Background
Transfemoral (TF) access is the safest, quickest, and most studied access route for transcatheter aortic valve replacement (TAVR).1 While TAVR has demonstrated excellent clinical outcomes, femoral access site complications remain one of the most common adverse events of TAVR,2 with attendant morbidity and even mortality.
Despite contemporary safety refinements in obtaining wide-bore TF access (e.g., the routine use of vascular ultrasound and micropuncture), high body mass index (BMI)3 and increased femoral arterial depth (FAD)4 are strong predictors of vascular complications during TF-TAVR. In such high BMI patients, panniculus retraction (by a variety of nonstandardized methods) may reduce the FAD and thus facilitate safer TF access.
Locally, we have standardized this technique by repurposed use of a dedicated adhesive panniculus retractor (APR) device, initially designed for use in obese patients during caesarean section. The FAST-ACCESS study reports our clinical experience using this APR device during TF-TAVR in high BMI patients. Specifically, we report (i) the reduction in FAD achieved using the dedicated APR device and (ii) the vascular complication rate in consecutive patients with high BMI undergoing TF-TAVR when using the dedicated APR device.
Method
Twenty-five consecutive clinically obese patients undergoing TF-TAVR with BMI >30 and large abdominal panniculi were included. Prior to TAVR, FAD was measured from the TAVR-computerised tomography (CT) as the perpendicular height from the skin to the optimal common femoral artery (CFA) puncture point (FADpre). The optimal CFA puncture point was determined by the performing TAVR operator, considering the following criteria: (i) the presence of an underlying femoral head, (ii) the absence of CFA anterior wall calcification or branching vessels, and (iii) a satisfactory CFA mean lumen diameter (MLD), defined as >5.5 mm.
During TAVR, the APR device (traxi - Laborie, Portsmouth, USA) was applied according to its instructions for use (https://www.laborie.com/product/traxi-panniculus-retractor/). Prior to obtaining arterial access, FAD was then remeasured as the perpendicular height from the skin to the optimal CFA puncture point using ultrasound (FADpost). TF-TAVR was performed according to routine clinical practice including vascular ultrasound and micropuncture in all cases. The APR device remained in situ for the duration of the TF-TAVR, including access site closure. Final digital subtraction angiography was performed of the CFA access site in all cases.
Following tests for normality, Student’s paired t-test was used to compare FADpre and FADpost. Applicable tests were 2-tailed and p < 0.05 was considered statistically significant. Vascular complication events post-TAVR were obtained from blinded review of final digital subtraction angiography images and hospital records until discharge. All analyses were performed using R version 3.2.1 (R, Vienna, Austria). The study was ethically approved by the UK Health Research Authority (ref: 23/HRA/1278).
Results
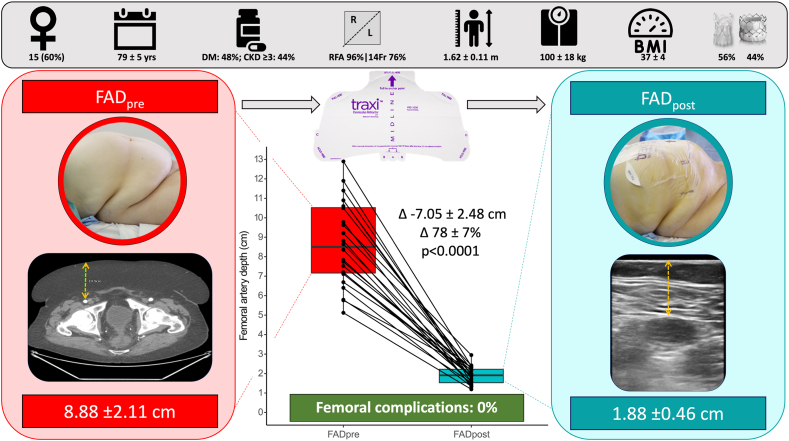
The mean age was 79 ± 5 years, and 60% were female. Mean BMI, height, and weight were 37 ± 4 kg/m2, 1.62 ± 0.11 meters, and 100 ± 18 kg, respectively. Right CFA access was used in 96% of patients. Femoral sheath sizes were 14Fr and 16Fr in 76% and 24%, respectively. CFA MLD at the site of puncture and the ipsilateral external iliac artery were 7.58 ± 1.17 mm and 7.83 ± 1.60 mm, respectively.
Implanted transcatheter heart valves were Edwards Sapien 3/Ultra and Acurate Neo2 in 44% and 56%, respectively. CFA access site vascular closure was achieved by 2 suture-mediated closure systems (Perclose ProStyle, 36%) ± a vascular closure device (8 Fr Angio-Seal, 64%). Antiplatelet therapy was aspirin (60%), clopidogrel (12%), or a return to usual-dose anticoagulant (28%).
Mean FADpre vs. FADpost was 8.93 ± 2.48 cm and 1.89 ± 0.44 cm, respectively (Δ-7.05 ± 2.48 cm, p < 0.0001 for difference, Figure 1). The procedural success rate was 100%. The vascular complication rate was 0%.
Figure 1.
Femoral artery depth measurements pre (left) and post (right) adhesive panniculus retractor device attached to elevate panniculus in patients with BMI >30 undergoing TAVR.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; FAD, femoral artery depth; RFA, right femoral artery; TAVR, transcatheter aortic valve replacement (device images used with permission).
Conclusion
In a consecutive series of clinically obese patients undergoing TF-TAVR with large abdominal panniculi and deep common femoral arteries, the use of a dedicated APR device significantly reduced femoral artery depth (Δ-7.05 ± 2.48 cm) and was associated with complete procedural success of TF-TAVR and a total absence of vascular complications.
Accordingly, in addition to the routine use of vascular ultrasound and micropuncture, panniculus retraction using a dedicated APR device may represent the new gold standard contemporary approach to wide-bore femoral access in such patients. However, the device may not be applicable to patients with abdominal wall injury, for example, stoma’s or an allergy to adhesives.
Conventional nonstandardized techniques for panniculus retraction include application of medical tape or manual elevation of the panniculus by staff members, albeit minimally costly, has multiple drawbacks. These include (i) the temporary nature of manual elevation, (ii) nonreproducibility, (iii) instability of anatomical structures during vascular access (and closure), (iv) risk of desterilization, (v) exposure of staff to excess ionizing radiation, and (vi) patient discomfort and loss of dignity. Conversely, a dedicated APR device circumvents all these limitations, is quick to apply (∼30 seconds) and is relatively inexpensive (∼$60).
Our study has several limitations. First, our study is a single-center, nonrandomized design with a modest sample size. However, zero vascular complications across the entire study population are encouraging for wider extrapolation to claims of general safety. Second, our primary comparison of FADpre vs. FADpost utilizes 2 different imaging modalities, namely TAVR-CT (for FADpre) and vascular ultrasound (for FADpost). For a truer comparison, paired measurements of FAD pre- and post-APR could be performed utilizing a single imaging modality. However, we elected to replicate the real-world clinical workflow of TAVR patients, in whom the TAVR-CT is an essential component of preprocedural planning. Accordingly, we identified patients for APR device usage (and thus inclusion in the FAST-ACCESS study) based upon the FAD determined on TAVR-CT, and hence this is reported. Third, despite high BMI and deep femoral artery anatomy, the MLDs in our study cohort were favorable for TF-TAVR. Therefore, our results may not be directly translatable into more borderline MLD TF-TAVR cases or in those with excessive calcification. Fourth, all patients underwent TF-TAVR using dedicated expandable sheaths. Accordingly, our safety results may not be directly translatable to so-called ‘sheathless’ transcatheter heart valve systems which involve multiple exchanges of equipment through the CFA access site, however, APR device usage may be expected to optimize the safety of this also. Randomized studies are required to robustly explore the device-specific impact on vascular complications.
Ethics Statement
The study adhered to the ethical principles underlying the Declaration of Helsinki and good practice guidelines on the proper conduct of research. The study was ethically approved by UK Health Research Authority (ref: 23/HRA/1278).
Funding
The authors have no funding to report.
Disclosure Statement
C. Cook is a consultant for Philips, Viz.ai and Cerebria.ai, speaker’s bureau for Boston Scientific. T. Keeble reports research grants from Boston Scientific, Volcano, Terumo, and Abbott Vascular. J. Davies reports research grant from Abbott Vascular and speaker fees from Bayer and Pfizer. C. Cook reports a relationship with Philips Healthcare US that includes: consulting or advisory. He reports a relationship with Cerebria.ai that includes: consulting or advisory. He also reports a relationship with Viz.ai, Inc and reports a relationship with Boston Scientific Corporation that includes: speaking and lecture fees. T. Keeble reports a relationship with Boston Scientific Corporation that includes: funding grants. He reports a relationship with Volcano Corporation that includes: funding grants. He reports a relationship with Terumo Corp that includes: funding grants and also reports a relationship with Abbott Vascular International BVBA that includes: funding grants. J. Davies reports a relationship with Abbott Vascular International BVBA that includes: funding grants. He reports a relationship with Bayer Corporation that includes: speaking and lecture fees. He also reports a relationship with Pfizer that includes: speaking and lecture fees. The other authors report no conflicts.
References
- 1.Rocha B., Nolasco T., Teles R., et al. TAVI via alternative access routes: patient selection and 10-year center experience. Eur Heart J. 2021;42(Supplement_1) [Google Scholar]
- 2.Scarsini R., De Maria G.L., Joseph J., et al. Impact of complications during transfemoral transcatheter aortic valve replacement: how can they be avoided and managed? J Am Heart Assoc. 2019;8(18) doi: 10.1161/JAHA.119.013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berti S., Bartorelli A.L., Koni E., et al. Impact of high body mass index on vascular and bleeding complications after transcatheter aortic valve implantation. Am J Cardiol. 2021;155:86–95. doi: 10.1016/j.amjcard.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Durand E., Penso M., Hemery T., et al. Standardized measurement of femoral artery depth by computed tomography to predict vascular complications after transcatheter aortic valve implantation. Am J Cardiol. 2021;145:119–127. doi: 10.1016/j.amjcard.2020.12.089. [DOI] [PubMed] [Google Scholar]