreased downstream activity GSK3 due to its higher phosphorylation, resulting in increased β-catenin translocation.63
Cu
Cu, a crucial micronutrient, functions as a cofactor in enzymes related to respiration, oxygen transport, and antioxidant defence. Cu ions can trigger oxidative damage, potentially leading to DNA damage and low-density lipoprotein peroxidation.64, 65 For instance, some research demonstrates the ability of Cu ions to catalyse the production of hydroxyl radicals via the Haber-Weiss reaction.66 Therefore, Cu ion levels in the intra and extracellular are tightly regulated to minimise these effects. Wilson’s disease, characterised by Cu accumulation, highlights the oxidative potential of Cu, which can cause organ damage and chronic inflammation.67 Recent studies have produced varied results regarding the influence of Cu ions on macrophages. Some studies indicate that lower Cu concentrations promote anti-inflammatory markers, while higher concentrations induce pro-inflammatory markers. Huang et al.68 showed that when macrophages were cultured on titanium plates containing 0.2 and 2 mM Cu ions, the pro-inflammatory markers of the cells increased in a concentration-dependent manner, and the anti-inflammatory factors (arginine, interleukin (IL)-4, IL-6) decreased in a concentration-dependent manner. Additionally, the incorporation of Cu into biomaterials has yielded conflicting findings. Wang et al.69 investigated the inflammatory response of stainless-steel biomaterials containing nano-Cu. When it was implanted in mice, the secretion of inflammatory factors was significantly increased at the early stage, but was significantly reduced after 2 weeks.69 Further research is essential to understand the factors governing Cu’s impact on macrophage polarisation.
F
F is an essential trace element, which can be an effective osteoimmunomodulatory agent. Wu et al.70 have shown that macrophages can be greatly influenced by F-mediated osteoimmunomodulation. F can stimulate macrophages to produce a bone immune environment conducive to osteogenesis and angiogenesis. In their research, 2.4 and 24 μM sodium F can promote osteogenesis by increasing polyamine production in macrophages.70, 71 And macrophages stimulated by F showed an inhibited inflammatory response. Fluorine can promote the expression of inhibitor of κB-α to inhibit the expression of pro-inflammatory genes such as tumour necrosis factor-α and IL-6. In vivo, fluorine also can influence other immune cells such as T cells and mast cells to create an immune environment suitable for bone regeneration.72
Gadolinium
Gadolinium (Gd) is a rare earth element. Zhao et al.73 developed multifunctional scaffolds composed of gadolinium phosphate (GdPO4), chitosan, and ferroferric oxide (Fe3O4) with a precise 100 nm pore size, and three-dimensional network of macropores. These scaffolds, embellished with hydrated GdPO4 nanorods in a c-axis orientation on macropore walls, were crafted for addressing breast cancer bone metastases.73 The structured architecture and macropores facilitated cell adhesion and new bone tissue formation. Gd ions released from the GdPO4/chitosan/Fe3O4 scaffolds initiated M2 macrophage polarisation, and led to a significant upsurge in anti-inflammatory cytokines as indicated by CD206 expression. Moreover, the GdPO4/chitosan/Fe3O4 and GdPO4/chitosan scaffolds markedly stimulated new blood vessel development, and supported osteogenesis through enhanced vascularisation.
Hafnium
Seweryn et al.74 investigated the immunomodulatory effects of hafnium (Hf) oxide (HfO2) by analysing macrophage M1/M2 polarisation utilizing reverse transcription quantitative polymerase chain reaction. Following 4 hours of lipopolysaccharide stimulation, an increase in anti-inflammatory IL-10 expression was observed in lipopolysaccharide-treated macrophages cultured on HfO2. The data underline the potential immunomodulatory capacity of HfO2, and herald promising application in bone regeneration.
Li
Bartnikowski et al.75 investigated the effect on bone by mixing Li carbonate with the biomaterial polymer polycaprolactone to achieve sustained release of Li. Experimental results showed that the released Li significantly polarised macrophages towards an immunomodulatory M2 phenotype, reduced pro-inflammatory M1 phase, and inhibited osteoclast activity, demonstrating an effective targeted tissue engineering system with the potential for further innovative ion release.
Mg
Mg is crucial for cellular metabolism, activating over 300 enzymes involved in the metabolism of carbohydrates, nucleic acids, and proteins.76 It is also vital for the structural and functional integrity of cellular organelles.77 In immunology, Mg modulates white blood cell functions, such as phagocytosis and lymphocyte production, and exhibits anti-inflammatory properties.78 Mg deficiency has been linked to heightened inflammatory responses, including the activation of white blood cells and macrophages, increased cytokine release, and excess free radical production.79 Qiao et al.80 found that Mg ions induce a distinct cytokine profile, characterised by elevated levels of C-C motif chemokine ligand-5, IL-8, and IL-1ra, and a decrease in IL-1. This profile suggests that Mg ions facilitate monocyte recruitment and maturation into macrophages, while concurrently inhibiting pro-inflammatory cytokines like IL-1 through the up-regulation of IL-1ra, thus contributing to bone formation.
Si
Si ions, emanating from silica-based materials, have been shown to suppress pro-inflammatory cytokines and M1 markers through the Wnt5a/Ca2+ signalling pathway, thereby promoting osteogenesis by enhancing mineralisation and alkaline phosphatase (ALP) activity in BMSCs.81, 82 Similarly, Si ions from titanium nanotube arrays also modulate the immune response by decreasing M1 markers and elevating anti-inflammatory markers like IL-10 and CD206.83 Moreover, Si ions derived from various titanium-based materials have demonstrated an ability to inhibit pro-inflammatory cytokine secretion, such as tumour necrosis factor-α, IL-1β, and IL-6, in vitro, likely via the attenuation of NF-κB activation.84, 85
Sr
Sr exhibits a notable potential in modulating macrophage polarisation, crucial for immune response in bone regeneration. Zhao et al.86 demonstrated that a Sr-Zn-phosphorus (P) coating on Ti substrates could preferentially steer macrophages toward the M2 phenotype by activating hypoxia-inducible factor-1 (HIF-1) signalling through the release of Sr ions and Zn ions, thereby bolstering bone integration in rat femur with titanium implants. Concurrently, Yu et al.87 delineated that Sr ions doped nanorods array expedited the phenotypic transition of macrophages to M2, consequently amplifying osteogenic cytokine and growth factors (TGF-β1 and BMP-2) expressions. Furthermore, the study elucidated that STSr7 (Sr-doped Ti coated with sodium titanate, prepared via ion exchange at 100°C for 24 hours in 1000 mM 10 mL Sr(CH3COO)2 solution) outperformed in vivo in terms of osseointegration, releasing a higher concentration of Sr ions compared to STSr4 (prepared under similar conditions but with 10 mM 10 mL Sr(CH3COO)2 solution). This enhanced Sr ions ion release from STSr7 demonstrated a more consistent and effective stimulation of macrophage transition to M2, establishing a conducive environment for bone regeneration.
Zn
Zn, a pivotal element in human physiology, partakes in diverse cellular responses, profoundly influencing immune function, cell division, and skeletal morphogenesis. As an essential trace element, it is indispensable for specific key enzymes and transcription factors, which is integral to immune responsiveness. Optimal Zn levels mitigate inflammatory cytokinesecretion by macrophages, enhance anti-inflammatory cytokineexpression, and maintain an anti-inflammatory milieu. Zhao et al.88 demonstrated that Zn ions, derived from Zn-coated materials, modulate macrophage polarisation, inducing anti-inflammatory and osteoblastic cytokine secretion, thereby augmenting osteogenic differentiation potential of BMSCs. According to another study, Zn ions proficiently modulate activated macrophage polarisation towards the M2 phenotype, exerting an anti-inflammatory effect.89 The release of Zn ions from Zn-doped tricalcium phosphate (TCP) has been noted to amplify tartrate-resistant acid phosphatase and ALP activity in human BMSCs, and regulate the formation of multi-nucleated giant cells and RAW264.7 macrophage activity.90
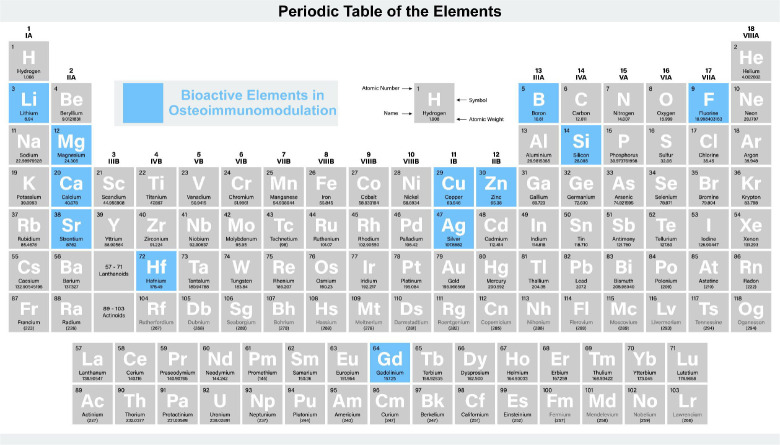
In summary, bioactive elements such as Zn, F, Mg, Ca, Gd, Hf, and Sr play critical roles in osteoimmunomodulation, contributing to bone regeneration (Figure 3). Zn ions aid in anti-inflammatory cytokine expression and osteogenic differentiation of bone marrow stem cells. F ions influence macrophages to create an osteogenic and angiogenic bone immune environment. Mg ions modulate monocyte recruitment and activation, impacting cytokine profiles favourable for bone healing. Ca ions, primarily through CaSR-mediated Wnt/β-catenin signalling, promote macrophage M2 polarisation and subsequent bone formation. Gd ions in scaffolds initiate M2 macrophage polarisation and enhance vascularisation. HfO2 shows promise in immunomodulation, particularly in increasing anti-inflammatory IL-10 expression. Sr ions in coatings and nanorods induce M2 macrophage polarisation, consequently amplifying osteogenic cytokines and growth factors, fostering bone integration and regeneration. These elements not only modulate immune responses but also activate signalling pathways crucial for bone tissue engineering and regeneration.
Figure 3. The role of bioactive elements in osteoimmunomodulation. Created with BioRender.com.
The role of bioactive elements in neuroregulation
Cu
In the neuroregulation of bone regeneration, Cu can act as an intracellular signalling molecule, binding with neurons to promote new bone formation, and affecting the process of bone repair. Cu can also regulate the excitability of neurons, having a significant neural regulation role in bone regeneration. Moreover, as one of the components of biodegradable metal implant, Cu can promote bone regeneration. The specific mechanisms and modes of action require further research to elucidate.28
A novel biohybrid biodegradable hydrogel (gelatin methacrylate, GelMA) that incorporates copper ion-modified germanium-phosphorus (GeP) nanosheets. These nanosheets exhibit properties that promote neuro-vascular regeneration and possess antibacterial activity. The modification of GeP nanosheets with copper ions improves their stability and enables sustained release of bioactive ions. The integrated hydrogel demonstrates a significant enhancement in the osteogenic differentiation of BMSCs, facilitates angiogenesis in human umbilical vein endothelial cells (HUVECs), and upregulates neural differentiation-related proteins in neural stem cells. In vitro studies with injectable electroactive GelMA/ GeP@Cu biohybrid hydrogel scaffolds show enhanced nerve differentiation and axon regeneration. These findings suggest that GelMA/GeP@Cu has great potential as a valuable biomaterial for neuro-vascularised bone regeneration and infection prevention in the field of bone tissue engineering.91
Mg
Mg ions can promote bone regeneration by stimulating the release of PGE2 through the activation of bone marrow macrophages. In the early stage of fracture healing (1st week), Mg ions can significantly increase the concentration of PGE2 in bone and serum, simultaneously activating sensory nerves, thereby stimulating PGE2 receptor-4 receptors. This induces the phosphorylation of cAMP-response element binding protein and upregulation of 5-hydroxytryptamine receptor 2C in the hypothalamus, ultimately downregulating sympathetic nerve activity related to bone repair. Under the influence of the medullary macrophage-sympathetic neuron-osteoblast neural circuit, this process promotes bone formation.28
A photosensitive conductive hydrogel by incorporating Mg-modified black phosphorus (BP@Mg) into GelMA. The combined effect of conductive nanosheets and bioactive ions released from BP@Mg enhances the migration and secretion of Schwann cells, which directly promote innerved bone regeneration through the secretion of nerve growth factor and brain-derived neurotrophic factor. In an infected skull defect model, the GelMA-BP@Mg hydrogel demonstrates effective antibacterial activity and enhances the regeneration of bone and calcitonin gene-related polypeptide-α (CGRP) nerve fibres. This phototherapy conductive hydrogel offers a novel approach to repairing infected bone defects, utilizing skeletal-associated innervation as a therapeutic strategy.92 Zhang et al.93 implanted a pure Mg pin into the intact distal femur of rats. This led to a notable increase in neuronal CGRP levels in the peripheral cortex of the femur and the ipsilateral dorsal root ganglia. CGRP promotes the activation of adenosine 3,5-cyclic monophosphate-responsive element binding protein 1 and SP7 (osterix) through calcitonin receptor-like receptor and receptor activity modifying protein 1. Consequently, this enhances the osteogenic differentiation of stem cells.93
Zn
Zn ions can promote the neural regulation of bone repair through modulating anti-inflammatory pathways. Specifically, under the influence of Zn, sensory nerve endings secrete PGE2, which can activate the PGE2 receptor-4 signalling in CGRP+ sensory nerve endings, becoming an intraneural sensory signal, and thereby activating sensory nerves. This activation, through the mutual regulation with the central nervous system, realizes the positive regulation function in bone repair. Additionally, research has indicated that under the influence of Zn ions, sensory nerve endings can also release CGRP, which can promote the proliferation and differentiation of osteoblasts, thereby better facilitating the process of bone repair.28
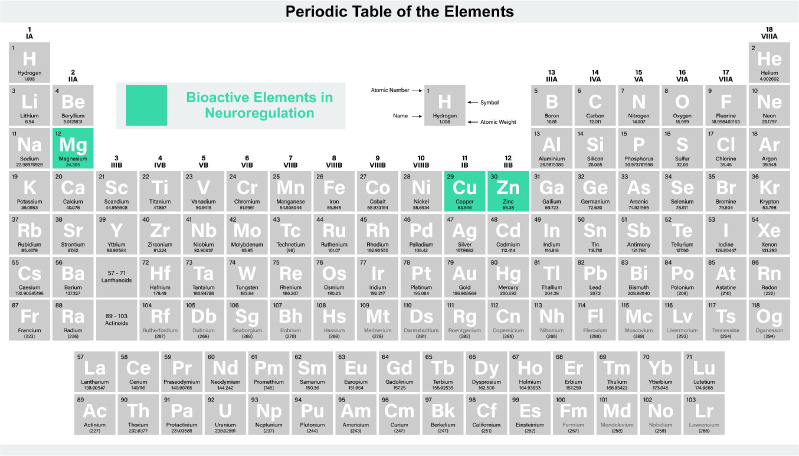
Briefly, bioactive elements such as Cu, Mg, and Zn play critical roles in neuroregulation and contribute to bone regeneration (Figure 4). Mg ions stimulate bone repair by increasing PGE2 levels through bone marrow macrophage activation, influencing the neural circuit encompassing medullary macrophages, sympathetic neurons, and osteoblasts, thereby enhancing bone formation. Zn ions enhance neuroregulation via anti-inflammatory pathways, facilitating sensory nerve activation and osteoblast function. Cu ions, an intracellular signalling agent in bone neuroregulation, binds with neurons to bolster new bone formation and modulate neuronal excitability. Its role in biodegradable metal implants suggests potential in bone regeneration, though detailed mechanisms warrant further investigation.
Figure 4. The role of bioactive elements in neuroregulation. Created with BioRender.com.
The role of bioactive elements in angiogenesis
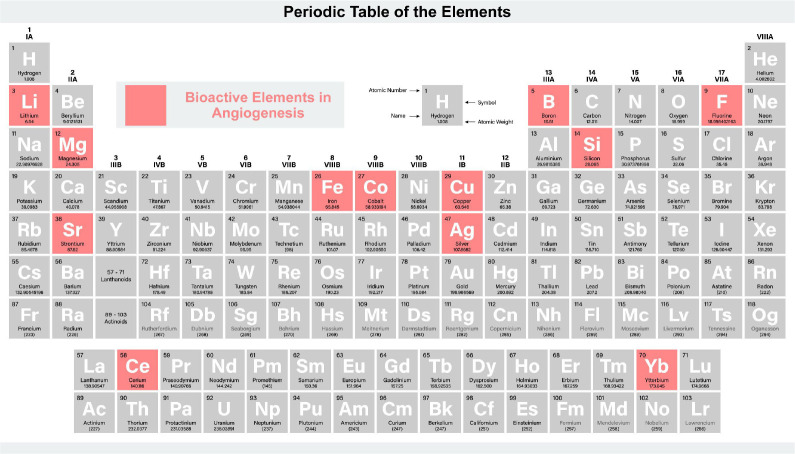
Ag
Ag has a long history for the use of antibiosis, which is a classic implant material. In recent years, scientists have discovered that Ag is a functional promoter of bone regeneration. According to Jeanmonod et al.’s study,94 Ag acetate can promote angiogenesis. They implanted Ag acetate-coated Dacron vascular grafts into the dorsal skinfold chamber in mice, and discovered that functional capillaries were more dense than uncoated grafts. Meanwhile, Ag acetate coating could stimulate the ingrowth of new microvessels into Dacron vascular grafts. Most importantly, due to the dacron Ag graft controlling the release of Ag ions, the concentration of Ag ions wouldn’t be too high to damage the normal cells. Therefore, Ag acetate-coated Dacron vascular grafts do not induce a severe inflammatory response.94, 95 Ag nanoparticles (AgNPs) also have the function of promoting angiogenesis. Kang et al.96 found that AgNPs induced tube formation on growth factor-reduced matrix glue, produced reactive oxygen species and released angiogenic factors vascular endothelial growth factor and nitric oxide via SVEC4-10 endothelial cells. Therefore, both Ag ions and nanoparticles can promote angiogenesis.96
B
B, like the previously discussed ions, also exhibits a beneficial effect on angiogenesis, although the specific mechanisms remain less explored. B is often integrated into ceramic biomaterials, such as B-doped bioactive glass scaffolds. Different concentrations of B in these scaffolds have been tested, with lower doses (up to 925 μM) demonstrating enhanced angiogenic effects compared to higher doses (around 3700 μM).97 A similar study showed that lower B concentrations enhanced endothelial cell proliferation and tubule formation.98 A in vivo study with B-containing bioactive glass demonstrated increased angiogenic gene expression patterns and vascular density.99 B has also been incorporated into composites of polymers and ceramics, Xia et al.97 studied the effects of polycaprolactone containing B-bioactive glass at different levels (0, 10, 20, 30 and 40 wt%) on angiogenesis, showing that optimal angiogenic effects were achieved at specific concentrations (30 wt%), while higher concentrations (40 wt%) proved cytotoxic to cells. Additionally, when combined with other ions, B has shown synergistic effects on vascular endothelial growth factor (VEGF) secretion. Chen et al.100 studied the effects of B-based glass and Cu-Zn doped B-based glass on human fibroblast cell lines. The results showed that VEGF secretion was increased in both cells, but the latter was more obvious.100
Cu
Cu is one of the most important elements in humans, which is the third most prevalent mineral present in the body. Copper is mainly found in the body as Cu2+ and Cu+, and they are involved in a number of important biological reactions. Cu has an effect of promoting angiogenesis. Kong et al.21 found that Cu2+ can positively affect the expression of angiogenic growth factors in HUVECs and human dermal fibroblasts. VEGF is one of the most important mediators of angiogenesis during the proliferation phase. Angiogenin (ANG) is a ribonuclease which is a strong stimulator of angiogenesis and interacts with endothelial cells. Cu2+ can not only promote the expression of VEGF, but also regulate the transcription of angiopoietin and influence the localization of ANG to enhance its function. Meanwhile, copper has a big effect on matrix metalloproteinases (MMPs), which can regulate the activity of VEGF and other growth factors and promote cell proliferation in angiogenesis.101 Researches have shown that low concentrations of Cu can stimulate the activity of MMPs, while high concentrations of copper can increase the expression of MMPs in fibroblasts. Therefore, Cu ions are important to angiogenesis in bone formation.102
Cerium
Xiang et al.103 modified a tissue engineering bone scaffold with cerium (Ce) oxide nanoparticles and evaluated the impact of Ce oxide nanoparticles on the growth and paracrine activity of MSCs on the scaffold surface. Ce oxide nanoparticles have the potential to increase MSC proliferation while also inhibiting apoptosis. Finally, large levels of the angiogenic factor VEGF were found. As a result of the improved paracrine of VEGF, endothelial progenitor cell proliferation, differentiation, and tube forming ability may be enhanced.
Co
Because of its ability to stabilise HIF-1 and hence activate VEGF, Co ions have been utilised to accelerate vascularisation.104 The addition of Co ions to silk fibroin/F/calcium phosphide (CaP) may aid adipose-derived stem cells in the formation of tube-like structures.105 However, because collagen type 1 matrix deposition was reduced, cell proliferation and endothelial network formation were inhibited. Chai et al.20 found that preconditioning human periosteum-derived mesenchymal stem cell-seeded tissue engineered constructs with a fine-tuned solution containing Co increased VEGF secretion and improved human periosteum-derived mesenchymal stem cell development and endothelial network formation.
F
F has been shown to augment angiogenesis in a dose-dependent manner, demonstrating synergetic regulation of osteogenesis and angiogenesis by modulating the expression of key factors such as BMP-2, oncostatin M (OSM), spermine/spermidine synthase, insulin-like growth factor-1, and VEGF. Wu et al.22 observed that active angiogenesis was consistently present in F-treated groups, and attributed this to VEGF expression under varying F treatments. Further, F was found to stimulate insulin-like growth factor-1 production, a critical factor in endothelial cell migration and tubular formation.106 Given the dose-dependent variations in its impact, F exerts multifaceted effects on osteogenesis, osteoclastogenesis, and angiogenesis.71
Iron
Shi et al.107 studied the effect of Fe3+ release on the angiogenic response of bone grafts. As a controlled ferric agent, iron (Fe)-doped octacalcium phosphate was produced. The affinity, survival, proliferation, and angiogenic differentiation of HUVECs were all greatly improved by Fe-doped octacalcium phosphate. The scaffold promoted endothelial cell adhesion and spreading, as well as angiogenesis, as evidenced by the development of additional blood vessels and increased expression of particular markers. Fe3+ can boost HIF-1α and VEGF levels, as well as nitric oxide secretion and endothelial nitric oxide synthase synthesis, all of which influence endothelial cell activity and angiogenesis.
Li
Li-incorporated bioactive glass ceramic has been shown to enhance the pro-angiogenic capabilities of HUVECs both in vitro and in vivo.23 This enhancement is mediated through the induction of miR-130a in BMSC-derived exosomes, leading to phosphatase and tensin homolog deleted on chromosome ten (PTEN) downregulation and AKT pathway activation. The resultant cellular activities include increased endothelial cell proliferation, migration, tube formation, and increased expression of pro-angiogenic genes.
Mg
Mg ions improved the proliferation, migration, and osteogenic differentiation of BMSCs, as well as having evident impacts on angiogenesis, by selectively activating the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. Directly promoting the migration of ECs and up-regulating the production of VEGF in BMSCs increase angiogenesis.108 The sensory nerve-endothelial cell interaction mediated by Mg through the CGRP-focal adhesion kinase-VEGF signalling pathway enhanced the repair of critical size bone defects.109 Lai et al.45 have developed a new bioactive porous scaffold using low temperature rapid prototyping technology, comprising of poly (lactide-co-glycolide), TCP, and Mg. By incorporating Mg into the scaffold, they observed that it not only provided a favourable template for vessel infiltration but also stimulated neo-angiogenesis. This ultimately led to new bone formation and remodelling in challenging bone defects, specifically those associated with steroid-induced osteonecrosis.45
Sr
Sr can play a great effect on bone regeneration, including immune response, angiogenesis and new bone formation. Previous studies have shown that Sr-containing materials have the ability to promote angiogenesis. Yan et al.27 have found that appropriate doses of Sr ions (0.2–1 mM) can enhance the secretion of VEGFA and ANG-1 in HUVECs and BMSCs co-culture systems, which has the potential to create an angiogenic microenvironment at an early stage. Sr not only promotes angiogenesis by stimulating osteoblasts to secrete angiogenic cytokines,110 but also significantly promotes early angiogenesis by regulating macrophage phenotype. Zhao et al.111 have found that the monodispersed Sr-containing bioactive glasses microspheres could stimulate macrophages to exhibit a tendency towards the M2 phenotype and express high levels of platelet-derived growth factor-BB in vitro. What is the most important is that this access can play a big role in the early vascularisation, which is different from the way of osteoblasts.111
Si
Si is one of the essential trace elements in human body, which is necessary in the development and growth of bone and connective tissue.112 Studies have shown that Si ions can stimulate angiogenesis. Li and Chang113 found that the 0.7–1.8 μg/mL Si ions provided by 1/64 and 1/256 diluted Ca silicate extracts stimulated the proliferation of HUVECs and upregulate the expression of pro-angiogenic factors (VEGF and basic fibroblast growth factor). Their receptors activate the expression of endothelial nitric oxide synthase and increase the expression of nitric oxide in HUVECs.113 Zhai et al.114 have shown that akermanite ceramics, which is a suitable source of Si ions, can promote angiogenesis. It can release and keep the suitable Si ions to induce angiogenesis by increasing gene expression of pro-angiogenic cytokine receptors and upregulating downstream signalling, such as nitric oxide synthase and nitric oxide.114 Their experiment successfully demonstrated that akermanite ceramics were the first silicone-containing ceramics capable of inducing angiogenesis during bone regeneration. Mao et al.115 have found that Sr and Si ions had synergistic effects on osteogenesis, osteoclast formation and angiogenesis. They use the SMS bioceramics, which has Sr and Si ionic compositions, to study the influence of angiogenesis. And they had made the conclusion that SMS bioceramics can enhance osteogenic differentiation and expression of preferred angiogenic factors in BMSC from ovariectomized rats, rebalance the osteoprotegerin/receptor activator of nuclear factor-κB ligand (RANKL) ratio in BMSC from ovariectomized rats at early stage, and inhibit RANKL-induced osteoclast formation at late stage. The effect of Si ions on promoting osteogenesis and Sr ions on enhancing angiogenesis and inhibiting osteoclast formation can be well combined by SMC.115
Ytterbium
Tang et al.116 used lyophilization and mineralisation techniques to successfully create magnetic Y-doped hydroxyapatite/chitosan nanohybrid scaffolds with ytterbium (Yb) dopants as well as magnetic SrFe12O19 nanoplates. Yb ions from magnetic Y-doped hydroxyapatite/chitosan nanohybrid scaffolds only reached a maximum concentration of 0.30 μM, according to in vitro tests. The osteogenic and angiogenic properties of the magnetic Y-doped hydroxyapatite/chitosan nanohybrid scaffolds were remarkably improved by the Yb dopants and magnetic SrFe12O19 nanoplates. For one thing, the bioactive components promoted the migration of endothelial cells, the up-regulation of angiogenic protein VEGFA expression, and macrophage polarisation toward M2 phenotype, leading to the rapid development of blood vessels in bone defects. In vivo bone mineralisation was made possible by the sufficient nutrients and oxygen that the newly created blood vessels provided. For another, by activating the BMP-2/Smad pathway in the scaffolds, both the Yb dopants and magnetic SrFe12O19 nanoplates enhanced osteogenic differentiation of rat BMSCs and in vivo bone tissue regeneration, suggesting a beneficial influence in the entire osteogenic process. The majority of bone abnormalities were filled with newly created bone tissues 12 weeks after surgery. In order to speed up the repair of bone defects, Yb dopants and magnetic SrFe12O19 nanoplates would be expected.
Zn
The influence of Zn on angiogenesis is primarily regulated via the Zn-sensing receptor/G protein-coupled receptor 39.117 Within a concentration range of 20–60 μM, Zn enhances human coronary artery endothelial cell viability, proliferation, and angiogenic marker expression.118 Biomaterials such as 5% Zn-Bioglass®-incorporated calcium phosphate cement have been shown to upregulate VEGF and induce tubule formation in endothelial cells.119 Likewise, polycaprolactone matrices with zinc oxide (ZnO) nanoparticles promote cell proliferation and elevate VEGF and fibroblast growth factor expression, with the nanoparticles also catalysing reactive oxygen species generation via hydrogen peroxide (H2O2), a ZnO byproduct.120 Elevated ZnO concentrations, however, inhibit angiogenesis due to excessive reactive oxygen species production. The angiogenic potential of Zn varies with the biomaterial’s morphological properties, such as nanoparticle configuration.121 Anti-angiogenic aspects of Zn are also being explored for cancer therapeutics by inhibiting tumour vasculature.122 When combined with Si, Zn can activate the p38 pathway, contributing to bone regeneration and angiogenesis via cytokine expression.123 Further investigations are warranted to comprehensively understand angiogenic roles and underlying mechanisms of Zn.
In summary, various bioactive elements such as Mg, Sr, Si, and Ag play pivotal roles in promoting angiogenesis, which is instrumental for bone regeneration (Figure 5). Ag, historically recognised for its antibiosis, now stands out as a bone regeneration stimulant, with Ag acetate-coated grafts showing enhanced microvessel ingrowth without inducing inflammation. B, integrated into ceramic biomaterials, demonstrates angiogenic effects at specific concentrations. Cu, vital for angiogenesis, influences angiogenic growth factor expression in key cellular components. Co ions, by stabilising HIF-1, expedite new vessel formation. Ce oxide nanoparticles on scaffolds enhance MSC proliferation, subsequently promoting VEGF secretion. Fe3+ boosts angiogenesis by modulating endothelial cell activity. F, in a dose-dependent manner, orchestrates angiogenesis through osteogenesis and angiogenic factor regulation. Li-incorporated bioactive glass ceramic shows enhanced angiogenic potential both in vivo and in vitro. Mg ions, channelling through the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway, have a positive impact on angiogenesis. Sr, through VEGF and ANG-1 modulation, plays a significant role in early vascularisation. Si ions stimulate angiogenesis via BMP-2/ Smad pathway activation. Yb dopants in scaffolds combined with magnetic particles promote angiogenesis and bone tissue regeneration. Lastly, Zn mediates its angiogenic effects through specific cellular interactions, with its potential varying based on the morphology of the biomaterial.
Figure 5. The role of bioactive elements in angiogenesis. Created with BioRender.com.
The role of bioactive elements in osteogenesis
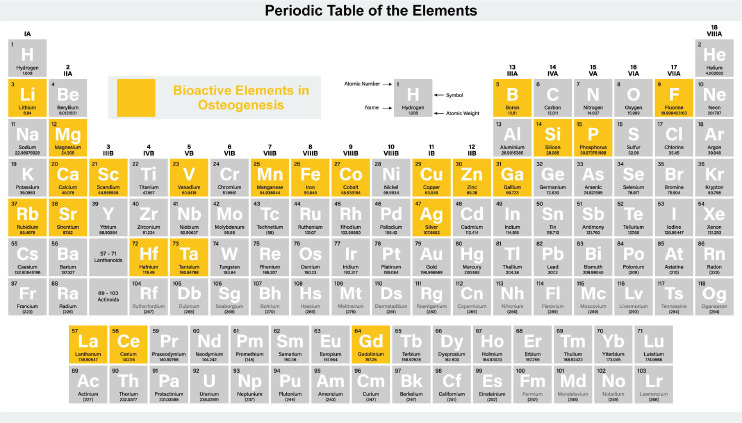
Ag
Ag could not only promote angiogenesis, but also promote new bone formation. It has been reported that AgNPs can promote osteogenic lineage induction and actin aggregation in new bone formation. These promotions can only be found in AgNPs, not silver nitrate (AgNO3). These promotions about AgNPs are achieved by stimulating the physiological activity of new bone-forming cells. AgNPs have been shown to promote mineralisation in MC3T3-E1 osteoblasts through microRNA-mediated upregulation of bone morphogenetic genes. Two studies revealed that, aside from the elevated expression of osteogenesis-critical bone morphogenetic proteins, the most significant transcriptional change was a decrease in osteoclast markers.124, 125 AgNPs also foster keratinocyte proliferation, fibroblast differentiation, and MSC osteogenic differentiation in vitro.126 While the underlying mechanisms remain unidentified, these activities collectively contribute to new bone cell proliferation and differentiation, thereby facilitating new bone formation.
B
B, an essential trace element, plays a pivotal role in various biological processes, notably bone growth and maintenance.127 Its supplementation has been linked to enhanced bone strength and microstructure.128 Mechanistically, B fosters osteogenesis via the activation of the Wnt/β-catenin pathway, particularly through the up-regulation of the transcription factor transcription factor 7-like 2.129 This suggests its potential in bone regeneration therapies. Experimentally, B ions, when released from B nitride nanotubes, have been observed toinduce osteogenesis, evidenced by enhanced protein adsorption, MSC attachment, and upregulation of osteogenic markers.130 Furthermore, B-doped bioactive-glass scaffolds, have displayed promising osteogenic outcomes,131 amplifying cell adhesion, differentiation markers, and mineralisation. Specifically, B-enriched bioactive glass scaffolds activated the Wnt/β-catenin pathway, influencing osteoblastic differentiation.132 However, while the role of Setd7 in B-mediated osteoblast differentiation has been identified, additional studies are required to uncover other potential contributing factors.
Ca
Doping Ca ions can notably accelerate adhesion, proliferation, and mineralisation of osteoblasts.133 Ca, one of the ions that form the bone matrix, affect cells and living systems in several ways. The calcium phosphates in bone tissues cause bone formation and maturation through calcification.134 Ca ions also influence bone repair via cellular communication. Ca causes bone tissue regeneration by accelerating mature bone cells and inducing bone growth precursor cells through the creation of nitric oxide.135, 136 Ca ions also activate the extracellular signal-regulated kinase1/2 pathway,137 which stimulates osteoblastic bone production, and the PI3K/Akt pathways,138 which prolongs the lifespan of osteoblasts. Ca ions also control the production and resorption activities of osteoclasts. Integrin β1 and vinculin proteins are important factors of focal adhesion complexes that prove effective on the adhesion, proliferation, and differentiation of BMSCs. Ca ions show helpful to the high expression of integrin β1 and vinculin proteins. Hence Ca ions increase the protein expression and support cell adherence. The incorporation of Ca accelerates the proliferation of stem cells and the early differentiation of osteoblasts in the cell. The expression of the related proteins like osteopontin (OPN),139 Runx2 and ALP would verify the conclusion. As a secretory phosphorylated glycoprotein, OPN adheres to HA via its distinct binding domain during osteogenesis and holds the formation of osteoid nodules in vivo, which directly influence the construction of the three-dimensional structure of newly formed bone.140 The introduction of Ca ions suggests the abundant expression of OPN protein and supports the osteogenic differentiation of BMSCs.
Ce
Ce-doped mesoporous bioactive glass nanoparticles used as vectors for local administration of Ce have shown to stimulate the expression of pro-osteogenic genes in Saos-2 cells, as well as the development and calcification of a primitive osseous ECM. Westhauser et al.141 studied the impact of the ionic dissolution products of mesoporous bioactive glass nanoparticle on cellular osteogenic differentiation and their ability to form and mature a primitive osseous ECM. In the presence of Ce-doped mesoporous bioactive glass nanoparticles, the development and calcification of a primitive osseous ECM was greatly enhanced in a positive concentration-dependent manner, as evidenced by an increased presence of collagen and increased ECM calcification.
Co
Co-TCP increased the vitality of BMSCs.104 In vitro, 2% Co-TCP increased ALP activity, matrix mineralisation, and osteogenic gene expression in BMSCs. Excessive Co doping, on the other hand, reduced TCP-induced osteogenesis. Additionally, Co2+ can inhibit the osteogenic differentiation of MSCs by mimicking hypoxia. Hsu et al.142 have shown that hypoxia caused by Co2+ activation of HIF impairing osteogenic differentiation, as evidenced by reduced ALP activity and expression of osteogenic markers core-binding factor alpha(1) (Cbfα1) and osteopontin. There are also some studies have shown that hypoxia caused by releasement of Co2+ can enhance the osteogenic potential of MSC after having a certain pretreatment.143 The specific influence mechanism of Co needs to be studied in the future.
Cu
Cu can play a big role in bone metabolism, and severe Cu deficiency can lead to bone abnormalities, such as decreased bone strength. Cu can enhance the osteogenic differentiation of MSCs. The early studies on the effect of Cu on MSCs in postmenopausal women showed that Cu ions reduced osteoblast proliferation, increased differentiation by 2 folds, and, particularly, increased calcium deposition. Cu ions can enhance cell activity and proliferation and enhance the expression of bone specific protein.95 Burghardt et al.144 produced a composite material that deposited copper on a titanium alloy. The proliferation of MSC was stimulated by 0.1 mM Cu ions, ALP activity was increased, and mineralisation was increased 144. And other studies found an increase in the osteogenic gene expression of collagen type 1, ALP, OPN and Runx2 from rat BMSCs cultured with Cu-containing calcium phosphate cement.145, 146
F
F-containing biomaterials have been shown to have osteogenic properties, which are usually used as implant coating. F-containing biomaterials also can stimulate the proliferation and differentiation of osteoblasts in vitro and even promote bone formation of osteoblasts in vivo.147 Chen et al.148 have shown that the coating consisting of F can improve the activity of adhesion, proliferation and differentiation of osteoblasts, and has a good biocompatibility. Meanwhile, F can enhance bone-associated glycoprotein synthesis in BMSCs, leading to increased mineral deposition. But excessive F intake over a long period of time can eventually lead to damage to bone structure. Optimal osteogenic differentiation is achieved with F ion concentrations of 50 and 500 μM72 and osteoblast mineralisation to be between 0.01 to 10 μM.149 Thus, the physiological impact of F on skeletal tissue is dose-dependent in the local environment.
Fe
The addition of Fe ions (Fe3+/Fe2+) to calcium phosphate cement can speed up osteoblast proliferation. Doping Fe into bone cement boosted MC3T3-E1 cell proliferation and migration while also increasing their ALP activity and expression of osteogenic-related genes. The expression of HIF-α increased after cells were grown with Fe-dicalcium phosphate dihydrate scaffold extract, enhancing cell chemokine receptor activation and promoting cell migration.150
Gallium
Gallium (Ga) is used in medicine mainly in antibacterial and cancer treatments. Ga nitrate is a drug that speeds up bone absorption. Short-term Ga therapy can effectively reduce bone turnover in vivo and increase bone calcium in patients with bone calcification to treat cancer-related hypercalcaemia.95 According to Strazic Geljic et al.151, bone cement containing Ga can significantly inhibit the expression of osteoclast- and osteoblast-associated genes. And they found that TCP/phosphate-based scaffolds with single Ga promoted cell proliferation and significantly inhibited osteogenic differentiation and osteoclast activity in vitro. For the current literature, Ga has a range of promising qualities for future applications in tissue engineering.
Gd
The Gd dopants in the scaffolds triggeredthe signalling pathway (Wnt/β-catenin,152 Smad/Runx2,153 Akt/GSK3β154), enabling BMSCs to proliferate and differentiate into osteoblasts.
Hf
HfO2 was found to encourage pre-osteoblasts while impairing the viability of pre-osteoclasts.74 HfO2 stimulates the expression of OPN, Runx2, and TGF-β in osteoblasts on the mRNA and protein levels. Meanwhile, the major regulators of osteoclast differentiation: c-Fos, MMP-9, PU.1, receptor activator of nuclear factor-κB, and tartrate-resistant acid phosphatase, are expressed less when HfO2 is present. HfO2 stimulates pre-osteoclast death while simultaneously protecting pre-osteoblast from apoptosis by increasing the expression of the Bcl-2 transcript. Thus, a technique to stop the overactivity of osteoclasts during bone repair was raised. Moreover, HfO2 protects pre-osteoblasts from apoptosis not only by activating specific genes relevant to osteogenesis but also by inducing the production of miR-17-5p and miR-7a-5p, two essential regulators of osteoblast proliferative activity and apoptosis. While miR-16 inhibits MSC production of important osteogenic markers and calcium mineral deposition during osteogenic differentiation, miR-21-5p is a well-known osteogenesis activator. Hopefully, HfO2 may be used as a bioactive agent to modify and activate osteogenesis when administered to implantable metallic materials.
Lanthanum
Lanthanum (La)-substituted MgAl layered double hydroxide scaffolds activate the Wnt/β-catenin pathway, thereby promoting BMSC proliferation and osteogenic differentiation due to the releasement of La ions. This activation leads to the upregulation of ALP, Runx2, collagen type 1, and OCN gene expressions. Additionally, the scaffolds act to obstruct the NF-κB signalling pathway, significantly diminishing RANKL-induced osteoclastogenesis.155
Li
Li increases bone regeneration via blocking GSK3 and thereby activating the β-catenin signalling pathway.156 The release of Li ions stimulated the adhesion and proliferation of BMSCs more effectively. Furthermore, Li doped mesoporous silica nanospheres may improve BMSC ALP activity as well as the expression of osteogenesis-related genes (OPN, ALP, Runx2, and OCN).157 Mo et al.158 investigated how low concentration Li stimulation caused BMSCs to proliferate and differentiate into osteoblasts. They also discovered that adding 500 μM of Li to the canonical Wnt signalling pathway and osteogenesis differentiation in BMSCs reversed the inhibitory effect of 10 μM of XAV-939.159 Huang et al.160 found the RANKL/ osteoprotegerin signalling axis was also involved in these effects.
Mg
Mg ions, via the PI3K/Akt signalling pathway, can influence the expression of bone-related genes such as Runx2, ALP, OCN, and OPN.161 In vitro, an appropriate concentration of Mg ions can greatly enhance pre-osteoblast proliferation and differentiation, as well as the up-regulation of osteogenic genes, and in vivo, significant new bone formation.24
Manganese
Manganese ion (Mn2+) has been shown to enhance the expression of osteogenic genes described in osteoblasts by increasing ALP activity, collagentype 1, OCN, BMP, and soluble intercellular adhesion molecule-1. Mn-doped implants have been shown to stimulate cell proliferation, cell differentiation and biological activity, they are promising materials for bone tissue regeneration.162 Additionally, the release of Mn2+ from bioactive glass caused hMSCs differentiation through a bone route and subsequent mineralisation, according to the findings.163 Mn2+ had a concentration-dependent effect on cell activities, and a lower Mn2+ concentration could encourage BMSCs to differentiate into osteoblasts. Wu et al.164 discovered that Mn2+ concentrations below 7.17 g/L stimulated the proliferation of BMSCs and increased the expression of osteogenesis-related genes.
Rubidium
Rb, recognised as a crucial trace element in the human body, is known for its low toxicity.165 Research indicates that biomaterials infused with rubidium significantly advance osteoblastic differentiation, particularly in the middle and late stages, and also enhance osteogenic capacities. A pivotal attribute of rubidium is its remarkable antibacterial properties, establishing it as an effective agent for enhancing biomaterials.166, 167 Tan et al.168 have developed glass-ceramics infused with varying levels of Rb. Within the glass matrix, hydroxyapatite crystals are formed. The incorporation of rubidium enhances the formation and growth of these crystals. These rubidium-enriched glass-ceramics demonstrate superior bending strength compared to their rubidium-free counterparts. Crucially, the presence of rubidium fosters the proliferation and adhesion of human hBMSCs and increases ALP activity. Exhibiting robust mechanical properties, exceptional bioactivity, and biocompatibility, these rubidium-modified glass-ceramics hold promise for applications in bone regeneration.168
Scandium (Sc)
ScCl3 promotes osteogenesis and inhibits adipogenesis in the Wnt/β-catenin signalling pathway when used at the optimal concentration.169 Moreover, ScCl3 can enhance bone remodelling by improving osteogenic differentiation in lineage commitment of rBMSCs.
Si
Si is an essential element for the development and growth of bone and connective tissue. In vivo, water-soluble forms of Si, chiefly as orthosilicic acid [OSA, Si(OH)4]. It can accelerate bone mineralisation by affecting collagen and inhibits bone resorption in postmenopausal women.170 And it can also play an important effect on stimulation of osteoblasts and bone formation and inhibition of osteoclast formation and bone resorption. A study has shown that OSA stimulated osteoblast differentiation at a physiological concentration of 20 μM.171 Dong et al.172 havefoundthat OSAcould promote the osteogenic differentiation of rat BMSCs through the BMP-2/Smad1/5/ Runx2 signalling pathway to participate in the induction of collagen type 1 and osteocalcin synthesis. Zhou et al.173 have found that OSA could accelerate bone formation in human osteoblast-like cells through the PI3K-Akt-mammalian target of rapamycin pathway. Zhou et al.174 have shown that OSA and Si(OH)4 could stimulate osteoblast differentiation in vitro through upregulating miR-146a to antagonize NF-κB activation. OSA can also play an effect on osteoclast. A study has shown that OSA inhibits RANKL-induced osteoclastogenesis by promoting the expression of miR-130b, which can counteract the negative effect of oophorectomy on miR-130b expression in rats.175 miR-130b plays a role in cell proliferation, differentiation, apoptosis, tumour progression and metastasis, and serves as a biomarker for recurrence and prognosis. You et al.176 have shown that the expression of miR-130b increased with the application of OSA. In vitro, the overexpression or inhibition of miR-130b significantly promoted or inhibited the osteogenic differentiation of osteoblasts under the application of OSA.176 And as the scaffold material, dicalcium silicate is a good choice used as containing bioactive coating materials for prosthetic bone implant. It can induce the deposition of carbonised hydroxyapatite at the material-tissue interface, form a strong binding with bone tissue, inhibit the formation of osteoclast, promote the proliferation and differentiation of human osteoblasts and the expression of bone-related genes.177
Sr
The addition of Sr in biomaterials reduced the capacity to generate apatite, but it also encouraged cell division, proliferation, differentiation into osteoblasts, and mineralisation of the ECM.178 Li et al.179 verified the influence of Sr2+ on MSC osteogenic differentiation. Sr2+ stimulated noncanonical Wnt signalling to regulate the production and distribution of the PAR complex, hence regulating cell division, and the increased cell population contributed to enhanced osteogenic differentiation. Meanwhile, Sr2+ release increased the expression of genes involved in hMSC osteogenic development; early indicators (Runx2, collagen type 1) were proportionate to the amount of Sr2+ released, and late markers (ALP, OCN) were higher.180 To some extent, Sr2+ could prevent osteoclastogenesis on its own. Sr2+ decreased osteoclast activity more than recombinant human BMP-2.181 By inhibiting the RANKL-activated p38 signalling and NF-κB signalling pathways, Sr-substituted sub-micron bioactive glass with the combined action of substituted sub-micron bioactive glass extract and Sr2+ demonstrated the most inhibitory effect on osteoclast differentiation.182
Vanadium
Vanadium (V), a ubiquitous trace element in both plants and animals, has intriguing biological effects.183 Found in a variety of oxidation states, from –1 to +5, certain forms like V III, IV, and V are noted for their insulin and growth factor mimicking properties at therapeutic levels. Notably, V compounds influence bone metabolism due to their primary storage in bone tissue.183 Recent studies have unveiled that a complex of V (IV) with ascorbic acid can promote osteoblast differentiation and mineralisation in a lab setting, underscoring its potential in bone formation.184 The amount of collagen type 1 produced in osteoblasts was found to be directly correlated with the dose of the V compound.
Zn
Zn is integral to bone development and is found in calcification sites such as osteons and calcified cartilage. Its levels in bone tissue rise with increasing mineralisation. Zn promotes osteoblast proliferation and elevates ALP activity within a narrow dose range (1–50 μM).185 Beyond this range, osteogenic activity diminishes. Kwun et al.186 reported that Zn-deficient media reduced ALP activity, matrix-related gene expression, and mineralised matrix deposition in MC3T3-E1 cells. Zn also safeguards osteoblasts from apoptosis and enhances their spreading, attachment, and chemotaxis. Furthermore, Zn is crucial for osteoclastogenesis. Reduced numbers of osteoclasts were observed in the distal femur growth plates of Zn-deficient rats.186, 187 Zn exhibits a dose-response effect on osteoclast formation and activity, and at certain concentrations, can induce osteoclast apoptosis.188, 189 A study by O’Connor et al.190 showed that Zn deficiency in Turkey poults and Sprague-Dawley rats led to reduced growth, bone shortening, and diminished ALP activity. The rats developed osteopenia, characterised by significant reductions in cancellous bone, osteoblast surface area, and osteoclast numbers.190 Thus, the multifaceted roles of Zn in bone metabolism warrant further investigation.
Tantalum
Due to its exceptional osteogenic activity, corrosion resistance, and antibacterial adhesion, biomedical tantalum (Ta) has attracted considerable attention as a promising implantable material for bone repair. Hu et al.191 developed Ta/ polyetheretherketone composites by blending Ta nanoparticles with polyetheretherketone for load-bearing bone repair applications. The incorporation of Ta nanoparticles in Ta/ polyetheretherketone composites resulted in favourable surface roughness, hydrophilicity, and surface energy, thereby enhancing the osteoconductivity of fibrous electrospun polylactic acid (PLA) membranes used in guided bone regeneration.191 A Ta coating was applied to electrospun PLA membranes through the deposition of sputtered Ta ions around the PLA fibres. This Ta-PLA coating greatly enhanced the attachment, proliferation, and differentiation of preosteoblasts on the membranes. In vivo studies demonstrated that within 6 weeks, a majority of calvarial defects treated with Ta-PLA membranes were completely covered with newly formed bone, while the defects treated with bare PLA membranes showed minimal bone coverage.192 In recent years, porous Ta has been extensively investigated for its regulatory effects on BMSCs. It plays a crucial role in regulating the proliferation, migration, and differentiation of BMSCs, making it a widely used material for bone defect repair.193-195
P
P is a vital element in the human body, comprising approximately 1% of total body weight as a constituent of bones.196 Culturing human tonsil-derived MSCs in osteogenic medium containing incremental concentrations of P (a mixture of Na2HPO4 and NaH2PO4) for 14 days increased their ability to undergo osteogenic differentiation.197 Black phosphorus exhibits the capability to regulate oxidation and degradation, resulting in the non-toxic byproduct PO43-. This PO43- acts as a mineralisation resource, promoting in the formation of calcium phosphate (CaP) deposits that facilitate bone repair.198, 199 Introduction of graphene oxide nanosheets enhances initial cell attachment and wraps around black phosphorus, enabling continuous release of PO43-. This stimulation of cell osteogenesis promotes the formation of new bone.200 Numerous studies have demonstrated that P-rich materials can stimulate mineralisation and promote bone regeneration.201
Bioactive elements, ranging from trace metals to rare earths, play a foundational role in bone metabolism and regeneration (Figure 6). Elements like Ag, Cu, and Zn actively influence osteogenesis, angiogenesis, and osteoblast proliferation. Ga and Si, traditionally known for antibacterial and bone mineralisation effects respectively, showcase potential in bone turnover and osteoclast modulation. Mn, F, and Co, though dose-dependent, exhibit promising osteogenic differentiation impacts. Li, Mg, and Ca ions enhance bone cell proliferation, differentiation, and overall bone tissue regeneration. Rarer elements like Rb, Sr, Sc, La, Ce, Gd, and Hf, when doped in biomaterials, activate specific pathways fostering bone cell proliferation, differentiation, and osteogenic gene expression. These elements, in synergy, offer avenues for innovative bone regeneration therapies.
Figure 6. The role of bioactive elements in osteogenesis. Created with BioRender.com.
Challenges and Perspectives
This review comprehensively explores the role and mechanisms of bioactive elements such as Zn, Mg, and Si in bone regeneration. Initially, the review underlines the complex biological attributes and regeneration process of bone tissue, involving multiple stages like osteoimmunomodulation, neuroregulation, angiogenesis, and osteogenesis. Traditional bone regeneration strategies, although effective, are fraught with limitations such as stability, immunogenicity, and cost-effectiveness, often relying on growth factors and pharmaceuticals. Bioactive elements not only offer therapeutic efficacy but also superior stability and reduced biotic risks. These elements are frequently integrated into biomaterials, serving as multifaceted bioactive components that facilitate bone regeneration through controllable release mechanisms. In summary, this review establishes bioactive elements as a robust and clinically viable strategy for advanced bone regeneration, contributing to the advancement of bone tissue engineering.
The exploration of diverse elements in bone regeneration reveals promising prospects, showcasing a preference for employing a mixture of multiple elements in contemporary research endeavors. The myriad combinations, encompassing considerations of concentration and proportion among other parameters, necessitate rigorous experimental validations and iterative optimisations, thereby representing a time and resource-intensive endeavor. The integration of machine learning (ML) emerges as a potential solution to mitigate this impasse. ML, representing a facet of artificial intelligence, flourishes owing to its ability to evolve through data comprehension over time. It holds the promise to significantly expedite the screening and optimisation of elemental combinations for therapeutic efficacy, adeptly understanding the high-dimensional parameter space, and pinpointing potent combinations with minimal empirical trials.
Transitioning to the emerging realm of organoids, it unveils a fertile domain for clinical translational research, albeit the field of bone organoids remains nascent, with its construction strategies yet to reach maturation. The architectural complexities of bone organoids frequently necessitate the incorporation of a spectrum of growth factors to stimulate stem cell differentiation, yet the prohibitive cost of these factors presents a substantial impediment. The prospective benefits of bioactive elements in bone organoid construction herald a paradigm shift. The venture into multi-element incorporation could outline an economical and efficacious avenue, harboring the potential to revolutionize the bone organoid research paradigm. The envisaged synergistic interactions among these bioactive elements could not merely mimic the complex biochemical cues indispensable for bone development but also potentially unveil novel mechanisms underpinning bone regeneration, thereby augmenting the therapeutic and scientific horizons in bone tissue engineering and regenerative medicine.
Footnotes
Author contributions: JCS conceived the idea, LB handled the design, writing, and revision of the paper, and PR Song contributed to the writing and revision of this work. All authors approved the final version of the manuscript.
Financial support: This work was financially supported by National Natural Science Foundation of China (Nos. 82230071, 82172098), and Laboratory Animal Research Project of Shanghai Committee of Science and Technology (No. 23141900600).
Acknowledgement: None.
Conflicts of interest statement: There are no conflicts of interest.
Editor note: Jiacan Su is an Editorial Board member of Biomaterials Translational. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and his research group.
References
- 1.Koushik T. M., Miller C. M., Antunes E. Bone tissue engineering scaffolds: function of multi-material hierarchically structured scaffolds. Adv Healthc Mater. 2023;12:e2202766. doi: 10.1002/adhm.202202766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl A., Yang Y. P. Regenerative approaches for the treatment of large bone defects. Tissue Eng Part B Rev. 2021;27:539–547. doi: 10.1089/ten.teb.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes T. A. P., Gonçalves L. M. L., Brito J. A. A. Relationships between bone turnover and energy metabolism. J Diabetes Res. 2017;2017:9021314. doi: 10.1155/2017/9021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan J., Maturavongsadit P., Metavarayuth K., Luckanagul J. A., Wang Q. Enhanced bone defect repair by polymeric substitute fillers of multiarm polyethylene glycol-crosslinked hyaluronic acid hydrogels. Macromol Biosci. 2019;19:e1900021. doi: 10.1002/mabi.201900021. [DOI] [PubMed] [Google Scholar]
- 5.Gyulay K. K., Karászi P., Rédei M., Sólymos P., Schandl K., Lacza Z., Horváthy D. B. Evaluation of serum albumin-coated bone allograft for bone regeneration: a seven-year follow-up study of 26 cases. Int J Mol Sci. 2023;24:9232. doi: 10.3390/ijms24119232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferraz M. P. Bone grafts in dental medicine: an overview of autografts, allografts and synthetic materials. Materials (Basel) 2023;16:4117. doi: 10.3390/ma16114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai L., Du Z., Du J., Yao W., Zhang J., Weng Z., Liu S., Zhao Y., Liu Y., Zhang X., Huang X., Yao X., Crawford R., Hang R., Huang D., Tang B., Xiao Y. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials. 2018;162:154–169. doi: 10.1016/j.biomaterials.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Bosch-Rué È., Díez-Tercero L., Buitrago J. O., Castro E., Pérez R. A. Angiogenic and immunomodulation role of ions for initial stages of bone tissue regeneration. Acta Biomater. 2023;166:14–41. doi: 10.1016/j.actbio.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Janmohammadi M., Nazemi Z., Salehi A. O. M., Seyfoori A., John J. V., Nourbakhsh M. S., Akbari M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact Mater. 2023;20:137–163. doi: 10.1016/j.bioactmat.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J., Cheng X., Wu J., Chen J., Pei X. The development of magnesium-based biomaterials in bone tissue engineering: A review. J Biomed Mater Res B Appl Biomater. 2023 doi: 10.1002/jbm.b.35326. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Yu R., Wang M., Wang S., Ouyang X., Yan Z., Chen S., Wang W., Wu F., Fan C. Insulin-like growth factor binding protein 4 loaded electrospun membrane ameliorating tendon injury by promoting retention of IGF-1. J Control Release. 2023;356:162–174. doi: 10.1016/j.jconrel.2023.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Youssef A., Aboalola D., Han V. K. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017;2017:9453108. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S. K. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100:354–387. doi: 10.1002/jps.22276. [DOI] [PubMed] [Google Scholar]
- 14.Jiskoot W., Randolph T. W., Volkin D. B., Middaugh C. R., Schöneich C., Winter G., Friess W., Crommelin D. J., Carpenter J. F. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J Pharm Sci. 2012;101:946–954. doi: 10.1002/jps.23018. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell A. C., Briquez P. S., Hubbell J. A., Cochran J. R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apostolopoulos V., Bojarska J., Chai T. T., Elnagdy S., Kaczmarek K., Matsoukas J., New R., Parang K., Lopez O. P., Parhiz H., Perera C. O., Pickholz M., Remko M., Saviano M., Skwarczynski M., Tang Y., Wolf W. M., Yoshiya T., Zabrocki J., Zielenkiewicz P., AlKhazindar M., Barriga V., Kelaidonis K., Sarasia E. M., Toth I. A global review on short peptides: frontiers and perspectives. Molecules. 2021;26:430. doi: 10.3390/molecules26020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamley I. W. Small bioactive peptides for biomaterials design and therapeutics. Chem Rev. 2017;117:14015–14041. doi: 10.1021/acs.chemrev.7b00522. [DOI] [PubMed] [Google Scholar]
- 18.Zou P., Chen W. T., Sun T., Gao Y., Li L. L., Wang H. Recent advances: peptides and self-assembled peptide-nanosystems for antimicrobial therapy and diagnosis. Biomater Sci. 2020;8:4975–4996. doi: 10.1039/d0bm00789g. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Klein T., Murray R. Z., Crawford R., Chang J., Wu C., Xiao Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19:304–321. [Google Scholar]
- 20.Chai Y. C., Mendes L. F., van Gastel N., Carmeliet G., Luyten F. P. Fine-tuning pro-angiogenic effects of cobalt for simultaneous enhancement of vascular endothelial growth factor secretion and implant neovascularization. Acta Biomater. 2018;72:447–460. doi: 10.1016/j.actbio.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Kong N., Lin K., Li H., Chang J. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J Mater Chem B. 2014;2:1100–1110. doi: 10.1039/c3tb21529f. [DOI] [PubMed] [Google Scholar]
- 22.De la Fuente B., Vázquez M., Rocha R. A., Devesa V., Vélez D. Effects of sodium fluoride on immune response in murine macrophages. Toxicol In Vitro. 2016;34:81–87. doi: 10.1016/j.tiv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Liu L., Liu Y., Feng C., Chang J., Fu R., Wu T., Yu F., Wang X., Xia L., Wu C., Fang B. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials. 2019;192:523–536. doi: 10.1016/j.biomaterials.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Shen J., Chen B., Zhai X., Qiao W., Wu S., Liu X., Zhao Y., Ruan C., Pan H., Chu P. K., Cheung K. M. C., Yeung K. W. K. Stepwise 3D-spatio-temporal magnesium cationic niche: nanocomposite scaffold mediated microenvironment for modulating intramembranous ossification. Bioact Mater. 2021;6:503–519. doi: 10.1016/j.bioactmat.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai L., Wu R., Wang Y., Wang X., Zhang X., Huang X., Qin L., Hang R., Zhao L., Tang B. Osteogenic and angiogenic activities of silicon-incorporated TiO(2) nanotube arrays. J Mater Chem B. 2016;4:5548–5559. doi: 10.1039/c6tb01109h. [DOI] [PubMed] [Google Scholar]
- 26.Bai L., Hang R., Gao A., Zhang X., Huang X., Wang Y., Tang B., Zhao L., Chu P. K. Nanostructured titanium–silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl Surf Sci. 2015;355:32–44. [Google Scholar]
- 27.Yan R., Li J., Wu Q., Zhang X., Hu L., Deng Y., Jiang R., Wen J., Jiang X. Trace element-augmented titanium implant with targeted angiogenesis and enhanced osseointegration in osteoporotic rats. Front Chem. 2022;10:839062. doi: 10.3389/fchem.2022.839062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao W., Pan D., Zheng Y., Wu S., Liu X., Chen Z., Wan M., Feng S., Cheung K. M. C., Yeung K. W. K., Cao X. Divalent metal cations stimulate skeleton interoception for new bone formation in mouse injury models. Nat Commun. 2022;13:535. doi: 10.1038/s41467-022-28203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai L., Liu Y., Du Z., Weng Z., Yao W., Zhang X., Huang X., Yao X., Crawford R., Hang R., Huang D., Tang B., Xiao Y. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018;76:344–358. doi: 10.1016/j.actbio.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Bai L., Chen P., Zhao Y., Hang R., Yao X., Tang B., Liu C., Xiao Y., Hang R. A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration. Biomaterials. 2021;278:121162. doi: 10.1016/j.biomaterials.2021.121162. [DOI] [PubMed] [Google Scholar]
- 31.Kusumbe A. P., Ramasamy S. K., Adams R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y., Hu C., Feng Y., Li D., Ai T., Huang Y., Chen X., Huang L., Tan J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen Biomater. 2020;7:233–245. doi: 10.1093/rb/rbaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Z., Wu X., Wang Y., Li M., Li Y., Liu X., Zhang X., Lan Z., Wang J., Du Y., Zhang S. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact Mater. 2022;10:195–206. doi: 10.1016/j.bioactmat.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Wang J., Gao R., Liu X., Feng Z., Zhang C., Huang P., Dong A., Kong D., Wang W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials. 2022;285:121538. doi: 10.1016/j.biomaterials.2022.121538. [DOI] [PubMed] [Google Scholar]
- 35.Schlundt C., Fischer H., Bucher C. H., Rendenbach C., Duda G. N., Schmidt-Bleek K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021;133:46–57. doi: 10.1016/j.actbio.2021.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Bai X., Liu W., Xu L., Ye Q., Zhou H., Berg C., Yuan H., Li J., Xia W. Sequential macrophage transition facilitates endogenous bone regeneration induced by Zn-doped porous microcrystalline bioactive glass. J Mater Chem B. 2021;9:2885–2898. doi: 10.1039/d0tb02884c. [DOI] [PubMed] [Google Scholar]
- 37.Lee J., Byun H., Madhurakkat Perikamana S. K., Lee S., Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthc Mater. 2019;8:e1801106. doi: 10.1002/adhm.201801106. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Lin Q., Zhang H., Wang S., Cui J., Hu Y., Liu J., Li M., Zhang K., Zhou F., Jing Y., Geng Z., Su J. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 2023;28:273–283. doi: 10.1016/j.bioactmat.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushioka J., Chow S. K., Toya M., Tsubosaka M., Shen H., Gao Q., Li X., Zhang N., Goodman S. B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm Regen. 2023;43:29. doi: 10.1186/s41232-023-00279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang X., Pathak J. L., Wu W., Li J., Huang W., Wu Q., Xin M., Wu Y., Huang Y., Ge L., Zeng S. Human serum-derived exosomes modulate macrophage inflammation to promote VCAM1-mediated angiogenesis and bone regeneration. J Cell Mol Med. 2023;27:1131–1143. doi: 10.1111/jcmm.17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv X., Gao F., Cao X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 2022;34:1914–1931. doi: 10.1016/j.cmet.2022.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao R., Mi B., Hu Y., Lin S., Xiong Y., Lu X., Panayi A. C., Li G., Liu G. Hallmarks of peripheral nerve function in bone regeneration. Bone Res. 2023;11:6. doi: 10.1038/s41413-022-00240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L., Li A., Zhu L., Gan L., Zuo L. Roxadustat promotes osteoblast differentiation and prevents estrogen deficiency-induced bone loss by stabilizing HIF-1α and activating the Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2022;17:286. doi: 10.1186/s13018-022-03162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nencini S., Ringuet M., Kim D. H., Chen Y. J., Greenhill C., Ivanusic J. J. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain. 2017;13:1744806917697011. doi: 10.1177/1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Y., Li Y., Cao H., Long J., Wang X., Li L., Li C., Jia Q., Teng B., Tang T., Peng J., Eglin D., Alini M., Grijpma D. W., Richards G., Qin L. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Yan Y., Wang L., Ge L., Pathak J. L. Osteocyte-mediated translation of mechanical stimuli to cellular signaling and its role in bone and non-bone-related clinical complications. Curr Osteoporos Rep. 2020;18:67–80. doi: 10.1007/s11914-020-00564-9. [DOI] [PubMed] [Google Scholar]
- 47.Testa U., Castelli G., Pelosi E. Role of endothelial progenitor cells in vascular development, homestatic maintenance of blood vessels and in injury-mediated reparative response. Stem Cell Investig. 2020;7:7. [Google Scholar]
- 48.Zhang Y., Zhang Y. Y., Pan Z. W., Li Q. Q., Sun L. H., Li X., Gong M. Y., Yang X. W., Wang Y. Y., Li H. D., Xuan L. N., Shao Y. C., Li M. M., Zhang M. Y., Yu Q., Li Z., Zhang X. F., Liu D. H., Zhu Y. M., Tan Z. Y., Zhang Y. Y., Liu Y. Q., Zhang Y., Jiao L., Yang B. F. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1α-VEGF/SDF-1α-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol Sin. 2023;44:999–1013. doi: 10.1038/s41401-022-01013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akatsu Y., Takahashi N., Yoshimatsu Y., Kimuro S., Muramatsu T., Katsura A., Maishi N., Suzuki H. I., Inazawa J., Hida K., Miyazono K., Watabe T. Fibroblast growth factor signals regulate transforming growth factor-β-induced endothelial-to-myofibroblast transition of tumor endothelial cells via Elk1. Mol Oncol. 2019;13:1706–1724. doi: 10.1002/1878-0261.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Li J. Current progress in growth factors and extracellular vesicles in tendon healing. Int Wound J. 2023;20:3871–3883. doi: 10.1111/iwj.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miron R. J., Bosshardt D. D. OsteoMacs: Key players around bone biomaterials. Biomaterials. 2016;82:1–19. doi: 10.1016/j.biomaterials.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q., Liu Y., Li J., Wang J., Liu C. Recapitulation of growth factor-enriched microenvironment via BMP receptor activating hydrogel. Bioact Mater. 2023;20:638–650. doi: 10.1016/j.bioactmat.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman M. S., Akhtar N., Jamil H. M., Banik R. S., Asaduzzaman S. M. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Z., Ren L., Zhang Z., Yang Z., Wu S., Liu G., Cheng B., Wu J., Xia J. A multifunctional neuromodulation platform utilizing Schwann cell-derived exosomes orchestrates bone microenvironment via immunomodulation, angiogenesis and osteogenesis. Bioact Mater. 2023;23:206–222. doi: 10.1016/j.bioactmat.2022.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu K., Shang Z., Yang X., Zhang Y., Cao L. Macrophage polarization and the regulation of bone immunity in bone homeostasis. J Inflamm Res. 2023;16:3563–3580. doi: 10.2147/JIR.S423819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veis D. J., O’Brien C. A. Osteoclasts, Master Sculptors of Bone. Annu Rev Pathol. 2023;18:257–281. doi: 10.1146/annurev-pathmechdis-031521-040919. [DOI] [PubMed] [Google Scholar]
- 57.Yang N., Liu Y. The role of the immune microenvironment in bone regeneration. Int J Med Sci. 2021;18:3697–3707. doi: 10.7150/ijms.61080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao W., Helder M. N., Bravenboer N., Wu G., Jin J., Ten Bruggenkate C. M., Klein-Nulend J., Schulten E. Is there a governing role of osteocytes in bone tissue regeneration? Curr Osteoporos Rep. 2020;18:541–550. doi: 10.1007/s11914-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Guan M., Ren R., Gao C., Cheng H., Li Y., Gao B., Wei Y., Fu J., Sun J., Xiong W. Improved immunoregulation of ultra-low-dose silver nanoparticle-loaded TiO(2) nanotubes via M2 macrophage polarization by regulating GLUT1 and autophagy. Int J Nanomedicine. 2020;15:2011–2026. doi: 10.2147/IJN.S242919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Routray I., Ali S. Boron induces lymphocyte proliferation and modulates the priming effects of lipopolysaccharide on macrophages. PLoS One. 2016;11:e0150607. doi: 10.1371/journal.pone.0150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X., Li K., Xie Y., Qi S., Shen Q., Yu J., Huang L., Zheng X. Improved osteogenesis of boron incorporated calcium silicate coatings via immunomodulatory effects. J Biomed Mater Res A. 2019;107:12–24. doi: 10.1002/jbm.a.36456. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Wu Q., Yin C., Jia X., Zhao Z., Zhang X., Yuan G., Hu H., Zhao Q. Sustained calcium ion release from bioceramics promotes CaSR-mediated M2 macrophage polarization for osteoinduction. J Leukoc Biol. 2021;110:485–496. doi: 10.1002/JLB.3MA0321-739R. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y., Ye Y. C., Chen Y., Zhao J. L., Gao C. C., Han H., Liu W. C., Qin H. Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balsano C., Porcu C., Sideri S. Is copper a new target to counteract the progression of chronic diseases? Metallomics. 2018;10:1712–1722. doi: 10.1039/c8mt00219c. [DOI] [PubMed] [Google Scholar]
- 65.Gaetke L. M., Chow-Johnson H. S., Chow C. K. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014;88:1929–1938. doi: 10.1007/s00204-014-1355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu P., Dong J., Cheng N., Yang R., Han Y., Han Y. Inflammatory cytokines expression in Wilson’s disease. Neurol Sci. 2019;40:1059–1066. doi: 10.1007/s10072-018-3680-z. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Jiang Y., Shi H., Peng Y., Fan X., Li C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch. 2020;472:1415–1429. doi: 10.1007/s00424-020-02412-2. [DOI] [PubMed] [Google Scholar]
- 68.Huang Q., Li X., Elkhooly T. A., Liu X., Zhang R., Wu H., Feng Q., Liu Y. The Cu-containing TiO(2) coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf B Biointerfaces. 2018;170:242–250. doi: 10.1016/j.colsurfb.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Wang L., Ren L., Tang T., Dai K., Yang K., Hao Y. A novel nano-copper-bearing stainless steel with reduced Cu(2+) release only inducing transient foreign body reaction via affecting the activity of NF-κB and Caspase 3. Int J Nanomedicine. 2015;10:6725–6739. doi: 10.2147/IJN.S90249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S., Xia B., Mai S., Feng Z., Wang X., Liu Y., Liu R., Li Z., Xiao Y., Chen Z., Chen Z. Sodium fluoride under dose range of 2.4-24 μM, a promising osteoimmunomodulatory agent for vascularized bone formation. ACS Biomater Sci Eng. 2019;5:817–830. doi: 10.1021/acsbiomaterials.8b00570. [DOI] [PubMed] [Google Scholar]
- 71.Lee M. J., Chen Y., Huang Y. P., Hsu Y. C., Chiang L. H., Chen T. Y., Wang G. J. Exogenous polyamines promote osteogenic differentiation by reciprocally regulating osteogenic and adipogenic gene expression. J Cell Biochem. 2013;114:2718–2728. doi: 10.1002/jcb.24620. [DOI] [PubMed] [Google Scholar]
- 72.Lee M., Arikawa K., Nagahama F. Micromolar levels of sodium fluoride promote osteoblast differentiation through Runx2 signaling. Biol Trace Elem Res. 2017;178:283–291. doi: 10.1007/s12011-017-0930-5. [DOI] [PubMed] [Google Scholar]
- 73.Zhao P. P., Ge Y. W., Liu X. L., Ke Q. F., Zhang J. W., Zhu Z. A., Guo Y. P. Ordered arrangement of hydrated GdPO4 nanorods in magnetic chitosan matrix promotes tumor photothermal therapy and bone regeneration against breast cancer bone metastases. Chem Eng J. 2020;381:122694. [Google Scholar]
- 74.Seweryn A., Alicka M., Fal A., Kornicka-Garbowska K., Lawniczak-Jablonska K., Ozga M., Kuzmiuk P., Godlewski M., Marycz K. Hafnium (IV) oxide obtained by atomic layer deposition (ALD) technology promotes early osteogenesis via activation of Runx2-OPN-mir21A axis while inhibits osteoclasts activity. J Nanobiotechnology. 2020;18:132. doi: 10.1186/s12951-020-00692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartnikowski M., Moon H. J., Ivanovski S. Release of lithium from 3D printed polycaprolactone scaffolds regulates macrophage and osteoclast response. Biomed Mater. 2018;13:065003. doi: 10.1088/1748-605X/aad916. [DOI] [PubMed] [Google Scholar]
- 76.Fiorentini D., Cappadone C., Farruggia G., Prata C. Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients. 2021;13:1136. doi: 10.3390/nu13041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf F. I., Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med. 2003;24:3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen F. H. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018;11:25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Orden R., Eggett D. L., Franz K. B. Influence of graded magnesium deficiencies on white blood cell counts and lymphocyte subpopulations in rats. Magnes Res. 2006;19:93–101. [PubMed] [Google Scholar]
- 80.Qiao W., Wong K. H. M., Shen J., Wang W., Wu J., Li J., Lin Z., Chen Z., Matinlinna J. P., Zheng Y., Wu S., Liu X., Lai K. P., Chen Z., Lam Y. W., Cheung K. M. C., Yeung K. W. K. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat Commun. 2021;12:2885. doi: 10.1038/s41467-021-23005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Z., Han S., Shi M., Liu G., Chen Z., Chang J., Wu C., Xiao Y. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: From nanoimmunotoxicity to nanoimmunotherapy. Appl Mater Today. 2018;10:184–193. [Google Scholar]
- 82.Huang Y., Wu C., Zhang X., Chang J., Dai K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018;66:81–92. doi: 10.1016/j.actbio.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 83.Bai L., Liu Y., Zhang X., Huang X., Yao X., Hang R., Tang B., Xiao Y. Favorable manipulation of macrophage/endothelial cell functionality and their cross-talk on silicon-doped titania nanotube arrays. Nanoscale. 2019;11:5920–5931. doi: 10.1039/c8nr08381a. [DOI] [PubMed] [Google Scholar]
- 84.Rithidech K. N., Reungpatthanaphong P., Tungjai M., Jangiam W., Honikel L., Whorton E. B. Persistent depletion of plasma gelsolin (pGSN) after exposure of mice to heavy silicon ions. Life Sci Space Res (Amst) 2018;17:83–90. doi: 10.1016/j.lssr.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Brown D. M., Beswick P. H., Donaldson K. Induction of nuclear translocation of NF-kappaB in epithelial cells by respirable mineral fibres. J Pathol. 1999;189:258–264. doi: 10.1002/(SICI)1096-9896(199910)189:2<258::AID-PATH410>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 86.Zhao D. W., Liu C., Zuo K. Q., Su P., Li L. B., Xiao G. Y., Cheng L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem Eng J. 2021;408:127362. [Google Scholar]
- 87.Yu D., Guo S., Yu M., Liu W., Li X., Chen D., Li B., Guo Z., Han Y. Immunomodulation and osseointegration activities of Na(2)TiO(3) nanorods-arrayed coatings doped with different Sr content. Bioact Mater. 2022;10:323–334. doi: 10.1016/j.bioactmat.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao D. W., Zuo K. Q., Wang K., Sun Z. Y., Lu Y. P., Cheng L., Xiao G. Y., Liu C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater Sci Eng C Mater Biol Appl. 2021;118:111512. doi: 10.1016/j.msec.2020.111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russell K. C., Phinney D. G., Lacey M. R., Barrilleaux B. L., Meyertholen K. E., O’Connor K. C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 90.Luo X., Barbieri D., Davison N., Yan Y., de Bruijn J. D., Yuan H. Zinc in calcium phosphate mediates bone induction: in vitro and in vivo model. Acta Biomater. 2014;10:477–485. doi: 10.1016/j.actbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Xu Y., Xu C., Yang K., Ma L., Li G., Shi Y., Feng X., Tan L., Duan D., Luo Z., Yang C. Copper ion-modified germanium phosphorus nanosheets integrated with an electroactive and biodegradable hydrogel for neuro-vascularized bone regeneration. Adv Healthc Mater. 2023;12:e2301151. doi: 10.1002/adhm.202301151. [DOI] [PubMed] [Google Scholar]
- 92.Jing X., Xu C., Su W., Ding Q., Ye B., Su Y., Yu K., Zeng L., Yang X., Qu Y., Chen K., Sun T., Luo Z., Guo X. Photosensitive and conductive hydrogel induced innerved bone regeneration for infected bone defect repair. Adv Healthc Mater. 2023;12:e2201349. doi: 10.1002/adhm.202201349. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y., Xu J., Ruan Y. C., Yu M. K., O’Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J. Q., Chow D. H., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H. C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22:1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeanmonod P., Laschke M. W., Gola N., von Heesen M., Glanemann M., Dold S., Menger M. D., Moussavian M. R. Silver acetate coating promotes early vascularization of Dacron vascular grafts without inducing host tissue inflammation. J Vasc Surg. 2013;58:1637–1643. doi: 10.1016/j.jvs.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 95.Glenske K., Donkiewicz P., Köwitsch A., Milosevic-Oljaca N., Rider P., Rofall S., Franke J., Jung O., Smeets R., Schnettler R., Wenisch S., Barbeck M. Applications of metals for bone regeneration. Int J Mol Sci. 2018;19:826. doi: 10.3390/ijms19030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang K., Lim D. H., Choi I. H., Kang T., Lee K., Moon E. Y., Yang Y., Lee M. S., Lim J. S. Vascular tube formation and angiogenesis induced by polyvinylpyrrolidone-coated silver nanoparticles. Toxicol Lett. 2011;205:227–234. doi: 10.1016/j.toxlet.2011.05.1033. [DOI] [PubMed] [Google Scholar]
- 97.Xia L., Ma W., Zhou Y., Gui Z., Yao A., Wang D., Takemura A., Uemura M., Lin K., Xu Y. Stimulatory effects of boron containing bioactive glass on osteogenesis and angiogenesis of polycaprolactone: in vitro study. Biomed Res Int. 2019;2019:8961409. doi: 10.1155/2019/8961409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haro Durand L. A., Góngora A., Porto López J. M., Boccaccini A. R., Zago M. P., Baldi A., Gorustovich A. In vitro endothelial cell response to ionic dissolution products from boron-doped bioactive glass in the SiO(2)-CaO-P(2)O(5)-Na(2)O system. J Mater Chem B. 2014;2:7620–7630. doi: 10.1039/c4tb01043d. [DOI] [PubMed] [Google Scholar]
- 99.Li K., Lu X., Liu S., Wu X., Xie Y., Zheng X. Boron-incorporated micro/nano-topographical calcium silicate coating dictates osteo/angio-genesis and inflammatory response toward enhanced osseointegration. Biol Trace Elem Res. 2021;199:3801–3816. doi: 10.1007/s12011-020-02517-w. [DOI] [PubMed] [Google Scholar]
- 100.Chen S., Yang Q., Brow R. K., Liu K., Brow K. A., Ma Y., Shi H. In vitro stimulation of vascular endothelial growth factor by borate-based glass fibers under dynamic flow conditions. Mater Sci Eng C Mater Biol Appl. 2017;73:447–455. doi: 10.1016/j.msec.2016.12.099. [DOI] [PubMed] [Google Scholar]
- 101.Jacobs A., Renaudin G., Forestier C., Nedelec J. M., Descamps S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta Biomater. 2020;117:21–39. doi: 10.1016/j.actbio.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y., Zhang W., Yao Q. Copper-based biomaterials for bone and cartilage tissue engineering. J Orthop Translat. 2021;29:60–71. doi: 10.1016/j.jot.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiang J., Li J., He J., Tang X., Dou C., Cao Z., Yu B., Zhao C., Kang F., Yang L., Dong S., Yang X. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8:4489–4499. doi: 10.1021/acsami.6b00158. [DOI] [PubMed] [Google Scholar]
- 104.Zheng Y., Yang Y., Deng Y. Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2019;99:770–782. doi: 10.1016/j.msec.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 105.Fani N., Farokhi M., Azami M., Kamali A., Bakhshaiesh N. L., Ebrahimi-Barough S., Ai J., Eslaminejad M. B. Endothelial and osteoblast differentiation of adipose-derived mesenchymal stem cells using a cobalt-doped cap/silk fibroin scaffold. ACS Biomater Sci Eng. 2019;5:2134–2146. doi: 10.1021/acsbiomaterials.8b01372. [DOI] [PubMed] [Google Scholar]
- 106.Delafontaine P., Bernstein K. E., Alexander R. W. Insulin-like growth factor I gene expression in vascular cells. Hypertension. 1991;17:693–699. doi: 10.1161/01.hyp.17.5.693. [DOI] [PubMed] [Google Scholar]
- 107.Shi H., Yang S., Zeng S., Liu X., Zhang J., Zhang J., Wu T., Ye X., Yu T., Zhou C., Ye J. Enhanced angiogenesis of biodegradable iron-doped octacalcium phosphate/poly(lactic-co-glycolic acid) scaffold for potential cancerous bone regeneration. Appl Mater Today. 2019;15:100–114. [Google Scholar]
- 108.Lin S., Yang G., Jiang F., Zhou M., Yin S., Tang Y., Tang T., Zhang Z., Zhang W., Jiang X. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv Sci (Weinh) 2019;6:1900209. doi: 10.1002/advs.201900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye L., Xu J., Mi J., He X., Pan Q., Zheng L., Zu H., Chen Z., Dai B., Li X., Pang Q., Zou L., Zhou L., Huang L., Tong W., Li G., Qin L. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials. 2021;275:120984. doi: 10.1016/j.biomaterials.2021.120984. [DOI] [PubMed] [Google Scholar]
- 110.Gu Z., Xie H., Huang C., Peng H., Tan H., Li L., Yu X. Effects of strontium-doped calcium polyphosphate on angiogenic growth factors expression of co-culturing system in vitro and of host cell in vivo. RSC Adv. 2014;4:2783–2792. [Google Scholar]
- 111.Zhao F., Lei B., Li X., Mo Y., Wang R., Chen D., Chen X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials. 2018;178:36–47. doi: 10.1016/j.biomaterials.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Srinath P., Abdul Azeem P., Venugopal Reddy K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int J Appl Ceram Technol. 2020;17:2450–2464. [Google Scholar]
- 113.Li H., Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater. 2013;9:5379–5389. doi: 10.1016/j.actbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 114.Zhai W., Lu H., Chen L., Lin X., Huang Y., Dai K., Naoki K., Chen G., Chang J. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012;8:341–349. doi: 10.1016/j.actbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 115.Mao L., Xia L., Chang J., Liu J., Jiang L., Wu C., Fang B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017;61:217–232. doi: 10.1016/j.actbio.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 116.Tang Y. Q., Wang Q. Y., Ke Q. F., Zhang C. Q., Guan J. J., Guo Y. P. Mineralization of ytterbium-doped hydroxyapatite nanorod arrays in magnetic chitosan scaffolds improves osteogenic and angiogenic abilities for bone defect healing. Chem Eng J. 2020;387:124166. [Google Scholar]
- 117.Zhu D., Su Y., Zheng Y., Fu B., Tang L., Qin Y. X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/ GPR39. Am J Physiol Cell Physiol. 2018;314:C404–C414. doi: 10.1152/ajpcell.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma J., Zhao N., Zhu D. Endothelial cellular responses to biodegradable metal zinc. ACS Biomater Sci Eng. 2015;1:1174–1182. doi: 10.1021/acsbiomaterials.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang J., Park Y. D., Bae W. J., El-Fiqi A., Shin S. H., Lee E. J., Kim H. W., Kim E. C. Effects of bioactive cements incorporating zinc-bioglass nanoparticles on odontogenic and angiogenic potential of human dental pulp cells. J Biomater Appl. 2015;29:954–964. doi: 10.1177/0885328214550896. [DOI] [PubMed] [Google Scholar]
- 120.Hassan A., Elebeedy D., Matar E. R., Fahmy Mohamed Elsayed A., Abd El Maksoud A. I. Investigation of angiogenesis and wound healing potential mechanisms of zinc oxide nanorods. Front Pharmacol. 2021;12:661217. doi: 10.3389/fphar.2021.661217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bosch-Rué E., Diez-Tercero L., Giordano-Kelhoffer B., Delgado L. M., Bosch B. M., Hoyos-Nogués M., Mateos-Timoneda M. A., Tran P. A., Gil F. J., Perez R. A. Biological roles and delivery strategies for ions to promote osteogenic induction. Front Cell Dev Biol. 2020;8:614545. doi: 10.3389/fcell.2020.614545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santos C. J., Ferreira Soares D. C., Ferreira C. A., de Barros A. L. B., Silva Cunha Junior A. D., Filho F. M. Antiangiogenic evaluation of ZnWO(4) nanoparticles synthesised through microwave-assisted hydrothermal method. J Drug Target. 2018;26:806–817. doi: 10.1080/1061186X.2018.1428810. [DOI] [PubMed] [Google Scholar]
- 123.Song Y., Wu H., Gao Y., Li J., Lin K., Liu B., Lei X., Cheng P., Zhang S., Wang Y., Sun J., Bi L., Pei G. Zinc silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl Mater Interfaces. 2020;12:16058–16075. doi: 10.1021/acsami.0c00470. [DOI] [PubMed] [Google Scholar]
- 124.Qing T., Mahmood M., Zheng Y., Biris A. S., Shi L., Casciano D. A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J Appl Toxicol. 2018;38:172–179. doi: 10.1002/jat.3528. [DOI] [PubMed] [Google Scholar]
- 125.Wu Y., Cheng Z., Hu W., Tang S., Zhou X., Dong S. Biosynthesized silver nanoparticles inhibit osteoclastogenesis by suppressing NF-κB signaling pathways. Adv Biol (Weinh) 2023:e2300355. doi: 10.1002/adbi.202300355. [DOI] [PubMed] [Google Scholar]
- 126.Liu X., Lee P. Y., Ho C. M., Lui V. C., Chen Y., Che C. M., Tam P. K., Wong K. K. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem. 2010;5:468–475. doi: 10.1002/cmdc.200900502. [DOI] [PubMed] [Google Scholar]
- 127.Nielsen F. H. The emergence of boron as nutritionally important throughout the life cycle. Nutrition. 2000;16:512–514. doi: 10.1016/s0899-9007(00)00324-5. [DOI] [PubMed] [Google Scholar]
- 128.Dessordi R., Spirlandeli A. L., Zamarioli A., Volpon J. B., Navarro A. M. Boron supplementation improves bone health of non-obese diabetic mice. J Trace Elem Med Biol. 2017;39:169–175. doi: 10.1016/j.jtemb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 129.Yin C., Jia X., Zhao Q., Zhao Z., Wang J., Zhang Y., Li Z., Sun H., Li Z. Transcription factor 7-like 2 promotes osteogenic differentiation and boron-induced bone repair via lipocalin 2. Mater Sci Eng C Mater Biol Appl. 2020;110:110671. doi: 10.1016/j.msec.2020.110671. [DOI] [PubMed] [Google Scholar]
- 130.Li X., Wang X., Jiang X., Yamaguchi M., Ito A., Bando Y., Golberg D. Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2016;104:323–329. doi: 10.1002/jbm.b.33391. [DOI] [PubMed] [Google Scholar]
- 131.Yin C., Jia X., Miron R. J., Long Q., Xu H., Wei Y., Wu M., Zhang Y., Li Z. Setd7 and its contribution to Boron-induced bone regeneration in Boron-mesoporous bioactive glass scaffolds. Acta Biomater. 2018;73:522–530. doi: 10.1016/j.actbio.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 132.Ying D., Ouyang Z., Liu T., Liu X., Huang Q. Boron-containing micro/nano-structured TiO2/bioceramics coatings with modulatory effects on SaOS-2 cell response. Mater Lett. 2018;228:29–32. [Google Scholar]
- 133.Cai B., Tan P., Jiang N., Guo Z., Ay B., Li S., Hou Y., Li Y., You Y., Zhang L., Zhu S. Bioinspired fabrication of calcium-doped TiP coating with nanofibrous microstructure to accelerate osseointegration. Bioconjug Chem. 2020;31:1641–1650. doi: 10.1021/acs.bioconjchem.0c00201. [DOI] [PubMed] [Google Scholar]
- 134.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S23–30. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 135.Foreman M. A., Gu Y., Howl J. D., Jones S., Publicover S. J. Group III metabotropic glutamate receptor activation inhibits Ca2+ influx and nitric oxide synthase activity in bone marrow stromal cells. J Cell Physiol. 2005;204:704–713. doi: 10.1002/jcp.20353. [DOI] [PubMed] [Google Scholar]
- 136.Riddle R. C., Taylor A. F., Genetos D. C., Donahue H. J. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290:C776–784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- 137.Liu D., Genetos D. C., Shao Y., Geist D. J., Li J., Ke H. Z., Turner C. H., Duncan R. L. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Danciu T. E., Adam R. M., Naruse K., Freeman M. R., Hauschka P. V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003;536:193–197. doi: 10.1016/s0014-5793(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 139.Lee M. N., Hwang H. S., Oh S. H., Roshanzadeh A., Kim J. W., Song J. H., Kim E. S., Koh J. T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp Mol Med. 2018;50:1–16. doi: 10.1038/s12276-018-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gaharwar A. K., Mihaila S. M., Swami A., Patel A., Sant S., Reis R. L., Marques A. P., Gomes M. E., Khademhosseini A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv Mater. 2013;25:3329–3336. doi: 10.1002/adma.201300584. [DOI] [PubMed] [Google Scholar]
- 141.Westhauser F., Rehder F., Decker S., Kunisch E., Moghaddam A., Zheng K., Boccaccini A. R. Ionic dissolution products of Cerium-doped bioactive glass nanoparticles promote cellular osteogenic differentiation and extracellular matrix formation of human bone marrow derived mesenchymal stromal cells. Biomed Mater. 2021;16:035028. doi: 10.1088/1748-605X/abcf5f. [DOI] [PubMed] [Google Scholar]
- 142.Hsu S. H., Chen C. T., Wei Y. H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2013;31:2779–2788. doi: 10.1002/stem.1441. [DOI] [PubMed] [Google Scholar]
- 143.Yoo H. I., Moon Y. H., Kim M. S. Effects of CoCl2 on multi-lineage differentiation of C3H/10T1/2 mesenchymal stem cells. Korean J Physiol Pharmacol. 2016;20:53–62. doi: 10.4196/kjpp.2016.20.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burghardt I., Lüthen F., Prinz C., Kreikemeyer B., Zietz C., Neumann H. G., Rychly J. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 145.Lin Z., Cao Y., Zou J., Zhu F., Gao Y., Zheng X., Wang H., Zhang T., Wu T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater Sci Eng C Mater Biol Appl. 2020;114:111032. doi: 10.1016/j.msec.2020.111032. [DOI] [PubMed] [Google Scholar]
- 146.Zhang J., Wu H., He F., Wu T., Zhou L., Ye J. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater Sci Eng C Mater Biol Appl. 2019;99:1199–1212. doi: 10.1016/j.msec.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 147.Chen M., Wang X. Q., Zhang E. L., Wan Y. Z., Hu J. Antibacterial ability and biocompatibility of fluorinated titanium by plasma-based surface modification. Rare Met. 2022;41:689–699. [Google Scholar]
- 148.Chen L., Song W., Markel D. C., Shi T., Muzik O., Matthew H., Ren W. Flow perfusion culture of MC3T3-E1 osteogenic cells on gradient calcium polyphosphate scaffolds with different pore sizes. J Biomater Appl. 2016;30:908–918. doi: 10.1177/0885328215608335. [DOI] [PubMed] [Google Scholar]
- 149.Qu W. J., Zhong D. B., Wu P. F., Wang J. F., Han B. Sodium fluoride modulates caprine osteoblast proliferation and differentiation. J Bone Miner Metab. 2008;26:328–334. doi: 10.1007/s00774-007-0832-2. [DOI] [PubMed] [Google Scholar]
- 150.Pan X., Huang J., Zhang K., Pei Z., Ding Z., Liang Y., Gu Z., Li G., Xie H. Iron-doped brushite bone cement scaffold with enhanced osteoconductivity and antimicrobial properties for jaw regeneration. Ceram Int. 2021;47:25810–25820. [Google Scholar]
- 151.Strazic Geljic I., Melis N., Boukhechba F., Schaub S., Mellier C., Janvier P., Laugier J. P., Bouler J. M., Verron E., Scimeca J. C. Gallium enhances reconstructive properties of a calcium phosphate bone biomaterial. J Tissue Eng Regen Med. 2018;12:e854–e866. doi: 10.1002/term.2396. [DOI] [PubMed] [Google Scholar]
- 152.Liao F., Peng X. Y., Yang F., Ke Q. F., Zhu Z. H., Guo Y. P. Gadolinium-doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Mater Sci Eng C Mater Biol Appl. 2019;104:109999. doi: 10.1016/j.msec.2019.109999. [DOI] [PubMed] [Google Scholar]
- 153.Zhao P. P., Hu H. R., Liu J. Y., Ke Q. F., Peng X. Y., Ding H., Guo Y. P. Gadolinium phosphate/chitosan scaffolds promote new bone regeneration via Smad/Runx2 pathway. Chem Eng J. 2019;359:1120–1129. [Google Scholar]
- 154.Zhu D. Y., Lu B., Yin J. H., Ke Q. F., Xu H., Zhang C. Q., Guo Y. P., Gao Y. S. Gadolinium-doped bioglass scaffolds promote osteogenic differentiation of hBMSC via the Akt/GSK3β pathway and facilitate bone repair in vivo. Int J Nanomedicine. 2019;14:1085–1100. doi: 10.2147/IJN.S193576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chu M., Sun Z., Fan Z., Yu D., Mao Y., Guo Y. Bi-directional regulation functions of lanthanum-substituted layered double hydroxide nanohybrid scaffolds via activating osteogenesis and inhibiting osteoclastogenesis for osteoporotic bone regeneration. Theranostics. 2021;11:6717–6734. doi: 10.7150/thno.56607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cheng H., Guo Q., Zhao H., Liu K., Kang H., Gao F., Guo J., Yuan X., Hu S., Li F., Yang Q., Fang Z. An injectable hydrogel scaffold loaded with dual-drug/sustained-release PLGA microspheres for the regulation of macrophage polarization in the treatment of intervertebral disc degeneration. Int J Mol Sci. 2022;24:390. doi: 10.3390/ijms24010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang J., Cai L., Tang L., Zhang X., Yang L., Zheng K., He A., Boccaccini A. R., Wei J., Zhao J. Highly dispersed lithium doped mesoporous silica nanospheres regulating adhesion, proliferation, morphology, ALP activity and osteogenesis related gene expressions of BMSCs. Colloids Surf B Biointerfaces. 2018;170:563–571. doi: 10.1016/j.colsurfb.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 158.Mo F., Jiang K., Zhao D., Wang Y., Song J., Tan W. DNA hydrogel-based gene editing and drug delivery systems. Adv Drug Deliv Rev. 2021;168:79–98. doi: 10.1016/j.addr.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 159.Tan Z., Zhou B., Zheng J., Huang Y., Zeng H., Xue L., Wang D. Lithium and copper induce the osteogenesis-angiogenesis coupling of bone marrow mesenchymal stem cells via crosstalk between canonical Wnt and HIF-1α signaling pathways. Stem Cells Int. 2021;2021:6662164. doi: 10.1155/2021/6662164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang T. B., Li Y. Z., Yu K., Yu Z., Wang Y., Jiang Z. W., Wang H. M., Yang G. L. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomater Sci. 2019;7:1101–1116. doi: 10.1039/c8bm01411f. [DOI] [PubMed] [Google Scholar]
- 161.Wang J., Ma X. Y., Feng Y. F., Ma Z. S., Ma T. C., Zhang Y., Li X., Wang L., Lei W. Magnesium ions promote the biological behaviour of rat calvarial osteoblasts by activating the PI3K/Akt signalling pathway. Biol Trace Elem Res. 2017;179:284–293. doi: 10.1007/s12011-017-0948-8. [DOI] [PubMed] [Google Scholar]
- 162.Pantulap U., Arango-Ospina M., Boccaccini A. R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J Mater Sci Mater Med. 2021;33:3. doi: 10.1007/s10856-021-06626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barrioni B. R., Norris E., Li S., Naruphontjirakul P., Jones J. R., Pereira M. M. Osteogenic potential of sol-gel bioactive glasses containing manganese. J Mater Sci Mater Med. 2019;30:86. doi: 10.1007/s10856-019-6288-9. [DOI] [PubMed] [Google Scholar]
- 164.Wu T., Shi H., Liang Y., Lu T., Lin Z., Ye J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped β-tricalcium phosphate. Mater Sci Eng C Mater Biol Appl. 2020;109:110481. doi: 10.1016/j.msec.2019.110481. [DOI] [PubMed] [Google Scholar]
- 165.Su Y., Chen L. J., He J. R., Yuan X. J., Cen Y. L., Su F. X., Tang L. Y., Zhang A. H., Chen W. Q., Lin Y., Wang S. M., Ren Z. F. Urinary rubidium in breast cancers. Clin Chim Acta. 2011;412:2305–2309. doi: 10.1016/j.cca.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 166.Ouyang S., Zheng K., Huang Q., Liu Y., Boccaccini A. R. Synthesis and characterization of rubidium-containing bioactive glass nanoparticles. Mater Lett. 2020;273:127920. [Google Scholar]
- 167.Chen M., Wu S., Tan Y., Li R., Liu Y., Huang Q. Rubidium-doped titanium surfaces with modulatory effects on MC3T3-E1 cell response and antibacterial capacity against Staphylococcus aureus. Biomed Mater. 2019;14:045016. doi: 10.1088/1748-605X/ab2585. [DOI] [PubMed] [Google Scholar]
- 168.Tan Y. N., Chen W. J., Wei W., Huang Q. L., He X. Rubidium-modified bioactive glass-ceramics with hydroxyapatite crystals for bone regeneration. Trans Nonferrous Met Soc China. 2021;31:521–532. [Google Scholar]
- 169.Ren N., Yu X., Wang A., Liang N., Feng Z., Sun C. Effects of scandium chloride on osteogenic and adipogenic differentiation of mesenchymal stem cells. J Rare Earths. 2022;40:161–168. [Google Scholar]
- 170.Millucci L., Minetti M., Orlandini M., Braconi D., Schiavone M. L., Galderisi S., Marzocchi B., Spiga O., Rappuoli R., Spreafico A., Perretti G., Bernardini G., Santucci A. Beer promotes differentiation and mineralization of human osteoblastic cells: Role of silicon. J Funct Foods. 2019;54:109–118. [Google Scholar]
- 171.Reffitt D. M., Ogston N., Jugdaohsingh R., Cheung H. F., Evans B. A., Thompson R. P., Powell J. J., Hampson G. N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 172.Dong M., Jiao G., Liu H., Wu W., Li S., Wang Q., Xu D., Li X., Liu H., Chen Y. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol Trace Elem Res. 2016;173:306–315. doi: 10.1007/s12011-016-0686-3. [DOI] [PubMed] [Google Scholar]
- 173.Zhou H., Jiao G., Dong M., Chi H., Wang H., Wu W., Liu H., Ren S., Kong M., Li C., Zhang L., Chen Y. Orthosilicic acid accelerates bone formation in human osteoblast-like cells through the PI3K-Akt-mTOR pathway. Biol Trace Elem Res. 2019;190:327–335. doi: 10.1007/s12011-018-1574-9. [DOI] [PubMed] [Google Scholar]
- 174.Zhou X., Moussa F. M., Mankoci S., Ustriyana P., Zhang N., Abdelmagid S., Molenda J., Murphy W. L., Safadi F. F., Sahai N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016;39:192–202. doi: 10.1016/j.actbio.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 175.Ma W., Wang F., You Y., Wu W., Chi H., Jiao G., Zhang L., Zhou H., Wang H., Chen Y. Ortho-silicic acid inhibits RANKL-induced osteoclastogenesis and reverses ovariectomy-induced bone loss in vivo. Biol Trace Elem Res. 2021;199:1864–1876. doi: 10.1007/s12011-020-02286-6. [DOI] [PubMed] [Google Scholar]
- 176.You Y., Ma W., Wang F., Jiao G., Zhang L., Zhou H., Wu W., Wang H., Chen Y. Ortho-silicic acid enhances osteogenesis of osteoblasts through the upregulation of miR-130b which directly targets PTEN. Life Sci. 2021;264:118680. doi: 10.1016/j.lfs.2020.118680. [DOI] [PubMed] [Google Scholar]
- 177.Liangjiao C., Ping Z., Ruoyu L., Yanli Z., Ting S., Yanjun L., Longquan S. Potential proinflammatory and osteogenic effects of dicalcium silicate particles in vitro. J Mech Behav Biomed Mater. 2015;44:10–22. doi: 10.1016/j.jmbbm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 178.Geng Z., Sang S., Wang S., Meng F., Li Z., Zhu S., Cui Z., Jing Y., Wang C., Su J. Optimizing the strontium content to achieve an ideal osseointegration through balancing apatite-forming ability and osteogenic activity. Biomater Adv. 2022;133:112647. doi: 10.1016/j.msec.2022.112647. [DOI] [PubMed] [Google Scholar]
- 179.Li Y., Yue J., Liu Y., Wu J., Guan M., Chen D., Pan H., Zhao X., Lu W. W. Strontium regulates stem cell fate during osteogenic differentiation through asymmetric cell division. Acta Biomater. 2021;119:432–443. doi: 10.1016/j.actbio.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 180.Stefanic M., Peroglio M., Stanciuc A. M., Machado G. C., Campbell I., Kržmanc M. M., Alini M., Zhang X. The influence of strontium release rate from bioactive phosphate glasses on osteogenic differentiation of human mesenchymal stem cells. J Eur Ceram Soc. 2018;38:887–897. [Google Scholar]
- 181.Montagna G., Cristofaro F., Fassina L., Bruni G., Cucca L., Kochen A., Divieti Pajevic P., Bragdon B., Visai L., Gerstenfeld L. An in vivo comparison study between strontium nanoparticles and rhBMP2. Front Bioeng Biotechnol. 2020;8:499. doi: 10.3389/fbioe.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang D., Zhao F., Gao W., Chen X., Guo Z., Zhang W. Strontium-substituted sub-micron bioactive glasses inhibit ostoclastogenesis through suppression of RANKL-induced signaling pathway. Regen Biomater. 2020;7:303–311. doi: 10.1093/rb/rbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barrio D. A., Etcheverry S. B. Vanadium and bone development: putative signaling pathways. Can J Physiol Pharmacol. 2006;84:677–686. doi: 10.1139/y06-022. [DOI] [PubMed] [Google Scholar]
- 184.Cortizo A. M., Molinuevo M. S., Barrio D. A., Bruzzone L. Osteogenic activity of vanadyl(IV)-ascorbate complex: evaluation of its mechanism of action. Int J Biochem Cell Biol. 2006;38:1171–1180. doi: 10.1016/j.biocel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 185.Jin G., Cao H., Qiao Y., Meng F., Zhu H., Liu X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 186.Kwun I. S., Cho Y. E., Lomeda R. A., Shin H. I., Choi J. Y., Kang Y. H., Beattie J. H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone. 2010;46:732–741. doi: 10.1016/j.bone.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 187.Moonga B. S., Dempster D. W. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J Bone Miner Res. 1995;10:453–457. doi: 10.1002/jbmr.5650100317. [DOI] [PubMed] [Google Scholar]
- 188.Guo B., Yang M., Liang D., Yang L., Cao J., Zhang L. Cell apoptosis induced by zinc deficiency in osteoblastic MC3T3-E1 cells via a mitochondrial-mediated pathway. Mol Cell Biochem. 2012;361:209–216. doi: 10.1007/s11010-011-1105-x. [DOI] [PubMed] [Google Scholar]
- 189.Hadley K. B., Newman S. M., Hunt J. R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J Nutr Biochem. 2010;21:297–303. doi: 10.1016/j.jnutbio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 190.O’Connor J. P., Kanjilal D., Teitelbaum M., Lin S. S., Cottrell J. A. Zinc as a therapeutic agent in bone regeneration. Materials (Basel) 2020;13:2211. doi: 10.3390/ma13102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu G., Zhu Y., Xu F., Ye J., Guan J., Jiang Y., Di M., Li Z., Guan H., Yao X. Comparison of surface properties, cell behaviors, bone regeneration and osseointegration between nano tantalum/PEEK composite and nano silicon nitride/PEEK composite. J Biomater Sci Polym Ed. 2022;33:35–56. doi: 10.1080/09205063.2021.1974812. [DOI] [PubMed] [Google Scholar]
- 192.Hwang C., Park S., Kang I. G., Kim H. E., Han C. M. Tantalum-coated polylactic acid fibrous membranes for guided bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;115:111112. doi: 10.1016/j.msec.2020.111112. [DOI] [PubMed] [Google Scholar]
- 193.Zhou Z., Liu D. Mesenchymal stem cell-seeded porous tantalum-based biomaterial: a promising choice for promoting bone regeneration. Colloids Surf B Biointerfaces. 2022;215:112491. doi: 10.1016/j.colsurfb.2022.112491. [DOI] [PubMed] [Google Scholar]
- 194.Mas-Moruno C., Garrido B., Rodriguez D., Ruperez E., Gil F. J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J Mater Sci Mater Med. 2015;26:109. doi: 10.1007/s10856-015-5445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shi L. Y., Wang A., Zang F. Z., Wang J. X., Pan X. W., Chen H. J. Tantalum-coated pedicle screws enhance implant integration. Colloids Surf B Biointerfaces. 2017;160:22–32. doi: 10.1016/j.colsurfb.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 196.Qing Y., Li R., Li S., Li Y., Wang X., Qin Y. Advanced black phosphorus nanomaterials for bone regeneration. Int J Nanomedicine. 2020;15:2045–2058. doi: 10.2147/IJN.S246336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim H. D., Jang H. L., Ahn H. Y., Lee H. K., Park J., Lee E. S., Lee E. A., Jeong Y. H., Kim D. G., Nam K. T., Hwang N. S. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 2017;112:31–43. doi: 10.1016/j.biomaterials.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 198.Eckart R. E., Shry E. A., Burke A. P., McNear J. A., Appel D. A., Castillo-Rojas L. M., Avedissian L., Pearse L. A., Potter R. N., Tremaine L., Gentlesk P. J., Huffer L., Reich S. S., Stevenson W. G., Department of Defense Cardiovascular Death Registry Group Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 199.Goretti Penido M., Alon U. S. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27:2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X., Miller A. L., 2nd, Park S., George M. N., Waletzki B. E., Xu H., Terzic A., Lu L. Two-dimensional black phosphorus and graphene oxide nanosheets synergistically enhance cell proliferation and osteogenesis on 3D printed scaffolds. ACS Appl Mater Interfaces. 2019;11:23558–23572. doi: 10.1021/acsami.9b04121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Avgoulas E. I., Sutcliffe M. P. F., Linderman S. W., Birman V., Thomopoulos S., Genin G. M. Adhesive-based tendon-to-bone repair: failure modelling and materials selection. J R Soc Interface. 2019;16:20180838. doi: 10.1098/rsif.2018.0838. [DOI] [PMC free article] [PubMed] [Google Scholar]