Abstract
Background
The Veterans Affairs (VHA) is working to establish a population-based colorectal cancer screening program for average-risk patients using mailed fecal immunochemical testing (FIT). However, low response rates to mailed FIT may hinder success. Key features of mailed FIT programs, including the use of reminders, differ among various national programs, with limited evidence among veterans.
Objective
We sought to test whether using reminders, either via telephone call or text message, was effective in improving mailed FIT response rates.
Design
We conducted a prospective, randomized quality improvement trial (ClinicalTrials.gov NCT05012007). Veterans who had not returned a FIT within 2 weeks of receiving the kit were randomized to one of three groups: (1) control (no reminder); (2) an automated telephone call reminder; or (3) an automated text message reminder.
Participants
A total of 2658 veterans enrolled at VA Puget Sound Health Care System who were aged 45–75 and had an average risk of colorectal cancer.
Interventions
A single automated telephone call or text message reminder prompting veterans to return the FIT kit.
Main Measures
Our primary outcome was FIT return at 90 days and our secondary outcome was FIT return at 180 days.
Key Results
Participant average age was 62 years, 88% were men, and 66% White. At 90 days, both the phone and text reminder interventions had higher FIT return rates compared to control (intention-to-treat results (ITT): control 28%, phone 39%, text 38%; p<0.001). At 180 days, FIT kit return remained higher in the reminder interventions (ITT: control 32%, phone 42%, text 40%; p<0.001).
Conclusions
Automated reminders increased colorectal cancer screening completion among average-risk veterans. An automated phone call or text message was equally effective. VHA facilities seeking to implement a mailed FIT program should consider using phone or text reminders, depending on available resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08409-8.
KEY WORDS: population health, preventative health, cancer screening
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the USA.1 CRC also has strong potential for prevention and reduction in mortality with universal screening, which is currently recommended for all average-risk Americans beginning at age 45.2 However, in 2018, only 67% of US adults reported being up-to-date with guideline-recommended CRC screening.3 Higher rates (over 80%) have historically been described in the Veterans Health Administration (VHA)4—an integrated health system of over 900 clinics with 6.2 million active patients.5 The COVID-19 pandemic profoundly disrupted access to health care, leading to an estimated CRC screening deficit of 3.8 million persons nationally in 2020.6 Due to the public health emergency, the VA mandated cessation of non-urgent medical procedures, including screening colonoscopy, in March 2020.7
Given the importance of CRC screening and the unavailability of screening colonoscopy, the VHA recommended primary fecal immunochemical testing (FIT) for screening in veterans considered average risk for CRC.8, 9 FIT is a recommended screening modality for CRC and offers an evidence-based, cost-effective, and convenient option.10 To complete FIT, patients are provided an at-home testing kit to return to the facility laboratory. To address the pandemic-induced CRC screening gap, VHA sought to implement mailed FIT programs rather than the previous standard of providing FIT kits to patients during in-person visits.11, 12 Outside VHA, mailed FIT programs have been shown to be a successful method of population-based CRC screening, with increases in screening rates of 28% higher than usual care.13–15
However, mailed FIT completion rates (i.e., returning tests to the lab) can be low, ranging from 26 to 59%.15 Features of mailed FIT programs such as patient reminders, sent after mailed FIT, are one strategy to increase FIT completion.14, 16–18 Reminders of all modalities, including text messages, letters, or calls after a FIT mailing, have been shown to increase the rate of return by 3–21% in non-veteran populations.13 However, the optimal method of the reminder is not clear, with limited evidence on the efficacy of reminders to return FIT tests among veterans.19 Veterans may respond differently to such outreach than the general population due to different economic, racial, and health demographics.4, 20
As an integrated health system, the VHA provides an opportunity to evaluate the efficacy of post-FIT reminders with less influence from the potential barriers (e.g., insurance coverage, access, cost) to screening completion that may arise in studies within fee-for-service systems. Understanding the impact of key features of a mailed FIT program such as a reminder program within the VHA would fill a knowledge gap about which elements of a mailed FIT are essential to improving return rates among veterans. Therefore, to assess if simple, automated reminder strategies could enhance CRC screening in a mailed FIT program among average-risk veterans, we conducted a randomized controlled trial comparing the effect of reminders and reminder modalities (automated phone or text message) on FIT return rate within a regional VHA medical center.
METHODS
This was a pragmatic, prospective, quality improvement randomized controlled trial evaluating the effectiveness of text and phone reminders to no reminders on FIT return rates for CRC screening. This study was conducted at VA Puget Sound Health Care System, an integrated regional network serving 112,000 veterans through two hospital-affiliated clinics, and seven community-based clinics in the Pacific Northwest. Patient eligibility, demographics, and outcomes were drawn from VHA electronic databases.21
This study was conducted as non-research quality improvement for evaluation of primary care operations under the designation of the VHA Office of Primary Care and was, therefore, not subject to IRB review nor exemption. This trial was prospectively registered under Clinical Trials Number 05012007.22 One update to the eligibility criteria (detailed below) in the pre-specified protocol was made on August 20, 2021, to align with operational workflows and updated clinical practice guidelines.2
Inclusion and Exclusion Criteria
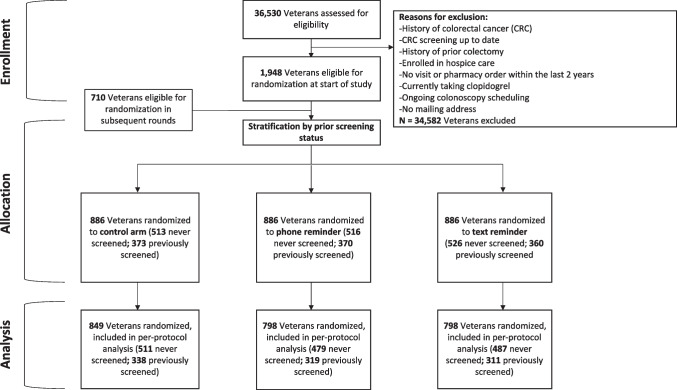
Eligible veterans were enrolled at VA Puget Sound Health Care System with at least 1 outpatient visit in the past 2 years (n = 20,857). Data for eligible veterans aged 50–75 years were drawn for trial participants starting on July 12, 2021. On August 20, 2021, the minimum age for our trial was reduced to 45 years to accommodate changes in national screening guidelines.2 Patients were excluded if they were already up-to-date with appropriate prior CRC screening (any VHA-approved modality, n = 12,101), not average risk for CRC according to VHA guidelines (n = 5586), were newly prescribed clopidogrel within the last 6 months (n = 95), enrolled in hospice (n = 24), already scheduled for upcoming colonoscopy or sigmoidoscopy within 90 days (n = 36), or lacked a mailing address (n = 111). A total of 1948 patients were eligible for inclusion (Fig. 1). New clopidogrel users within the last 6 months were excluded as these individuals were determined to be likely unable to hold anticoagulation in order to complete the recommended follow-up colonoscopy. These inclusion and exclusion criteria were re-applied to eligible patients before each subsequent weekly round of FIT kit mailings, for a total of 9 rounds, until October 28, 2021.
Fig. 1.
Consort diagram
Sample Size and Power
Based on VA Puget Sound Health Care System administrative data in 2020, 43% of patients who had an order for an annual FIT for CRC screening within the electronic health record had completed testing via laboratory records. Existing literature shows that reminders increase rates of FIT return between 3 and 21% in non-veteran populations.13 We powered our study to detect a 7% absolute difference as it was the smallest between-group difference that we could detect given the size of the available eligible population, and this difference fell within the expected effect size based on prior reports. 13A sample size of 2653 individuals allowed for the detection of an absolute difference of 7% between groups, using an 80% power and a 5% two-sided significance level. The sample size also allowed for 10% attrition in the event of returned mail or non-working phone numbers.
Randomization
From all eligible patients, 2653 patients were selected and randomized in a 1:1:1 allocation to the intervention arms or control arm using permuted block randomization, with random block sizes of 3 and 6.23 Randomization occurred 2 weeks after the FIT kit was mailed, and only among patients who had not returned the FIT. Computerized randomization was conducted with R (version 4.0.1).11 No blinding was applied, and patients were not notified of their enrollment nor allocation in the trial.
Intervention
This trial was conducted in concert with the system-wide implementation of a mailed FIT program. The overall implementation of the mailed screening program has been described.12 For this trial, all patients were mailed a printed primer postcard introducing FIT for CRC screening 2 weeks prior to being mailed the FIT kit (Supplemental Figure 1). All participants were then mailed a FIT kit with an included prepaid return envelope, an introductory letter, and instructions for completing the FIT. Two weeks after the FIT kit mailing, we identified veterans who had no FIT result in our electronic health record (EHR). These veterans were randomized to receive either a standardized SMS-text message from a secure VHA software platform (VEText system24) or an automated phone call (AudioCARE, PA) prompting them to return their test. The text scripts were developed in partnership with VEText developers to meet text messaging privacy standards, and the phone script was modified to closely match the language in the text message (Supplemental Text Box). The text system sent one SMS to a patient phone number using the primary number on file in the EHR, and if undeliverable, then attempted to send the message to any additional back-up numbers on file. The automated phone call system made three attempts to deliver the message to the primary number on file. Those assigned to the usual care arm received the postcard and mailed FIT kit and no reminder.
Outcomes
The primary outcome was the FIT return rate at 90 days post-randomization. Our secondary outcome was the FIT return rate at 180 days post-randomization.
Statistical Analysis
We used chi-square and Student’s t-test to compare characteristics between patients who completed FIT and those who did not. We used multivariable logistic regression models to assess the outcomes of interest. For increased precision, we specified a priori adjusted models for patient age, sex (male/female), race and ethnicity (American Indian/Alaska Native, Asian/Pacific Islander/Native Hawaiian, Hispanic, multi-race, non-Hispanic Black, non-Hispanic White, Other),25 rurality (urban/rural), Gagne comorbidity index,26 neighborhood socioeconomic index (by decile of population),27 and prior history of FIT completion within last 5 years.
An analysis of futility was completed as an interim analysis was completed after 50% (n=1327) of the estimated sample size had been enrolled and had reached the primary endpoint.28 A z-statistic was calculated from the proportional difference between the combined treatment groups (calls and texts) versus control and this z-statistic was compared to the futility boundary to determine if it was crossed. A one-sided non-binding futility boundary of 0.50 (using an O’Brien-Fleming alpha spending design and Pocock-type beta spending function) was used.28–30
The intention-to-treat (ITT) analyses examined the association between the randomization group and FIT test return, stratifying by prior screening and other demographic characteristics known to be associated with the likelihood of CRC screening completion. The per-protocol analysis excluded veterans whose mailed FIT tests were marked as undeliverable and returned by the post office (n = 10), who had returned a FIT kit after cohort generation and prior to the study kit mailing date (n = 75), or who we were unable to reach by text (n = 91) or by phone (n = 59). P-values were adjusted via the Hochberg method31 to account for multiple comparisons, as well as the O’Brien-Fleming method to account for the interim analysis. Where indicated, results reported as predicted probabilities were estimated by marginal standardization.
Statistical analyses were performed using R version 4.0.1.11
RESULTS
A total of 2658 eligible patients were randomized. Of these, 886 patients were randomized to each of the three groups: usual care, automated telephone reminder, or automated text reminder. Of all included patients, the average age was 62 years old (mean, SD = 8.5) and the majority were male (88%) and non-Hispanic White (66%). Forty-one percent of patients had previously completed FIT testing within 5 years prior to study participation. Patient characteristics were well balanced between both intervention and control arms (Table 1), although slightly more racial and ethnic minority patients, and patients living closer to primary care sites, were in the control arm than either text or call arms.
Table 1.
Patient Characteristics at Baseline. Mean (SD), Except Where Noted as n (%)
| Randomized group | |||||
|---|---|---|---|---|---|
| Variable | Overall, N = 2658 | Usual care, n = 886 | Phone reminder, n = 886 | Text reminder, n = 886 | P1 |
| Age (years) | 62.1 (8.6) | 61.8 (8.6) | 62.1 (8.6) | 62.3 (8.5) | 0.6 |
| Female, n (%) | 319 (12.0) | 105 (11.9) | 112 (12.6) | 102 (11.5) | 0.8 |
| Race/ethnicity, n (%) | 0.06 | ||||
| American Indian/Alaska Native | 39 (1.5) | 13 (1.5) | 14 (1.6) | 12 (1.4) | |
| Asian/Pac Islander/Native Hawaiian | 153 (5.8) | 56 (6.3) | 51 (5.8) | 46 (5.2) | |
| Hispanic | 118 (4.4) | 29 (3.3) | 39 (4.4) | 50 (5.6) | |
| Multi-race/other/missing | 172 (6.5) | 77 (8.7) | 53 (6.0) | 42 (4.7) | |
| Non-Hispanic Black | 426 (16.0) | 158 (17.8) | 132 (14.9) | 136 (15.3) | |
| Non-Hispanic White | 1750 (65.8) | 553 (62.4) | 597 (67.4) | 600 (67.7) | |
| Married, n (%) | 1206 (45.4) | 384 (43.3) | 416 (47.0) | 406 (45.8) | 0.3 |
| Gagne score | 0.4 (1.9) | 0.5 (1.3) | 0.5 (1.3) | 0.4 (1.2) | 0.6 |
| Socioeconomic status index < 4th decile, n (%)2 | 923 (40.9) | 320 (42.6) | 314 (41.2) | 289 (38.7) | 0.8 |
| Rural status, n (%)3 | 579 (22.5) | 184 (21.4) | 193 (22.4) | 202 (23.7) | 0.6 |
| Drive distance to primary care (miles) | 17.0 (13.7) | 16.3 (13.4) | 17.3 (14.2) | 17.5 (13.4) | 0.04 |
| PC visit in prior year (%)3 | 1908 (72.5) | 642 (73.3) | 636 (72.2) | 630 (71.9) | 0.8 |
| Prior FIT screening (%)4 | 1103 (41.5) | 373 (42.1) | 370 (41.8) | 360 (40.6) | 0.8 |
PC primary care, CRC colorectal cancer
1Kruskal-Wallis rank sum test; Pearson’s chi-squared test
2Included in adjusted models as by decile, shown as aggregate for brevity. Veterans in the 4th or lower decile by socioeconomic status index have been shown to have greater mortality
3Missing data (n): rural/urban status (control = 26, arm 2 = 26, arm 3 = 33); PC visits in prior year (control = 10, arm 2 = 5, arm 3 = 10)
4Lab evidence of prior screening with FIT in last 5 years
In unadjusted ITT analysis at 90 days, we observed significantly higher absolute rates of returned kits among both phone and text reminder groups compared to usual care (Table 2). After adjustment for confounders, both the intervention groups had significantly higher predicted probability of returning the FIT kit compared to usual care; patients who received a phone reminder had an 11.3% (95% CI: 6.9–15.7%) absolute increase in the probability of kit return, while patients who received a text reminder had a 10.3% (5.9–14.7%) increase in the probability of kit return compared to usual care. Results were similar in the per-protocol analyses (Table 3).
Table 2.
Unadjusted Rate of Mailed Fecal Immunochemical Testing (FIT) Kit Return, Comparing Reminder Modalities to Usual Care at 90 and 180 days
| Randomized group (N = 2658) | ||||
|---|---|---|---|---|
| Outcomes | Usual care, n = 886 | Phone reminder, n = 886 | Text reminder, n = 886 | P1 |
| Intention-to-treat, n (%) | ||||
| Return, 90d | 250 (28.2) | 345 (38.9) | 334 (37.7) | <0.001 |
| Return, 180d | 283 (31.9) | 371 (41.9) | 352 (39.7) | <0.001 |
| Per-protocol, n (%)2 | ||||
| Return, 90d | 247 (29.1) | 323 (40.5) | 303 (38.0) | <0.001 |
| Return, 180d | 279 (32.9) | 347 (43.5) | 319 (40.0) | <0.001 |
1Pearson’s chi-squared test
2Excluded veterans with undeliverable addresses, unreachable by phone or text, and those who returned a FIT after cohort generation but prior to study mailing date. Denominators: usual care (n = 849); phone (n = 798); text (n = 798)
Table 3.
Difference in Return of Mailed Screening Kit at 90 and 180 Days Among Patients Randomized to Phone or Text Reminder Groups, Compared to Usual Care
| Difference in return rate (predicted probability (95% CI)) | aOR (95% CI)) | P1 | Q2 | |
|---|---|---|---|---|
| Intention-to-treat | ||||
| 90 days | <0.001 | <0.001 | ||
| Usual care | Ref | Ref | ||
| Phone | 11.3 (6.9–15.7) | 1.7 (1.4–2.1) | ||
| Text | 10.3 (5.9–14.7) | 1.6 (1.3–2.0) | ||
| 180 days | <0.001 | <0.001 | ||
| Usual care | Ref | Ref | ||
| Phone | 10.3 (5.8–14.8) | 1.6 (1.3–2.0) | ||
| Text | 8.6 (4.1–13.1) | 1.5 (1.2–1.8) | ||
| Per-protocol | ||||
| 90 days | <0.001 | <0.001 | ||
| Usual care | Ref | Ref | ||
| Phone | 11.9 (7.3–16.4) | 1.8 (1.4–2.2) | ||
| Text | 9.5 (5.0–14.1) | 1.6 (1.3–2.0) | ||
| 180 days | <0.001 | <0.001 | ||
| Usual care | Ref | Ref | ||
| Phone | 10.9 (6.2–15.5) | 1.6 (1.3–2.0) | ||
| Text | 7.7 (3.1–12.4) | 1.4 (1.2–1.8) | ||
CI confidence interval
1Test for differences across all groups, including usual care
2False discovery rate correction for multiple testing
Models are adjusted for age, sex, race/ethnicity, Gagne, and prior FIT screening
In the unadjusted ITT analysis at 180 days, we observed higher rates of returned kits at 180 days among both the phone and text groups compared to the no-reminder group (Table 2). In the adjusted model, the predicted probability of returning the FIT kit was higher in both intervention groups compared to usual care (phone, 10.3% absolute increase in return rate (95% CI: 5.8–14.8%); text, 8.6% absolute increase (4.1–13.1%)). Results remained consistent in the per-protocol analysis (Table 3).
DISCUSSION
We found that automated text and phone call reminders both increased the return of mailed FIT kits in a regional CRC screening program for average-risk veterans compared to usual care. Both reminder modalities similarly led to higher rates of returned kits at 90 and 180 days than patients who did not receive a reminder.
Mailed FIT programs are an expanding population health strategy to increase access to CRC screening.13 Prior studies have shown the benefit of reminders as a key component of mailed FIT programs to enhance screening completion, and reminders are now recommended by the Centers for Disease Control and Prevention.13 As the VHA seeks to expand its mailed FIT efforts,12 examining key components of such programs among veterans is essential to implementation. Ours is the first study to evaluate the impact of reminders within a systematic mailed FIT program at VHA. Prior studies within the VHA that have used more labor-intensive approaches to deliver mailed FIT and reminder outreach have been successful, but these approaches are unlikely to be widely implemented due to time and labor cost.19, 32 Our study presents a more feasible, low-effort automated intervention which improves CRC screening and increases FIT return rates by at least 10%.
Our work adds important insights on effective and scalable automated strategies to improve FIT return within resource and time-limited settings. Prior systematic reviews showed that different reminder modalities—phone, text, live calls, letters, navigators—can incrementally improve the FIT return rate by as much as 3–6%.14, 16 Few studies have looked at text messaging specifically head-to-head with other automated strategies, and these previous studies evaluating text-based reminder strategies, each using different methods and comparison groups, have shown mixed results.18, 33–37 Interestingly, our study found similar effectiveness of a text and automated call reminder strategy. Given high rates of cell phone access (over 90%), even among older and rural populations,38 our results support the use of scalable low-effort reminder solutions to increase CRC screening rates in a mailed FIT program among veterans. Our work provides needed information to VHA and other health care facilities looking to adopt mailed FIT programs and incorporate reminders that fit within their operational workflow.
Increasing mailed FIT return rates remains an important challenge. Even with the automated reminders, nearly 60% of FIT kits were not returned, which is comparable to mailed FIT return programs in other populations.15 Future work should consider testing the frequency of automated reminders or adding step-wise resources, such as live calls or patient navigators to increase FIT return rates, as has been deployed by other health care systems.39 Lastly, due to expanded guidelines on eligibility for CRC screening, we included patients between the ages of 45 and 49. Our trial included only 186 patients who met this expanded screening eligibility. Newer trials may need to explore whether this new-to-screening cohort responds differently to outreach would be a valuable area of future research.
Limitations
Our randomized controlled trial was conducted in partnership with primary care leadership at VA Puget Sound Health Care System, and interest was in reminder strategies that were both effective and operationally sustainable. As such, we did not investigate live-person calls, letters, or multi-component reminders, limiting generalizability to health systems interested in these strategies. While reminder strategies are not “one-size-fits-all” and should be tailored to the specific patient population, our trial was not powered to detect differences between text and phone reminders, nor to assess their effectiveness in specific subpopulations of veterans. Other limitations exist related to our eligibility criteria. Finally, our findings among veterans may not generalize to non-veteran populations.
CONCLUSIONS
In this randomized trial of FIT reminder interventions, we found that either an automated text or an automated phone call significantly increased FIT returned rates among average-risk veterans due for CRC screening. Given growing interest in mailed FIT as a strategy to increase CRC screening, this study adds to the literature supporting reminders overall and provides additional insight into the effectiveness of automated and highly scalable reminder modalities.
Supplementary Information
(DOCX 84 kb)
Acknowledgements
This work was made possible through operational support from VA Puget Sound Health Care System Leadership.
Funding
This work was made possible through operational funding from VA Puget Sound Health Care System Leadership. Additionally, this work was funded by the Primary Care Analytics Team through the Veterans Health Administration Office of Primary Care. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US government, the Department of Veterans Affairs, and the University of Washington.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 3.QuickStats: Percentage of Adults Aged 50-75 Years Who Met Colorectal Cancer (CRC) Screening Recommendations*,† - National Health Interview Survey, United States, 2018§. MMWR Morb Mortal Wkly Rep. 2020;69(11):314. 10.15585/mmwr.mm6911a7 [DOI] [PMC free article] [PubMed]
- 4.May FP, Yano EM, Provenzale D, Steers WN, Washington DL. Race, Poverty, and Mental Health Drive Colorectal Cancer Screening Disparities in the Veterans Health Administration. Med Care. 2019;57(10):773–780. doi: 10.1097/MLR.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Veterans Affairs. VA Overall: VA in Fiscal Year 2021. Published 2021. Accessed October 13, 2022. https://www.accesstocare.va.gov/Healthcare/Overall
- 6.Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of Cancer Screening Deficit in the United States With the COVID-19 Pandemic. JAMA Oncol. 2021;7(6):878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshinski R. Coronavirus (COVID-19) – Guidance for Elective Procedures. Published online March 15, 2020. https://www.navao.org/wp-content/uploads/2020/03/03152020-COVID-19-Elective-Procedures-1.pdf. Accessed October 13, 2022.
- 8.Gawron AJ, Kaltenbach T, Dominitz JA. The Impact of the Coronavirus Disease-19 Pandemic on Access to Endoscopy Procedures in the VA Healthcare System. Gastroenterology. 2020;159(4):1216–1220.e1. doi: 10.1053/j.gastro.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidance to Avoid All Routine or Non-urgent Face to Face Visits, Memorandum, Department of Veterans Affairs. Published March 31, 2020. Accessed November 10, 2021. https://www.navao.org/wp-content/uploads/2020/04/Guidance-to-Avoid-All-Routine-or-Non-urgent-Face-to-Face-Visits-Signed.pdf
- 10.Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement | Cancer Screening, Prevention, Control | JAMA | JAMA Network. Accessed January 13, 2022. https://jamanetwork.com/journals/jama/fullarticle/2779985
- 11.U.S. Department of Veterans Affairs. Colorectal Cancer Screening with Programmatic Mailed FIT: A program to mail FIT kits for CRC screening directly to Veterans. Published 2021. Accessed October 13, 2022. https://marketplace.va.gov/innovations/colorectal-cancer-screening-with-programmatic-mailed-fit
- 12.Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11(4):e001927. doi: 10.1136/bmjoq-2022-001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Coronado GD, Argenbright K, et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention-sponsored Summit. CA Cancer J Clin. 2020;70(4):283–298. doi: 10.3322/caac.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178(12):1645–1658. doi: 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager M, Demb J, Asghar A, et al. Mailed Outreach Is Superior to Usual Care Alone for Colorectal Cancer Screening in the USA: A Systematic Review and Meta-analysis. Dig Dis Sci. 2019;64(9):2489–2496. doi: 10.1007/s10620-019-05587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: A systematic review. Prev Med. 2019;118:113–121. doi: 10.1016/j.ypmed.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin BC, Ireland MJ, March S, et al. Strategies for increasing participation in mail-out colorectal cancer screening programs: a systematic review and meta-analysis. Syst Rev. 2019;8(1):257. doi: 10.1186/s13643-019-1170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman SN, Liss DT, Brown T, et al. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med. 2015;30(8):1178–1184. doi: 10.1007/s11606-015-3234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldshore MA, Mehta SJ, Fletcher W, Tzanis G, Doubeni CA, Paulson EC. An RCT of Fecal Immunochemical Test Colorectal Cancer Screening in Veterans Without Recent Primary Care. Am J Prev Med. 2020;59(1):41–48. doi: 10.1016/j.amepre.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert PL, Batten AS, Gunnink E, et al. Reliance on Medicare Providers by Veterans after Becoming Age-Eligible for Medicare is Associated with the Use of More Outpatient Services. Health Serv Res. 2018;53 Suppl 3(Suppl Suppl 3):5159–5180. doi: 10.1111/1475-6773.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price LE, Shea K, Gephart S. The Veterans Affairs’s Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs Adm Q. 2015;39(4):311–318. doi: 10.1097/NAQ.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuttner L. Reminders for FIT (Fecal Immunochemical Test) Kits Via Different Modalities: A Randomized Controlled Trial. clinicaltrials.gov; 2021. Accessed February 2, 2022. https://clinicaltrials.gov/ct2/show/NCT05012007
- 23.Snow G. blockrand: Randomization for Block Random Clinical Trials. Published online April 6, 2020. Accessed April 21, 2022. https://CRAN.R-project.org/package=blockrand
- 24.U.S. Department of Veterans Affairs. VEText. Accessed October 13, 2022. https://www.va.gov/HEALTH/VEText.asp
- 25.Hernandez S, Sylling P, Mor M, et al. Developing an Algorithm for Combining Race and Ethnicity Data Sources in the Veterans Health Administration. Mil Med. 2019;185. 10.1093/milmed/usz322 [DOI] [PubMed]
- 26.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson K, Schwartz G, Hernandez S, Simonetti J, Curtis I, Fihn SD. The Association Between Neighborhood Environment and Mortality: Results from a National Study of Veterans. J Gen Intern Med. 2017;32(4):416–422. doi: 10.1007/s11606-016-3905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wassmer G, Pahlke F. rpact: Confirmatory Adaptive Clinical Trial Design, Simulation, and Analysis. Published online 2021. https://www.rpact.org/
- 29.O’Brien P, Fleming T. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. doi: 10.2307/2530245. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Hermann C, Rauch G. Optimality criteria for futility stopping boundaries for group sequential designs with a continuous endpoint. BMC Med Res Methodol. 2020;20:274. doi: 10.1186/s12874-020-01141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 32.Schlichting JA, Mengeling MA, Makki NM, et al. Increasing colorectal cancer screening in an overdue population: participation and cost impacts of adding telephone calls to a FIT mailing program. J Community Health. 2014;39(2):239–247. doi: 10.1007/s10900-014-9830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uy C, Lopez J, Trinh-Shevrin C, Kwon SC, Sherman SE, Liang PS. Text Messaging Interventions on Cancer Screening Rates: A Systematic Review. J Med Internet Res. 2017;19(8):e296. doi: 10.2196/jmir.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirst Y, Skrobanski H, Kerrison RS, et al. Text-message Reminders in Colorectal Cancer Screening (TRICCS): a randomised controlled trial. Br J Cancer. 2017;116(11):1408–1414. doi: 10.1038/bjc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagoel L, Neter E, Stein N, Rennert G. Harnessing the Question–Behavior Effect to Enhance Colorectal Cancer Screening in an mHealth Experiment. Am J Public Health. 2016;106(11):1998–2004. doi: 10.2105/AJPH.2016.303364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coronado GD, Rivelli JS, Fuoco MJ, et al. Effect of Reminding Patients to Complete Fecal Immunochemical Testing: A Comparative Effectiveness Study of Automated and Live Approaches. J Gen Intern Med. 2018;33(1):72–78. doi: 10.1007/s11606-017-4184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huf SW, Asch DA, Volpp KG, Reitz C, Mehta SJ. Text Messaging and Opt-out Mailed Outreach in Colorectal Cancer Screening: a Randomized Clinical Trial. J Gen Intern Med. 2021;36(7):1958–1964. doi: 10.1007/s11606-020-06415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pew Research Center. Mobile Fact Sheet: Mobile phone ownership over time. Pew Research Center: Internet, Science & Tech. Accessed October 13, 2022. https://www.pewresearch.org/internet/fact-sheet/mobile/
- 39.Selby K, Jensen CD, Levin TR, et al. Program Components and Results From an Organized Colorectal Cancer Screening Program Using Annual Fecal Immunochemical Testing. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2022;20(1):145–152. doi: 10.1016/j.cgh.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 84 kb)