Abstract
Background
Baseline left ventricular diastolic dysfunction (LVDD) is associated with poor health status in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement (TAVR), but health status improvement after TAVR appears similar across all grades of LVDD. Here, we aim to examine the relationship between changes in LVDD severity and health status outcomes following TAVR.
Methods
Patients who underwent TAVR and had evaluable LVDD at both baseline and 1 year in the PARTNER (Placement of Aortic Transcatheter Valves) 2 SAPIEN 3 registries and PARTNER 3 trial were analyzed. LVDD grade was evaluated using echocardiography core lab data and an adapted definition of American Society of Echocardiography guidelines. Health status was assessed using the Kansas City Cardiomyopathy Questionnaire Overall Summary (KCCQ-OS) score. The association between ΔLVDD severity and ΔKCCQ-OS was examined using linear regression models adjusted for baseline KCCQ-OS.
Results
Of 1100 patients, 724 (65.8%), 283 (25.7%), and 93 (8.5%) had grade 0/1, 2, and 3 LVDD at baseline, respectively. At 1 year, LVDD severity was unchanged in 790 (71.8%) patients, improved in 189 (17.2%), and worsened in 121 (11.0%). Among 376 patients with baseline grade 2 or 3 LVDD, 50.3% had improvement in LVDD. In the overall cohort, KCCQ-OS score improved by 21.9 points at 1 year. There was a statistically significant association between change in LVDD severity (improved, unchanged, and worsened) and ΔKCCQ-OS at 1 year (p = 0.007).
Conclusions
Change in LVDD grade was associated with change in health status 1 year following TAVR.
Keywords: Aortic stenosis, Diastolic dysfunction, Health status, Transcatheter aortic valve replacement
Graphical abstract
Graphical Abstract:Association between change in LVDD and change in health status. (a) The change in LVDD severity from baseline to 1 year following TAVR. The thickness of the arrows reflects the proportion of the total number of patients in the study (N = 1100). Counts and percentages above the arrows reflect the proportion of patients within each baseline LVDD grade that improved, worsened, or remained unchanged after TAVR. Majority of patients were either improved or unchanged. (b) The least squares mean difference in change in health status in patients with improved LVDD or worsened LVDD vs. unchanged LVDD. Change in LVDD severity at 1 year was independently associated with change in KCCQ-OS at 1 year. The analysis was adjusted for baseline KCCQ-OS score, age, sex, hypertension, chronic obstructive pulmonary disease, and stroke.Abbreviations: KCCQ-OS, Kansas City Cardiomyopathy Questionnaire–Overall Summary; LVDD, left ventricular diastolic dysfunction; TAVR, transcatheter aortic valve replacement

.
Introduction
As the indications for transcatheter aortic valve replacement (TAVR) expand across the spectrum of operative risk, the pool of patients with severe symptomatic aortic stenosis (AS) eligible for TAVR continues to grow, including younger patients with longer life expectancies. As a result, there is a growing need to better understand predictors of long-term outcomes, one of which is left ventricular diastolic dysfunction (LVDD).
LVDD has been shown to predict poor outcomes in patients with AS,1 including those undergoing TAVR.2, 3, 4, 5 The presence of LVDD at baseline is associated with increased risks of mortality and cardiovascular hospitalization 1 year following TAVR.2, 3, 4 Conversely, after the elimination of AS-induced afterload following TAVR, improvement in LVDD is associated with a reduction in adverse events at 1 year.3,4 While there is accumulating evidence regarding the impact of LVDD on clinical outcomes, there remains a paucity of knowledge about the associations of LVDD before and after TAVR with patients’ symptoms, functional status, and quality of life. Health status outcomes are an important endpoint for patient populations considering TAVR.6
Previous studies have demonstrated that patients with baseline LVDD have worse health status at the time of TAVR; however, health status improvement after TAVR appears similar across all grades of LVDD.7 Whether changes in LVDD are associated with health status recovery after TAVR remains unclear. Accordingly, we evaluated the association between change in LVDD and change in health status following TAVR.
Materials and Methods
Study Population and Echocardiographic Acquisition
Patients enrolled in the PARTNER (Placement of Aortic Transcatheter Valves) 2 SAPIEN 3 registries and the PARTNER 3 trial were included. The design and results of these trials have been previously reported.8, 9, 10 In brief, these were prospective multicenter studies that enrolled patients with severe symptomatic AS across the surgical risk spectrum, that is, inoperable/high-risk, intermediate-risk, and low-risk. Both trials were approved by the individual site institutional review boards, and written informed consent was provided by all patients. The trials were registered with ClinicalTrials.gov: #NCT03222141 (P2 S3 high-risk/inoperable cohort), #NCT03222128 (P2 S3 intermediate-risk cohort), and #NCT02675114 (P3). Patients with severe mitral regurgitation (MR), mitral stenosis (MS) of any severity, or missing data necessary for assessing LVDD grade in this study (e.g., peak E and A velocities) were excluded from the analytic cohort.
Assessment of Diastolic Dysfunction
All echocardiograms were analyzed by independent echocardiography core laboratories in accordance with American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) standards.11 Image acquisition and analysis quality assurance were ensured by the use of a detailed acquisition protocol, intraobserver and interobserver variability testing, and by comprehensive training. LVDD was assessed at baseline and 1-year post-TAVR. The diagnosis and severity of LVDD were examined using definitions adapted from guidelines set forth by ASE/EACVI.12 The 4 variables required for identifying the presence of LVDD and their abnormal cutoff values are: (1) annular e’ velocity (septal e’ <7 cm/s or lateral e’ <10 cm/s); (2) average E/e’ ratio >14; (3) left atrial volume index >34 mL/m2; and (4) peak tricuspid regurgitation (TR) velocity >2.8 m/s.12 LVDD was considered present if 3 or more variables were abnormal. Grade 0 LVDD or normal diastolic dysfunction was considered present if only one variable was abnormal. The required variables to grade the severity of LVDD were the mitral inflow pattern (E/A ratio) and peak E velocity. Grade 1 LVDD was defined as an E/A ratio ≤0.8 and peak E velocity <50 cm/s. Grade 3 LVDD was defined as an E/A ratio ≥2.
If the E/A ratio was ≤0.8 and E > 50 cm/s or E/A ratio was >0.8 but <2, then additional parameters were necessary. To further characterize LVDD severity, 3 recommended variables and their abnormal cutoffs were required: (1) peak TR continuous-wave Doppler velocity >2.8 m/s; (2) E/e’ ratio (septal E/e’ ratio >15, lateral E/e’ ratio >13, or average E/e’ ratio >14); and (3) left atrial volume index >34 ml/m2. If 2 or 3 of these variables met their cutoff values, grade 2 LVDD was identified. If 0 or 1 of the 3 variables met their cutoff values, grade 1 LVDD was identified. If only 2 variables were available to analyze, 0, 1, and 2 abnormal variables classified LVDD grade as grade 0/1, grade indeterminate, and grade 2, respectively. The indeterminate group was excluded from this analysis. The same methodology was used to assess LVDD at follow-up echocardiograms. Change in LVDD was evaluated between baseline, 30 days, and 1-year post-TAVR. “Improved” LVDD was defined as a decrease of ≥1 LVDD grade at 30 days or 1 year. Conversely, “worsened” LVDD was defined as an increase of ≥1 LVDD grade at 30 days or 1 year. Otherwise, LVDD was considered “unchanged.”
Measurement of Health Status
Patient-level health status was assessed using the 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline, 30 days, and 1 year after TAVR. The 23-item KCCQ13 is a self-administered questionnaire in patients with heart failure that has been shown to be a reliable and valid instrument in patients with AS.14 A KCCQ Overall Summary (KCCQ-OS) score is calculated as the average of 5 domain areas: physical limitation, symptom frequency, quality of life, self-efficacy, and social limitation. The range of KCCQ-OS scores is 0 to 100 categorized as very poor (KCCQ-OS < 25), poor (KCCQ-OS 25-49), fair (KCCQ-OS 50-74), and good health status (KCCQ-OS ≥75). Changes of 5, 10, and 20 points on the KCCQ-OS indicate a small, moderate, and large change in health status.15 Six-minute walk test (6MWT) distance (in meters) was analyzed as a secondary measurement of health status at baseline, 30 days, and 1 year after TAVR.
Statistical Analysis
Baseline characteristics and echocardiographic parameters were compared by LVDD grade severity. Continuous variables were compared using analysis of variance, and categorical variables were compared using Fisher exact test. The mean KCCQ-OS at baseline, 30 days, and 1 year following TAVR and the change from baseline at follow-up visit were compared across LVDD grades using analysis of variance. In addition, from baseline to the 1-year time point, we presented the frequencies of different magnitudes of change in KCCQ-OS across the change of LVDD (unchanged, improved, worsened) and across the number of grades changed (i.e., improved 2 grades and worsened 2 grades). Lastly, a linear regression model was used to examine the association between change in LVDD grade and change in KCCQ-OS at 1 year following TAVR. The model was adjusted for baseline KCCQ-OS, age, sex, hypertension, chronic obstructive pulmonary disease, and stroke.
Results
Study Population
Between October 2013 and October 2017, a total of 2253 TAVR patients were enrolled in the PARTNER 2 SAPIEN 3 registries and PARTNER 3 trial. Of these, 593 patients were excluded from analysis for the following reasons: severe MR (n = 17), MS (n = 155), and missing data (e.g., missing E/A ratio; n = 421). Baseline LVDD grade was evaluated in 1660 patients; LVDD grade was indeterminate in 85 patients who were excluded from analysis. Between baseline and 1 year, 475 patients were also excluded due to missing LVDD grade (n = 117 died; n = 48 exited the study; n = 257 had unevaluable echocardiograms; n = 53 had indeterminate LVDD grade). The final paired analytic cohort comprised 1100 patients (Figure 1).
Figure 1.
Algorithm for assessment of baseline LVDD. Derivation of the final analytic cohort by LVDD severity. Patients with severe aortic stenosis undergoing TAVR from the PARTNER 2 SAPIEN 3 high- and intermediate-risk registries and PARTNER 3 low-risk trial were assessed for baseline LVDD. LVDD grade was evaluated using echocardiography core lab data and an adapted definition of American Society of Echocardiography guidelines. Patients who had evaluable data at both baseline and 1 year were analyzed.
Abbreviations: LA, left atrium; LVDD, left ventricular diastolic dysfunction; MR, mitral regurgitation; MS, mitral stenosis; PARTNER, Placement of Aortic Transcatheter Valves; TAVR, transcatheter aortic valve replacement; TR, tricuspid regurgitation.
Baseline characteristics of the study patients according to LVDD grade are provided in Table 1. According to adapted ASE/EACVI classification, the patients were stratified as LVDD grade 0/1 (n = 724, 65.8%), grade 2 (n = 283, 25.7%), and grade 3 (n = 93, 8.5%). The mean age was 79.8 years, 57.5% were male, the average Society of Thoracic Surgeons Predictive Risk of Mortality (STS PROM) score was 4.9 ± 2.9, and 65.8% had mild baseline LVDD. Age, race, STS PROM score, and gait speed were the only significant differences in baseline characteristics between the grades of LVDD. The median duration of follow-up was 1292.4 ± 488.2 days.
Table 1.
Baseline characteristics according to baseline LVDD grade
| Characteristics | Grade 0/1 (N = 724) | Grade 2 (N = 283) | Grade 3 (N = 93) | p value∗ |
|---|---|---|---|---|
| Age, y | 79.3 ± 7.62 | 80.7 ± 7.31 | 81.3 ± 7.78 | 0.0036 |
| Female sex | 307/724 (42.4%) | 130/283 (45.9%) | 30/93 (32.3%) | 0.0674 |
| Non-Caucasian | 48/724 (6.6%) | 34/283 (12.0%) | 7/93 (7.5%) | 0.0227 |
| STS PROM | 4.5 ± 2.83 | 5.5 ± 2.79 | 6.1 ± 3.20 | <0.0001 |
| HTN | 641/724 (88.5%) | 255/283 (90.1%) | 88/93 (94.6%) | 0.1791 |
| Home O2 | 23/437 (5.3%) | 12/233 (5.2%) | 4/81 (4.9%) | >0.9999 |
| ESRD on HD | 49/724 (6.8%) | 23/283 (8.1%) | 7/93 (7.5%) | 0.7090 |
| Stroke | 54/724 (7.5%) | 23/283 (8.1%) | 8/93 (8.6%) | 0.8306 |
| Sleep apnea† | 45/281 (16.0%) | 9/47 (19.1%) | 1/9 (11.1%) | 0.8827 |
| Gait speed‡ | ||||
| Normal ≥0.83m/s | 284/712 (39.9%) | 70/280 (25.0%) | 22/92 (23.9%) | <0.0001 |
| Slow 0.5-0.83m/s | 224/712 (31.5%) | 98/280 (35.0%) | 25/92 (27.2%) | |
| Slowest <0.5m/s | 204/712 (28.7%) | 112/280 (40.0%) | 45/92 (48.9%) | |
Values presented as mean ± SD or n/N (%).
Abbreviations: ESRD on HD, end-stage renal disease on hemodialysis; HTN, hypertension; LVDD, left ventricular diastolic dysfunction; STS PROM, Society of Thoracic Surgeons Predictive Risk of Mortality.
p values were computed by analysis of variance for continuous variables and Fisher exact test for categorical variables.
Sleep apnea was not collected in the SAPIEN 3 registries.
Gait speed was calculated based on six-minute walk test; slowest group includes those unable to walk.
Baseline Echocardiographic Parameters
At baseline, higher LVDD grade was associated with larger left atrial volume indices, higher rates of MR or TR, and higher right ventricular systolic pressures (p < 0.001). Conversely, higher baseline LVDD grades were associated with smaller aortic valve areas and lower left ventricular ejection fractions (p < 0.001) (Table 2).
Table 2.
Comparison of baseline echocardiographic characteristics in patients according to LVDD grade
| Characteristics | Grade 0/1 (N = 724) | Grade 2 (N = 283) | Grade 3 (N = 93) | p value∗ |
|---|---|---|---|---|
| Mean aortic valve gradient | 47.9 ± 13.32 | 48.6 ± 14.32 | 44.3 ± 11.44 | 0.0682 |
| Aortic valve area | 0.72 ± 0.172 | 0.69 ± 0.160 | 0.65 ± 0.151 | <0.0001 |
| LVEF | 64.2 ± 11.88 | 60.2 ± 14.22 | 53.4 ± 14.43 | <0.0001 |
| RVSP | 29.1 ± 7.75 | 37.3 ± 12.82 | 41.8 ± 12.18 | <0.0001 |
| Mitral regurgitation | 260/713 (36.5%) | 176/282 (62.4%) | 73/93 (78.5%) | <0.0001 |
| Mild | 243/713 (34.1%) | 152/282 (53.9%) | 55/93 (59.1%) | NA |
| Moderate | 17/713 (2.4%) | 24/282 (8.5%) | 18/93 (19.4%) | |
| Severe | 0/713 (0.0%) | 0/282 (0.0%) | 0/93 (0.0%) | |
| Aortic regurgitation | 35/719 (4.9%) | 23/283 (8.1%) | 5/93 (5.4%) | 0.2691 |
| Moderate | 34/719 (4.7%) | 23/283 (8.1%) | 5/93 (5.4%) | NA |
| Severe | 1/719 (0.1%) | 0/283 (0.0%) | 0/93 (0.0%) | |
| Tricuspid regurgitation | 5/696 (0.7%) | 18/277 (6.5%) | 13/92 (14.1%) | <0.0001 |
| Moderate | 5/696 (0.7%) | 16/277 (5.8%) | 9/92 (9.8%) | NA |
| Severe | 0/696 (0.0%) | 2/277 (0.7%) | 4/92 (4.3%) | |
| E/A ratio | 0.7 ± 0.20 | 1.1 ± 0.28 | 2.7 ± 0.57 | <0.0001 |
| A, cm/s | 109.8 ± 27.24 | 102.8 ± 27.62 | 43.1 ± 11.60 | <0.0001 |
| E/e’ ratio | 13.9 ± 5.81 | 21.4 ± 7.68 | 20.0 ± 6.81 | <0.0001 |
| Average e’, cm/s | 7.7 ± 1.97 | 11.1 ± 2.08 | 11.3 ± 1.93 | <0.0001 |
| TR velocity (cm/s) | 253.3 ± 35.08 | 295.9 ± 50.50 | 317.1 ± 48.74 | <0.0001 |
| LAVI (mL.m2) | 35.2 ± 11.16 | 45.1 ± 11.59 | 52.6 ± 15.60 | <0.0001 |
| LVOT stroke volume | 75.8 ± 15.72 | 76.6 ± 18.33 | 67.2 ± 16.80 | <0.0001 |
Values presented as (mean ± SD) or n/N (%).
Abbreviations: LAVI, left atrial volume index; LVDD, left ventricular diastolic dysfunction; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
p values were computed by analysis of variance for continuous variables and Fisher exact test for categorical ones.
Change in LVDD at 30 Days and 1 Year Following TAVR
Of 1100 patients who had baseline and 1-year LVDD assessments, 997 also had completed a 30-day LVDD assessment. The changes in LVDD grades between baseline, 30 days, and 1 year are shown in Supplemental Figure 1. At 30 days following TAVR, LVDD severity was unchanged in 709 (71.1%) patients, improved in 177 (17.8%), and worsened in 111 (11.1%) patients. At 1 year, LVDD severity was unchanged in 790 (71.8%) patients, improved in 189 (17.2%), and worsened in 121 (11.0%) patients. Among patients with grade 2 or grade 3 LVDD at baseline, 53.2% had an improvement in LVDD grade at 30 days and 50.3% demonstrated an improvement at 1 year. Out of the 177 patients with improved LVDD from baseline to 30 days, 126 (71.2%) patients showed sustained improvements at 1 year (Supplemental Table 1). There was no significant interaction between STS PROM and change in LVDD grade.
Association Between Change in LVDD and Health Status
In the overall cohort, the KCCQ-OS score improved by 21.9 points at 1-year follow-up. Changes in KCCQ-OS score from baseline to 30 days and 1 year stratified by baseline LVDD grade are summarized in Table 3. The proportion of patients experiencing different magnitudes of change in KCCQ-OS at 1-year follow-up is shown in Figure 2, stratified according to change in LVDD (unchanged, improved, worsened). In the unadjusted analysis, the mean (95% CI) KCCQ-OS increased by 24.1 (21.1, 27.2) points among patients with improved LVDD; 22.1 (20.6, 23.6) points among patients with unchanged LVDD, and 17.4 (13.5, 21.3) points change in KCCQ-OS at 1-year post-TAVR among patients whose LVDD worsened (p = 0.0278). In the adjusted analysis, there was a statistically significant association between change in LVDD severity (improved, unchanged, and worsened) and ΔKCCQ-OS at 1 year (p = 0.007). In comparison to unchanged LVDD, worsened LVDD was associated with worsening KCCQ-OS at 1 year (ΔKCCQ-OS -4.0 [95% CI -7.1, -0.9]), whereas the association between improved LVDD and change in KCCQ-OS did not reach statistical significance (ΔKCCQ-OS +1.9 [95% CI -0.6, 4.4]). Sensitivity analyses were performed in which the 85 patients from the indeterminate baseline LVDD group were included as having grade 1 or grade 2 LVDD at baseline, and no significant differences in health status at 30 days and 1 year were observed. The addition of aortic valve mean gradient as a covariate in adjusted analyses did not significantly change these results (Supplemental Table 2).
Table 3.
Comparison of KCCQ according to baseline LVDD grade by analysis of variance
| Visit | Grade 0/1 (N = 724) | Grade 2 (N = 283) | Grade 3 (N = 93) | p value |
|---|---|---|---|---|
| Baseline | 61.8 ± 21.88 (711) | 57.9 ± 22.74 (277) | 56.1 ± 23.82 (90) | 0.0096 |
| 30 d | 80.0 ± 18.50 (718) | 78.4 ± 18.91 (280) | 76.2 ± 20.16 (89) | 0.1342 |
| 30 d change from baseline | 18.6 ± 19.78 (705) | 20.5 ± 21.33 (274) | 20.0 ± 21.96 (87) | 0.4134 |
| 1 y | 82.7 ± 18.34 (708) | 81.2 ± 18.29 (277) | 81.7 ± 17.23 (91) | 0.5105 |
| 1 y change from baseline | 20.9 ± 20.28 (696) | 23.3 ± 22.14 (271) | 26.3 ± 24.71 (88) | 0.0364 |
Values presented as mean ± SD (N).
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; LVDD, left ventricular diastolic dysfunction.
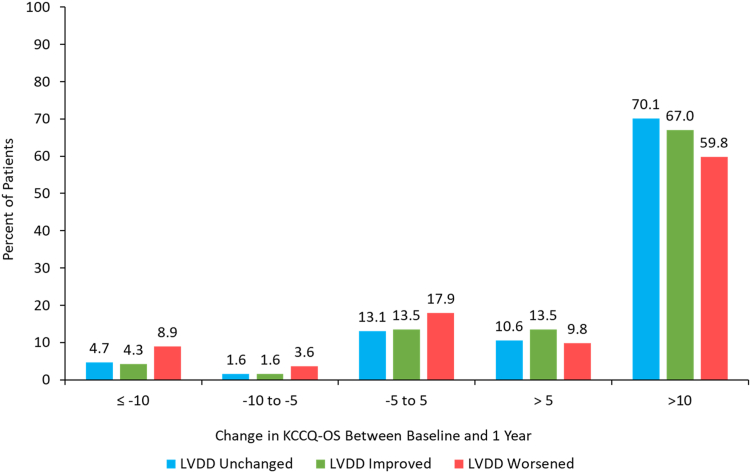
Figure 2.
Health status change 1-year post-TAVR by change in LVDD severity. Unadjusted change in KCCQ-OS score from baseline to 1 year by patients whose LVDD grade stayed the same (N = 758), improved (N = 185), or worsened (N = 112) from baseline to 1 year. Majority of patients across the LVDD spectrum had an improvement in KCCQ-OS score after TAVR, however, the largest improvement in health status was seen in patients whose LVDD grade also improved.
Abbreviations: KCCQ-OS, Kansas City Cardiomyopathy Questionnaire–Overall Summary; LVDD, left ventricular diastolic dysfunction; TAVR, transcatheter aortic valve replacement.
In unadjusted analyses, 6MWT distance was lower in patients with LVDD at baseline (Table 4). However, in adjusted analyses, the association between change in LVDD at 1 year and change in 6MWT distance did not reach statistical significance (p = 0.13).
Table 4.
Comparison of 6MWT distance according to baseline LVDD grade by analysis of variance
| Visit | Grade 0/1 (N = 724) | Grade 2 (N = 283) | Grade 3 (N = 93) | p value |
|---|---|---|---|---|
| Baseline (m) | 250.3 ± 134.01 | 207.0 ± 125.84 | 190.7 ± 121.80 | <0.0001 |
| 30 d (m) | 277.5 ± 133.78 | 244.2 ± 127.70 | 234.9 ± 130.20 | 0.0001 |
| 30 d change from baseline (m) | 24.4 ± 102.66 | 37.6 ± 99.10 | 43.2 ± 128.67 | 0.0866 |
| 1 y (m) | 271.7 ± 141.23 | 235.3 ± 138.29 | 229.2 ± 127.01 | 0.0002 |
| 1 y change from baseline (m) | 18.4 ± 114.95 | 31.5 ± 128.56 | 38.0 ± 139.05 | 0.1621 |
Values presented as mean ± SD.
6MWT, six-minute walk test; LVDD, left ventricular diastolic dysfunction.
Discussion
Given that the treatment of AS can improve health status by both directly relieving cardiac outflow gradients and by improving the diastolic function of the left ventricle (LV), we sought to understand the association of the latter with health status improvements after TAVR. The main findings were that: (1) approximately 50% of patients with severe AS and at least moderate (grade 2) LVDD at baseline demonstrated an improvement in LVDD 1 year following TAVR, and (2) change in LVDD at 1 year was associated with differences in the extent of health status improvement after TAVR, that is, improvement in LVDD was associated with greater improvement in health status and worsening LVDD was associated with less improvement in health status. The trend between 6MWT distance and change in LVDD following TAVR was similar to the trend observed between KCCQ-OS and change in LVDD but did not reach statistical significance. This may be because KCCQ-OS is a comprehensive measurement of health status encompassing physical limitation, symptom frequency, quality of life, self-efficacy, and social limitation whereas 6MWT distance is a measurement of exercise capacity alone. While prior studies have established the significance of improvement in LVDD following TAVR with respect to cardiovascular death and rehospitalization,4 these results extend the prior findings to patients’ health status—their symptoms, functional status, and quality of life.
In patients with cardiovascular disease, optimization of health status and disease-specific quality of life has been recognized as an important goal of treatment.16 Using the PARTNER 2 SAPIEN 3 registry, Ong et al.4 demonstrated that improvement in LVDD was associated with a larger improvement in KCCQ-OS at 30 days, but health status outcomes at 1 year were not reported. A prior single-center study of 304 patients demonstrated that baseline LVDD grade was inversely associated with baseline health status; however, improvement in LVDD was not associated with 30-day or 1-year health status.7 In contrast, the present study reveals a significant association between change in LVDD and change in health status 1 year after TAVR.
There are several potential explanations for these divergent findings. First, the larger sample size in the current study may have allowed for better detection of changes in KCCQ during follow-up. Second, the use of a consortium of core laboratories in a clinical trial cohort may have improved the ability to detect changes in LVDD grade on follow-up and reduced variability in echocardiographic interpretation. Third, the definition of severe AS required for enrollment in trial cohorts may be more strict than what is seen in clinical practice. Irrespective of the exact mechanism for these differences, our findings can be further explained by exploring the pathophysiology of LVDD with severe AS.
Progressive mechanical obstruction due to AS imposed on the LV leads to increased afterload with the eventual development of hypertrophic remodeling and reactive interstitial fibrosis.17, 18, 19 Consequently, impairment in myocardial relaxation and increased cardiomyocyte stiffness culminate in the development of LVDD.20 As LVDD progresses, transmittance of elevated end-diastolic pressures to the pulmonary vasculature leads to increased pulmonary capillary pressure which manifests as exertional dyspnea or overt heart failure, the most common symptoms of severe AS.21,22 Elimination of AS-associated afterload by TAVR has been shown to induce regression of LV hypertrophy23,24 and subsequently improve LVDD.25 To this end, it is plausible that improvement in LVDD following TAVR contributes to improvement in symptomology, and hence, health status as seen in the present study.
In agreement with previous observations,2, 3, 4,24 our study demonstrates that LVDD grade may not improve or may even worsen following TAVR in a substantial group of patients. Worsened LVDD was found to be associated with less improvement in health status at 1 year following TAVR. Our study shows that increasing severity of baseline LVDD is associated with worsening echocardiographic changes in cardiac structure and function (ie, left atrial volume, MR, TR, LV ejection fraction) and worsening LV filling and relaxation (i.e., E/A ratio, average e’ velocity, TR velocity), indicating progression of disease and compromised cardiac reserve. How these factors impact health status following TAVR is unknown. Despite reduction of afterload with TAVR, there may be slow nonlinear regression of LV hypertrophy23,25 and/or sustained myocardial fibrosis26,27 that may contribute to the irreversibility of LVDD. Furthermore, particularly in the elderly population, concomitant pathologies contributing to persistent or residual LVDD may be underrecognized, such as hypertension28 or cardiac amyloidosis.29 The nonuniformity of change in LVDD post-TAVR highlights its complex mechanisms and multifactorial etiologies and underscores the need for further diagnostic investigation.
The use of multimodality imaging is essential in the evaluation of cardiac function and structure during follow-up after TAVR.30 Novel echocardiographic parameters such as LV diastolic strain31,32 and left atrial strain33,34 may offer additional diagnostic and prognostic value in refining LVDD assessment. In a single-center study of 100 patients undergoing TAVR, Sabatino et al.35 found left atrial strain, which is correlated with elevated LV end-diastolic pressures,33 to be predictive of mortality and heart failure hospitalization. Cardiac magnetic resonance imaging can also provide a direct examination of myocardial fibrosis as measured by extracellular volume and late gadolinium enhancement.36,37 Chin et al.18 demonstrated, in a prospective cohort of 166 AS patients and 37 healthy volunteers, that there was progressive LV decompensation, including worsening LVDD, across stages of fibrosis. Early reversible diffuse myocardial fibrosis, quantified by extracellular volume on T1-mapping, has been found to be associated with LVDD in patients with AS38 and may potentially enhance LVDD assessment post-TAVR. Further studies are needed to determine whether these tools can help inform the assessment of LVDD following TAVR.
Study Limitations
The findings of this study should be interpreted in the light of several potential limitations. First, 51.2% of the initial cohort were excluded due to various reasons such as missing baseline data, MR or MS, unevaluable echocardiogram for LVDD at 1 year, or LVDD indeterminant grade. Consequently, selection bias may have been introduced in our observational study. The majority of missing E/A data was secondary to the presence of atrial fibrillation. Second, the presence of mitral annular calcification was not collected. Mitral annular calcification is known to influence diastolic assessment leading to falsely elevated transmitral velocities and decreased lateral and posterior annular velocities. We excluded patients with MS to overcome this potential confounding factor. Third, although the importance of myocardial fibrosis in understanding the longitudinal change in LVDD and health status following TAVR, data on myocardial fibrosis were not available for our study. Finally, advanced echo imaging parameters such as left atrial strain and left ventricular global longitudinal strain were not available in this data set and could not be analyzed.
Conclusions
Approximately half of patients with severe AS and ≥ moderate LVDD at baseline demonstrated improvement in LVDD 1 year following TAVR. Moreover, improvement in LVDD was associated with greater improvement in patient-level health status. Further investigation is needed with respect to multimodal imaging in the assessment of LVDD after TAVR and clinical management.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines. The PARTNER 2 S3 Registry and PARTNER 3 Trial protocols were approved by the institutional review boards of participating sites and all patients provided written informed consent.
Funding
The PARTNER 2 SAPIEN 3 registries and PARTNER 3 trial were sponsored by Edwards Lifesciences (Irvine, California). Rayan S El-Zein and Ali Malik are supported by the National Heart, Lung, and Blood Institutes of Health under Award Number T32H110837. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health.
Review Statement
Given their roles as editors, Philippe Pibarot, DVM, PhD, Rebecca T. Hahn, MD, Maria Alu, MS, Martin B. Leon, and Michael J. Mack, MD, had no involvement in the peer review of this article and had no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Julius Gardin, MD.
Disclosure Statement
David J. Cohen receives research grant support from Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic and Consulting income from Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic. John A. Spertus is the PI of grants from NIH, Abbott Vascular, and the American College of Cardiology Foundation; is a consultant to Janssen, Novartis, Amgen, Bristol Meyers Squibb, AstraZeneca, Bayer and Merck; serves on the Scientific Advisory Board of United Healthcare and the Board of Directors for Blue Cross Blue Shield of Kansas City; and owns the copyright to the KCCQ, SAQ, and PAQ. Philippe Pibarot has received funding from Edwards Lifesciences, Medtronic, Pi-Cardia, and Cardiac Phoenix for echocardiography core laboratory analyses and research studies in the field of transcatheter valve therapies, for which he received no personal compensation. Philippe Pibarot has received lecture fees from Edwards Lifesciences and Medtronic. Rebecca T. Hahn reports speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; she has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic and Novartis; she has stock options with Navigate; and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. Maria C. Alu reports institutional research funding from Edwards Lifesciences (no direct compensation). Kan Shang is an employee of Edwards Lifesciences. Susheel K. Kodali reports institutional research grants from Edwards Lifesciences, Medtronic, and Abbott, consulting fees from Abbott, Admedus, and Meril Lifesciences, and equity options from Biotrace Medical and Thubrikar Aortic Valve Inc. Vinod H Thourani reports research and/or consulting from Abbott Vascular, Artivion, Atricure, Boston Scientific, Edwards Lifesciences, HighLife, Jenavalve, Medtronic, and Shockwave. Martin B. Leon serves on the PARTNER Trial Executive Committee - Edwards Lifesciences (nonpaid) and reports institutional research grants and being a nonpaid advisor for Abbott, Boston Scientific, and Medtronic; nonpaid advisor for Sinomed, and equity in Medinol. Michael J. Mack served as co-primary investigator for the PARTNER trial for Edwards Lifesciences and COAPT trial for Abbott; served as study chair for the APOLLO trial for Medtronic (all activities unpaid). Adnan K. Chhatriwalla Speakers Bureau: Abbott Vascular, Edwards Lifesciences, Medtronic Inc.; Proctor: Edwards Lifesciences, Medtronic Inc.; Research Grant: Boston Scientific. The other authors had no conflicts to declare.
Guest Editor: Julius Gardin, MD
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Gjertsson P., Caidahl K., Farasati M., Oden A., Bech-Hanssen O. Preoperative moderate to severe diastolic dysfunction: a Novel Doppler echocardiographic long-term prognostic factor in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2005;129:890–896. doi: 10.1016/j.jtcvs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Asami M., Lanz J., Stortecky S., et al. The impact of left ventricular diastolic dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11:593–601. doi: 10.1016/j.jcin.2018.01.240. [DOI] [PubMed] [Google Scholar]
- 3.Blair J.E.A., Atri P., Friedman J.L., et al. Diastolic function and transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2017;30:541–551. doi: 10.1016/j.echo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ong G., Pibarot P., Redfors B., et al. Diastolic function and clinical outcomes after transcatheter aortic valve replacement: PARTNER 2 SAPIEN 3 registry. J Am Coll Cardiol. 2020;76:2940–2951. doi: 10.1016/j.jacc.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Anantha-Narayanan M., Malik U., Mbai M., et al. Impact of diastolic dysfunction on long-term mortality and quality of life after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2020;95:1034–1041. doi: 10.1002/ccd.28444. [DOI] [PubMed] [Google Scholar]
- 6.Arnold S.V., Spertus J.A., Lei Y., et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–597. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik A.O., Omer M., Pflederer M.C., et al. Association between diastolic dysfunction and health status outcomes in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:2476–2484. doi: 10.1016/j.jcin.2019.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 9.Kodali S., Thourani V.H., White J., et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37:2252–2262. doi: 10.1093/eurheartj/ehw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thourani V.H., Kodali S., Makkar R.R., et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. doi: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 11.Douglas P.S., DeCara J.M., Devereux R.B., et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22:755–765. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 13.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 14.Arnold S.V., Spertus J.A., Lei Y., et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 15.Butler J., Khan M.S., Mori C., et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:999–1005. doi: 10.1002/ejhf.1810. [DOI] [PubMed] [Google Scholar]
- 16.Rumsfeld J.S., Alexander K.P., Goff D.C., Jr., et al. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 17.Everett R.J., Treibel T.A., Fukui M., et al. Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol. 2020;75:304–316. doi: 10.1016/j.jacc.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin C.W.L., Everett R.J., Kwiecinski J., et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hein S., Arnon E., Kostin S., et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 20.Kampaktsis P.N., Kokkinidis D.G., Wong S.C., Vavuranakis M., Skubas N.J., Devereux R.B. The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart. 2017;103:1481–1487. doi: 10.1136/heartjnl-2017-311506. [DOI] [PubMed] [Google Scholar]
- 21.Dahl J.S., Christensen N.L., Videbaek L., et al. Left ventricular diastolic function is associated with symptom status in severe aortic valve stenosis. Circ Cardiovasc Imaging. 2014;7:142–148. doi: 10.1161/CIRCIMAGING.113.000636. [DOI] [PubMed] [Google Scholar]
- 22.Park S.J., Enriquez-Sarano M., Chang S.A., et al. Hemodynamic patterns for symptomatic presentations of severe aortic stenosis. JACC Cardiovasc Imaging. 2013;6:137–146. doi: 10.1016/j.jcmg.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Tzikas A., Geleijnse M.L., Van Mieghem N.M., et al. Left ventricular mass regression one year after transcatheter aortic valve implantation. Ann Thorac Surg. 2011;91:685–691. doi: 10.1016/j.athoracsur.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Dahiya G., Kyvernitakis A., Joshi A.A., et al. Impact of transcatheter aortic valve replacement on left ventricular hypertrophy, diastolic dysfunction and quality of life in patients with preserved left ventricular function. Int J Cardiovasc Imaging. 2021;37:485–492. doi: 10.1007/s10554-020-02015-z. [DOI] [PubMed] [Google Scholar]
- 25.Vizzardi E., D'Aloia A., Fiorina C., et al. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25:1091–1098. doi: 10.1016/j.echo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Treibel T.A., Kozor R., Schofield R., et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71:860–871. doi: 10.1016/j.jacc.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biederman R.W., Magovern J.A., Grant S.B., et al. LV reverse remodeling imparted by aortic valve replacement for severe aortic stenosis; is it durable? A cardiovascular MRI study sponsored by the American Heart Association. J Cardiothorac Surg. 2011;6:53. doi: 10.1186/1749-8090-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imanaka K., Kohmoto O., Nishimura S., Yokote Y., Kyo S. Impact of postoperative blood pressure control on regression of left ventricular mass following valve replacement for aortic stenosis. Eur J Cardiothorac Surg. 2005;27:994–999. doi: 10.1016/j.ejcts.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Castano A., Narotsky D.L., Hamid N., et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bax J.J., Delgado V., Hahn R.T., et al. Transcatheter aortic valve replacement: role of multimodality imaging in common and complex clinical scenarios. JACC Cardiovasc Imaging. 2020;13:124–139. doi: 10.1016/j.jcmg.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Dokainish H., Sengupta R., Pillai M., Bobek J., Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–1509. doi: 10.1016/j.amjcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Khoury D.S., Thohan V., Torre-Amione G., Nagueh S.F. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 33.Wakami K., Ohte N., Asada K., et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847–851. doi: 10.1016/j.echo.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Nagueh S.F. Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail. 2018;20:38–48. doi: 10.1002/ejhf.971. [DOI] [PubMed] [Google Scholar]
- 35.Sabatino J., De Rosa S., Leo I., et al. Early reduction of left atrial function predicts adverse clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bing R., Cavalcante J.L., Everett R.J., Clavel M.A., Newby D.E., Dweck M.R. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2019;12:283–296. doi: 10.1016/j.jcmg.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehdipoor G., Chen S., Chatterjee S., et al. Cardiac structural changes after transcatheter aortic valve replacement: systematic review and meta-analysis of cardiovascular magnetic resonance studies. J Cardiovasc Magn Reson. 2020;22:41. doi: 10.1186/s12968-020-00629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H.J., Lee H., Kim S.M., et al. Diffuse myocardial fibrosis and diastolic function in aortic stenosis. JACC Cardiovasc Imaging. 2020;13:2561–2572. doi: 10.1016/j.jcmg.2020.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.