Abstract
This study was carried out from February 2020 to September 2021 in Parque Nacional das Emas (PNE), a national park located in the Cerrado biome, midwestern Brazil, as well as in surrounding rural properties. Serum and tick samples were collected from dogs, terrestrial small mammals, and humans. Ticks were also collected from the environment. Dogs were infested with Rhipicephalus linnaei adults, whereas small mammals were infested by immature stages of Amblyomma spp., Amblyomma triste, Amblyomma dubitatum, and Amblyomma coelebs. Ticks collected from vegetation belonged to several species of the genus Amblyomma, including A. coelebs, A. dubitatum, Amblyomma naponense, Amblyomma sculptum, and A. triste. Two Rickettsia species were molecularly detected in ticks: Rickettsia parkeri in A. triste from the vegetation and a Rickettsia sp. (designated Rickettsia sp. strain PNE) in A. sculptum and A. triste collected from lowland tapirs (Tapirus terrestris). Based on short gltA gene fragments, this rickettsial organism showed 99.7–100% to Rickettsia tillamookensis. Seroreactivity to Rickettsia antigens was detected in 21.9% of dogs, 15.4% of small mammals, and 23.5% of humans. The present study reveals the richness of ticks and demonstrates the circulation of rickettsial agents in one of the largest conservation units in the Cerrado biome in Brazil. To our knowledge, this is the first report of a rickettsial phylogenetically related to R. tillamookensis in Brazil.
Keywords: Amblyomma sculptum, Amblyomma triste, Rickettsia parkeri, Rickettsia tillamookensis
1. Introduction
The Cerrado biome is a vast ecoregion of tropical savanna, being the second largest biome in South America and the most diverse savanna in the world. In fact, it is one of the 35 hotspots of global biodiversity, harboring a considerable richness of endemic animals and plants [1,2,3]. In Brazil, it extends over a continuous area in the central region, plus discontinuous areas in the south and north of the country [2]. Despite the importance of this biome, only 6.5% of its natural vegetation cover is currently protected [4].
Parque Nacional das Emas (PNE) is one of the most important national parks within the Cerrado biome. Around 85 species of native mammals have been recorded in the PNE and its surroundings [5,6]. This national park has been the scene of several scientific research studies on animals and their associated pathogens [7,8,9]. For instance, previous studies reported the presence of at least 10 different species of ticks infesting medium- to large-sized mammals in PNE [7,10]. Among the reported tick species, Amblyomma sculptum (referred to as ‘Amblyomma cajennense’) is of great importance, not only because of its broad host range but also because it is the primary vector of Rickettsia rickettsii, a spotted fever group rickettsiae (SFGR). In Brazil, R. rickettsii causes Brazilian spotted fever, the deadliest tick-borne disease of the western hemisphere [11]. Nonetheless, the circulation of SFGR in A. sculptum and other tick species of the PNE has never been investigated.
The PNE, its ticks, and their associated pathogens represent an interesting research model to study considering the limited human influence on this area. Indeed, studies in such a preserved area could provide novel information on the tick-borne rickettsial agents circulating in Brazil. Therefore, the present study was designed to investigate the circulation of Rickettsia spp. in ticks from animals, humans, and the environment in PNE. We also assessed the exposure of dogs, terrestrial small mammals, and humans to rickettsiae.
2. Materials and Methods
2.1. Study Area
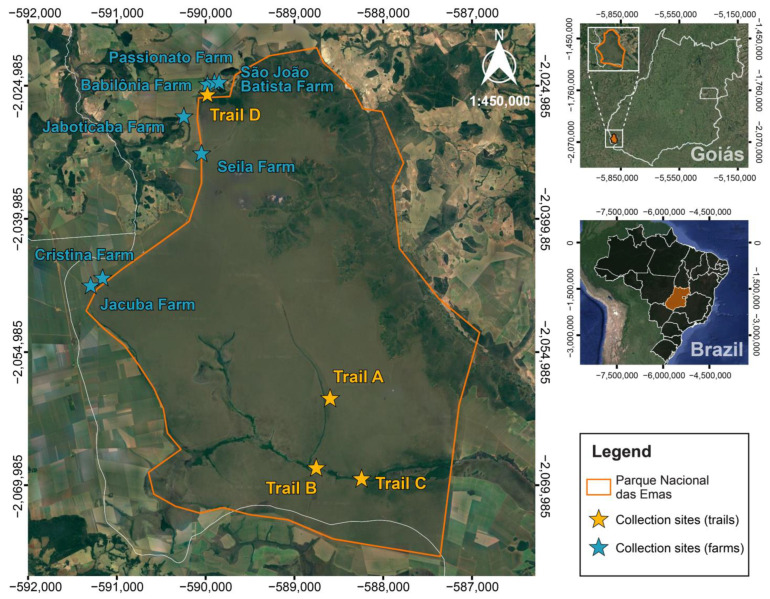
PNE is in the southwestern portion of Goiás state, midwestern Brazil (Figure 1). The park is named after the large number of rheas (Rhea americana) that inhabit the region, which comprises an area of ~132,642 hectares. The climate type is Aw (Köppen climate classification), characterized as a tropical savanna climate with dry winter and rainy summer, with up to five months of drought. The average annual temperature is around 25 °C, ranging from 20 °C to 31 °C during the autumn–winter and spring–summer, respectively. The park is surrounded by rural properties with extensive soy and corn plantations.
Figure 1.
Locations of farms surrounding the park and trails within Parque Nacional das Emas (PNE) in the state of Goiás, midwestern Brazil, where animals, humans, and ticks were sampled in the present study.
Tick collection sites included trails A (18°11′38″ S and 52°52′29″ W, altitude 824 m), B (18°15′38″ S and 52°53′20″ W, altitude 779 m), C (18°16′16″ S and 52°50′35″ W, altitude 778 m), and D (17°54′06″ S and 52°59′56″ W, altitude 815 m) (Figure 1). Trails A, B, and C were established in a seasonal forest with marsh areas (seasonally flooded forest) and Cerrado sensu stricto (typical savanna) (Figure 1). Trail D was in an open field [12]. Tick collections from the environment and small mammal trappings were carried out at the four pre-established trails during three expeditions: (1) February 2020 (summer), (2) October 2020 (spring), and (3) September 2021 (winter). Blood samples were collected from terrestrial small mammals trapped inside the PNE and from dogs and humans in the surrounding rural areas. Ticks were sampled from dogs, terrestrial small mammals, researchers, and the environment. This study was previously approved by the Chico Mendes Institute for Biodiversity (ICMBio Permit No. 70143-1), the Institutional Animal Care and Use Committee (CEUA/UFG) of the Federal University of Goiás (protocol no. 121/19), and the Research Ethics Committee (CEP/UFG) (protocol no. 3.857.973).
2.2. Terrestrial Small Mammal Trapping
To capture small mammals, 126 cage-type traps (97 Sherman and 29 Tomahawk) were used with different baits (mortadella, smoked sausage, bacon, peanut butter, pumpkin, banana, and apple). Traps were installed for five consecutive nights during three collection campaigns. Additionally, three pitfall stations (with six buckets of 42.5 cm diameter and 65 cm height in each station) connected with a plastic fence (20 m long and 50 cm high) [13] were installed during the same period as the cage-type traps. The total capture effort was 1890 cage-type trap-nights and 270 fall-type trap-nights. The traps were inspected daily in the morning, and the captured animals were placed in cloth bags and properly anesthetized with 90 mg/kg of ketamine +50 mg/kg of xylazine intramuscularly to collect material (ticks and blood) [14].
Blood samples from small mammals were obtained with facial or caudal venipuncture using 1 mL syringes and 13 × 0.45 mm hypodermic needles. Each animal was marked with a numbered earring (fish and small animal tag size 1; National Band and Tag, Newport, KY, USA) and identified using different identification keys and species descriptions [15]. Some captured animals, whose species identification in the field was not possible, were euthanized, fixed, and sent for later identification by a mammal taxonomist.
2.3. Samples from Dogs and Humans
During the field campaigns in the surroundings of the PNE, seven farms (Figure 1) were visited, and blood samples were obtained from dogs and humans. Dogs were also examined for tick infestations on the entire body and humans on clothing, arms, legs, neck, and hands. A brief questionnaire-based interview was carried out with the dog owners to select animals 4 months or older and with free access to the forest environment.
Human blood samples were collected with intermediate cubital venipuncture and dog blood samples with jugular or cephalic venipuncture. Right after collection, the blood samples were placed into tubes without anti-coagulant and kept at room temperature (25 °C) until visible clot retraction and then centrifuged at 3000× g for 5 min. The obtained sera were transferred into 1.5 mL tubes and stored at –20 °C until processing.
2.4. Detection of Antibodies to Rickettsia spp.
Sera from dogs, rodents, marsupials, and humans were tested using an immunofluorescence assay (IFA) targeting four Rickettsia antigens isolated from Brazil (R. rickettsii strain Pampulha, Rickettsia parkeri strain Atlantic rainforest, Rickettsia amblyommatis strain Ac37, and Rickettsia bellii strain Mogi), as previously described [16,17,18]. Briefly, sera were diluted in two-fold increments with phosphate-buffered saline (PBS) from an initial dilution of 1:64. The slides were incubated with fluorescein isothiocyanate-labelled rabbit anti-dog IgG (Sigma, St Louis, MO, USA), goat anti-mouse IgG (Sigma), sheep anti-opossum IgG (CCZ, São Paulo, Brazil), rabbit anti-human IgG (IgG, Sigma, St. Louis, MO, USA), and rabbit anti-guinea pig IgG (Sigma) for canine, Cricetidae rodent, marsupial, human, and Echymidae rodent (Clyomys laticeps) sera, respectively. For each sample, the endpoint IgG titer reacting with each of the four Rickettsia antigens was determined. An endpoint titer at least four-fold higher for a Rickettsia species than those observed for the other Rickettsia species was considered probably homologous to the first Rickettsia species or a very closely related species [17,19]. On each slide, a non-reactive serum (negative control) and reactive serum (positive control) from dogs, rodents, marsupials, or humans from the other studies were tested at a 1:64 dilution [14,19,20].
2.5. Tick Collection and Identification
Each small mammal and dog was carefully inspected for the presence of ticks for five minutes. Host-seeking ticks were collected from the environment using the cloth dragging technique and by searching visually for ticks on the vegetation, as previously described [21,22,23].
Ticks were also collected from a ‘domesticated’ tapir (Tapirus terrestris) that inhabited the premises of the PNE and from an anteater (Myrmecophaga tridactyla) found dead on the GO-341 highway (17°39′26.49″ S and 52°55′48.49″ W) during the first expedition to the PNE, as well as from the researchers’ skin or clothing during field collections.
The ticks were removed with the aid of toothless tweezers, placed in 50 mL conical tubes containing isopropyl alcohol, and kept at room temperature until taxonomic identification in the laboratory. The ticks were identified to the species level under a stereomicroscope using descriptions and taxonomic keys [24,25,26,27]. Because of the absence of taxonomic keys for Amblyomma larvae from Brazil, they were identified to the genus level only [28].
2.6. DNA Extraction and Molecular Detection of Rickettsia
The ticks collected in this study were randomly selected and individually processed for DNA extraction using the guanidine isothiocyanate and phenol/chloroform technique [29]. In addition to ticks, blood samples collected from small terrestrial mammals and humans were also subjected to DNA extraction using the DNAeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s recommendations.
DNA from ticks and blood samples were tested with a TaqMan real-time qPCR assay targeting a 147 bp fragment of the rickettsial citrate synthase (gltA) gene [30,31]. The qPCR-positive samples were additionally tested using a panel of conventional PCR assays targeting four rickettsial genes and two intergenic regions. PCR assays were performed using primers targeting fragments of the following protein-coding genes and intergenic regions: a 401 bp fragment of the gltA gene; a 532 bp fragment of the ompA gene; a 449 bp fragment of the atpA gene; a fragment (unknown size) of the coxA gene [30,32,33,34]; 351 bp and 144 bp fragments of the intergenic regions RC1027-xthA2 and rpmE-tRNAfmet, respectively [35].
Conventional PCR products (gltA and ompA genes) were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and sequenced using the BigDyeTM Terminator v3.1 Matrix Standards Kit (Applied Biosystems, Foster City, CA, USA) at the Núcleo de Plataformas Tecnológicas (Fiocruz PE). Sequencing reactions were carried in both directions in a 3500× L Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the same primers as for the conventional PCR assays.
PCR-negative samples were further tested using PCR protocols targeting the 16S rDNA gene of ticks [36] or the cytochrome b (cytB) gene of mammals [37,38] to validate the DNA extraction protocol. If a sample did not produce any product in these PCR assays, the sample was discarded.
2.7. Phylogenetic Analysis and Molecular Identification
We assembled and analyzed consensus sequences using the Sequencher® v. 5.4.6 (http://www.genecodes.com (accessed on 10 October 2023)), considering a Phred quality score of ≥30. Then, consensus sequences were subjected to similarity searches against the GenBank Rickettsiales database (taxid:766) using the Basic Local Alignment Search Tool (BLASTn; http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 October 2023)). For phylogenetic reconstruction, sequences of the gltA gene were manually retrieved from genomes of Rickettsia spp. available in GenBank [39]. We used the MAFFT algorithm’s integrative refinement method FFT-NS-I for sequence alignment [40]. Dataset ends were trimmed, and a final alignment of 350 bp in length was used for phylogenetic reconstruction, as previously performed [41]. In brief, we reconstructed the Rickettsia phylogenetic tree using maximum likelihood inference with IQ-TREE 2 [42] with an ultrafast bootstrap (1000 replicates) for branch support. The best-fit evolutionary model was determined using ModelFinder [43], implemented in IQ-TREE, and selected based on the Bayesian Information Criterion (BIC). We utilized iTOL v.5 [44] to visualize and edit the phylogenetic tree.
3. Results
3.1. Collected Samples
Samples were collected from 41 dogs, of which 53.6% (22/41) were females and 46.3% (19/41) were males. Of these, 17% (7/41) were infested with 57 ticks, all identified as adults of Rhipicephalus linnaei (32 females and 25 males).
A total of 26 terrestrial small mammals belonging to 12 species (two marsupials and ten rodents) was captured. In particular, 11.5% (3/26) were didelphid marsupials (including one Didelphis albiventris and two Gracilinanus agilis), and 88.5% (23/26) were rodents belonging to the Cricetidae (one Calomys tener, one Oecomys cleberi, one Oecomys roberti, four Nectomys squamipes, six Necromys lasiurus, six Oligoryzomys cf. mattogrossae, one Cerradomys scotti, one Cerradomys maracajuensis, and one Oxymycterus delator), and Echimyidae families (one Clyomys laticeps). Of these, 46.1% (12/26) were females, and 53.8% (14/26) were males. Considering the three trails separately, the highest capture success was on trail C, totaling 53.8% (14/26), followed by trail A at 26.9% (7/26) and trail B at 19.2% (5/26).
Out of 28 humans enrolled in this study, 35.7% (10/28) were females, and 64.3% (18/28) were males. Of the total, 17 were blood-sampled and 11 were examined for the presence of ticks on their clothing, arms, legs, necks, and hands.
A total of 3211 ticks were sampled, of which 1.8% (57/3211) were from dogs, 8.4% (271/3211) from terrestrial small mammals, 0.5% (15/3211) from a tapir, 0.4% (13/3211) from an anteater, 0.5% (15/3211) from humans, and 88.4% (2840/3211) from the environment.
3.2. Serology of Animals and Humans
Among dogs, 21.9% (9/41) reacted to one or more rickettsial antigens, with 7.3% (3/41) to R. rickettsii (endpoint titer: 64), 17.1% (7/41) to R. bellii (endpoint titers: 64–512) and 4.9% (2/41) to R. amblyommatis (endpoint titers: 64). No dog reacted to R. parkeri. Four dogs (9.7%) showed a homologous reaction to R. bellii, whereas another two dogs (4.9%) showed inconclusive reactivities to R. rickettsii and R. amblyommatis. Concerning small mammals, 8.7% (2/23) of the rodents reacted to R. parkeri (endpoint titer: 128), while 66.7% (2/3) of the marsupials (one G. agilis and one D. albiventris) reacted to R. rickettsii (endpoint titer: 128) and R. amblyommatis (endpoint titer: 64), respectively. Among humans, 23.5% (4/17) reacted to at least one Rickettsia spp. antigen, with 17.6% (3/17) of them showing a homologous reaction to R. bellii (endpoint titers: 128–256) (Table 1).
Table 1.
Seroreactivity to four Rickettsia species in humans, dogs, and terrestrial small mammals from Parque Nacional das Emas (PNE), state of Goiás, from February 2020 to September 2021.
| Humans, Dogs, and Terrestrial Small Mammal Species (Tested Specimens) |
Number of Animals Reactive to Each Rickettsia spp./% Seroreactivity (Endpoint Titers) | No. Individuals with PAIHR a | |||
|---|---|---|---|---|---|
| R. rickettsii | R. parkeri | R. bellii | R. ammblyommatis | ||
| Humans (17) | 0/0 | 0/0 | 3/17.6 (128–256) | 1/5.9 (64) | 3 R. bellii |
| Dogs (41) | 3/7.3 (64) | 0/0 | 7/17.1 (64–512) | 2/4.9 (64) | 4 R. bellii |
| Calomys tener (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Cerradomys maracajuensis (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Cerradomys scotti (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Clyomys laticeps (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Didelphis albiventris (1) | 0/0 | 0/0 | 0/0 | 1/100 (64) | |
| Gracilinanus agilis (2) | 1/50 (128) | 0/0 | 0/0 | 0/0 | 1 R. rickettsii |
| Necromys lasiurus (6) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Nectomys squamipes (4) | 0/0 | 1/25 (128) | 0/0 | 0/0 | 1 R. parkeri |
| Oecomys cleberi (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Oecomys roberti (1) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Oligoryzomys cf. mattogrossae (6) | 0/0 | 0/0 | 0/0 | 0/0 | |
| Oxymycterus delator (1) | 0/0 | 1/100 (128) | 0/0 | 0/0 | 1 R. parkeri |
| TOTAL (84) | 4/4.8 | 2/2.4 | 10/12 | 4/4.8 | |
a PAIHR, possible antigen involved in a homologous reaction. A homologous reaction was determined when the endpoint titer to a Rickettsia species was at least four-fold higher than the endpoint titers observed for the other three Rickettsia species. In this case, the Rickettsia species (or a very closely related genotype) involved in the highest endpoint titer was considered the PAIHR.
3.3. Tick Identification
Of the 3211 ticks collected, 5.9% (189/3211) were larvae, 71.2% (2287/3211) were nymphs, and 22.9% (735/3211) were adults. The collected ticks were identified as A. sculptum (2200 nymphs, 286 females, 364 males), R. linnaei (32 females, 25 males), Amblyomma spp. (189 larvae), Amblyomma coelebs (two nymphs), Amblyomma dubitatum (11 nymphs), Amblyomma naponense (one nymph, one female), Amblyomma parvum (one male), and Amblyomma triste (73 nymphs, 16 females, 10 males) (Table 2).
Table 2.
Ticks collected from humans and animals from Parque Nacional das Emas (PNE), state of Goiás, from February 2020 to September 2021.
| Tick Host (Individuals) | A. sp. | A. coe | A. dub | A. par | A. scu | A. tri | R. lin | ||
|---|---|---|---|---|---|---|---|---|---|
| L | N | N | A | N | A | N | A | A | |
| Humans (11) | 1 (1) | 12 (5) | 2 (1) | ||||||
| Dogs (41) | 57 (7) | ||||||||
| Didelphis albiventris (1) | 5 (1) | 1 (1) | 4 (1) | ||||||
| Cerradomys maracajuensis (1) | 4 (1) | 1 (1) | |||||||
| Cerradomys scotti (1) | 41 (1) | 1 (1) | |||||||
| Necromys lasiurus (6) | 2 (2) | 3 (1) | |||||||
| Nectomys squamipes (4) | 38 (4) | 5 (1) | 4 (1) | ||||||
| Oligoryzomys cf. mattogrossae (6) | 66 (4) | 9 (3) | |||||||
| Oxymycterus delator (1) | 32 (1) | 55 (1) | |||||||
| Tapirus terrestris (1) | 2 (1) | 13 (1) | |||||||
| Myrmecophaga tridactyla (1) | 13 (1) | ||||||||
| TOTAL | 188 (14) | 1 (1) | 10 (3) | 1 (1) | 12 (5) | 17 (3) | 72 (7) | 13 (1) | 57 (7) |
A. sp.: Amblyomma sp.; A. coe: Amblyomma coelebs; A. dub: Amblyomma dubitatum; A. nap: Amblyomma naponense; A. par: Amblyomma parvum; A. scu: Amblyomma sculptum; A. tri: Amblyomma triste; R. lin: Rhipicephalus linnaei; L: larvae; N: nymph; A: adult.
Most ticks (88.5%) were found on the vegetation (trails A, B, C, and D). These included A. sculptum (2188 nymphs and 633 adults), A. triste (one nymph and 13 adults), A. coelebs (one nymph), A. dubitatum (one nymph), and A. naponense (one nymph and one adult).
Amblyomma sculptum was found on the researchers’ clothing (0.4%; 13/3211) and other animals (0.5%, 15/3211). In total 11.5% (371/3211) of the ticks were collected from animals, including 8.4% from rodents and marsupials, 1.8% from dogs, 0.9% from the anteater and tapir, and 0.5% from humans (Table 2).
Among the terrestrial small mammals captured, 57.7% (15/26) were infested with a total of 271 ticks (188 larvae and 83 nymphs) that were identified as Amblyomma spp. (188 larvae), A. triste (72 nymphs), A. dubitatum (ten nymphs), and A. coelebs (one nymph). Four species of ticks were found infesting the terrestrial small mammals, with a spy hocicudo (O. delator) being the most infested animal with 32 Amblyomma larvae and 55 A. triste nymphs (Table 2).
Ticks collected opportunistically from the researchers were identified as A. sculptum (12 nymphs, two females) and A. parvum (one male). Finally, the tapir was infested with A. triste (eight females and five males) and A. sculptum (two females), whereas the anteater was infested with A. sculptum (four females and nine males) (Table 2).
The following voucher tick specimens were deposited in the tick collection “Coleção Nacional de Carrapatos do Cerrado” (CNCC) of the Veterinary and Animal Science School, Federal University of Goiás (CNCC 041–CNCC 072): 189 Amblyomma spp. larvae (CNCC 045, CNCC 060–062, CNCC 064–066, CNCC 068–070, CNCC 072), 1.798 A. sculptum nymphs (CNCC 045, CNCC 055–059), 406 A. sculptum adults (CNCC 047, CNCC 050, CNCC 052–053, CNCC 055), nine A. dubitatum nymphs (CNCC 041, CNCC 066, CNCC 068–069), two A. coelebs nymphs (CNCC 042, CNCC 067), 39 A. triste nymphs (CNCC 063, CNCC 066, CNCC 071), 11 A. triste adults (CNCC 043–044, CNCC 048, CNCC 051), one A. naponense nymph (CNCC 046), one A. naponense adult (CNCC 046), one A. parvum adult (CNCC 049), and 24 R. linnaei adults (CNCC 0054).
3.4. Molecular Detection of Rickettsia spp.
Upon qPCR testing, all samples from small mammals and humans were negative for the gltA gene of rickettsiae.
The ticks (n = 550) that were randomly selected for molecular analysis are described in Table 3. Of these, 1.4% (8/550) were positive for the gltA gene and 0.2% (1/550) for the ompA gene. Ticks that were positive only for the gltA gene were collected from the tapir (A. triste and A. sculptum), while the A. triste adult positive for both the gltA and ompA genes was collected from the environment (Table 3).
Table 3.
Molecular detection of rickettsial DNA in ticks collected from vegetation, humans, and animals from Parque Nacional das Emas (PNE), state of Goiás, a non-endemic area for Brazilian spotted fever, from February 2020 to September 2021.
| Tick Species | Tick Stages | Source | No. Ticks with Rickettsial DNA/No. Tested Ticks (% Positive) |
Rickettsia Species Identified by DNA Sequencing (GenBank Accession Number) |
|---|---|---|---|---|
| Amblyomma sculptum | Nymph | Vegetation | 0/255 (0) | |
| Adult | Vegetation | 0/207 (0) | ||
| Human | 0/1 (0) | |||
| Tapirus terrestris | 1/1 (100) | Rickettsia sp. strain PNE (OR289682) | ||
| Myrmecophaga tridactyla | 0/4 (0) | |||
| Amblyomma triste | Nymph | Vegetation | 0/1 (0) | |
| Cerradomys maracajuensis | 0/1 (0) | |||
| Necromys asiurus | 0/2 (0) | |||
| Nectomys squamipes | 0/2 (0) | |||
| Oligoryzomys cf. mattogrossae | 0/6 (0) | |||
| Oxymycterus delator | 0/19 (0) | |||
| Adult | Vegetation | 1/9 (11.1) | R. parkeri (OR289686, OR728038) | |
| Tapirus terrestris | 6/6 (100) | Rickettsia sp. strain PNE (OR289684, OR289685, OR728036, OR728037) | ||
| Amblyomma dubitatum | Nymph | Cerradomys scotti | 0/1 (0) | |
| Nectomys squamipes | 0/2 (0) | |||
| Didelphis albiventris | 0/2 (0) | |||
| Rhipicephalus linnaei | Adult | Dog | 0/31 (0) | |
| TOTAL | 8/550 (1.4) |
A total of seven sequences (five gltA, one ompA, and one rpmE-tRNAfmet) were successfully obtained and analyzed with BLAST. gltA sequences presented high percent identity (range 99.7–100% with 100% coverage) with Rickettsia tillamookensis strain Tillamook (GenBank accession number: CP060138.2), whereas the ompA sequence was 100% identical to Rickettsia parkeri strain RS (MN114096.1). Attempts to sequence other genes (atpA and coxA) or intergenic regions (RC1027-xthA2 and rpmE-tRNAfmet) for the gltA-positive samples were unsuccessful (short fragments, no consensus, poor reading). However, a rpmE-tRNAfmet sequence was successfully generated for the ompA-positive tick, and the sequence was 100% identical to R. parkeri isolate Sandhill Crane (CP101541.1).
The rickettsial sequences generated in the present study were deposited in GenBank under the accession numbers: OR289682, OR289684, OR289685, OR728036, and OR728037 for the gltA of Rickettsia sp. strain PNE, OR289686, and OR728038 for ompA and rpmE-tRNAfmet of R. parkeri, respectively (Table 3).
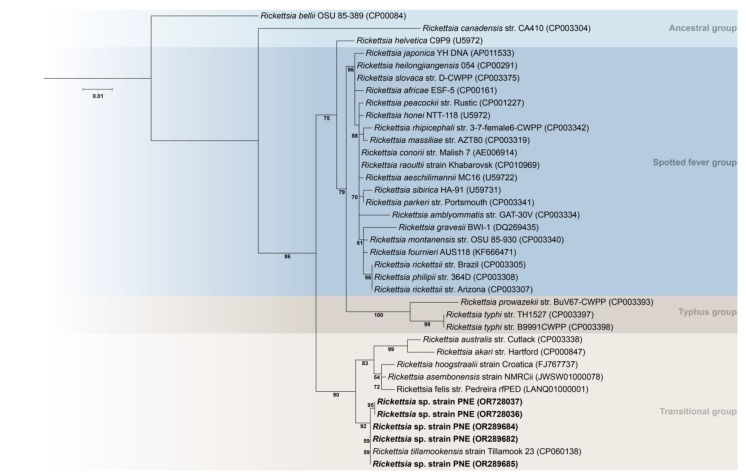
Our phylogenetic analysis based on obtained partial gltA gene fragments placed all five of the Rickettsia sp. strain PNE in a clade containing R. tillamookensis within the transitional group (Figure 2).
Figure 2.
Phylogenetic tree reconstruction of the Rickettsia genus based on a fragment of the gltA gene using a dataset of 37 sequences (31 species) and 350 bp in length. The tree was inferred using the maximum-likelihood method with 1000 replicates of ultrafast bootstrap (numbers at the nodes; values < 50 were omitted) and the substitution model K3Pu + F + I. Rickettsia bellii OSU 85-389 (CP00084) was used as an outgroup. GenBank accession numbers are in parentheses. Sequences originally generated in the present study are in bold.
4. Discussion
The present study identified seven tick species and two rickettsiae and provided serological evidence of rickettsial exposure in domestic dogs, small mammals, and humans in a national park in the Cerrado biome and surrounding farms.
All terrestrial small mammals and humans tested negative for Rickettsia spp. using qPCR, which was somewhat expected considering that the bacteremia due to pathogenic Rickettsia spp. is relatively short and of low magnitude [45]. On the other hand, the production of anti-Rickettsia antibodies tends to increase over time and may be long-lasting [45], which increases their usefulness for epidemiological studies. In fact, 21.9% of the dogs were seroreactive to at least one rickettsial species, with 44.4% showing homologous reactions to R. bellii. This rickettsia infects a wide range of tick species in the Americas, including those that parasitize dogs, such as A. aureolatum, A. ovale, and A. sculptum [46]. The overall seropositivity found is higher than previously reported in dogs from Goiás state [47,48] and other areas in the northeast and south regions of Brazil [49,50,51]. However, the seropositivity was lower than that detected in areas where SFGR are endemic, such as in the south and southeast regions of Brazil [18,29]. Regarding humans, 23.5% were reactive for at least one rickettsial species, the probable homologous antigen involved in the reaction also being R. bellii. This rickettsia is considered non-pathogenic for animals and humans, but some studies suggest that this same species could play an important role in the ecoepidemiology of SFGR impairing the maintenance and transmission of pathogenic rickettsiae, such as R. rickettsii, in ticks [11,52].
Concerning small mammals, 15.4% were seroreactive to at least one rickettsial species. In another national park in the Cerrado biome, 14%, 28%, and 17% of the small mammals were seroreactive to R. rickettsii, R. parkeri, and R. amblyommatis, respectively [53]. Higher seropositivity has been reported in the south and southeast regions of Brazil [14,51,54]. Concerning rodents, C. maracajuensis and O. roberti were previously reported only in Serranópolis (south of Goiás) and the region known as Mato Grosso de Goiás (central portion of Goiás), respectively [55]. We identified both species (C. maracajuensis and O. roberti) in our study, thus extending their known distribution range in this state. Tick species collected from small mammals were identified in the following decreasing order of frequency: A. triste nymphs, A. dubitatum nymphs, and A. coelebs nymph. Previous studies on small rodents in the Cerrado biome indicated a predominance of immature stages of A. coelebs, A. parvum, and A. triste [53,56,57].
Most ticks collected from small mammals were from trail C, which has a floodplain vegetation type, as similarly observed by other studies that also reported larger amounts of ticks in wet and flooded areas [53,58,59]. Studies carried out in other areas of the Cerrado in Brazil and Pampa in Uruguay and Argentina reported an association between A. triste adults and swampy areas [53,58,60,61]. Among the 10 rodent species collected, five were infested with A. triste immature ticks; a single spy hocicudo (O. delator) harbored 76.4% of the ticks collected from small mammals. This aggregation pattern of infestation has been demonstrated for Amblyomma fuscum on the São Lourenço punaré (Thrichomys laurentius) [62]. Oxymycterus rodents have already been reported in the Atlantic Forest biome as hosts for other species of the genus Amblyomma [51]. Thus, we can suggest that O. delator may play an important role as a host of A. triste larvae and nymphs in PNE. However, further studies should be conducted to confirm this hypothesis.
The reduced number of A. dubitatum ticks on rodents and marsupials in this study suggests that small mammals are occasional hosts [63,64]. The absence of A. parvum on terrestrial small mammals may reflect its low density in the study area, in contrast to another study in the Cerrado biome where immature stages of A. parvum were frequently collected [53,56].
Although humans do not act as primary hosts for ticks, tick infestations on humans may be very common in some areas, which may increase the risk of pathogen transmission. Amblyomma sculptum is associated with riparian forests close to anthropized environments, where their preferred hosts (i.e., capybaras) are commonly found. These areas are often frequented by humans in search of leisure, thus increasing the risk of tick exposure [23,65]. Rhipicephalus linnaei can act as a competent vector of R. rickettsii, and in the present study, it was found infesting dogs, which are their preferred hosts. Popularly known as the brown dog tick, R. linnaei is an endophilic tick that is well adapted to indoor environments, thus living in very close contact with humans. This species is distributed throughout Brazilian biomes and has been reported on humans in the states of Pernambuco, Goiás, Rio de Janeiro, Pará, Mato Grosso do Sul, and Rio Grande do Sul [66,67].
Our DNA sequencing data confirmed the circulation of R. parkeri and a rickettsial organism (Rickettsia sp. strain PNE) phylogenetically related to R. tillamookensis in PNE. In Brazil, R. parkeri has already been reported in A. triste ticks collected from vegetation in the state of São Paulo and from wild animals in the state of Mato Grosso do Sul [68,69]. This tick is highly competent in transmitting R. parkeri [61,69], the agent of a milder form of spotted fever rickettsiosis in Brazil [70,71].
This is the first published report of a rickettsial organism related to R. tillamookensis in Brazil infecting A. triste and A. sculptum collected from the same tapir. Attempts to obtain other sequences from other DNA targets were unsuccessful. While we found a very high identity (99.7–100%) between Rickettsia sp. strain PNE and R. tillamookensis, we only obtained a short fragment (350 bp) of the gltA gene, which is very conserved among some rickettsial species. For this reason, we designated this organism as Rickettsia sp. strain PNE until new molecular data are available to confirm the identity of this species. R. tillamookensis was isolated in 1976 from a pool of Ixodes pacificus ticks collected in 1967 in Tillamook County Oregon, USA. It was first described in 1978, but only recently formally characterized as a new species belonging to the transitional group of Rickettsia [72]. The isolate produced low-grade fever and mild scrotal edema after intraperitoneal injection in guinea pigs (Cavia porcellus), but its pathogenicity is still unknown [72,73].
5. Conclusions
The present study confirmed the circulation of two rickettsiae in the PNE, including the pathogenic R. parkeri and a rickettsial organism (Rickettsia sp. strain PNE) phylogenetically related to R. tillamookensis. The circulation of other rickettsial agents in this national park cannot be ruled out considering the typical low prevalence of R. rickettsii in A. sculptum ticks, even in endemic areas [74]. Finally, visitors to PNE should be advised to protect themselves against ticks while visiting the park to reduce the risk of exposure to rickettsial organisms, including R. parkeri.
Author Contributions
Conceptualization, R.L.d.R.P. and F.d.S.K.; methodology, R.L.d.R.P., W.V.d.F.P., L.C.N., L.G.F.d.P., N.J.d.L., B.B.F.d.S., B.G.P., G.T.P., F.D.-T., M.B.L., T.F.M., J.S., L.C.d.S.-P., W.H. and F.d.S.K.; software, L.C.d.S.-P., F.D.-T. and F.d.S.K.; validation, M.B.L., F.D.-T. and F.d.S.K.; formal analysis, M.B.L., L.C.d.S.-P., F.D.-T. and F.d.S.K.; investigation, R.L.d.R.P., W.V.d.F.P., M.B.L., F.D.-T. and F.d.S.K.; resources, R.L.d.R.P., M.B.L., F.D.-T. and F.d.S.K.; data curation, W.V.d.F.P., M.B.L., T.F.M., F.D.-T. and F.d.S.K.; writing—original draft preparation, R.L.d.R.P. and F.d.S.K.; writing—review and editing and visualization, W.V.d.F.P., G.T.P., M.B.L., F.D.-T. and F.d.S.K.; supervision, F.d.S.K.; project administration, F.d.S.K.; funding acquisition, R.L.d.R.P. and F.d.S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was previously approved by the Chico Mendes Institute for Biodiversity (ICMBio Permit no 70143-1), the Ethics Commission on the Use of Animals of the Federal University of Goiás (CEUA/UFG—protocolo:121/19), and the Research Ethics Committee (CEP protocol: 3.857.973).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG; process number: 202110267000287). LCSP is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov (accessed on 10 October 2023)). F.S.K. received a productivity scholarship from CNPq (process number: 317557/2021-1). TFM was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process numbers: 2019/03167-0; 2020/05987-1).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cincotta R.P., Wisnewski J., Engelman R. Human population in the biodiversity hotspots. Nature. 2000;404:990–992. doi: 10.1038/35010105. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho M.L. O conceito de bioma. Acta Bot. Bras. 2006;20:13–23. doi: 10.1590/S0102-33062006000100002. [DOI] [Google Scholar]
- 3.Sloan S., Jenkins C.N., Joppa L.N., Gaveau D.L.A., Laurence W.F. Remaining natural vegetation in the global biodiversity hotspots. Biol. Conserv. 2014;177:12–24. doi: 10.1016/j.biocon.2014.05.027. [DOI] [Google Scholar]
- 4.Françoso R.D., Brandão R., Nogueira C.C., Salmona Y.B., Machado R.B., Colli G.R. Habitat loss and the effectiveness of protected areas in the Cerrado Biodiversity Hotspot. Nat. Conserv. 2015;13:35–40. doi: 10.1016/j.ncon.2015.04.001. [DOI] [Google Scholar]
- 5.Marinho-Filho J.S., Rocrigues F.H.G., Juarez K.M. The Cerrado mammals: Diversity ecology and natural history. In: Oliveira P.S., Marques R.J., editors. The Cerrado of Brazil: Ecology and Natural History of Neotropical Savana. Columbia University Press; Irvington, NY, USA: 2002. pp. 266–289. [Google Scholar]
- 6.Jácomo A.T.A., Kashivakura C.K., Ferro C., Furtado M.M., Asteste S., Tôrres N.M., Sollmann R., Silveira L. Home range and spatial organization of maned wolves in the Brazilian grasslands. J. Mammal. 2009;90:150–157. doi: 10.1644/07-MAMM-A-380.1. [DOI] [Google Scholar]
- 7.Bechara G.H., Szabó M.P., Almeida Filho W.V., Bechara J.N., Pereira R.J., Garcia J.E., Pereira M.C. Ticks associated with armadillo (Euphractus sexcinctus) and anteater (Myrmecophaga tridactyla) of Emas National Park, State of Goiás, Brazil. Ann. N. Y. Acad. Sci. 2002;969:290–293. doi: 10.1111/j.1749-6632.2002.tb04394.x. [DOI] [PubMed] [Google Scholar]
- 8.Vynne C., Keim J.L., Machado R.B., Marinho-Filho J., Silveira L., Groom M.J., Wasser S.K. Resource selection and its implications for wide-ranging mammals of the Brazilian cerrado. PLoS ONE. 2011;6:e28939. doi: 10.1371/journal.pone.0028939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furtado M.M., Hayashi E.M., Allendorf S.D., Coelho C.J., de Almeida Jácomo A.T., Megid J., Ramos Filho J.D., Silveira L., Tôrres N.M., Ferreira Neto J.S. Exposure of Free-Ranging Wild Carnivores and Domestic Dogs to Canine Distemper Virus and Parvovirus in the Cerrado of Central Brazil. Ecohealth. 2016;13:549–557. doi: 10.1007/s10393-016-1146-4. [DOI] [PubMed] [Google Scholar]
- 10.Martins T.F., Furtado M.M., de Almeida Jácomo A.T., Silveira L., Sollmann R., Torres N.M., Labruna M.B. Ticks on free-living wild mammals in Emas National Park, Goiás State, central Brazil. Syst. Appl. Acarol. 2011;16:201–206. doi: 10.11158/saa.16.3.2. [DOI] [Google Scholar]
- 11.Parola P., Paddock C.D., Socolovschi C., Labruna M.B., Mediannikov O., Kernif T., Abdad M.Y., Stenos J., Bitam I., Fournier P.-E., et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministério do Meio Ambiente, Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, Fundação Pró-Natureza . Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) Plano de Manejo: Parque Nacional das Emas; Brasília, Brazil: 2004. [(accessed on 10 October 2023)]. pp. 142–235. Available online: https://www.gov.br/pt-br/orgaos/instituto-brasileiro-do-meio-ambiente-e-dos-recursos-naturais-renovaveis. [Google Scholar]
- 13.Umetsu F., Naxara L., Pardini R. Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. J. Mammal. 2006;87:757–765. doi: 10.1644/05-MAMM-A-285R2.1. [DOI] [Google Scholar]
- 14.Krawczak F.S., Binder L.C., Oliveira C.S., Costa F.B., Moraes-Filho J., Martins T.F., Sponchiado J., Melo G.L., Gregori F., Polo G., et al. Ecology of a tick-borne spotted fever in Southern Brazil. Exp. Appl. Acarol. 2016;70:219–229. doi: 10.1007/s10493-016-0070-1. [DOI] [PubMed] [Google Scholar]
- 15.Bonvicino C.R., Oliveira J.A., Andrea P.S. Guia dos Roedores do Brasil, com Chaves para Gêneros Baseados em Caracteres Externos. OPAS/OMS; Rio de Janeiro, Brasil: 2008. p. 120. [Google Scholar]
- 16.Horta M.C., Labruna M.B., Sangioni L.A., Vianna M.C.B., Gennari M.S., Galvão M.A.M. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever endemic area in the state of São Paulo, Brazil: Serological evidence for infection by Rickettsia rickettsii and another spotted fever groups Rickettsia. Am. J. Trop. Med. Hyg. 2004;71:93–97. [PubMed] [Google Scholar]
- 17.Labruna M.B., Horta M.C., Aguiar D.M., Cavalcante G.T., Pinter A., Gennari S.M., Camargo C.M.A. Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro municipality, western Amazon, Brazil. Vector Borne Zoonotic Dis. 2007;7:249–255. doi: 10.1089/vbz.2006.0621. [DOI] [PubMed] [Google Scholar]
- 18.Barbieri A.R.M., Filho J.M., Nieri-Bastos F.A., Souza J.C., Szabó M.P.J., Labruna M.B. Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever endemic area of southern Brazil. Ticks Tick Borne Dis. 2014;5:848–853. doi: 10.1016/j.ttbdis.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Szabó M.P.J., Nieri-Bastos F.A., Spolidorio M.G., Martins T.F., Barbieri A.M., Labruna M.B. In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecologiacal aspects of the Atlantic rain forest Rickettsia, the causative agent of a now spotted fever rickettsiosis in Brazil. Parasitology. 2013;140:719–728. doi: 10.1017/S0031182012002065. [DOI] [PubMed] [Google Scholar]
- 20.Kmetiuk L.B., Paula W.V.F., Pádua G.T., Delai R.R., Freitas A.R., Farinhas J.H., de Paula L.G.F., Giuffrida R., Pimpão C.T., Santarém V.A., et al. Epidemiology of Rickettsia spp. in Atlantic rainforest areas of island and seashore mainland, southern Brazil. Transbound. Emerg. Dis. 2022;69:3597–3605. doi: 10.1111/tbed.14723. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira P.R., Borges L.M.F., Lopes C.M.L., Leite R.C. Population dynamics of the free-living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo. Minas Gerais State, Brazil. Vet. Parasitol. 2000;92:295–301. doi: 10.1016/S0304-4017(00)00322-8. [DOI] [PubMed] [Google Scholar]
- 22.Terassini F.A., Barbieri F.S., Albuquerque S., Szabó M.P.J., Camargo L.M.A., Labruna M.M. Comparison of two methods for collecting free-living ticks in the Amazonian Forest. Ticks Tick Borne Dis. 2010;1:194–196. doi: 10.1016/j.ttbdis.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 23.de Paula L.G.F., do Nascimento R.M., Franco A.D., Szabó M.P.J., Labruna M.B., Monteiro C., Krawczak F.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasites Vectors. 2022;15:193. doi: 10.1186/s13071-022-05311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros-Battesti D.M., Arzua M., Bechara G.H. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um Guia Ilustrado para Identificação de Espécies. Vox/ICTTD-3,/Butantan; São Paulo, Brasil: 2006. p. 223. [Google Scholar]
- 25.Martins T.F., Onofrio V.C., Barros-Battesti D.M., Labruna M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification Key. Ticks Tick-Borne Dis. 2010;1:75–99. doi: 10.1016/j.ttbdis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Dantas-Torres F., Martins T.F., Murioz-Leal S., Onofrio V.C., Barros-Battesti D.M. Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick-Borne Dis. 2019;10:101252. doi: 10.1016/j.ttbdis.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Slapeta J., Halliday B., Chandra S., Alanazi A.D., Abdel-Shafy S. Rhipicephalus linnaei (Audouin 1826) recognised as the “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato: Neotype designation, redescription, and establishment of morphological and molecular reference. Ticks Tick-Borne Dis. 2022;13:102024. doi: 10.1016/j.ttbdis.2022.102024. [DOI] [PubMed] [Google Scholar]
- 28.Clifford C.M., Anastos G., Elbl A. The Larval Ixodid Ticks of the Eastern United States (Acarina-Ixodidae) Volume 2. Publications of the Entomological Society of America; College Park, MD, USA: 1961. pp. 213–237. Miscellaneous. [Google Scholar]
- 29.Sangioni L.A., Horta M.C., Vianna M.C., Gennari S.M., Soares R.M., Galvão M.A., Schumaker T.T., Ferreira F., Vidotto O., Labruna M.B. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg. Infect. Dis. 2005;11:265–270. doi: 10.3201/eid1102.040656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labruna M.B., Whitworth T., Horta M.C., Bouyer D.H., Mcbride J.W., Pinter A., Popov V., Gennari S.M., Walker D.H. Rickettsia species infecting Amblyomma cooperi Ticks from an area in the state of São Paulo Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004;42:90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guedes E., Leite R.C., Prata M.C.A., Pacheco R.C., Walker D.H., Labruna M.B. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever endemic area in the state of Minas Gerais. Mem. Inst. Oswaldo Cruz. 2005;100:841–845. doi: 10.1590/S0074-02762005000800004. [DOI] [PubMed] [Google Scholar]
- 32.Regnery R.L., Spruil C.L., Plikaytis B.D. Genotipic identification of rickettsiae and estimation of intraspecific sequences divergence for portion of two Rickettsial gene. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinert L.A., Werren J.H., Aebi A., Stone G.N., Jiggins F.M. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7:6. doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitorino L., Chelo I.M., Bacellar F., Zé-Zé L. Rickettsiae phylogeny: A multigenic approach. Microbiology. 2007;153:160–168. doi: 10.1099/mic.0.2006/001149-0. [DOI] [PubMed] [Google Scholar]
- 35.Fournier P.E., Zhu Y., Ogata H., Raoult D. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J. Clin. Microbiol. 2004;42:5757–5766. doi: 10.1128/JCM.42.12.5757-5766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangold A.J., Bargues M.D., Mas-Coma S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae) Parasitol. Res. 1998;84:478–484. doi: 10.1007/s004360050433. [DOI] [PubMed] [Google Scholar]
- 37.Kocher T.D., Thomas W.K., Meyer A., Edwards S.V., Pablo S., Villablancatt F.X., Wilson A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Nati. Acad. Sci. USA. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steuber S., Abdel-Rady A., Clausen P.H. PCR-RFLP analysis: A promising technique for host species identification of blood meals from tsetse flies (Diptera: Glossinidae) Parasitol. Res. 2005;97:247–254. doi: 10.1007/s00436-005-1410-y. [DOI] [PubMed] [Google Scholar]
- 39.Diop A., El Karkouri K., Raoult D., Fournier P.E. Genome sequence-based criteria for demarcation and definition of species in the genus Rickettsia. Int. J. Syst. Evol. Microbiol. 2020;70:1738–1750. doi: 10.1099/ijsem.0.003963. [DOI] [PubMed] [Google Scholar]
- 40.Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa-Paula L.C., Silva L.G., Junior W.J.S., Júnior C.A.S.F., Costa C.H.N., Pessoa F.A.C., Dantas-Torres F. Genetic structure of allopatric populations of Lutzomyia longipalpis sensu lato in Brazil. Acta Trop. 2021;222:106031. doi: 10.1016/j.actatropica.2021.106031. [DOI] [PubMed] [Google Scholar]
- 42.Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., Von Haeseler A., Lanfear R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. Erratum in Mol. Biol. Evol. 2020, 37, 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., Von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letunic I., Bork P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos F.C.P., Nascimento E.M.M., Katz G., Angerami R.N., Colombo S., Souza E.R., Labruna M.B., Silva M.V. Brazilian spotted fever: Real-time PCR for diagnosis of fatal cases. Ticks Tick-Borne Dis. 2012;3:312–314. doi: 10.1016/j.ttbdis.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 46.Krawczak F.S., Labruna M.B., Hecht J.A., Paddock C.D., Karpathy S.E. Genotypic characterization of Rickettsia bellii reveals distinct lineages in the United States and South America. BioMed Res. Int. 2018;2018:8505483. doi: 10.1155/2018/8505483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins M.E.P., Brito W.M.E.D., Labruna M.B., Moraes J.F., Sousa-Martins K.C., Vieira R.P. Epidemiological survey of supposed spotted fever outbreak. Cienc. Anim. Bras. 2016;17:459–471. doi: 10.1590/1089-6891v17i334947. [DOI] [Google Scholar]
- 48.Neves L.C., Barreto A.L.G., Souza M.X., Martins D.B., Barbieri A.R.M., Serpa M.C.A., Muñoz-Leal S., Labruna M.B., Krawczak F.S. Serosurvey on rickettsiae of the spotted fever group and Rickettsia bellii among dogs in the state of Goiás, Brazil. Braz. J. Vet. Parasitol. 2020;29:e021419. doi: 10.1590/s1984-29612020018. [DOI] [PubMed] [Google Scholar]
- 49.Rotondano T.E.F., Krawczak F.S., Barbosa W.O., Moraes J.F., Bastos F.N., Labruna M.B., Azevedo S.S., Melo M.A., Almeida A.M.P. Ehrlichia canis and Rickettsia spp. in dogs from urban areas in Paraíba state, northeastern Brazil. Rev. Bras. Parasitol. Vet. 2017;26:211–215. doi: 10.1590/s1984-29612017030. [DOI] [PubMed] [Google Scholar]
- 50.Lopes M.G., Krawczak F.S., Lima J.T.R., Fournier G.F.S.R., Acosta I.C.L., Ramirez D.G., Marcili A., Labruna M.B., Gennari S.M. Occurrence of Ehrlichia canis and Hepatozoon canis and probable exposure to Rickettsia amblyommatis in dogs and cats in Natal, RN. Rev. Bras. Parasitol. Vet. 2019;28:151–156. doi: 10.1590/s1984-296120180065. [DOI] [PubMed] [Google Scholar]
- 51.Krawczak F.S., Binder L.C., Sobotyk C., Costa F.B., Gregori F., Martins T.F., Pádua G.T., Sponchiado J., Melo G.L., Polo G., et al. Rickettsial infection in ticks from a natural area of Atlantic Forest biome in southern Brazil. Exp. Appl. Acarol. 2022;88:371–386. doi: 10.1007/s10493-022-00754-3. [DOI] [PubMed] [Google Scholar]
- 52.Sakai R.K., Costa F.B., Ueno T.E.H., Ramirez D., Soares J.F., Fonseca A.H., Labruna M.B., Barros-Battesti D.M. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected by Rickettsia bellii. Ticks Tick-Borne Dis. 2014;5:917–923. doi: 10.1016/j.ttbdis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Barbieri A.R.M., Szabó M.P.J., Costa F.B., Martins T.F., Soares H.S., Pascoli G., Torga K., Saraiva D.G., Ramos V.N., Osava C., et al. Species richness and seasonal dynamics of ticks with notes on rickettsial infection in a Natural Park of the Cerrado biome in Brazil. Ticks Tick-Borne Dis. 2019;10:442–453. doi: 10.1016/j.ttbdis.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Horta M.C., Labruna M.B., Pinter A., Linardi P.M., Shumaker T.T.S. Rickettsia infection in five areas of the state of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 2007;102:793–801. doi: 10.1590/S0074-02762007000700003. [DOI] [PubMed] [Google Scholar]
- 55.Hannibal W., Zortéa M., Calaça A.M., Carmignotto A.P., Bezerra A.M.R., Carvalho H.G., Bonvicino C.R., Martins A.C.M., Aguiar L.M.S., de Souza M.B., et al. Checklist of mammals from Goiás, central Brazil. Biota Neotrop. 2021;21:e20201173. doi: 10.1590/1676-0611-BN-2020-1173. [DOI] [Google Scholar]
- 56.Szabó M.P., Castro M.B., Ramos H.G., Garcia M.V., Castagnolli K.C., Pinter A., Veronez V.A., Magalhães G.M., Duarte J.M., Labruna M.B. Species diversity and seasonality of free-living ticks (Acari: Ixodidae) in the natural habitat of wild Marsh deer (Blastocerus dichotomus) in Southeastern Brazil. Vet. Parasitol. 2007;143:147–154. doi: 10.1016/j.vetpar.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Sponchiado J., Melo G.L., Martins T.F., Krawczak F.S., Labruna M.B., Cáceres N.C. Association patterns of ticks (Acari: Ixodida: Ixodidae, Argasidae) of small mammals in Cerrado fragments, western Brazil. Exp. Appl. Acarol. 2015;65:389–401. doi: 10.1007/s10493-014-9877-9. [DOI] [PubMed] [Google Scholar]
- 58.Nava S., Mangold A.J., Mastropaolo M., Venzal J.M., Fracassi N., Guglielmone A.A. Seasonal dynamics and hosts of Amblyomma triste (Acari: Ixodidae) in Argentina. Vet. Parasitol. 2011;181:301–308. doi: 10.1016/j.vetpar.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 59.Colombo V.C., Fasano A., Beldomenico P.M., Nava S. Ticks host specificity: An analysis based on host phylogeny and tick ecological features using Amblyomma triste and Amblyomma tigrinum immature stages. Ticks Tick-Borne Dis. 2018;9:781–787. doi: 10.1016/j.ttbdis.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Szabó M.P.J., Olegário M.M.M., Santos A.L.Q. Tick fauna from two locations in the Brazilian savannah. Exp. Appl. Acarol. 2007;43:73–84. doi: 10.1007/s10493-007-9096-8. [DOI] [PubMed] [Google Scholar]
- 61.Venzal J.M., Estrada-Peña A., Castro O., de Souza C.G., Félix M.L., Nava S., Guglielmone A.A. Amblyomma triste Koch, 1844 (Acari: Ixodidae): Hosts and seasonality of the vector of Rickettsia parkeri in Uruguay. Vet. Parasitol. 2008;155:104–109. doi: 10.1016/j.vetpar.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 62.Aléssio F.M., Dantas-Torres F., Siqueira D.B., Lizée M.H., Marvulo M.F., Martins T.F., Labruna M.B., Silva J.C., Mauffrey J.F. Ecological implications on the aggregation of Amblyomma fuscum (Acari: Ixodidae) on Thrichomys laurentius (Rodentia: Echimyidae), in northeastern Brazil. Exp. Appl. Acarol. 2012;57:83–90. doi: 10.1007/s10493-012-9531-3. [DOI] [PubMed] [Google Scholar]
- 63.Nava S., Venzal J.M., Labruna M.B., Mastropaolo M., González E.M., Mangold A.J., Guglielmone A.A. Hosts, distribution and genetic divergence (16S rDNA) of Amblyomma dubitatum (Acari: Ixodidae) Exp. Appl. Acarol. 2010;51:335–351. doi: 10.1007/s10493-009-9331-6. [DOI] [PubMed] [Google Scholar]
- 64.Dantas-Torres F., Aléssio F.M., Siqueira D.B., Mauffrey J.F., Marvulo M.F., Martins T.F., Moraes-Filho J., Camargo M.C., D’Auria S.R., Labruna M.B., et al. Exposure of small mammals to ticks and rickettsiae in Atlantic Forest patches in the metropolitan area of Recife, North-eastern Brazil. Parasitology. 2012;139:83–91. doi: 10.1017/S0031182011001740. [DOI] [PubMed] [Google Scholar]
- 65.Szabó M.P.J., Pinter A., Labruna M.B. Ecology, biology, and distribution of spotted fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013;3:27. doi: 10.3389/fcimb.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dantas-Torres F., Figueredo L.A., Brandão-Filho S.P. Rhipicephalus sanguineous (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev. Soc. Bras. Med. Trop. 2006;38:64–67. doi: 10.1590/S0037-86822006000100012. [DOI] [PubMed] [Google Scholar]
- 67.Mentz M.B., Trombka M., Silva G.L., Silva C.E. Rhipicephalus sanguineus (Acari: Ixodidae) biting a human being in Porto Alegre city, Rio Grande do Sul, Brazil. Rev. Inst. Med. Trop. 2016;58:35. doi: 10.1590/s1678-9946201658035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silveira I., Pacheco R.C., Szabó M.P., Ramos H.G., Labruna M.B. Rickettsia parkeri in Brazil. Emerg. Infect. Dis. 2007;13:1111–1113. doi: 10.3201/eid1307.061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nieri-Bastos F.A., Szabó M.P., Pacheco R.C., Soares J.F., Soares H.S., Moraes-Filho J., Dias R.A., Labruna M.B. Comparative evaluation of infected and noninfected Amblyomma triste ticks with Rickettsia parkeri, the agent of an emerging rickettsiosis in the New World. BioMed Res. Int. 2013:402737. doi: 10.1155/2013/402737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krawczak F.S., Muñoz-Leal S., Guztzazky A.C., Oliveira S.V., Santos F.C.P., Angerami R.N., Moraes J.F., de Souza J.C., Jr., Labruna M.B. Case report: Rickettsia sp. strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am. J. Trop. Med. Hyg. 2016;95:551–553. doi: 10.4269/ajtmh.16-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.da Paixão Sevá A., Martins T.F., Muñoz-Leal S., Rodrigues A.C., Pinter A., Luz H.R., Angerami R.N., Labruna M.B. A human case of spotted fever caused by Rickettsia parkeri strain Atlantic rainforest and its association to the tick Amblyomma ovale. Parasites Vectors. 2019;12:471. doi: 10.1186/s13071-019-3730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gauthier D.T., Karpathy S.E., Grizzard S.L., Batra D., Rowe L.A., Paddock C.D. Characterization of a novel transitional group Rickettsia species (Rickettsia tillamookensis sp. nov.) from the western black-legged tick, Ixodes pacificus. Int. J. Syst. Evol. Microbiol. 2021;71:004880. doi: 10.1099/ijsem.0.004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paddock C.D., Slater K., Zambrano A.S.M.L., Kleinian J.E., Padgett K.A., Saunders M.E.M., Andrews E.S., Trent E., Zhong J., Sambado S., et al. Detection and Isolation of Rickettsia tillamookensis (Rickettsiales: Rickettsiaceae) From Ixodes pacificus (Acari: Ixodidae) from Multiple Regions of California. J. Med. Entomol. 2022;59:1404–1412. doi: 10.1093/jme/tjac038. [DOI] [PubMed] [Google Scholar]
- 74.Krawczak F.S., Nieri-Bastos F.A., Nunes F.P., Soares J.F., Moraes J.F., Labruna M.B. Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever-endemic area. Parasites Vectors. 2014;7:7. doi: 10.1186/1756-3305-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within this article.