Abstract
Glucan-binding protein A (GbpA) of Streptococcus mutans has been hypothesized to promote sucrose-dependent adherence and the cohesiveness of plaque and therefore to contribute to caries formation. We have analyzed the adherence properties and virulence of isogenic gbpA mutants relative to those of wild-type S. mutans. Contrary to expectations, the gbpA mutant strains displayed enhanced sucrose-dependent adherence in vitro and enhanced cariogenicity in vivo. In vitro, S. mutans was grown in the presence of [3H]thymidine and sucrose within glass vials. When grown with constant rotation, significantly higher levels of gbpA mutant organisms than of wild type remained adherent to the vial walls. Postgrowth vortexing of rotated cultures significantly decreased adherence of wild-type organisms, whereas the adherence of gbpA mutant organisms was unaffected. In the gnotobiotic rat model, the gbpA mutant strain was hypercariogenic though the colonization levels were not significantly different from those of the wild type. The gbpA mutant strain became enriched in vivo with organisms that had undergone a recombination involving the gtfB and gtfC genes. The incidence of gtfBC recombinant organisms increased as a function of dietary sucrose availability and was inversely correlated with caries development. We propose that the absence of GbpA elevates the cariogenic potential of S. mutans by altering the structure of plaque. However, the hypercariogenic plaque generated by gbpA mutant organisms may be suboptimal for S. mutans, leading to the accumulation of gtfBC recombinants whose reduced glucosyltransferase activity restores a less cariogenic plaque structure.
The primary virulence traits of Streptococcus mutans are sucrose-dependent adherence, aciduricity, and acidogenicity (7). Sucrose-dependent adherence is mediated by glucans, the products of the enzymatic glucosyltransferases (GTFs) (5). GTF-I, GTF-SI, and GTF-S, encoded by the gtfB, gtfC, and gtfD genes, respectively, are extracellular enzymes which polymerize the glucose moieties of sucrose into glucans. The capacity of the GTFs to bind glucans is prerequisite for their enzymatic activity (18). The importance of the GTFs in cariogenicity, particularly of the products of the gtfB and gtfC genes, has been established by many laboratories (11, 25, 26).
S. mutans also synthesizes three nonenzymatic glucan-binding proteins (GBPs) whose contribution to virulence is uncertain. These are GBP74, GBP59, and GbpC. Although no comprehensive survey has been reported, work from several laboratories suggests that these GBPs are common to many, if not all, strains of S. mutans (3, 14, 16, 19). GBP74 was identified by Russell (14) and was so designated based on an apparent size of 74 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). However, the deduced size of the mature protein following DNA sequencing was 59 kDa (2), which is similar to the sizes subsequently reported by Smith et al. for GBP59 (19) and by Sato et al. for GbpC (16). To avoid confusion and to follow the initiative of Sato (16), we propose to designate the GBPs in the order of their discovery as GbpA (GBP74), GbpB (GBP59), and GbpC.
Like the GTFs, GbpA is a secreted protein found both in association with the cell surface and in the extracellular medium. The carboxyl-terminal three-quarters of GbpA has homology to the carboxyl-terminal repeat domains of the GTFs (2). Functionally, GbpA has been proposed to contribute to S. mutans adherence and to the cohesiveness of plaque based on experiments which demonstrated that antisera raised against a preparation of GbpA inhibited sucrose-dependent adherence (4) and on the observation that a chemically generated S. mutans GbpA mutant formed a softer, more brittle plaque in vitro (15). GbpA is antigenically distinct from both GbpB and GbpC. Although immunization with GbpB has been reported to be protective against caries (20), the actual function of GbpB is unknown, and the gene encoding it has yet to be cloned. GbpC appears to be anchored to the cell wall, is partially similar to the Spa family of oral streptococcal proteins, and is involved in dextran-dependent aggregation under defined, stressful growth conditions (16). The need for the multiplicity, and potentially the balance, of the three GTFs and three GBPs in plaque formation and structure is unknown. In this report, we compare properties of wild-type (wt) S. mutans with those of isogenic gbpA mutants. We show that GbpA− mutants are elevated in sucrose-dependent adherence in vitro, are hypercariogenic in vivo, and accumulate in vivo recombinations between the gtfB and gtfC genes. The data presented here are the first demonstration of a GBP affecting the virulence of S. mutans.
MATERIALS AND METHODS
Bacterial strains.
Construction of the gbpA mutant strains of S. mutans 3209 and UA130 was achieved by allelic replacement of the gbpA gene with an erythromycin resistance gene flanked by the 5′ and 3′ portions of the gbpA gene as previously described (1). Southern and Western blotting analyses were used to confirm strain constructions.
S. mutans 3209 and UA130 (both serotype c) and their respective gbpA isogenic mutant strains were routinely grown anaerobically at 37°C in either of the chemically defined media FMC (21) and CDM (JRH Biosciences, Lexena, Kans.). Growth on Todd-Hewitt agar (Difco Laboratories, Grand Island, N.Y.) and mitis salivarius (MS) agar (Difco Laboratories) plates was used to confirm culture purity and colony morphology, respectively. The gbpA mutant strains were maintained in vitro with 25 μg of erythromycin per ml.
In vitro adherence assays.
Overnight cultures of S. mutans 3209 wt and gbpA mutant were diluted in FMC and grown to a cell density of 0.05 at 540 nm. One milliliter of each culture was placed in 15-by-45-mm, 3.7-ml flat-bottomed cylindrical glass vials (Fisher) to which sterile sucrose and [3H]thymidine (New England Nuclear) were added to 1% and 0.30 μCi/ml, respectively. The vials were sealed and incubated at 37°C either standing upright or rotating on a variable-angle hematological rotator at a rotation speed of 10 rpm and an angle with the vial top 30° greater than horizontal. After 5 h of incubation, the vials were either gently rinsed twice with 3 ml of phosphate-buffered saline (PBS), pH 7.2, delivered to the vial walls via peristaltic pump (2 ml/min), or vortexed (Fisher Vortex Genie 2) at top speed for 30 s prior to rinsing. Supernatants were gently decanted along the edge of a toothpick held perpendicular to the mouth of the vial. Adherence was quantified by scintillation counting.
The preparation of hydroxyapatite-coated microtiter plates has been previously described (17). Overnight cultures of rough and smooth S. mutans 3209 wt and gbpA mutant were diluted in fresh CDM and grown to a cell density of 0.35 at 540 nm. A total of 25 μl of 50% sucrose (final concentration, 5%) and 225 μl of each culture were placed in the wells of a hydroxyapatite-coated microtiter plate which was incubated at 37°C while rotating, as described above. After 3 h of incubation, the contents of the wells were aspirated and the wells were rinsed with 300 μl of PBS and stained with a crystal violet solution (Becton Dickinson, Cockeysville, Md.) for 2 min. The contents of the wells were aspirated, and the wells rinsed twice with 300 μl of PBS prior to release of retained crystal violet. Following the addition of 300 μl of Gram stain decolorizer (ethanol/acetone ratio of 3:1), the absorbance at 610 nm was measured by a plate reader. The signal which was derived from non-sucrose-containing wells was considered background and was subtracted.
Virulence testing of S. mutans in germfree rats.
The cariogenic potentials of S. mutans UA130 and UA130 gbpA mutant were determined in gnotobiotic Fischer rats as previously described (9). Specifically, 21-day-old weanling rats were provided the caries-promoting diet 305 (5% sucrose) ad libitum (24 h/day) for 7 days. On days 1, 2, and 3 of this period, the rats were orally challenged with saturated swabs of UA130 or UA130 gbpA mutant (2 × 108 CFU/ml). On day 8, the rats were either switched to a 6-h/day feeding schedule or continued on the ad libitum diet. Colonization was assessed on days 8, 16, and 36 by culturing oral swabs on MS and blood agar plates. The rats were sacrificed on day 36, and plaque microbiology (10) and caries scores were determined by the method of Keyes (6). Colony morphology of postmortem cultures from infected animals was assessed by plating cultures on MS agar with erythromycin where appropriate.
Determination of GTF activity.
To visualize GTF activity of S. mutans, cell-associated proteins were resolved by SDS-PAGE followed by incubation with Triton X-100 (Fisher Biotech) and sucrose (13). Specifically, overnight CDM broth cultures of UA130 and gbpA mutant were diluted into 20 ml of fresh CDM, grown to an optical density at 540 nm of 1.0, and harvested by centrifugation. Cells were resuspended in 150 μl of 2× cracking buffer (0.038 M Tris HCl [pH 6.8], 1% SDS, 2.5% β-mercaptoethanol, 15% glycerol) and incubated at 37°C for 1 h. Twenty microliters of cell-free supernatant was resolved by SDS-PAGE. Following electrophoresis, SDS was eluted from the gel by two 30-min washes with PBS–2% Triton X-100. In situ GTF activity was visualized by incubation of the gel for 16 h in PBS (pH 6.5) containing 1% sucrose and 0.05% 11,000-molecular-weight dextran (Sigma). The gels were briefly rinsed with PBS and incubated in a 45% methanol–10% acetic acid solution for 10 min to enhance the glucan bands. Gels were photographed against a black background.
To quantify GTF activity, cell-associated proteins from rough and smooth colonies of UA130 wt were resolved by SDS-PAGE. The 84-kDa molecular mass standard was used to separate the gel into the top half, which was developed for GTF activity as described above, and the bottom half, which was analyzed for GbpA content by Western blotting. Scanning densitometry was used to quantify the glucan and GbpA signals, and GTF activity was normalized to GbpA content.
Isolation of chromosomal DNA.
S. mutans chromosomal DNA was isolated by a modification of the method of Marmur (8). Specifically, 20 ml of S. mutans overnight broth culture was harvested by centrifugation, resuspended in 100 μl of 10 mM Tris HCl (pH 8) containing 5 mg of lysozyme and 50 U of mutanolysin, and incubated with agitation at 37°C for 1 h. Proteinase K (200 μg) was added, and incubation was continued for 1 h. Cell lysis was achieved by the addition of SDS to 2.5% and gentle mixing by inversion. The lysed-cell solution was phenol extracted, and the aqueous phase was subjected to four rounds of organic-phase extraction with phenol-chloroform (1:1), phenol-chloroform-isoamyl alcohol (125:24:1), chloroform-isoamyl alcohol (24:1), and finally chloroform. Two volumes of 95% ethanol was added to the aqueous phase, mixed by gentle inversion, and incubated overnight at −20°C. Nucleic acids were gently pelleted by brief (25-s) centrifugation (5,800 × g), rinsed with 70% ethanol, and resuspended in 300 μl of sterile 10 mM Tris HCl (pH 8) containing 2 μg of RNase A.
Long-range PCR.
Amplification of the gtfB-gtfC region was achieved with the primers gtfB.Prom.F (5′-GGCTTGTTGCTGGAATCAATGC-3′) and gtfC-R (5′-TTCTTCTTTTGAAAAACGGGTACG-3′) (Life Technologies) with the Boehringer Mannheim Expand Long Template PCR system as per the manufacturer’s instructions with a slight modification. Specifically, the use of buffer 3 and Hot-Start tubes (Molecular Bio-Products) increased yield and specificity. Amplification conditions were 3 min at 94°C followed by 10 cycles of 94°C for 30 s, 55°C for 90 s, and 68°C for 8 min, followed by 20 cycles of 94°C for 30 s, 55°C for 90 s, and 68°C for 8 min 20 s plus 20 s/cycle, followed by a final extension at 68°C for 7 min. The MJ Research MiniCycler thermal cycler was used for all amplification reactions. PCR products were resolved by gel electrophoresis through 0.7% agarose and visualized by ethidium bromide staining.
Statistical analysis.
All numerical data presented here are expressed as the means ± the standard deviations. Data analysis and determination of significance were performed by the unpaired, two-tailed Student t test or, if appropriate, the unpaired, two-tailed nonparametric Mann-Whitney test. Differences were considered significant when a value of P ≤ 0.05 was obtained.
RESULTS
In vitro adherence.
To ensure that growth rate differences would not be an additional factor in the interpretation of in vitro adherence assay data, [3H]thymidine uptake and culture optical density increase of wt and gbpA mutant S. mutans 3209 were measured. No differences in growth rate were found under any of the culture conditions employed in the in vitro adherence assays.
To investigate the contribution of GBP to sucrose-dependent adherence, cultures of S. mutans 3209, either wt or gbpA mutant, were allowed to grow in a stationary, upright position or with rotation for 5 h in the presence of sucrose and [3H]thymidine, after which the unincorporated label and nonadherent bacteria were removed. The plaque produced by the stationary cultures was heavily deposited on the bottom of the vials, whereas the plaque produced by the cultures grown with rotation was evenly dispersed over the bottom and the wall of the vials and covered four times more surface area than did the stationary-growth plaques. Visual inspection of the vials prior to the addition of scintillation cocktail revealed that markedly higher levels of adherent gbpA mutant plaque than of wt plaque were retained in the rotated cultures. Adherent bacteria were quantified by scintillation counting, and the results are shown in Table 1. The effect of GbpA on sucrose-dependent adherence was clearly seen in the rotated cultures. No effect was apparent in the stationary cultures. Among the rotated cultures, the adherent counts per minute were significantly higher for the gbpA mutant group (P < 0.05). These differences are significant at the 95% confidence interval according to the Student t test. Also as shown in Table 1, the adherence of rotated wt S. mutans decreased significantly in response to the increased mechanical agitation of vortexing whereas the adherence of rotated gbpA mutant S. mutans was unaffected, suggesting that plaque developed in the absence of GbpA may be structurally different from wt S. mutans plaque.
TABLE 1.
In vitro sucrose-dependent adherence of S. mutansa
| Genotype | Adherent cpm for culture
|
|||
|---|---|---|---|---|
| Rotating
|
Stationary
|
|||
| Rinsed | Vortexed | Rinsed | Vortexed | |
| gbpA mutant | 4,882 ± 584 | 4,867 ± 1,009 | 15,013 ± 1,659 | 2,995 ± 329 |
| wt | 3,151b ± 292 | 2,031b ± 545 | 13,804 ± 1,598 | 3,233 ± 487 |
S. mutans 3209 cultures, either wt or gbpA mutant, were allowed to grow, stationary or while rotating, for 5 h in the presence of sucrose and [3H]thymidine, after which the vials were either rinsed or vortexed prior to rinsing. Adherent bacteria were quantified by scintillation counting and reported here as counts per minute ± standard deviations. Boldface numbers are statistically higher (P < 0.05) than others in the same column (n = 9).
Numbers bearing the superscript b are significantly different (P < 0.05) from each other.
Virulence in germfree rats.
Table 2 contains the pooled data from two separate determinations of the cariogenicity of S. mutans UA130 wt and gbpA mutant in the gnotobiotic rat model. The levels of colonization of both the wt and gbpA mutant groups were significantly higher in the animals fed ad libitum (24 h) than in the animals on a 6-h feeding schedule. GbpA plays no apparent role in colonization as evident by the similar colonization levels of the wt and gbpA mutant groups within each dietary regimen.
TABLE 2.
Virulence of S. mutans UA130 wt and gbpA mutant in monoinfected germfree ratsa
| Diet and genotype | Cell recoveryc
|
Mean caries score ± SDb
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buccal
|
Sulcal
|
Proximal
|
||||||||||||
| CFU/ml | % Smoothd | E | Ds | Dm | Dx | E | Ds | Dm | Dx | E | Ds | Dm | Dx | |
| 6 h | ||||||||||||||
| gbpA mutant | 1.3 × 106 ± 2.7 × 106 | 14.9 ± 12.9 | 16.08 ± 2.7 | 12.50 ± 2.1 | 8.83 ± 2.2 | 6.08 ± 1.5 | 20.17 ± 3.1 | 17.50 ± 2.7 | 10.33 ± 2.7 | 5.67 ± 3.1 | 4.17 ± 3.2 | 1.50 ± 2.0 | 0 | 0 |
| wt | 5.4 × 106 ± 1.6 × 107 | 0.3 ± 0.9 | 13.42 ± 1.4 | 10.33 ± 1.4 | 6.58 ± 1.2 | 5.33 ± 0.9 | 19.50 ± 2.1 | 15.08 ± 2.7 | 9.92 ± 2.2 | 4.00 ± 2.3 | 4.50 ± 2.4 | 1.63 ± 1.7 | 0 | 0 |
| 24 h | ||||||||||||||
| gbpA mutant | 1.6 × 109 ± 2.2 × 109 | 31.6 ± 17.7 | 16.27 ± 1.7 | 12.73 ± 1.8 | 8.91 ± 1.9 | 5.73 ± 2.2 | 19.72 ± 1.6 | 16.36 ± 1.9 | 10.73 ± 1.9 | 6.73 ± 1.3 | 4.18 ± 1.4 | 1.50 ± 1.4 | 0.50 ± 0.8 | 0 |
| wt | 9.6 × 108 ± 1.4 × 109 | <0.1 | 15.64 ± 1.2 | 13.09 ± 1.3 | 9.73 ± 1.5 | 6.73 ± 1.5 | 21.09 ± 2.0 | 17.18 ± 2.2 | 10.82 ± 2.6 | 6.73 ± 1.9 | 6.36 ± 1.5 | 2.57 ± 1.4 | 0.17 ± 0.4 | 0 |
For each experiment, groups of six rats were tested for each strain within each diet group (animals fed for 6 h [6 h] or ad libitum [24 h]). Data shown here are the culmination of two separate experiments. Boldface numbers are statistically higher (P < 0.05) than those of the isogenic counterpart in the same diet group.
Caries scores as determined by the method of Keyes (6) indicate the relative depths of the carious lesions and the relative areas of the molar surfaces affected by the lesions (numerical score). Abbreviations: E, enamel involvement; Ds, slight dentinal involvement; Dm, moderate dentinal involvement; Dx, extensive dentinal involvement.
S. mutans recovered from rat molar surfaces prior to caries scoring.
Percentage of S. mutans organisms recovered from rat molar surfaces displaying a smooth morphology on MS agar.
It had been predicted that GbpA was a virulence factor whose inactivation would result in a reduction of caries. In contrast, Table 2 shows that under a restricted feeding regimen the gbpA mutant strain had an increased virulence that reached statistical significance on the buccal surfaces. The sulcal caries scores tended to be higher for the gbpA mutant strain, but these differences were not statistically significant according to the Student t test. With the exception of enamel involvement of the proximal surfaces, in which the caries score of the gbpA mutant group was significantly lower than that of the wt, no significant differences in cariogenicity were observed when the animals had unrestricted access to the caries-promoting diet. A likely explanation for the different results obtained from the restricted versus the unrestricted diets will be addressed below.
Postinfection colony morphology of S. mutans.
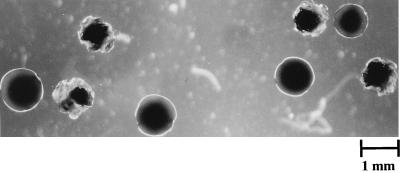
At the termination of the cariogenicity experiment, oral flora of the rats was plated on MS agar for enumeration. Examination of colony morphology revealed that a significant portion of the colonies recovered from the gbpA mutant-infected rats displayed a smooth morphology (Fig. 1), whereas the incidence of recoverable smooth colonies from the wt-infected rats was less than 1%. Retrospective analysis of the wt and gbpA mutant cultures from which the initial rat inocula were generated revealed negligible levels of smooth colonies in either population, suggesting that the accumulation of the smooth colonies was an in vivo event. To confirm this hypothesis, the cariogenicity experiment was repeated with inocula generated from a single isolated rough colony of either UA130 gbpA mutant or wt. Examination of postinfection colony morphology again revealed that smooth colonies accumulated in vivo, specifically in the gbpA mutant populations. This phenomenon was observed in both the restricted and the unrestricted feeding groups, and the differences between wt and gbpA mutant smooth colony frequencies were statistically significant (Table 2). Repeated streaking of these smooth colonies on MS agar revealed that the smooth phenotype was stable, with no smooth-to-rough reversions observed.
FIG. 1.
Colony morphology of gbpA mutant S. mutans UA130 recovered 36 days postinfection from monoinfected gnotobiotic rats fed ad libitum.
Molecular basis of the smooth colony phenotype.
We compared the relative contributions of GbpA absence and the smooth phenotype to sucrose-dependent adherence in a modification of the hydroxyapatite-coated microtiter plate adherence assay described by Schilling et al. (17). As was found with adherence to glass (Table 1, data for rinsed, rotated group), the rough wt strain displayed 68% of the adherence of the rough gbpA mutant strain. No significant differences in adherence between the smooth gbpA mutant strain and the smooth wt strain were found, suggesting that the effect of GbpA absence is dependent upon the presence of the rough phenotype. The smooth strains of S. mutans wt and gbpA mutant were reduced in sucrose-dependent adherence by 72 and 78%, respectively, relative to their rough counterparts.
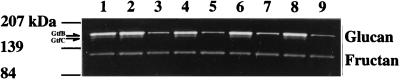
Investigation by quantitative activity gel electrophoresis revealed smooth colony variants to have 15 to 20% of the GTF activity of their rough counterparts. This decrease in enzyme activity correlated with a specific decrease of GTF protein levels as revealed by Coomassie blue staining of SDS-PAGE gels (data not shown). Figure 2 shows the GTF activity of gbpA mutant S. mutans UA130 rough and smooth colonies. Two bands of GTF activity (GtfB and GtfC) were found in cell-associated proteins of rough colonies, whereas one GTF activity band was found in smooth colony cell-associated protein preparations. Because the enzymatic activity of GtfB produces only insoluble glucan, whereas the activity of GtfC produces both soluble and insoluble glucan (23), the GtfC glucan band is fainter than that of GtfB. No differences in fructosyltransferase activity were observed between rough and smooth colonies. To date, all smooth colony variants of a given strain appear to possess similar levels of GTF activity.
FIG. 2.
GTF activities of gbpA mutant S. mutans UA130 rough and smooth colonies. Equivalent levels of cell-associated proteins were resolved by SDS-PAGE and incubated at 37°C in PBS, pH 6.5, containing sucrose and Triton X-100. Gels were photographed against a black background. Positions of SDS-PAGE molecular mass standards and GTF activities are indicated in the left margin. Positions of glucan and fructan are indicated in the right margin. Lane 1, preinoculum UA130. Lanes 2 to 9, colonies recovered from four rats (A2 to A5) 36 days postinoculation; 2, A2-1 rough; 3, A2-1 smooth; 4, A3-1 rough; 5, A3-1 smooth; 6, A4-1 rough; 7, A4-1 smooth; 8, A5-1 rough; 9, A5-1 smooth.
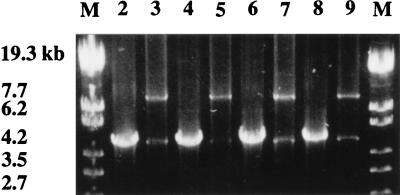
Kuramitsu and colleagues have reported that smooth, colonization-defective colonies arise in vitro at a frequency of 1 × 10−3 to 3 × 10−3 as the result of recombination events involving the highly homologous, tandemly arranged gtfB and gtfC genes (12, 22, 25). On the basis of these reports, we examined the gtfBC region of our rough and smooth colonies by long-range PCR. Specific primers were designed to hybridize upstream of gtfB and directly downstream of gtfC with a predicted PCR product of 10 kb from an intact gtfB-gtfC template. Primer design was based on the published sequence of the gtfB and gtfC genes of S. mutans GS-5 (18, 23). Two PCR products, one of 10 and one of 5.3 kb, were amplified from chromosomal DNA isolated from rough colonies, whereas one product of 5.3 kb was amplified from the chromosomal DNA of smooth colonies (Fig. 3). Increasing the annealing temperature did not reduce the yield of the 5.3-kb product, suggesting that the 5.3-kb product was not the result of false priming. Both the 10- and the 5.3-kb products hybridized with a gtfB-derived probe when subjected to Southern hybridization analysis (data not shown). Southern analysis of SphI-digested chromosomal DNAs probed with a gtfB-derived probe revealed a 5.8-kb band in DNA from smooth colonies and a 10.8-kb band present in DNA from rough colonies (data not shown). From these results, we concluded that the 10-kb product was amplified from an intact gtfB-gtfC template and the 5.3-kb product was amplified from the recombinant fusion gene gtfBC. With 0.1 to 0.3% of the S. mutans population being gtfBC recombinant (12), it seemed logical that, with the inherent preferential amplification of smaller PCR products, the 5.3-kb PCR product would be found even in a population arising from a single isolated rough colony. Significantly, the 10-kb product has not been amplified from the chromosomal DNA of smooth colonies even under the least stringent conditions tested. All smooth colony isolates to date have displayed the reduced GTF phenotype and the recombinant gtfBC genotype.
FIG. 3.
Amplification of the gtfB-gtfC region of gbpA mutant S. mutans UA130 rough and smooth colonies. Long-range PCR and electrophoresis were carried out as described in the text. The sizes (in kilobases) of StyI-digested lambda DNA (lanes M) are indicated in the left margin. Lanes 2 to 9, colonies recovered from four rats (A2 to A5) 36 days postinoculation; 2, A2-1 smooth; 3, A2-1 rough; 4, A3-1 smooth; 5, A3-1 rough; 6, A4-1 smooth; 7, A4-1 rough; 8, A5-1 smooth; 9, A5-1 rough.
Ueda and Kuramitsu have reported that, within Escherichia coli, recombination involving plasmid-borne gtfB and gtfC genes was mediated by homology (RecA dependent) and yielded gtfBC gene fusions with a variety of recombination sites (22). Preliminary work in our laboratory suggests that several independently isolated gtfBC fusion genes from various S. mutans strains and genetic backgrounds (including recA) are homogeneous.
gtfBC recombination and the absence of GbpA have opposing effects.
The caries data in Table 2 suggested that the absence of GbpA promoted virulence only when sucrose intake was restricted. Since gtfBC recombinant organisms have been shown to be less cariogenic (25), it seemed likely that the absence of GbpA potentiated caries development in both diets but that this effect was mitigated in the 24-h diet by the increased frequency of gtfBC recombinants. To investigate whether a high incidence of gtfBC recombination could suppress the cariogenic potential of gbpA mutant organisms, we reexamined the data from gbpA mutant-infected rats on the restricted diet. Reevaluation of this data set was possible because the incidence of recombination ranged from less than 1% to 35%. By segregating the data at the mean level of recombination for this data set (14.9% [Table 2]), it was possible to examine the 6-h gbpA mutant data set as a subgroup with high levels (mean, 22.33%) and a subgroup with low levels (mean, 0.17%) of recombination. A similar dissection of the data from the unrestricted diet groups was not possible because too few of the gbpA mutant groups had low levels of gtfBC recombination. Table 3 shows the caries scores of restricted-diet-fed rats infected with UA130 wt, highly recombinant gbpA mutant, and gbpA mutant with a wt level of recombination. No significant differences in colonization were found among these groups. In the absence of significant levels of gtfBC recombination, gbpA mutant S. mutans was significantly more cariogenic than wt S. mutans on buccal and sulcal surfaces. As expected, a high incidence of gtfBC recombinants within the gbpA mutant group reduced cariogenicity to near-wt levels. With the exception of enamel involvement of the proximal surfaces, the caries scores for the wt and gbpA mutant highly recombinant groups are not statistically different. It appears that the absence of GbpA increases cariogenicity and that gbpA mutant plaque accumulates gtfBC recombinant organisms which have reduced cariogenic potential.
TABLE 3.
Virulence of S. mutans UA130 as a function of GbpA and gtfBC recombinant incidencea
| Strain | Cell recoveryc (mean ± SD)
|
Mean caries score ± SDb
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buccal
|
Sulcal
|
Proximal
|
||||||||||||
| % Smooth | CFU/ml | E | Ds | Dm | Dx | E | Ds | Dm | Dx | E | Ds | Dm | Dx | |
| wt | 0.3 ± 0.9 | 5.4 × 106 ± 1.6 × 107 | 13.42 ± 1.4 | 10.33 ± 1.4 | 6.58d ± 1.2 | 5.33 ± 0.9 | 19.50 ± 2.1 | 15.08 ± 2.7 | 9.92 ± 2.2 | 4.00 ± 2.3 | 4.50e ± 2.4 | 1.63 ± 1.7 | 0 | 0 |
| gbpA mutant | ||||||||||||||
| High gtfBC | 22.33 ± 8.3 | 5.7 × 105 ± 2.9 × 105 | 14.50 ± 2.1 | 11.17 ± 1.5 | 7.83 ± 1.9 | 5.83 ± 0.8 | 17.83 ± 2.6 | 15.67 ± 2.4 | 8.67d ± 2.6 | 3.33 ± 1.9 | 1.67d,e ± 2.6 | 0 | 0 | 0 |
| Low gtfBC | 0.17 ± 0.3 | 4.0 × 106 ± 5.0 × 106 | 17.67 ± 2.3 | 13.83 ± 1.7 | 9.83d ± 2.1 | 6.3 ± 2.0 | 22.50 ± 0.8 | 19.33 ± 1.3 | 12.00d ± 1.5 | 8.00 ± 2.1 | 6.67d ± 1.0 | 0 | 0 | 0 |
Data from the S. mutans UA130 wt- and gbpA mutant-infected rats from the restricted diet group. Data from the gbpA mutant-infected rats were divided at the mean level of recombination (14.9%) into a group with a high proportion of gtfBC recombinants (High gtfBC) (n = 6) and a group with a low incidence of recombinants (Low gtfBC) (n = 6). Boldface numbers are statistically higher (P < 0.05) than all others in the same column.
Caries scores as determined by the method of Keyes (6) indicate the relative depths of the carious lesions and the relative areas of the molar surfaces affected by the lesions (numerical score). Abbreviations: E, enamel involvement; Ds, slight dentinal involvement; Dm, moderate dentinal involvement; Dx, extensive dentinal involvement.
S. mutans organisms recovered from rat molar surfaces prior to caries scoring.
Scores within the same column bearing the superscript d are statistically different from each other (P < 0.05).
Scores within the same column bearing the superscript e are statistically different from each other (P < 0.05).
DISCUSSION
The nonenzymatic GBPs of S. mutans have been shown to have properties presumed to be associated with cariogenicity; however, there has been no experimental verification of a role in virulence. To our knowledge, this report is the first direct demonstration of a role for a nonenzymatic GBP in the cariogenicity of S. mutans.
Based on the fact that the C terminus of GbpA bears homology to the C termini of the GTFs and shares the capacity to bind glucan, it has been hypothesized that GbpA may promote sucrose-dependent adherence and the cohesiveness of plaque and therefore contribute to caries formation. Several reports have provided evidence for these hypotheses. Anti-GbpA serum was found to reduce sucrose-dependent adherence of S. mutans (4), and a chemically generated GbpA mutant which displayed a smooth phenotype was reduced in sucrose-dependent adherence (15). On the basis of these observations, it was expected that a gbpA mutant strain of S. mutans would be reduced in cariogenicity. Surprisingly, our data show that the absence of GbpA enhanced virulence.
We propose that the enhanced virulence of gbpA mutant S. mutans results from a change in plaque structure. Several observations from this and other laboratories are consistent with this hypothesis. It has previously been observed that the sucrose-induced aggregation of a gbpA mutant results in a fluffier, less densely packed plaque (1). Preliminary results suggest that overexpression of GbpA in a gtfBC recombinant background restores the rough colony morphology phenotype (unpublished results). These observations suggest that GbpA contributes to the development of cohesive, densely packed plaque. The observation that the sucrose-dependent adherence of rotationally grown wt S. mutans is susceptible to the increased mechanical stress of vortexing whereas the adherence of rotationally grown gbpA mutant S. mutans is resistant (Table 1) suggests that GbpA affects the mechanical integrity of plaque. We believe that wt organisms were more susceptible to the shearing forces of rotation and vortexing due to the formation of larger and more cohesive aggregates and that organisms associated with a less cohesive gbpA mutant plaque were not as prone to dislodgment by these shearing forces, because smaller, less cohesive aggregates could break away without affecting adjacent bacteria. The fact that the gbpA mutant strain colonized the rat molars to a similar degree as the wt but was more cariogenic (Tables 2 and 3) suggests that the gbpA mutant strain is hypercariogenic. We have found no differences in the acidogenicities of gbpA mutant and wt S. mutans in batch culture, suggesting that it is the gbpA mutant plaque and not the gbpA mutant cell that imparts hypercariogenicity.
On the basis of these data, we believe that GbpA contributes to the cell density and cohesiveness of S. mutans plaque. We suspect that the hypercariogenicity of gbpA mutant plaque is a result of greater plaque porosity which facilitates more rapid and sustainable nutrient influx to deeper levels of the plaque biofilm, allowing for sustained generation of an acidic, cariogenic microenvironment. Work by Van Houte et al. (24) has shown both in vitro and in vivo that a decrease in S. mutans cell density resulted in an increased porosity which was associated with an increase in the acidogenicity and demineralization capacity of the S. mutans cell mass. Assays to directly measure the acidogenic potential of wt and gbpA mutant plaque are being carried out.
Perhaps the most striking result presented in this work was the propensity of gbpA mutant plaque to become enriched in vivo with organisms which had undergone recombination of the gtfB and gtfC genes. For us to be absolutely confident of this result, the cariogenicity studies were repeated with inocula specifically generated from single isolated rough (nonrecombinant) colonies. Both trials of the animal studies revealed an enrichment of gtfBC recombinant organisms specifically in gbpA mutant S. mutans. The increased incidence of recombinant organisms recovered from gbpA mutant plaque could have resulted from either an increased frequency of recombination or a selection for recombinant organisms. Analysis of the recombination frequencies of recA mutant, gbpA mutant, and wt S. mutans in batch culture and in a plaque model is in progress. In either case, the gbpA mutant plaque favored the accumulation of gtfBC organisms whereas the incidence of recombinant organisms recovered from the wt plaque was in agreement with the previously reported in vitro spontaneous recombination frequency of 0.1 to 0.3% (12, 22). The present report is the first to describe an in vivo accumulation of the virulence-attenuating recombinations involving the gtfB and grfC genes. Although at this time we cannot rule out other unknown functions of GbpA, whose absence may have favored the accumulation of recombinant organisms, we hypothesize that recombinant organisms accumulated in response to the altered plaque deposited by gbpA mutant S. mutans. Perhaps the hypercariogenic conditions of the gbpA mutant plaque (putatively a sustained lower pH) were also detrimental for the growth and metabolism of S. mutans such that organisms with reduced association with the gbpA mutant plaque were selected for. Alternately, an optimal balance between glucan and GBPs may exist, such that loss of a GBP favors accumulation of organisms producing reduced levels of glucan. Obviously, many questions about the causes, mechanisms, and effects of recombinant organism generation-selection remain, yet the intriguing fact that S. mutans gbpA mutant populations containing significant proportions of recoverable recombinant organisms were not decreased in colonization (Tables 2 and 3) suggests the possibility that recombinant organisms may be more than molecular mistakes and may in fact fill an ecological niche in S. mutans colonization.
ACKNOWLEDGMENTS
We thank Mary M. Vickerman for providing a recA mutant strain of S. mutans 3209, Joseph E. Mazurkiewicz for help with photography, Cecily C. Harmon for expertise with the gnotobiotic rat model, and Charlotte J. Hammond for help with the rat microbiology.
The research efforts of J.A.B. and K.R.O.H. were supported by grant DE10058 from the National Institute of Dental Research. The research efforts of S.M.M. were supported by grants DE09081 and DE08182 from the National Institute of Dental Research.
REFERENCES
- 1.Banas J A, Gilmore K S. Analysis of Streptococcus mutans and Streptococcus downei mutants insertionally inactivated in the gbp and gtfS genes. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 281–283. [Google Scholar]
- 2.Banas J A, Russell R R B, Ferretti J J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas J A, Russell R R B, Ferretti J J. Distribution of the gbp gene of Streptococcus mutans and properties of a GBP mutant. Zentbl Bakteriol Suppl. 1992;22:377–378. [Google Scholar]
- 4.Douglas C W I, Russell R R B. Effects of specific antisera on adherence properties of the oral bacterium Streptococcus mutans. Arch Oral Biol. 1982;27:1039–1045. doi: 10.1016/0003-9969(82)90009-7. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons R L, van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- 6.Keyes P. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958;37:1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- 7.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 9.Michalek S M, McGhee J R, Navia J M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975;12:69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalek S M, McGhee J R, Shiota T, Devenyns D. Low sucrose levels promote extensive Streptococcus mutans-induced dental caries. Infect Immun. 1977;16:712–714. doi: 10.1128/iai.16.2.712-714.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro C L, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry D, Wondrack L M, Kuramitsu H K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983;41:722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell R R B. Glucosyltransferase of Streptococcus mutans. Microbios. 1979;23:135–146. [Google Scholar]
- 14.Russell R R B. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol. 1979;112:197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- 15.Russell R R B, Donald A C, Douglas C W I. Fructosyltransferase activity of a glucan-binding protein from Streptococcus mutans. J Gen Microbiol. 1983;129:3243–3250. doi: 10.1099/00221287-129-10-3243. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Yamamoto Y, Kizaki H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1997;65:668–675. doi: 10.1128/iai.65.2.668-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilling K M, Carson R G, Bosko C A, Golikeri G D, Bruinooge A, Hoyberg K, Waller A M, Hughes N P. A microassay for bacterial adherence to hydroxyapatite. Colloids Surf B. 1994;3:31–38. [Google Scholar]
- 18.Shiroza T, Ueda S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith D J, Akita H, King W F, Taubman M A. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1994;62:2545–2552. doi: 10.1128/iai.62.6.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith D J, Taubman M A. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–3073. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terleckyj B, Willett N P, Shockman G D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda S, Kuramitsu H K. Molecular basis for the spontaneous generation of colonization-defective mutants of Streptococcus mutans. Mol Microbiol. 1988;2:135–140. doi: 10.1111/j.1365-2958.1988.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 23.Ueda S, Shiroza T, Kuramitsu H K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988;69:101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- 24.Van Houte J, Russo J, Prostak K S. Increased pH-lowering ability of Streptococcus mutans cell masses associated with extracellular glucan-rich matrix material and the mechanisms involved. J Dent Res. 1989;68:451–459. doi: 10.1177/00220345890680030301. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita Y, Bowen W H, Kuramitsu H K. Molecular analysis of a Streptococcus mutans strain exhibiting polymorphism in the tandem gtfB and gtfC genes. Infect Immun. 1992;60:1618–1624. doi: 10.1128/iai.60.4.1618-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]