Abstract
In this study, we tested the hypothesis that the immunogenicity and protective efficacy of polysaccharide-protein conjugate vaccines are influenced by three variables: (i) molecular size of the conjugate, (ii) molecular size of the polysaccharide used for conjugation, and (iii) extent of polysaccharide-to-protein cross-linking. Type III group B Streptococcus capsular polysaccharide was linked by reductive amination at multiple sites to tetanus toxoid to create a polysaccharide-protein conjugate (III-TT). A single lot of III-TT was fractionated into small, medium, and large Mr pools. Whereas all three conferred protection in a maternal immunization-neonatal challenge model in mice, the smallest Mr conjugate evoked less polysaccharide-specific immunoglobulin G (IgG) than the two larger Mr conjugates. To test whether the molecular size of the polysaccharide used for conjugation also affected the immunogenicity of the conjugate, vaccines were synthesized using capsular polysaccharides with Mrs of 38,000, 105,000, and 349,000. Polysaccharide-specific IgG responses in mice increased with the Mr of the polysaccharides, and protective efficacy was lower for the smallest polysaccharide conjugate compared to the other two vaccines. Immunogenicity testing of a series of vaccines prepared with different degrees of polysaccharide-to-protein cross-linking demonstrated higher polysaccharide-specific antibody responses as the extent of cross-linking increased. However, opsonic activity was greatest in mouse antiserum raised to a moderately cross-linked conjugate, suggesting that some antibodies evoked by highly cross-linked conjugates were directed to a nonprotective epitope. We conclude that conjugate size, polysaccharide size, and degree of polysaccharide-protein cross-linking influence the immunogenicity and protective efficacy of III-TT conjugate vaccines.
Immunity to infection with many encapsulated bacteria is conferred by antibodies to the capsular polysaccharide. Bacterial polysaccharides have properties of thymus-independent type 2 antigens, namely, restricted immunoglobulin G (IgG) subclass responses, relatively poor generation of memory B cells, and failure to stimulate antibody responses in neonates and in xid mice. Coupling a polysaccharide or component oligosaccharide to a carrier protein to produce a conjugate molecule may result in immunogenic properties more like those of thymus-dependent antigens, including stimulation of higher levels of IgG antibodies, enhanced memory responses, and immunogenicity in infants. However, immunogenicity has varied widely among polysaccharide-protein conjugates, even for conjugates based on the same polysaccharide, an observation that suggests that specific physical-chemical properties of the conjugate affect the polysaccharide-specific immune responses.
In previous studies, the immunogenicity of group B Streptococcus (GBS) capsular polysaccharide antigens has been increased by covalent coupling to proteins to form polysaccharide-protein conjugate vaccines. A vaccine consisting of GBS serotype III polysaccharide coupled to tetanus toxoid (III-TT) elicited a greater antibody response to the type III polysaccharide both in animals (11, 23) and in humans (9) than did uncoupled type III polysaccharide. The type III GBS capsular polysaccharide is a polymer with a large relative molecular mass (Mr) consisting of multiple repeating units of a backbone trisaccharide of glucose–N-acetylglucosamine–galactose and a side chain (linked to the N-acetylglucosamine residue) of sialic acid-galactose. The bonds between the sialic acid carbons 7 and 8 and between carbons 8 and 9 are susceptible to cleavage by periodate, which results in the formation of an aldehyde function at carbon 7 or 8 (6). Aldehyde groups created in this manner on polysaccharides of GBS serve as sites for covalent bonding to free amino groups on proteins (14, 18, 23, 24).
In the present study, we utilized this well-characterized coupling method to synthesize a variety of type III GBS capsular polysaccharide-tetanus toxoid (TT) conjugate vaccines that differed with respect to defined physical-chemical parameters. By comparing immune responses to the vaccines, we determined the effects of molecular size of the conjugate, molecular size of the polysaccharide used for coupling, and degree of capsular polysaccharide-to-protein cross-linking on the immunogenicity and protective efficacy of GBS conjugate vaccines.
MATERIALS AND METHODS
Bacterial strain.
GBS type III strain M781 (23) was used as a source of type III polysaccharide, as the target strain for in vitro opsonophagocytosis assays, and as the challenge strain in vaccine efficacy experiments.
Purification of type III GBS polysaccharide.
All conjugate vaccines were prepared with type III polysaccharide purified from cells or spent culture supernatant fluids of strain M781 using procedures described previously (23).
Preparation of conjugates of different molecular sizes.
Aldehyde groups were formed on approximately 50% of sialic acid residues of a single lot of type III polysaccharide by oxidation with sodium meta-periodate as described previously (23). The oxidation reaction proceeded for 90 min in the dark at room temperature and was stopped by the addition of ethylene glycol. Oxidized polysaccharide was dialyzed against water, filter sterilized, and dried by lyophilization. The degree of sialic acid oxidation was determined by gas-liquid chromatography–mass spectrometry (GC–MS) of trimethylsilyl derivatives (23).
Purified, oxidized type III polysaccharide (36 mg) was coupled to an equal amount of TT by reductive amination with sodium cyanoborohydride, as described previously (23). The progress of conjugation was monitored by gel filtration analysis on a Superose 6 HR 10/30 column by using a fast protein liquid chromatography (FPLC) system (Pharmacia Biotech Inc., Piscataway, N.J.). Sample aliquots of the conjugation reaction mixture were analyzed before and at several points after the addition of sodium cyanoborohydride. Conjugation of polysaccharide to TT was indicated by the progressive increase in the void-volume peak monitored by measurement of UV absorbance at 277 nm. The coupling reaction was considered to be complete when the magnitude of the void-volume peak remained constant for two successive samples. The conjugate was purified and size fractionated into three pools with a Sephacryl S-500 column (1.6 by 90 cm; Pharmacia) equilibrated with phosphate-buffered saline containing 0.025% NaN3. Each of the three size pools was treated with 2 mg of sodium borohydride and incubated at room temperature for 60 min to reduce any remaining aldehydes, dialyzed against sterile water at 4°C, and lyophilized to dryness. The carbohydrate content of each conjugate vaccine was determined in the phenol-sulfuric acid assay (4) with purified type III polysaccharide as the standard, and their protein content was determined by the method of Larson et al. (12) with purified TT monomer as the standard.
Generation of conjugate vaccines using different sizes of type III polysaccharide.
Purified type III polysaccharide (100 mg in 3 ml of 0.1 M sodium phosphate–0.15 M sodium chloride [pH 7.0] [PBS] containing 0.025% NaN3) was loaded on an S-300 HR gel filtration column (2.6 by 91.5 cm; Pharmacia) and fractionated into three equal pools. Each pool was dialyzed against deionized water and lyophilized to dryness. The Mrs of each of the three type III polysaccharide fractions were estimated by FPLC on a Superose 6 HR 10/30 column equilibrated with PBS and standardized with dextrans. Peak Mrs for the three fractions were 349,000, 105,000, and 38,000. Aldehyde groups were formed on approximately 30% of sialic acid residues by oxidation with sodium meta-periodate as described above. The degrees of sialic acid oxidation as determined by GC–MS of trimethylsilyl derivatives (23) for polysaccharides with Mrs of 349,000, 105,000, and 38,000 were 27, 30, and 34%, respectively.
Each size-fractionated, oxidized type III polysaccharide was coupled in a separate reaction to TT by reductive amination with sodium cyanoborohydride, as described above. Conjugates were separated from uncoupled TT and polysaccharide with an S-300 HR column. The void-volume peak was collected, reduced with sodium borohydride, dialyzed against water, passed through a filter (pore size, 0.45 μm), and dried by lyophilization. The carbohydrate and protein contents of each conjugate vaccine were determined as described above.
Preparation of conjugates with different degrees of type III polysaccharide oxidation.
Samples of a single lot of type III polysaccharide were oxidized to various degrees to prepare III-TT conjugates with various degrees of polysaccharide-protein cross-linking: 20-mg samples of polysaccharide were dissolved in 2 ml of water containing 1, 2.5, 5, or 7.5 mM sodium periodate. The reaction mixtures were stirred in the dark for 2 h at room temperature, and each oxidation reaction was quenched with ethylene glycol. The oxidized polysaccharides were dialyzed against water, filter sterilized, and dried by lyophilization. The proportions of oxidized sialic acid residues for the samples were 18, 35, 66, and 89%, as determined by GC–MS of trimethylsilyl derivatives as described above.
Each of the samples of oxidized type III polysaccharide was coupled to TT by reductive amination with sodium cyanoborohydride, as described above. Sodium borohydride (10 mg) was added to each reaction mixture to reduce any remaining aldehydes after conjugation was complete. Conjugates were purified by passage over a Superdex 200 PG column (1.6 by 90 cm; Pharmacia) eluted with 0.15 M NaCl containing 0.01% thimerosal. Fractions corresponding to the void-volume peak were pooled.
Immunization of mice with conjugate vaccines.
Immunization of mice with III-TT conjugates of different Mrs and III-TT conjugates prepared by using polysaccharides of different Mrs is described below in the section on efficacy testing of vaccines. For the four vaccines used to evaluate the effect of cross-linking on immunogenicity (type III polysaccharide in which 18% of the sialic acid residues had been previously oxidized [III18%]-TT, III35%-TT, III66%-TT, and III89%-TT), groups of ten female Swiss Webster mice (4 to 6 weeks old) (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) were injected subcutaneously with 2 μg of native type III polysaccharide or with one of the four III-TT conjugate vaccines (containing approximately 2 μg of conjugated polysaccharide) in a total volume of 0.2 ml in a suspension of aluminum hydroxide gel (Alhydrogel; Superfos Biosector, Vedbaek, Denmark) at 1.0 mg of elemental Al/ml in 0.15 M NaCl containing 0.01% thimerosal. Ten control mice were injected with 0.2 ml of the adjuvant suspension alone. Booster doses in the same amounts were given 21 and 35 days after the initial doses, and the mice were exsanguinated after 45 days. Sera were collected, pooled by groups, and stored at −70°C.
Determination of type III polysaccharide-specific antibody by direct ELISA.
The concentrations of polysaccharide-specific IgG in sera from mice immunized with III-TT conjugate vaccines were determined by a quantitative enzyme-linked immunosorbent assay (ELISA) (5). For some samples, concentrations of polysaccharide-specific IgG subclasses and titers of type III-specific IgM in these sera were also determined. Microtiter plates (Immulon 4; Dynatech Laboratories, Chantilly, Va.) were coated with 0.1 ml of a solution (5 μg/ml) of type III GBS polysaccharide coupled to human serum albumin (III-HSA) in 0.1 M sodium carbonate buffer, pH 9.8. The secondary antibody used in this test was alkaline phosphatase-labeled goat-anti mouse IgG, IgM, IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotechnology Associates Inc., Birmingham, Ala.). The A405 was determined after 1 h of incubation. The IgM titers were reported as the reciprocal dilutions of serum that gave an A405 of 1.0. The levels of type III polysaccharide-specific total IgG and IgG-subclass antibodies in each serum pool were determined by comparison of the A405 for mouse serum with a standard curve calculated from the results of a separate ELISA that used antibody to mouse IgG (Fab specific) coated onto microtiter wells and known concentrations of IgG and IgG-subclass standards (Southern Biotechnology Associates) (5). Standard curves were constructed for IgG and for the different IgG subclasses by binding various concentrations of the appropriate Ig standard to ELISA wells coated with anti-IgG capture antibody. Such a standard curve permitted translation of an absorbance value obtained in the ELISA to an antibody concentration determined from the standard curve.
Competitive ELISA.
Competitive inhibition of the binding of mouse antibody elicited by III18%-TT, III35%-TT, III66%-TT, and III89%-TT vaccines to GBS type III-HSA-coated microtiter plates was assessed by using type III polysaccharide, III66%, III33%, or III14%, or III-HSA as an inhibitor. Antisera were diluted to give an A405 reading of 1.0 in the absence of inhibitor. The percentage of binding inhibition was determined by comparison of the A405 in the presence of an inhibitor with that obtained in its absence.
In vitro opsonophagocytosis assay.
The ability of pooled sera obtained 45 days after the primary vaccination with III18%-TT, III35%-TT, III66%-TT, or III89%-TT to opsonize GBS strain M781 for in vitro killing by human peripheral blood leukocytes was tested as described previously (2). The assay was performed in the presence of 10% human serum previously adsorbed with strain M781 cells and stored at −80°C to preserve complement activity. Results were expressed as the reduction in CFU after 1 h of incubation with mixing at 37°C.
Efficacy of GBS III-TT vaccines in mice.
The protective efficacy of III-TT conjugate vaccines was measured with the maternal immunization-neonatal mouse challenge model of GBS infection, as described previously (15). Each group of three to five adult female CD-1 mice (Charles River Breeding Laboratories, Inc., Wilmington, Mass.) was vaccinated intraperitoneally with 2 μg of one of the nine vaccines in PBS mixed 1:1 with aluminum hydroxide gel (3%) (Alhydrogel) in a total volume of 0.5 ml. Control mice received 2 μg of uncoupled type III polysaccharide mixed with aluminum hydroxide gel or 0.5 ml of saline-aluminum hydroxide gel suspension. Mice received a booster dose of the appropriate vaccine with adjuvant 3 weeks after the primary vaccination. Female mice were mated after receiving the second dose of vaccine (or control preparation). The litters were born 21 to 31 days after mating. Newborn pups less than 48 h old were challenged intraperitoneally with an ordinarily lethal concentration (3.5 × 105 to 7.4 × 105 CFU, or approximately 10,000 × the 50% lethal dose) of GBS strain M781 in 0.05 ml. The survival of pups was assessed 48 h after challenge. Serum for antibody measurements was collected from dams before vaccination and 41 to 49 days after the first dose of vaccine.
Statistical analysis.
The significance of differences in survival among groups in the protection studies was assessed by using Fisher’s exact test; significance of differences in antibody levels among groups was assessed by using the Kruskal-Wallis nonparametric analysis of variance (Instat version 2.0 software; Graphpad Software, Inc., San Diego, Calif.). Results of in vitro opsonophagocytic assays of antisera elicited by III-TT conjugates with different degrees of cross-linking were analyzed, after log10 transformation, by two-way analysis of variance: antiserum preparation and dilution level were main effects, and preparation by dilution interaction was included in the model. Main effect and interaction terms were all significant (P < 0.001), and Bonferroni pairwise comparison t tests indicated that reductions in CFU obtained with the III66%-TT antiserum were significantly greater (total type I error rate for all comparisons fixed at 0.05) than those obtained with any other antiserum (20).
RESULTS
Effect of conjugate size on immunogenicity.
To test the influence of conjugate size on immunogenicity, a III-TT conjugate vaccine was prepared by using type III GBS capsular polysaccharide with an Mr of ∼200,000. Controlled treatment with sodium periodate resulted in oxidation of 50% of the sialic acid residues of the polysaccharide. TT was coupled to the oxidized polysaccharide by reductive amination in the presence of sodium cyanoborohydride. The resulting conjugate vaccine was divided into three fractions by gel filtration chromatography, designated III-TTL (large), III-TTM (medium), and III-TTS (small) (Table 1). FPLC analysis of the purified, size-fractionated conjugate vaccine preparations on an S-500 HR column showed a relative distribution coefficient (Kav) of 0.21 for vaccine III-TTL, 0.41 for vaccine III-TTM, and 0.56 for vaccine III-TTS (Fig. 1). The three vaccines were similar with respect to carbohydrate content, containing 39 to 56% type III polysaccharide and 44 to 61% TT (percent dry weight).
TABLE 1.
Immunogenicity and protective efficacy in mice of GBS type III-TT conjugate vaccines of different molecular sizes
| Vaccine | Polysaccharide size (Mr) | Kava of III-TT conjugate | Concn of polysaccharidespecific IgG (μg/ml)b | No. of survived/challenged mice (%)c |
|---|---|---|---|---|
| III-TTL | 200,000 | 0.21 | 3.6 (2.6 to 46.5) | 64/67 (96) |
| III-TTM | 200,000 | 0.41 | 20.1 (2.1 to 61.3) | 59/62 (95) |
| III-TTS | 200,000 | 0.56 | 1.5 (0.4 to 2.8) | 57/59 (97) |
| Saline | 1/47 (2) |
Determined by gel filtration chromatography using an S-500 HR column.
Median (range) of values from 5 mice per group. Sera from mice (n = 3) obtained before vaccination had a polysaccharide-specific IgG (μg/ml) median level of 0.0.
Survival of pups born to vaccinated dams. Pups were challenged with a single intraperitoneal injection of GBS strain M781 (3.6 × 105 CFU to 6.0 × 105 CFU per pup).
FIG. 1.
Elution profiles (A277) of GBS type III-TT conjugate vaccines on Sephacryl S-500 gel filtration column. Purified III-TTL (upper profile), III-TTM (middle profile), and III-TTS (lower profile) had peak Kav values of 0.21, 0.41, and 0.56, respectively. The void and bed volumes of the column were 74 and 181 ml, respectively.
Antibody responses in mice were greater for the two larger Mr conjugates, III-TTL and III-TTM, than for the smallest conjugate, III-TTS (Table 1) (P = 0.008, Kruskal-Wallis test for variation among group medians). However, all three vaccines were sufficiently immunogenic to elicit protective immunity in a maternal vaccination-neonatal mouse challenge model of GBS infection. Of pups born to dams vaccinated with any of the three conjugate vaccines, 95 to 97% survived lethal challenge with type III GBS, while only 2% of pups born to control dams survived (Table 1).
Effect of polysaccharide size on immunogenicity of the conjugate vaccine.
The experiments described above documented that larger Mr conjugates were more immunogenic. To test whether the size of the polysaccharide used for conjugation also influences immunogenicity, we prepared conjugate vaccines by using polysaccharides of different Mrs. Type III GBS polysaccharide was fractionated into small, medium, and large Mr pools by gel filtration chromatography. The average Mrs of the three pools was determined by FPLC to be 38,000, 105,000, and 349,000, respectively. Each of the size-fractionated polysaccharides was treated with sodium periodate to modify approximately 30% of the sialic acid residues on the polysaccharide. GC–MS analysis of the oxidized polysaccharides confirmed oxidation of 27 to 34% of the sialic acid residues of the three polysaccharide preparations. Conjugation of each, separately, to TT produced conjugate vaccines with similar compositions (54 to 62% carbohydrate) but polysaccharides of different lengths: small (IIIS-TT), medium (IIIM-TT), or large (IIIL-TT). Each of the conjugate vaccines eluted as a broad peak on an S-500 HR column, consistent with a polydisperse molecular size distribution. Peak Kav values were 0.58 for IIIS-TT, 0.56 for IIIM-TT, and 0.52 for IIIL-TT. These Kav values are similar to that of a thyroglobulin standard (Mr, 669,000; Kav = 0.58).
Immunogenicity testing in mice revealed a trend of increasing antibody responses with increasing Mr of the polysaccharide used in the conjugates: the median serum concentration of polysaccharide-specific IgG in mice vaccinated with IIIS-TT was 1.1 μg/ml, while responses were higher to IIIM-TT (1.6 μg/ml) and to IIIL-TT (5.5 μg/ml) (P < 0.001, Kruskal-Wallis test for variation among group medians) (Table 2). The IIIM-TT and IIIL-TT vaccines were 100% protective in the maternal vaccination-neonatal challenge model of GBS disease, whereas only 26 of 44 pups (59%) born to dams vaccinated with IIIS-TT survived challenge (P < 0.001, Fisher’s exact test).
TABLE 2.
Immunogenicity and protective efficacy in mice of GBS type III-TT conjugate vaccines prepared with type III polysaccharides of different molecular sizes
| Vaccine | Polysaccharide size (Mr)a | Concn of polysaccharide-specific IgG (μg/ml)b | No. of survived/challenged (%)c |
|---|---|---|---|
| IIIL-TT | 349,000 | 5.5 (2.4 to 6.9) | 47/47 (100) |
| IIIM-TT | 105,000 | 1.6 (0.7 to 7.7) | 51/51 (100) |
| IIIS-TT | 38,000 | 1.1 (0.01 to 2.2) | 26/44 (59) |
| Saline | 0.0 (0.0 to 0.0) | 1/47 (2) |
Determined by gel filtration chromatography using an S-500 HR gel filtration column.
Median (range) of values from five mice per group.
Survival of pups born to vaccinated dams. Pups were challenged with a single intraperitoneal injection of GBS strain M781 (3.9 × 105 to 7.4 × 105 CFU per pup).
Effects of polysaccharide-protein cross-linking on immunogenicity.
Sialic acid residues present as side chain termini on the type III GBS polysaccharide serve as sites for coupling to the carrier protein. By varying the concentration of sodium periodate, it is possible to control the fraction of sialic acid residues that are oxidized (17). Since each oxidized sialic acid residue can serve as a site for coupling, increasing oxidation of the polysaccharide results in a more highly cross-linked conjugate.
To test the effect of polysaccharide-protein cross-linking on immunogenicity, a series of vaccines was constructed by using type III GBS polysaccharide in which various proportions of the sialic acid residues were modified by periodate oxidation: 18, 35, 66, or 89%. Each of the oxidized polysaccharides was coupled separately to TT to produce a series of III-TT conjugates with different degrees of polysaccharide-protein cross-linking. All four conjugates contained 42 to 50% polysaccharide. Immunogenicity testing of these vaccines in mice demonstrated a striking relationship between the extent of polysaccharide-protein cross-linking and polysaccharide-specific antibody response to the conjugate (Table 3). The log10 polysaccharide-specific IgG level in immune sera was strongly correlated with the degree of sialic acid oxidation, i.e., extent of cross-linking, in the vaccines (r = 0.98). All four vaccines were protective (94 to 100% survival) in the maternal immunization-neonatal mouse challenge model of GBS infection (Table 3).
TABLE 3.
Immunogenicity and protective efficacy in mice of GBS type III-TT conjugate vaccines prepared with different degrees of polysaccharide-protein cross-linking
| Vaccine | Sialic acid residues oxidized (%)a | Concn of polysaccharide-specific IgG (μg/ml)b | No. of survived/challenged mice (%)c |
|---|---|---|---|
| III18%-TT | 18 | 4.0 | 29/29 (100) |
| III35%-TT | 35 | 30.7 | 28/28 (100) |
| III66%-TT | 66 | 143.8 | 32/32 (100) |
| III89%-TT | 89 | 478.8 | 32/34 (94) |
| Saline | 0.1 | 0/34 (0) |
The percentage of sialic acid residues oxidized was determined by GC–MS of trimethylsilyl derivatives. The number of oxidized sialic acid residues determines the extent of polysaccharide-protein cross-linking.
Measurements were made on pooled serum samples (n = 10 mice per group).
Survival of pups born to vaccinated dams. Pups were challenged with a single intraperitoneal injection of GBS strain M781 (3.9 × 105 to 7.4 × 105 CFU per pup).
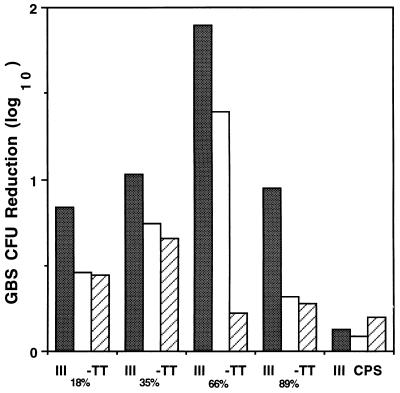
As an additional means of assessing the functional activity of vaccine-induced antibodies, we tested the opsonic power of immune mouse serum in an in vitro opsonophagocytic assay. Pairwise comparison of the extent of opsonophagocytic killing of type III GBS mediated by each of three serum dilutions (1:400, 1:800, and 1:1,600) revealed significantly greater opsonic killing activity in antiserum to III66%-TT than in antiserum to III18%-TT, III35%-TT, or III89%-TT (P < 0.001) (Fig. 2). Thus, while opsonic activity exhibited a pattern similar to the antibody levels measured by ELISA for antisera evoked by the first three conjugates, the opsonic activity of III89%-TT antiserum was unexpectedly low in relation to the concentration of type III polysaccharide-specific antibody in the serum as determined by ELISA.
FIG. 2.
In vitro opsonophagocytic killing of GBS type III strain M781 in the presence of 10% normal human serum as a complement source, human peripheral blood leukocytes, and serum obtained on day 45 from mice immunized with III18%-TT, III35%-TT, III66%-TT, or III89%-TT vaccine or with uncoupled type III polysaccharide (III CPS). Final assay serum dilutions were 1:400 (shaded bars), 1:800 (open bars), and 1:1,600 (hatched bars). Values are the means of duplicate determinations.
The finding that antiserum to a highly cross-linked vaccine had less opsonic power than would have been predicted on the basis of the level of specific IgG antibodies in the serum led us to examine not only IgG but also relative amounts of specific IgM and IgG subclasses in these antisera. The titers of polysaccharide-specific IgM in sera from immunized mice demonstrated a pattern similar to that observed for specific IgG, that is, a progressive increase with increased cross-linking (Table 4). Analysis of the subclass distribution of specific IgG indicated that the increase in specific IgG with increasing cross-linking was attributable primarily to antibodies of the IgG1 subclass. The concentration of specific IgG2 was greater for antiserum from III66%-TT recipients than for III89%-TT. However, it seems unlikely that a relative deficiency of IgG2 antibodies explains the reduced opsonic activity of III89%-TT antiserum since III35%-TT antiserum had fourfold less IgG2 than III89%-TT antiserum but a similar opsonic titer (Table 4).
TABLE 4.
Polysaccharide-specific IgG subclass levels and IgM titers, as determined by ELISA, in sera from mice vaccinated with GBS type III-TT conjugate vaccines with different degrees of polysaccharide-protein cross-linking or with III CPS
| Vaccinea | Polysaccharide-specific IgG subclasses (μg/ml)
|
IgM titerb | |||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | ||
| III18%-TT | 8.0 | 0.3 | 8.9 | 5.5 | 2,090 |
| III35%-TT | 33.8 | 7.9 | 8.0 | 9.2 | 2,650 |
| III66%-TT | 88.0 | 47.9 | 27.3 | 31.2 | 3,340 |
| III89%-TT | 454.4 | 31.7 | 34.2 | 32.5 | 4,530 |
| III CPS | 3.3 | 0.0 | 0.4 | 0.1 | 4,440 |
Vaccines are the same as those described in Table 3.
Reciprocal serum dilution that resulted in an A405 of 1.0, 1 h after addition of substrate.
Effect of conjugate cross-linking on epitope specificity of vaccine-induced antibodies.
The discrepancy between antibody levels measured by ELISA and the opsonic activity of the highly cross-linked III89%-TT vaccine suggested that antibodies evoked by this vaccine had reduced functional activity compared to those evoked by conjugates with a lower degree of cross-linking. A possible mechanism for such a phenomenon is alteration of the polysaccharide epitope in highly cross-linked vaccines such that antibodies evoked by the conjugate bind less avidly to GBS than do antibodies stimulated by less highly cross-linked preparations. To test this hypothesis, ELISA competition experiments were done with either unconjugated GBS type III polysaccharide or III-HSA to compete for binding of vaccine-induced antibodies to III-HSA-coated ELISA wells. For III89%-TT antiserum, unconjugated polysaccharide failed to inhibit antibody binding to III-HSA, even at concentrations as high as 10 μg/ml, consistent with the presence in the III89%-TT antiserum of antibodies directed to an epitope in the conjugate but not in the uncoupled polysaccharide (Fig. 3A). Unconjugated type III polysaccharide in which 66% of the sialic acid residues had been oxidized (III66%) inhibited antibody binding to III-HSA as efficiently as III-HSA itself. The inhibitory activity of type III polysaccharide in which 33 or 14% of the sialic acid residues were oxidized (III33% or III14%) was intermediate between that of III66% and untreated type III polysaccharide. By contrast, for antisera evoked by III18%-TT, by III35%-TT, and by III66%-TT, untreated and oxidized type III polysaccharides were equally efficient inhibitors of antibody binding to III-HSA (Fig. 3B, C, and D, respectively). Thus, antibodies evoked by III89%-TT recognize a novel epitope in oxidized type III polysaccharide, whether coupled to a protein or uncoupled, that is not present in untreated type III polysaccharide.
FIG. 3.
Inhibition of binding of mouse antiserum raised to GBS type III-TT conjugate vaccine III89%-TT (A), III66%-TT (B), III35%-TT (C), or III18%-TT (D) by III CPS (solid triangles), by type III polysaccharide in which 14% (open triangles), 33% (solid squares), or 66% (open squares) of the sialic acid residues were oxidized, or by III-HSA (solid circles) on microtiter wells coated with III-HSA. Values are the means of duplicate determinations.
DISCUSSION
The immunogenicity of several bacterial polysaccharides has been enhanced by coupling of the polysaccharide to a protein (7). However, relatively little is known about the physical-chemical parameters responsible for this effect. Coupling methods have been of two general types (3). In the first method, the polysaccharide is coupled to the protein at a single site, usually with use of a spacer molecule to form a neoglycoprotein. In the second, attachment of polysaccharide to proteins at several sites results in high-molecular-weight lattice-like structures cross-linking several polysaccharide and protein molecules. Both neoglycoproteins and lattice-structured conjugates have been prepared with oligo- or polysaccharides of type III GBS, and both designs have resulted in immunogenicity in animals that is superior to that of uncoupled type III polysaccharide (16, 23). The immunogenicity and efficacy of either type of glycoconjugate may be influenced by several factors, including the size of the polysaccharide, the size of the resulting conjugate, and, in the case of lattice-structured conjugate vaccines, the degree of cross-linking between the polysaccharide and protein components.
Among the parameters that may affect immunogenicity, the molecular size of the saccharide component is the one that has received the most attention. In a study of dextran-protein conjugates in mice, optimum antibody responses were evoked by saccharides with Mrs of 1,000 to 4,000 linked to a carrier protein via the reducing end of the saccharide, while conjugates of larger saccharides, whether end-linked or cross-linked, were less immunogenic (21). Glycoconjugate vaccines against Haemophilus influenzae type b have utilized full-length polysaccharide or derivative oligosaccharides of various sizes. Among end-linked oligosaccharide conjugates, immunogenicity in infants and adults was similar for saccharides of 4 to 12 ribosyl ribitol phosphate repeating units (1). Similarly, in a recent study of end-linked oligosaccharide-protein conjugates against Streptococcus pneumoniae, there was no clear relationship between the molecular size of the saccharide and the immunogenicity of the conjugate in rabbits (10). Paoletti et al. synthesized oligosaccharide-TT conjugates of a similar design using fragments corresponding to 6, 14, or 25 repeating units of the type III GBS polysaccharide (16). Antisera raised in rabbits to the intermediate-size conjugate had the highest protective activity in vivo, reflecting, perhaps, an optimum between opposing trends of enhanced IgG class responses to shorter-chain-length conjugates and increased expression of the conformational epitope of the native polysaccharide in longer-chain-length conjugates (16).
The studies cited above provide some information on the influence of saccharide size on immunogenicity of conjugate vaccines constructed by single-site coupling of a polysaccharide or oligosaccharide to a carrier protein. However, many polysaccharide-protein conjugates are constructed, rather, by linking the polysaccharide to the carrier protein at multiple sites along the saccharide chain. For GBS polysaccharide conjugates, this approach has yielded vaccines that are easier to synthesize than oligosaccharide conjugates with single-site coupling and that are highly immunogenic in animals and human volunteers (9, 16, 23–25). The design of such glycoconjugates results in a number of differences in the structure of the vaccine compared to those prepared by single-site coupling, including the use of a polysaccharide with a higher Mr, close apposition of polysaccharide and protein epitopes at several sites in the conjugate, constraint of polysaccharide flexibility, and creation of lattice-like structures linking several molecules of each vaccine component. Each of these effects may influence immunogenicity by altering epitopes of one of both components or by modifying the interactions between antigen-specific B and T cells.
In the present study, we analyzed the influence of each of three variables on immune responses to GBS type III polysaccharide-protein conjugates constructed by using multisite coupling. To test directly the effect of these three parameters on immunogenicity and efficacy in animals, we synthesized a series of type III GBS polysaccharide-TT conjugate vaccines, utilizing defined components and a standardized coupling technique. Results obtained with conjugate vaccines of differing sizes demonstrated that the larger conjugates were more immunogenic. An effect of molecular size on immunogenicity has been well known for pure polysaccharide antigens; optimal antibody responses generally require immunogens with Mrs of 90,000 or higher (8). Results of the present study support, as well, an effect of molecular size on immunogenicity of polysaccharide-protein conjugate vaccines.
The size of the polysaccharide component of the conjugate also influenced antibody responses, with conjugates constructed by using polysaccharides with higher Mrs evoking higher levels of polysaccharide-specific antibodies. While this effect may be due in part to the fact that larger polysaccharides produced larger conjugates, the effect of polysaccharide size on the overall Mr of the conjugate was quite modest, suggesting that the size of the polysaccharide also influences immunogenicity, independent of effects on conjugate size. Peeters et al. reported similar findings in a study that found superior immunogenicity in mice of a type 4 S. pneumoniae polysaccharide compared to that of a 12-repeating-unit oligosaccharide conjugate vaccine, both prepared by a multisite coupling method (19). Enhancement of immunogenicity with increasing size of the polysaccharide component may reflect optimal expression of the chain-length-dependent epitope of the polysaccharide (22).
An important step in the coupling of GBS polysaccharides to proteins is the formation of aldehyde groups on a selected number of sialic acid residues by oxidation with sodium meta-periodate—sites that are then coupled to amino groups on proteins (23). Since the number of sialic acid residues oxidized determines the number of attachment sites to the carrier protein, it was possible to synthesize III-TT conjugates with different degrees of polysaccharide-protein cross-linking. More highly cross-linked vaccines were more immunogenic, an effect that may be due to stabilization of the conformational epitope of the polysaccharide or to effects on processing, presentation, and recognition of the polysaccharide-protein complex by B and T cells.
Although antibody responses in mice increased with the extent of polysaccharide-protein cross-linking, opsonic activity was greatest in antisera from mice that received a moderately cross-linked conjugate (III66%-TT). This result suggested that both moderately (III66%-TT) and highly (III89%-TT) cross-linked conjugates evoked protective antibodies but that the III89%-TT construct also elicited antibodies that bound to the polysaccharide-protein conjugate but either were not directed to a protective epitope or blocked binding of opsonic antibodies. ELISA competition experiments using native or oxidized type III polysaccharide or III-HSA as an inhibitor supported the former hypothesis, demonstrating that a portion of the antibodies elicited by the highly cross-linked conjugate recognized an epitope induced by extensive oxidation of side chain sialic acid residues of the polysaccharide but not present on the native type III polysaccharide.
Results of these studies indicate that immunogenicity and protective efficacy of GBS III-TT vaccines are influenced by the molecular size of the conjugate, the molecular size of the polysaccharide, and the extent of polysaccharide-protein cross-linking. Although increasing Mr of either the polysaccharide or the conjugate resulted in enhanced immunogenicity, in practice an upper limit is imposed by the Mr of naturally occurring GBS polysaccharides and by the formation of insoluble gels when polysaccharides with very high Mrs are incorporated into highly cross-linked conjugates (13, 26). For GBS III-TT conjugates, cross-linking enhanced immunogenicity, but extensive oxidation of side chain sialic acid residues appeared to distort the epitope of the polysaccharide in highly cross-linked vaccines. We conclude that specific physical-chemical attributes of polysaccharide-protein conjugate vaccines influence immunogenicity and protective efficacy. Systematic study of the relationship between such parameters and immune responses can improve the design of polysaccharide conjugate vaccines.
ACKNOWLEDGMENTS
The GC–MS analysis of oxidized type III polysaccharide was performed by Wei Zou in the laboratory of Harold J. Jennings, National Research Council of Canada, Ottawa. We thank April Blodgett, Julieanne Pinel, Kenneth Johnson, Paul Concannon, and Samuel Moore for expert technical assistance and Vincent J. Carey for assistance with the statistical evaluation of the data.
This work was supported by Public Health Service grant AI23339 and contract AI25152, both from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Anderson P W, Pichichero M E, Stein E C, Porcelli S, Betts R F, Connuck D M, Korones D, Insel R A, Zahradnik J M, Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines of Haemophilus influenzae type b capsular antigen uniterminally coupled to the diphtheria protein crm 197. J Immunol. 1989;142:2464–2468. [PubMed] [Google Scholar]
- 2.Baltimore R S, Kasper D L, Baker C J, Goroff D K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977;118:673–678. [PubMed] [Google Scholar]
- 3.Dick W E, Beurret M. Glycoconjugates of bacterial carbohydrate antigens. In: Cruse J M, Lewis R E Jr, editors. Conjugate vaccines. S. Basel, Switzerland: Karger A. G.; 1989. pp. 48–114. [PubMed] [Google Scholar]
- 4.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 5.Guttormsen H-K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 6.Jennings H J, Lugowski C, Kasper D L. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochemistry. 1981;20:4511–4518. doi: 10.1021/bi00519a001. [DOI] [PubMed] [Google Scholar]
- 7.Jennings H J, Sood R K. Synthetic glycoconjugates as human vaccines. In: Lee Y C, Lee R T, editors. Neoglycoconjugates: preparation and applications. New York, N.Y: Academic Press; 1994. pp. 325–361. [Google Scholar]
- 8.Kabat E A, Bezer A E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958;78:306–318. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- 9.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H-K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laferriere C A, Sood R K, de Muys J-M, Michon F, Jennings H J. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- 11.Lagergard T, Shiloach J, Robbins J B, Schneerson R. Synthesis and immunological properties of conjugates composed of group B Streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990;58:687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson E, Howlett B, Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986;155:243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- 13.Lees A, Nelson B L, Mond J J. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14:190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 14.Madoff L, Paoletti L, Tai J, Kasper D. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J Clin Invest. 1994;94:286–292. doi: 10.1172/JCI117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madoff L C, Michel J L, Gong E W, Rodewald A K, Kasper D L. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect Immun. 1992;60:4989–4994. doi: 10.1128/iai.60.12.4989-4994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paoletti L C, Kasper D L, Michon F, DiFabio J, Jennings H J, Tosteson T D, Wessels M R. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Invest. 1992;89:203–209. doi: 10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paoletti L C, Wessels M R, Kasper D L. Optimization of group B streptococcal glycoconjugate vaccines. In: Chanock R M, Brown F, Ginsberg H S, Norrby E, editors. Vaccines 95. Molecular approaches to the control of infectious diseases. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 213–217. [Google Scholar]
- 18.Paoletti L C, Wessels M R, Michon F, DiFabio J, Jennings H J, Kasper D L. Group B Streptococcus type II polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1992;60:4009–4014. doi: 10.1128/iai.60.10.4009-4014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters C C A M, Tenbergen-Meekes A-M, Poolman J T, Beurret M, Zegers B J M, Rijkers G T. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun. 1991;59:3504–3510. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SAS Institute. SAS STAT user’s guide, version 6. Vol. 1. Cary, N.C: SAS Institute; 1990. [Google Scholar]
- 21.Seppala I, Makela O. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989;143:1259–1264. [PubMed] [Google Scholar]
- 22.Wessels M R, Munoz A, Kasper D L. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci USA. 1987;84:9170–9174. doi: 10.1073/pnas.84.24.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessels M R, Paoletti L C, Pinel J, Kasper D L. Immunogenicity and protective activity in animals of a type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Infect Dis. 1995;171:879–884. doi: 10.1093/infdis/171.4.879. [DOI] [PubMed] [Google Scholar]
- 25.Wessels M R, Paoletti L C, Rodewald A K, Michon F, DiFabio J, Jennings H J, Kasper D L. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–4766. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wessels, M. R., L. C. Paoletti, H.-K. Guttormsen, F. Michon, A. J. D’Ambra, and D. L. Kasper. Unpublished data.