Abstract
Burn patients suffer a break in the physical barrier (skin), which, when combined with their generalized state of immunodeficiency, creates an open window for opportunistic infections, mainly with Pseudomonas aeruginosa. Infection of the burn wound has always been a major factor in retardation of wound healing, and sepsis remains the leading cause of death in burn patients. Because studies have shown that topical treatment with antiexotoxin A (ETA) antibodies significantly increases survival in rats infected with toxin-producing strains of P. aeruginosa, we examined 11 synthetic peptides encompassing 12 to 45 amino acid (aa) residues, representing what were predicted by computer analysis to be the most hydrophilic and antigenic regions of ETA. These synthetic peptides were injected into rabbits for antibody production. Different groups of rabbits were immunized with a combination of peptides, with each combination representing one of the three distinct domains of ETA. Animals immunized with various peptide combinations produced peptide-specific antibodies that exhibited cross-reactivity to ETA. Two major epitopes were identified on the ETA molecule by experiments with peptide-specific antibodies in enzyme-linked immunosorbent assay and immunoprecipitation. One of these epitopes was located in the translocation domain (II) (aa 297 to 310), while the other was mapped to the last 13 aa residues at the carboxy-terminal end of the enzymatic domain (III) (aa 626 to 638). Of these two regions, the epitope in the enzymatic domain induced a much higher level of neutralizing antibodies that abrogated the cytotoxic activity of ETA in vitro. Antibodies to this epitope blocked the ADP-ribosyltransferase activity of ETA and appeared to interfere with binding of the substrate elongation factor 2 to the enzymatic active site of the ETA molecule. We conclude that polyclonal, as well as monoclonal, antibodies to short peptides, representing small regions of ETA, may have therapeutic potential in passive immunization or topical treatment of burn patients infected with toxin-producing strains of P. aeruginosa.
Pseudomonas aeruginosa is an opportunistic pathogen that causes serious and sometimes fatal infections in the compromised host, especially in patients with major trauma or thermal injuries (9, 29, 32, 37). Several extracellular products of P. aeruginosa are implicated in its pathogenicity, including the heat-labile exotoxin A (ETA) (22, 23), several proteases (14, 21, 28), and hemolysins (43). P. aeruginosa ETA is 10,000 times more toxic than lipopolysaccharide (LPS) isolated from the outer membrane of P. aeruginosa (3, 24). The mature structural ETA is a single-chain polypeptide with a molecular weight of 66,583 that consists of 613 amino acid (aa) residues. X-ray crystallographic studies (1) identified three structural domains: the receptor binding domain I (aa 1 to 252 and 365 to 404) (18), the translocation domain II (aa 253 to 364) (4), and the enzymatic domain III (aa 405 to 613) (13). The cytotoxic activity of ETA is attributed to the enzymatic domain, which inhibits protein synthesis through ADP-ribosylation of eukaryotic elongation factor 2 (eEF-2) in a manner similar to that of diphtheria toxin (19). When cultured in vitro, 80 to 90% of all P. aeruginosa clinical isolates produce ETA (34), and over 90% of all P. aeruginosa strains harbor the chromosomal gene for ETA (42). ETA is believed to be the most toxic virulence factor produced by P. aeruginosa (24), and its cytotoxic activity extends to a wide variety of mammalian cells (25). ETA has been shown to inhibit proliferation of human granulocyte and macrophage progenitor cells (33, 39) to alter the production of tumor necrosis factor alpha (TNF-α) by human leukocytes (38), and to interfere with murine interleukin-1 production by peritoneal macrophages in vitro (26). These results suggest a role for ETA in the pathophysiology of P. aeruginosa septicemia, a major cause of death among burn patients (11, 35, 40, 44).
Wound healing is a major concern in treatment of traumatic injuries (17). We have previously examined the effect of ETA on wound healing in an acute wound model in rats (16). Our study showed a direct correlation between inoculation of the wound with ETA and the delay in the healing process, as measured by the rate of wound closure and the tensile strength of skin (16). In the present study, synthetic peptides corresponding to predicted immunogenic regions on the surface of the ETA molecule were generated to identify an epitope or epitopes capable of eliciting neutralizing antibodies. Our studies showed that one of the peptides, encompassing a region within the enzymatic domain of ETA (aa 610 to 638), represented an immunodominant epitope on the surface of ETA. Antibodies specific for the carboxy-terminal portion of this peptide (aa 626 to 638) were capable of conferring protection to the target cells against the cytotoxic effect of ETA, as well as inhibiting the ADP-ribosyltransferase activity of ETA in a cell-free system in vitro.
MATERIALS AND METHODS
Synthetic peptides.
Specific amino acid sequences within ETA were selected for production of antibodies. Amino acid sequence selection for synthetic peptide synthesis was based on the analysis of hydrophilicity (Kyte-Doolittle), antigenic index (Jameson-Wolf), and surface probability (Emini) (Fig. 1 and Table 1). Peptides were synthesized by the Synthetic Antigen Laboratory at the University of Texas, M. D. Anderson Cancer Center, Houston. Individual peptides were 12 to 45 aa long. Peptides, supplied as lyophilized powder, were reconstituted with distilled water to a stock solution of 10 mg/ml. A dilute solution of each peptide was conjugated to keyhole limpet hemocyanin (KLH) (Pierce, Rockford, Ill.) according to the manufacturer’s recommendations. Because of the relatively large size of peptides 9 (45 aa) and 11 (29 aa), and based on our studies with mice, which showed these two peptides to be very immunogenic (data not shown), peptides 9 and 11 were not conjugated. Briefly, peptides were conjugated in a conjugation buffer [0.1 M 2-(N-morpholino)-ethanesulfonic acid, 0.9 M NaCl, 0.02% NaN3 (pH 4.7)], in the presence of the coupling reagent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Pierce).
FIG. 1.
The 11 synthetic peptides were synthesized, corresponding to different regions within the three structural domains of ETA. The position of the individual peptides in the diagram reflects the location and the overlap between some of them. The sizes of ETA and synthetic peptides were not drawn to scale. The sequences of various synthetic peptides are shown in Table 1.
TABLE 1.
Various synthetic peptides generated to binding, translocation, and enzymatic domains of ETA and rabbit group designation for the immunization protocol
| Rabbit group | Domain of ETA | Peptide designation | Amino acid residues corresponding to ETA sequencea |
|---|---|---|---|
| I | Binding (Ia) | Peptide 1 | 99–110 |
| Peptide 2 | 201–215 | ||
| Peptide 7 | 143–157 | ||
| Peptide 10 | 26–41 | ||
| II | Translocation (II) | Peptide 3 | 297–310 |
| Peptide 8 | 352–362 | ||
| Peptide 9 | 289–333 | ||
| IIIa | Enzymatic (III) | Peptide 4 | 508–520 |
| Peptide 5 | 598–618 | ||
| Peptide 6 | 626–638 | ||
| IIIb | Enzymatic (III) | Peptide 11 | 610–638 |
| IV | Holotoxin | 1–638b | |
| Vc | KLH |
Peptides were synthesized to match regions within the three different domains of ETA which have high surface probability.
Amino acid residues 1 through 638 represent the ETA amino acid sequence, including the leader peptide sequence, which corresponds to aa 1 to 25.
Group of two rabbits immunized with KLH (the carrier protein used in peptide conjugation).
Animal immunization and bleeding.
Female, albino New Zealand rabbits (Myrtle’s Rabbitry, Thompson Station, Tenn.) were immunized with 25 μg of ETA (List Biological, Campbell, Calif.). For peptide immunization, the immunizing dosage was 1 mg of each peptide per rabbit. The initial dose was delivered subcutaneously along with Freund’s complete adjuvant (Sigma Chemical Co., St. Louis, Mo.). Subsequent injections (12.5 μg of ETA and 500 μg of individual peptides) were given in conjunction with an equal volume of Freund’s incomplete adjuvant. Immunizations were given 1 month apart, and blood samples were collected 15 days after each immunization. Rabbits were sedated with an appropriate dose of a cocktail consisting of acepromazine (Fort Dodge Laboratories, Fort Dodge, Iowa) and Buprenex (Reckitt & Colman Pharmaceutical, Inc., Richmond, Va.) 20 min before the blood was drawn. Blood samples were stored overnight at 4°C, and the following day, serum was separated by centrifugation. Serum samples were kept at −70°C for prolonged storage.
Affinity purification.
Individual peptides were coupled to Sepharose-6B (Pharmacia, Piscataway, N.J.) in 0.1 M carbonate–bicarbonate buffer (pH 11) overnight. Later, the peptide-Sepharose slurry was packed in a 15-cm glass column (Fisher Scientific Co., Houston, Tex.) after appropriate washing with 0.01 M sodium phosphate buffer (pH 7.0). Columns were stored in the same buffer containing 0.02% sodium azide at 4°C. For antibody purification, serum was passed through the column under a slow, regulated flow (0.5 ml/min). The column was then washed with phosphate buffer, and peptide-specific antibodies were eluted with 0.5 M glycine-HCl buffer (pH 2.5). The eluant was immediately neutralized with 10% (vol/vol) Tris base buffer (pH 8.0), desalted, and concentrated with Centriprep 30 columns (Amicon, Inc., Beverly, Mass.).
ELISA.
Immulon 2 96-well plates (Dynatech, Inc., Chantilly, Va.) were coated with the desired antigen by addition of 100 ng of the protein per well or peptide at a concentration of 1 μg/well in 0.5 M carbonate–bicarbonate buffer at pH 9.6 and incubation of the plates overnight at 4°C. For some experiments, microtiter plates were coated with 100 μl of equimolar concentrations of ETA and individual peptides (1.5 × 10−8 M). The plates were washed with phosphate-buffered saline (PBS) containing 0.05 M Tween 20 to reduce the background, and the serum specimens or affinity-purified antibodies to be tested were then diluted in the same buffer. Horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit) (Pierce), diluted in the PBS-Tween 20 buffer, were used to probe the primary antibodies. The substrate 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Sigma) was then added for 10 min, and the optical density at 405 nm (OD405) was measured with an ELISA plate reader (Molecular Device Corp., Menlo Park, Calif.). For ETA capture ELISA experiments, the plates were coated with 100 ng of eEF-2 per well as described above. ETA (or ETA preincubated with affinity-purified antibodies) was activated with 4 M urea and 1% dithiothreitol (DTT) (Sigma) prior to incubation on the plates. Polyclonal rabbit anti-ETA and HRP-conjugated goat anti-rabbit immunoglobulin G were then used to measure the level of ETA captured on the plates.
Immunoprecipitation with protein G.
ETA was preincubated for 1 h at 37°C with immune serum diluted 5× in 0.01 M phosphate buffer (pH 7.5)–150 mM NaCl prior to the addition of 25 μl of recombinant protein G (Amersham Co., Arlington Heights, Ill.). The mixture was then incubated for 1 h at 37°C, and the pellet was washed several times with phosphate buffer and boiled for 5 min in 20 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) buffer (20). Samples then were loaded onto a 12% polyacrylamide gel (20). The proteins from the gel were transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif.), which was then probed with goat anti-ETA antibodies (List Biological) and HRP-labeled rabbit anti-goat immunoglobulin G (Sigma). The blots were developed by the enhanced chemiluminescence technique (Amersham). For eEF-2 precipitation experiments, ETA was activated in 4 M urea and 1% DTT at room temperature for 15 min before addition of eEF-2. ETA–eEF-2 complex was then precipitated by sequential addition of the affinity-purified anti-ETA antibodies and recombinant protein G to the mixture. When interference with ETA binding to eEF-2 was determined, the toxin was preincubated with affinity-purified antibodies prior to activation.
Cytotoxicity assay.
Overnight confluent monolayers of 3T3 Swiss Albino fibroblasts were trypsinized, harvested, and counted (27). Fibroblasts (>90% viability) were then seeded into 96-well plates (100 μl/well) at a concentration of 2 × 105 cells/ml in Eagle’s minimum essential medium (EMEM) (Sigma) plus 10% fetal bovine serum (Intergen, Purchase, N.Y.). The next day, the cells were washed twice with PBS and incubated in leucine-deficient EMEM (2% serum) in the presence of [3H]leucine (Amersham) and ETA or ETA preincubated with preimmune serum, immune serum, or affinity-purified antibodies to ETA. Incorporation of [3H]leucine in 3T3 cells was stopped by placing the plate at 4°C for 10 min. The cells were lysed in potassium hydroxide, and then protein, precipitated by trichloroacetic acid (TCA), was collected on filter paper with a cell harvester (Flow Laboratories, Rockville, Md.). The level of [3H]leucine incorporation then was measured with a beta counter (Beckman, Fullerton, Calif.).
Binding of ETA to target cells.
ETA was labeled with 125I (Amersham) by employing the Iodo-Beads reagent (Pierce). Monolayers of 3T3 fibroblasts were incubated with 125I-ETA at a specific activity of 2.4 × 104 cpm/ng of ETA in the presence or absence of antibodies. Cultures were incubated for 6 h at 37°C, and the radioactivity of the cells was measured with a gamma counter after appropriate washing with PBS (3×).
Purification of eEF-2.
eEF-2 was extracted from wheat germ according to the procedure originally described by Chung and Collier (6) and modified by Beattie et al. (2).
ADP-ribosylation assay.
For the ADP-ribosylation inhibition assay, 20 ng of ETA was preincubated with 20 μl of affinity-purified antibodies (2 mg/ml) to ETA or synthetic peptides for 30 min at 37°C. ETA then was activated by incubation of the mixture with 6.7 μl of 16 M urea and 4% DTT at 25°C for 15 min. The ADP-ribosylation reaction mixture was allowed to incubate for 15 min at 25°C in the presence of 60 μl of buffer containing 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, 20 mM DTT, 50 μg of bovine serum albumin per ml, along with 0.125 μCi of 14C-NAD (Amersham) and 5 μg of eEF-2. The reaction was stopped by applying the mixture to a Whatman 3MM paper already ruled into 1.5-in. squares and presoaked in 10% TCA in ether. The filter paper then was washed four times (10 min each), twice in 5% TCA and twice in absolute methanol. The filter paper was air dried, and individual squares were cut and counted with 10 ml of Scintiverse II (Fisher) in a Beckman LS5000 scintillation counter (Beckman). Values from reactions performed in the absence of eEF-2 were considered as background. Spontaneous ADP-ribosylation of eEF-2 in the absence of activated ETA was deducted from all experimental values.
RESULTS
Generation of antipeptide antibodies.
ETA consists of three structural domains that have different functions during the cytotoxicity process (1). The various peptides we synthesized were divided into three groups based on the domains (I to III) of ETA (Fig. 1 and Table 1). Peptide 11 (encompassing aa 610 to 638 within the enzymatic domain of ETA) was assigned to a separate group (IIIb) for rabbit immunization (Table 1). This was done on the basis of a report by Roscoe et al. (36) that the region consisting of aa 600 to 638 of ETA contains an immunodominant epitope(s) and also because of our previous studies, which indicated that peptide 11 is capable of perpetuating and maintaining a high level of ETA-neutralizing antibodies in mice primed with ETA (data not shown).
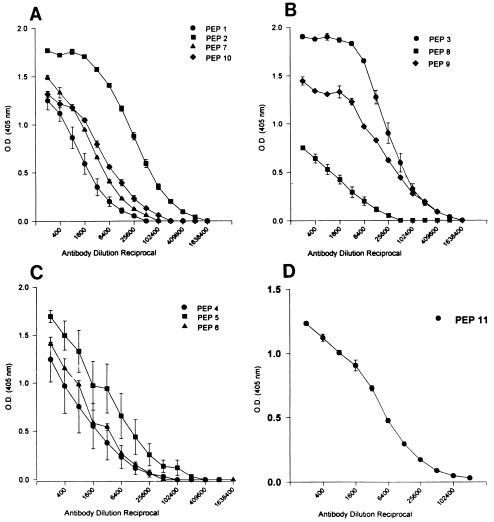
The peptide combinations (represented by peptides in one particular domain of ETA) were mixed, and immunogens were injected into separate groups of rabbits with two rabbits per group (Table 1). Sera collected during the immunization protocol, after the antibody response reached a plateau, were assayed for peptide-specific antibodies in ELISA. Figure 2 shows the level of antibodies generated to individual peptides within the rabbit group immunized with a particular peptide combination. All peptides induced a reasonable peptide-specific antibody response; however, the highest antibody titers were to peptides 2, 3, 5, 9, and 11 (Fig. 2). Peptides 3 and 9 (notice overlap between the two peptides in Fig. 1) were of special interest, because they encompass the region within ETA where arginine 304 is located. ETA is believed to be cleaved at this residue prior to translocation into the cytoplasm across the membrane of the endocytic vesicle.
FIG. 2.
Antibody responses of rabbits immunized with different combinations of peptides (PEP). Serial dilution of serum from individual rabbits was tested by ELISA. The microtiter plates were coated with 1 μg of the corresponding peptide per well. Serum from group I rabbits, immunized with a combination of peptides 1, 2, 7, and 10 (binding domain), was tested against the corresponding peptides (A). Group II rabbits were immunized with a combination of peptides 3, 8, and 9 (translocation domain). The sera from these rabbits were titrated against peptides 3, 8, and 9 (B). Group IIIa rabbits were immunized with a combination of peptides 4, 5, and 6 (enzymatic domain). Their sera were titrated against peptides 4, 5, and 6 (C). Group IIIb rabbits were immunized with peptide 11 (enzymatic domain), and their sera were tested against peptide 11 (D). For each rabbit, serum reactivity to individual peptides was tested in triplicate, and the arithmetic mean ± standard error was plotted for each group of rabbits.
Anti-ETA reactivity to synthetic peptides.
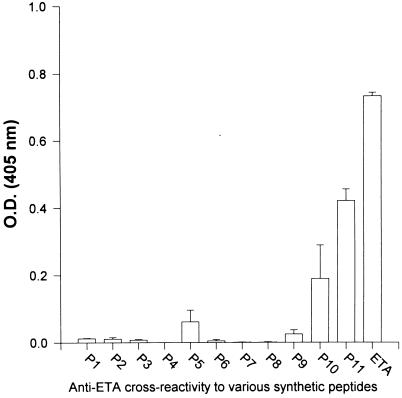
Serum samples (1:400 dilution) from rabbits immunized with native ETA (group IV) were tested for reactivity to various synthetic peptides in an ELISA. Antibodies specific for the regions represented by peptides 9 and 11 corresponded to a major portion of the polyclonal antibodies generated against ETA (data not shown). Although the antibodies to ETA reacted at a significant level with peptide 3, the latter represented a region that overlapped with peptide 9 (Fig. 1). Because of the limited ability of synthetic peptides to adhere to assay plates, compared to that of larger protein molecules such as ETA, excess peptides were used to coat the microtiter plates in initial experiments. However, when the experiment described above was repeated with ELISA plates coated with equimolar amounts of peptides and ETA (1.5 × 10−8 M), the immunodominance of the region represented by peptide 11 was still apparent (Fig. 3). These findings did not, however, indicate the role of this peptide in inducing neutralizing antibodies. Furthermore, other neutralizing epitopes may plausibly be located within a region or regions of ETA that were not considered antigenic by available prediction programs and hence were not selected for peptide synthesis.
FIG. 3.
Level of peptide (P1, P2, etc.)-cross-reacting antibodies in serum from group IV rabbits (immunized with ETA), as determined by ELISA. Plates were coated with equimolar amounts of individual peptides and ETA (1.5 × 10−8 M). Serum was tested at a dilution of 1:400. All tests were performed in triplicate, and results were plotted as the mean from two rabbits ± standard error.
Cross-reactivity of peptide-specific antibodies to ETA in ELISA.
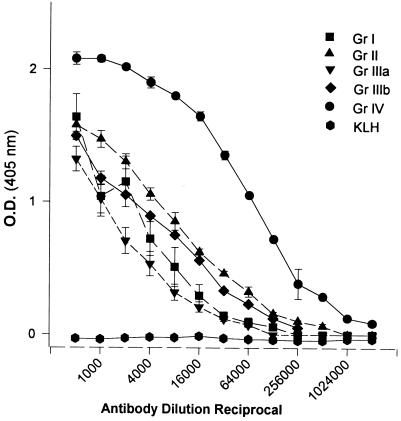
As shown in Fig. 4, sera from all four groups of peptide-immunized rabbits contained a significant level of antibodies that could cross-react with ETA in ELISA. However, it was not determined whether every single peptide injected into rabbits induced ETA-cross-reacting antibodies. ETA-cross-reacting antibodies in serum samples from group I (binding domain) rabbits could be induced by all or any of the four peptides (1, 2, 7, and 10) injected into the rabbits (Fig. 2A and 4). By comparing Fig. 2B and 4, it is apparent that the level of ETA-cross-reacting antibodies in the serum samples from group II (translocation domain) rabbits (immunized with peptides 3, 8, and 9) is higher than the level of peptide 8-specific antibodies. We also noted that peptide 8 failed to maintain and propagate an ETA-specific immune response in mice primed with a single dose of ETA (data not shown). These data suggested low cross-reactivity of antipeptide 8 antibodies in group II rabbits to native ETA (Fig. 4). The high level of ETA cross-reacting antibodies in serum samples from group IIIa and IIIb (enzymatic domain) rabbits (Fig. 4) emphasized the importance of the region represented by peptide 11 (aa 610 to 638) as an immunodominant region within the enzymatic domain of ETA. As indicated in Fig. 1, peptide 11 contained all of the amino acid residues of peptides 5 and 6 used to immunize group IIIa rabbits. Animals immunized with a carrier protein (KLH) alone did not elicit ETA-cross-reacting antibodies (Fig. 4).
FIG. 4.
Titers of sera from different groups (Gr) of rabbits tested against ETA in ELISA plates coated with 100 ng of ETA per well. Group I rabbits were immunized with peptides 1, 2, 7, and 10 (binding domain); group II rabbits were immunized with peptides 3, 8, and 9 (translocation domain); group IIIa rabbits were immunized with peptides 4, 5, and 6 (enzymatic domain); group IIIb rabbits were immunized with peptide 11 only (enzymatic domain); and group IV rabbits were immunized with native ETA. KLH represents a group of two rabbits immunized with KLH (1 mg per rabbit), the carrier protein used in peptide conjugation. Serum samples from each rabbit were tested in triplicate, and the data are plotted as the mean ± standard error for each group of rabbits.
Immunoprecipitation of ETA with antipeptide antibodies.
Serum samples from all of the different groups of rabbits contained antibodies that coprecipitated with ETA in a recombinant protein G immunoprecipitation experiment (Fig. 5). These studies indicated that antipeptide antibodies cross-reacted with the native holotoxin in solution and negated the possibility that cross-reactivity of ETA with peptide-specific antibodies in ELISA was an artifact due to partial unfolding of the toxin caused by the charged nature of the assay plates and the detergent used to reduce background in ELISA. This experiment further indicated the antigenicity of domain II (specifically the region encompassed by peptides 3 and 9) and that of the region within domain III (enzymatic domain) represented by peptide 11 (Fig. 5, lanes 3 and 5). Interestingly, antibodies specific to peptide 11, which represented sequences of both peptides 5 and 6 combined (Fig. 5, lane 5), demonstrated a high level of cross-reactivity to ETA compared to antibodies specific to peptides 4, 5, and 6 (Fig. 5, lane 4). This observation is intriguing, because peptides 5 and 6 represented parts of peptide 11 which generated a significant level of ETA cross-reacting antibodies (Fig. 5, lane 5). One possible explanation is that the dominant epitope in this region may be conformational rather than linear, and all 29 aa residues encompassed by peptide 11 may be necessary for proper folding to mimic the structure of the native ETA molecule epitope.
FIG. 5.
Immunoprecipitation of ETA with peptide-specific antibodies. ETA preincubated with serum from group IV (immunized with ETA) rabbits (lane 1), group I (binding domain) rabbits (lane 2), group II (translocation domain) rabbits (lane 3), group IIIa (enzymatic domain) rabbits (lane 4), group IIIb (enzymatic) rabbits (lane 5), preimmune rabbit serum (lane 6), and PBS (lane 7) was precipitated with recombinant protein G, separated on a 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and stained with anti-ETA antibodies (0.25 μg/ml). Lane 8 represents pure ETA (2 μg) directly loaded onto the gel.
In vitro cytotoxicity protection with crude serum containing antipeptide antibodies.
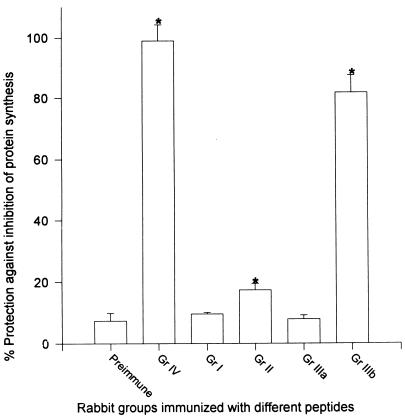
The lethal dose of ETA that caused 50% inhibition of protein synthesis in monolayer cultures of 3T3 Swiss Albino fibroblasts was determined to be 15 ng/ml. Serum samples from all five groups of rabbits were tested for their capacity to protect monolayers of 3T3 fibroblasts against two 50% lethal doses (LD50s) of ETA (30 ng/ml) (Fig. 6). Serum was tested at a dilution of 1:10, and the level of protection against inhibition of protein synthesis was measured by incorporation of [3H]leucine in the target cells. Among all groups of peptide-immunized rabbits, group II and group IIIb (immunized with peptides 3, 8, and 9 and peptide 11, respectively) showed significant protection against ETA-induced inhibition of protein synthesis compared to the protection provided with the control preimmune serum (P < 0.05) (Fig. 6). However, the level of protection afforded by serum samples from group IIIb (enzymatic domain) rabbits immunized with peptide 11 was much higher than that provided by serum samples from group II (translocation domain) rabbits used at the same dilution (Fig. 6). These data reaffirmed the immunodominance of the region represented by peptide 11 and the role of this epitope in inducing protective antibodies.
FIG. 6.
Protection against ETA-induced inhibition of protein synthesis by antisera raised to different synthetic peptides. Serum samples from different groups (Gr) of rabbits were diluted 1:10 in leucine-deficient EMEM supplemented with [3H]leucine. Two LD50s of ETA (final concentration, 30 ng/ml) were added to the diluted serum, and the mixture was incubated for 1 h at 37°C, before being added to monolayers of 3T3 fibroblasts. After 4 h of incubation at 37°C in the presence of 5% CO2, [3H]leucine incorporated into protein extracted from the cells was measured with a beta counter. Protection was expressed as the percentage of [3H]leucine incorporated in the absence of ETA. Serum samples from each rabbit were tested in triplicate, and the data were plotted as the mean ± standard error for each group. Asterisks indicate statistical significance compared to toxin mixed with preimmune serum (P < 0.05) by one-way analysis of variance.
In vitro cytotoxicity protection with affinity-purified antibodies to synthetic peptides.
Antibodies in serum samples from group IIIb rabbits (immunized with peptide 11) were purified with separate Sepharose 6B affinity purification columns conjugated with peptides 5, 6, and 11 (peptide 11 encompassed the sequences of both peptides 5 and 6) (Fig. 1). Similarly, serum from group II rabbits (translocation domain) was purified with separate affinity columns conjugated with peptides 3 and 9 (peptide 9 encompassed the region represented by peptide 3 plus additional amino acid residues flanking this region) (Fig. 1 and Table 1). Serum from group IIIb rabbits (immunized with peptide 11) contained a relatively low level of peptide 5-specific antibodies. Passing serum samples from group IIIb rabbits through a peptide 6 column absorbed the majority of peptide 11-specific and ETA-cross-reacting antibodies. However, passing the same sample through a peptide 5-specific column did not absorb any antibodies (data not shown). Also, sera from group IIIb rabbits (immunized with peptide 11) contained a much lower level of peptide 5-specific antibodies than peptide 6-specific antibodies, as determined by ELISA (data not shown). Therefore, affinity-purified antibodies specific for peptides 6, 11, 3, and 9 were tested for the capacity to protect against inhibition of protein synthesis induced by ETA. Table 2 shows the concentration of peptide-specific antibodies necessary to confer 50% protection against two LD50s of ETA. Peptide 6-specific antibodies were capable of conferring 50% protection at 29 μg/ml, a concentration less than half that of peptide 11-specific antibodies needed to provide the same level of protection (63 μg/ml). Peptide 9-specific antibodies were not as efficient at neutralizing the cytotoxic activity of ETA. Antipeptide 9 antibodies at a concentration of 176 μg/ml provided 50% protection. Finally, peptide 3-specific antibodies failed to confer any protection, even at a concentration as high as 250 μg/ml. Based on these observations, it was concluded that we have identified an immunodominant epitope capable of inducing neutralizing antibodies which could abrogate the cytotoxic activity of ETA in vitro (Table 2). Although all 29 aa residues represented by peptide 11 (aa 610 to 638) seemed necessary to elicit the production of protective antibodies, we were able to localize this epitope to the last 13 aa residues at the carboxy terminus of ETA, as represented by peptide 6 (Fig. 1 and Table 1).
TABLE 2.
Concentration of affinity-purified antibodies conferring 50% protection against 2 LD50s of ETA in an in vitro cytotoxicity assaya
| Antibody target | Antibody concn (μg/ml)b |
|---|---|
| ETA | 00.28 ± 0.02 |
| Peptide 6 | 29.00 ± 3.50 |
| Peptide 11 | 63.00 ± 5.00 |
| Peptide 9 | 176.00 ± 17.7 |
| Peptide 3 | >250.00 (no neutralization) |
Serial dilutions of affinity-purified anti-ETA or antipeptide antibodies were tested for their capacity to protect monolayers of fibroblasts from inhibition of [3H]leucine incorporation into protein in the presence of 30 ng of ETA per ml. The level of [3H]leucine incorporation into cells not treated with ETA was considered to be 100%.
Data represent the mean ± standard deviation from two independent experiments, with each experiment performed in triplicate.
The effect of antipeptide antibodies in altering ETA binding to target cells.
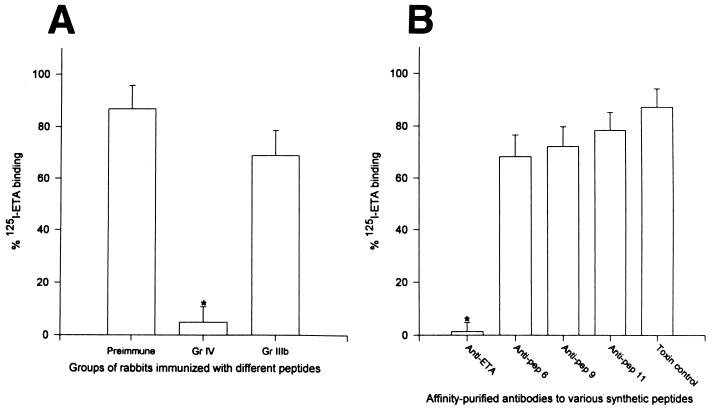
In order to understand the mechanism by which these antipeptide antibodies protected cells from the cytotoxic effect of ETA, we conducted experiments to study the effect of these antibodies on the binding to target cells and on the ADP-ribosyltransferase enzymatic activity of the toxin. ETA was radiolabeled with 125I, and our control experiment indicated that increasing concentrations of unlabeled ETA competed with the binding of 125I-ETA to 3T3 fibroblast target cells (data not shown). Maximum binding was observed in the absence of any unlabeled ETA. Preimmune serum and serum samples from group IV (immunized with ETA) and group IIIb (immunized with peptide 11) rabbits were tested at a 1:10 dilution. Only serum from group IV rabbits, immunized with ETA, was able to effectively prevent ETA binding to 3T3 fibroblasts (Fig. 7A) (P < 0.05 compared to preimmune serum). Rabbit preimmune serum had no effect on the binding of ETA to the fibroblasts (Fig. 7A). We subsequently tested the effect of affinity-purified, antipeptide antibodies on the binding of ETA to target cells. We used a concentration of affinity-purified, antipeptide antibodies four times that required to provide 50% protection against inhibition of protein synthesis caused by 2 LD50s of ETA (30 ng/ml). Anti-ETA antibodies completely abrogated binding of ETA to target cells, whereas antipeptide 6, 9, and 11 antibodies did not significantly interfere with 125I-ETA binding compared to that of the toxin control (Fig. 7B).
FIG. 7.
125I-ETA binding to 3T3 fibroblasts in the presence of immune rabbit serum. 125I-ETA was preincubated with a 1:10 dilution of serum from group (Gr) IV rabbits (immunized with ETA) or group IIIb rabbits (immunized with peptide 11) and preimmune serum. The mixture was preincubated for 1 h at 37°C and added to monolayers of 3T3 fibroblasts, which were then incubated for 6 h at 37°C. The level of 125I-ETA remaining after washing was measured with a gamma scintillation counter (A). (B) 125I-ETA was preincubated with affinity-purified antibodies at a concentration 4× the amount necessary to provide 3T3 fibroblasts with 50% protection against inhibition of protein synthesis caused by the same amount of ETA (30 ng/ml). Data were expressed as the percentage of 125I-ETA binding in the absence of antibody. Results represent the mean from two rabbits ± standard error. All experiments were performed in duplicate. An asterisk indicates statistical significance compared to toxin mixed with preimmune serum (A) or PBS (B) (P < 0.05). pep, peptide.
Inhibition of ADP-ribosyltransferase activity by antipeptide antibodies.
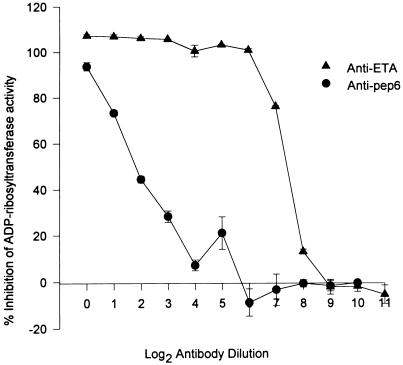
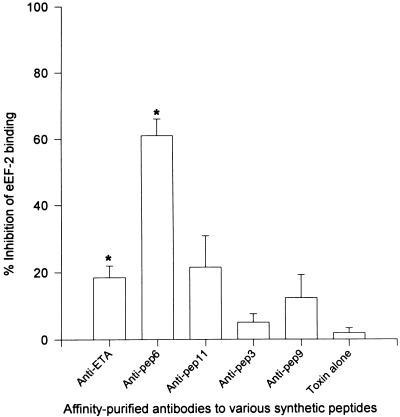
In order to confirm that inhibition of the enzymatic activity of ETA is a mechanism by which peptide 6 and peptide 11 antibodies neutralized the lethal effect of ETA, we examined the effect of these antibodies on the capacity of ETA to transfer an ADP-ribose moiety from NAD to eEF-2. Before initiating these studies, the identity of the 100-kDa purified eEF-2 protein was confirmed. We noted that active ETA (ETA treated with 4 M urea and 1% DTT) coprecipitated with the 100-kDa eEF-2 protein in the presence of anti-ETA antibodies and recombinant protein G (data not shown). Also, we observed that active ETA, but not inactive ETA, specifically bound to eEF-2 in the ELISA. When tested for the capacity to inhibit ADP-ribosyltransferase activity, serum from group I rabbits (immunized with peptides 1, 2, 7, and 10 [within the binding domain]) and group II rabbits (immunized with peptides 3, 8, and 9 [within the translocation domain]) did not interfere with the enzymatic activity of ETA (Fig. 8A). Serum from group IIIa rabbits (immunized with peptides 4, 5, and 6) and group IIIb rabbits (immunized with peptide 11) caused 28.8 and 39.2% inhibition of ETA enzymatic activity, respectively (P < 0.05 compared to preimmune serum) (Fig. 8A). Serum from group IV rabbits (immunized with ETA) completely blocked the enzymatic activity of ETA (Fig. 8A). Affinity-purified anti-ETA and anti-peptide 6 antibodies (2 mg/ml) conferred 103 and 94% inhibition of the ADP-ribosyltransferase activity of ETA, respectively (Fig. 8B). Antipeptide 11 antibodies, although not as potent as antipeptide 6 antibodies, caused 79% inhibition of the enzymatic activity of ETA (P < 0.05 compared to toxin alone) (Fig. 8B). Antipeptide 3 provided a significant, but very low level of inhibition of ADP-ribosylation (P < 0.05 compared to toxin alone) (Fig. 8B). Antipeptide 9 antibodies did not cause any significant interference with the enzymatic activity of ETA (Fig. 8B). When twofold serial dilutions of anti-ETA and antipeptide 6 antibodies were tested for inhibition of ETA enzymatic activity, anti-ETA antibodies maintained a high level of inhibition even at very low antibody concentrations (50% inhibition of enzymatic activity at approximately 12 ng of antibody per ml) (Fig. 9). On the other hand, a low concentration of antipeptide 6 antibodies was not as effective in neutralizing the enzymatic activity of ETA (Fig. 9). Almost 50-fold more of antipeptide 6 antibodies compared to the amount of anti-ETA antibodies was needed to provide a similar level of neutralization (50% inhibition at 600 ng of antibody per ml). Antipeptide 6 antibodies at 2 mg/ml significantly interfered with the binding of ETA to immobilized eEF-2 on ELISA plates (P < 0.05 compared to toxin alone) (Fig. 10). Anti-ETA antibodies at the same concentration (2 mg/ml) were not as efficient as antipeptide 6 antibodies in interfering with the interaction between ETA and eEF-2 compared to that of the PBS control (Fig. 10).
FIG. 8.
Inhibition of ADP-ribosyltransferase activity with immune rabbit serum. ETA (20 ng per reaction mixture) was preincubated for 1 h at 37°C with a 1:5 dilution of preimmune serum or serum from rabbits from group (Gr) I (immunized with peptides [pep] 1, 2, 7, and 10), group II (immunized with peptides 3, 8, and 9), group IIIa (immunized with peptides 4, 5, and 6), group IIIb (immunized with peptide 11), or group IV (immunized with ETA). ETA was then activated, and the reaction mixture was assayed for ADP-ribosylation in the presence of 14C-labeled NAD and eEF-2. The reaction was stopped after 15 min at 25°C with 10% TCA. Samples were then counted with a beta counter (A). (B) ETA (20 ng/ml) was preincubated with 40 μg of affinity-purified peptide-specific antibodies. Inhibition of activity was expressed as the percentage of activity measured in the absence of antibody. Results represent the mean for each group of rabbits ± standard error. Spontaneous ADP-ribosylation of eEF-2 in the absence of ETA was deducted from values obtained in all experiments. Experiments were repeated twice, and each was performed in duplicate. Asterisks indicate statistical significance compared to toxin mixed with preimmune serum (A) or PBS (B) (P < 0.05).
FIG. 9.
Titration of ADP-ribosyltransferase-inhibiting antibodies. Anti-ETA and antipeptide 6 (Anti-pep6) antibodies (2 mg/ml) were serially diluted and incubated with ETA (20 ng per reaction mixture) for 1 h at 37°C. ETA was then activated, and the reaction mixture was incubated for 15 min at 25°C. The reaction was stopped with 10% TCA, and the samples were counted with a beta scintillation counter. Inhibition of activity was expressed as the percentage of activity measured in the absence of antibody. Spontaneous ADP-ribosylation of eEF-2 in the absence of ETA was deducted from the actual values obtained. Results represent the mean ± standard error of two independent experiments, with each experiment performed in duplicate.
FIG. 10.
Inhibition of ETA binding to immobilized eEF-2. ETA (50 ng/ml) was incubated with affinity-purified antibodies (40 μg/well) for 1 h at 37°C. ETA was then activated, and the mixture was added to microtiter plates coated with eEF-2. ETA captured on the plates was then probed with rabbit anti-ETA and HRP-labeled goat anti-rabbit antibodies. The reaction was developed with ABTS, and the OD was determined with an ELISA plate reader. Inhibition of binding was expressed as the percentage of binding measured in the absence of antibody. Bars represent the mean ± standard error from three experiments, with each experiment performed in triplicate. Asterisks represent statistical significance compared to toxin alone (P < 0.05). pep, peptide.
DISCUSSION
In 1994, Roscoe et al. (36) used serum samples derived from primates that had been immunized with ETA-derived immunotoxins for immunological analysis. The serum samples examined were obtained from two different groups of primates. One group was immunized with LMB-1, an immunotoxin which consisted of NLysPE38 (a 38-kDa derivative of ETA lacking domain Ia and part of domain Ib) chemically coupled to B3 (a monoclonal antibody specific for B antigen expressed on the surface of carcinoma cells). The other group was immunized with LMB-7, a single-chain recombinant immunotoxin consisting of the Fv domain of B3 fused with NLysPE38 (36). Serum samples from the two different groups of rabbits were tested by ELISA for cross-reactivity with ETA or synthetic peptides encompassing overlapping regions within the amino acid sequence of ETA. Based on this study, the authors concluded that the region between aa 616 and 637 of ETA constituted an immunodominant-neutralizing epitope. Our studies with immune serum derived from rabbits immunized with ETA and synthetic peptides confirmed their findings and narrowed the stretch of neutralizing epitope to only 13 aa at the carboxy terminus of the molecule. In a previous study by Olson et al. (31), investigators failed to induce neutralizing antibodies by using a synthetic peptide almost identical in sequence to peptide 11 used in our study. The current study shows that the region encompassed by peptide 11 is highly immunogenic and that peptide 11 by itself is enough to induce a high level of antibodies capable of neutralizing ETA. Although Olson et al. (31) conjugated their peptide prior to immunization, whereas we did not, this does not seem to be the reason for the difference between our results and the results they reported. In another related study, we immunized two different groups of BALB/c mice with conjugated and unconjugated peptide 11. Mice immunized with conjugated peptide 11 developed a higher level of circulating antipeptide 11 and ETA-cross-reacting antibodies in serum than the mice immunized with the unconjugated peptide 11. Also, serum from the mice receiving conjugated peptide 11 conferred a higher level of neutralization of the enzymatic activity of ETA than did serum from mice immunized with unconjugated peptide (data not shown). The difference in the method of conjugation (we used the EDC method, whereas, they used the glutaraldehyde method) or the animal model used for immunization (they used rats) might be responsible for the difference in the results obtained. Glutaraldehyde is a homobifunctional reagent that works by coupling two proteins or peptides via amino groups. This method often fails to induce peptide-specific antibodies because of ineffective conjugation, homopolymerization, or even precipitation during coupling. On the other hand, EDC is a very efficient heterobifunctional reagent that couples an amino group with a carboxyl group to form a peptide bond (45). Thus far, no other studies have succeeded in inducing ETA-neutralizing antibodies by using synthetic peptides. Peptides 5 and 6, encompassing the two ends of peptide 11 (the amino terminus and carboxy terminus, respectively) failed to induce neutralizing antibodies. Peptide 11-specific antibodies contained mostly antibodies that cross-reacted with peptide 6, and affinity-purified peptide 6-specific antibodies were more efficient in conferring protection against ETA (Table 2). Therefore, we believe that the amino acid sequence within peptide 11 constitutes an important neutralizing epitope, located within the 13 aa residues at the carboxy terminus of ETA (Fig. 6 and 8B). Additional amino acid residues (aa 596 to 625) could be essential to present this epitope in its native form to the immune system. Our data obtained with 125I-labeled ETA indicated that antibodies to this epitope did not interfere with the binding of ETA to the target cells. Antipeptide 11 and antipeptide 6 antibodies were used at concentrations 4× that necessary to provide 3T3 fibroblasts with 50% protection against 2 LD50s of ETA, and still a substantial amount of 125I-ETA bound to the cells (Fig. 7B). The capacity of antipeptide 6 and antipeptide 11 antibodies to block the ADP-ribosyltransferase activity of ETA in a cell-free system in vitro was a direct indication of the mechanism by which antibodies to this epitope conferred protection against ETA and hence the role of the carboxy terminus of the toxin in the toxic activity of ETA (Fig. 8B). The fact that antipeptide 6 antibodies interfered with the binding of active ETA to eEF-2 immobilized on ELISA plates is additional evidence regarding the location of this amino acid sequence within the active site of the molecule (Fig. 10). Anti-ETA antibodies, although very potent in neutralizing the enzymatic activity of ETA, did not seem to markedly interfere with the binding of eEF-2 to the toxin (Fig. 10). Conversely, antipeptide 6 antibodies did not inhibit ADP-ribosyltransferase activity of ETA as efficiently as did anti-ETA antibodies (Fig. 9). One possible explanation is that the region represented by peptide 6 may not be the only neutralizing epitope within the enzymatic domain of ETA and that other important epitopes, probably conformational, are yet to be identified. This hypothesis may explain why sera from group IV rabbits (immunized with ETA) nearly completely inhibited ETA’s enzymatic activity, whereas sera from group IIIb rabbits (immunized with peptide 11) conferred only a 39.2% inhibition.
Our study indicated the presence of a highly immunodominant region within the translocation domain (aa 289 to 333) based on ELISA and immunoprecipitation experiments. This region, although potently immunogenic, did not constitute an important neutralizing epitope. The translocation domain of ETA is thought to undergo partial unfolding and cleavage prior to translocation of the enzymatic domain to the cytoplasm of the target cell (8). One possible explanation for the low efficiency of peptide 9-specific antibodies in conferring protection against ETA could be that antibodies to this region fall off their target epitope(s) during the activation process that ETA undergoes in the endolysosomal vesicle. Ogata et al. (30) reported that two monoclonal antibodies generated against a 40-kDa truncated form of ETA (PE40) were capable of binding to the native soluble ETA with strong affinity. The binding site for one of these antibodies (M40-1) was mapped to aa 289 to 333 within the translocation domain, corresponding to the exact region encompassed by peptide 9 in our study. They reported the high affinity for ETA of this antibody and its capability for neutralizing the cytotoxic effect of ETA in vitro without affecting binding or the enzymatic activity of ETA. They concluded this region could be important for other functions of ETA. Our findings are concurrent with regard to the presence of a highly immunogenic epitope(s) within this region of ETA. A synthetic peptide (peptide 9) encompassing this amino acid sequence was capable of eliciting a high level of ETA cross-reacting antibodies, which were not, however, very potent in neutralizing ETA cytotoxic activity (Table 2). When compared to peptide 11-specific antibodies, a relatively higher concentration of peptide 9-specific antibodies was needed to protect against inhibition of protein synthesis caused by ETA (Table 2). Antipeptide 9 antibodies had no effect on the enzymatic activity of ETA (Fib. 8B).
Although previous studies indicated monoclonal antibodies generated to a toxoid form of ETA were neutralizing and mapped within the binding domain of ETA (5), we were unable to develop neutralizing antibodies specific for this region by using the synthetic peptide strategy. Neutralizing epitopes located in this region of the toxin may plausibly be three-dimensional conformational epitopes. Once a better model for the three-dimensional structure of ETA becomes available, this model will be very helpful in selecting potential antigenic, surface-exposed regions within the sequence of ETA. Synthetic peptides encompassing these regions may prove more effective in inducing neutralizing antibodies. Active immunization with toxoids to generate antitoxic immunity has been employed with significant success in treating tetanus and diphtheria. Passive administration of antitoxin has clinical application in both diseases, as well as in treating botulism, and providing some protection against snake venom toxicity. Generally, antitoxin therapy has not been applied routinely to combat other infections in which bacterial exotoxins have been demonstrated to participate, probably because of the risk of antigenic cross-reactivity and serum sickness. We have previously shown that ETA from P. aeruginosa impairs the wound healing process (16). With the emergence of multiple antibiotic resistance in many bacteria, a supplemental therapy is needed to combat burn wound sepsis (15). On one hand, the apparent low affinity and low neutralizing capability of antipeptide antibodies compared to anti-ETA polyclonal antibodies could have negative implications in an immunotherapeutic approach using these antibodies. On the other hand, the use of polyclonal antibodies generated against peptides which represent neutralizing epitopes of ETA has the advantage of minimum risk of cross-reactivity. Furthermore, the combination of antipeptide antibodies, or antipeptide antibodies along with antibodies specific to P. aeruginosa LPS, could be more effective than anti-ETA antibodies alone. In addition, identification of neutralizing epitopes by the peptide strategy may create an opportunity to develop a vaccine with defined specificity. Concern about serum sickness can be addressed by the development of either human monoclonal antibodies or chimeric antibodies consisting of murine monoclonal variable-chain regions fused to human constant-chain regions. The latter strategy is being used with encouraging success in the administration of antibodies specific for TNF-α to patients with rheumatoid arthritis (10), anti-alpha interferon for patients with HIV-induced immunosuppression (12), and anti-TNF-α for treatment of chronic bowel inflammation in patients with Crohn’s disease (41).
ACKNOWLEDGMENTS
This study was supported by grant 8520 from the Shriners Hospitals for Children.
We thank James C. Thompson for wise and most appreciated counsel in the preparation of the manuscript and Mardelle Susman for editorial review.
REFERENCES
- 1.Allured V S, Collier R J, McKay D B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0 angstrom resolution. Proc Natl Acad Sci USA. 1986;83:1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie B K, Prentice G A, Merrill A R. Investigation into the catalytic role for the tryptophan residues within domain III of Pseudomonas aeruginosa exotoxin A. Biochemistry. 1996;35:15134–15142. doi: 10.1021/bi961985t. [DOI] [PubMed] [Google Scholar]
- 3.Bryan C S, Reynolds X L, Berner E R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effect of antimicrobial therapy. Rev Infect Dis. 1983;5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary V K, Xu Y H, FitzGerald D, Adhya S, Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium of Escherichia coli. Proc Natl Acad Sci USA. 1988;85:2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia J K S, Pollack M, Avigan D, Steinbach S. Functionally distinct monoclonal antibodies reactive with enzymatically active and binding domains of Pseudomonas aeruginosa toxin A. Infect Immun. 1986;52:756–762. doi: 10.1128/iai.52.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung D W, Collier R J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977;16:832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick, J. D., V. Shull, J. E. Karp, and J. Valentine. 1988. Bacterial and host factors affecting Pseudomonas aeruginosa colonization versus bacteremia in granulocytopenic patients. Eur. J. Cancer Clin. Oncol. 25(Suppl. 1):S47–54. [PubMed]
- 8.Farahbakhsh Z T, Wisnieski B J. The acidic triggered entry pathway of Pseudomonas exotoxin A. Biochemistry. 1987;28:580–585. doi: 10.1021/bi00428a025. [DOI] [PubMed] [Google Scholar]
- 9.Feigin R D, Shearer W T. Opportunistic infection in children. In the compromised host. J Pediatr. 1975;87:677–694. doi: 10.1016/s0022-3476(75)80289-7. [DOI] [PubMed] [Google Scholar]
- 10.Feldman M. What is the mechanism of action of anti-tumor necrosis factor alpha antibody in rheumatoid arthritis? Int Arch Allergy Immunol. 1996;111:362–365. doi: 10.1159/000237393. [DOI] [PubMed] [Google Scholar]
- 11.Furuya N, Hirakata Y, Tomono K, Matsumoto T, Takeda K, Kaker M, Yamaguchi K. Comparison of mortality rates in mice with endogenous septicemia due to Pseudomonas aeruginosa isolates from different clinical sources. J Med Microbiol. 1993;39:141–146. doi: 10.1099/00222615-39-2-141. [DOI] [PubMed] [Google Scholar]
- 12.Gingeri A, Santagostino E, Cusini M, Muca-Perja M, Marinoni A, Mannucci P H, Burny A, Griscuolo M, Lu W, Anderieru J M, Mebika J P, Luchgar A, Fall L S, Chams V, Feldman M, Hermens P, Zagury J F, Bizzini B, Musicco M, Zagury D. Absence of clinical, virological, immunological signs of progression in HIV-1-infected patients receiving active anti-interferon-alpha immunization: a 30-month follow-up report. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:55–67. doi: 10.1097/00042560-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gray G L, Smith D H, Baldridge J S, Harkins R N, Vasil M L, Chan E Y, Heyneker H L. Cloning, nucleotide sequence and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1984;81:2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedberg M, Miller J K, Tompkins V N. Elastase activity of Pseudomonas aeruginosa isolates from hospital patients. Am J Clin Pathol. 1969;52:631–633. [PubMed] [Google Scholar]
- 15.Heggers J P, Robson M C, editors. Quantitative bacteriology: its role in the armamentarium of the surgeon. 1st ed. Boca Raton, Fla: CRC Press, Inc.; 1991. [Google Scholar]
- 16.Heggers J P, Haydon S, Ko F, Hayward P G, Carp S, Robson M. Pseudomonas aeruginosa exotoxin A: its role in retardation of wound healing: the 1992 Lindberg award. J Wound Care Rehabil. 1992;13:512–518. [PubMed] [Google Scholar]
- 17.Heggers J P, et al. Treatment of infections in burns. In: Herndon D N, editor. Total burn care. W. B. London, England: Saunders Co., Ltd.; 1996. pp. 98–135. [Google Scholar]
- 18.Hwang J, Fitzgerald D J P, Adhya S, Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987;48:129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- 19.Iglewski B H, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci USA. 1975;72:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;226:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Liu P V. The role of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966;116:112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- 22.Liu P V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973;128:506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- 23.Liu P V, Yoshii S, Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1974;128:514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- 24.Liu, P. V. 1974. Extracellular toxins of Pseudomonas aeruginosa. J. Infect. Dis. 130(Suppl.):S94–99. [DOI] [PubMed]
- 25.Middlebrook J L, Dorland R B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977;23:183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- 26.Misfeldt M L, Legaard P X, Howell S E, Fornella M H, LeGrand R D. Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990;58:978–982. doi: 10.1128/iai.58.4.978-982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller P C, Evans M J, Faden R C, Henson L C, Rogers B, Heggers J P. The effect of anti-exotoxin A on the adherence of Pseudomonas aeruginosa to hamster tracheal epithelial cells in vitro. Tissue Cell. 1994;26:181–188. doi: 10.1016/0040-8166(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 28.Morihara K. Production of elastase and proteinase by Pseudomonas aeruginosa. J Bacteriol. 1964;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan P, Holder I A, MacMillan B G. Burn wounds: microbiology, local host defenses and current therapy. Crit Rev Clin Lab Sci. 1973;4:61–100. doi: 10.3109/10408367309151684. [DOI] [PubMed] [Google Scholar]
- 30.Ogata M, Pastan I, Fitzgerald D. Analysis of Pseudomonas exotoxin activation and conformational changes by using monoclonal antibodies as probes. Infect Immun. 1991;59:407–414. doi: 10.1128/iai.59.1.407-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson J C, Hammod A N, Vincent T S, Beachey E H, Iglewsky B H. Identification of functional epitopes of Pseudomonas aeruginosa exotoxin A using synthetic peptides and subclone products. Mol Immunol. 1990;27:981–993. doi: 10.1016/0161-5890(90)90121-f. [DOI] [PubMed] [Google Scholar]
- 32.Polk H C. Consensus summary on infection. J Trauma. 1979;19:894. [PubMed] [Google Scholar]
- 33.Pollack M, Anderson S E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978;19:1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack M, Taylor N S, Callahan L T., III Exotoxin production by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1975;15:776–780. doi: 10.1128/iai.15.3.776-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack M. The role of exotoxin A in disease and immunity. Rev Infect Dis. 1983;5:s979–s984. doi: 10.1093/clinids/5.supplement_5.s979. [DOI] [PubMed] [Google Scholar]
- 36.Roscoe D M, Jung S-H, Benhar I, Pai L, Lee B K, Pastan I. Primate antibody response to immunotoxin: serological and computer-aided analysis of epitopes on a truncated form of Pseudomonas exotoxin. Infect Immun. 1994;62:5055–5065. doi: 10.1128/iai.62.11.5055-5065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabath L D, editor. P. aeruginosa, the organism, the diseases it causes, and their treatment. Bern, Switzerland: Hans Huber; 1980. pp. 264–280. [Google Scholar]
- 38.Staugas R E M, Harvey D P, Ferrante A, Nandoskar M, Allison A C. Induction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) by Pseudomonas aeruginosa and exotoxin A-induced suppression of lymphoproliferation and TNF, lymphotoxin, gamma interferon, and Il-1 production in human leukocytes. Infect Immun. 1992;60:3162–3168. doi: 10.1128/iai.60.8.3162-3168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart R K, Pollack M. Pseudomonas aeruginosa exotoxin A inhibits proliferation of human bone marrow progenitor cells in vitro. Infect Immun. 1982;38:206–211. doi: 10.1128/iai.38.1.206-211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teplitz C. The pathology of burns and the fundamentals of burn wound sepsis. In: Artz C P, et al., editors. Burns: a team approach. W. B. Philadelphia, Pa: Saunders; 1979. pp. 45–94. [Google Scholar]
- 41.van Dullemen H M, van Deventer S J, Hommes D W, Bijl H A, Jansen J, Tytgat G N, Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 42.Vasil M L, Gray G L, Grant C C. Abstracts of the 84th Annual Meeting of the American Society for Microbiology 1984. Washington, D.C: American Society for Microbiology; 1984. Molecular analysis of the exotoxin A gene of Pseudomonas species, abstr. D70; p. 62. [Google Scholar]
- 43.Vymola F, Lochmann O. Characteristics of Pseudomonas haemolysin. J Hyg Epidemiol Microbiol Immunol. 1974;18:302–309. [PubMed] [Google Scholar]
- 44.Young L S, Stevens P, Kaijser B. Gram-negative pathogens in septicemia infections. Scand J Infect Dis. 1982;31:78–94. [PubMed] [Google Scholar]
- 45.Zeggers N, Gerritse K, Deen C, Boersma W, Claasen E. An improved conjugation method for controlled covalent coupling of synthetic peptides to proteins using glutaraldehyde in dialysis method. J Immunol Methods. 1990;130:195–200. doi: 10.1016/0022-1759(90)90048-z. [DOI] [PubMed] [Google Scholar]