Abstract
Endogenous interleukin-12 (IL-12) mediates protection against Yersinia enterocolitica in C57BL/6 mice by triggering gamma interferon (IFN-γ) production in NK and CD4+ T cells. Administration of exogenous IL-12 confers protection against yersiniae in Yersinia-susceptible BALB/c mice but exacerbates yersiniosis in resistant C57BL/6 mice. Therefore, we wanted to dissect the different mechanisms exerted by IL-12 during Yersinia infections by using different models of Yersinia-resistant and -susceptible mice, including resistant C57BL/6 mice, susceptible BALB/c mice, intermediate-susceptible wild-type 129/Sv mice, 129/Sv IFN-γ-receptor-deficient (IFN-γR−/−) mice and C57BL/6 tumor necrosis factor (TNF) receptor p55 chain-deficient (TNFR p55−/−) mice. IFN-γR−/− mice turned out to be highly susceptible to infection by Y. enterocolitica compared with IFN-γR+/+ mice. Administration of IL-12 was protective in IFN-γR+/+ mice but not in IFN-γR−/− mice, suggesting that IFN-γR-induced mechanisms are essential for IL-12-induced resistance against yersiniae. BALB/c mice could be rendered Yersinia resistant by administration of anti-CD4 antibodies or by administration of IL-12. In contrast, C57BL/6 mice could be rendered more resistant by administration of transforming growth factor β (TGF-β). Furthermore, IL-12-triggered toxic effects in C57BL/6 mice were abrogated by coadministration of TGF-β. While administration of IL-12 alone increased TNF-α levels, administration of TGF-β or TGF-β plus IL-12 decreased both TNF-α and IFN-γ levels in Yersinia-infected C57BL/6 mice. Moreover, IL-12 did not induce toxicity in Yersinia-infected TNFR p55−/− mice, suggesting that TNF-α accounts for IL-12-induced toxicity. Taken together, IL-12 may induce different effector mechanisms in BALB/c and C57BL/6 mice resulting either in protection or exacerbation. These results are important for understanding the critical balance of proinflammatory and regulatory cytokines in bacterial infections which is decisive for beneficial effects of cytokine therapy.
Yersinia enterocolitica is a gram-negative, predominantly extracellularly located pathogen which causes enteritis and enterocolitis in humans and rodents (17, 24, 40). Moreover, systemic infection including abscesses and granulomatous lesions in the spleen and liver occur, particularly in immunocompromised individuals (9, 36). As in infections with intracellular pathogens, T cells, particularly CD4+ Th1 cells, in cooperation with activated macrophages are required for clearance of primary Yersinia infection (1, 2, 5). The protective host response to yersiniosis is mediated by various proinflammatory cytokines. Thus, neutralization of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), or interleukin-12 (IL-12) abrogates resistance against this pathogen, suggesting that T-cell-activated macrophages are important effector cells in the protective host response to yersiniae (1, 4, 8).
Previous studies showed that C57BL/6 mice are resistant against Y. enterocolitica while BALB/c mice are susceptible (16). Administration of IFN-γ, IL-12, or anti-IL-4 antibodies (Abs) rendered BALB/c mice resistant to yersiniae. In contrast, in Yersinia-resistant C57BL/6 mice, administration of IFN-γ and anti-IL-4 Abs did not significantly affect yersiniosis. However, IL-12 treatment of Yersinia-infected C57BL/6 mice increased bacterial numbers in the spleen and caused toxic effects in the liver (1, 8). Experimental viral infection revealed that TNF-α is involved in IL-12-triggered toxicity (31). Furthermore, it was shown that the higher and predominantly IL-12-dependent IFN-γ levels produced by CD4+ T and NK cells of C57BL/6 mice correlate with resistance against yersiniae compared to BALB/c mice. In line with these results, IL-12-mediated protection of BALB/c mice was partially abrogated by administration of anti-IFN-γ Abs (8).
In various infection models, it was shown that transforming growth factor β (TGF-β) counteracts IL-12-mediated resistance. TGF-β is an immunoregulatory molecule which inhibits the activation of macrophages (30, 45) and the generation and cytolytic activities of cytotoxic T cells, natural killer cells, and lymphokine-activated killer cells (23, 37, 38). The immunosuppressive effect of TGF-β appears to be mainly caused by inhibition of IFN-γ, TNF-α, IL-1, IL-2, and IL-12 production (12, 21, 37). TGF-β plays an important role in the progression of infections including infections with Mycobacterium avium (11), Leishmania amazonensis (7), Leishmania braziliensis (6, 7), Trypanosoma cruzi (39), and Toxoplasma gondii (21). In contrast to these investigations, TGF-β plays a beneficial role in acquired resistance against Candida albicans (41) and Listeria monocytogenes (29) infection.
The aim of this study was to analyze the mechanisms of IL-12 exerted in Y. enterocolitica infections in different strains of mice. For this purpose, we have used various mouse models including Yersinia-resistant C57BL/6 mice, Yersinia-susceptible BALB/c mice, Yersinia-intermediate-susceptible 129/Sv mice, and 129/Sv IFN-γ-receptor-deficient (IFN-γR−/−) mice, and C57BL/6 TNF receptor p55 chain-deficient (TNFR p55−/−) mice to dissect the differential and ambiguous IL-12-mediated mechanisms which cause either protection or exacerbation.
MATERIALS AND METHODS
Mice.
Female, 6- to 8-week-old C57BL/6 and BALB/c mice were purchased from Charles River Wiga (Sulzfeld, Germany) and kept under specific-pathogen-free conditions (positive-pressure cabinet). Female, 6- to 8-week-old 129/Sv/Ev IFN-γ receptor type II+/+, 129/Sv/Ev IFN-γ receptor type II−/− mice (20), and C57BL/6 TNFR p55−/− (34) were bred under specific-pathogen-free conditions.
Infection of animals.
Freshly thawed, plasmid-harboring Y. enterocolitica WA-314 serotype O:8 organisms suspended in 0.1 ml of sterile phosphate-buffered saline (PBS), pH 7.4, were used for intravenous and oral infection as described previously (1). The actual number of bacteria administered was determined by plating serial dilutions of the inoculum on Mueller-Hinton agar and counting CFU after an incubation period of 36 h at 26°C. In kinetic studies, five mice per group were killed by carbon dioxide asphyxiation on days 1, 3, and 7 postinfection (p.i.) with 5 × 103 bacteria for both mouse strains if not otherwise stated. The spleens were aseptically removed, and single-cell suspensions were prepared by using 5 ml of PBS containing 0.1% bovine serum albumin. Duplicates of 0.1 ml of serial dilutions of these preparations were plated on Mueller-Hinton agar. The limit of detectable CFU was 25 (log1025 = 1.4). All animal experiments were repeated at least three times and gave comparable results.
Abs.
The Abs used in this study were anti-CD4 (GK1.5 [Dianova, Hamburg, Germany)] and YTS 191), anti-CD8 (53-6.7 [Dianova] and YTS 169), anti-CD3 (145 2C11), anti-IFN-γ (R4-6A2 and AN 18.17.24), anti-IL-4 (BVD6 24G2 [Pharmingen, San Diego, Calif.] and 11B11), anti-TNF-α (MP6-XT22 [Pharmingen] and polyclonal anti-TNF-α [Pharmingen]), polyclonal anti-asialo GM1 (Wako, Neuss, Germany), and EE5 (control Ab; kindly provided by W. Bohne, Würzburg, Germany). Abs were purified from hybridoma supernatants by protein G-Sepharose 4 Fast Flow (Pharmacia-LKB, Uppsala, Sweden) and fast-performance liquid chromatography (Pharmacia-LKB), and then coupled to normal human serum-biotin (Sigma, Deisenhofen, Germany) or fluorescein isothiocyanate (FITC) (Sigma) by standard methods (15).
Lymphocyte preparation.
A part of the splenic single-cell suspensions described above was used for in vitro cultures. Erythrocytes were lysed by a short incubation in 0.15 M NH4Cl, washed three times with Hanks balanced salt solution and resuspended in Click/RPMI 1640 cell culture medium (Biochrom, Berlin, Germany) supplemented with 2 mM l-glutamine (Biochrom), 10 mM HEPES (Biochrom), 5 × 10−5 M 2-mercaptoethanol (Biochrom), 10 μg of streptomycin (Biochrom) per ml, 100 U of penicillin (Biochrom) per ml, and 10% heat-inactivated fetal calf serum (FCS) (Roth, Karlsruhe, Germany) at a final cell concentration of 2 × 106 per ml of Click/RPMI 1640 medium containing 10% FCS.
Cytokine assays.
For determination of cytokine production, 2 × 106 splenocytes were cultured in 2 ml of cell culture medium in 12-well macroculture plates (Nunc, Roskilde, Denmark) in the presence of 10 μg of heat-killed yersiniae (HKY) per ml or 3 μg of concanavalin A (ConA) per ml. Cytokines were modulated by incubation with recombinant IL-12 (kindly provided by M. Gately; 0.1 ng per ml of medium), and recombinant TGF-β2 (kindly provided by G. Zenke, Sandoz Pharma-Ag, Basel, Switzerland, 1 to 4 ng per ml of medium). After 48 h, supernatants were harvested and used in the cytokine assays.
(i) IFN-γ.
IFN-γ levels were determined by using a capture enzyme-linked immunosorbent assay (ELISA) (3). Briefly, microtiter plates (Greiner, Solingen, Germany) were coated with anti-IFN-γ monoclonal antibody (MAb) (AN-18.17.24). After blocking of nonspecific binding sites, supernatants were added to the wells and incubated overnight. After several wash steps, biotin-labeled anti-IFN-γ MAb (R4-6A2) was added. Finally, an avidin-biotin-alkaline phosphatase complex (Strept ABC-AP kit; DAKO, Glostrup, Denmark) was added. For signal development, the wells were incubated with p-nitrophenyl phosphate disodium (Sigma), and the optical density was determined at wavelengths of 405 and 490 nm with an ELISA reader. The levels of IFN-γ from spleen cell cultures were calculated from the straight-line portion of the standard curve by using recombinant IFN-γ (1).
(ii) TNF-α.
TNF-α levels were determined by using a capture ELISA including polyclonal rabbit anti-murine TNF-α antiserum (Genzyme, Boston, Mass.) and biotin-labeled anti-TNF-α MAb (Dianova) as described above for IFN-γ ELISA.
(iii) IL-4.
IL-4 levels were determined by using a capture ELISA including anti-IL-4 MAb (BVD6 24G2) and biotin-labeled anti-IL-4 MAb (11B11) as described above for IFN-γ ELISA.
In vivo administration of Abs and cytokines.
In various experiments, the course of infection was modulated by intraperitoneal (i.p.) administration of (i) human TGF-β2 (1 to 4 μg), (ii) recombinant murine IL-12 (20 to 100 ng) on days −1, 0, 1, 2, and 3 p.i., (iii) anti-CD4 (YTS 191), (iv) anti-CD8 (YTS 169), and (v) anti-asialo GM1 on day −1 and day 3 p.i. Control animals were i.p. injected with the appropriate volume of PBS containing control MAb or serum.
Flow cytometry.
Success of T-cell depletion was confirmed by flow cytometry. Splenocytes were suspended in PBS containing 2% FCS and stained with one of the following MAbs: an FITC-conjugated anti-CD3 MAb and phycoerythrin-conjugated anti-CD4 (GK1.5) and anti-CD8 (53-6.7) MAbs. Labeling procedures were conducted at 4°C. From each sample, 10,000 cells were analyzed in a FACScan (Becton Dickinson, Heidelberg, Germany). Depletion efficiency was >95% after CD4 or CD8 T-cell depletion measured in the spleen after 3 and 7 days.
Cytotoxicity assays.
Success of NK cell depletion was confirmed by cytotoxicity assays (8). YAC-1 cells (5 × 106; American Type Culture Collection, Rockville, Md.) as target cells were labeled with 200 μCi of Na[51Cr]O4 by incubation for 1 h at 37°C, and then washed three times in Click/RPMI-5% FCS. One hundred microliters of YAC-1 cells was added to each well in 96-well microtiter plates (Nunc) and incubated for 6 h at 37°C. Then, 25 μl of supernatant was harvested from each well and counted with a beta plate scintillation counter (Pharmacia-LKB). The percentage of specific 51Cr release was calculated by the following formula: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Experimental release was obtained from wells that contained target and effector cells, and maximum release was obtained by resuspending target cells before harvesting. Values are the means of six wells. After NK cell depletion, no specific lysis was detectable when the effector/target ratio was 100:1.
Statistics.
Differences between mean values were analyzed with Student’s t test. A P value of <0.05 was considered statistically significant.
RESULTS
What are the effector cells for IL-12-mediated protection against Y. enterocolitica?
To evaluate which cell populations are the effectors for IL-12-mediated protection against Y. enterocolitica in BALB/c mice, CD4+ T cells, CD8+ T cells, or NK cells were depleted by administration of anti-CD4, anti-CD8, or anti-asialo GM1 Abs before and after infection with Y. enterocolitica. Parallel groups of mice were additionally treated with 20 ng of IL-12. Bacterial numbers in spleens were determined 7 days p.i. As depicted in Table 1, CD4+ T-cell depletion rendered BALB/c mice resistant against Y. enterocolitica (P < 0.001), whereas CD8+ T cell or NK cell depletion had no impact on the numbers of bacteria in spleens.
TABLE 1.
Modulation of Y. enterocolitica infection in BALB/c mice by administration of anti-CD4, anti-CD8, or anti-asialo GM1 Abs and IL-12a
| In vivo modulation treatment
|
No. of bacteria (log CFU per spleen) on day 7 p.i. | Cytokine level (ng/ml) in:
|
||||
|---|---|---|---|---|---|---|
| Ab | IL-12b | HKY-stimulated spleen cells
|
ConA-stimulated spleen cells
|
|||
| IFN-γ | IL-4 | IFN-γ | IL-4 | |||
| None (control) | − | 6.6 ± 0.2 | 1.08 ± 0.53 | <1.2 | 6.5 ± 2.2 | 2.7 ± 0.2 |
| + | <1.4c | 0.43 ± 0.23 | <1.2 | 12.0 ± 7.0 | <1.2c | |
| Anti-CD4 | − | <1.4c | <0.3c | <1.2 | 6.2 ± 3.3 | <1.2 |
| + | <1.4 | 0.43 ± 0.11 | <1.2 | 9.2 ± 4.2 | <1.2 | |
| Anti-CD8 | − | 6.6 ± 0.6 | 0.94 ± 0.09 | <1.2 | 4.8 ± 2.4 | 2.8 ± 0.4 |
| + | 2.1 ± 0.6c | 0.63 ± 0.35 | <1.2 | 10.2 ± 5.1 | <1.2c | |
| Anti-NK | − | 6.2 ± 0.8 | 0.52 ± 0.15 | <1.2 | 5.0 ± 7.0 | 2.1 ± 0.3 |
| + | 3.8 ± 0.6c | 1.70 ± 0.9c | <1.2 | 7.0 ± 3.5 | <1.2c | |
Mice were infected with 2 × 103 bacteria and injected with anti-CD4, anti-CD8, or anti-asialo GM1 (NK) Abs on day −1 and +3 p.i. Additional groups of mice were treated with 20 ng of IL-12 on days −1, 0, +1, +2, and +3 p.i. After 7 days, spleens were removed and single-cell suspensions were prepared. One aliquot of this suspension was used to determine bacterial numbers in the spleen, and another part was used to culture 2 × 106 splenocytes per 2 ml of medium with HKY (10 μg per ml) or ConA (3 μg per ml). The supernatants were collected 48 h after stimulation. IFN-γ and IL-4 production were determined by ELISA. Results are the means and standard deviations for four animals.
−, without IL-12; +, with IL-12.
Statistically significantly different from values obtained with control group (P < 0.05).
CD4+ T-cell depletion in IL-12-treated mice did not influence bacterial numbers in spleens compared to IL-12-treated control mice (Table 1). In contrast, NK cell depletion partially abrogated IL-12-mediated protection in Yersinia-infected BALB/c mice (P < 0.01). After CD8+ T-cell depletion of IL-12-treated BALB/c mice, there was also a slight increase in bacterial numbers in spleens (P < 0.05). Therefore, we conclude that NK cells and possibly CD8+ T cells are involved in IL-12-mediated protection in BALB/c mice.
In parallel experiments, spleen cells were stimulated with HKY, and production of IFN-γ and IL-4 was determined in cell culture supernatants (Table 1). After stimulation with HKY, a slight decrease in the IFN-γ level after NK or CD4+ T-cell depletion was observed. Administration of IL-12 in vivo caused only a marginal increase of IFN-γ in NK cell-depleted mice. Comparison of IFN-γ levels after ConA stimulation showed no significant decrease after NK, CD8+, or CD4+ T-cell depletion. Likewise, after ConA stimulation, IFN-γ levels were only slightly increased in IL-12-treated Yersinia-infected controls and in IL-12-treated CD4+ T-cell- or NK-cell-depleted mice (Table 1). IL-4 production was detectable only after ConA stimulation and was reduced in IL-12-treated or CD4+ T-cell-depleted mice (P < 0.01), suggesting that IL-4 production could be involved in the inhibitory effect of CD4+ T cells on elimination of yersiniae (Table 1).
IL-12-activated CD8 T cells and NK cells are essential to protect against yersiniae in CD4 T-cell-depleted mice.
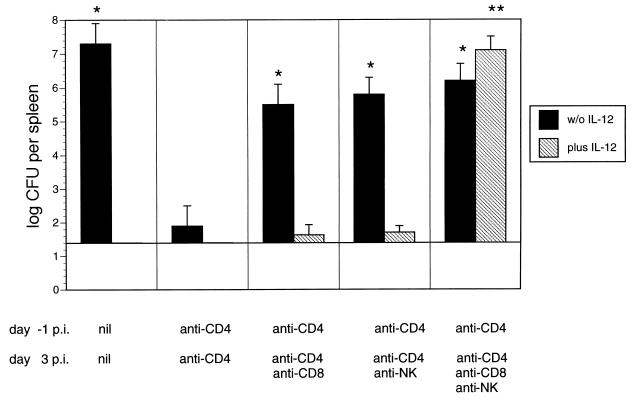
To identify the effector cells for protection against yersiniae in CD4+ T-cell-depleted BALB/c mice, animals were treated with anti-CD4 Abs prior to Yersinia infection and on day 3 p.i. with anti-CD4 Abs in the presence or absence of anti-CD8 or anti-asialo GM1 Abs. In addition, some groups of mice were treated with 20 ng of IL-12 each day. The results in Fig. 1 show that additional CD8+ T-cell or NK cell depletion abrogated resistance mediated by CD4+ T-cell depletion (P < 0.001). These results indicate that both CD8+ T and NK cells are essential for protection against yersiniae in CD4+ T-cell-depleted BALB/c mice. Abrogation of resistance in IL-12-treated, CD4+ T-cell-depleted mice was detected only in mice additionally depleted of both CD8+ T cells and NK cells, indicating that either of these two cell populations upon administration of IL-12 is sufficient to mediate protection against yersiniosis.
FIG. 1.
Bacterial numbers in spleens of BALB/c mice 7 days p.i. with 2 × 103 Y. enterocolitica and administration of PBS containing control Abs or anti-CD4 Abs 1 day prior to infection. On day +3 p.i., mice were treated with PBS containing control or anti-CD4 Abs without or in combination with anti-CD8, anti-asialo GM1 (NK), or anti-CD8 plus anti-asialo GM1 Abs. Results are the means and standard deviations for four mice. The asterisks indicate statistically significant differences (P < 0.01) compared with the values obtained with the control group (∗ for group of mice treated with anti-CD4 MAb alone; ∗∗ for group of mice treated with anti-CD4 MAb plus IL-12). w/o IL-12, without IL-12.
IL-12 has no protective effect against yersiniae in IFN-γ receptor knockout mice.
Administration of anti-IFN-γ Abs in BALB/c mice abrogates IL-12-mediated protection against yersiniosis only partially (8). Therefore, we studied whether IL-12 can protect mice against yersiniae in an IFN-γ-independent manner. 129/Sv IFN-γR+/+ and 129/Sv IFN-γR−/− mice were infected with Y. enterocolitica, and bacterial counts in the spleen were determined 5 days p.i. As shown in Table 2, yersinia-infected IFN-γR−/− mice had significant higher bacterial counts in the spleen 5 days p.i. than the wild-type mice did, indicating that IFN-γR−/− mice are much more susceptible to Yersinia infection than wild-type mice are. The results of these experiments confirmed previous data that IFN-γ is essential for clearance of Yersinia infection (8).
TABLE 2.
Bacterial numbers in the spleens of IFN-γR−/− and IFN-γR+/+ micea
| Mouse strain | Inoculum | No. of bacteria (log CFU per spleen) |
|---|---|---|
| IFN-γR+/+ | 4 × 102 | 3.5 ± 0.4 |
| 4 × 103 | 5.0 ± 0.6 | |
| 4 × 104 | 7.5 ± 0.5 | |
| IFN-γR−/− | 4 × 102 | 7.1 ± 0.3 |
Mice were infected with different numbers of Y. enterocolitica, and bacterial numbers in the spleen were determined on day 5 p.i.
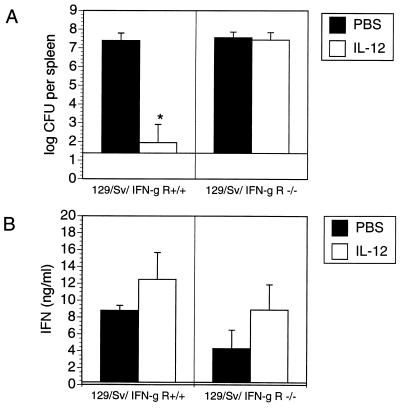
To study the effect of IL-12 on the course of Yersinia infection in IFN-γR−/− and IFN-γR+/+ mice, these mice were treated with PBS or IL-12 prior to and after Yersinia infection, and bacterial counts in spleens were determined on day 5 p.i. Administration of IL-12 rendered IFN-γR+/+ mice resistant to infection, while IL-12 treatment had no impact on yersiniosis in IFN-γR−/− mice (Fig. 2). These results suggest that IL-12 is not able to protect against yersiniae in the absence of IFN-γ-induced mechanisms. In parallel experiments, spleen cells of infected mice were isolated and exposed to HKY in vitro, and IFN-γ and IL-4 levels were determined in cell culture supernatants. The results show that both IFN-γR+/+ and IFN-γR−/− mice produced comparable IFN-γ levels (Fig. 2). In vivo administration of IL-12 slightly increased IFN-γ production in both mouse strains, suggesting that IL-12 may be partially involved in the amplification cascade leading to IFN-γ production. However, we found no evidence that IL-4 is upregulated in IFN-γR−/− mice, because IL-4 levels were not detectable in HKY- or ConA-stimulated spleen cells of either in vivo untreated or IL-12-treated IFN-γR+/+ or IFN-γR−/− mice (data not shown).
FIG. 2.
Bacterial numbers (A) and IFN-γ production (B) in the spleen cells from Yersinia-infected (day 5 p.i.) 129/Sv IFN-γR+/+ (2 × 104 Y. enterocolitica) or 129/Sv IFN-γR−/− mice (2 × 102 Y. enterocolitica) treated with PBS or 20 ng of IL-12 each day. Spleen cells were stimulated with HKY (10 μg per ml). Results are the means and standard deviations for four mice. The asterisk indicates a statistically significant difference (P < 0.01) compared with the values obtained with the control group.
Modulation of Yersinia infection by TGF-β.
C57BL/6 mice produce high levels of IFN-γ dependent on endogenous IL-12 production, which in this particular case correlates with higher resistance against Yersinia compared to BALB/c mice. Treatment with exogenous IL-12, however, is toxic in C57BL/6 mice. We therefore tested whether TGF-β might influence IL-12-triggered exacerbation of yersiniosis.
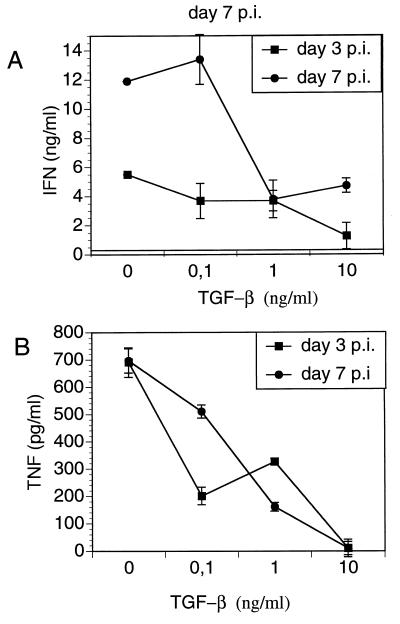
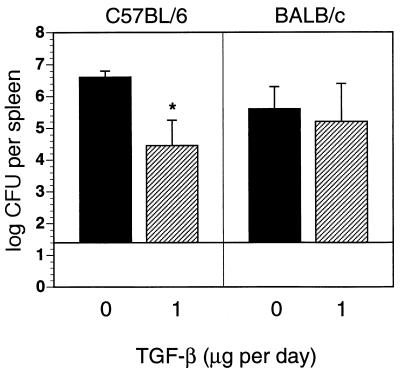
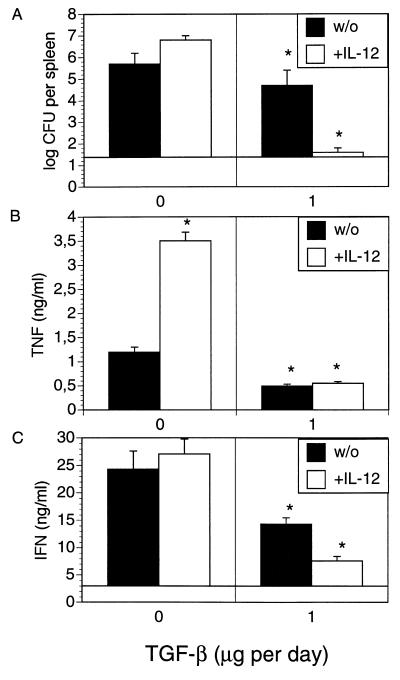
To determine whether TGF-β alters production of cytokines in Yersinia infection, spleen cells from Yersinia-infected C57BL/6 mice were stimulated with HKY in the presence of TGF-β and production of IFN-γ and TNF-α was determined in culture supernatants. TGF-β reduced IFN-γ and TNF-α levels in a dose-dependent manner (Fig. 3). Therefore, we hypothesized that administration of TGF-β might exacerbate yersiniosis. Both C57BL/6 and BALB/c mice were treated with various amounts of TGF-β prior to and after Y. enterocolitica infection. Administration of 1 μg of TGF-β each day decreased bacterial numbers (P < 0.01) in Yersinia-infected C57BL/6 mice, while administration of 4 μg of TGF-β (data not shown) had no effect on clearance of yersinae (Fig. 4). TGF-β treatment had no impact on the numbers of bacteria in BALB/c mice.
FIG. 3.
TGF-β-modulated IFN-γ and TNF-α production by HKY-stimulated spleen cells from C57BL/6 mice prepared 3 and 7 days p.i. with 5 × 103 Y. enterocolitica. Results are the means and standard deviations for three mice.
FIG. 4.
Bacterial numbers in the spleen from C57BL/6 and BALB/c mice 5 days after infection with 2 × 104 or 2 × 103 Y. enterocolitica, respectively, and i.p. administration of PBS or 1 μg of TGF-β2 on days −1, 0, +1, +2, and +3 p.i. The results are the means and standard deviations for four mice. The asterisk indicates a statistically significant difference (P < 0.01) compared with the values obtained with the control group.
To test whether TGF-β can modulate toxicity of IL-12, C57BL/6 mice were treated with TGF-β plus IL-12 prior to and after Yersinia infection. As shown in Fig. 5A, the combination of IL-12 and TGF-β decreased bacterial numbers (P < 0.001) compared to PBS and IL-12 treatments in mice. In parallel experiments, spleen cells were isolated and exposed to HKY and production of TNF-α and IFN-γ was determined (Fig. 5B and C). HKY-stimulated spleen cells of IL-12-treated Yersinia-resistant mice produced significant higher TNF-α levels (Fig. 5B) than those of control mice (P < 0.001), while IL-12 treatment had no significant impact on IFN-γ production (Fig. 5C). TGF-β treatment in the presence or absence of IL-12 in vivo led to decreased TNF-α and IFN-γ levels compared to those of control mice (P < 0.001). These results suggest that IL-12-mediated toxic effects could be due to increased TNF-α levels and that the protective effects of IL-12 can be restored in C57BL/6 mice by TGF-β, possibly via a moderate downregulation of TNF-α production.
FIG. 5.
Bacterial numbers in the spleen (A) and TNF-α (B) and IFN-γ production (C) by spleen cells from C57BL/6 mice 5 days after infection with 2 × 104 Y. enterocolitica and i.p. administration of PBS or 1 μg of TGF-β2 on days −1, 0, +1, +2, and +3 p.i. with (+) or without (w/o) the addition of 100 ng of IL-12 on days −1, 0, +1, +2, and +3 p.i. (A). Spleen cells were stimulated with HKY (10 μg per ml), and the presence of IFN-γ (B) and TNF-α (C) in cell culture supernatants was determined. The results are the means and standard deviations for four mice. The asterisks indicate statistically significant differences (P < 0.01) compared with the values obtained with the control group.
TNF-α accounts for IL-12-mediated toxicity in yersiniosis.
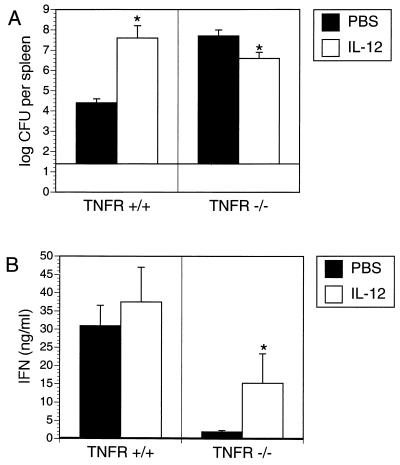
To investigate whether TNF-α is involved in IL-12-mediated exacerbation of Yersinia infection in C57BL/6 mice, TNFR p55−/− mice were used. C57BL/6 TNFR p55−/− and TNFR p55+/+ mice were treated with PBS or IL-12 before and after infection with yersiniae. As indicated in Fig. 6, bacterial numbers in the spleens of Yersinia-infected TNFR p55−/− mice were more than 1,000-fold higher than in the spleens of TNFR p55+/+ mice, even when hundredfold-less yersiniae were used to infect TNFR p55−/− mice. These data show that TNFR-mediated mechanisms are essential for clearance of Yersinia infection. Consequently, both TNFR p55+/+ and TNFR p55−/− mice were treated with IL-12 before and after Yersinia infection. IL-12 increased bacterial numbers in the spleens of Yersinia-infected TNFR p55+/+ mice. In contrast, IL-12 treatment slightly decreased (P < 0.01) bacterial numbers in the spleen of Yersinia-infected TNFR p55−/− mice (Fig. 6A), suggesting that TNFR-mediated mechanisms are involved in the toxic effects of IL-12 in Yersinia-infected C57BL/6 mice.
FIG. 6.
Bacterial numbers in the spleen (A), and IFN-γ production by spleen cells (B) from C57BL/6 TNFR p55+/+ and C57BL/6 TNFR p55−/− mice 5 days after infection with 2 × 104 or 2 × 102 Y. enterocolitica, respectively, and i.p. administration of PBS or 1 μg of IL-12 on days −1, 0, +1, +2, and +3 p.i. The asterisks indicate statistically significant differences (P < 0.01) compared with the values obtained with the control group.
In parallel experiments, spleen cells were isolated and exposed to HKY, and production of IFN-γ was determined (Fig. 6B). HKY-stimulated spleen cells derived from PBS-treated Yersinia-infected TNFR p55−/− mice produced only low levels of IFN-γ compared to wild-type mice. IFN-γ production of HKY stimulated spleen cells derived from TNFR p55−/− mice was partially restored by IL-12 treatment in vivo.
DISCUSSION
TNF-α, IL-12, and IFN-γ are essential for clearance of Yersinia infection (1, 8, 35). Comparison of Yersinia-resistant C57BL/6 and Yersinia-susceptible BALB/c mice revealed that the two mouse strains differ in their ability to produce IFN-γ (8, 35). BALB/c mice are IFN-γ low producers, while C57BL/6 mice are IFN-γ high producers. Furthermore, BALB/c and C57BL/6 mice have different responses to the administration of IL-12 (8). Thus, IL-12 confers protection against yersiniae in susceptible BALB/c mice but exacerbates yersiniosis and toxicity in resistant C57BL/6 mice. Extending these studies, we wanted to dissect the mechanisms of the ambiguous IL-12 effects in different mouse strains.
CD4+ T cells play a crucial but ambiguous role for clearance of Yersinia infection. Thus, CD4+ T cells promote clearance of Y. enterocolitica in C57BL/6 mice (8) but promote exacerbation of Yersinia infection in BALB/c mice. In line with these data, previous studies showed that depletion of CD4+ T cells improved resistance of BALB/c mice against Leishmania (26, 27). Depletion of NK cells or CD8+ T cells had no impact on clearance of Yersinia infection in BALB/c mice. However, we showed that both CD8+ T and NK cells are crucial for clearance of Yersinia infection in CD4+ T-cell-depleted BALB/c mice, suggesting that CD4+ T cells may inhibit both CD8+ T and NK cells in their potential ability to eliminate yersinae.
To identify the effector cells for IL-12-mediated protection against yersinae, BALB/c mice were simultaneously treated with IL-12 and antibodies against different cell populations. These experiments showed that CD8+ T-cell and NK cell depletion increased bacterial numbers in Yersinia-infected IL-12-treated BALB/c mice, indicating that in these mice, CD8+ T or NK cells may be sufficient to control Yersinia infection, probably in concert with remaining cell populations like macrophages.
In C57BL/6 mice, there is a clear-cut correlation of IL-12-dependent IFN-γ production of NK cells (early phase of infection) or CD4 T cells (late phase) and clearance of Yersinia. In contrast, CD4 T-cell depletion or IL-12 mediates protection but does not increase IFN-γ production in BALB/c mice. In experimental leishmaniasis, CD4 T-cell depletion caused a decrease in the frequency of IL-4-secreting T-cell clones without a concomitant increase of IFN-γ-secreting T-cell clones (26). Likewise, HKY-stimulated spleen cells of CD4 T-cell-depleted Yersinia-infected BALB/c mice showed no significant increase of IFN-γ production compared to control mice. In contrast to leishmaniasis, no significant levels of IL-4 were found in yersiniosis. However, after ConA stimulation, no significant differences in IFN-γ levels after depletion of CD4 T cells, CD8 T cells, or NK cells were observed, whereas IL-4 production was significantly decreased after CD4 T-cell depletion or IL-12 administration. These results suggest that IL-4 produced by CD4 T cells might be involved in promoting Yersinia susceptibility of BALB/c mice. In keeping with this assumption, in both Yersinia and Leishmania infections, administration of anti-IL-4 Abs rendered BALB/c mice resistant (1, 18). These data suggest that although IFN-γ is crucial for IL-12-mediated protection, alternative mechanisms seem to be involved in clearance of Yersinia infection.
Previous work showed that the protective effect of IL-12 in BALB/c mice can be only partially abrogated by the addition of anti-IFN-γ Abs, suggesting that IL-12 might cause protective effects in an IFN-γ-independent manner (8). Herein we show that IFN-γR-deficient mice are more susceptible to Yersinia infection than wild-type mice. Similar results were demonstrated in other infection models (10, 20, 22). Administration of IL-12 increased resistance against yersinae in IFN-γ-R+/+ but not in IFN-γR−/− mice. These data confirm that IFN-γ is essential for clearance of Yersinia infection and suggest that IL-12 has no protective effect in the absence of IFN-γR-mediated mechanisms. On the other hand, we cannot yet exclude the possibility that the lack of IFN-γR in knockout mice might have affected the development of the immune system per se.
Yersinia-triggered IFN-γ production was only slightly affected in IFN-γR−/− mice compared to wild-type mice, indicating that initial upregulation of IFN-γ is not affected by IFN-γR-mediated mechanisms. This result is in keeping with reports demonstrating that IFN-γ−/− mice produce IL-12 during murine endotoxemia and show higher susceptibility to Mycobacterium bovis (10). Since IFN-γ seems to be essential for upregulation of IL-12R β2 expression, IFN-γR deficiency may also cause a low IL-12R β2 expression on T cells, leading to IL-12 unresponsiveness (44) and thereby inhibiting effector mechanisms mediated directly by IL-12. However, it is not yet clear whether IFN-γ is required for both initial mechanisms inducing anti-Yersinia host responses and for the resulting effector mechanisms leading to resolution of Y. enterocolitica infection.
A virus infection model revealed that TNF-α accounts for IL-12-induced toxicity (31). As IL-12 triggers toxicity and exacerbates yersiniosis in C57BL/6 mice, the course of Yersinia infection in C57BL/6 TNFR p55−/− mice was investigated and revealed that IL-12-triggered toxicity was not observed in the absence of TNFR. Moreover, bacterial numbers were slightly decreased in IL-12-treated TNFR p55−/− mice, suggesting that TNF-α is involved in IL-12-triggered toxicity in yersiniosis. Nevertheless, IL-12 had only a slight beneficial effect on Yersinia infection in TNFR p55−/− mice, probably because TNF-α is crucial for clearance of Yersinia infection (4). Moreover, as mentioned above, it should be considered that the lymphoid organogenesis is altered in TNFR p55−/− mice (25) and that this fact might partially account for the results obtained in this study.
In Leishmania infection, CD4 T-cell depletion elevated tissue expression of inducible nitric oxide synthetase (iNOS) in susceptible BALB/c mice to a level comparable with resistant C57BL/6 mice (42). Furthermore, it was suggested that the relative lack of iNOS in susceptible mice could be due to a higher expression of TGF-β in tissues of susceptible mice than in resistant mice (42). In this study, we found that TGF-β improved resistance against yersiniae in C57BL/6 mice but had no impact on the course of infection in BALB/c mice. Administration of IL-12 plus TGF-β rendered C57BL/6 mice more resistant to Yersinia infection and abrogated toxic effects of IL-12.
It was recently shown that TNF-α in synergism with IL-2 is essential for triggering IL-12-induced toxicity (14, 31–33). Likewise, administration of IL-12 led to increased TNF-α levels in HKY-stimulated spleen cells from Yersinia-infected C57BL/6 mice. TGF-β had an opposing effect to IL-12 by moderate downregulation of TNF-α production, suggesting that in Yersinia-resistant C57BL/6 mice which induce a strong Th1 response, there might be a deficit of negative immunoregulatory mechanisms. Therefore, we speculate that both IL-12-induced toxicity mediated by TNF-α and IL-12-induced protection mediated by IFN-γ may be balanced by TGF-β, which seems to be critical to obtain an optimal immune response against yersinae.
The immunoregulatory role of TGF-β, however, is not yet clear. Contradictory data on TGF-β-mediated Th1 responses have been reported, indicating that TGF-β stimulates (13, 28, 43) or downregulates Th1 development (19, 46). TGF-β with or without combination of IL-12 decreases IFN-γ production by CD4+ T cells from C57BL/6 or BALB/c mice but increases IFN-γ production by CD4+ T cells from CBA/J and C3H/He mice (19).
There are also contradictory reports about the role of TGF-β in infections. TGF-β promotes infection in L. braziliensis (6) and T. gondii (21) in correlation with downregulation of IFN-γ (6, 21) and upregulation of IL-4 and IL-10 (6). Moreover, in T. gondii infection, TGF-β counteracts IL-12 protective effect in mice with severe combined immunodeficiency disorder (21). On the other hand, TGF-β plays a beneficial role in resistance against C. albicans (41) and L. monocytogenes (29) by upregulating IL-4 and IL-10 production (41) and downregulating IFN-γ, TNF-α, and IL-6 production (29). Thus, TGF-β-mediated downregulation of various cytokines correlates with either resolution or excerbation of infections, suggesting that balancing of certain cytokine production levels is decisive for the outcome in different infectious diseases.
Taken together, cytokine regulation of effector mechanisms leading either to CD4+ T cells promoting or suppressing immune responses against Yersinia play a central role in the different abilities of C57BL/6 and BALB/c mice to clear Yersinia infection. These data are important for understanding the critical balance of proinflammatory and regulatory cytokines during bacterial infections which is decisive for beneficial effects of cytokine therapy.
ACKNOWLEDGMENTS
We are grateful to K. Pfeffer (Technical University of Munich, Munich, Germany) for providing TNFR p55 knockout mice, M. Gately (Hoffmann-LaRoch Inc., Nutley, New Jersey) for generously providing us with IL-12, G. Zenke (NOVARTIS, Pharma-AG, Basel, Switzerland) for generously providing us with TGF-β2, and S. Preger and N. Bücheler for expert technical assistance.
REFERENCES
- 1.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Beer M, Hantschmann P, Preger S, Vogel U, Heymer B, Heesemann J. The cellular immune response against Yersinia enterocolitica in different inbred strains of mice: evidence for an important role of T lymphocytes. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;278:383–395. doi: 10.1016/s0934-8840(11)80855-8. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Bohn E, Ewald J H, Heesemann J. Deferoxamine B but not deferoxamine G1 inhibits cytokine production in murine bone marrow macrophages. J Infect Dis. 1995;172:490–496. doi: 10.1093/infdis/172.2.490. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth I B, Heesemann J. In vivo neutralization of tumor necrosis factor alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth I B, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barral A, Barral Netto M, Yong E C, Brownell C E, Twardzik D R, Reed S G. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barral Netto M, Barral A, Brownell C E, Skeiky Y A, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 8.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 9.Bouza E, Dominguez A, Meseguer M, Buzon L, Boixeda D, Revillo M J, de Rafael L, Martinez Beltran J. Yersinia enterocolitica septicemia. Am J Clin Pathol. 1980;74:404–409. doi: 10.1093/ajcp/74.4.404. [DOI] [PubMed] [Google Scholar]
- 10.Dalton D K, Pitts Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 11.Denis M, Ghadirian E. Transforming growth factor beta (TGF-β1) plays a detrimental role in the progression of experimental Mycobacterium avium infection: in vivo and in vitro evidence. Microb Pathog. 1991;11:367–372. doi: 10.1016/0882-4010(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 12.Espevik T, Figari I S, Shalaby M R, Lackides G A, Lewis G D, Shepard H M, Palladino M A. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fargeas C, Wu C Y, Nakajima T, Cox D, Nutman T, Delespesse G. Differential effect of transforming growth factor beta on the synthesis of Th1- and Th2-like lymphokines by human T lymphocytes. Eur J Immunol. 1992;22:2173–2176. doi: 10.1002/eji.1830220833. [DOI] [PubMed] [Google Scholar]
- 14.Gately M K, Warrier R R, Honasoge S, Carvajal D M, Faherty D A, Connaughton S E, Anderson T D, Sarmiento U, Hubbard B R, Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994;6:157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- 15.Goding J W. Monoclonal antibodies: principle and practice. London, United Kingdom: Academic Press, Inc.; 1986. pp. 262–265. [Google Scholar]
- 16.Hancock G E, Schaedler R W, MacDonald T T. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun. 1986;53:26–31. doi: 10.1128/iai.53.1.26-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoehn P, Goedert S, Germann T, Koelsch S, Jin S, Palm N, Ruede E, Schmitt E. Opposing effects of TGF-beta 2 on the Th1 cell development of naive CD4+ T cells isolated from different mouse strains. J Immunol. 1995;155:3788–3793. [PubMed] [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in the regulation of T cell-independent resistence to Toxoplasma gondii. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 22.Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez Mon M, Derynck R, Sporn M B, Fauci A S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian C J, Hwang W S, Pai C H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987;55:1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Fu Y X, Molina H, Chaplin D D. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 26.Morris L, Aebischer T, Handman E, Kelso A. Resistance of BALB/c mice to Leishmania major infection is associated with a decrease in the precursor frequency of antigen-specific CD4+ cells secreting interleukin-4. Int Immunol. 1993;5:761–767. doi: 10.1093/intimm/5.7.761. [DOI] [PubMed] [Google Scholar]
- 27.Muller I, Kropf P, Etges R J, Louis J A. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993;61:3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagelkerken L, Gollob K J, Tielemans M, Coffman R L. Role of transforming growth factor-beta in the preferential induction of T helper cells of type 1 by staphylococcal enterotoxin B. Eur J Immunol. 1993;23:2306–2310. doi: 10.1002/eji.1830230938. [DOI] [PubMed] [Google Scholar]
- 29.Nakane A, Asano M, Sasaki S, Nishikawa S, Miura T, Kohanawa M, Minagawa T. Transforming growth factor beta is protective in host resistance against Listeria monocytogenes infection in mice. Infect Immun. 1996;64:3901–3904. doi: 10.1128/iai.64.9.3901-3904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson B J, Ralph P, Green S J, Nacy C A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991;146:1849–1857. [PubMed] [Google Scholar]
- 31.Orange J S, Salazar Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C A. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orange J S, Wolf S F, Biron C A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 33.Ozmen L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Gately M, Garotta G. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 Kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 35.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 36.Rabson A R, Hallett A F, Koornhof H J. Generalized Yersinia enterocolitica infection. J Infect Dis. 1975;131:447–451. doi: 10.1093/infdis/131.4.447. [DOI] [PubMed] [Google Scholar]
- 37.Ranges G E, Figari I S, Espevik T, Palladino M A. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166:991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rook A H, Kehrl J H, Wakefield L M, Roberts A B, Sporn M B, Burlington D B, Lane H C, Fauci A S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136:3916–3920. [PubMed] [Google Scholar]
- 39.Silva J S, Twardzik D R, Reed S G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta) J Exp Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaccapelo R, Romani L, Tonnetti L, Cenci E, Mencacci A, Del Sero G, Tognellini R, Reed S G, Puccetti P, Bistoni F. TGF-beta is important in determining the in vivo patterns of susceptibility or resistance in mice infected with Candida albicans. J Immunol. 1995;155:1349–1360. [PubMed] [Google Scholar]
- 42.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swain S L, Huston G, Tonkonogy S, Weinberg A. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 44.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL-)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 46.Wu C Y, Demeure C E, Gately M, Podlaski F, Yssel H, Kiniwa M, Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. I. Induction of IL-4-producing cells after long-term culture in the presence of IL-4 plus either IL-2 or IL-12. J Immunol. 1994;152:1141–1153. [PubMed] [Google Scholar]