Abstract
Epithelial cells are the first point of host contact for invasive intestinal pathogens and may initiate mucosal inflammatory responses via production of proinflammatory cytokines and mediators. The aim of the present study was to investigate in vitro the initial invasion of a parasitic nematode (Trichinella spiralis), to measure the early production of specific epithelial cytokines and inflammatory mediators after invasion, and to compare these responses with those to invasive bacteria. Monolayers of human colonic epithelial cell lines (HT29, T84, and Caco-2) were infected by T. spiralis or Listeria monocytogenes. Bile-activated infective larvae of T. spiralis invaded and migrated into the epithelial cell monolayers, leaving trails of dead cells. Transmission electron microscopy studies of damaged cells along the trail showed a progressive increase in size, disruption of cell membranes, loss or dilution of cytoplasmic proteins, and swelling of mitochondria and nuclei. However, no nuclear fragmentation was observed. With reverse transcription-PCR and an enzyme-linked oligonucleotide chemiluminescent assay, mRNA transcripts of interleukin-1β (IL-1β), IL-8, and epithelial neutrophil-activating peptide 78 were shown to increase in epithelial cells invaded by T. spiralis or L. monocytogenes, but only L. monocytogenes elicited increased inducible nitric oxide synthase (iNOS) mRNA. No increase in tumor necrosis factor alpha or transforming growth factor β mRNA was seen after T. spiralis invasion. Increased levels of IL-8 were also released from the basolateral surfaces of infected monolayers as detected by sandwich enzyme-linked immunosorbent assay. Induction and secretion of proinflammatory cytokines in epithelial cells after nematode or bacterial invasion may initiate the acute inflammatory response of the small intestine. The upregulation of iNOS in bacterial infections may contribute to mucosal defense and may also be associated with subsequent cell death, whereas different mechanisms appear to operate after nematode invasion.
The response of the intestine to invasion by pathogens represents a complex interaction between nonspecific inflammatory mechanisms and immunologically specific adaptive events. Epithelial cells are the first site of entry for invasive intestinal pathogens and may provide early signals for the acute mucosal inflammatory response via the release of proinflammatory cytokines and inflammatory mediators. A number of studies have examined the process in vitro with human colonic epithelial cell lines. These have been shown to produce a wide range of proinflammatory cytokines (IL-1α, IL-6, IL-8, GM-CSF, GRO-α, MCP-1, and TNF-α) in response to invasive microbial pathogens (Entamoeba histolytica, Escherichia coli, Listeria monocytogenes, Salmonella dublin, Shigella dysenteriae, and Yersinia enterocolitica) (8, 14). However, the patterns of epithelial cytokine response vary with the site of infection and type of pathogen (3, 14, 21).
Intestinal inflammation is mediated by proinflammatory cytokines and inflammatory mediators. NO is an important mediator and is also known to have antimicrobial activity (13). Excessive formation of NO by iNOS has been associated with cellular toxicity and tissue damage in experimental models of active intestinal inflammation (24). With immunoperoxidase staining, iNOS was localized in inflamed epithelium in ulcerative colitis, Crohn’s disease, and diverticulitis (27). Therefore, epithelial iNOS may be important in acute mucosal inflammatory responses after invasion by pathogens.
Recently, an in vitro model of epithelial invasion by intestinal nematodes has been developed (20). Analysis of epithelial invasion by these parasites may broaden our understanding of epithelial cytokine responses to invasion by pathogens. Trichinella spiralis is a human intestinal pathogen that is becoming increasingly prevalent in Europe and North America because of changes in dietary habits and consumption of ethnic food. It has been used as an experimental model to study mucosal and inflammatory responses in animals. T. spiralis initiates infection by penetrating the columnar epithelium of the small intestine. During the early stages of infection, the inflammatory response is characterized by infiltration of neutrophils and macrophages/monocytes in the lamina propria (18). However, during the later stages of infection mast cells increase in number both in the lamina propria and between epithelial cells (26). In the murine model, the mucosal inflammatory response, which mediates expulsion of adult worms from the small intestine, is T-cell dependent (32). Moreover, data from many studies have shown that protective T-cell responses against intestinal nematodes are dependent on T helper 2 cytokines (10). However, the inductive and regulatory mechanisms of mucosal inflammatory responses to invasion by T. spiralis are still largely unknown.
In this paper, we describe the epithelial responses to invasion by the nematode T. spiralis with a recently described in vitro model (20) and compare these to responses elicited by invasive bacteria. The invasion and migration into epithelial monolayers by infective larvae produced significant damage to cell membranes and organelles. Invasion by infective larvae or bacteria resulted in increases in mRNA transcripts of IL-1β, IL-8, and ENA-78, but only bacteria elicited increased iNOS mRNA levels. Expression and secretion of proinflammatory cytokines in epithelial cells after microbial invasion may initiate the acute inflammatory response of the small intestine. The upregulation of iNOS mRNA in bacterial infections may be important in mucosal defense and subsequent cell death.
MATERIALS AND METHODS
Abbreviations.
The abbreviations used in this paper are as follows: DIG, digoxigenin; DMEM, Dulbecco’s minimal essential medium; ELISA, enzyme-linked immunosorbent assay; ELOCA, enzyme-linked oligonucleotide chemiluminescent assay; ENA-78, epithelial neutrophil-activating peptide 78; FCS, fetal calf serum; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO, growth-related protein; IL, interleukin; iNOS, inducible nitric oxide synthase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; l-NAME, l-nitro-l-arginine methyl ester; MCP-1, monocyte chemotactic protein 1; PBS, phosphate-buffered saline; RT, reverse transcription; TEM, transmission electron microscopy; TGF, transforming growth factor; TNF, tumor necrosis factor.
Parasitological techniques.
The intestinal parasitic nematode T. spiralis was maintained as described previously (34). Infective muscle larvae were isolated from CFLP mice infected at least 35 days earlier. The infected mice were killed, skinned, and eviscerated. The muscles containing the encysted larvae were finely minced and digested in 0.6% pepsin A (P-7000; Sigma Chemical Co., St. Louis, Mo.) and 1% HCl for 3 h at 37°C. The isolated infective larvae were washed several times with 0.8% NaCl and subsequently activated in 5 mg of porcine bile extract (Sigma B-8531) per ml in Hanks’ balanced salt solution (Gibco, Basel, Switzerland) for 1 h at 37°C.
Intestinal epithelial cell culture.
Human colonic epithelial cell lines HT29, T84, and Caco-2 were obtained from the European Collection of Animal Cell Cultures (Porton Down, United Kingdom) and grown to confluency in 60-mm2 culture dishes (Costar, High Wycombe, United Kingdom). HT29 cells (passage 180-5) were cultured in DMEM (Gibco) containing 10% FCS (Gibco), 2 mmol of glutamine (Sigma G-5763) per liter, 100 U of penicillin G per ml, and 0.1 mg of streptomycin (Sigma P-0906) per ml at 37°C and 5% CO2. T84 cells (passage 80-5) were grown similarly in 50% DMEM–50% Ham’s F-12 medium (Gibco) supplemented with 10% FCS, 2 mmol of glutamine per liter, and antibiotics (as for HT29 cells). Caco-2 cells (passage 32-7) were cultured in DMEM supplemented with 10% FCS, 10 μg of human transferrin (Sigma T-8158) per ml, 2 mmol of glutamine per liter, and antibiotics (as for the above cell lines). Monolayers of HT29 and T84 cells were grown to confluency for the Trichinella invasion assay, and Caco-2 cells were used 3 weeks after confluency, by which time the cells were differentiated with dome regions and apical microvilli as well as sucrase isomaltase activity (determined by an assay from Sigma).
Trichinella invasion assay.
The Trichinella invasion assay was carried out as described previously, with some modifications (20). Briefly, bile-activated larvae were resuspended in 5 ml of 1.75% agar (Difco, East Molesey, United Kingdom) in the culture medium appropriate for each cell line at 45°C. The agar-larvae mixture was immediately overlaid on the epithelial monolayers at room temperature until the agar was solidified, and the culture dish was then incubated for 5 h at 37°C and 5% CO2. The choice of this time span, which gave significant changes in mRNA levels, was based on data from preliminary experiments carried out to optimize the protocol. After the agar was removed with a blunt needle, the epithelial cells were processed for light and electron microscopy and RNA extraction, as described below.
Bacterial infection experiments.
Monolayers were also infected by L. monocytogenes to provide a positive control of pathogen invasion. After incubation with 107 CFU of L. monocytogenes per ml for 1 h at 37°C, the cells were washed twice in culture medium to remove extracellular bacteria. The cells were then incubated with culture medium containing 50 μg of gentamicin (Sigma G-1397) per ml for 4 h at 37°C to kill the remaining extracellular bacteria. After a total of 5 h of infection, the cells were harvested for RNA extraction, as described below. The culture supernatants were filtered by a 0.22-μm-pore-size filter and stored at −20°C until use.
Light and electron microscopy.
Changes in epithelial monolayers caused by Trichinella invasion were studied by light and electron microscopy. After incubation with infective larvae, the epithelial cells were stained with 0.5% trypan blue (Sigma T-6146) and examined with an Olympus CK2 inverted microscope. The ultrastructural changes in infected epithelial cells were studied by TEM. Epithelial monolayers on culture dishes were fixed in 2.5% (vol/vol) glutaraldehyde (in 0.1 M cacodylate buffer, pH 7.4) for 2 h. All samples were subsequently washed in PBS and postfixed in 1% osmium tetroxide for 1 h before dehydration in ethanol and embedding in Epon resin, according to standard procedures. Suitable areas for TEM were selected from 0.5 μm toluidine blue-stained sections. They were stained with uranyl acetate and lead citrate before being observed with a JEOL 1200 EX transmission electron microscope.
RT-PCR.
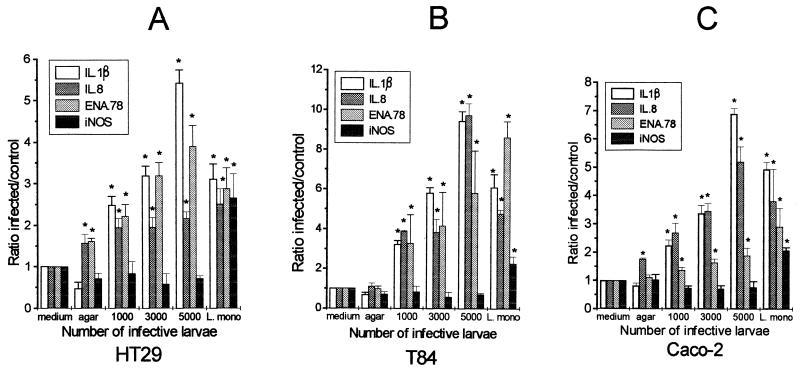
RT-PCR was carried out as described previously (23). Briefly, total RNA was obtained by lysing of the cells in RNAzol B (Biogenesis Ltd., Poole, United Kingdom), extraction with chloroform, and precipitation with isopropanol. After being washed and dried, the RNA pellet was resuspended in 50 μl of diethyl pyrocarbonate-treated H2O. Random hexamer-primed RT was carried out with 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco). Control tubes without reverse transcriptase were set up with half of the RNA from each sample. Primers and probes for ENA-78, GAPDH, IL-1β, IL-8, and iNOS were kindly designed by R. Seth and R. A. Robins (Immunology, QMC Nottingham), and the primers were synthesized in-house (Table 1). GAPDH was used as a reference marker to correct for any variation in procedure. Specific oligonucleotide probes to detect PCR products were synthesized and labelled with DIG by R&D Systems Europe Ltd. Reaction conditions are given in Table 1. PCR products were quantified by ELOCA with the labelled probes to identify PCR products after blotting onto nylon membranes. After incubation with rabbit anti-DIG antibody labelled with alkaline phosphatase (Boehringer Mannheim, Lewes, United Kingdom), the membranes were incubated with chemiluminescent substrate (CSP-Star; Boehringer Mannheim) and the photons emitted were measured by a microplate scintillation spectrophotometer. The signal for the cytokine PCR product was recorded as counts per second and expressed as a ratio of the counts per second for the GAPDH product. Expression of mRNA of IL-1β, IL-8, ENA-78, or iNOS in each sample was obtained by dividing the counts per second of IL-1β, IL-8, ENA-78, or iNOS by the corresponding value for GAPDH expression. The result obtained was then used to calculate the relative ratio of expression of cytokines or inflammatory mediators in infected cells to the medium control. This final ratio was used to construct the graphs presented in Fig. 3. The data were calculated as means ± standard errors of the means, and the medium control value was normalized as 1.
TABLE 1.
Primer and probe sequences for PCR and ELOCA
| Primer or probe | Sequence(s)a | PCR product size (bp) |
|---|---|---|
| Primers | ||
| IL-1β | 5′ CTC CGG GAC TCA CAG CAA AAA 3′ | 327 |
| 5′ GGG GAA CTG GGC AGA CTC A 3′ | ||
| IL-8 | 5′ TTG GCA GCC TTC CTG ATT TCT 3′ | 220 |
| 5′ TTT CCT TGG GGT CCA GAC AGA 3′ | ||
| ENA-78 | 5′ TGT TGG TGC TGC TGC TG 3′ | 212 |
| 5′ ACA AAT TTC CTT CCC GTT CTT 3′ | ||
| iNOS | 5′ ATG GAA CAT CCC AAA TAC GA 3′ | 453 |
| 5′ CAG CAT CTC CTG GTG AAA CA 3′ | ||
| GAPDH | 5′ GGT GAA GTT CGG AGT CAA CGG A 3′ | 240 |
| 5′ GAG GGA TCT CGC TCC TGG AAG A 3′ | ||
| Probesb | ||
| IL-1β | 5′ CCA CCT CCA GGG ACA 3′ | |
| IL-8 | 5′ TCA TTG AGA GTG GAC C 3′ | |
| ENA-78 | 5′ ACG CAA CGC AGC TCT 3′ | |
| iNOS | 5′ TTC TCC TGC CCA CTT C 3′ | |
| GAPDH | 5′ GCC TTG ACG GTG CCA T 3′ |
For each primer pair, the sense primer is given above the antisense primer.
Labelled directly with DIG via a six-carbon spacer.
FIG. 3.
Ratios of cytokine PCR products of intestinal epithelial cell lines infected with T. spiralis and L. monocytogenes obtained by RT-PCR and ELOCA. Monolayers of HT29 (A), T84, (B), and Caco-2 (C) cells were infected with 1 × 103, 3 × 103, and 5 × 103 infective larvae of T. spiralis or 107 CFU of L. monocytogenes per ml. After 5 h of infection, total RNA extracted from each sample was used for semiquantitative RT-PCR and ELOCA to examine the levels of expression of mRNA of IL-1β, IL-8, ENA-78, and iNOS. The experiments were repeated twice, and results are presented as ratios of infected to control cell lines, as described in Materials and Methods. *, statistically significant compared with uninfected medium control (P < 0.05).
Sandwich ELISA for IL-8.
To confirm whether increased mRNA expression correlated with protein secretion, levels of one representative cytokine (IL-8, a marker for neutrophilic inflammation) were measured in the culture supernatants of infected cells. The epithelial cell lines were grown to confluency in 24-mm-diameter Transwell plates (Costar). Seven hours after infection with T. spiralis or L. monocytogenes, the culture supernatants from the lower compartments of the cluster plate were collected and stored at −20°C. IL-8 was measured by sandwich ELISA using Mab618-biotinylated BAF218 (R&D Systems) as the cytokine-specific antibodies. Cytokine levels were quantified against recombinant human IL-8 standard (208-IL-010) (R&D Systems). The lower sensitivity of the assay was 32 pg/ml, calculated as the mean optical density value of [20 replicated medium controls plus two times the standard deviations]. Any test wells with optical density values above this sensitivity were considered positive for IL-8.
Statistics.
The results were analyzed by one-way analysis of variance and Student’s t test. A P value of <0.05 was considered statistically significant.
RESULTS
Behavior of T. spiralis in human intestinal epithelial monolayers.
Light microscopy studies showed that bile-activated infective larvae invaded and migrated within the epithelial monolayers shortly after the agar overlay was applied (Fig. 1A). The presence of the agar was critical in providing initial mechanical support for the larvae. Without the agar, infective larvae could move only over the epithelial monolayers and did not penetrate the epithelial cells. However, once the infective larvae were embedded in the epithelial monolayers, they continued to invade and migrate even when the agar was removed.
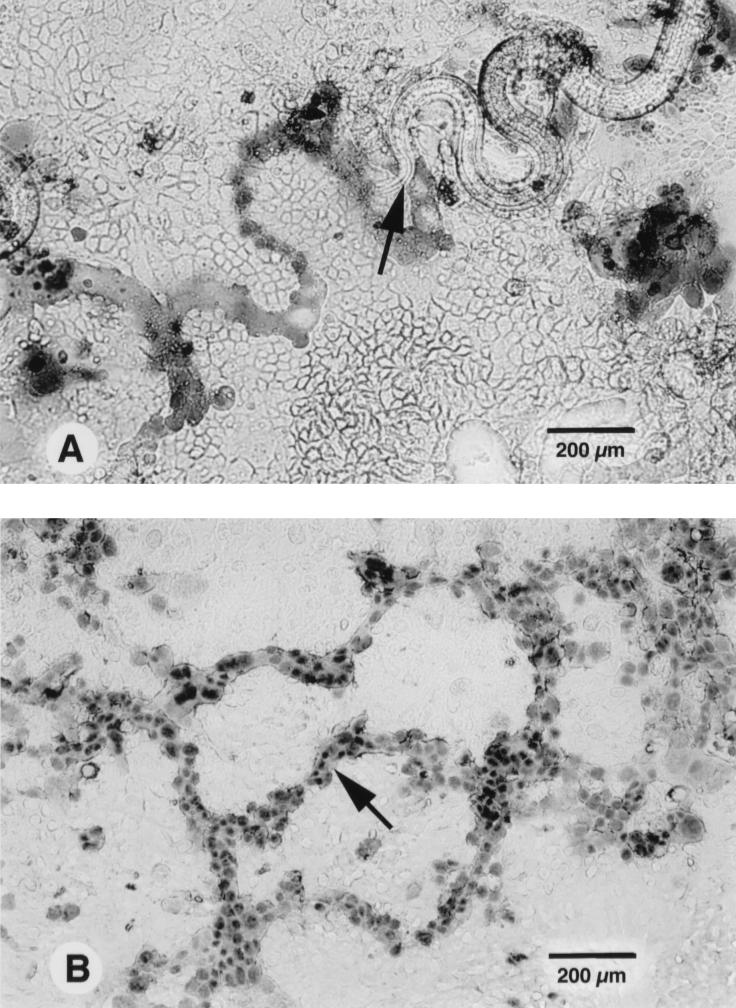
FIG. 1.
Light microscopy of human colonic epithelial monolayers infected by T. spiralis. (A) With trypan blue staining, the infective larva (arrow) can be seen to have left a trail of dead cells after invasion and migration in Caco-2 epithelial monolayers. (B) Trails of damaged T84 epithelial cells consist of many dead cells (arrow), which are larger than adjacent cells and have enlarged nuclei.
The invasive behavior of T. spiralis varied with the state of the larvae and the nature of the epithelial cell lines. Bile-treated infective larvae invaded epithelial cells more rapidly than freshly pepsin-digested infective larvae. Moreover, the degree of invasion of infective larvae in epithelial cells was in the order of Caco-2, T84, and HT29 cells, as indicated by the speed of invasion of the larvae and the extent of damage to epithelial cells (data not shown).
The morphology of the damaged epithelial cells after Trichinella invasion was studied by trypan blue and toluidine blue staining. With trypan blue staining, it was observed that the infective larvae left behind trails of dead cells (Fig. 1B). These cells were larger in size than adjacent cells. With toluidine blue staining, these dead cells were lightly stained whereas unaffected cells were stained deeply blue. The zigzag pattern of the trails reflected the serpentine movement of the larvae in the monolayers.
Ultrastructural changes in epithelial cells after Trichinella invasion.
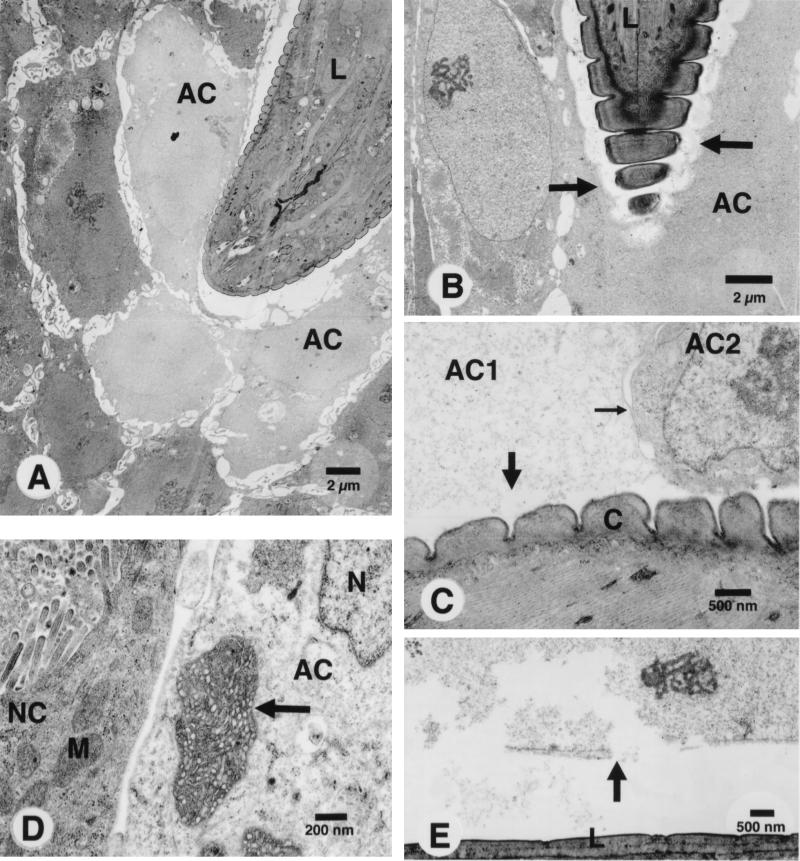
TEM studies showed that the epithelial cells were seriously damaged after invasion by T. spiralis. The infective larvae injured the cells sequentially as they moved through the monolayers, and only the cells in direct contact with the larvae were damaged (Fig. 2A). The infected cells were larger in size, and their cytoplasm and nuclei were less electron dense. The cell membranes of infected cells were severely disrupted (Fig. 2B). However, only the membrane in direct contact with the cuticular surface of the larva was disrupted (Fig. 2C), the apical and lateral membranes of the same cell remaining intact. Moreover, the infected cells showed either loss or dilution of cytoplasmic proteins. Other ultrastructural changes in the infected cells included swelling of nuclei and mitochondria (Fig. 2D). Disruption of cell membranes also extended to the nuclear membrane. When this was in direct contact with the larval surface, the nuclear membrane ruptured and the nucleus disintegrated (Fig. 2E). However, no nuclear fragmentation was observed. Therefore, it appeared that the larvae damaged the cells by disrupting the cell membranes. In consequence, the cells lost osmotic control, their organelles became swollen, and the infected cells eventually burst.
FIG. 2.
TEM of human colonic epithelial cells infected by T. spiralis. (A) Infective larva (L) penetrating an HT29 epithelial monolayer. The cytoplasm and nuclei of adjacent cells (AC) are less electron dense than intact cells. (B) Invasion of infective larva (L) into HT29 cells. Note the disruption (arrows) of the membranes of adjacent cells. (C) Damage of cells along the path of the larva. Note the disruption of the membrane (large arrow) and the loss of cytoplasmic proteins of the cell (AC1). Only the cell membrane in direct contact with the cuticle (C) of the larva is disrupted, whereas the lateral membrane (small arrow) of the same cell remains intact. (D) The mitochondrion (arrow) and nucleus (N) of the infected HT29 cell (AC) are swollen compared to the adjacent normal cell (NC) and its mitochondrion (M). (E) The nuclear membrane (arrow) of a Caco-2 cell in direct contact with the infective larva (L) is disrupted.
Production of cytokines and iNOS in epithelial cells in response to invasion by pathogens.
Three different intestinal epithelial cell lines were used to study the production of proinflammatory cytokines and inflammatory mediators in response to invasion. The response to T. spiralis was compared to that elicited by bacterial invasion, with L. monocytogenes used as a positive control.
By RT-PCR and ELOCA, it was shown that invasion by T. spiralis caused an elevation of mRNA of IL-1β, IL-8, and ENA-78 in the epithelial cells after 5 h of infection (Fig. 3). The observed increase was dose dependent. Levels of IL-1β, IL-8, and ENA-78 were increased even when 1,000 larvae were used, but much greater increases were seen with 5,000 larvae. However, no increase in iNOS mRNA was seen after Trichinella invasion at any dose level. No changes in mRNA for TNF-α or TGF-β were seen (data not shown). Infection with L. monocytogenes also caused an increase of IL-1β, IL-8, and ENA-78 in all cell lines, but the iNOS mRNA transcript was also upregulated. Similar results were obtained with other enteroinvasive bacteria (Salmonella enteritidis and E. coli [data not shown]).
Elevated mRNA expression for IL-8 correlated with increased secretion of IL-8 by infected epithelial cells. By sandwich ELISA, secreted IL-8 was found to be increased in all cell lines after infection with T. spiralis or L. monocytogenes (Table 2). Compared with medium and agar controls, epithelial cells produced significantly higher levels of IL-8 in the lower compartments of the cluster plate after 7 h of infection with T. spiralis or L. monocytogenes. The levels of IL-8 released by epithelial cells were similar whether they were infected with T. spiralis or L. monocytogenes.
TABLE 2.
Secretion of IL-8 by human colonic epithelial monolayers after infection with T. spiralis and L. monocytogenesa
| Infecting organism | IL-8 (pg/ml)b
|
||
|---|---|---|---|
| HT29 | T84 | Caco-2 | |
| None (medium control) | 24.31 ± 0.2 | 885.2 ± 64 | 24.34 ± 0.1 |
| None (agar control) | 31.42 ± 0.2* | 1,057 ± 120 | 25.64 ± 0.2 |
| T. spiralis | 45.56 ± 0.1* | 1,467 ± 106* | 35.97 ± 0.5* |
| L. monocytogenes | 41.89 ± 0.3* | 1,557 ± 112* | 33.75 ± 0.3* |
Monolayers of HT29, T84, and Caco-2 cells were grown on microporous membranes (Transwell; Costar) and infected with 103 infective larvae of T. spiralis or 107 CFU of L. monocytogenes per ml. After 7 h of infection, culture supernatants in the lower compartments were collected and levels of secreted IL-8 were measured by sandwich ELISA. The experiments were repeated twice.
Values are means ± standard errors of the means. ∗, significantly different from medium control (P < 0.05).
DISCUSSION
The outcome of infection of the gastrointestinal tract is determined by the host’s ability to mount innate and adaptive immune responses. The inflammatory changes which accompany these responses may play an important role in host defense but can also have pathological consequences. Studies with gastrointestinal nematodes have contributed to a greater understanding of the capacity of the intestine to mount immune and inflammatory responses to infection, especially those mediated by T cells (33). The majority of studies, however, have concentrated on the development and expression of these responses rather than on their induction. Recent studies of host defense against other pathogens have shown that early expression of innate, nonspecific immunity may determine the outcome of later adaptive, specific immune responses (9). For many invasive intestinal nematodes, epithelial cells are the first point of host contact. Data from many studies have shown that epithelial cells play an integral role in mucosal immune responses (1, 15). Epithelial responses to invasion by nematodes may therefore provide data relevant to the induction of mucosal immune and inflammatory responses.
Invasion by T. spiralis produced significant damage in epithelial cells at the ultrastructural level and resulted in increased mRNA for IL-1β, IL-8, and ENA-78. Cell damage was associated with disruption of the cell membrane. With the loss of osmotic control, the damaged cells showed increased size, dilution of cytoplasmic proteins, and swelling of organelles, eventually becoming necrotic and bursting. This is very different from bacterial infections in which epithelial cells die by apoptosis. For example, in Clostridium difficile infection, bacterial toxin A induced cell rounding, detachment, and apoptosis in epithelial cell lines and organ cultures of human colonic biopsy specimens (19). Moreover, apoptosis in HT29 epithelial cell lines induced by invasive S. dublin and E. coli was related to NO production (16).
Epithelial cell invasion is necessary for most intestinal pathogens to establish infection. Although various molecules which mediate adherence and invasion have been identified in some intracellular pathogens, in many cases the mechanism of invasion is still unclear. The in vitro model used here suggests that T. spiralis may injure epithelial cells by mechanical and biochemical means. Based on the speed of invasion, mechanical movement is likely to be the major method. However, the loss of membrane integrity of epithelial cells in direct contact with the larvae suggests that there may be molecules either on the surface or released in secretions which facilitate invasion. Excretory-secretory (ES) products of T. spiralis are known to contain serine proteases, cysteine proteases, and metalloproteinases (2, 30) and may contain pore-forming proteins similar to those of Trichuris nematodes, which also develop exclusively within the intestinal epithelium (6).
Data from many sources, with a variety of cell lines and pathogens, suggest that cell line models of pathogen invasion provide data relevant to in vivo events (8, 14, 25). Although these cell lines are all transformed, they are well established in studies of infection with bacteria, viruses, and protozoa. Primary cell lines, although closer to in vivo characteristics, are not suitable for experiments involving invasion of worm parasites, as they are more difficult to establish and maintain for long periods. The damage to epithelial cells by T. spiralis described here in vitro is similar to that obtained in vivo. By using TEM, it was shown that the membranes of invaded cells became increasingly convoluted the closer they were to the larvae (7). Damaged epithelial cells showed degenerative changes, such as loss of microvilli, disruption of apical membranes, and swelling of mitochondria. In 1979, Wright showed that the larvae lie within the cytoplasm of epithelial cells (36); however, this was not seen in the present study. The infective larvae appeared to invaginate rather than penetrate the cell membranes and came to lie in the cytoplasm after the cell membranes were disrupted. This difference may be due to differences in the three-dimensional architecture of the epithelium in vivo and in vitro.
Both nematode and bacterial invasions resulted in transcriptional activation of IL-1β, IL-8, and ENA-78, but only bacteria elicited increased iNOS mRNA. The upregulation of these proinflammatory cytokines may contribute to the initiation of acute inflammatory responses and restitution (epithelial cell repair). IL-1β is known to attract and activate macrophages, NK cells, and T and B cells and has been found by RT-PCR in primary epithelial cells from rats after 2 days of infection with T. spiralis (28). IL-8 and ENA-78 are C-X-C chemokines that are potent chemoattractants and activators of neutrophils (31). Epithelial cell-derived IL-8 released as a result of bacterial invasion was shown to attract neutrophils by transforming the matrix components of epithelial cells (22). Moreover, IL-8 was recently shown to attract T cells and monocytes via degranulation of neutrophils (29). Both IL-1β and IL-8 were shown to stimulate intestinal restitution in IEC-6 cell lines (4, 5). ENA-78 is a newly discovered neutrophil chemoattractant initially isolated from the conditioned media of the IL-1- or TNF-activated A549 human lung type II alveolar epithelial cell line (35). The amino acid sequence of ENA-78 shows 22% similarity to that of IL-8 and 48 to 51% similarity to GRO-α/β/γ. Based on cross-desensitization experiments, it has been suggested that ENA-78 activity is mediated through the IL-8 receptor type (17). Increases in the level of ENA-78 have been detected in epithelial cells in patients with inflammatory bowel diseases (38). Recent studies of activated human colonic epithelial cells showed that the patterns of production of IL-8 and ENA-78 were different (37). IL-8 production was very short-lived, whereas ENA-78 was long lasting but delayed in onset, occurring only when IL-8 secretion was downregulated. This suggested that IL-8 and ENA-78 may be important in both early and later stages of mucosal inflammation and could therefore contribute to the pattern of inflammatory responses seen in T. spiralis infection.
The upregulation of mRNA transcripts of iNOS in epithelial cells after invasion by bacteria suggests that NO may be important in mucosal defense. NO from activated macrophages is known to control and inhibit intracellular pathogens, and NO from epithelial cells has recently been shown to inhibit the intraepithelial growth of Chlamydia species (12). The differences in the expression of iNOS in epithelial cells injured by bacteria and T. spiralis may be due to the expression of iNOS in epithelial cells that undergo apoptosis. NO has been shown to induce apoptosis in intestinal epithelial cells after bacterial invasion (16). Epithelial NO may not be important in innate defense mechanisms against multicellular parasites, and this may explain the lack of iNOS induction in T. spiralis-injured epithelial cells. However, other animal studies showed that when rats were given l-NAME, a nonselective inhibitor of nitric oxide synthase, orally for 6 days, there was increased NO activity in the mucosa and a reduced number of adult worms of T. spiralis in the small intestine (11). Therefore, it appears that iNOS from other cellular sources may be involved in the acquired effector mechanism against intestinal nematodes.
In conclusion, the data presented here show that transcriptional activation of proinflammatory cytokines follows invasion of intestinal epithelial cells by both prokaryotic and eukaryotic pathogens. In the case of T. spiralis, these cytokines may initiate the protective acute inflammatory response by recruiting and activating cells in the lamina propria. The patterns of production of proinflammatory cytokines and inflammatory mediators are different between different groups of invasive pathogens.
ACKNOWLEDGMENTS
This work was supported by grants from the Hong Kong Croucher Foundation and the University of Nottingham. The electron microscopy unit of the Histopathology Department was generously supported by Wellcome Trust (grant 048326). Chris Li is a postdoctoral research fellow of the Hong Kong Croucher Foundation.
Judy Appleton gave helpful advice.
REFERENCES
- 1.Christ A D, Blumberg R S. The intestinal epithelial cells: immunological aspects. Springer Semin Immunopathol. 1996;18:449–461. doi: 10.1007/BF00824052. [DOI] [PubMed] [Google Scholar]
- 2.Criado-Fornelio A, Armas-Serra C, Gimenez-Pardo C, Casado-Escribano N, Jimenez-Gonzalez A, Rodriguez-Caabeiro F. Proteolytic enzymes from Trichinella spiralis larvae. Vet Parasitol. 1992;45:133–140. doi: 10.1016/0304-4017(92)90035-8. [DOI] [PubMed] [Google Scholar]
- 3.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin-8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 4.Dignass A U, Podolsky D K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 5.Dignass A U, Hotz A, Becker A, Goebell H. Interleukin-8 (IL-8) stimulates intestinal epithelial restitution in vitro. Gastroenterology. 1997;112:A960. [Google Scholar]
- 6.Drake L, Korchev Y, Bashford L, Djamgoz M, Wakelin D, Ashall F, Bundy D. The major secreted product of the whipworm, Trichuris, is a pore-forming protein. Proc R Soc Lond B. 1994;257:255–261. doi: 10.1098/rspb.1994.0123. [DOI] [PubMed] [Google Scholar]
- 7.Dunn I J, Wright K A. Cell injury caused by Trichinella spiralis in the mucosal epithelium of B10A mice. J Parasitol. 1985;71:757–766. [PubMed] [Google Scholar]
- 8.Eckmann L, Reed S L, Smith J R, Kagnoff M F. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J Clin Invest. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 10.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 11.Hogaboam C M, Collins S M, Blennerhassett M G. Effects of oral L-NAME during Trichinella spiralis infection in rats. Am J Physiol. 1996;271:G338–G346. doi: 10.1152/ajpgi.1996.271.2.G338. [DOI] [PubMed] [Google Scholar]
- 12.Igietseme J U, Uriri I M, Chow M, Abe E, Rank R. Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem Biophys Res Commun. 1997;232:595–601. doi: 10.1006/bbrc.1997.6335. [DOI] [PubMed] [Google Scholar]
- 13.James S. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J M, Witthoft T, Eckmann L, Kagnoff M F. Bacterial invasion of intestinal epithelial cells induces programmed cell death. Gastroenterology. 1997;112:A1013. [Google Scholar]
- 17.Koch A E, Kunkel S L, Larlow L A, Mazarakis D D, Haines G K, Burdick M D, Pope R M, Strieter R M. Macrophage inflammatory protein-1α. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–928. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsh J E, Race G J. Allergic inflammation as a hypothesis for the expulsion of worms from tissues: a review. Exp Parasitol. 1975;37:251–266. doi: 10.1016/0014-4894(75)90077-6. [DOI] [PubMed] [Google Scholar]
- 19.Mahida Y R, Makh S, Hyde S, Gray T, Borriello S P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ManWarren T, Gagliardo L, Geyer J, McVay C, Pearce-Kelling S, Appleton J. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect Immun. 1997;65:4806–4812. doi: 10.1128/iai.65.11.4806-4812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick B A, Colgan S P, Delp-Archer C, Miller G, Blaser M J, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlan J M, Seth R, Vautier G, Robins A, Scott B, Hawkey C J, Jenkins D. Interleukin-8 and inducible nitric oxide synthase mRNA levels in inflammatory bowel disease at first presentation. J Pathol. 1997;81:87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Miller M J S, Thompson J H, Zhang X J, Sadowska-Krowicka H, Kakkis J, Munshi U K, Sandoval M, Rossi J L, Eloby-Childress S, Beckman J S, Ye Y Z, Rodi C P, Manning P T, Currie M G, Clark D A. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;190:1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruitenberg E J, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature (London) 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 27.Singer I I, Kawka E W, Scott S, Weidner J R, Mumford R A, Riehl T E, Stenson W F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 28.Stadnyk A W, Kearsey J A. Pattern of proinflammatory cytokine mRNA expression during Trichinella spiralis infection of the rat. Infect Immun. 1996;64:5138–5143. doi: 10.1128/iai.64.12.5138-5143.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taub D D, Anver M, Oppenheim J J, Longo D L, Murphy W J. T lymphocyte recruitment by interleukin-8 (IL-8): IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97:1931–1941. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todorova V K, Knox D P, Kennedy M W. Proteinases in the excretory/secretory products (ES) of adult Trichinella spiralis. Parasitology. 1995;111:201–208. doi: 10.1017/s0031182000064957. [DOI] [PubMed] [Google Scholar]
- 31.Van Damme J. Interleukin-8 and related chemotactic cytokines. In: Thomson A, editor. The cytokine handbook. 2nd ed. London, United Kingdom: Academic Press; 1994. pp. 184–208. [Google Scholar]
- 32.Wakelin D, Denhem D A. The immune responses. In: Campbell W C, editor. Trichinella and trichinosis. New York, N.Y: Plenum Press; 1983. pp. 265–308. [Google Scholar]
- 33.Wakelin D, Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976;72:173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]
- 34.Wakelin D, Grencis R K. T-cell and genetic control of inflammatory cells. In: Moqbel R, editor. Allergy and immunity to helminths. London, United Kingdom: Taylor and Francis; 1992. pp. 107–136. [Google Scholar]
- 35.Walz A, Burgener R, Car B, Baggiolini M, Kunkel S L, Strieter R M. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin-8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright K A. Trichinella spiralis: an intracellular parasite in the intestine phase. J Parasitol. 1979;65:441–445. [PubMed] [Google Scholar]
- 37.Yang S K, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 38.Z’Graggen K, Walz A, Mazzucchelli L, Strieter R M, Muller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113:808–816. doi: 10.1016/s0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]