Abstract
A major outer membrane protein band of approximately 25 to 27 kDa is commonly observed in strains of Haemophilus influenzae. This study has investigated the potential of a 26-kDa protein (OMP26) from nontypeable H. influenzae (NTHI) as a vaccine candidate. OMP26 was used to immunize rats via intestinal Peyer’s patches, followed by an intratracheal boost. Immunization was found to significantly enhance bacterial clearance following pulmonary challenge with both the homologous NTHI strain and a different NTHI strain. Significant levels of anti-OMP26 were found in the serum and bronchoalveolar lavage from immunized rats, and isotypes of immunoglobulin G (IgG) were also measured in serum. Analysis of IgG isotypes present in serum following OMP26-immunization suggest that predominantly a T-helper 1-type response was induced. The OMP26 protein was amino-terminally sequenced and found to have no homology with the P5 of H. influenzae type b P5 or the fimbrin protein of NTHI, both can migrate upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis at similar molecular masses but OMP26 has 100% homology with a segment of the H. influenzae Rd genome. The results of this study suggest that OMP26 may be a suitable vaccine candidate against NTHI infection and warrants continued investigation and characterization.
Nontypeable Haemophilus influenzae (NTHI) is a gram-negative bacterium that is a common cause of otitis media (2, 23), pneumonia (3, 25), exacerbation of chronic bronchitis (reviewed in reference 23), sinusitis (13, 33), meningitis, postpartum and neonatal infections (27, 35), osteomyelitis, septicemia, bacteremia, and other invasive bacterial diseases (reviewed in references 23 and 31). Currently, there is no vaccine available that can prevent the occurrence of these NTHI infections.
Several outer membrane proteins (OMP) have been assessed as potential vaccine candidates. The OMP P6 is highly conserved among strains (26). Immunization studies with recombinant P6 in a mixture of other proteins failed to protect chinchillas against otitis media (10); however, mucosal immunization with P6 resulted in enhanced pulmonary clearance in rats that differed in rate among strains of NTHI (16). The major porin protein from NTHI, P2 has significant variability in surface loop regions between strains (6, 11, 20). Mucosal immunization with P2 resulted in significant pulmonary clearance in rats (15); however, the degree of clearance was dependent on the specificity of the T- and B-cell responses to the P2 protein and was less than the clearance reported previously following immunization with P6 (16).
A major OMP corresponding to the classified P5 band at approximately 26 kDa (non-heat modified) has been investigated in H. influenzae type b (Hib) (22) and, more recently, NTHI (5). This OMP was one of two lower-molecular-mass bands on sodium dodecyl sulfate (SDS)-polyacrylamide gels used to subtype H. influenzae strains (1) and has an apparent molecular mass of 25 to 27 kDa. The protein, when purified from a Hib strain (21), was found to be heat modifiable, demonstrating an apparent molecular mass of 35 kDa after heating for 30 min at 100°C in the presence of β-mercaptoethanol. A fimbrin protein of a similar molecular mass and expressed by NTHI has been characterized (28) and found to have 92% amino acid sequence homology with the Hib P5 and the same heat-modifiable characteristic. The NTHI fimbrin was capable of conferring partial protection against NTHI in a chinchilla otitis media model (28).
This study was undertaken to characterize and assess the potential of another 26-kDa OMP, called OMP26. The results demonstrate that this protein enhanced pulmonary clearance of both homologous and heterologous strains of NTHI and suggest that OMP26 warrants further investigation as a potential vaccine candidate. (OMP26 is the subject of an international patent [15a].)
(Part of this study was presented at the 8th International Congress of Mucosal Immunology, 17 to 20 July 1995, San Diego, Calif.)
MATERIALS AND METHODS
Bacterial strain and culture.
NTHI strains of biotype I (NTHI-I; isolate 289) and biotype II (NTHI-II) were isolated from the sputum of adult patients with chronic bronchitis. HI-CD was obtained from the Swiss Serum and Vaccine Institute, Berne, Switzerland, as an NTHI strain; however, it was positive (unpublished data) for the cap gene following hybridization using the pU038 probe (14). Hib-II (biotype II) was isolated from the sputum of a chronic bronchitic. The bacteria were prepared by overnight growth at 37°C in 5% CO2 on brain heart infusion agar plates supplemented with 50 ml of defibrinated horse blood per liter of agar (Hunter AntiSera, Callaghan, New South Wales, Australia).
Purification of OMP26.
A crude outer membrane preparation was obtained (24) from bacteria grown overnight on agar plates, and OMP26 was purified by preparative polyacrylamide gel electrophoresis (PAGE) as previously described (17). Preparative SDS-PAGE to purify OMP26 was performed with a Bio-Rad model 491 Prep Cell, using a 60-ml 14% T-1.42% C acrylamide-BIS (N,N′-methylenebisacrylamide) separating gel with a 10-ml 4% T-0.36% C acrylamide-BIS stacking gel polymerized in a 37-mm (internal diameter) column (17). Fractions were concentrated by lyophilization and analyzed for protein content by analytical SDS-PAGE. OMP26 isolated under these conditions contained SDS, which was subsequently removed (29). Fractions containing OMP26 were pooled and dialyzed prior to determination of protein concentration. The presence of lipooligosaccharide (LOS) was assessed by both silver staining of SDS-PAGE minigels and assaying with the E-TOXATE Limulus lysate test (Sigma, Castle Hill, New South Wales, Australia).
Analytical SDS-PAGE.
A 10-μl fraction sample of purified OMP26 was added to an equal volume of sample buffer containing SDS and β-mercaptoethanol and boiled for 5 min. Electrophoresis was performed with minigels of a gradient of 10 to 15%, using the Pharmacia PhastSystem followed by silver staining using the PhastSystem staining unit.
Protein concentration determination.
Protein concentration was determined with the Pierce Micro BCA (bicinchoninic acid) protein assay reagent and the Pierce albumin standard (Laboratory Supplies, Marrickville, New South Wales, Australia).
Immunization.
Specific-pathogen-free DA male rats aged between 8 and 10 weeks were used, immunized, and challenged as previously described (16). The immunization protein was prepared by emulsifying 200 or 800 μg of protein per ml in a 1:1 ratio of incomplete Freund’s adjuvant (IFA; Difco Laboratories, Detroit, Mich.), and phosphate-buffered saline (PBS), and a total inoculum of 10 or 40 μg of protein, respectively, was administered to each animal via subserosal injection of intestinal Peyer’s patches (IPP). Control groups included (i) a sham-immunized group receiving IFA and PBS only and (ii) a positive group immunized with killed bacteria of the homologous NTHI strain. Bacteria were killed by suspension in 1% (wt/vol) paraformaldehyde in PBS and incubated at 37°C for 2 h. The bacteria were washed four times in PBS, and the concentration was adjusted to a bacterial equivalent of 2 × 1010 per ml. Killing was verified, and bacteria were then emulsified in a 1:1 ratio with IFA so that each animal received approximately 5 × 108 bacteria. Rats received an intratracheal (i.t.) boost on day 14 post-IPP immunization. The animals were sedated with halothane, and 10 μg of OMP26 at a concentration of 200 μg per ml in PBS was introduced into the lungs via an i.t. cannula and dispersed with two 5-ml volumes of air. The nonimmune group received 50 μl of PBS, while the killed bacterium-immunized group received 50 μl of killed bacteria (bacteria count of 1010 per ml).
Bacterial challenge.
Bacteria were prepared by overnight culture as described above and resuspended in PBS. The concentration of inoculum was estimated by optical density at 405 nm and confirmed by counting CFU of the overnight plating of serial dilutions of the inoculum.
Pulmonary challenge with live bacteria was performed on day 21 post-IPP immunization. The animals were sedated with halothane, and a bolus inoculum of 5 × 108 CFU of live H. influenzae in 50 μl of PBS was introduced into the lungs via an i.t. cannula and dispersed with two 5-ml volumes of air. Four hours after lung inoculation, animals were killed by an intraperitoneal injection of pentobarbital sodium. Blood was collected by heart puncture, and aliquots of serum stored at −20°C for antibody analysis. Lungs were lavaged with five 2-ml volumes of PBS via the trachea, which had been exposed through an incision in the neck, and the pooled bronchoalveolar lavage (BAL) was serially diluted to determine CFU. Lungs were also removed following lavage and homogenized in 10 ml of PBS, and bacterial counts were determined.
Cytospin and BAL cell counts.
Cytospin slides (Shandon Inc., Pittsburgh, Pa.) were prepared to determine percentages of polymorphonuclear neutrophils, macrophages, and others cells present in the BAL. Percentages were determined from three differential cell counts on each slide, and mean percentages ± standard errors of the means (SEM) were calculated. The BAL was centrifuged, the pellet was resuspended in PBS, and the total number of cells present in the BAL was determined by using a hemocytometer and staining with methylene blue.
Antigen-specific ELISAs.
Polysorb microtiter wells (Nunc, Roskilde, Denmark) were coated with purified OMP26 in 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]) overnight at 4°C. The concentrations of OMP26 were 1 μg per ml for assay of immunoglobulin G (IgG), IgG2a, IgA, and IgM and 10 μg per ml for IgG1, IgG2b, IgG2c, and IgE. The plates were washed five times in washing buffer (PBS containing 0.05% Tween 20). The wells were blocked with 100 μl of blocking buffer (5% skim milk in PBS–0.05% Tween 20) for 60 min at room temperature. Plates were washed five times, and serum (1/10 to 1/3,200) or BAL (1/2 to 1/16) samples were serially diluted in blocking buffer, added to the wells, and incubated at room temperature for 90 min. After removal of the samples by washing five times, 100 μl of horseradish peroxidase-conjugated anti-rat immunoglobulin diluted in blocking buffer was added to the wells and incubated at room temperature for 90 min. Conjugated immunoglobulins used were goat anti-rat IgG (1/2,000), IgA (1/1,000), and IgM (1/4,000) (Fc specific; Nordic Immunological Laboratories, Tilberg, Netherlands); and mouse anti-rat IgG1 (1/500), IgG2a (1/1,000), IgG2b (1/500), and IgG2c (1/500) (ICN Biochemicals Inc., Costa Mesa, Calif.). The plates were washed five times, and the wells were developed with 100 μl of the substrate tetramethylbenzidine (Fluka, Buchs, Switzerland) in phosphate citrate buffer (pH 5) containing 0.05% (vol/vol) H2O2. The reaction was stopped with 100 μl of 0.5 M H2SO4. Plates were read at 450 nm on a Titertek Multiscan MCC/340 plate reader. Plate background was determined by rows coated with coating buffer alone and treated the same as test wells. Between-plate variation was assessed by comparison of one immune and one control sample repeated on each plate. Serum or BAL was diluted until an optical density reading in a range between 0.4 and 0.9 was obtained. The enzyme-linked immunosorbent assay (ELISA) titer was calculated by multiplying the reciprocal of the dilution factor by the optical density value.
Antigen-specific lymphocyte assay.
The assay was performed essentially as described previously (7, 17). Briefly, lymphocytes from mesenteric lymph nodes were resuspended in culture medium (Multicel RPMI 1640 [Cytosystem, Castle Hill, New South Wales, Australia] containing 0.01 M HEPES [pH 7.2], 5 × 10−5 M β-mercaptoethanol, 2 mM l-glutamine [ICN, Sydney, Australia], 5% fetal calf serum, and penicillin-streptomycin-amphotericin B [as described above]) to obtain a final concentration of 106 cells per ml. The antigen (OMP26) or bacterial extract prepared as described elsewhere (17) was suspended in culture medium in a 10-fold dilution series and sterile filtered. The cell suspension and antigen were added in triplicate to flat-bottom multiwell microculture plates (Nunc) to give a final volume of 0.2 ml per well. Lymphocyte proliferation was estimated by [3H]thymidine (Amersham Australia, North Ryde, New South Wales, Australia) incorporation for the last 8 h of a 4-day culture. Results were calculated by subtraction of background from the geometric means of triplicate wells and then the geometric mean ± SEM of the entire treatment group.
Immunoblot assay.
Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose (0.2-μm pore size; Bio-Rad Laboratories, North Ryde, NSW, Australia) (30) for 55 min in 25 mM Tris–192 mM glycine (pH 8.8) buffer. Membranes were soaked in Tris-buffered saline (TBS; 20 mM Tris, 500 mM NaCl [pH 7.5]) for 10 min, blocked for 30 min with TBS containing 5% (wt/vol) skim milk, washed twice (5 min) in TTBS (TBS, 0.05% Tween [pH 7.5]), and incubated at room temperature for 90 min in rat serum diluted 10-fold in TTBS–5% (wt/vol) skim milk. After washing, the membrane was incubated for 90 min with goat horseradish peroxidase-conjugated anti-rat IgG (Fc specific; Nordic Immunology) diluted 1/500 in TTBS–5% skim milk. Before development with 0.05% (wt/vol) 4-chloro-1-naphthol (Bio-Rad Laboratories)–16.7% (vol/vol) methanol–0.015% (vol/vol) H2O2 in TBS, the membrane was washed twice in TTBS and once in TBS.
Electron microscopy.
Immunoelectron microscopy with goat anti-rat immunoglobulin bound to 12-nm gold spheres was used to determine if OMP26 was surface exposed. Bacteria from strain NTHI-I were washed and suspended in distilled water (approximately 109 CFU/ml), loaded onto nickel grids, and incubated at room temperature for 60 min. The grids were rinsed for 15 s in PBS, blocked by incubation for 1 h with 0.5% (wt/vol) bovine serum albumin in PBS, and then incubated for 1 h with 1:10 dilution of rat antiserum. After washing, the grids were incubated for 1 h with 12-nm colloidal gold conjugated goat anti-rat IgG (Pierce, Rockford, Ill.) diluted 1:40, washed, negatively stained, and viewed by an electron microscope technical expert on a Philips electron microscope (at the John Curtin School of Medical Research, Canberra, Australian Capital Territory, Australia).
Preparation of OMP26 for amino acid sequencing.
OMP26 was prepared for amino (N)-terminal sequencing by SDS-PAGE, using a Protean II electrophoresis unit (Bio-Rad) and a 12% acrylamide resolving gel. The protein was then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, Mass.), using a TransBlot (Bio-Rad). The location of the protein band on the PVDF membrane was determined by Coomassie staining, and the PVDF membrane was destained with methanol following excision of the protein section. This was sequenced by Cortecs International, Deeside, Clwyd, United Kingdom.
Statistical analysis.
The data have been expressed as means ± SEM. The pulmonary clearance data, total numbers of phagocytic cells, and differential cell count data were compared for statistical significance between groups by one-way analysis of variance, followed by Tukey’s test for multiple-comparison analysis (Macintosh Systat). Antibody data were assessed for between-group significance by an unpaired t test, and lymphocyte proliferation data were assessed by a fully factorial analysis of variance (Macintosh Systat). Linear correlation between two variables was determined using the Pearson correlation coefficient (Macintosh Systat).
RESULTS
Purification of OMP26.
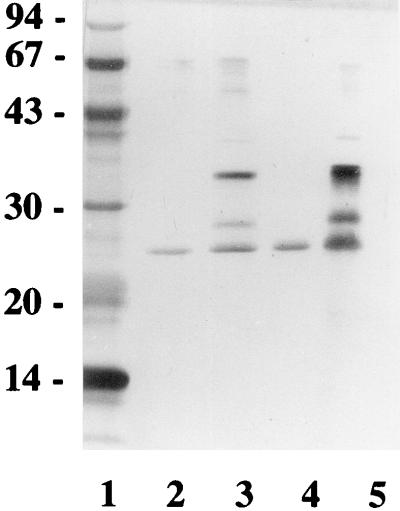
OMP26 was successfully separated from a group of three proteins with molecular masses of between 26 and 30 kDa. Figure 1 shows the position of this protein in relation to the other two, and the silver-stained gel indicates the high degree of purity following the final purification step. Assessment of the heat-modifiable characteristic of this protein found that after 30 min, the boiled protein sample still migrated with the same molecular mass (Fig. 1), as did the other neighboring protein bands. This indicated that none of the three proteins in the molecular mass range of 26 to 30 kDa obtained in the semipurified extraction was likely to be the heat-modifiable P5 or fimbrin proteins. Assessment of the protein for the presence of LOS contamination was performed with a E-TOXATE assay kit and found to be less than 0.6 μg of endotoxin per mg of protein. This represented the detection limit of the assay and is substantially less than LOS contamination reported in other immunization studies with proteins (10).
FIG. 1.
SDS-PAGE analysis of OMP26 and two other proteins with higher molecular masses. Proteins were separated on a 12% acrylamide-BIS gel by SDS-PAGE. Lane 1, molecular mass standards (molecular masses shown in kilodaltons on the left). Lanes 2 and 3, the protein samples were mixed with SDS reducing buffer but were not boiled. Lane 2, purified OMP26; lane 3, mixed protein sample of the three main proteins isolated in this molecular weight range. Lanes 4 and 5, same samples as in lanes 2 and 3, respectively, but heated for 30 min at 100°C in SDS reducing buffer containing β-mercaptoethanol.
Amino acid sequence identification.
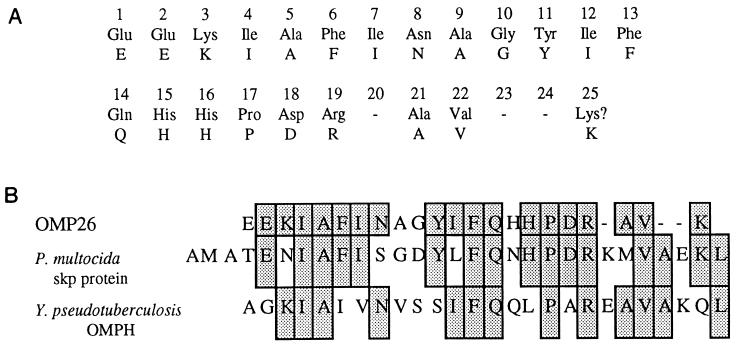
An N-terminal amino acid sequence was obtained from the protein band transferred to a PVDF membrane. Amino acid sequence analysis for the first 25 peptides is shown in Fig. 2A. The sequence analysis indicates no sequence homology with the N-terminal sequence of either Hib P5 (22) or the fimbrin protein (28), which have similar molecular masses if not heat modified. The N-terminal amino acid sequence does show 100% homology with a segment of the recently sequenced H. influenzae Rd genome (9), 56% homology with a 21.4-kDa protein from Pasteurella multocida (18), and 44% sequence homology with a 19-kDa outer membrane protein from Yersinia pseudotuberculosis (32).
FIG. 2.
(A) N-terminal sequence of the first 25 amino acid residues of OMP26. (B) Comparison of the amino acid sequences of OMP26 and the proteins from H. influenzae Rd genome, P. multocida, and Y. pseudotuberculosis. The shaded areas represent identical amino acids.
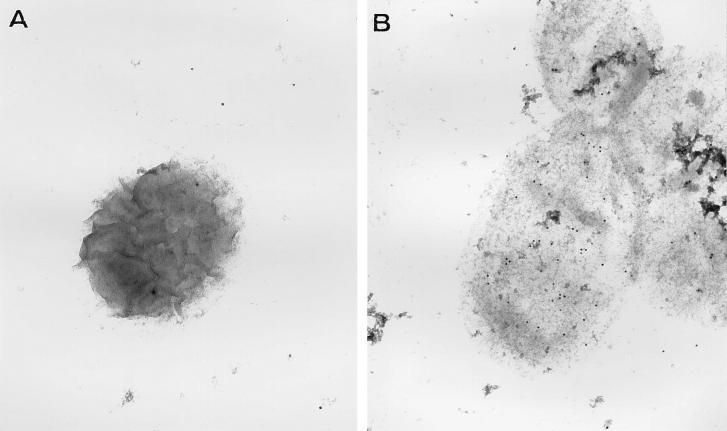
Polyclonal antisera against OMP26 immunolabeled a surface epitope on NTHI-I (Fig. 3A) which was not seen following incubation of NTHI-I with nonimmune serum (Fig. 3B). These results suggest that OMP26 has at least one surface-exposed domain and that following mucosal immunization with OMP26, some antibody is directed against this region.
FIG. 3.
Electron microscopy view of an NTHI-I strain immunolabeled with nonimmune rat serum (A) and polyclonal anti-OMP26 rat serum (B) and detected with anti-rat IgG-conjugated gold spheres. Gold spheres associated with NTHI-I immunolabeled with nonimmune serum were equivalent to the background, nonspecific binding of the gold-labeled conjugate, whereas there was consistent labeling above background binding to NTHI-I immunolabeled with the polyclonal anti-OMP26 serum (B).
Bacterial clearance.
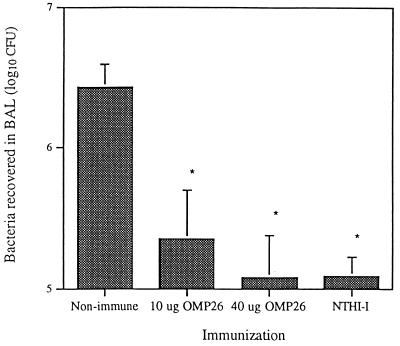
Rats immunized with OMP26 and challenged with live bacteria of the NTHI-I homologous strain on day 21 showed significant bacterial clearance (P < 0.005). Rats immunized and boosted with 10 μg of OMP26 had significantly fewer bacteria in the lung than the nonimmune group after 4 h. Rats receiving 40 μg of OMP26 in the IPP immunization and boosted with 10 μg of OMP26 had slightly further enhanced bacterial clearance which was equivalent to the clearance observed for killed-bacteria-immunized rats (Fig. 4).
FIG. 4.
NTHI-I bacteria recovered in the BAL 4 h after challenge with live bacteria. Nonimmune rats (n = 8) were sham treated, 10-μg OMP26 group was IPP immunized and i.t. boosted with 10 μg of OMP26 (n = 7), 40-μg OMP26 group was IPP immunized with 40 μg of OMP26 and i.t. boosted with 10 μg of OMP26 (n = 4), NTHI-I group was IPP immunized and i.t. boosted with killed bacteria (n = 5). *, P < 0.005 compared to nonimmune group. Live bacteria inoculum contained 7 × 109 CFU per ml as determined by plating of serial dilution.
Rats immunized with OMP26 and challenged with bacteria from a second nontypeable strain, NTHI-II (Table 1), exhibited clearance of this strain similar to the clearance for the homologous challenge and significantly different from that for the nonimmune rats (P < 0.005).
TABLE 1.
Pulmonary clearance and phagocytic cell counts following mucosal immunization with OMP26 and challenge by homologous and nonhomologous NTHI strains
| Rat groupb | Sample | Mean H. influenzae recovered 4 h postchallenge (log10 CFU)a ± SEM (% clearance)
|
Total no. of cells (106) in BAL (mean ± SEM)
|
||
|---|---|---|---|---|---|
| NTHI-I | NTHI-II | NTHI-I | NTHI-II | ||
| Nonimmune | BAL | 7.01 ± 0.11 | 6.28 ± 0.07 | 15.9 ± 1.1 | 17.5 ± 3.0 |
| Lung | 7.76 ± 0.05 | 6.69 ± 0.05 | |||
| OMP26 immunized | BAL | 6.15 ± 0.15 (87)c | 5.15 ± 0.16 (93)c | 26.1 ± 1.1c | 28.2 ± 1.6c |
| Lung | 6.81 ± 0.17 (89)c | 6.01 ± 0.19 (80)c | |||
Values for rats challenged for 4 h with live bacteria from either NTHI-I or NTHI-II strains on day 21 after IPP immunization with 10 μg of OMP26. All rats received an i.t. boost (10 μg) on day 14. Nonimmune rats were a combination of sham-treated and untreated animals, with no difference in response observed between animals. The concentration of live bacteria in the challenge inoculum (as determined by plating of serial dilutions) was 10.38 (log10) CFU per ml for NTHI-I and 10.17 (log10) CFU per ml for NTHI-II.
n = 4 per group for challenge with NTHI-I; n = 4 for nonimmune and n = 6 for OMP26-immunized groups for challenge with NTHI-II.
P < 0.005 compared to nonimmune group.
Greater numbers of phagocytic cells were present in the BAL of OMP26-immunized animals and correlated with the enhanced bacterial clearance in these animals (Table 1), although differential cell counts for polymorphonuclear neutrophils and macrophages were the same for immune and nonimmune groups (data not shown).
OMP26-specific immune responses.
Antibody to OMP26 was measured in the serum and BAL samples of rats immunized with OMP26 as well as from rats that had been immunized with killed bacteria from four different strains of H. influenzae. High OMP26-specific antibody titers for IgG, IgA, and IgM were found in the serum, and for IgG and IgA in the BAL, of rats immunized with OMP26, with the highest levels observed for the group receiving the higher immunization dosage of 40 μg (Table 2). Low levels of OMP26-specific IgG, IgA, and IgM in the serum and IgG and IgA in the BAL were also found in rats that had been immunized with different strains of H. influenzae (Table 2). OMP26-specific IgE could not be detected (data not shown).
TABLE 2.
Comparison of OMP26-specific antibodies in serum and BAL following immunization with either OMP26 or killed bacteria from H. influenzae strains
| Groupb | n | ELISA titera of antibody to OMP26a
|
|||||
|---|---|---|---|---|---|---|---|
| Serum
|
BAL
|
||||||
| IgG | IgA | IgM | IgG | IgA | IgM | ||
| Nonimmune | 7 | NDd | ND | 10.5 ± 1.6 | ND | ND | ND |
| Immunized | |||||||
| OMP26 | |||||||
| 10 μg | 5 | 1,209 ± 255e | 936 ± 161e | 30.5 ± 4e | 4.3 ± 1.0e | 6.1 ± 1.9e | ND |
| 40 μg | 5 | 2,806 ± 405e | 2,440 ± 410e | 142.5 ± 30.6e | 8.3 ± 1.9e | 29.1 ± 7.9e | ND |
| Killed bacteriac | |||||||
| NTHI-Ic | 4 | 58 ± 17e | 37 ± 4e | 39.1 ± 0.8e | 0.5 ± 0.1 | 1.2 ± 0.5 | ND |
| NTHI-IIc | 4 | 22 ± 4e | 26 ± 5e | 39.3 ± 5.0e | 0.6 ± 0.1 | 0.4 ± 0.1 | ND |
| HI-CDc | 4 | 18 ± 1e | 20 ± 1e | 42.2 ± 3.1e | 0.6 ± 0.1 | 0.8 ± 0.3 | ND |
| Hib-IIc | 4 | 27 ± 4e | 38 ± 3e | 34.4 ± 2.3e | 0.6 ± 0.1 | 1.6 ± 0.4 | ND |
Calculated as described in Materials and Methods.
Rats were immunized via IPP on day 0, received an i.t. boost on day 14, and were challenged with live bacteria on day 21. Serum and BAL samples were prepared as described in Materials and Methods.
Rats were immunized with killed bacteria from the strain of H. influenzae indicated and had received a live challenge with bacteria from the homologous strain.
ND, OMP26-specific antibody could not be detected at the lowest sample dilution.
P < 0.05 compared to nonimmune group.
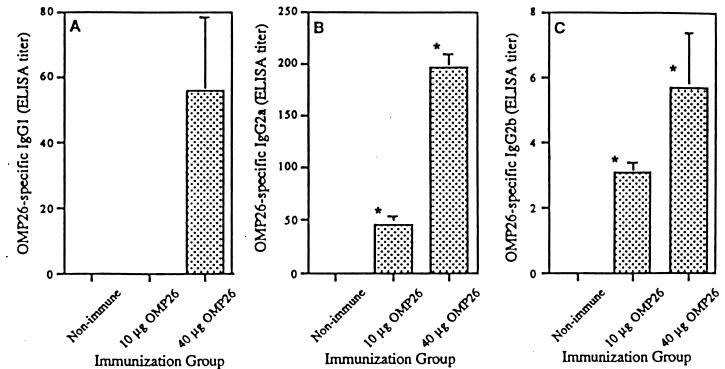
OMP26-specific IgG1 was detectable only following the 40-μg immunization, whereas IgG2a and IgG2b subclasses were found for both 10- and 40-μg OMP26 immunization groups (Fig. 5). Levels of both IgG2a and IgG2b increased significantly (P < 0.05) with the increase in concentration of OMP26 from 10 to 40 μg in the IPP inoculum. IgG2c was also measured; however, significant levels of OMP26-specific antibody from this subclass could not be detected (data not shown).
FIG. 5.
OMP26-specific levels of IgG subclasses in serum samples of rats immunized with OMP26. (A) IgG1 antibody; (B) IgG2a antibody; (C) IgG2b antibody. Values represent means ± SEM for serum from four to seven rats per group. ∗, P < 0.05 compared to nonimmune group.
Recognition of OMP26 by antibodies present in the serum of OMP26-immunized and H. influenzae (four strains)-immunized rats was shown by immunoblot analysis (data not shown). There was no recognition of OMP26 by nonimmune serum.
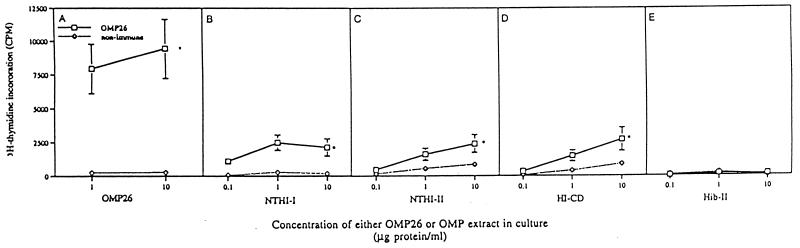
Cells from the OMP26-immunized group responded significantly to OMP26, extracts from strains NTHI-I, NTHI-II, and HI-CD, but not the extract from the Hib-II strain, in culture in vitro (Fig. 6).
FIG. 6.
Antigen-specific proliferation of lymphocytes isolated from the mesenteric lymph nodes of OMP26-immunized and nonimmune rats. Cells were cultured for 4 days with either OMP26 or OMP extract from four different H. influenzae strains. Shown are lymphocyte responses to culture with OMP26 (A), NTHI-I OMP extract (B), NTHI-II OMP extract (C), HI-CD OMP extract (D), and Hib-II OMP extract (E). Values represent means ± SEM of combined responses for lymphocytes from four rats per group and triplicate cultures of lymphocytes per rat. ∗, P < 0.05 compared to nonimmune response.
DISCUSSION
This study has identified a protein from strain NTHI-I that is different from the heat-modifiable P5 from Hib and fimbrin protein from NTHI but has a corresponding molecular mass. OMP26 was the lower-molecular-mass band on SDS-PAGE of a group of three proteins with molecular masses of between 26 and 30 kDa and corresponded to a major protein band between P6 and a band corresponding to P4, the next major OMP for H. influenzae strains. Studies of P5 in Hib strains had identified P5 as being heat modifiable when boiled for 30 min under reducing conditions, shifting from an apparent molecular mass of 25 to 27 kDa to one of 35 to 36.5 kDa (21). OMP26 isolated from NTHI-I was not heat modifiable. N-terminal sequence analysis of OMP26 identified the sequence of this 26-kDa protein as distinctly different from those of the Hib P5 (22) and a fimbrin protein (28).
The N-terminal sequence had 100% homology with a segment of the H. influenzae Rd genome (9) encoding a protein nominated as an export factor homolog (HI 0916) based on apparent homology to other skp transporter proteins (76% similarity). A protein band corresponding to OMP26 was present in all strains of H. influenzae used in this study (data not shown). The sequence has a high homology with that reported for the H. influenzae Rd genome (9), with variation that resulted in an alteration of amino acids 102 and 170 (unpublished data).
The sequence for OMP26 had similarities to those of the proteins from two other gram-negative bacteria, P. multocida and Y. pseudotuberculosis. skp-encoded proteins are believed to play a role either as chaperones in extracytoplasmic compartments or as folding catalysts (19). In P. multocida, the skp gene and the firA gene are part of an operon governing the first steps of lipid A synthesis (4). In the H. influenzae Rd genome (9), the location on the gene encoding OMP26 is also adjacent to the region homologous to the firA gene. The relationship of OMP26 to these proteins and any role of OMP26 in protein transport have yet to be determined.
Primary inoculation of OMP26 into the IPP followed by a boost in the lungs induced immune responses that were highly protective against not only pulmonary challenge by the homologous strain but also from challenge by a different unrelated NTHI strain. This immunization targets the mucosal immune system and has been shown to be effective in inducing an effective immune response in the lungs (15, 16, 34). Immunization with whole bacteria results in an immune response to a range of antigens (12) including P2, P6, and LOS (our unpublished data) and is sufficient to enhance bacterial clearance of the homologous NTHI strain (34). Immunization with P2 (15), the most abundant OMP, did not result in bacterial clearance of this magnitude; however, immunization with P6 enhanced pulmonary clearance to a similar degree in some strains (16). Immunization with OMP26 resulted in clearance similar to that for whole-bacterium immunization, suggesting that OMP26 may be a suitable vaccine candidate.
OMP26-specific antibody levels were measured in serum and BAL from OMP26-immunized rats and from rats immunized with killed bacteria from four H. influenzae strains. Significant titers of IgG, IgA, and IgM were found in OMP26-immunized serum, and these levels increased with an increase in concentration of OMP26 in the IPP inoculum. Low levels of OMP26-specific IgG, IgA, and IgM were also found in serum from whole-bacterium-immunized rats. The levels were lower than in the purified-protein-immunized rats but were similar in magnitude for the different H. influenzae strains. OMP26-specific immunoglobulin in BAL was significant for IgG and IgA in OMP26-immunized groups but barely detectable in whole-bacterium-immunized groups. This finding suggests that immunization with killed bacteria results in a poor immune response to OMP26.
Antigen-specific T-helper (Th)-induced secretion of lymphokines during antigen processing is believed to influence IgG isotypes (8), and measurement of isotypes of IgG is indicative of the type of Th response induced during immunization. OMP26-specific IgG1 was detected only after immunization with 40 μg of OMP26, suggesting that this was not the predominant isotype induced. OMP26-specific IgG2a and IgG2b were detectable, with IgG2a appearing to be the dominant isotype. These isotype responses suggest that the Th and lymphokine response following immunization with OMP26 may be a Th1 type response.
Lymphocytes from OMP26-immunized rats proliferated in vitro in response to OMP26 and OMP extracts from H. influenzae strains. This finding demonstrates the cross-reactivity of the lymphocytes to T-cell epitopes in antigen in the different extracts. The magnitude of response by the OMP26-specific lymphocytes to the OMP extracts was significantly less than that observed for the purified protein since OMP26 is present as a small percentage of the total protein concentration in the extracts.
The identification of this 26-kDa protein, called OMP26, as having the capacity to enhance bacterial clearance following mucosal immunization has been an important finding. OMP26 may have significant potential as a candidate for a vaccine against respiratory infections caused by NTHI. Future studies will be undertaken to further characterize this protein by the development of clones, determine the degree of conservation at a molecular level, and identify its role on the bacterial surface.
ACKNOWLEDGMENTS
This research was supported by the National Health and Medical Research Council, Australia, and by Cortecs International. J. Kyd was supported for part of the study by an Australian Postgraduate Research Award.
We thank Melissa Musicka for excellent microbiology assistance and Katherine Gillespie at the John Curtin School of Medical Research for the electron microscopy work.
REFERENCES
- 1.Barenkamp S J, Munson R S, Jr, Granoff D M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981;143:668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone C D, Klein J O. Otitis media with effusion, atelectasis and eustachian tube dysfunction. In: Bluestone C D, Stool S E, editors. Pediatric otolaryngology. Philadelphia, Pa: The W. B. Saunders Co.; 1983. p. 356. [Google Scholar]
- 3.Claesson B A, Lagergard T, Trollfors B. Antibody response to outer membrane of noncapsulated Haemophilus influenzae isolated from the nasopharynx of children with pneumonia. Pediatr Infect Dis J. 1991;10:104–108. doi: 10.1097/00006454-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Delamarche C, Manoha F, Behar G, Houlgatte R, Hellman U, Wroblewski H. Characterisation of the Pasteurella multocida skp and firA genes. Gene. 1995;161:39–43. doi: 10.1016/0378-1119(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 5.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duim B, Dankert J, Jansen H M, van Alphen L. Genetic analysis of the diversity in outer membrane P2 of non-encapsulated Haemophilus influenzae. Microb Pathog. 1993;14:451–462. doi: 10.1006/mpat.1993.1044. [DOI] [PubMed] [Google Scholar]
- 7.Dunkley M L, Husband A J. The induction and migration of antigen-specific helper cells for IgA responses in the intestine. Immunology. 1986;57:379–385. [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelman F D, Holmes J, Katona I M, Urban J J F, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 10.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase E M, Campagnari A A, Sarwar J, Shero M, Wirth M, Cumming C U, Murphy T F. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1991;59:1278–1284. doi: 10.1128/iai.59.4.1278-1284.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen E J, Hart D A, McGehee J L, Toews G B. Immune enhancement of pulmonary clearance of nontypeable Haemophilus influenzae. Infect Immun. 1988;56:182–190. doi: 10.1128/iai.56.1.182-190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirschmann J V, Everett E D. Haemophilus influenzae infections in adults: report of nine cases and a review of the literature. Medicine (Baltimore) 1979;58:80–94. doi: 10.1097/00005792-197901000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kroll J S, Moxon E R. Capsulation and gene copy number at the cap locus of Haemophilus influenzae type b. J Bacteriol. 1988;170:859–864. doi: 10.1128/jb.170.2.859-864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyd J M, Cripps A W. Modulation of antigen-specific T and B cell responses influence bacterial clearance of nontypeable Haemophilus influenzae from the lung in a rat model. Vaccine. 1996;14:1471–1478. doi: 10.1016/s0264-410x(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 15a.Kyd, J. M., A. W. Cripps, C. Smith, and Cortecs International Ltd. 1996. Patent PCT/GB96/01549. Novel Haemophilus influenzae antigen.
- 16.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyd J M, Taylor D, Cripps A W. Conservation of immune responses to proteins isolated by preparative polyacrylamide gel electrophoresis from the outer membrane of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:5652–5658. doi: 10.1128/iai.62.12.5652-5658.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manoha F, Behar G, Houlgarre R, Hellman U, Wroblewski H, Delamarche C. skp protein-Pasteurella multocida. Heidelburg, Germany: EMBL data library, EMBL; 1993. [Google Scholar]
- 19.Missiakas D, Betton J M, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli surA, fkpA and skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 20.Munson R S, Jr, Bailey C, Grass S. Diversity of the outer membrane protein P2 gene from major clones of Haemophilus influenzae type b. Mol Microbiol. 1989;3:1797–1803. doi: 10.1111/j.1365-2958.1989.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 21.Munson R S, Jr, Granoff D M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae. Infect Immun. 1985;49:544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munson R S, Jr, Grass S, West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993;61:4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy T F, Apicella M A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Murphy T F, Bartos L C. Purification and analysis with monoclonal antibodies of P2, the major outer membrane protein of nontypeable Haemophilus influenzae. Infect Immun. 1988;56:1084–1089. doi: 10.1128/iai.56.5.1084-1089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musher D M, Kubitschek K R, Crennan J, Baughn R E. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med. 1983;99:444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 26.Nelson M B, Munson R S, Jr, Apicella M A, Sikkema D J, Molleston J P, Murphy T F. Molecular conservation of the P6 outer membrane among strains of Haemophilus influenzae: analysis of antigenic determinants, gene sequences, and restriction fragment length polymorphisms. Infect Immun. 1991;59:2658–2663. doi: 10.1128/iai.59.8.2658-2663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quentin R, Goudeau A, Wallace R J, Jr, Smith A L, Selander R K, Musser J M. Urogenital, maternal and neonatal isolates of Haemophilus influenzae: identification of unusually virulent serologically non-typable clone families and evidence for new Haemophilus species. J Gen Microbiol. 1990;136:1203–1209. doi: 10.1099/00221287-136-7-1203. [DOI] [PubMed] [Google Scholar]
- 28.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Terada T. Removal of dodecyl sulfate from protein solution. Anal Biochem. 1988;172:259–263. doi: 10.1016/0003-2697(88)90440-x. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4355. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk D C. The pathogenicity to Haemophilus influenzae. J Med Microbiol. 1984;18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 32.Vuoria R, Hirvas L, Raybourne R B, Yu D T Y, Vaara M. OMPH-Yersinia pseudotuberculosis. Heidelburg, Germany: EMBL data library, EMBL; 1991. [Google Scholar]
- 33.Wald E R, Milmoe G J, Bowen A D, Ledesma-Medina J, Salmon N, Bluestone C D. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 34.Wallace F J, Clancy R L, Cripps A W. An animal model demonstration of enhanced clearance of nontypable Haemophilus influenzae from respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989;140:311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- 35.Wallace R J, Jr, Musher D M, Septimus E J, McGowan J E, Quinones F J, Wiss K, Vance P H, Trier P A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and β lactamase production. J Infect Dis. 1981;144:101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]