Abstract
Use of FDG-PET to detect vascular inflammation is increasingly common in the clinical management of patients with large-vessel vasculitis (LVV). In this review, the role of FDG-PET to diagnose and monitor vascular disease activity will be detailed. Suggestions how to incorporate FDG-PET imaging into a clinical workflow will be provided with emphasis on patient preparation, image acquisition, and image interpretation. If FDG-PET imaging is obtained, multimodal imaging assessment, whereby FDG-PET and non-invasive angiography are obtained concurrently, and correlation of imaging findings with clinical assessment is generally advisable. Consideration of the clinical scenario and treatment status of the patient is important when interpreting vascular FDG-PET findings.
Keywords: PET-CT, Fluorodeoxyglucose, imaging, Takayasu’s arteritis, Giant cell arteritis, Large-Vessel Vasculitis
Clinical Application of FDG-PET in Systemic Vasculitis
Giant cell arteritis (GCA) and Takayasu’s arteritis (TAK) are the two major forms of large-vessel vasculitis (LVV) (1). Vasculitis of the large arteries is also a disease feature of several other conditions, including IGG4-RD, sarcoidosis, Behcet’s disease, relapsing polychondritis, and Cogan’s syndrome. For each of these conditions, clinical assessment of vascular inflammation in the aorta and its primary branches often poses challenges to health care providers because large artery inflammation does not necessarily always produce clinical symptoms. When a patient with LVV is symptomatic, clinical complaints are often the result of ischemia from damage to the large arteries with compromise to the vascular lumen, rather than directly attributable to vascular inflammation. Once a patient experiences significant luminal damage, the therapeutic window to preserve undamaged vasculature may have been missed.
In addition to diagnostic challenges, accurately monitoring these diseases over time can be problematic. Late in the disease course, a patient may complain of chronic symptoms such as vascular claudication, and it may not be apparent to the treating physician whether these symptoms represent ongoing vascular inflammation that may benefit from additional medical therapy versus chronic damage not amenable to therapeutic response (2). Traditionally, acute phase reactant levels, including the erythrocyte sedimentation rate and c-reactive protein, have been used to complement clinical assessment and help guide a physician to differentiate symptoms attributable to active disease versus damage. However, for most forms of LVV, acute phase reactants are not sensitive to represent vascular inflammation and are not always specific for vasculitis (3). Patients with vasculitis can develop progressive vascular damage in absence of clinical symptoms and abnormalities in acute phase reactants (4). Direct assessment of the vasculature by imaging studies in conjunction with clinical and laboratory assessment is generally advisable.
Catheter-based fluoroscopic angiography and non-invasive angiography has been used to visualize vascular damage in LVV. Historically, in practice, use of angiography was largely confined to patients with Takayasu’s arteritis, despite increasing knowledge that conditions like giant cell arteritis may similarly affect the aorta and branch arteries in addition to causing cranial arteritis. In recent years, however, several prominent societies have endorsed expansion of the vascular imaging toolbox for the clinical management of different forms of LVV. The 2021 American College of Radiology Appropriateness Criteria for noncerebral vasculitis designated FDG-PET as “usually appropriate” which is the same designation applied to magnetic resonance angiography or computed tomographic angiography (5). The 2021 American College of Rheumatology/Vasculitis Foundation guidelines for giant cell arteritis and Takayasu’s arteritis conditionally recommended noninvasive vascular imaging of the large arteries, but do not specify a preferential type of imaging, to aid in clinical diagnosis of giant cell arteritis when temporal artery biopsy is not diagnostic (6). The 2018 EULAR recommendations for the management of LVV endorse use of vascular ultrasound over temporal artery biopsy to confirm a diagnosis of giant cell arteritis in cases where there is a high index of clinical suspicion but also recommend that all cases of LVV should be confirmed by biopsy or imaging, including either by ultrasound, angiography, or FDG-PET (7). In 2021, the Center for Medicare and Medicaid Services in the United States removed a clause preventing reimbursement of FDG-PET for non-oncological indications, and clinical access to PET scans to manage patients with LVV is increasing around the country (8). More recently, vascular imaging, including FDG-PET, has been incorporated into the 2022 ACR/EULAR Classification Criteria for Takayasu’s arteritis and giant cell arteritis, meaning that vascular imaging can be used to help classify specific subgroups of patients who have been diagnosed with LVV (9, 10). Diffuse FDG uptake throughout the large arteries or bilateral involvement of the axillary arteries has been associated with giant cell arteritis, whereas FDG PET activity tends to be more focal in patients with Takayasu’s arteritis and typically spares the axillary and vertebral arteries (FIGURE 1) (11).
Figure 1. Typical Patterns of Arterial FDG Uptake in Takayasu’s Arteritis Compared to Giant Cell Arteritis.
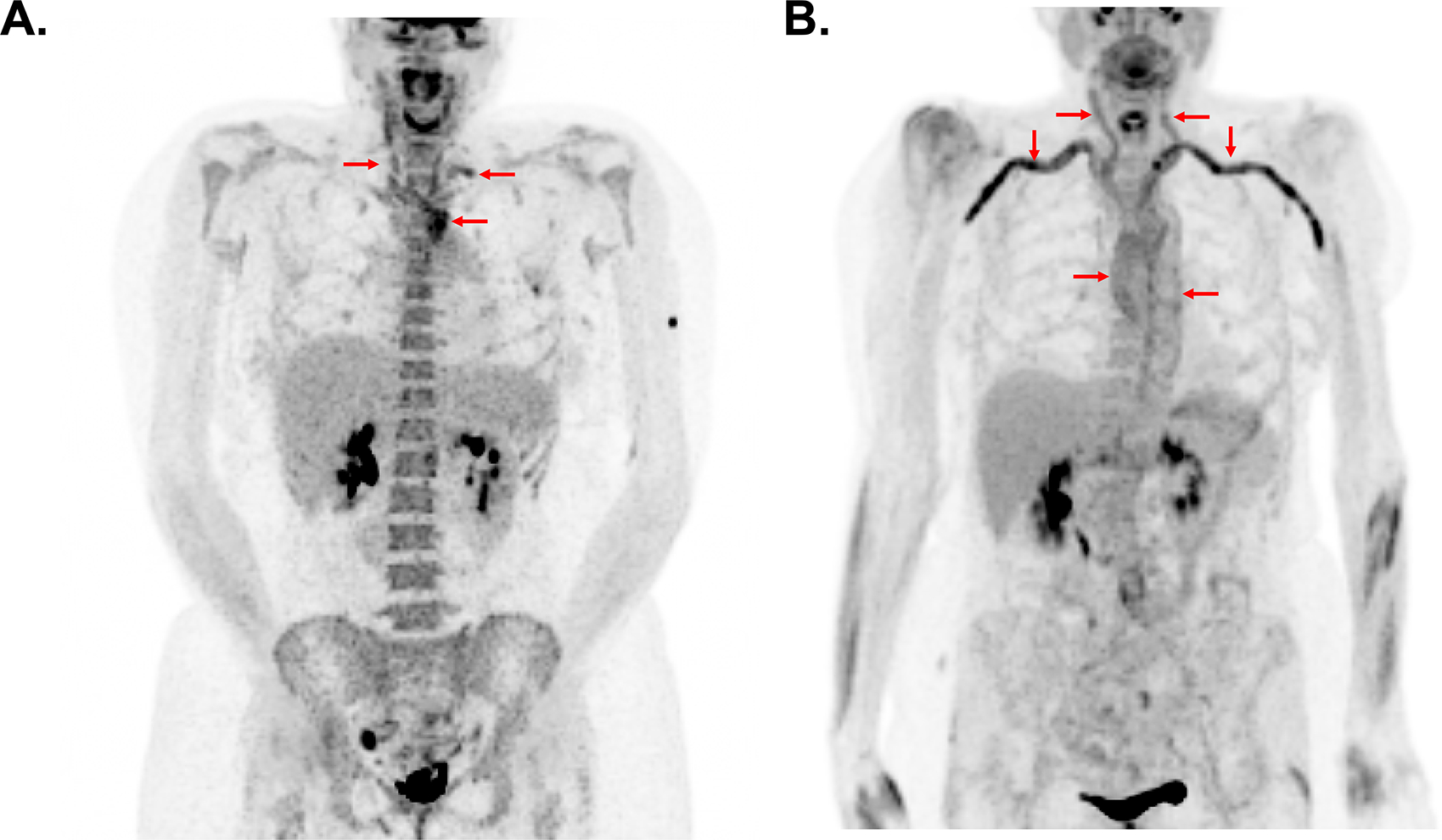
While there is considerable variability in the pattern of vascular inflammation among patients with large-vessel vasculitis, patients with Takayasu’s arteritis commonly have focal areas of FDG uptake in the large arteries and patients with giant cell arteritis frequently have a more diffuse pattern of vascular FDG uptake. In panel A, a patient with Takayasu’s arteritis has intense FDG uptake confined to the aortic arch carotid arteries, and left subclavian artery (arrows). In panel B, a patient with giant cell arteritis has diffuse activity throughout the large arteries with particularly prominent signal in the subclavian and axillary arteries (arrows).
Use of FDG-PET To Diagnose Large-Vessel Vasculitis
Simultaneous acquisition of PET and computed tomographic (CT) or magnetic resonance (MR) imaging provides functional and structural information. PET/MRI may be particularly attractive in younger populations to minimize radiation exposure; however, both PET/CT or PET/MR in general provide equivalent information about vascular FDG uptake. The clinical application of vascular imaging to manage patients with LVV has largely focused on the use of imaging to diagnose these conditions. Three clinical scenarios are commonly encountered when considering use of FDG-PET to diagnose LVV. In the first scenario, a patient presents with clinical symptoms that strongly suggest a diagnosis of LVV and FDG-PET imaging is used to confirm the diagnosis. In this scenario, performance characteristics of FDG-PET are excellent. A recent meta-analysis about the diagnostic role of FDG-PET reported a pooled sensitivity of 76% and specificity of 93% (12). Glucocorticoid use may reduce the sensitivity of FDG-PET; thus, it is generally advisable to perform a PET scan as expeditiously as possible when these diagnoses are considered (13).
In the second clinical scenario, an FDG-PET scan may be ordered to help determine a diagnosis in a patient who has clinical symptoms that are not highly specific for a diagnosis of LVV. For example, an increasing body of literature supports use of FDG-PET in cases where there is fever of unknown origin or inflammation of unknown origin. Results from those studies demonstrate that a large proportion of patients who present with non-specific constitutional symptoms including unexplained fever may eventually be diagnosed with LVV (14, 15). However, interpretation of borderline abnormalities on vascular PET in this context must be performed with caution, as increased FDG uptake in the arterial wall can be seen in atherosclerosis or vascular wall remodeling (16). A clinical diagnosis of LVV that is entertained in this clinical context should be supported whenever possible with complimentary imaging studies such as angiography, or in cases of suspected giant cell arteritis, potentially with a temporal artery biopsy.
Finally, in a time where imaging is increasingly incorporated into diagnostic algorithms, incidental discovery of vascular abnormalities may occur (17). For patients with structural abnormalities of large arteries incidentally noted on non-invasive imaging, use of FDG-PET may help to define these abnormalities further. Similarly, in patients with oncologic conditions who routinely undergo FDG-PET for cancer surveillance, vascular PET abnormalities may be discovered and prompt additional diagnostic considerations. Checkpoint inhibitors and granulocyte-colony stimulating factors, which are routinely used in oncology, can cause aortitis that may be incidentally detected by FDG-PET during routine cancer surveillance (18, 19).
Use of FDG-PET To Monitor LVV
Although FDG-PET can play an important role to diagnose LVV, the utility of serial PET imaging to monitor disease activity is less well defined. In theory, FDG-PET could be useful to detect vascular inflammation and inform treatment decisions prior to the onset of irreversible vascular damage (FIGURE 2). Complicating matters, cross sectional studies demonstrate that a substantial proportion of patients with LVV have imaging evidence of active vasculitis by FDG-PET during periods of otherwise apparent clinical remission (20). These studies are congruent with older autopsy findings that most patients with LVV have vascular inflammation even when vasculitis is believed to be in clinical remission at the time of death (21). Whether therapy should be modified solely based on subclinical imaging findings is unclear, and few prospective observational studies address this important question.
Figure 2. A Conceptual Schema For Changes in Vascular Involvement Over Time in Patients with Large-Vessel Vasculitis.
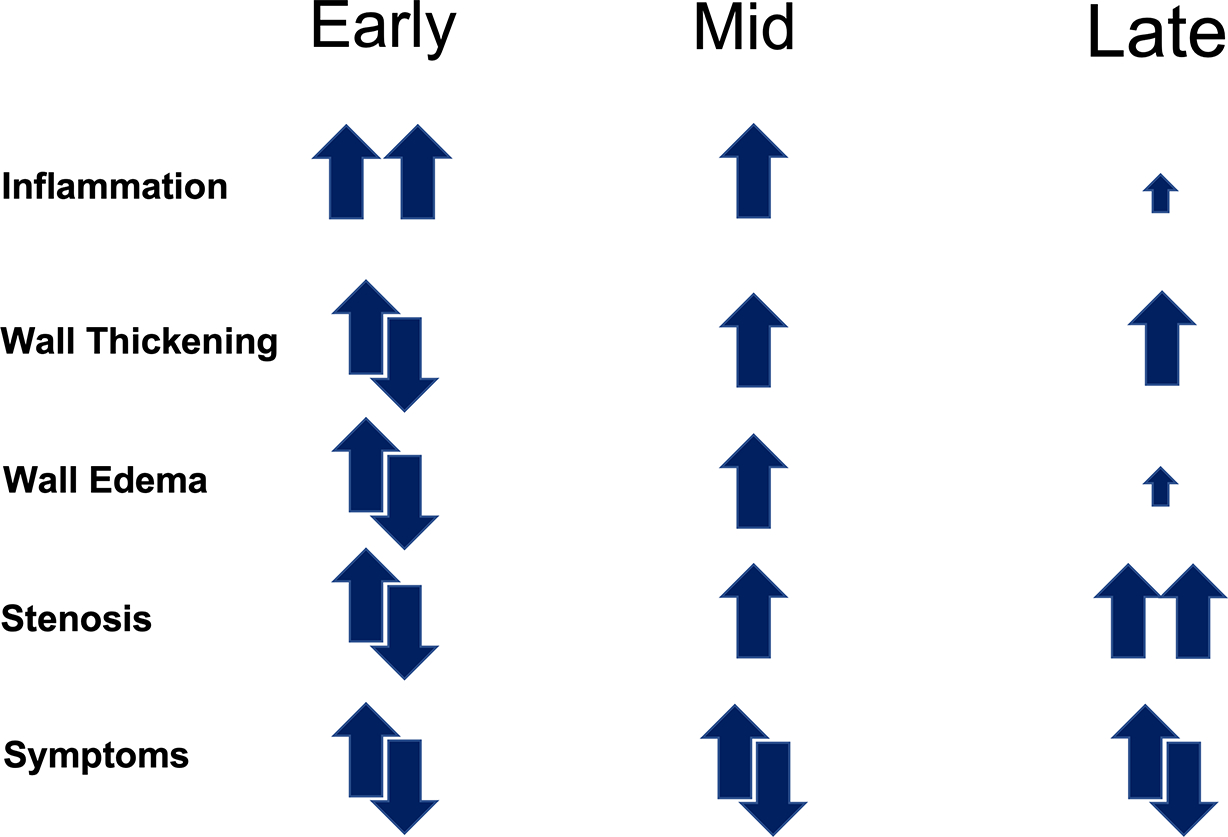
Vascular inflammation is a prominent feature of early disease, while resultant stenosing vascular damage defines the later stages of disease. At all points during the disease course, a patient may or may not experience clinical symptoms.
Recently, a prospective study examined the relationship between FDG-PET activity in specific arterial territories and future risk of angiographic progression of disease defined by stenosis, occlusion, or aneurysm (22). In 38 patients with Takayasu’s arteritis and 32 patients with giant cell arteritis followed for a median of 1.6 years, new areas of luminal pathology only developed in 8 out of 1,091 arterial territories. Most areas where angiographic change occurred were preceded by active vasculitis by FDG-PET; however, angiographic damage did not develop in 92% of arterial regions that had active vasculitis by FDG-PET on baseline imaging. Concomitant vascular edema and wall thickness in arterial regions where there was severe FDG uptake confirmed additional risk for angiographic damage. Although this was a single-center study that should be confirmed in independent cohorts, these findings suggest that development of new areas of vascular damage in patients with LVV is a relatively infrequent occurrence. Thus, reflexive treatment of patients based solely on FDG-PET findings is generally not advisable, and multimodal imaging with complementary FDG-PET and angiography may best delineate high risk vascular lesions (FIGURE 3).
Figure 3. Multimodal Imaging with Angiography and FDG-PET Identifies High Risk Vascular Lesions.
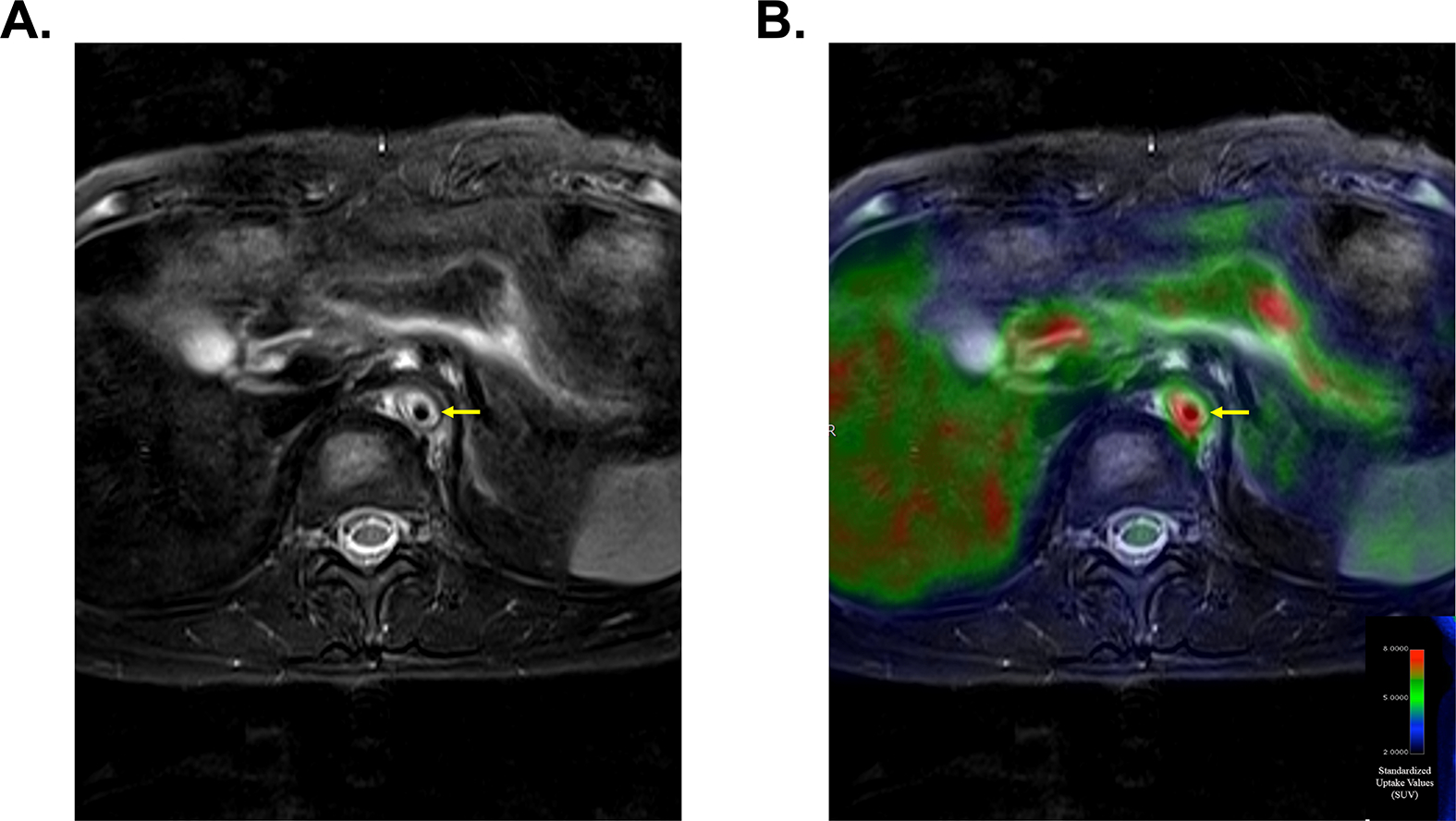
Magnetic resonance imaging demonstrates vascular wall thickening and severe edema involving the descending aorta (Panel A). Concomitant FDG-PET-MR imaging demonstrates severe FDG uptake within the same vascular lesion (Panel B). This vascular area is at high risk for progressive vascular damage.
While guidelines regarding use of serial FDG-PET to monitor patients with LVV are lacking, emerging data suggests that FDG-PET may be useful to monitor treatment response. PETVAS, a summary score of FDG uptake throughout the large arteries, has been increasingly incorporated as an outcome measure of vascular inflammation in observational cohort studies conducted by groups around the world (23–27). Several observational cohort studies have shown that tocilizumab reduces vascular inflammation as measured by PETVAS (26, 28). Incorporation of FDG-PET into future trials may pose logistic challenges, as imaging protocols need to be standardized, but offers potential to measure treatment effect directly at the vascular level. To date, few randomized controlled trials in LVV have met the primary endpoint due in part to the subjective nature of clinical disease activity assessment for these diseases.
Observational studies suggest that FDG-PET may be useful to detect treatment efficacy in an objective way with fewer numbers of patients and may be useful to study to optimal duration of therapy in these patients (28). Whether FDG-PET provides useful prognostic information is unclear. For example, studies have reported conflicted results about whether FDG-PET activity during clinical remission predicts future relapse (20, 24, 29–32).
Novel Radiotracers
Lack of specificity is a potential drawback to the clinical application of FDG in vascular PET imaging. Enhanced glycolysis within the arterial wall could be secondary to inflammation from invading immune cells, vascular remodeling and repair, or other factors including secondary atherosclerosis. Novel radiotracers that target specific immune subsets offer the promise of a more specific in vivo surrogate to histologic assessment. Immune cell subsets contribute to disease pathogenesis in LVV (33). Macrophages and activated T helper cell subsets have been implicated in disease in both TAK and GCA. Various radiotracers beyond FDG have been proposed and are in different stages of clinical and pre-clinical testing across a variety of indications (34, 35). Advanced molecular imaging of macrophage and T cell markers makes theoretical sense in LVV, as these cells are the predominant leukocytes seen in arterial biopsies from patients. Macrophage-targeted imaging includes radiotracers directed against folate receptors, translocator protein (TSPO), mannose receptor CD206, vascular endothelial growth factor (VEGF), and various other chemokine receptors. T-cell targeted imaging includes radiotracers directed against the IL-2 receptor. Whether any of these novel radiotracers will have clinical application in LVV remains to be determined. Two main challenges exist as potential barriers to the preferential use of novel radiotracers in vascular PET: 1) non-specific blood pool activity may be problematic in radiotracers that target hematopoietic cells; and 2) similar to FDG, differentiation of vasculitis from other conditions such atherosclerosis and determining active disease from vascular remodeling may still be challenging even with immune-specific radiotracers.
Practical Application of FDG-PET into a Clinical Workflow
Initial challenges for the routine adoption of FDG PET in the clinical evaluation of LVV involve experience of the referring clinician to order the test in the appropriate clinical scenario, the technical aspects required to perform the studies, and the skills of the reader to interpret the scans accurately. FDG PET requires multi-day patient preparation and a high level of attention to detail. For clinical use, as well as for clinical trials, we follow the guidelines set forth by SNMMI, ACR, and EANM for patient preparation and imaging techniques (36). For image interpretation, we have observed an initial sharp learning curve for the reader when attention is drawn away from the typical disease presentation of hypermetabolic tumors, to instead focus on FDG uptake in the vasculature. Without appropriate patient selection, adequate patient preparation, and training of the reader, there can be increases in cost, effort in scheduling, patient frustration, and diagnostic error.
Patient preparation – Hydration
FDG can be considered a good analogue of glucose. However, one main difference is that FDG is readily eliminated from the human body by the kidneys, like a diabetic patient with hyperglycemia shedding glucose into the urine. The effect is to quicken the biological half-life or “residence time” that FDG exists in the body. Thus, the overall radiation dose is kept low for most patients. We can encourage the elimination of radiation from the patient by assuring good hydration. However, excessive hydration should be avoided to reduce the chance that the patient must leave the imaging table to urinate. A simple explanation to the patient is to consume 12–16 oz only water on the day of their exam. One noteworthy advantage of FDG-PET in comparison to non-invasive angiography with contrast media is that PET can be performed in patients with renal insufficiency (37).
Patient preparation – Diet
Nuclear medicine and radiology departments will likely have different ways to approach diet preparation. It is important to learn how your institution conducts the patient preparation process and to be consistent in how the instructions are presented to the patient. The common instruction is for the patient to consume a “low carbohydrate” diet the day before the exam in addition to fasting on the day of the exam. However, for scheduling purposes it is often necessary to have imaging in the latter part of the day. In such cases, a minimum of six hours fasting prior to injection is recommended. In our experience, more misconceptions occur when the patient decides what a “low carbohydrate diet” is. For instance, some patients may not realize that sauces contain high carbohydrate levels. We often see patients doing well with the instructions to stay well hydrated but misunderstand how to avoid carbohydrates from fluids. There is limited evidence about what artificial sweeteners do to FDG biodistribution, which leads us to recommend elimination of all of them. On the day of the exam, most centers ask patients not to eat at least six hours prior to the FDG injection.
In a fed state, insulin is released to maintain serologic glucose homeostasis and by removing glucose from the bloodstream and storing it within cells. If a patient did not understand the diet instructions or was unable to strictly follow them for any reason, a common image finding will be the appearance of physiologic cardiac activity, as myocytes prefer glucose for ATP production in the fed state. A proper diet preparation will most often result in myocardial suppression. Another common finding in the fed state is strong effect of insulin on GLUT1 receptors in the muscles, which results in sometimes increased diffuse uptake best seen throughout the skeletal muscle. In any case, we attempt to minimize the availability of carbohydrates (simple as well as complex) to mimic a ketogenic diet. Ideal diet preparation results in suppression of normal uptake in the heart and minimal to no uptake in the muscles. This strategy has two primary goals; 1) to make sure that other normal cells in the body are not siphoning FDG away from the target cells of interest (e.g., leukocytes), and 2) to ensure that the normal and abnormal cells are in the same metabolic state each time we image the patient. To evaluate change in FDG uptake at different time points, potential differences in physiologic variables that alter FDG distribution must be reduced or eliminated if possible. There is a spectrum of opinion how strict one needs to be with patient preparation and how the prep should be conducted. To help simplify, we’ve found greater success through telling the patient exactly what they should eat rather than telling them what they should not eat. For example, we have a short list of recommended food items to have the day before the study and on the day of their study. Particularly for clinical trials, but also for routine clinical practice, this diet/fluid strategy is simple to follow and produces high quality quantitative and qualitative results.
Glucocorticoids
Glucocorticoids curtail inflammatory activity visible with FDG PET. However, withholding this often-important medication for imaging purposes should be approached with great caution. Our workflow is to image without modification of medication use, but to thoroughly document and consider potential confounding effect of glucocorticoids during image interpretation. The patient’s medication list should be readily available to the reader at the time of interpretation. Interpretation should be approached differently, as treatment effect may significantly decrease sensitivity of findings. For instance, a technically normal “borderline” study might be reconsidered as consistent with LVV if it is known that the patient was taking high doses of glucocorticoids for an extended duration at the time of the study. One small, randomized study demonstrated that diagnostic utility of FDG-PET for giant cell arteritis declines precipitously after taking prednisone 60mg daily for ten days (13); however, a diagnosis may still be established even in patients on chronic glucocorticoids (38).
Laterality
Issues around the intravenous catheter placement on the day imaging are a source of diagnostic error in many medical scenarios but are particularly important for LVV imaging. Because FDG PET diagnosis is best conducted along with CT or MR angiography, patients will often have multiple modality imaging studies done on the same day, where the same intravenous catheter will serve each modality. We previously reported on the importance of diagnostic errors in MR due to the laterality of intravenous contrast injection (39). These artifacts are due to the interfering high concentration of intravenous contrast in the upper extremity vein immediately adjacent to the artery of interest. The mechanisms for the artifacts in MRI and CT are different, but the detrimental effect on diagnostic confidence is the same. Additionally, if there is the common occurrence of mild extravasation of FDG, lymphatic drainage of FDG can track along the vessels and lead to diagnostic uncertainty (FIGURE 4). The FDG uptake in these cases is often low and is accentuated in nuclear imaging due to the prominence of even very mild/trace extravasations. The volume of extravasations is over-emphasized in the images due to the long period of time (an hour or more) between injection and imaging and the very high sensitivity of PET technology. If you want to evaluate an area where LVV often causes significant issues, such as the subclavian arteries, it is most effective to plan on intravenous catheter placement in the arm that is least affected by disease. If possible, it is best to get all imaging exams on a single day to make sure that each imaging acquisition uses the thoughtful placement of a single intravenous catheter.
Figure 4. Pitfalls to FDG-PET Interpretation.
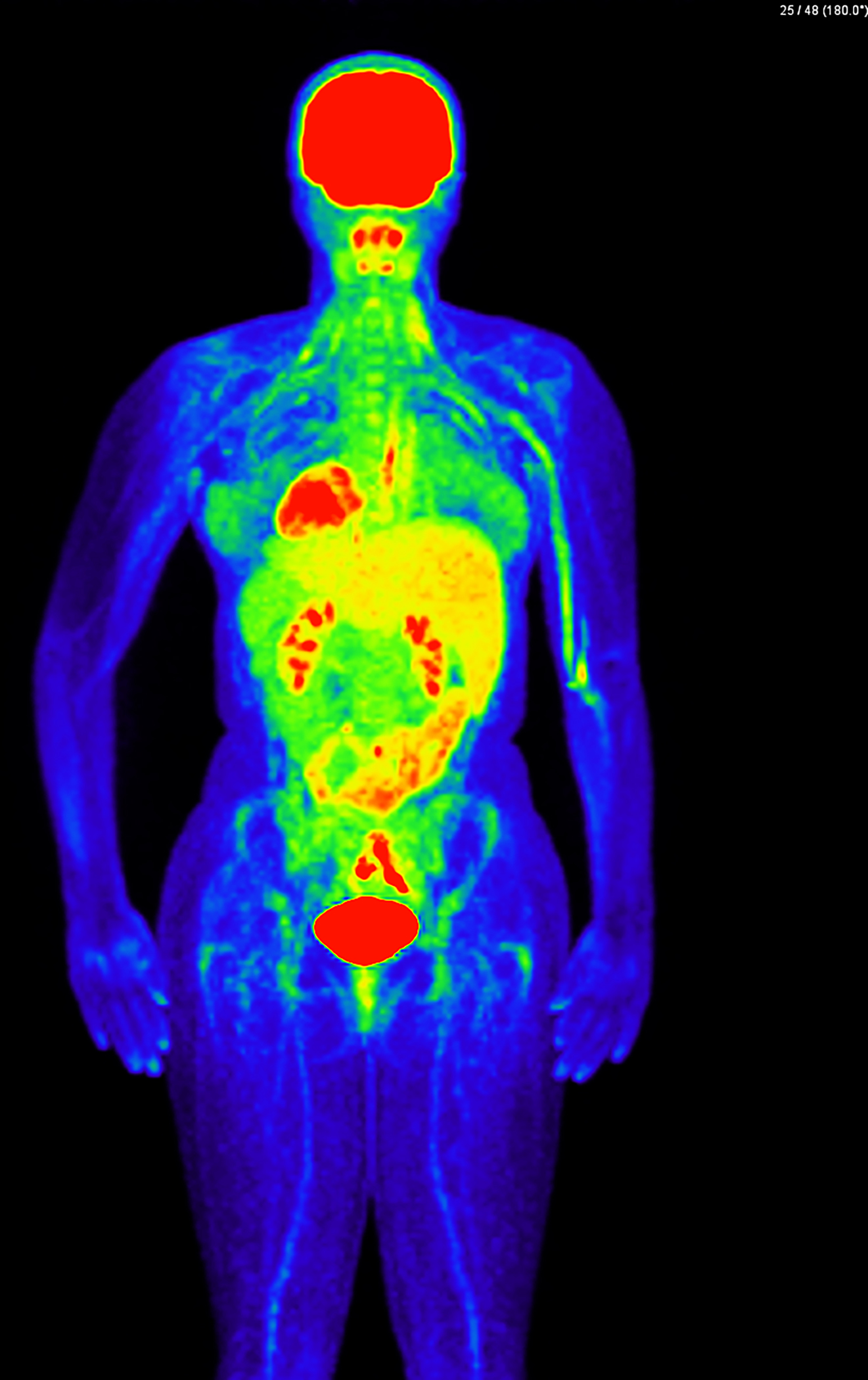
FDG-PET imaging demonstrating extravasation of FDG at the left arm injection site with lymphatic drainage of FDG along the upper extremity vasculature. This finding could be misinterpreted as active vasculitis or interfere with evaluation of true disease of the adjacent axillary and subclavian arteries.
FDG Uptake Time
Uptake time refers to the time interval between FDG injection and image acquisition. We have experience with 60-minute as well as 120-minute uptake times for LVV FDG PET (40). Though most PET imaging facilities are used to 60-minute uptake times for oncologic-based FDG imaging, 120 minutes is preferred in vasculitis to reduce adjacent blood pool activity that can interfere with visualization of the vascular wall (40). Standardizing uptake time across different studies will be essential when comparing qualitative and quantitative results. Given potential limitations of different PET imaging facilities to accommodate alternate uptake times, diagnostic information using either method is more important than strict adherence to a 120-minute uptake time for clinical purposes, but research studies should strongly consider delayed imaging acquisition to generate the highest quality information within a common reference standard. We caution the referring clinician and the image interpreter to take care when comparing scans utilizing differing uptake times and scans performed at different imaging facilities. Without the widespread use of LVV FDG PET in the community, only readers experienced in LVV PET are adequately equipped to reduce (not eliminate) diagnostic issues in this situation. Thus, it is important to prioritize consistency for longitudinal qualitative and quantitative imaging evaluation of chronic diseases.
Image Interpretation
A barrier to routine clinical adoption of LVV FDG PET is that formal standardized image interpretation for FDG LVV does not exist, as it commonly does for new PET radiotracers coming to the market in order to smooth the initial transition to clinical interpretation. We observe that even senior clinicians benefit from a training phase dedicated to LVV FDG PET interpretation showing the variety of disease presentations and methods for image normalization. This remains a needed area of scientific exploration. However, here we outline a few common pitfalls for image interpretation.
If sufficiently FDG-avid, some things can be seen on PET which are too small or obscured to visualize on CT, which may lead some readers to disregard the PET activity. However, our experience with new FDG PET LVV readers and referring clinicians of all specialties and levels of seniority, is that the more common mistake is attributing abnormal uptake when the normal variation of expected vessel wall activity is seen. We find that most PET readers are more comfortable with tumor-level high uptake (SUV greater than 3–5) and less familiar with grading a disease that may be in the 1–2 range of SUV. PET is excellent at depicting higher level of uptake, but low-level uptake can lead the eye to intrinsic image noise that is mistaken for disease and leads to known issues for quantification, particularly when maximum standardized uptake value (SUV) values are used with or without a target-to-background ratio (TBR) (41).
Heeding warnings that low-level activity can be difficult to interpret, inexperienced readers may disregard true abnormal PET activity if there is not a corresponding abnormality on CT or MR imaging. However, we have many examples of profound vascular abnormalities identified by FDG PET where the vessels appear normal on concomitant angiography (FIGURE 5). Some mistakes can be avoided through the understanding of image reproduction at your individual institution. For the purposes of reproducibility of image display and to follow the relative intensity of lesions over time, we commonly rely on the standardized uptake value (SUV). The most common way SUV is calculated is SUV = (pixel intensity * patient body weight) / dose. Pixel intensity represents the number of photons coming out of a volume of imaging space, typically measured as megabecquerels per mL (MBq/mL). Some calculate SUV in terms of patient lean body mass instead of body weight. Others argue that SUV should not be used, and target-to-background (TBR) radios better serve patients. Whatever method is used, it is very important to respect that the SUV or TBR is highly specific to the machine doing the imaging and the technique of image reconstruction from that machine, which will vary according to institution preference. Therefore, scientific literature that proposes specific SUV or TBR thresholds to define active disease in LVV should be interpreted with caution as these values are likely not generalizable to other institutions unless the exact same imaging equipment and acquisition protocols from the source studies are employed. Metrics that report the total volume of inflamed arterial tissue or the total glycolytic activity level within an inflamed region of artery have also been proposed as additional potential quantitative outcome measures (42).
Figure 5. Discordance between angiography and FDG-PET.
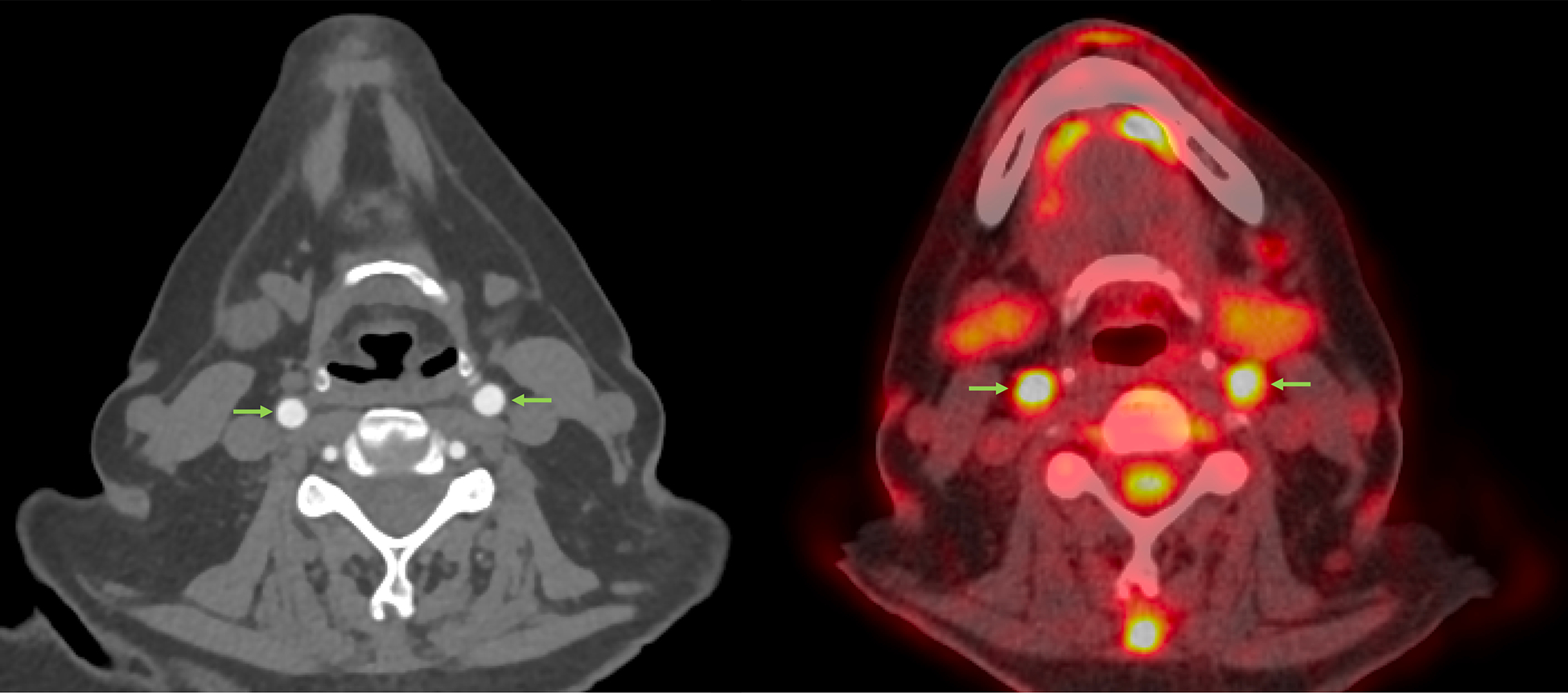
In these axial images obtained at the time of diagnosis in patient with giant cell arteritis, computed tomographic angiography does not demonstrate luminal damage or vascular wall pathology in the carotid arteries; whereas, FDG-PET detects severe abnormal arterial uptake. (arrows).
FIGURE 6 shows two coronal image reconstructions centered over the thoracic descending aorta. The images are reconstructed from the same raw PET data. However, the image on the left (FIGURE 6A) is reconstructed with much more data input (~3–4x more photons) than the image on the right (FIGURE 6B) but from the same raw data source. The differences in image noise in these images are only due to density of data reconstructed, which has a direct result in quantitative values, where higher TBR and SUV are seen with the noisier data. Many reconstruction strategies are employed to mitigate the problem of image noise with PET data. In FIGURE 6B, heterogeneity of FDG uptake in the aorta with higher image noise is seen, which may be mistaken for numerous foci of abnormal activity. However, FIGURE 6A demonstrates that the heterogeneity is due to image noise and not true focal activity. If one were able to give 100x the dose, which is not feasible, we would see significantly more uniform PET images. Issues related to image noise are compounded for overweight patients, which often results in systematically higher measured SUV and TBR for structures within these patients when imaged and measured with contemporary methodology. Thus, when using quantitative information such as SUV and TBR, it is likely only accurate when comparing the same patient longitudinally, if that patient was scanned with the same machine, imaging technique, uptake time, and image processing method.
Figure 6. Image processing influences quantification of arterial FDG uptake.
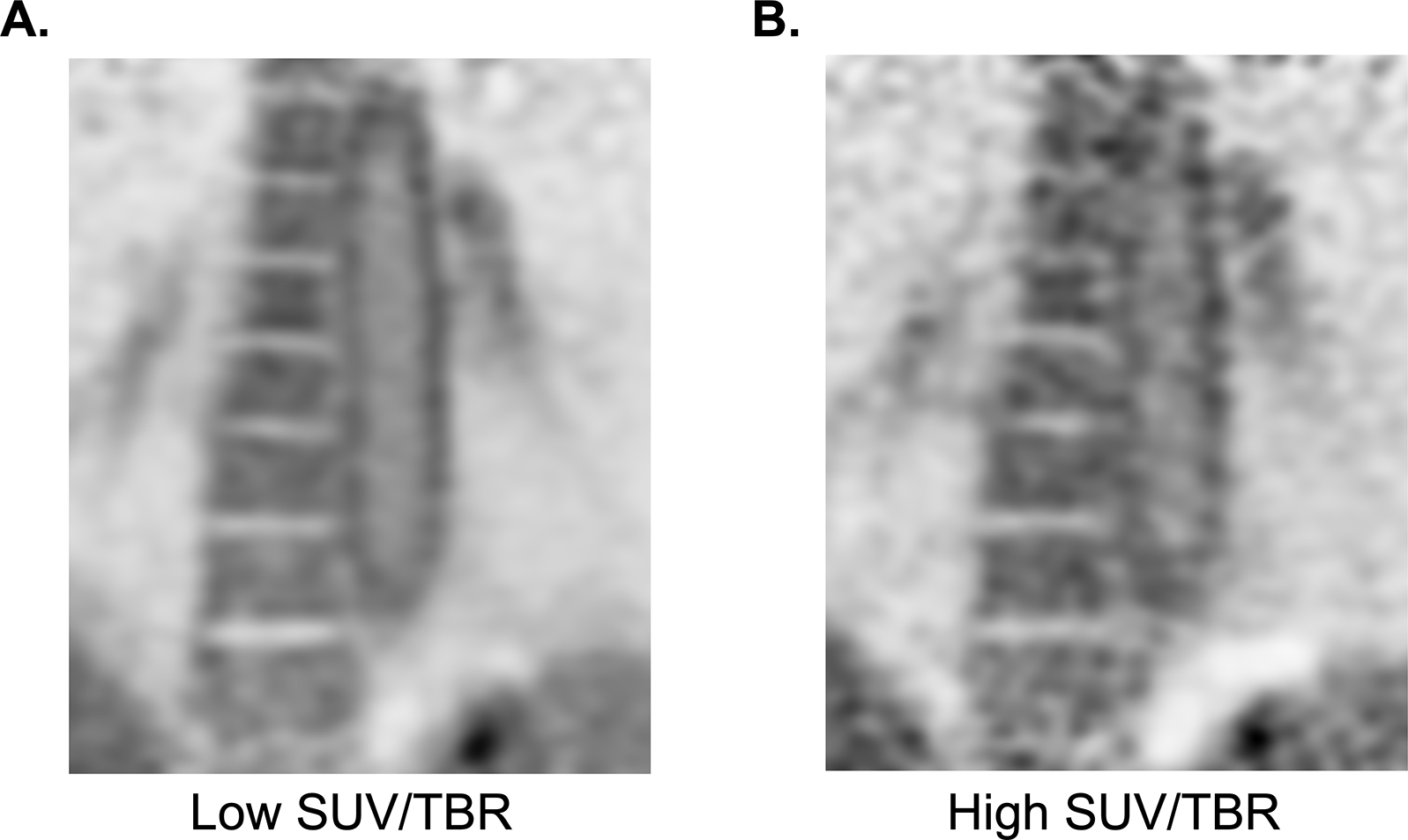
Two coronal reconstructions of the same raw FDG PET data of the descending thoracic aorta. Left frame (A) showing reconstruction of the full time of an extended data acquisition (~20 minutes) compared to the right frame (B) showing the typical acquisition in clinical PET, of no more than 5 minutes per image frame. The typical acquisition results in higher maximum SUV and TBR of structures due to image noise alone because it is sampling the highest point in high statistical noise. This patient does not have a history of LVV, and the activity in the aorta is likely due only to physiologic smooth muscle cell activity. Differentiation with pathologic uptake in this case is due the pan-arterial uptake that scales with relative size of the arteries throughout the patient’s body.
In part because image noise has a great effect on quantitative measurement and because whole-vessel inflammation measurement is a tedious process that may not be feasible for clinical applications, we investigated the use of a qualitative PET Vascular Activity Score (PETVAS) (20). PETVAS has advantages and disadvantages (43). It relies on interpretation of the images, which is subject to the experience and bias of the user. Despite these problems, similar techniques have been validated and adopted for clinical practice for FDG in oncology with lymphoma, where the Deauville score is now a recommended tool for routine clinical FDG PET in that disease (Table 1) (44). Although similar, Deauville has long-standing application within lymphoma and other malignancies, is validated to long-term outcomes and histology, and pulls boolean logic into its calculation. Also dissimilar to PETVAS, the highest score in Deauville can represent very intensely FDG avid disease or that there is new lymphoma or new disease anywhere in the body regardless of intensity. Importantly, these elements are validated in Deauville because both the intensity of the lesion (e.g., tumor grade) and the existence of new disease are directly tied to patient outcomes. Such a multitool is needed in a clinically challenging disease like LVV. Thus far, neither the lesion intensity nor the presence of abnormal FDG-uptake strongly predicts angiographic outcomes. Thus, FDG-PET findings must be interpreted in clinical context and reflexive changes in treatment based on vascular PET abnormalities alone are generally not advisable.
Table 1.
Comparison between the Deauville score for lymphoma and the PETVAS score for large-vessel vasculitis
| Deauville Score for Lymphoma | PETVAS for Large-Vessel Vasculitis* |
|---|---|
|
| |
| 1 no uptake above background | 0 no uptake above background |
| 2 uptake ≤ mediastinum | 1 uptake ≤ mediastinum |
| 3 uptake > mediastinum but ≤ liver | 2 uptake > mediastinum but ≤ liver |
| 4 uptake moderately > liver | 3 uptake > liver |
| 5 uptake markedly > liver or new lesions | No similar score |
| x new areas of uptake unlikely to be related to lymphoma | No similar score |
PETVAS is scored by determining FDG uptake on a 0–3 scale in nine specific arterial territories: ascending aorta, aortic arch, descending thoracic aorta, abdominal aorta, right and left carotid arteries, right and left subclavian arteries, and the branchiocephalic artery. PETVAS is therefore a summary score that ranges from 0–27. A specific scoring threshold does not define “active disease” because vasculitis can be focal or diffuse in an individual patient.
Summary
Incorporation of advanced molecular imaging into a clinical workflow to diagnose and manage patients with LVV is an increasingly important way to complement clinical assessment in these complex diseases. Given the technical challenges with image acquisition and interpretation, close collaboration between the treating physician and the interpreting reader is required to ensure that vascular imaging studies are successfully incorporated into the clinical management of these patients. Advancements in technology and increased research efforts in this area will continue to improve assessment strategies for LVV.
Practice Points:
FDG-PET is useful to assess metabolic activity as a surrogate for vascular inflammation
Patient preparation, image acquisition, and image processing can affect interpretation of FDG-PET images in patients with large-vessel vasculitis
Interpretation of arterial FDG uptake can be challenging for readers of all levels of experience, and understanding diagnostic pitfalls is important to reduce clinical errors
Clinical collaboration between rheumatology and nuclear medicine physicians is important when integrating advanced molecular imaging into the clinical management of patients with large-vessel vasculitis
Research Agenda:
Prospective observational cohort data is needed to address how vascular imaging should be used to monitor patients with large-vessel vasculitis over time
Image acquisition and interpretation should be standardized if FDG-PET is used as an outcome measure in a randomized clinical trial to test therapeutic effect of medications directly at the vascular level
Acknowledgements and Funding:
This study was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH). The authors declare no conflicts of interest.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2.Quinn KA, Alessi HD, Ponte C, Rose E, Ahlman MA, Redmond C, et al. Use of 18F-fluorodeoxyglucose positron emission tomography to standardize clinical trial recruitment in Takayasu’s arteritis. Rheumatology (Oxford). 2022;61(10):4047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56(3):1000–9. [DOI] [PubMed] [Google Scholar]
- 4.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Vascular I, Aghayev A, Steigner ML, Azene EM, Burns J, Chareonthaitawee P, et al. ACR Appropriateness Criteria(R) Noncerebral Vasculitis. J Am Coll Radiol. 2021;18(11S):S380–S93. [DOI] [PubMed] [Google Scholar]
- 6.Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021;73(8):1349–65. [DOI] [PubMed] [Google Scholar]
- 7.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. [DOI] [PubMed] [Google Scholar]
- 8.Wahl RL, Dilsizian V, Palestro CJ. At Last, (18)F-FDG for Inflammation and Infection! J Nucl Med. 2021;62(8):1048–9. [DOI] [PubMed] [Google Scholar]
- 9.Ponte C, Grayson PC, Robson JC, Suppiah R, Gribbons KB, Judge A, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74(12):1881–9. [DOI] [PubMed] [Google Scholar]
- 10.Grayson PC, Ponte C, Suppiah R, Robson JC, Gribbons KB, Judge A, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Takayasu Arteritis. Arthritis Rheumatol. 2022;74(12):1872–80. [DOI] [PubMed] [Google Scholar]
- 11.Gribbons KB, Ponte C, Carette S, Craven A, Cuthbertson D, Hoffman GS, et al. Patterns of Arterial Disease in Takayasu Arteritis and Giant Cell Arteritis. Arthritis Care Res (Hoboken). 2020;72(11):1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YH, Choi SJ, Ji JD, Song GG. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis : A meta-analysis. Z Rheumatol. 2016;75(9):924–31. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P, Hauge EM. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2018;45(7):1119–28. [DOI] [PubMed] [Google Scholar]
- 14.Betrains A, Boeckxstaens L, Moreel L, Wright WF, Blockmans D, Van Laere K, et al. Higher diagnostic yield of 18F-FDG PET in inflammation of unknown origin compared to fever of unknown origin. Eur J Intern Med. 2023;110:71–6. [DOI] [PubMed] [Google Scholar]
- 15.Schonau V, Vogel K, Englbrecht M, Wacker J, Schmidt D, Manger B, et al. The value of (18)F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): data from a prospective study. Ann Rheum Dis. 2018;77(1):70–7. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Bural GG, Torigian DA, Rader DJ, Alavi A. Emerging role of FDG-PET/CT in assessing atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2009;36(1):144–51. [DOI] [PubMed] [Google Scholar]
- 17.Quinn KA, Gribbons KB, Carette S, Cuthbertson D, Khalidi NA, Koening CL, et al. Patterns of clinical presentation in Takayasu’s arteritis. Semin Arthritis Rheum. 2020;50(4):576–81. [DOI] [PubMed] [Google Scholar]
- 18.Parodis I, Dani L, Notarnicola A, Martenhed G, Fernstrom P, Matikas A, et al. G-CSF-induced aortitis: Two cases and review of the literature. Autoimmun Rev. 2019;18(6):615–20. [DOI] [PubMed] [Google Scholar]
- 19.Boland P, Heath J, Sandigursky S. Immune checkpoint inhibitors and vasculitis. Curr Opin Rheumatol. 2020;32(1):53–6. [DOI] [PubMed] [Google Scholar]
- 20.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. (18) F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018;70(3):439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostberg G Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand Suppl. 1972;533:135–59. [PubMed] [Google Scholar]
- 22.Quinn KA, Ahlman MA, Alessi HD, LaValley MP, Neogi T, Marko J, et al. Association of (18) F-Fluorodeoxyglucose-Positron Emission Tomography Activity With Angiographic Progression of Disease in Large Vessel Vasculitis. Arthritis Rheumatol. 2023;75(1):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahra SK, Ozguven S, Unal AU, Oner FA, Ones T, Erdil TY, et al. Assessment of Takayasu arteritis in routine practice with PETVAS, an 18F-FDG PET quantitative scoring tool. Turk J Med Sci. 2022;52(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli E, Muratore F, Mancuso P, Boiardi L, Marvisi C, Besutti G, et al. The role of PET/CT in disease activity assessment in patients with large vessel vasculitis. Rheumatology (Oxford). 2022;61(12):4809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang F, Han Q, Zhou X, Zheng Z, Wang S, Ma W, et al. Performance of the PET vascular activity score (PETVAS) for qualitative and quantitative assessment of inflammatory activity in Takayasu’s arteritis patients. Eur J Nucl Med Mol Imaging. 2020;47(13):3107–17. [DOI] [PubMed] [Google Scholar]
- 26.Schonau V, Roth J, Tascilar K, Corte G, Manger B, Rech J, et al. Resolution of vascular inflammation in patients with new-onset giant cell arteritis: data from the RIGA study. Rheumatology (Oxford). 2021;60(8):3851–61. [DOI] [PubMed] [Google Scholar]
- 27.Park EH, Lee EY, Lee YJ, Ha YJ, Yoo WH, Choi BY, et al. Infliximab biosimilar CT-P13 therapy in patients with Takayasu arteritis with low dose of glucocorticoids: a prospective single-arm study. Rheumatol Int. 2018;38(12):2233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn KA, Dashora H, Novakovich E, Ahlman MA, Grayson PC. Use of 18F-fluorodeoxyglucose positron emission tomography to monitor tocilizumab effect on vascular inflammation in giant cell arteritis. Rheumatology (Oxford). 2021;60(9):4384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7. [DOI] [PubMed] [Google Scholar]
- 30.Sammel AM, Hsiao E, Schembri G, Bailey E, Nguyen K, Brewer J, et al. Cranial and large vessel activity on positron emission tomography scan at diagnosis and 6 months in giant cell arteritis. Int J Rheum Dis. 2020;23(4):582–8. [DOI] [PubMed] [Google Scholar]
- 31.Janes ALF, Castro MF, Arraes AED, Savioli B, Sato EI, de Souza AWS. A retrospective cohort study to assess PET-CT findings and clinical outcomes in Takayasu arteritis: does 18F-fluorodeoxyglucose uptake in arteries predict relapses? Rheumatol Int. 2020;40(7):1123–31. [DOI] [PubMed] [Google Scholar]
- 32.Ma L, Wu B, Sun Y, Ding Z, Dai X, Wang L, et al. PET vascular activity score for predicting new angiographic lesions in patients with Takayasu arteritis: a Chinese cohort study. Rheumatology (Oxford). 2023. [DOI] [PubMed] [Google Scholar]
- 33.Pugh D, Karabayas M, Basu N, Cid MC, Goel R, Goodyear CS, et al. Large-vessel vasculitis. Nat Rev Dis Primers. 2022;7(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Geest KSM, Sandovici M, Nienhuis PH, Slart R, Heeringa P, Brouwer E, et al. Novel PET Imaging of Inflammatory Targets and Cells for the Diagnosis and Monitoring of Giant Cell Arteritis and Polymyalgia Rheumatica. Front Med (Lausanne). 2022;9:902155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corovic A, Wall C, Nus M, Gopalan D, Huang Y, Imaz M, et al. Somatostatin Receptor PET/MR Imaging of Inflammation in Patients With Large Vessel Vasculitis and Atherosclerosis. J Am Coll Cardiol. 2023;81(4):336–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slart R, Writing g, Reviewer g, Members of EC, Members of EI, Inflammation, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campochiaro C, Misra DP. PET in Takayasu arteritis: onwards and upwards towards a future of robust multimodality disease activity assessment? Rheumatology (Oxford). 2022;61(SI):SI4–SI5. [DOI] [PubMed] [Google Scholar]
- 38.Narvaez J, Estrada P, D LL, Vidal-Montal P, Brugarolas E, Maymo-Paituvi P, et al. Efficacy and safety of leflunomide in the management of large vessel vasculitis: A systematic review and metaanalysis of cohort studies. Semin Arthritis Rheum. 2023;59:152166. [DOI] [PubMed] [Google Scholar]
- 39.Marinelli KC, Ahlman MA, Quinn KA, Malayeri AA, Evers R, Grayson PC. Stenosis and Pseudostenosis of the Upper Extremity Arteries in Large-Vessel Vasculitis. ACR Open Rheumatol. 2019;1(3):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn KA, Rosenblum JS, Rimland CA, Gribbons KB, Ahlman MA, Grayson PC. Imaging acquisition technique influences interpretation of positron emission tomography vascular activity in large-vessel vasculitis. Semin Arthritis Rheum. 2020;50(1):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodge MA, Chaudhry MA, Wahl RL. Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med. 2012;53(7):1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ora M, Misra DP, Kavadichanda CG, Singh K, Rathore U, Jain N, et al. Metabolic inflammatory volume and total inflammatory glycolysis: novel parameters to evaluate PET-CT disease activity in Takayasu arteritis. Clin Rheumatol. 2023;42(7):1855–61. [DOI] [PubMed] [Google Scholar]
- 43.Dashora HR, Rosenblum JS, Quinn KA, Alessi H, Novakovich E, Saboury B, et al. Comparing Semiquantitative and Qualitative Methods of Vascular (18)F-FDG PET Activity Measurement in Large-Vessel Vasculitis. J Nucl Med. 2022;63(2):280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


