Abstract
Background
The Internet could provide a means of delivering secondary prevention programmes to people with coronary heart disease (CHD).
Objectives
To determine the effectiveness of Internet‐based interventions targeting lifestyle changes and medicines management for the secondary prevention of CHD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, in December 2014. We also searched six other databases in October 2014, and three trials registers in January 2015 together with reference checking and handsearching to identify additional studies.
Selection criteria
Randomised controlled trials (RCTs) evaluating Internet‐delivered secondary prevention interventions aimed at people with CHD.
Data collection and analysis
Two review authors independently assessed risk of bias and extracted data according to the Cochrane Handbook for Systematic Reviews of Interventions. We assessed evidence quality using the GRADE approach and presented this in a 'Summary of findings' table.
Main results
Eighteen trials met our inclusion criteria. Eleven studies are complete (1392 participants), and seven are ongoing. Of the completed studies, seven interventions are broad, targeting the lifestyle management of CHD, and four focused on physical activity promotion. The comparison group in trials was usual care (n = 6), minimal intervention (n = 3), or traditional cardiac rehabilitation (n = 2).
We found no effects of Internet‐based interventions for all‐cause mortality (odds ratio (OR) 0.27, 95% confidence interval (CI) 0.04 to 1.63; participants = 895; studies = 6; low‐quality evidence). There was only one case of cardiovascular mortality in a control group (participants = 895; studies = 6). No incidences of non‐fatal re‐infarction were reported across any of the studies. We found no effects for revascularisation (OR 0.69, 95% CI 0.37 to 1.27; participants = 895; studies = 6; low‐quality evidence).
We found no effects for total cholesterol (mean difference (MD) 0.00, 95% CI ‐0.27 to 0.28; participants = 439; studies = 4; low‐quality evidence), high‐density lipoprotein (HDL) cholesterol (MD 0.01, 95% CI ‐0.06 to 0.07; participants = 437; studies = 4; low‐quality evidence), or triglycerides (MD 0.01, 95% CI ‐0.17 to 0.19; participants = 439; studies = 4; low‐quality evidence). We did not pool the data for low‐density lipoprotein (LDL) cholesterol due to considerable heterogeneity. Two out of six trials measuring LDL cholesterol detected favourable intervention effects, and four trials reported no effects. Seven studies measured systolic and diastolic blood pressure; we did not pool the data due to substantial heterogeneity. For systolic blood pressure, two studies showed a reduction with the intervention, but the remaining studies showed no effect. For diastolic blood pressure, two studies showed a reduction with the intervention, one study showed an increase with the intervention, and the remaining four studies showed no effect.
Five trials measured health‐related quality of life (HRQOL). We could draw no conclusions from one study due to incomplete reporting; one trial reported no effect; two studies reported a short‐ and medium‐term effect respectively; and one study reported both short‐ and medium‐term effects.
Five trials assessed dietary outcomes: two reported favourable effects, and three reported no effects. Eight studies assessed physical activity: five of these trials reported no physical activity effects, and three reported effectiveness. Trials are yet to measure the impact of these interventions on compliance with medication.
Two studies measured healthcare utilisation: one reported no effects, and the other reported increased usage of healthcare services compared to a control group in the intervention group at nine months' follow‐up. Two trials collected cost data: both reported that Internet‐delivered interventions are likely to be cost‐effective.
In terms of the risk of bias, the majority of studies reported appropriate randomisation and appropriate concealment of randomisation processes. A lack of blinding resulted in a risk of performance bias in seven studies, and a risk of detection bias in five trials. Two trials were at risk of attrition bias, and five were at risk for reporting bias.
Authors' conclusions
In general, evidence was of low quality due to lack of blinding, loss to follow‐up, and uncertainty around the effect size. Few studies measured clinical events, and of those that did, a very small number of events were reported, and therefore no firm conclusions can be made. Similarly, there was no clear evidence of effect for cardiovascular risk factors, although again the number of studies reporting these was small. There was some evidence for beneficial effects on HRQOL, dietary outcomes, and physical activity, although firm conclusions cannot yet be made. The effects on healthcare utilisation and cost‐effectiveness are also inconclusive, and trials are yet to measure the impact of Internet interventions on compliance with medication. The comparison groups differed across trials, and there were insufficient studies with usable data for subgroup analyses. We intend to study the intensity of comparison groups in future updates of this review when more evidence is available. The completion of the ongoing trials will add to the evidence base.
Keywords: Aged, Humans, Middle Aged, Internet, Life Style, Coronary Artery Disease, Coronary Artery Disease/prevention & control, Coronary Artery Disease/rehabilitation, Diet, Exercise, Quality of Life, Randomized Controlled Trials as Topic, Risk Reduction Behavior, Secondary Prevention, Secondary Prevention/methods
Plain language summary
Internet‐based programmes for people with heart disease
Review question
Are Internet‐based support programmes for people with heart disease helpful in improving their heart disease condition?
Background
Heart disease is the most common cause of ill health and preventable death. Cardiac rehabilitation is a programme that helps people with heart disease gain better health. It is held in group classes that take place at hospitals or within the community. People attend these classes once or twice a week for around six to eight weeks. The classes usually involve exercising, and receiving advice on ways to improve their health. People needing these programmes are not always able to attend them. An alternative is to provide this programme through the Internet. In this review we looked at whether programmes delivered through the Internet are helpful in improving death rates, the need for surgery, repeated heart attacks, cholesterol levels, blood pressure, health‐related quality of life (HRQOL), diet, physical activity, medication compliance, healthcare usage, and costs.
Study characteristics
The evidence is current to December 2014. We included 18 studies. Eleven are complete, and seven are ongoing. In the completed studies, 1392 people with coronary heart disease were recruited. The average age of participants ranged from 54.9 to 66.27 years. The majority of people recruited were men. Studies were carried out worldwide, and in a variety of healthcare settings. Seven studies tested broad programmes targeting multiple lifestyle factors related to heart disease. Four studies tested programmes focused only on increasing levels of physical activity. The length of the programmes in the included studies ranged from six weeks to one year. These programmes were compared to no intervention in six studies, some support in three studies, and full traditional rehabilitation in two studies.
Key results
There is no evidence to date to suggest that Internet‐delivered programmes help reduce rates of death or future cardiac surgery, but this was based on a small number of studies. There is also no strong evidence to date suggesting a benefit of these programmes for lipid levels or blood pressure. There is some evidence to suggest improvements in HRQOL and behaviour change, but there is insufficient evidence to date to draw firm conclusions. Studies have not yet measured the impact of Internet‐delivered programmes on medication compliance. There was very limited information on healthcare utilisation and cost of interventions. The reporting of the seven ongoing studies will add to the evidence base.
Quality of the evidence
The evidence was generally of low quality. The included studies were at some risk of bias, with six studies judged at high risk of bias for some risk of bias domains. The results of this review therefore need to be interpreted cautiously.
There is currently limited evidence on the effects of Internet‐based interventions for the treatment of coronary heart disease. We identified seven ongoing trials, which we will incorporate into this review when the results are available.
Summary of findings
Summary of findings for the main comparison. Internet‐based interventions compared to usual care or no care for prevention of coronary heart disease.
| Internet‐based interventions compared to usual care or no care for prevention of coronary heart disease: clinical outcomes | ||||||
| Patient or population: patients with coronary heart disease Settings: healthcare settings Intervention: Internet‐based interventions Comparison: usual care or no care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care or no care | Internet‐based interventions | |||||
| Total mortality | Study population | OR 0.27 (0.04 to 1.63) | 895 (6 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 9 per 1000 | 2 per 1000 (0 to 15) | |||||
| Moderate risk population | ||||||
| 3 per 1000 | 1 per 1000 (0 to 5) | |||||
| Revascularisation | Study population | OR 0.69 (0.37 to 1.27) | 895 (6 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 58 per 1000 | 41 per 1000 (22 to 73) | |||||
| Moderate risk population | ||||||
| 18 per 1000 | 12 per 1000 (7 to 23) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Maddison 2014, Reid 2012, Zutz 2007 ‐ outcomes may have been influenced by lack of blinding. 2Confidence interval crosses line of no effect (uncertainty around the magnitude of effect).
Summary of findings 2. Internet‐based interventions compared to usual care or no care for prevention of coronary heart disease.
| Internet‐based interventions compared to usual care or no care for prevention of coronary heart disease: cardiovascular risk factors | ||||||
| Patient or population: patients with coronary heart disease Settings: healthcare settings Intervention: Internet‐based interventions Comparison: usual care or no care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care or no care | Internet‐based interventions | |||||
| Total cholesterol | The mean total cholesterol in the intervention groups was 0 higher (0.27 lower to 0.28 higher) | 439 (4 studies) | ⊕⊕⊝⊝ low1 | |||
| LDL cholesterol | See comment | See comment | Not pooled | 437 (4 studies) | ⊕⊕⊝⊝ low1 | High levels increase risk, while low levels reduce risk. Data not pooled due to unexplained considerable (I2 = 77%) heterogeneity |
| HDL cholesterol | The mean HDL cholesterol in the intervention groups was 0.01 higher (0.06 lower to 0.07 higher) | 437 (4 studies) | ⊕⊕⊝⊝ low1 | High levels reduce risk, while low levels increase risk | ||
| Triglycerides | The mean triglycerides in the intervention groups was 0.01 higher (0.17 lower to 0.19 higher) | 439 (4 studies) | ⊕⊕⊝⊝ low1 | High levels increase risk, while low levels reduce risk | ||
| Systolic blood pressure | See comment | See comment | Not pooled | 623 (5 studies) | ⊕⊕⊝⊝ low2 | Systolic blood pressure measured in 5 studies. Data not pooled due to unexplained substantial (I2 = 63%) heterogeneity |
| Diastolic blood pressure | See comment | See comment | Not pooled | 622 (5 studies) | ⊕⊕⊝⊝ low2 | Diastolic blood pressure measured in 5 studies. Data not pooled due to unexplained substantial (I2 = 58%) heterogeneity |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;HDL: High‐density lipoprotein ; LDL: Low‐density lipoprotein | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Zutz 2007 ‐ outcomes may have been influenced by lack of blinding; Varnfield 2014 ‐ loss to follow‐up > 20% in both experimental arms. 2Devi 2014, Zutz 2007 ‐ outcomes may have been influenced by lack of blinding; Varnfield 2014 ‐ loss to follow‐up > 20% in both experimental arms.
Background
Description of the condition
Cardiovascular diseases are the number one cause of death globally. It has been estimated that the cumulative cost of cardiovascular disease to the European Union economy is EUR 196 billion a year (European Society of Cardiology 2012). Secondary prevention interventions can favourably modify cardiac risk factors in people with coronary heart disease (CHD) (McAlister 2001), and have a positive effect on physical activity, exercise training, and overall cardiorespiratory fitness (Lavie 2009). Furthermore, there is also strong evidence showing benefits in terms of clinical events and health‐related quality of life (HRQOL) (Cole 2011; McAlister 2001; O'Connor 1989; Oldridge 1988). Previous Cochrane reviews have highlighted the potential benefits in terms of effectiveness of both exercise‐based interventions and psychological interventions for CHD (Heran 2011; Whalley 2011). A further Cochrane review illustrates that smoking cessation effectively alters the course of CHD (Critchley 2003).
Not all individuals with CHD take part in secondary prevention programmes. A recent annual survey found that only 43% of people with CHD took part in cardiac rehabilitation in England, Wales, and Northern Ireland between 2012 and 2013 (NACR 2014). Referral, uptake, and adherence to traditional cardiac rehabilitation programmes are often poor, particularly in older patients, women, ethnic minorities, and in patients with angina or heart failure (Beswick 2004). The reasons for this lack of uptake are complex, some related to the organisation and system of delivery (O'Driscoll 2007), and others to individual choice. Factors related to rehabilitation non‐attendance that appear frequently in the literature are employment commitments, difficulties with transport, lack of time, distance to travel to rehabilitation, and embarrassment related to attending rehabilitation (De Vos 2013; McKee 2013; Neubeck 2012).
Home‐based interventions are an alternative way to improve access to secondary prevention programmes. A meta‐analysis of 36 trials of home‐based secondary prevention interventions demonstrated that they improve HRQOL, lower systolic blood pressure, lower total cholesterol, reduce smoking rates, and reduce depression in people with CHD (Clark 2010). A Cochrane review confirmed the effectiveness of home‐based compared with centre‐based cardiac rehabilitation programmes(Taylor 2010), reporting no differences between programmes in the number of clinical events, exercise capacity, blood pressure, total cholesterol, proportion of smokers, or HRQOL. A recent approach has been to embrace technology and use the Internet as an option to support lifestyle change important for the secondary prevention of CHD.
Description of the intervention
Using the Internet offers an alternative way to deliver secondary prevention interventions. Internet interventions can overcome inconveniences such as the time and expense involved in travelling to intervention locations (Griffiths 2006; Neville 2009; Nguyen 2004). Users also benefit from having information and support available 24 hours per day. With advanced website programming, it is also possible to create highly interactive interventions that incorporate theoretical constructs of health behaviour change and evidence‐based 'behaviour change techniques' (Ciccolo 2008; Michie 2013). Patients are also able to communicate with health professionals through the use of various communication channels such as email, instant chat, or discussion forums (Griffiths 2006; Murray 2008). Interventions may not include all of these features, however there is considerable potential to design interventions with as many features as possible. Further practical advantages for service providers include cost‐effectiveness, in Murray 2008, and the ability to reach large, geographically dispersed populations without time or location restrictions (Eng 1999; Eysenbach 2001; Griffiths 2006). Web‐based interventions also have the potential to store large volumes of information and can be easily updated as new research becomes available (Murray 2008).
How the intervention might work
Online interventions have been shown to be effective for general health behaviour change (Wantland 2004). Several trials have examined the effectiveness of Internet‐based interventions for promoting healthy nutrition and weight loss and increasing physical activity (Moore 2008; Sternfeld 2009). These findings are consistent with reviews that also report considerable benefit of Internet‐based interventions in increasing physical activity (Davies 2012).
Internet‐based interventions have been shown to be effective for people with multiple sclerosis in reducing medication discontinuation and increasing patients’ intentions towards medication persistency (Liang 2006). A review has examined telehealth interventions for secondary prevention of CHD (Neubeck 2009). The review included 11 studies, of which two used Internet‐based interventions; the remaining nine studies evaluated interventions delivered via telephone. The overall findings suggest that telehealth interventions are useful in the secondary prevention of CHD, showing improvements in the risk factor profile of patients with the intervention. The two Internet‐based studies included in this review present positive findings both in terms of clinical events, in Southard 2003, and cardiovascular risk factor profile (Zutz 2007). Munro 2013 assessed interventions for heart disease populations that compared Internet‐based cardiac rehabilitation to usual care in a systematic review. This review was broad, including studies of heart failure populations, and cohort study designs. The review included nine studies, which demonstrated improvements in clinical outcomes, physical activity, and psychosocial outcomes. A review recently carried out by Widmer 2015 assessed the benefit of digital health interventions on cardiovascular disease outcomes, and included studies delivering interventions through various digital technologies. Thirteen studies included in this review reported no significant improvements in weight, diastolic blood pressure, triglyceride levels, total cholesterol, low‐density lipoprotein cholesterol, or glucose in secondary prevention populations, but did demonstrate significant reductions in body mass index. A recent study on the feasibility of using Internet‐based interventions in this patient population showed that over 60% of participants surveyed who were eligible for cardiac rehabilitation had Internet access and were confident in opening links and navigating websites (Neubeck 2010), therefore demonstrating potential to reach this population via the Internet. No adverse effects of Internet‐based interventions have been observed in populations studied to date.
Why it is important to do this review
To date, evidence suggests that traditional secondary prevention interventions are effective in reducing adverse outcomes in people with CHD (Heran 2011; Taylor 2010), but that access to services in terms of provision, uptake, and adherence is limited (NACR 2014). Internet‐based interventions may address some of these limitations and be an effective alternative method of providing secondary prevention to this patient group (Griffiths 2006). No systematic reviews have specifically focused on examining the effectiveness of Internet‐based interventions for the secondary prevention of CHD, nor has there been an assessment of Internet intervention effects on HRQOL, lifestyle factors related to CHD, or cost‐effectiveness of these interventions.
Objectives
To determine the effectiveness of Internet‐based interventions targeting lifestyle changes and medicines management for the secondary prevention of CHD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults (18 years of age or older) with CHD, including those having experienced a myocardial infarction, a revascularisation procedure (including stent, coronary artery bypass grafting, or percutaneous transluminal coronary angioplasty), those with angina, or angiographically defined CHD.
Types of interventions
We considered all Internet‐based interventions designed to promote a healthy lifestyle and medicines management and reduce cardiovascular risk in people with CHD.
We defined Internet‐based interventions as individually targeted interactive computer‐mediated applications available via the Internet. We only included interventions delivered via the Internet, and therefore studies considered for this review were primarily computer based, although we did consider that Internet‐based interventions may also be delivered via smartphone technology.
We excluded interventions delivered via other technologies that did not require an Internet connection.
We specifically excluded Internet‐based interventions that focused on smoking cessation, as this was the subject of a Cochrane review registered with the Tobacco Addictions group (Civljak 2010). This did not include trials where smoking cessation formed part of a package of care.
We only considered trials where the comparison group was usual care or no intervention, and where follow‐up was reported at least three months postintervention.
Types of outcome measures
Primary outcomes
Clinical outcomes:
Mortality (cardiovascular and overall)
Non‐fatal re‐infarction
Revascularisation
Cardiovascular risk factors:
Lipid levels (total cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), and triglycerides)
Blood pressure (systolic blood pressure and diastolic blood pressure)
HRQOL
Secondary outcomes
Lifestyle changes in diet and physical activity. Where possible, we focused on objective measures of lifestyle change.
Compliance with medication
Healthcare utilisation and costs
Adverse intervention effects
Search methods for identification of studies
Electronic searches
We searched the following electronic databases between October and December 2014:
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11 of 12, 2014) on the Cochrane Library
MEDLINE (OVID, 1946 to November week 4 2014)
EMBASE Classic and EMBASE (OVID, 1947 to 2014 December 22)
PsycINFO (OVID, 1806 to October week 3 2014)
CINAHL on EBSCOhost (to 17 October 2014)
Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), and Conference Proceedings Citation Index‐Science (CPCI‐S) on Web of Science (Thomson Reuters, 1970 to 15 October 2014)
Health Technology Assessment (HTA), Database of Abstracts of Reviews of Effects (DARE), and NHS Economic Evaluation Database (NEED) on the Cochrane Library (Issue 3 of 4, 2014)
We also used medical subject headings (MeSH) or equivalent and text word terms. We applied no language restrictions.
We have listed the search strategies in Appendix 1. We used the Cochrane sensitivity‐maximising RCT filter for MEDLINE and adaptations of it for use in the other databases (Lefebvre 2011), except CENTRAL and PsycINFO.
Searching other resources
We checked reference lists of reviews and retrieved articles for additional studies.
In January 2015, we further searched the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), ClinicalTrials.gov (www.clinicaltrials.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing trials. We used different combinations of the following search terms: coronary heart disease, cardiovascular disease, Internet, web‐based, world wide web, and online.
We handsearched the Journal of Medical Internet Research and proceedings from the World Congress on Medical and Health Informatics (MEDINFO) for additional studies from the last five years. We contacted authors where necessary for additional information.
Data collection and analysis
Selection of studies
Two review authors (RD and shared between EI, EF, and SS) independently screened the titles and abstracts of all records using a checklist to identify relevant papers. We then obtained the full‐text reports of potentially relevant studies and applied our inclusion criteria to select studies for inclusion. Other review authors (JP or KR) were consulted when there were disagreements between review authors about study selection.
Data extraction and management
Two review authors (RD and shared between EI, EF, and SS) independently extracted data using a proforma. We contacted chief investigators for additional information if necessary. We extracted details regarding the study methodology, participant characteristics, study setting, intervention design (frequency, duration, intensity, level of interactivity, and the focus of the intervention), outcome data (including details of outcome assessment), adverse effects, and methodological quality (randomisation, blinding, attrition) from each of the included studies. Other review authors (JP or KR) were consulted when there were disagreements between review authors about data extraction.
Assessment of risk of bias in included studies
Two review authors (RD and shared between EF, SS, and JP) assessed risks of selection, performance, detection, attrition, and reporting bias using the Cochrane 'Risk of bias' tool (Higgins 2011).
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We expressed dichotomous outcomes as odds ratios or risk ratios, and we calculated 95% confidence intervals for each study. We compared net changes for continuous variables (that is intervention group minus control group differences) and calculated a weighted mean difference or standardised mean difference and 95% confidence intervals for each study.
Assessment of heterogeneity
We carried out tests of heterogeneity (using the Chi2 test of heterogeneity and I2 statistic) for each outcome. In the case of no heterogeneity, we performed a fixed‐effects meta‐analysis. If we detected significant heterogeneity, we looked for possible explanations (for example participants and intervention). If the heterogeneity was not explainable, the review authors considered the following options: provide a narrative overview and not aggregate the studies at all, or use a random‐effects model with appropriate cautious interpretation. We used the Cochrane Handbook for Systematic Reviews of Interventions as a guide to interpret the I2 statistic where taken in consideration with the magnitude and direction of effect and strength of evidence for heterogeneity from the confidence interval for the I2 statistic or P value from the Chi2 test are as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity.
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analyses on:
Multi‐component Internet‐based interventions versus single‐component interventions, however all the interventions contained multiple components, and none were single‐component interventions. Therefore we were unable to carry out this subgroup analysis.
2. Internet interventions as part of a broader package of care including non‐Internet‐based interventions versus Internet‐only interventions.
We planned to examine the effect of intensity and duration of the intervention (in terms of number of contacts, support given, and interactivity) and period of follow‐up using stratified analyses or meta‐regression. However, the number of studies with usable data was insufficient to explore this formally.
Usual care was defined by the study and included some measures focused on secondary prevention. We intended to examine the intensity of secondary prevention measures in the comparison group compared to that in the experimental group, but there were insufficient trials included for us to do this.
Sensitivity analysis
We intended to conduct sensitivity analyses excluding studies at high risk of bias and to produce funnel plots and tests of asymmetry to assess possible publication bias (Egger 1997), but the number of included trials was insufficient for us to do this.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The searches yielded a total of 21,459 potentially relevant studies, which we reduced to 14,841 after removing duplications. From these we short‐listed 111 studies. We examined the full papers to these studies, which resulted in including 16 published papers (11 completed trials reported in 12 publications and four trial protocols reported in four published articles).
We searched the reference lists of included studies and relevant review articles, which resulted in no additional studies being included. We also searched trial registers and identified three additional ongoing trials. We have summarised this process in Figure 1.
1.
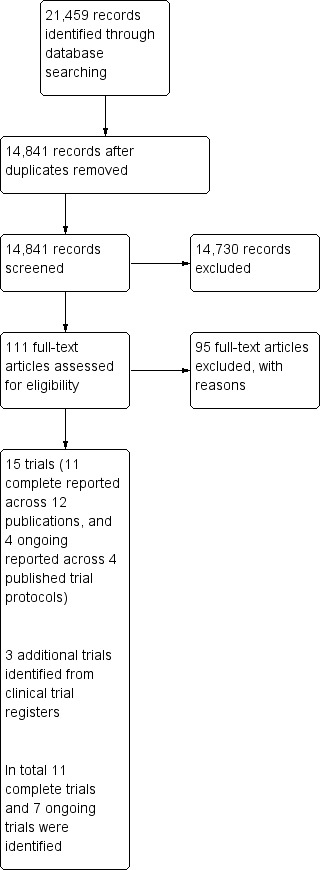
Study flow diagram.
Overall, we included 11 completed trials (12 publications), Antypas 2014, Devi 2014, Frederix 2015, Lear 2014, Lindsay 2009, Maddison 2014, Reid 2012, Southard 2003, Varnfield 2014, Vernooij 2012, and Zutz 2007, and seven ongoing trials, Dale 2014, ISRCTN29243064, NCT02228603, NCT02350192, Redfern 2014, Reinwand 2013, and Shah 2011. We have outlined full study details and risks of bias in completed trials in the Characteristics of included studies table. We have outlined details of the ongoing trials in the Characteristics of ongoing studies table.
Recruitment
Included studies that are complete with data available were conducted in the USA (Southard 2003; Zutz 2007), Canada (Lear 2014; Reid 2012), the UK (Devi 2014; Lindsay 2009), the Netherlands (Vernooij 2012), Belgium (Frederix 2015), Norway (Antypas 2014), New Zealand (Maddison 2014), and Australia (Varnfield 2014). Four trials recruited participants from conventional cardiac rehabilitation programmes (Antypas 2014; Frederix 2015; Varnfield 2014; Zutz 2007). One trial was set in tertiary care and recruited people who had undergone percutaneous coronary revascularisation and were not planning on taking part in cardiac rehabilitation (Reid 2012). One trial recruited cardiac inpatients from both a tertiary and a regional hospital (Lear 2014). One trial recruited participants from two metropolitan hospitals, through outpatient clinics (Maddison 2014). Two trials recruited participants from primary care general practitioner practices (Devi 2014; Lindsay 2009), one trial recruited from primary and community health services (Varnfield 2014), and two trials recruited from both primary and secondary care (Southard 2003; Vernooij 2012).
A total of 1392 participants were recruited, with sample sizes ranging from 15 to 330 participants. Ten studies reported the mean age of study participants, which ranged from 54.9 to 66.27 years, and one trial reported the median age of participants in the control and experimental group as 58.4 years and 61.7 years, respectively (Lear 2014). In six studies, over 80% of participants were male (Frederix 2015; Lear 2014; Maddison 2014; Reid 2012; Varnfield 2014; Zutz 2007), and in five studies over 70% of participants were male (Antypas 2014; Devi 2014; Lindsay 2009; Southard 2003; Vernooij 2012).
Southard 2003 recruited participants diagnosed with CHD, congestive heart failure, or both. The medical characteristics of participants recruited are described, amalgamating those with multiple diagnoses. In the sample 9.6% had a past medical history of congestive heart failure, and therefore the sample consisted of mostly people with CHD. Reid 2012 recruited a mixture of percutaneous coronary intervention (PCI), acute myocardial infarction (AMI), and coronary artery bypass grafting (CABG) patients. Vernooij 2012 sample included a mixture of coronary artery disease, cerebrovascular disease, abdominal aortic aneurysm, and peripheral vascular disease, and Frederix 2015 sample included post‐PCI and post‐CABG patients. Lear 2014 recruited cardiac inpatients admitted for either acute coronary syndrome or revascularisation procedure. Zutz 2007 recruited a mixture of myocardial infarction (MI), PCI, CABG, and diabetes mellitus patients. Devi 2014 recruited a stable angina population, Maddison 2014 recruited both angina and MI patients, and Varnfield 2014 recruited post‐MI patients. Lindsay 2009 and Antypas 2014 did not provide specific details of CHD diagnosis. Three trials described participants' ethnicity: Southard 2003 and Devi 2014 samples consisted of 97.1% and 91% white participants, respectively, and Maddison 2014 recruited predominately European New Zealanders (76%).
Interventions
Of the 11 completed trials, seven interventions were broad, targeting the general management of CHD (Devi 2014; Lear 2014; Lindsay 2009; Southard 2003; Varnfield 2014; Vernooij 2012; Zutz 2007), and four interventions were focused on physical activity promotion (Antypas 2014; Frederix 2015; Maddison 2014; Reid 2012). Seven interventions were delivered using the Internet only (Devi 2014; Lear 2014; Lindsay 2009; Reid 2012; Southard 2003; Vernooij 2012; Zutz 2007), and four interventions were delivered through both the Internet and mobile telephone technology (Antypas 2014; Frederix 2015; Maddison 2014; Varnfield 2014).
The intervention evaluated by Lindsay 2009 was an online heart care support community, where participants interacted with each other in one of five discussion forums moderated by researchers. The intervention also contained information resources about CHD, diet, exercise, and smoking. The web‐based programme evaluated by Southard 2003 was based around educational modules and involved interactive features such as multiple‐choice self test questions, an online discussion group, and a feature that allowed participants to upload health information, for example exercise and blood pressure. The health information provided by the user was then used to produce graphic feedback which showed the user's progress over time. The intervention evaluated by Vernooij 2012 was a personalised website containing an overview of participants' risk factors and self management information about different CHD risk factors. The intervention evaluated by Devi 2014 involved tailored goal‐setting for exercise, diet, anxiety and emotions, and smoking. Depending on the participant’s performance, these goals were modified/made increasingly difficult throughout the programme. Programme users also had to complete an online interactive exercise diary, uploading the daily number of exercise minutes carried out. The intervention evaluated by Varnfield 2014 used a smartphone to monitor health and exercise and to deliver motivational and educational materials via text messages and through audio or video files. The health and exercise daily diary entries were synchronised to a web portal, which mentors could access and review to give feedback during a weekly scheduled telephone consultation. The web‐based programme evaluated by Zutz 2007 comprised weekly education sessions, scheduled one‐on‐one chat sessions with various healthcare professionals, and monthly 'ask an expert' group chat sessions. Participants were also required to upload exercise levels, heart rate, weight, blood pressure, and glucose levels (if diabetic) data to the website. The trial carried out by Lear 2014 evaluated an intervention consisting of weekly education sessions (in the form of interactive slide presentations), a feature to upload participant health data, progress notes (for healthcare professionals), scheduled one‐on‐one chat sessions with a healthcare professional, and monthly 'ask an expert' group chat sessions.
Trials carried out by Antypas 2014, Frederix 2015, Maddison 2014, and Reid 2012 focused on promoting physical activity only. Both Antypas 2014 and Frederix 2015 investigated the effectiveness of an Internet‐ and mobile phone‐based intervention offered to participants after completing traditional cardiac rehabilitation. Antypas 2014 offered participants tailored motivation and support through both a website and text messaging, physical activity goal‐setting, and access to generic information. In Frederix 2015, participants wore a physical activity monitor, and uploaded weekly data via a USB connection to an online participant account, from which they received automated personalised feedback weekly via email or text messages. Similar to both Antypas and Frederix, Maddison 2014 aimed to increase moderate and vigorous aerobic exercise in those with CHD with an intervention comprised of personalised automated text messages, pedometer‐based step counts feedback, personalised feedback on a website, video messages, motivational messages, and weekly health and exercise tips. Reid 2012 aimed to increase levels of physical activity in those not taking part in traditional cardiac rehabilitation; intervention participants uploaded their daily physical activity data onto the intervention website and completed a series of online tutorials, which generated new physical activity plans.
The length of the interventions ranged from six weeks, in Devi 2014, to one year (Antypas 2014; Vernooij 2012). The duration of the other interventions was three months (Zutz 2007), four months (Lear 2014), four and a half months (Frederix 2015), six months (Maddison 2014; Reid 2012; Southard 2003), and nine months (Lindsay 2009). The intervention evaluated by Varnfield 2014 consisted of a six‐week intervention, which was followed by a six‐month self management phase. Participants received training on how to use the intervention in 10 trials (Antypas 2014; Devi 2014; Frederix 2015; Lear 2014; Lindsay 2009; Maddison 2014; Reid 2012; Varnfield 2014; Vernooij 2012; Zutz 2007). Details of how the intervention was introduced to participants was not described by Southard 2003.
In eight interventions, participants were able to initiate communication with a healthcare professional (Antypas 2014; Devi 2014; Lear 2014; Reid 2012; Southard 2003; Varnfield 2014; Vernooij 2012; Zutz 2007). Communication was through email access (Devi 2014; Reid 2012; Zutz 2007), private‐messaging function on the website (Antypas 2014; Southard 2003; Vernooij 2012; Zutz 2007), one‐to‐one chat facility (Lear 2014), a synchronised group chat (Devi 2014; Lear 2014; Zutz 2007), an online discussion forum (Lindsay 2009; Southard 2003), or telephone consultations (Varnfield 2014). The healthcare professionals communicating with participants were exercise specialists (Lear 2014; Reid 2012; Zutz 2007), dietitians (Lear 2014; Southard 2003; Zutz 2007), nurse practitioners (Lear 2014; Vernooij 2012; Zutz 2007), cardiac rehabilitation specialists (Devi 2014), or a physiotherapist (Antypas 2014). Varnfield 2014 did not describe the professional background of those delivering the telephone consultation component of the intervention. Only one intervention included a prompt feature, in which a nurse practitioner could message a participant, or telephone participants who had not recently logged on to the programme (Vernooij 2012).
The intervention user was able to communicate with other intervention users in four studies, either through online discussion forums (Lindsay 2009; Southard 2003), messaging on other users' profile pages (Antypas 2014), via an online synchronised group chat (Devi 2014; Lear 2014), or through email (Southard 2003).
Control groups
In all studies, the web‐based intervention was evaluated in two‐arm trials.
Usual care was the comparison group in six trials. One study did not provide details of usual care (Southard 2003), and in the others it was usual general practitioner care (Devi 2014; Lear 2014; Vernooij 2012), no intervention (Frederix 2015), or wait‐list control (Zutz 2007).
In three trials, control group participants received a minimal intervention consisting of a static, non‐tailored web‐based programme (Antypas 2014), weekly drop‐in sessions (Lindsay 2009), or general physical activity guidance and an educational booklet (Reid 2012). In two trials, the comparison group was traditional cardiac rehabilitation (Maddison 2014; Varnfield 2014).
Use of the intervention by participants
Five trials reported the frequency of website login. Southard 2003 reported that on average participants logged into the website 58 times over the six‐month intervention period, which was equivalent to twice per week. Devi 2014 reported an average of 19 logins per participant over the six‐week intervention, with an average of three logins per week. In Zutz 2007, the average number of logins over the 12‐week programme was 50, which averaged to 4.2 times per week. Vernooij 2012 and Lear 2014 did not report average values, instead reporting median and range values, respectively. In Vernooij 2012, participants logged in a median of 56 (interquartile range 35 to 83) times during the 12‐month intervention, and in Lear 2014, weekly logins ranged from one participant not logging in to the website at all to other participants logging in more than eight times per week.
Excluded studies
We have presented reasons for study exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We judged the risk of bias in the 11 completed trials; Figure 2 and Figure 3 outline summaries of our judgements presented as percentages across all studies, and for each included study, respectively.
2.
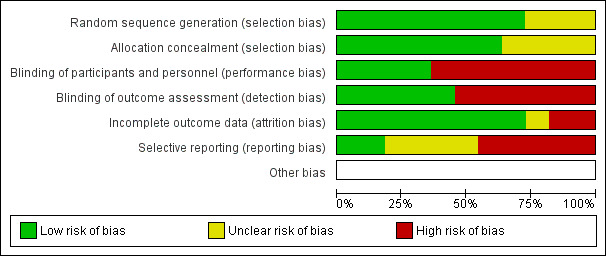
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
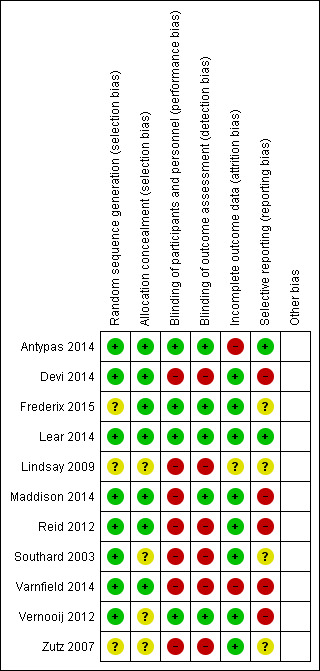
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies randomly allocated participants to study conditions.
In eight studies there was a low risk of bias in the method used to generate randomisation sequence (Antypas 2014; Devi 2014; Lear 2014; Maddison 2014; Reid 2012; Southard 2003; Varnfield 2014; Vernooij 2012), and in three studies there was an unclear risk (Frederix 2015; Lindsay 2009; Zutz 2007). The unclear risk was due to insufficient details provided in reports to judge adequate randomisation.
In seven studies there was a low risk of bias in the methods used to conceal participant allocation (Antypas 2014; Devi 2014; Frederix 2015; Lear 2014; Maddison 2014; Reid 2012; Varnfield 2014). In four studies there was an unclear risk (Lindsay 2009; Southard 2003; Vernooij 2012; Zutz 2007), as they did not describe the measures taken to ensure concealment of group allocation.
Blinding
Participants were not blinded in 10 trials (Devi 2014; Frederix 2015; Lear 2014; Lindsay 2009; Maddison 2014; Reid 2012; Southard 2003; Varnfield 2014; Vernooij 2012; Zutz 2007). We judged this lack of blinding to be at high risk of bias for study outcomes in seven studies (Devi 2014; Lindsay 2009; Maddison 2014; Reid 2012; Southard 2003; Varnfield 2014; Zutz 2007). In contrast, we judged this to be at low risk of causing bias in three studies (Frederix 2015; Lear 2014; Vernooij 2012), as the study outcome assessments were not likely to be influenced by the lack of blinding. One trial compared two Internet intervention conditions, the web‐based intervention that was under trial and the control group, which received a non‐tailored version of the programme, and therefore in this study it was possible to blind participants to study conditions (Antypas 2014).
In terms of blinding outcome assessors, five studies blinded outcome assessors to group allocation (Antypas 2014; Lear 2014; Maddison 2014; Southard 2003; Vernooij 2012), of which one, Southard 2003, reported inadequate blinding. The outcome assessor was not blinded in six trials (Devi 2014; Frederix 2015; Lindsay 2009; Reid 2012; Varnfield 2014; Zutz 2007), of which five were judged to be at high risk of bias as study outcomes may have been influenced (Devi 2014; Lindsay 2009; Reid 2012; Varnfield 2014; Zutz 2007), whereas this may have not been the case in Frederix 2015 due to the nature of physiological outcome measures used.
Incomplete outcome data
We were unable to judge attrition bias in Lindsay 2009 due to a discrepancy in participant drop‐out reported in the published paper. Attempts made to contact the authors were unsuccessful.
In the remaining 10 trials, participant drop‐out varied, ranging from 4%, in Southard 2003, to 72%, in Antypas 2014.
Six trials achieved follow‐up of 80% or more (Frederix 2015; Lear 2014; Maddison 2014; Southard 2003; Vernooij 2012; Zutz 2007), of which four reported reasons for participant drop‐out and were judged as unlikely to be at risk of bias (Frederix 2015; Lear 2014; Maddison 2014; Southard 2003). Two of these trials did not report reasons for participant drop‐out (Vernooij 2012; Zutz 2007), although due to the low level of attrition this was unlikely to have caused bias.
We also judged other trials with attrition rates of 23%, in Devi 2014, and 31%, in Reid 2012, to be at low risk of attrition bias. In Devi 2014, the number of and reasons for dropouts were balanced across groups. In Reid 2012, the number of dropouts was balanced across groups, and missing data was replaced using multiple imputations.
In contrast, we judged both Antypas 2014 and Varnfield 2014 to be at high risk of attrition bias. Antypas 2014 reported a high attrition rate of 72%, and does not describe reasons for missing data, and the drop‐out rate in Varnfield 2014 was 40% and judged to be related to the trial's primary outcome measure (uptake, adherence, and completion rates of the intervention).
Selective reporting
The risk of selective reporting was unclear in four studies where the study protocol was not available (Frederix 2015; Lindsay 2009; Southard 2003; Zutz 2007). We judged two studies to be at low risk (Antypas 2014; Lear 2014), as all prespecified outcomes outlined in the protocol were reported. We judged five studies to be at high risk, as some variables described in trial protocols were not reported (Devi 2014; Maddison 2014; Reid 2012; Varnfield 2014; Vernooij 2012), although four studies did report the primary outcome (Devi 2014; Maddison 2014; Reid 2012; Vernooij 2012), and in one trial the reported primary outcome measure differed from the primary outcome measure described in the study protocol (Varnfield 2014).
Other potential sources of bias
The information provided in the included studies was insufficient to determine other potential sources of bias.
Effects of interventions
Effects of interventions on clinical outcomes
Seven studies reported clinical outcomes (Frederix 2015; Lear 2014; Maddison 2014; Reid 2012; Southard 2003; Vernooij 2012; Zutz 2007). One group reported overall cardiovascular events but did not break this down further (Southard 2003), so we were unable to combine data from this study in the meta‐analyses (contact was made with the authors, however they no longer have access to the data). This study reported that two and eight participants in the intervention group and control group, respectively, experienced a cardiovascular event. The difference between groups was of borderline statistical significance, P = 0.053.
Mortality
Six trials reported this as an outcome, with a total of 895 participants randomised (Analysis 1.1). A total of four deaths were reported across three of these trials, all in the control groups (Lear 2014; Reid 2012; Vernooij 2012). Vernooij 2012 reported one death described as a "fatal cerebrovascular event", Lear 2014 reported one death as a non‐cardiovascular disease death, and Reid 2012 reported two cases of mortality with no reasons provided. The odds ratio (OR) was 0.27 (95% confidence interval (CI) 0.04 to 1.63), P = 0.15 (Analysis 1.1), with low‐quality evidence (Table 1).
1.1. Analysis.
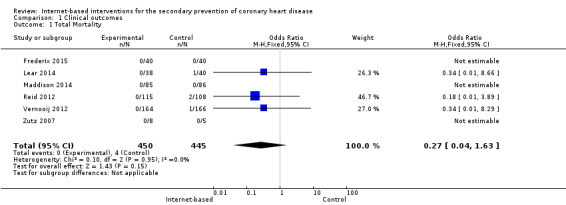
Comparison 1 Clinical outcomes, Outcome 1 Total Mortality.
Only one trial reported a case of cardiovascular‐related mortality; Vernooij 2012 reported one cardiovascular‐related death in the control group.
Non‐fatal re‐infarction
No studies reported any incidences of non‐fatal re‐infarction.
Revascularisation
Six studies contributed to the analysis with 895 participants randomised (Analysis 1.2). In total, 18 revascularisations were reported amongst the intervention groups in three studies (Lear 2014; Maddison 2014; Vernooij 2012), and 26 in the control groups across five studies (Frederix 2015; Lear 2014; Maddison 2014; Reid 2012; Vernooij 2012), with no evidence of an effect of the intervention (OR 0.69 (95% CI 0.37 to 1.27), P = 0.23) (Analysis 1.2, Figure 4), and with low‐quality evidence (Table 1).
1.2. Analysis.

Comparison 1 Clinical outcomes, Outcome 2 Revascularisation.
4.

Forest plot of comparison: 1 Cardio events, outcome: 1.1 Revascularisation.
Effects of interventions on cardiovascular risk factors
Lipid levels
Six trials assessed the impact of web‐based interventions on total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides (Frederix 2015; Lear 2014; Southard 2003; Varnfield 2014; Vernooij 2012; Zutz 2007). We were unable to combine data from Southard 2003 and Lear 2014. Southard 2003 did not report variance values, and we were unable to obtain these from the authors, and Lear 2014 reported data in terms of medians and interquartile ranges. At six months' follow‐up, Southard 2003 reported no statistically significant changes in cholesterol levels, and Lear 2014 reported statistically significant group differences between the control and intervention group for total cholesterol (P = 0.026) and LDL cholesterol (P = 0.022), although not for HDL cholesterol (P = 0.075) and triglycerides (P = 0.715). For total cholesterol, the median values in the control group at baseline, four months, and 16 months were 3.45 mmol, 3.77 mmol, and 3.66 mmol, respectively, and experimental group median values were 3.54 mmol, 3.68 mmol, and 3.60 mmol, respectively, P = 0.026. For LDL cholesterol, the median values in the control group at baseline, four months, and 16 months were 1.79 mmol, 1.99 mmol, and 1.82 mmol, respectively, and experimental group values were 1.74 mmol, 1.79 mmol, and 1.69 mmol, respectively, P = 0.022.
There were four studies where data could be combined, there was moderate heterogeneity for the outcome total cholesterol and a random‐effects model was used (I2 = 41%) showing no effect of the intervention on total cholesterol (mean difference (MD) 0.00 (95% CI ‐0.27 to 0.28) mmol/L, P = 0.98, four studies, 439 participants) (Analysis 2.1, Figure 5) with low‐quality evidence (Table 2). Similarly, there were no intervention effects for HDL cholesterol (MD 0.01 (95% CI ‐0.06 to 0.07) mmol/L, P = 0.82, four studies, 437 participants) (Analysis 3.1, Figure 6) with low‐quality evidence (Table 2). There was considerable heterogeneity for the LDL cholesterol outcome, and results were not pooled statistically (I2 = 77%) (Analysis 4.1, Figure 7), low‐quality evidence (Table 2). Of these studies, one reported a difference in LDL cholesterol between groups at 12 months' follow up (MD ‐0.3 (95% CI ‐0.5 to ‐0.1) (Vernooij 2012) other trials reported no effect on LDL levels (Frederix 2015; Varnfield 2014; Zutz 2007). For triglycerides, there were again no intervention effects (MD 0.01 (95% CI ‐0.17 to 0.19) mmol/L, P = 0.91, four studies, 439 participants) (Analysis 5.1, Figure 8).
2.1. Analysis.

Comparison 2 Total cholesterol, Outcome 1 Total Cholesterol.
5.
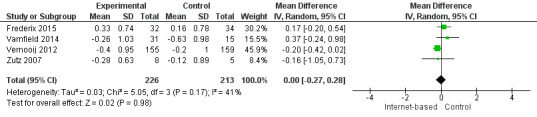
Forest plot of comparison: 2 Total cholesterol, outcome: 2.1 Total Cholesterol.
3.1. Analysis.
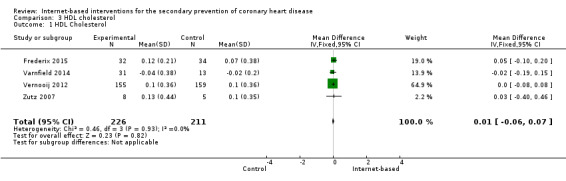
Comparison 3 HDL cholesterol, Outcome 1 HDL Cholesterol.
6.
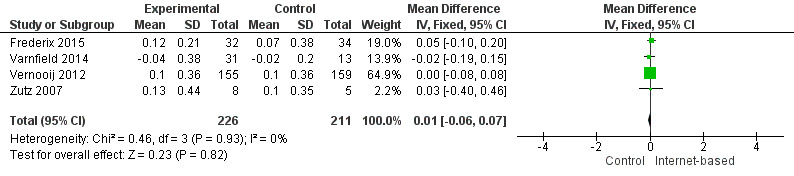
Forest plot of comparison: 3 HDL cholesterol, outcome: 3.1 HDL Cholesterol.
4.1. Analysis.
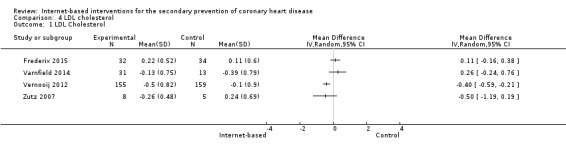
Comparison 4 LDL cholesterol, Outcome 1 LDL Cholesterol.
7.
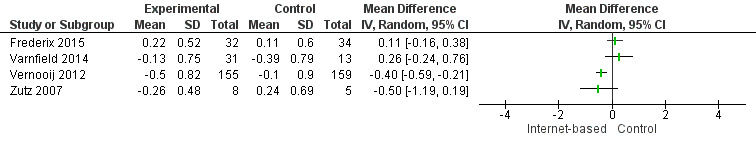
Forest plot of comparison: 4 LDL cholesterol, outcome: 4.1 LDL Cholesterol.
5.1. Analysis.
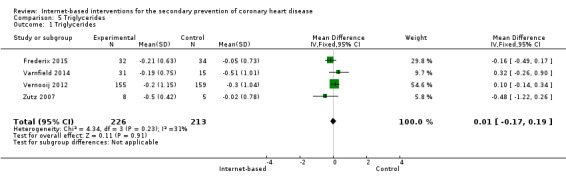
Comparison 5 Triglycerides, Outcome 1 Triglycerides.
8.
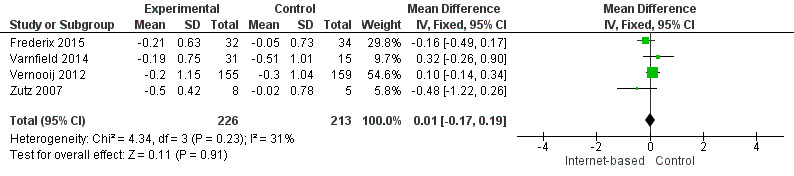
Forest plot of comparison: 5 Triglycerides, outcome: 5.1 Triglycerides.
Blood pressure
In total, seven studies measured systolic (SBP) and diastolic (DBP) blood pressure (Devi 2014; Lear 2014; Maddison 2014; Southard 2003; Varnfield 2014; Vernooij 2012; Zutz 2007). We were unable to combine data from two studies due to variance data not being reported (Southard 2003), and due to data being presented using median values (Lear 2014). Southard 2003 reported no effects for SBP or DBP between groups at a six months' follow‐up (P values not provided), and Lear 2014 reported a between‐group difference over time for SBP (P = 0.051), although not for DBP (P = 0.776). The median SBP values in the control group at baseline, four months', and 16 months' follow‐up were 112 mmHg, 114 mmHg, and 117 mmHg, and the experimental group median values were 121 mmHg, 126 mmHg, and 121 mmHg, respectively.
For the remaining five trials, heterogeneity was substantial (I2 = 63% for SBP, 58% for DBP), and so we did not pool results statistically (Analysis 6.1, Figure 9; Analysis 7.1, Figure 10). In Vernooij 2012, the difference in SBP at 12 months' follow‐up in the intervention group was ‐3 (standard deviation (SD) = 17.52) mmHg, and in the control group the difference was 2 (SD = 18.52) mmHg; this was reported within a 95% CI of ‐7.6 to 0.2. For DBP, the difference at a 12 months' follow‐up in the intervention group and the control group was ‐1 (SD = 9.54) mmHg, and 1 (SD = 10) mmHg, respectively; this was reported with a 95% CI of ‐4.4 to 0.4 (Vernooij 2012). Varnfield 2014 reported six weeks' follow‐up data in the published findings; we contacted the authors for the six months' follow‐up data, however this was not made available. At six weeks' follow‐up, Varnfield 2014 reported an intervention effect for DBP (P = 0.03), while the intervention effect on SBP was not significant (P = 0.4). Maddison 2014 measured DBP and SBP, which is not reported in the published findings; the authors were contacted and the findings provided. At six months' follow‐up, the change in SBP in the intervention group was 4.77 (SD = 13.39) mmHg, and in the control group 0.29 (SD = 13.37) mmHg. The change in DBP at six months' follow‐up in the intervention group was 1.23 (SD = 9.27) mmHg and in the control group ‐1.73 (SD = 10.16) mmHg. Both Devi 2014 and Zutz 2007 reported no significant SBP and DBP effects between the intervention and control groups at six months', and 12 weeks' follow‐up, respectively.
6.1. Analysis.
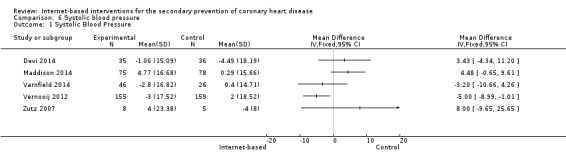
Comparison 6 Systolic blood pressure, Outcome 1 Systolic Blood Pressure.
9.
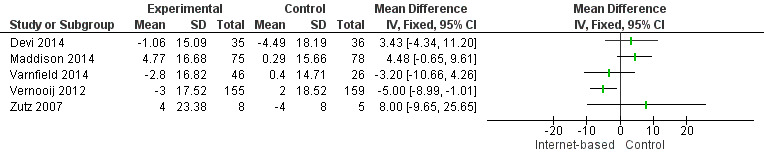
Forest plot of comparison: 6 Systolic blood pressure, outcome: 6.1 Systolic Blood Pressure.
7.1. Analysis.
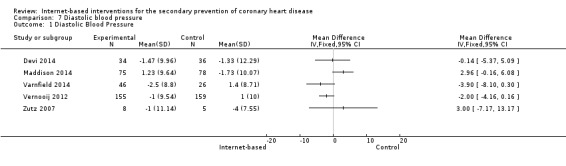
Comparison 7 Diastolic blood pressure, Outcome 1 Diastolic Blood Pressure.
10.
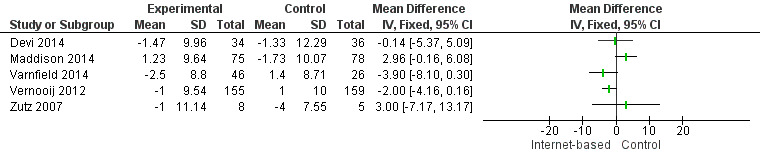
Forest plot of comparison: 7 Diastolic blood pressure, outcome: 7.1 Diastolic Blood Pressure.
Health‐related quality of life
Five studies measured changes in health‐related quality of life (HRQOL) (Devi 2014; Maddison 2014; Reid 2012; Southard 2003; Varnfield 2014). Due to a lack of homogeneity in instruments used across studies, we could not combine these findings in a meta‐analysis. Southard 2003 used the Dartmouth COOP, however only baseline data was described, and no follow‐up findings reported. We contacted the authors, and unfortunately this data is no longer available. Both Devi 2014 and Reid 2012 used the MacNew to measure HRQOL; we were unable to combine this data as Reid 2012 did not report baseline scores. Devi 2014 showed that compared to a control group the experimental group demonstrated statistically significant improvements in emotional HRQOL (P = 0.04, 95% CI 0.01 to 0.54) at six weeks' follow‐up and statistically significant improvements in social HRQOL at six months' follow‐up (P = 0.018, 95% CI 0.05 to 0.54). In addition, Maddison 2014 reported statistically significant intervention effects in the general health domain of the 36‐Item Short Form Health Survey at 24 weeks (mean difference 2.1, 95% CI 0.1 to 4.1; P = 0.03), while there were no statistically significant differences in other HRQOL domains. Varnfield 2014 reported a statistically significant improvement between groups in HRQOL measured using the EQ‐5D index at six weeks' follow‐up (adjusted MD ‐0.08, 95% CI ‐0.14 to ‐0.02, P = 0.01), however this improvement was not maintained at six months' follow‐up. Reid 2012 reported that the differences between groups over time at six and 12 months' follow‐up in HRQOL domains were not statistically significant.
Effects of interventions on lifestyle changes
Diet
Five trials assessed dietary outcomes (Devi 2014; Lear 2014; Lindsay 2009; Southard 2003; Varnfield 2014), and findings were inconclusive. Southard 2003 used MEDFICTS, a measure of fat and cholesterol intake, and reported no statistically significant changes at six months' follow‐up. Devi 2014 measured diet using the DINE (Dietary Instrument for Nutrition Education) and reported no statistically significant dietary effects at both six weeks' and six months' follow‐up. Varnfield 2014 assessed diet using Dietary Habits Questionnaire; this study did not report six months' follow‐up data, and at six weeks reported no statistically significant differences between groups in fat intake (P = 0.4), fibre intake (P = 0.7), sodium (P = 0.4), or alcohol (P = 0.6). Lear 2014 reported no significant group differences over time in carbohydrate (P = 0.224) and fat (P = 0.451) intake, but reported statistically significant intervention effects in protein (P = 0.044) and saturated fat (P = 0.018) intake. Lindsay 2009 also demonstrated a positive finding. This study measured the frequency of unhealthy foods eaten using variables from the Health Survey for England and reported that after six months of using the intervention, the experimental group ate unhealthy foods less often compared to the control group (P = 0.014); this change was not sustained at nine months' follow‐up (P = 0.517).
Physical activity
Eight trials assessed physical activity effects. Six studies used self report measures (Antypas 2014; Lear 2014; Lindsay 2009; Maddison 2014; Southard 2003; Zutz 2007), one used an objective measure (Devi 2014), and one used both an objective and a self report measure (Reid 2012).
Of the studies using self report measures, two used the International Physical Activity Questionnaire (IPAQ) (Antypas 2014; Maddison 2014), two used the Minnesota Leisure Time Physical Activity Questionnaire (Lear 2014; Zutz 2007), and two used unstandardised measures (Lindsay 2009; Southard 2003). In both Southard 2003 and Lindsay 2009, there were no significant physical activity effects.
Even though Maddison 2014 and Antypas 2014 both used the IPAQ, we could not combine the data, as Maddison 2014 reported means and standard deviations, and Antypas 2014 reported median and interquartile range values. Maddison 2014 reported a statistically significant increase in self reported leisure time physical activity (mean difference 110.2 min/week, 95% CI ‐0.8 to 221.3; P = 0.05) and walking (mean difference 151.4 min/week, 95% CI 27.6 to 275.2; P = 0.02) at 24 weeks in favour of the intervention group, which represents increases of 40% and 42%, respectively. Maddison 2014 reported no statistically significant differences for the other activity domains (total activity, active transport, domestic/gardening, and reduced sitting time). Antypas 2014 reported that at three months' follow‐up the intervention group had a significantly higher IPAQ score than the control group, P = 0.02, and higher levels of walking than the control group (P value not reported). There were no significant differences between groups in moderate and vigorous activity or time spent sitting at a one and three months' follow‐up (Antypas 2014).
Although Lear 2014 and Zutz 2007 both used the Minnesota Leisure Time Physical Activity Questionnaire, we were unable to combine their findings as Zutz 2007 reported mean values, and Lear 2014 reported median values. Zutz 2007 reported no statistically significant differences between groups (P value not reported). Similarly, Lear 2014 reported that the group differences over time in leisure time physical activity were not statistically significant (P = 0.191).
Two of the included studies used an objective measure to evaluate physical activity. Devi 2014 used an accelerometer to measure a range of outcomes, and reported statistically significant improvements in daily step count (intervention group n = 35, control group n = 40, P = 0.016, 95% CI 263 to 2451), energy expenditure (intervention group n = 35, control group n = 40, P = 0.01, 95% CI 43.93 to 309.98), duration of sedentary activity (intervention group n = 35, control group n = 40, P = 0.012, 95% CI ‐55.01 to ‐7.01), and duration of moderate activity (intervention group n = 35, control group n = 40, P = 0.014, 95% CI 6.01 to 51.20) at six weeks' follow‐up. There were no statistically significant effects at a six months' follow‐up (Devi 2014). Reid 2012 used both an objective (pedometer) and a self reported measure (a modified version of the Godin Leisure‐Time Exercise Questionnaire); however this study did not collect pedometer data at baseline, and only collected data at the six‐ and 12‐month follow‐ups. This study reported that the difference between groups over time in pedometer‐measured activity (P = 0.656) and self reported moderate and vigorous physical activity levels (P = 0.782) was not significant.
Effects of interventions on compliance with medication
No studies have yet measured the impact of web‐based interventions on compliance with medication.
Effects of interventions on healthcare utilisation and cost
Two studies collected data on healthcare utilisation. One study reported that at six months' follow‐up there were no differences between study groups in healthcare utilisation (P = 0.757), and at nine months' follow‐up the intervention group had statistically significantly higher levels of health visits than the control group (P = 0.044) (Lindsay 2009). The other study reported no statistically significant differences between groups in emergency room visits (P = 0.349) (Lear 2014).
Two studies reported on intervention cost‐effectiveness. Maddison 2014 collected information on the cost of implementing and delivering the intervention and described the intervention as likely to be cost‐effective in increasing metabolic equivalent (MET) hours (walking and leisure activity) per week, and for improving HRQOL. Southard 2003 also reported cost‐effectiveness data, and described the estimated cost of the intervention as USD 453 per participant. Based on the medical cost associated with the cardiovascular events that occurred in both study groups (USD 104,684 and USD 31,110 in the control and intervention group, respectively), there was a gross cost savings of USD 1418 per person, and the net cost savings was USD 965 per person. These figures project an estimated return of 213% on the investment.
Adverse intervention effects
An adverse intervention effect was reported in 1 trial. Lindsay 2009 reported statistically significant higher levels of health visits to a GP, nurse, specialist or other health provider in the intervention group at a 9 month follow up, compared to the control group (P = 0.044) (Lindsay 2009).
Interventions including non‐Internet‐based components versus Internet‐only interventions
The Internet‐based interventions tested in Antypas 2014 and Frederix 2015 were provided to patients after they had completed traditional CR. Antypas 2014 aimed to enhance the maintenance of PA, and Frederix 2015 aimed to improve patients' physical fitness. In relation to the outcomes of interest in this review, Antypas 2014 reported the effects on physical activity, and Frederix 2015 reported the effects on clinical outcomes and cardiovasular risk factors. Due to heterogeneity in reported outcomes between both of these studies we were unable to do any meaningful comparisons of these with Internet‐only interventions at this stage. This will be further examined in an update of this review when more evidence has accrued.
Discussion
With the rising prevalence of heart disease and economic pressures to produce low‐resource‐intensive/cost‐saving solutions, Internet‐delivered interventions have the potential to produce high impact. Internet interventions are not restrained by time or geographical location, and an increasing proportion of retired people over the age of 65 are using the Internet (Dutton 2013), reflecting the typical CHD population.
Summary of main results
We identified 11 completed trials with data available. In terms of study outcomes, seven studies measured clinical outcomes, eight assessed cardiovascular risk factors, five measured HRQOL, five measured impact on diet, and eight assessed physical activity. Six of the eight studies that measured physical activity relied upon self reported measures, which could have been affected by social desirability or poor recall. There was heterogeneity between studies, which prevented statistical pooling for some outcomes. For each analysis there were few studies that contributed, and no overall effects were seen for clinical events, although follow‐up was relatively short. In terms of cardiovascular risk factor outcomes, there were no statistically significant effects for total cholesterol, HDL cholesterol, and triglycerides. It was not possible to pool results from studies measuring LDL cholesterol. Of the four trials measuring LDL cholesterol, one reported favourable intervention effects (Vernooij 2012). Five studies measured HRQOL, with three studies finding evidence for improvements (Devi 2014; Maddison 2014; Varnfield 2014), demonstrating positive effects at six weeks, in Devi 2014 and Varnfield 2014, and at six months, in Devi 2014 and Maddison 2014. In terms of diet, one trial found an effect at six months, which was not maintained at a longer follow‐up (Lindsay 2009), and another trial demonstrated effects in protein and saturated fat intake (Lear 2014). There was some evidence to show that Internet‐based interventions have positive effects on physical activity. Eight studies measured physical activity effects, of which three reported improvements. Maddison 2014 reported improved self reported leisure time physical activity and walking at a six months' follow‐up, Antypas 2014 reported improved IPAQ score and walking at a three months' follow‐up, and Devi 2014 reported improved steps, energy expenditure, duration of sedentary activity, and duration of moderate activity at six weeks' follow‐up. No studies have been conducted yet that measure the effects of web‐based interventions on compliance with medication. Two studies measured healthcare utilisation (Lear 2014; Lindsay 2009). One study reported higher levels of healthcare visits in the intervention group compared to the control group at nine months' follow‐up (Lindsay 2009), the other study reported no differences between groups in emergency room visits (Lear 2014). Two studies measured the cost‐effectiveness of the intervention and reported positive findings in favour of the intervention (Maddison 2014; Southard 2003). There was one adverse intervention effect detected, and this was higher levels of healthcare visits at a 9 month follow up in comparison to a control group (Lindsay 2009).
Overall completeness and applicability of evidence
This is a relatively new area of research, with the first trial published in 2003 (Southard 2003), and then 2007 thereafter (Zutz 2007). A variety of interventions were studied, of which seven were broad, targeting the general management of coronary risk factors, and four focused on promoting physical activity. The length of follow‐up and participant characteristics varied between trials. Three trials had a long‐term follow‐up of 12 months, in Lear 2014, Reid 2012, and Vernooij 2012, six trials had a medium‐term follow‐up of six months, in Devi 2014, Maddison 2014, Reid 2012, Southard 2003, and Varnfield 2014, and nine months, in Lindsay 2009, and three trials had short‐term follow‐ups of three months, in Antypas 2014 and Zutz 2007, and 4.5 months, in Frederix 2015. Participant types varied across studies. Three trials recruited participants with a relatively recent manifestation of heart disease. Maddison 2014 recruited participants who in the last three to 12 months had a diagnosis of ischaemic heart disease; in the study by Reid 2012, over half (64.6%) of the sample had their first cardiac event; and in the study by Varnfield 2014, all participants were post‐myocardial infarction patients. One study recruited a primary care angina population (Devi 2014). Two studies recruited mixed CHD populations (Southard 2003; Vernooij 2012). Frederix 2015 recruited a post‐percutaneous coronary intervention and post‐coronary artery bypass grafting population, and, similarly, Lear 2014 recruited cardiac inpatients for either acute coronary syndrome or revascularisation. Zutz 2007 recruited a mixture of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, and diabetes mellitus patients, Antypas 2014 and Lindsay 2009 provided no specific details of CHD diagnosis.
We found no trials of that combined the Internet intervention with a face‐to‐face intervention component. In 10 out of 11 trials, participants were introduced to the intervention using a face‐to‐face consultation, and one trial did not describe how the intervention was introduced to participants (Southard 2003). We found no trials that recruited participants online, and therefore the feasibility of recruitment through the Internet is not yet known.
Studies were conducted in a wide range of countries, and therefore the structure of health care would have differed, limiting our ability to draw generalisable conclusions. The majority of participants were male, with mean ages across 10 studies ranging from 54.9 to 66.27 years, and studies providing details on ethnicity reported that the majority of participants were white, in Devi 2014 and Southard 2003, or New Zealand European (Maddison 2014). This again limits the extent to which these results can be generalised widely. Some studies did provide details on participant marital status (Lear 2014; Southard 2003), education level (Antypas 2014; Lear 2014; Reid 2012; Southard 2003), income (Lear 2014; Lindsay 2009; Southard 2003), and employment (Devi 2014; Southard 2003). Future studies should collect more participant demographics data to enable us to draw conclusions regarding applicability of evidence to wider populations in future updates of this review.
Due to unrestricted access to the Internet, a challenge remaining with web‐based intervention trials is the difficulty in determining the contribution of a specific web‐based programme. Intervention users may use multiple websites to search for information related to the disease, and therefore participants may well be using the intended intervention in conjunction with other sites. Similarly, the control group may also be using the Internet to search for information related to the disease.
The interventions evaluated varied. Seven interventions were delivered through the Internet only, and in four trials the intervention was delivered using both the Internet and mobile phone technology. With the increased use of smartphones and tablets, it is likely that future web‐based interventions will be used through a combination of different technologies, such as through computers, smartphones, and tablets. It is also possible that future interventions may also incorporate and capitalise on the rise in social networking.
In terms of participant engagement in interventions, five trials reported the frequency of participant logins. For a six‐month, three‐month, and six‐week intervention, the average numbers of weekly logins were 2 (Southard 2003), 4.2 (Zutz 2007), and 3 (Devi 2014), respectively. The study by Vernooij 2012 reported a median of 56 logins during a 12‐month intervention, and the study by Lear 2014 reported a range of 0 to greater than 8 logins per week over a four‐month intervention. It is possible that with longer‐length interventions there is a reduction in participant engagement. More trials assessing user engagement are required to enable firm conclusions.
Quality of the evidence
All of the studies included in this review were randomised controlled trials, and we assessed the quality of evidence using the GRADE approach for evidence synthesis. The evidence for the outcomes analysed was generally of low quality as a result of lack of blinding, uncertainty around the magnitude of effect, and loss to follow‐up (Table 1; Table 2). The majority of studies provided details about the generation of random sequence and the appropriate concealment of allocation. We were unable to judge the quality of the randomisation method used in three studies (Frederix 2015; Lindsay 2009; Zutz 2007), as these details were not provided. In addition, four trials did not describe the method used to conceal treatment allocation (Lindsay 2009; Southard 2003; Vernooij 2012; Zutz 2007). Ten studies did not blind participants to study groups; we judged this to be likely to cause bias in seven studies (Devi 2014; Lindsay 2009; Maddison 2014; Reid 2012; Southard 2003; Varnfield 2014; Zutz 2007). The outcome assessor was blinded in five trials (Antypas 2014; Lear 2014; Maddison 2014; Southard 2003; Vernooij 2012), of which one was judged to be at high risk of detection bias due to inadequate blinding (Southard 2003). In the six trials where the outcome assessor was not blinded, we judged five to be at high risk of bias as study outcomes may have been influenced (Devi 2014; Lindsay 2009; Reid 2012; Varnfield 2014; Zutz 2007). We also judged the likelihood of attrition bias. We judged two studies to be at high risk of attrition bias due to large attrition rates with no reasons for missing data provided (Antypas 2014), and because attrition was likely to be related to the trial's primary outcome measure (Varnfield 2014). In contrast, we judged eight studies judged to be at low risk of attrition bias (Devi 2014; Frederix 2015; Lear 2014; Maddison 2014; Reid 2012; Southard 2003; Vernooij 2012; Zutz 2007). We were unable to assess attrition bias in one trial due to a discrepancy detected in the published findings (Lindsay 2009). In terms of reporting bias, we judged five studies to be at high risk, as not all the measures outlined in the study protocol had been reported (Devi 2014; Maddison 2014; Reid 2012; Varnfield 2014; Vernooij 2012), although Devi 2014, Maddison 2014, Reid 2012, and Vernooij 2012 did report their primary outcome measures. Antypas 2014 and Lear 2014 were at low risk of reporting bias as all prespecified outcomes outlined in the protocol were described in the trial write‐up. It was not possible to judge risk in Frederix 2015, Lindsay 2009, Southard 2003, and Zutz 2007, as trial protocols were not available.
Potential biases in the review process
The searching for this review was extensive involving a number of different databases, and all review processes were conducted in duplicate to minimise bias. Although we looked for unpublished data, we were unable to find any unpublished randomised controlled trials that fulfilled our inclusion criteria, therefore the review contains published data only. Interventions targeting the secondary prevention of heart disease are often multi‐componential and complex, and due to the nature of cardiac risk factors involve changing lifestyle. When participants make lifestyle changes, various cognitions and psychological aspects are involved, and the complex nature of this means there are a large number of primary and secondary outcomes within trials that are of interest. However, we have only reported on prespecified outcomes as described in the protocol.
The comparison groups differed across trials, consisting of usual care (n = 6), minimal intervention (n = 3), or traditional cardiac rehabilitation (n = 2). We intended where possible to examine the intensity of secondary prevention measures in the comparison group compared to that in the experimental group. However, the number of studies with usable data for meta‐analyses was insufficient to explore this formally in subgroup analyses. The review authors intend to formally study the intensity of the comparison group in subgroup analysis in an update of this review when more evidence has accrued.
The protocol for this review was constructed at a time when smartphone technology was not as widely used as it is today, and therefore the review authors' primary focus was on interventions delivered using Internet websites. In our search we found that more recently conducted trials delivered interventions that combined smartphone and Internet site technology. This shows that these interventions are evolving, and in future updates of this review we intend to distinguish between the level of smartphone and Internet site contributions in the design of interventions.
The conclusions we can draw from this review are currently limited by the small number of included studies and the heterogeneity between studies in terms of the intervention and participant characteristics and length of follow‐up. More trials with longer follow‐ups are required to be able to determine the effects of the interventions on clinical events and whether effects on intermediate outcomes are sustained following the end of the intervention period.
Agreements and disagreements with other studies or reviews
A previous review conducted by Munro 2013 examined the impact of patient Internet‐based approaches to cardiac rehabilitation. This review included nine randomised controlled trials and cohort studies and reported positive findings for clinical outcomes and physical activity with the intervention. Due to the heterogeneity between studies, the authors stated that their results should be interpreted with caution. Widmer 2015 assessed the benefit of digital health interventions on cardiovascular disease outcomes and reported no significant improvements in weight, systolic blood pressure, triglyceride levels, total cholesterol, or low‐density lipoprotein cholesterol in secondary prevention populations. This current review reports similar findings to Widmer 2015.
Authors' conclusions
Implications for practice.
Due to the low‐quality evidence in study outcomes and limited findings to date, there are no implications for practice at present.
Implications for research.
The current evidence on the use of the Internet in the secondary prevention of heart disease is still evolving and has shown mixed results. A number of questions have been raised. We particularly need to investigate the long‐term effects of web‐based interventions used in the secondary prevention of CHD on cardiovascular risk factor profiles and clinical events. There is also a need to determine the intensity and duration of the intervention required to achieve effective secondary prevention of CHD and the effective components of behavioural changes.
With regards to the socio‐demographic characteristics of the CHD population, future studies should focus interventions on a wide range of participants so that findings are generalisable and can also be tailored to specific populations if differences are found.
More rigorous studies comparing the long‐term effects of Internet interventions are needed in order to determine long‐term effectiveness of Internet interventions for the secondary prevention of CHD. There is also a need to measure outcomes objectively. Physical activity was assessed using self report measures in six out of eight trials. These measures are susceptible to overestimations, which the use of accelerometer technology would reduce. Future trials should also include cost‐effectiveness outcomes.
Acknowledgements
The search strategy was developed in collaboration with Nicole Martin (Cochrane Heart Group). We would like to thank and acknowledge the following authors who provided clarification and data when asked: Antypas 2014; Frederix 2015; Lear 2014Maddison 2014; Reid 2012; Varnfield 2014; Vernooij 2012.
Appendices
Appendix 1. Search strategy
CENTRAL
#1MeSH descriptor: [Myocardial Ischemia] explode all trees #2MeSH descriptor: [Coronary Artery Bypass] explode all trees #3coronary near/2 disease* #4isch?emi* next heart #5myocard* next isch?emi* #6myocard* next infarct* #7heart next infarct* #8coronary next thrombo* #9coronary near/3 angioplast* #10angina* #11coronary next bypass* #12CABG #13PTCA #14MeSH descriptor: [Angioplasty, Balloon, Coronary] this term only #15coronary next arter?oscleros #16#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17MeSH descriptor: [Computer Systems] explode all trees #18computer* #19microcomputer* #20laptop* #21ipad* #22pc #23internet* #24local next area next network* #25lan #26world next wide next web #27www:ti,ab #28web*:ti,ab #29worldwide next web #30website*:ti,ab #31MeSH descriptor: [Medical Informatics] this term only #32health next information next technolog* #33medical next information next science* #34(medical or clinical or health) near/2 informatics #35MeSH descriptor: [Telemedicine] this term only #36telemedicine #37tele next medicine #38tele next health* #39telehealth* #40MeSH descriptor: [Educational Technology] this term only #41education* near/4 technolog* #42MeSH descriptor: [Software] this term only #43software* #44MeSH descriptor: [Software Design] this term only #45MeSH descriptor: [Telecommunications] this term only #46MeSH descriptor: [Computer‐Assisted Instruction] this term only #47MeSH descriptor: [Public Health Informatics] this term only #48MeSH descriptor: [User‐Computer Interface] this term only #49MeSH descriptor: [Telephone] explode all trees #50phone* #51telephone*:ti,ab #52MeSH descriptor: [Wireless Technology] this term only #53wireless #54MeSH descriptor: [Electronic Mail] this term only #55electronic next mail* #56e‐mail*:ti,ab #57email*:ti,ab #58e‐health #59electronic next health #60ehealth #61online:ti,ab #62on‐line #63chat next room* #64chatroom* #65blog* #66web next log* #67weblog* #68bulletin next board* #69bulletinboard* #70messageboard* #71message next board* #72interactive near/5 (health or medic*) #73MeSH descriptor: [Consumer Health Information] this term only #74twitter #75tweet* #76facebook #77yahoo:ti,ab #78skype #79youtube #80itunes #81mp3* #82podcast* #83iphone* #84(app or application) near/10 (internet or online or web*) #85#17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 #86#27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 #87#37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 #88#47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 #89#57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 #90#67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 #91#77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 #92#85 or #86 or #87 or #88 or #89 or #90 or #91 #93#16 and #92
MEDLINE (OVID)
1. exp Myocardial Ischemia/ 2. exp Coronary Artery Bypass/ 3. (coronary adj2 disease*).tw. 4. isch?emi* heart.tw. 5. myocard* isch?emi*.tw. 6. myocard* infarct*.tw. 7. heart infarct*.tw. 8. coronary thrombo*.tw. 9. (coronary adj3 angioplast*).tw. 10. angina*.tw. 11. coronary bypass*.tw. 12. CABG.tw. 13. PTCA.tw. 14. Angioplasty, Balloon, Coronary/ 15. coronary arter?oscleros*.tw. 16. or/1‐15 17. exp Computer Systems/ 18. computer*.tw. 19. microcomputer*.tw. 20. laptop*.tw. 21. ipad*.tw. 22. pc.tw. 23. internet*.tw. 24. local area network*.tw. 25. lan.tw. 26. world wide web.tw. 27. www.tw. 28. web*.tw. 29. worldwide web.tw. 30. website*.tw. 31. Medical Informatics/ 32. health information technolog*.tw. 33. medical information science*.tw. 34. ((medical or clinical or health) adj2 informatics).tw. 35. Telemedicine/ 36. telemedicine.tw. 37. tele medicine.tw. 38. tele health*.tw. 39. telehealth*.tw. 40. Educational Technology/ 41. (education* adj4 technolog*).tw. 42. Software/ 43. software*.tw. 44. Software Design/ 45. Telecommunications/ 46. Computer‐Assisted Instruction/ 47. Public Health Informatics/ 48. user‐computer interface/ 49. exp Telephone/ 50. phone*.tw. 51. telephone*.tw. 52. Wireless Technology/ 53. wireless.tw. 54. Electronic Mail/ 55. electronic mail*.tw. 56. e‐mail*.tw. 57. email*.tw. 58. e‐health.tw. 59. electronic health.tw. 60. ehealth.tw. 61. online.tw. 62. on‐line.tw. 63. chat room*.tw. 64. chatroom*.tw. 65. blog*.tw. 66. web log*.tw. 67. weblog*.tw. 68. bulletin board*.tw. 69. bulletinboard*.tw. 70. messageboard*.tw. 71. message board*.tw. 72. (interactive adj5 (health or medic*)).tw. 73. Consumer Health Information/ 74. twitter.tw. 75. tweet*.tw. 76. facebook.tw. 77. yahoo.tw. 78. skype.tw. 79. youtube.tw. 80. itunes.tw. 81. mp3*.tw. 82. podcast*.tw. 83. iphone*.tw. 84. ((app or application) adj10 (internet or online or web*)).tw. 85. or/17‐84 86. 16 and 85 87. randomized controlled trial.pt. 88. controlled clinical trial.pt. 89. randomized.ab. 90. placebo.ab. 91. clinical trials as topic.sh. 92. randomly.ab. 93. trial.ti. 94. 87 or 88 or 89 or 90 or 91 or 92 or 93 95. exp animals/ not humans.sh. 96. 94 not 95 97. 86 and 96
EMBASE OVID
1. heart muscle ischemia/ 2. coronary artery bypass graft/ 3. (coronary adj2 disease*).tw. 4. isch?emi* heart.tw. 5. myocard* isch?emi*.tw. 6. myocard* infarct*.tw. 7. heart infarct*.tw. 8. coronary thrombo*.tw. 9. (coronary adj3 angioplast*).tw. 10. angina*.tw. 11. coronary bypass*.tw. 12. CABG.tw. 13. PTCA.tw. 14. transluminal coronary angioplasty/ 15. coronary arter?oscleros*.tw. 16. or/1‐15 17. exp mass communication/ 18. exp computer/ 19. computer interface/ 20. computer*.tw. 21. microcomputer*.tw. 22. laptop*.tw. 23. ipad*.tw. 24. pc.tw. 25. internet*.tw. 26. local area network*.tw. 27. lan.tw. 28. world wide web.tw. 29. www.tw. 30. web*.tw. 31. worldwide web.tw. 32. website*.tw. 33. medical informatics/ 34. health information technolog*.tw. 35. medical information science*.tw. 36. ((medical or clinical or health) adj2 informatics).tw. 37. educational technology/ 38. (education* adj4 technolog*).tw. 39. exp computer program/ 40. software*.tw. 41. patient education/ 42. phone*.tw. 43. telephone*.tw. 44. wireless.tw. 45. electronic mail*.tw. 46. e‐mail*.tw. 47. email*.tw. 48. exp telehealth/ 49. e‐health.tw. 50. electronic health.tw. 51. ehealth.tw. 52. online system/ 53. online.tw. 54. on‐line.tw. 55. chat room*.tw. 56. chatroom*.tw. 57. blog*.tw. 58. web log*.tw. 59. weblog*.tw. 60. electronic bulletin board/ 61. bulletin board*.tw. 62. bulletinboard*.tw. 63. messageboard*.tw. 64. message board*.tw. 65. (interactive adj5 (health or medic*)).tw. 66. consumer health information/ 67. twitter.tw. 68. tweet*.tw. 69. facebook.tw. 70. yahoo.tw. 71. skype.tw. 72. youtube.tw. 73. itunes.tw. 74. mp3*.tw. 75. podcast*.tw. 76. iphone*.tw. 77. ((app or application) adj10 (internet or online or web*)).tw. 78. or/17‐77 79. 16 and 78 80. random$.tw. 81. factorial$.tw. 82. crossover$.tw. 83. cross over$.tw. 84. cross‐over$.tw. 85. placebo$.tw. 86. (doubl$ adj blind$).tw. 87. (singl$ adj blind$).tw. 88. assign$.tw. 89. allocat$.tw. 90. volunteer$.tw. 91. crossover procedure/ 92. double blind procedure/ 93. randomized controlled trial/ 94. single blind procedure/ 95. 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 96. (animal/ or nonhuman/) not human/ 97. 95 not 96 98. 79 and 97
PsycINFO
1. ischemia/ 2. heart surgery/ 3. exp heart disorders/ 4. (coronary adj2 disease*).tw. 5. isch?emi* heart.tw. 6. myocard* isch?emi*.tw. 7. myocard* infarct*.tw. 8. heart infarct*.tw. 9. coronary thrombo*.tw. 10. (coronary adj3 angioplast*).tw. 11. angina*.tw. 12. coronary bypass*.tw. 13. CABG.tw. 14. PTCA.tw. 15. coronary arter?oscleros*.tw. 16. or/1‐15 17. exp computers/ 18. computer*.tw. 19. microcomputer*.tw. 20. laptop*.tw. 21. ipad*.tw. 22. pc.tw. 23. internet/ 24. internet*.tw. 25. local area network*.tw. 26. lan.tw. 27. world wide web.tw. 28. www.tw. 29. web*.tw. 30. worldwide web.tw. 31. website*.tw. 32. websites/ 33. information technology/ 34. health knowledge/ 35. health information technolog*.tw. 36. medical information science*.tw. 37. ((medical or clinical or health) adj2 informatics).tw. 38. telemedicine/ 39. telemedicine.tw. 40. tele medicine.tw. 41. tele health*.tw. 42. telehealth*.tw. 43. technology/ 44. (education* adj4 technolog*).tw. 45. computer software/ 46. exp computer applications/ 47. software*.tw. 48. exp communications media/ 49. computer assisted instruction/ 50. exp human computer interaction/ 51. phone*.tw. 52. telephone*.tw. 53. wireless.tw. 54. exp Electronic Communication/ 55. electronic mail*.tw. 56. e‐mail*.tw. 57. email*.tw. 58. e‐health.tw. 59. electronic health.tw. 60. ehealth.tw. 61. online social networks/ 62. online.tw. 63. on‐line.tw. 64. chat room*.tw. 65. chatroom*.tw. 66. blog*.tw. 67. web log*.tw. 68. weblog*.tw. 69. bulletin board*.tw. 70. bulletinboard*.tw. 71. messageboard*.tw. 72. message board*.tw. 73. (interactive adj5 (health or medic*)).tw. 74. health promotion/ 75. health education/ 76. twitter.tw. 77. tweet*.tw. 78. facebook.tw. 79. yahoo.tw. 80. skype.tw. 81. youtube.tw. 82. itunes.tw. 83. mp3*.tw. 84. podcast*.tw. 85. iphone*.tw. 86. ((app or application) adj10 (internet or online or web*)).tw. 87. exp instructional media/ 88. or/17‐87 89. 16 and 88
CINAHL
S42 S24 and S41 S41 S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 S40 (TI crossover* OR cross‐over*) OR (AB crossover* OR cross‐over*) S39 (TI volunteer*) OR (AB volunteer*) S38 (MH "Crossover Design") S37 (TI allocat*) OR (AB allocat*) S36 (TI control*) OR (AB control*) S35 (TI assign*) OR (AB assign*) S34 (TI placebo*) OR (AB placebo*) S33 (MH "Placebos") S32 (TI random*) OR (AB random*) S31 (TI doubl* N1 mask*) OR (AB doubl* N1 mask*) S30 (TI singl* N1 mask*) OR (AB singl* N1 mask*) S29 (TI doubl* N1 blind*) OR (AB doubl* N1 blind*) S28 (TI singl* blind*) OR (AB singl* blind*) S27 PT clinical trial S26 (MH "Clinical Trials+") S25 (TI clinic* trial*) OR (AB clinic* trial*) S24 S11 and S23 S23 S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 S22 (TI interactive N3 health OR interactive N3 medic*) OR (AB interactive N3 health OR interactive N3 medic*) S21 (TI bulletin board* OR bulletinboard* OR messageboard* or message board*) OR (AB bulletin board* OR bulletinboard* OR messageboard* or message board*) S20 (TI online OR on‐line Or on line OR chat room* OR chatroom* OR blog* OR web log* OR weblog*) OR (AB online OR on‐line Or on line OR chat room* OR chatroom* OR blog* OR web log* OR weblog*) S19 (TI electronic health* OR ehealth OR e‐health OR e health) OR (AB electronic health* OR ehealth OR e‐health OR e health) S18 (TI electronic mail* OR email* OR e‐mail* OR e mail*) OR (AB electronic mail* OR email* OR e‐mail* OR e mail*) S17 (TI software* OR phone* OR telephone* OR wireless) OR (AB software* OR phone* OR telephone* OR wireless) OR (MH "Computers and Computerization+") S16 (TI education* N2 technolog*) OR (AB education* N2 technolog*) OR (MH "Educational Technology") S15 (TI telemedicine OR telehealth*) OR (AB telemedicine OR telehealth*) S14 (TI health* N5 inform* OR medic* N5 inform OR clinical N5 inform*) OR (AB health* N5 inform* OR medic* N5 inform OR clinical N5 inform*) OR (MH "Health Informatics+") S13 (TI internet* OR network* OR www OR web* OR website*) OR (AB internet* OR network* OR www OR web* OR website*) OR (MH "Telecommunications+") S12 (TI computer* or microcomputer* or laptop* or ipad* or pc) OR (AB computer* or microcomputer* or laptop* or ipad* or pc) OR (MH "Communications Software+") OR (MH "Computer Assisted Instruction") OR (MH "User‐Computer Interface") S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 S10 (TI coronary arter#oscleros*) OR (AB coronary arter#oscleros*) S9 (TI coronary bypass* OR CABG OR PTCA) OR (AB coronary bypass* OR CABG OR PTCA) OR (MH "Coronary Artery Bypass") S8 (TI angina*) OR (AB angina*) S7 (TI coronary N3 angioplast*) OR (AB coronary N3 angioplast*) OR (MH "Angioplasty, Transluminal, Percutaneous Coronary") S6 (TI coronary thrombo*) OR (AB coronary thrombo*) S5 (TI heart infarct*) OR (AB heart infarct*) S4 (TI myocard* infarct*) OR (AB myocard* infarct*) S3 (TI myocard* isch#emi*) OR (AB myocard* isch#emi*) OR (MH "Myocardial Ischemia+") S2 (TI isch#emi* heart) OR (AB isch#emic* heart) S1 (TI coronary N3 disease*) OR (AB coronary N3 disease*)
Web of Science – SCI‐EXPANDED, SSCI, CPCI‐S
RCT filter terms adapted from Cochrane RCT filter used for MEDLINE/EMBASE strategy. #29 #28 AND #27 #28 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) #27 #26 AND #12 #26 #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 #25 TS=(interactive SAME (health or medic*)) #24 TS=("bulletin board*" OR bulletinboard* OR messageboard* or "message board*") #23 TS=(online OR on‐line Or "on line" OR "chat room*" OR chatroom* OR blog* OR "web log*" OR weblog*) #22 TS=("electronic health*" OR ehealth OR e‐health OR "e health") #21 TS=("electronic mail*" OR email* OR e‐mail* OR "e mail*") #20 TS=wireless #19 TS=(phone* OR telephone*) #18 TS=software* #17 TS=(education* SAME technolog*) #16 TS=(telemedicine OR telehealth*) #15 TS=((health* OR medic* OR clinical) SAME inform*) #14 TS=(internet* OR network* OR www OR web* OR website*) #13 TS=(computer* or microcomputer* or laptop* or ipad* or pc) #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #11 TS=("coronary arteroscleros*" OR "coronary arterioscleros*") #10 TS=(CABG OR PTCA) #9 TS="coronary bypass*" #8 TS=angina* #7 TS=(coronary SAME angioplast*) #6 TS="coronary thrombo*" #5 TS="heart infarct*" #4 TS="myocard* infarct*" #3 TS=("myocard* ischemi*" OR "myocard* ischaemi*") #2 TS=("ischemi* heart" OR "ischaemi* heart") #1 TS=(coronary SAME disease*)
Data and analyses
Comparison 1. Clinical outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Mortality | 6 | 895 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 1.63] |
| 2 Revascularisation | 6 | 895 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.27] |
Comparison 2. Total cholesterol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Cholesterol | 4 | 439 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.27, 0.28] |
Comparison 3. HDL cholesterol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HDL Cholesterol | 4 | 437 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.07] |
Comparison 4. LDL cholesterol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL Cholesterol | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Triglycerides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Triglycerides | 4 | 439 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.19] |
Comparison 6. Systolic blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic Blood Pressure | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Diastolic blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diastolic Blood Pressure | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antypas 2014.
| Methods |
Study design: Randomised controlled trial. Control group: Access to a static, non‐tailored version of the web‐based intervention. |
|
| Participants |
Study location: Norway. CHD diagnosis/treatment: Participants with a history of cardiovascular disease taking part in cardiac rehabilitation. Mean age: Intervention group: 59.5 Control group: 58.8 Percentage men: Intervention group: 76% (n = 22) Control group: 79% (n = 30) All participants: 75% (n = 52) Number of participants recruited: 69 Participant ethnicity: Not reported. Recruited online or offline? Offline, conventional cardiac rehabilitation. |
|
| Interventions |
Name of the intervention: No name. Intervention aim: Enhance the maintenance of physical activity after cardiac rehabilitation. Intervention features: Participants were reminded through email and SMS text messages to complete intervention tasks and to log in to the programme. Participants also received tailored messages through both the website and SMS text messages. The programme encouraged participants to plan physical activities, and set themselves goals. The programme also contained an activity calendar for participants to log physical activity levels. How was the intervention introduced to the sample? A physiotherapist presents the intervention to all the participants and provides training on how to use the website. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Participants could message a physiotherapist through the website. Duration of the intervention? 1 year. |
|
| Outcomes |
Outcomes: 1. Physical activity. Measurement tool: International Physical Activity Questionnaire. Time points: 1 and 3 months' follow‐up. |
|
| Notes | Trial was registered with ClinicalTrials.gov. Registration number: NCT01223170 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online random number generator service was used |
| Allocation concealment (selection bias) | Low risk | Concealed using an online service |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There were 2 web‐based intervention conditions: 1 received the tailored version of the website, and the control group received a static, non‐tailored version of the programme |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large attrition rate (72%), with no reasons provided for missing data |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes outlined in the protocol have been reported |
Devi 2014.
| Methods |
Study design: Randomised controlled trial. Control group: Usual care. |
|
| Participants |
Study location: UK CHD diagnosis/treatment: Primary care angina patients. Mean age (SD): Intervention group: 66.27 (8.35) Control group: 66.20 (10.06) Percentage men: Intervention group: 71% (n = 34) Control group: 78% (n = 36) All participants: 78% (n = 74) Number of participants recruited: 94 Participant ethnicity: White British 91%, other white background 5%, other 4%. Recruited online or offline? Participants recruited offline, from primary care; GP practices. |
|
| Interventions |
Name of the intervention: ActivateYourHeart Intervention aim: To improve health behaviours related to CHD. Intervention features: The programme contained 4 stages; at each stage the user was set individualised goals focused on exercise, diet, emotions, and smoking. Compliance with these goals was checked at the end of each stage, and then goals were reset/modified accordingly. Participants uploaded data related to physical activity, emotions/mood, and smoking. Regular feedback on these behaviours was provided. The website also contained tailored information about the secondary prevention of CHD. How was the intervention introduced to the sample? The researcher provided face‐to‐face training on how to use the intervention, which involved registering the individual (creating a unique username and password), and demonstrating how to use the programme. Was there any contact between the researcher/healthcare professional and the sample during the intervention? A cardiac nurse was available for advice/support throughout the programme through either an online email link or by joining a scheduled synchronised chat room held on a weekly basis. Duration of the intervention? 6 weeks. |
|
| Outcomes |
Outcomes: 1. Physical activity. Measurement tool: an accelerometer, SenseWear Pro 3 armband 2. Blood pressure 3. Fat and fibre intake. Measurement tool: Dietary Instrument for Nutrition Education (DINE) 4. HRQOL. Measurement tool: MacNew Time points: 6 weeks and 6 months. |
|
| Notes | Trial was registered with the ISRCTN registry. Registration number: ISRCTN90110503 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and some of the outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and some of the outcomes may have been influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 23%, with number of dropouts balanced across groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Not all the measures reported in the protocol have been reported: cost and level of positivity |
Frederix 2015.
| Methods |
Study design: Randomised controlled trial. Control group: Participants wore the motion sensors, which were modified to hide information from the participant; these participants did not upload any physical activity information and did not receive feedback. |
|
| Participants |
Study location: Belgium. CHD diagnosis/treatment: Post‐PCI or ‐CABG patients. Mean age (SD): Intervention group: 58 (9) Control group: 63 (10) Percentage men: Intervention group: 81% (n = 32) Control group: 85% (n = 34) All participants: 82.5% (n = 66) Number of participants recruited: 80 Participant ethnicity: Not reported. Recruited online or offline? Offline, recruited after week 6 of their conventional cardiac rehabilitation programme. |
|
| Interventions |
Name of the intervention: No name is provided. Intervention aim: To continue to further improve the participant's physical fitness, quality of life, and cardiovascular risk factors after the completion of a traditional cardiac rehabilitation programme with telemonitoring support. Intervention features: Participants wore a motion sensor all day for 18 weeks that registered activity data during all the exercise sessions. Participants carried out a weekly upload of their physical activity data via USB‐connection to an online participant account and then received weekly personalised feedback on their physical activity by email or SMS. How was the intervention introduced to the sample? Face‐to‐face training session provided. Was there any contact between the researcher/healthcare professional and the sample during the intervention? No. Duration of the intervention? 18 weeks. |
|
| Outcomes |
Outcomes: 1. Rehospitalisation rates 2. Total cholesterol 3. HDL cholesterol 4. LDL cholesterol 5. Triglycerides Time points: 6‐ and 18‐week follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, however the study outcomes are not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, however the study outcomes are not likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 17.5%, with reasons for drop‐out provided, which were similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
Lear 2014.
| Methods |
Study design: Randomised controlled trial. Control group: Care from a primary care physician, simple guidelines for safe exercising and healthy eating, and a list of Internet resources. |
|
| Participants |
Study location: Canada. CHD diagnosis/treatment: Cardiac inpatients admitted for either acute coronary syndrome or revascularisation procedure. Median age (interquartile ranges): Intervention group: 61.7 (51.3, 65.2) Control group: 58.4 (52.8, 64.7) Perentage men: Intervention group: 90% (n = 34) Control group: 80% (n = 32) All participants: 85% (n = 66) Number of participants recruited: 78 Participant ethnicity: Not reported. Recruited online or offline? Offline, the study recruited cardiac inpatients from a tertiary and regional hospital in Canada. |
|
| Interventions |
Name of the intervention: No name is provided. Intervention aim: To reduce risk factors, CVD events, and premature mortality. Intervention features: The programme involved scheduled one‐on‐one chat sessions (with either a nurse, exercise specialist, or dietitian), weekly education sessions via interactive slide presentations, data recording (exercise stress test, blood test, progress notes (for health professionals)), and monthly ask‐an‐expert group chat sessions. How was the intervention introduced to the sample? Face‐to‐face training session provided. Was there any contact between the researcher/healthcare professional and the sample during the intervention? The participant could communicate with a nurse, exercise specialist, or dietitian through a one‐on‐one chat facility, and there was also a monthly ask‐an‐expert group chat facility. Duration of the intervention? 4 months. |
|
| Outcomes |
Outcomes: 1. Major cardiovascular events (revascularisation, unstable angina requiring hospitalisation, stroke, and death of any kind). 2. Total cholesterol 3. HDL cholesterol 4. LDL cholesterol 5. Triglycerides 6. Blood pressure. 7. Physical activity. Measurement tool: the 4‐week modified Minnesota Leisure Time Physical Activity Questionnaire. 8. Diet. Measurement tool: a 3‐day food record analysed by a dietitian, and reported as percent daily kilocalories consumed of fat, protein, and carbohydrates. 9. Healthcare utilisation. Measurement tool: emergency room visits. Time points: 4 months (postintervention) and 12 months' follow‐up. |
|
| Notes | Trial was registered with ClinicalTrials.gov. Registration number: NCT00683813 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by site using variable block sizes, and computer generated by a statistician |
| Allocation concealment (selection bias) | Low risk | Only the statistician had access to the randomisation list, and treatment allocation was revealed to the researcher via telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, however the study outcomes are not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was small (9%), and reasons for drop‐out reported. Attrition was balanced between groups and unlikely to introduce bias |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes outlined in the protocol have been reported |
Lindsay 2009.
| Methods |
Study design: Randomised controlled trial. Control group: The control group received new computers and broadband access, although they did not have access to the portal. Weekly drop‐in sessions and phone‐in support was available. |
|
| Participants |
Study location: UK. CHD diagnosis/treatment: No CHD history/diagnosis details provided, although it is described that participants were drawn from GPs CHD registries. Mean age: 62.9 Percentage men: 72.66% Number of participants recruited: 108 Participant ethnicity: Not reported. Recruited online or offline? Offline, from GP practices. |
|
| Interventions |
Name of the intervention: Hearts of Salford. Intervention aim: Improve management of heart disease and influence health behaviours. Intervention features: The main focus was 5 discussion forums, which were moderated by a researcher for 6 months and then unmoderated for 3 months. During the 6‐month moderated phase, the moderator would stimulate discussions and encourage participants to join in. During the unmoderated phase, the moderators still examined the discussion forum, although they did not start new threads. The website also contained a glossary, information resources about CHD, diet, exercise, and smoking. In addition, links and information about local resources were given. How was the intervention introduced to the sample? Participants received training on how to use the portal, however it is unclear who offered this training and whether it was a face‐to‐face introduction. Was there any contact between the researcher/healthcare professional and the sample during the intervention? During the moderated phase (first 6 months) participants had access to 2 forms of communication with moderators via either the discussion forum or one‐to‐one instant messaging. Duration of the intervention? 6 months moderated discussion forum and 3 months unmoderated discussion forum, 9 months in total. |
|
| Outcomes |
Outcomes: 1. Physical activity. Measurement tool: Authors describe that this was assessed in terms of asking ‘how many days during a typical week do you spend in moderate exercise?' 2. Diet. Measurement tool: Items taken from Health Survey for England. 3. Healthcare utilisation. Measurement tool: All visits to a GP, nurse, specialist, and other healthcare providers in the past month. Time points: At baseline, 6 months and 9 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and the outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and the outcomes may have been influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The results tables in the paper suggest no participant dropouts, however the text reports that 4 participants dropped out. Attempts to contact the authors for an explanation were unsuccessful |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available |
Maddison 2014.
| Methods |
Study design: Randomised controlled trial. Control group: Usual community‐based cardiac rehabilitation. |
|
| Participants |
Study location: New Zealand. CHD diagnosis/treatment: Clinically documented diagnosis of ischaemic heart disease within the previous 3 to 12 months. Mean age (SD): Intervention group: 61.4 (8.9) Control group: 59.0 (9.5) Percentage men: Intervention group: 81% (n = 69) Control group: 81% (n = 70) All participants: 81% (n = 139) Number of participants recruited: 171 Participant ethnicity: New Zealand Maori 13 (8%), Pacific 10 (6%), Asian 17 (10%), New Zealand European/other 131 (76%). Recruited online or offline? Offline, recruited from 2 metropolitan hospitals. |
|
| Interventions |
Name of the intervention: No name is provided. Intervention aim: Increasing physical activity. Intervention features: Personalised automated programme of SMS text messages delivered over 6 months. The messages were sent to participants outlining their prescribed exercise for each week, including duration, frequency, and intensity of exercise. Participants were provided a pedometer and step counts were used to indicate volume of activity for each given week. This was provided alongside a website containing personalised feedback on progress with goals, and exercise prescription. The website contained information on various forms of exercise, links to other websites/cardiac rehabilitation‐related information, video messages, motivational messages, and weekly health and exercise tips. How was the intervention introduced to the sample? Face‐to‐face training session provided. Was there any contact between the researcher/healthcare professional and the sample during the intervention? No. Duration of the intervention? 6 months. |
|
| Outcomes |
Outcomes: 1. Blood pressure. 2. Physical activity. Measurement tool: International Physical Activity Questionnaire. 3. HRQOL. Measurement tool: SF36. 4. Cost. Measurement tool: Cost of the programme, direct medical costs (cost of treatment, primary care, secondary care, and over‐the‐counter medications) are collected for the cost‐effective analysis. Time points: Baseline and 24‐week follow‐up (postintervention). |
|
| Notes | Trial was registered with ANZCTR (Australian New Zealand Clinical Trials Registry). Registration number: ACTRN12611000117910 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed using a central computerised system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and some of the outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small attrition rate (11%), which was balanced between groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Not all the outcomes described in the protocol have been reported: 6 minute walk test |
Reid 2012.
| Methods |
Study design: Randomised controlled trial. Control group: General physical activity guidance and an educational booklet. |
|
| Participants |
Study location: Canada. CHD diagnosis/treatment: This was the first cardiac event for 64.6% of participants, 98.2% of the sample had undergone a PCI procedure, and 29.1% of the sample had been admitted to hospital for AMI. Participants who had experienced cardiac events previously had had an AMI (18.8%), PCI (27.4%), or CABG (9.0%). Mean age (SD): Intervention group: 56.7 (9.0) Usual care group: 56.0 (9.0) Percentage men: Intervention group: 82.6% (n = 95) Usual care group: 86.1% (n = 93) All participants: 84.3% (n = 188) Number of participants recruited: 223 Participant ethnicity: This information is not provided. Recruited online or offline? Offline, participants were recruited during hospitalisation after successful percutaneous coronary revascularisation and began the programme at hospital discharge. |
|
| Interventions |
Name of the intervention: CardioFit Intervention aim: Promote physical activity in people with CHD who were not participating in a cardiac rehabilitation programme. Intervention features: An exercise specialist presented the participant in hospital with a personally tailored physical activity plan generated by the intervention. After hospital discharge, participants logged their daily activity on the CardioFit website and completed a set of 5 online tutorials. These tutorials were carried out at weeks 2, 4, 8, 14, and 20. Each tutorial took between 10 and 20 minutes to complete and developed a new physical activity plan for the participant to complete. How was the intervention introduced to the sample? Participants were given instructions on how to use the CardioFit website. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Between tutorials participants received emails from the exercise specialist providing motivational feedback on their progress. Participants were also able to email the exercise specialist questions concerning their progress. Duration of the intervention? 6 months. |
|
| Outcomes |
Outcomes: 1. Clinical adverse outcomes. 2. Physical activity. Measurement tool: a pedometer (Yamax DIGI‐WALKER, Yamasa) over a 7‐day period and a modified version of the Godin Leisure‐Time Exercise Questionnaire. 3. HRQOL. Measurement tool: MacNew Time points: Baseline, 6 months and 12 months following randomisation. |
|
| Notes | Trial was registered with ClinicalTrials.gov. Registration number: NCT00265525 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and the outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and the outcomes may have been influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 31%, numbers are balanced between groups. Reasons for drop‐out are not provided, however missing outcome values were replaced by multiple imputations |
| Selective reporting (reporting bias) | High risk | Not all the outcomes described in the protocol have been reported: use of secondary prevention medications |
Southard 2003.
| Methods |
Study design: Randomised controlled trial. Control group: Usual care, details not provided. |
|
| Participants |
Study location: USA. CHD diagnosis/treatment: Past medical history: MI (57.7%), congestive heart failure (9.6%), CABG (59.6%), percutaneous transluminal coronary angioplasty (38.5%), diabetes (17.3%), transient ischaemic attack/cerebrovascular accident (6.7%), peripheral vascular disease (12.5%), pacemaker (4.8%), and implanted coronary defibrillator (1.0%). Mean age (SD): Intervention group: 61.8 (10.6) Control group: 62.8 (10.6) Percentage men: Intervention group: 68% (n = 36) Control group: 82% (n = 42) All participants: 75% (n = 78) Number of participants recruited: 104 Participant ethnicity: White (97.1%), black (1.0%), and other (1.9%). Recruited online or offline? Offline. Participants were recruited from both primary care providers and hospital settingas well as ads in local newspapers throughout the same geographic area. |
|
| Interventions |
Name of the intervention: No name is provided. Intervention aim: To provide risk factor management support, education, and monitoring services to people with CVD. Intervention features: Participants were expected to complete education modules assigned by a case manager and enter data online into progress graphs (e.g. number of exercise minutes, blood pressure measurements). Graphic feedback and progress over time was then provided. Each educational module was interactive and contained multiple‐choice self tests, on which feedback was given. The programme also had links to related sites on the Internet, and participants could communicate with a dietitian, who provided feedback on diet. How was the intervention introduced to the sample? This information is not provided. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Case managers interacted with participants using a format similar to email. Participants also had the option of using an online discussion group and a list of participants’ email addresses. If necessary, telephone and mail contact from healthcare provider was also provided. Duration of the intervention? 6 months. |
|
| Outcomes |
Outcomes: 1. Major cardiovascular events. Measurement tool: Identified through patient record and verified via review of medical office or hospital records. 2. Blood pressure. 3. Cholesterol. 4. LDL cholesterol. 5. HDL cholesterol. 6. Triglycerides. 7. HRQOL. Measurement tool: Dartmouth COOP. 8. Diet. Measurement tool: MEDFICTS. 9. Physical activity. Measurement tool: Minutes of weekly exercise. Time points: Baseline and 6 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and some of the outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Inadequate blinding, the outcome assessor was aware of group assignment during exit visit but not during entry visit |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (4%), reasons for drop‐out are provided and unlikely to be related to the outcome |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available |
Varnfield 2014.
| Methods |
Study design: Randomised controlled trial. Control group: Usual community‐based cardiac rehabilitation. |
|
| Participants |
Study location: Australia. CHD diagnosis/treatment: Post‐MI. Mean age (SD): Intervention group: 54.9 (9.6) Control group: 56.2 (10.1) Percentage men: Intervention group: 91% (n = 48) Control group: 83% (n = 34) All participants: 87% (n = 82) Number of participants recruited: 120 Participant ethnicity: This information is not provided. Recruited online or offline? Offline, recruited through cardiac rehabilitation referral. |
|
| Interventions |
Name of the intervention: The Care Assessment Platform (CAP). Intervention aim: Improving patient empowerment and overcoming the barriers to uptake and adherence of traditional cardiac rehabilitation programmes. Intervention features: The intervention was delivered using a smartphone and a web portal. The smartphone had an integrated accelerometer and diary application for recording exercise and health information and for delivering motivational and educational messages. The data from the smartphone could be synchronised to a web portal, where participants uploaded data on weight, blood pressure, sleep duration/quality, exercise, stress, diet, and if relevant, alcohol and smoking. Mentors could have access to this information when speaking with participants during weekly telephone consultations. How was the intervention introduced to the sample? Participants received face‐to‐face training on how to use the intervention. Was there any contact between the researcher/healthcare professional and the sample during the intervention? The case mentor provided weekly telephone consultations. Duration of the intervention? 6 weeks, with a 6‐month maintenance phase. |
|
| Outcomes |
Outcomes: 1. Total cholesterol. 2. HDL cholesterol. 3. LDL cholesterol. 4. Triglycerides. 5. Blood Pressure. 6. Diet: Measurement tool: Dietary Habits Questionnaire. 7. HRQOL. Measurement tool: EQ‐5D. Time points: Baseline, 6 weeks, 6 months. |
|
| Notes | Trial was registered with ANZCTR (Australian New Zealand Clinical Trials Registry). Registration number: ACTRN12609000251224 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and some outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and some outcomes may have been influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large attrition rate (40%), reasons given for participant drop‐out are likely to be related to the study's primary outcome measure (uptake, adherence and completion rates of the intervention) |
| Selective reporting (reporting bias) | High risk | The primary outcome differs from the protocol, and not all outcomes have been described |
Vernooij 2012.
| Methods |
Study design: Randomised controlled trial. Control group: Usual care, participants were asked to contact their physician (vascular surgeon, cardiologist, neurologist) at the hospital or the general practitioner for risk factor management. The treating physician was then free to determine the frequency of control. |
|
| Participants |
Study location: Netherlands. CHD diagnosis/treatment: All participants had been diagnosed with a manifestation of vascular disease, coronary artery disease (46%), cerebrovascular disease (27%), abdominal aortic aneurysm (4%), and peripheral vascular disease (23%). Mean age (SD): Intervention group: 60.7 (7.8) Usual care group: 59.2 (8.9) Percentage men: Intervention group: 78% (n = 128) Control group: 71% (n = 118) All participants: 74.55% (n = 246) Number of participants recruited: 330 Participant ethnicity: This information is not provided. Recruited online or offline? Offline, participants were recruited through referral from a vascular specialist/GP. |
|
| Interventions |
Name of the intervention: No name. Intervention aim: Manage vascular risk factors in people with clinically manifest vascular disease. Intervention features: The website was personalised for each participant. CHD risk factors were displayed on separate web pages, and described a history of risk factor measurements (e.g. blood pressure, cholesterol), drug use, treatment goal, advice from the nurse, correspondence between the nurse and participant, and news items for that particular risk factor. How was the intervention introduced to the sample? There was a face‐to‐face introduction to the programme, participants were invited for an hour visit to the clinic where the participant received information on their risk factor levels, instructions on how to use the programme, and a username/password for access to the website. Was there any contact between the researcher/healthcare professional and the sample during the intervention? There was contact between the participant and the nurse practitioner through the website. In a case of non‐response, the nurse practitioner would contact the participant. Duration of the intervention? 1 year. |
|
| Outcomes |
Outcomes: 1. Cardiovascular events. 2. Total cholesterol. 3. HDL cholesterol. 4. LDL cholesterol. 5. Triglycerides. 6. Blood pressure. Time points: Baseline and 12‐month follow‐up (postintervention). |
|
| Notes | Trial was registered with ClinicalTrials.gov. Registration number: NCT00785031 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, however the study outcomes are not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (5%), reasons for drop‐out are not provided, however this small drop‐out rate is unlikely to have a major influence on the results in this study |
| Selective reporting (reporting bias) | High risk | Not all the measures reported in the protocol have been reported: additional costs per additional patient achieving treatment goal, and cost per life year gained |
Zutz 2007.
| Methods |
Study design: Randomised controlled trial. Control group: Observational control group. All participants were on a waiting list to receive hospital‐based cardiac rehabilitation after the 12‐week study. |
|
| Participants |
Study location: USA. CHD diagnosis/treatment: Participants in the intervention were categorised as MI (38%), PCA (38%), CABG (14%), or diabetes mellitus (13%). Participants in the control group were categorised as MI (29%), PCA (57%), CABG (50%), or diabetes mellitus (14%). Mean age (SD): Intervention group: 58 (4) Usual care group: 59 (12) Percentage men: Intervention group: 87.5% (n = 7) Control group: 71% (n = 5) All participants: 87% (n = 13) Number of participants recruited: 15 Participant ethnicity: This information is not provided. Recruited online or offline? Offline, participants were recruited through hospital‐based cardiac rehabilitation. |
|
| Interventions |
Name of the intervention: No name. Intervention aim: Deliver cardiac rehabilitation from a distance. Intervention features: Weekly education sessions, and participants were required to upload data on their exercise levels, heart rate, weight, blood pressure, and glucose levels (if diabetic). How was the intervention introduced to the sample? Training was provided on how to use the intervention. Was there any contact between the researcher/healthcare professional and the sample during the intervention? One‐on‐one chat sessions with various healthcare professionals including a nurse, dietitian, and an exercise specialist, and monthly 'ask an expert' group chat sessions were scheduled. Programme users were also given the email addresses of a nurse, an exercise specialist, and a dietitian if they had any questions, and a research assistant was available for technical support. Duration of the intervention? 12 weeks. |
|
| Outcomes |
Outcomes: 1. Clinical adverse outcomes. 2. Total cholesterol. 3. LDL cholesterol. 4. HDL cholesterol. 5. Triglycerides. 6. Blood pressure. 7. Physical activity: Measurement tool: Minnesota Leisure Time Physical Activity Questionnaire. Time points: Baseline and 12‐week follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and some outcomes may have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and some outcomes may have been influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 13%. After 12 weeks, 2/7 control group participants were lost to follow‐up. All intervention group participants (8/8) were followed up. Unlikely to have major influence on results in this small pilot study |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available |
AMI: acute myocardial infarction CABG: coronary artery bypass grafting CHD: coronary heart disease CVD: cardiovascular disease GP: general practitioner HDL: high‐density lipoprotein HRQOL: health‐related quality of life LDL: low‐density lipoprotein MI: myocardial infarction PCA: primary cardiac arrest PCI: percutaneous coronary intervention SD: standard deviation SF36: 36‐Item Short Form Health Survey
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ades 2000 | Not an RCT |
| Appelgate 2008 | Non‐CHD sample |
| Bailey 2006 | Intervention targeted at physicians |
| Bailey 2007 | Intervention targeted at physicians |
| Barley 2014 | Non‐Internet based |
| Barnason 2003 | Non‐Internet based |
| Barnason 2006 | Non‐Internet based |
| Barnason 2009a | Non‐Internet based |
| Barnason 2009b | Non‐Internet based |
| Bates 2003 | Not an RCT |
| Bavry 2008 | Non‐Internet based |
| Bell 2000 | Non‐CHD sample |
| Bennett 2011 | Non‐CHD sample |
| Berwanger 2012 | Intervention targeted at physicians |
| Blasco 2012 | Non‐Internet based |
| Bowles 2009 | Non‐Internet based |
| Brennan 2010 | Non‐CHD sample |
| Carling 2009 | Non‐CHD sample |
| Chiantera 2005 | Non‐Internet based |
| Cockayne 2011 | Non‐CHD sample |
| Coll 2011 | Non‐Internet based |
| Cooper‐DeHoff 2001 | Not an RCT |
| Coskun 2006 | Not an RCT |
| Cutrona 2010 | Not an RCT |
| Dalleck 2011 | Non‐Internet based |
| Danish 2002 | Not an RCT |
| Dedoncker 2012 | Non‐Internet based |
| Deligiannis 2010 | Non‐Internet based |
| DeVon 2010 | Non‐Internet based |
| Di 2000 | Non‐Internet based |
| Eccles 2002 | Decision support tool, not a lifestyle intervention |
| Feldman 2005 | Non‐CHD sample |
| Fletcher 1984 | Non‐Internet based |
| Frederix 2011 | Not an RCT |
| Giannuzzi 2006 | Non‐Internet based |
| Giannuzzi 2008 | Non‐Internet based |
| Gilutz 2009 | Non‐Internet based |
| Goessens 2008 | Not an RCT |
| Goff 2002 | Not an RCT |
| Goff 2003 | Non‐Internet based |
| Guzik 2001 | Not an RCT |
| Hetlevik 1999 | Non‐Internet based |
| Janssen 2010 | Non‐CHD sample |
| Jelinek 2009 | Not an RCT |
| Jenny 2001 | Non‐Internet based |
| Kashem 2006 | Non‐CHD sample |
| Katalinic 2008 | Non‐Internet based |
| Keeping‐Burke 2011 | Non‐Internet based |
| Kerr 2008 | Not an RCT |
| Kothe 2012 | Non‐CHD sample |
| Kukafka 2002 | Intervention not based on promoting healthy lifestyle or medicines management; addressed response to myocardial infarction symptoms |
| Körtke 2005 | Not an RCT |
| Körtke 2006 | Non‐Internet based |
| Lee 2011 | Non‐CHD sample |
| Lehmann 2011 | Non‐Internet based |
| Lester 2006 | Intervention targeted at physicians |
| Levine 2011 | No clinical or behaviour change outcomes |
| Liu 2010 | Non‐CHD sample |
| Mattera 2012 | Not an RCT |
| McGillion 2008 | Non‐Internet based |
| Michal 2013 | Non‐Internet based |
| Mohammady 2011 | Non‐Internet based |
| Moore 2001 | Non‐Internet based |
| Murtaugh 2005 | Non‐CHD sample |
| Nolan 2011 | Non‐Internet based |
| Nolan 2012 | Non‐CHD sample |
| O'Neil 2011 | Non‐Internet based |
| Oranta 2011 | Non‐Internet based |
| Oranta 2012 | Non‐Internet based |
| Parekh 2012 | Non‐CHD sample |
| Park 2014 | Non‐Internet based |
| Pogosova 2008 | Non‐Internet based |
| Richardson 2010 | Non‐CHD sample |
| Rollman 2009 | Non‐Internet based |
| Ross 2004 | Non‐CHD sample |
| Rossi 1997 | Intervention targeted at healthcare providers |
| Ruffin 2011 | Non‐CHD sample |
| Saffi 2014 | Non‐Internet based |
| Scalvini 2009 | Not an RCT |
| Schweier 2014 | Not an RCT |
| Sequist 2005 | Study assessed physician attitudes towards an electronic clinical reminder system |
| Sheridan 2010 | Non‐CHD sample |
| Sheridan 2011 | Non‐CHD sample |
| Stewart 2011 | Non‐CHD sample |
| Thompson 2008 | Non‐Internet based |
| Thomsen 2001 | Non‐Internet based |
| Vandelanotte 2010 | Not an RCT |
| Verheijden 2004 | Non‐CHD sample |
| Wakefield 2008 | Non‐Internet based |
| Waldmann 2008 | Non‐Internet based |
| Waldron 2010 | Non‐CHD sample |
| Wister 2007 | Non‐Internet based |
| Woodend 2008 | Non‐Internet based |
| Wu 2012 | Non‐Internet based |
| Yehle 2012 | Not an RCT |
CHD: coronary heart disease RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Dale 2014.
| Trial name or title | Improving coronary heart disease self‐management using mobile technologies (TEXT4HEART) |
| Methods |
Study design: Randomised controlled trial. Study location: New Zealand. |
| Participants |
CHD diagnosis/treatment: People with a documented diagnosis of CHD, and met the criteria for usual cardiac rehabilitation care. Recruited online or offline? Offline, recruited during hospital admission. |
| Interventions |
Name of the intervention: No name is provided. Intervention aim: Improving adherence to lifestyle change. Intervention features: A tailored programme of text messages per week for 6 months and access to an interactive website that contains a blog, a graph of physical activity progress, role model video messages, and weekly healthy lifestyle tips. How was the intervention introduced to the sample? Participants offered brief training in how to use SMS and the Internet if necessary. Was there any contact between the researcher/healthcare professional and the sample during the intervention? The participant is able to 'text an expert' with questions on lifestyle change, these questions are then answered within 24 hours. Intended duration of the intervention? 6 months. Control group: Usual cardiac rehabilitation. |
| Outcomes |
Measures: 1. Physical activity. Measurement tool: Godin Leisure‐Time Physical Activity Questionnaire. 2. Fruit and vegetable intake. Measurement tool: 2 specific questions used in the 2007 New Zealand Health Survey. 3. Medication adherence. Measurement tool: 8‐item Morisky Medication Adherence Scale. Time points: 6‐month follow‐up. |
| Starting date | May 2013 |
| Contact information | Principal Investigator and contact: Leila Pfaeffli Dale, lpfaeffli@nihi.auckland.ac.nz |
| Notes | Trial is registered with ANZCTR (Australian New Zealand Clinical Trials Registry). Registration number: ACTRN12613000901707 |
ISRCTN29243064.
| Trial name or title | Long‐term effectiveness of a comprehensive cardiac telerehabilitation program (Telerehab III): a randomised controlled trial |
| Methods |
Study design: Multicentre randomised controlled trial. Study location: Belgium. |
| Participants |
CHD diagnosis/treatment: Coronary artery disease and heart failure. Recruited online or offline? Offline, recruited from hospitals. |
| Interventions |
Name of the intervention: The intervention does not have a name. Intervention aim: To improve the long‐term physical fitness of people with heart disease. Intervention features: The program consisted of dietary, smoking cessation, and activity telecoaching. Participants also received prescribed exercise training, and wore a physical activity monitor throughout the program. Data from the monitor had to be regularly uploaded to the web‐based program, which then generated personalised feedback designed to encourage participants to achieve predefined goals. How was the intervention introduced to the sample? Participants receive training on how to use the programme. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Participants were provided with weekly feedback on physical activity levels, which was sent by email or SMS, or both, depending on participant preference. Intended duration of the intervention? 6 months. Control group: Usual cardiac rehabilitation. |
| Outcomes |
Measures: 1. Physical activity. Measurement tool: an accelerometer and the International Physical Activity Questionnaire. 2. Blood pressure. 3. Blood lipids. 4. Quality of life. Measurement tool: HeartQoL and EQ‐5D. Time points: 6‐week and 6‐month follow‐up. |
| Starting date | February 2013 |
| Contact information | Principal Investigator: Professor Paul Dendale, paul.dendale@uhasselt.be Contact: Dr Ines Frederix, ines.frederix@gmail.com |
| Notes | Trial is registered with the ISRCTN registry. Registration number: ISRCTN29243064 |
NCT02228603.
| Trial name or title | How to enhance physical activity after cardiac rehabilitation? A randomised controlled study comparing two follow‐up training exercise programs |
| Methods |
Study design: Randomised controlled trial. Study location: Norway. |
| Participants |
CHD diagnosis/treatment: myocardial infarction
and stable angina. Recruited online or offline? Offline, recruited from a hospital cardiac rehabilitation programme. |
| Interventions |
Experimental group: High‐intensity exercise, carried out in a group‐based format for 8 weeks, followed by group‐based counselling every 3rd month for 12 months. Active comparator group: Web‐based follow‐up program. Name of the intervention: No name reported. Intervention aim: Improve exercise adherence and healthy lifestyle changes. Intervention features: Not reported. How was the intervention introduced to the sample? Not reported. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Not reported. Intended duration of the intervention? 8 weeks. Control group 2: Usual care, information about recommended physical activity and healthy lifestyle. |
| Outcomes |
Measures: 1. Physical activity. Measurement tool: an accelerometer, SenseWear Pro 3 armband. 2. Quality of life. Measurement tool: MacNew. Time points: 2‐year follow‐up. |
| Starting date | August 2014 |
| Contact information | Principal Investigator: Professor Asbjørn Støylen, asbjorn.stoylen@ntnu.no Contact: Inger Lise Aamot, inger.lise.aamot@ntnu.no |
| Notes | Trial is registered with ClinicalTrials.gov. Registration number: NCT02228603 |
NCT02350192.
| Trial name or title | A randomized controlled trial of the effectiveness of a home‐based interactive e‐health educational intervention for middle‐aged cardiovascular disease adults in improving total exercise, adherence rate, exercise efficacy and outcomes |
| Methods |
Study design: Randomised controlled trial. Study location: Hong Kong. |
| Participants |
CHD diagnosis/treatment: Cardiovascular disease adults. Recruited online or offline? Not reported. |
| Interventions |
Name of the intervention: e‐health educational intervention (eHEI). Intervention aim: Improve total physical activity, exercise adherence, and quality of life. Intervention features: The programme provides culture‐specific information related to cardiovascular disease and information on how risk factors can be modified. It allows participants to self monitor their health and exercise behaviours and track their progress over time. How was the intervention introduced to the sample? A demonstration is provided to participants by a trained nurse. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Participants are telephoned at 2 weeks. Intended duration of the intervention? Not reported. Control group: Usual care, and an educational leaflet about coronary heart disease. |
| Outcomes |
Measures: 1. Physical activity. Measurement tool: the Godin‐Shephard Leisure‐Time Physical Activity Questionnaire. 2. Quality of life. Measurement tool: Chinese version of the 12‐Item Short Form Health Survey. Time points: 3 months' and 6 months' follow‐up. |
| Starting date | June 2013 |
| Contact information | Principal Investigator: Eliza Mi Ling Wong, elizawong@cuhk.edu.hk |
| Notes | Trial is registered with ClinicalTrials.gov. Registration number: NCT02350192 |
Redfern 2014.
| Trial name or title | A randomised controlled trial of a consumer‐focused e‐health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study protocol |
| Methods |
Study design: Randomised controlled trial. Study location: Australia. |
| Participants |
CHD diagnosis/treatment: Participants diagnosed with CVD or who are at risk of CVD. Recruited online or offline? Offline, recruited from general practice. |
| Interventions |
Name of the intervention: CONNECT. Intervention aim: The programme focuses on cardiovascular risk assessment, medication adherence, lifestyle change, and patient‐provider communication. Intervention features: Facility to view personal health records (information such as medicines, test results, blood pressure, weight), medication and healthy lifestyle reminders, motivational message prompts, interactive goal‐setting, and social media feature. How was the intervention introduced to the sample? Face‐to‐face training of participants. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Participants contacted at month 1 and 2 via telephone, and additional support provided as needed. Intended duration of the intervention? Participants will be assigned to the e‐health intervention for an average of 18 months (minimum 12 months and maximum 24 months). Control group: Usual health care. |
| Outcomes |
Measures: 1. All‐cause mortality. 2. Health‐related quality of life. Measurement tool: EQ‐5D. 3. Blood pressure. 4. LDL cholesterol. 5. Physical activity. Measurement tool: WHO Global Physical Activity Questionnaire. 6. Diet. Measurement tool: Self reported portions of fruit, vegetable intake, fish, salt and saturated fat intake consumed in 7 days. 7. Cardioprotective medication adherence. Measurement tool: Self report and verified by medical records and pharmaceutical benefits scheme data. Time points: minimum 12 months, maximum 24 months. |
| Starting date | October 2014 |
| Contact information | Principal investigator: Professor Julie Redfern, jredfern@georgeinstitute.org.au Other contacts: Ms Genevieve Coorey, gcoorey@georgeinstitute.org.au. |
| Notes | Trial is registered with ANZCTR (Australian New Zealand Clinical Trials Registry). Registration number: ACTRN12613000715774 This trial includes both participants with CVD and those at increased risk for CVD. Our review is concerned only with those with a CVD diagnosis. However, the authors state that "Prespecified analyses will be conducted on the subgroup: established CVD versus high‐risk non‐CVD", so we will be able to include these data in our review |
Reinwand 2013.
| Trial name or title | Designing a theory based and evidence based tailored eHealth rehabilitation aftercare program in Germany and the Netherlands |
| Methods |
Study design: Quasi‐experimental randomised controlled trial. Study location: Germany and the Netherlands. |
| Participants |
CHD diagnosis/treatment: People who have successfully completed cardiac rehabilitation. Recruited online or offline? Offline, recruited from cardiac rehabilitation classes. |
| Interventions |
Name of the intervention: RENATA 'Rehabilitation aftercare program for an optimal transfer into daily life' Intervention aim: The intervention encouraged participants to reflect on their own health goals, action plans, and coping plans. Intervention features: Involves 8 modules designed to increase risk perception of CVD and support positive outcome expectancies towards physical activity and vegetable consumption. How was the intervention introduced to the sample? Not reported. Was there any contact between the researcher/healthcare professional and the sample during the intervention? Not reported. Intended duration of the intervention? 8 weeks. Control group: Waiting‐list control. |
| Outcomes |
Measures: 1. Self reported physical activity. Measurement tool: International Physical Activity Questionnaire (short version). 2. Diet. Measurement tool: Participants will be asked to count the number of fruits and vegetables consumed over the past 7 days. 3. Quality of life. Measurement tool: WHOQOL‐BREF. Time points: Postintervention follow‐up (8 weeks), and further 4 weeks', 6 months', and 12 months' follow‐ups thereafter. |
| Starting date | July 2013 |
| Contact information | Principal Investigator: Prof. Dr. Sonia Lippke, s.lippke@jacobs‐university.de Other contact: Dominque Reinwand, d.reinwand@jacobs‐university.de |
| Notes | Trial is registered with ClinicalTrials.gov. Registration number: NCT01909349 |
Shah 2011.
| Trial name or title | Secondary Prevention Risk Intervention Via Telemedicine and Tailored Patient Education (SPRITE). A randomised trial to improve post MI management |
| Methods |
Study design: Randomised controlled trial (3 arms). Study location: North Carolina, USA. |
| Participants |
CHD diagnosis/treatment: MI and hypertension patients. Recruited online or offline? Participants are being recruited from a tertiary‐care healthcare system in a suburban setting. |
| Interventions |
Name of the intervention: Heart360 and HealthVault. Intervention aim: Provide patient education regarding disease management, participants to upload blood pressure and glucose measurements to allow self management and tracking and to communicate these measurements to the study team. Intervention features: Multifaceted, tailored approach. Monthly assessments performed through a web‐based interaction. Participants provided with evidence‐based recommendations regarding lifestyle behaviours and advised on how to achieve their goals. Financial barriers addressed by suggesting low‐cost diet and cheaper ways to exercise. Designed to be culturally sensitive. The behavioural modules included diet, exercise, smoking, alcohol, stress reduction, memory, literacy, social environment, patient‐provider relationship, missed appointments, medication management, side effects, and knowledge/risk perception. Tailored feedback regarding disease and lifestyle management is also provided. How was the intervention introduced to the sample? Training demonstrations and written instructions provided. In addition, verbal and written instructions on how to upload blood pressure measurements are also provided. Was there any contact between the researcher/healthcare professional and the sample during the intervention? No. Intended duration of the intervention? 12 months. Control group 1: Received CHD educational handouts at baseline, then continued with regular medical care throughout the remainder of the study. Control group 2: A self management programme provided by a nurse using a tailored telephone‐based intervention. |
| Outcomes |
Measures: 1. Systolic blood pressure. 2. LDL cholesterol. 3. Physical activity. Measurement tool: details not provided. 4. Diet. Measurement tool: details not provided. Time points: 12 months' follow‐up. |
| Starting date | June 2009 |
| Contact information | Principal Investigator: Dr Hayden Barry, boswo001@mc.duke.edu Other contact: Bimal Shah, bimal.shah@duke.edu |
| Notes | Trial is registered with ClinicalTrials.gov. Registration number: NCT00901277 |
CHD: coronary heart disease CVD: cardiovascular disease LDL: low‐density lipoprotein MI: myocardial infarction WHO: World Health Organization WHOQOL: World Health Organization Quality of Life
Differences between protocol and review
We planned to search Google Scholar. However, this was not done due to limited resources.
We intended where possible to examine the intensity of secondary prevention measures in the comparison group compared to that in the experimental group, but there were insufficient trials included for us to do this.
We also intended to conduct sensitivity analyses excluding studies of low methodological quality and to produce funnel plots and tests of asymmetry to assess possible publication bias (Egger 1997), but again the number of included trials was insufficient for us to do this. We will address this in future updates of this review when further evidence accrues.
We used the GRADE methodology to assess the quality of evidence and included 'Summary of findings' tables, although this was not specified in the protocol.
Contributions of authors
RD: drafting the protocol, selecting studies for inclusion, extracting data from relevant studies, judging trial risk of bias, providing input for the meta‐analysis, interpreting the findings, and drafting the final review.
SS: drafting the protocol, selecting studies for inclusion, extracting data from relevant studies, judging trial risk of bias, interpreting the findings, and drafting the final review.
JP: drafting the protocol, developing the search strategy, judging trial risk of bias, interpreting the findings, and drafting the final review.
EF: selecting studies for inclusion, extracting data from relevant studies, and judging trial risk of bias.
EI: selecting studies for inclusion and extracting data from relevant studies.
KR: drafting the protocol, developing the search strategy, selecting studies for inclusion, extracting data from relevant studies, leading on the meta‐analysis, judging trial risk of bias, interpreting the findings, and drafting the final review.
Sources of support
Internal sources
Division of Health Sciences, Warwick Medical School, University of Warwick, UK.
Health and Life Sciences, Coventry University, UK.
Department of Cardio‐Respiratory Medicine, University Hospitals of Leicester NHS Trust, UK.
External sources
Karen Rees is also supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
RD: RD is also an author of one of the included trials (Devi 2014).
SS: SS is also an author of one of the included trials (Devi 2014).
JP: JP is also an author of one of the included trials (Devi 2014).
EF: None declared.
EI: None declared.
KR: None declared.
New
References
References to studies included in this review
Antypas 2014 {published data only}
- Antypas K, Wangberg SC. An Internet‐ and mobile‐based tailored intervention to enhance maintenance of physical activity after cardiac rehabilitation: short‐term results of a randomized controlled trial. Journal of Medical Internet Research 2014;16(3):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devi 2014 {published data only}
- Devi R, Powell J, Singh S. A web‐based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. Journal of Medical Internet Research 2014;16(9):e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frederix 2015 {published data only}
- Frederix I, Driessche N, Hansen D, Berger J, Bonne K, Alders T, et al. Increasing the medium‐term clinical benefits of hospital‐based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. European Journal of Preventive Cardiology 2015;22(2):150‐8. [DOI] [PubMed] [Google Scholar]
Lear 2014 {published data only}
- Lear SA, Singer J, Banner‐Lukaris D, Horvat D, Park JE, Bates J, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the internet. Circulation: Cardiovascular Quality and Outcomes 2014;7(6):952‐9. [DOI] [PubMed] [Google Scholar]
Lindsay 2009 {published data only}
- Lindsay S, Bellaby P, Smith S, Baker R. Enabling healthy choices: is ICT the highway to health improvement?. Health (London, England: 1997) 2008;12(3):313‐31. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Smith S, Bellaby P, Baker R. The health impact of an online heart disease support group: a comparison of moderated versus unmoderated support. Health Education Research 2009;24(4):646‐54. [DOI] [PubMed] [Google Scholar]
Maddison 2014 {published data only}
- Maddison R, Pfaeffli L, Whittaker R, Stewart R, Kerr A, Jiang Y, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. European Journal of Preventive Cardiology 2014 May 9 [Epub ahead of print]. [DOI] [PubMed]
Reid 2012 {published data only}
- Reid RD, Morrin LI, Beaton LJ, Papadakis S, Kocourek J, McDonnell L, et al. Randomized trial of an internet‐based computer‐tailored expert system for physical activity in patients with heart disease. European Journal of Preventive Cardiology 2012;19(6):1357‐64. [DOI] [PubMed] [Google Scholar]
Southard 2003 {published data only}
- Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet‐based case management system for secondary prevention of heart disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2003;23(5):341‐8. [DOI] [PubMed] [Google Scholar]
Varnfield 2014 {published data only}
- Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone‐based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100(22):1770‐9. [DOI] [PubMed] [Google Scholar]
Vernooij 2012 {published data only}
- Vernooij JW, Kaasjager HA, Graaf Y, Wierdsma J, Grandjean HM, Hovens MM, et al. Internet based vascular risk factor management for patients with clinically manifest vascular disease: randomised controlled trial. BMJ (Clinical research ed.) 2012;344:e3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zutz 2007 {published data only}
- Zutz A, Ignaszewski A, Bates J, Lear SA. Utilization of the Internet to deliver cardiac rehabilitation at a distance: a pilot study. Telemedicine Journal and e‐Health 2007;13(3):323‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ades 2000 {published data only}
- Ades PA, Pashkow FJ, Fletcher G, Pina IL, Zohman LR, Nestor JR, et al. A controlled trial of cardiac rehabilitation in the home setting using electrocardiographic and voice transtelephonic monitoring. American Heart Journal 2000;139(3):543‐8. [DOI] [PubMed] [Google Scholar]
Appelgate 2008 {published data only}
- Appelgate W, Brown‐Connolly NE, Hickman D. Low‐cost web‐based clinical decision support and case management for congestive heart failure patients in Iowa. Telemedicine and e‐Health 2008;14. [Google Scholar]
Bailey 2006 {published data only}
- Bailey TC, Noirot LA, Gage BF, Li X, Shannon WD, Waterman B, Sinha S, Bouselli DA, Reichley RM, Goldberg AC, Dunagan WC. Improving adherence to coronary heart disease secondary prevention medication guidelines at a community hospital. AMIA Annual Symposium Proceedings, American Medical Informatics Association. Vol. 2006:p. 850. [PMC free article] [PubMed]
Bailey 2007 {published data only}
- Bailey TC, Noirot LA, Blickensderfer A, Rachmiel E, Schaiff R, Kessels A, et al. An intervention to improve secondary prevention of coronary heart disease. Archives of Internal Medicine 2007;167(6):586‐90. [DOI] [PubMed] [Google Scholar]
Barley 2014 {published data only}
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT nurse‐delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: a randomised controlled pilot study. PLoS One 2014;9(6):e98704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnason 2003 {published data only}
- Barnason S, Zimmerman L, Nieveen J, Schmaderer M, Carranza B, Reilly S, et al. Impact of a home communication intervention for coronary artery bypass graft patients with ischemic heart failure on self‐efficacy, coronary disease risk factor modification, and functioning. Heart & Lung 2003;32(3):147‐58. [DOI] [PubMed] [Google Scholar]
Barnason 2006 {published data only}
- Barnason S, Zimmerman L, Nieveen J, Hertzog M. Impact of a telehealth intervention to augment home health care on functional and recovery outcomes of elderly patients undergoing coronary artery bypass grafting. Heart & Lung 2006;35(4):225‐33. [DOI] [PubMed] [Google Scholar]
Barnason 2009a {published data only}
- Barnason S, Zimmerman L, Nieveen J, Schulz P, Miller C, Hertzog M, et al. Influence of a symptom management telehealth intervention on older adults' early recovery outcomes after coronary artery bypass surgery. Heart & Lung 2009;38(5):364‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnason 2009b {published data only}
- Barnason S, Zimmerman L, Schulz P, Tu C, Barnason S, Zimmerman L, et al. Influence of an early recovery telehealth intervention on physical activity and functioning after coronary artery bypass surgery among older adults with high disease burden. Heart & Lung 2009;38(6):459‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 2003 {published data only}
- Bates DW, Gawande AA. Patient safety: Improving safety with information technology. The New England Journal of Medicine 2003;348(25):2526‐34. [DOI] [PubMed] [Google Scholar]
Bavry 2008 {published data only}
- Bavry AA. Nurse‐coordinated multidisciplinary, family‐based cardiovascular disease prevention programme (EUROACTION). American College of Cardiology 2008;17:49. [Google Scholar]
Bell 2000 {published data only}
- Bell DS, Fonarow GC, Hays RD, Mangione CM, Bell DS, Fonarow GC, et al. Self‐study from web‐based and printed guideline materials. A randomized, controlled trial among resident physicians. Annals of Internal Medicine 2000;132(12):938‐46. [DOI] [PubMed] [Google Scholar]
Bennett 2011 {published data only}
- Bennett JB, Broome KM, Schwab‐Pilley A, Gilmore P. A web‐based approach to address cardiovascular risks in managers. Results of a randomized trial. Journal of Occupational and Environmental Medicine 2011;53:911‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berwanger 2012 {published data only}
- Berwanger O, Guimaraes HP, Laranjeira LN, Cavalcanti AB, Kodama A, Zazula AD, et al. A multifaceted intervention to narrow the evidence‐based gap in the treatment of acute coronary syndromes: Rationale and design of the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes (BRIDGE‐ACS) cluster‐randomized trial. American Heart Journal 2012;163:323‐9.e1. [DOI] [PubMed] [Google Scholar]
Blasco 2012 {published data only}
- Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation & Prevention 2012;32:25‐31. [DOI] [PubMed] [Google Scholar]
Bowles 2009 {published data only}
- Bowles KH, Holland DE, Horowitz DA. A comparison of in‐person home care, home care with telephone contact and home care with telemonitoring for disease management. Journal of Telemedicine and Telecare 2009;15(7):344‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 2010 {published data only}
- Brennan PF, Casper GR, Burke LJ, Johnson KA, Brown R, Valdez RS, et al. Technology‐enhanced practice for patients with chronic cardiac disease: Home implementation and evaluation. Heart & Lung: The Journal of Acute and Critical Care 2010;39(6):S34‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carling 2009 {published data only}
- Carling CL, Kristoffersen DT, Montori VM, Herrin J, Schuenemann HJ, Treweek S, et al. The effect of alternative summary statistics for communicating risk reduction on decisions about taking statins: a randomized trial. PLoS Medicine 2009:e1000134. [DOI] [PMC free article] [PubMed]
Chiantera 2005 {published data only}
- Chiantera A, Scalvini S, Pulignano G, Pugliese M, Lio L, Mazza A, et al. Role of telecardiology in the assessment of angina in patients with recent acute coronary syndrome. Journal of Telemedicine and Telecare 2005;11(Suppl 1):93‐4. [DOI] [PubMed] [Google Scholar]
Cockayne 2011 {published data only}
- Cockayne NL, Glozier N, Naismith SL, Christensen H, Neal B, Hickie IB. Internet‐based treatment for older adults with depression and co‐morbid cardiovascular disease: protocol for a randomised, double‐blind, placebo controlled trial. BMC Psychiatry 2011;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coll 2011 {published data only}
- Coll J, Sanjoaquin AC, Lopez M, Romero D, Pinilla R, Ochoa P. Does a preventive telemonitoring service improve the quality of life of frail elderly? Preliminary results of the European project dreaming. European Geriatric Medicine 2011;2:S128‐9. [Google Scholar]
Cooper‐DeHoff 2001 {published data only}
- Cooper‐DeHoff R, Handberg E, Heissenberg C, Johnson K. Electronic prescribing via the internet for a coronary artery disease and hypertension megatrial. Clinical Cardiology 2001;24(11):V14‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coskun 2006 {published data only}
- Coskun O, Eren A, Eren M. A computer based telemedicine protocol to predict acute coronary syndrome in patients with chest pain at home. International Heart Journal 2006;47(4):491‐500. [DOI] [PubMed] [Google Scholar]
Cutrona 2010 {published data only}
- Cutrona SL, Choudhry NK, Fischer MA, Servi A, Liberman JN, Brennan TA, et al. Modes of delivery for interventions to improve cardiovascular medication adherence. American Journal of Managed Care 2010;16(12):929‐42. [PMC free article] [PubMed] [Google Scholar]
Dalleck 2011 {published data only}
- Dalleck LC, Schmidt LK, Lueker R. Cardiac rehabilitation outcomes in a conventional versus telemedicine‐based programme. Journal of Telemedicine and Telecare 2011;17:217‐21. [DOI] [PubMed] [Google Scholar]
Danish 2002 {published data only}
- Danish Centre for Evaluation and Health Technology Assessment. Telemedical prehospital diagnostic of patients with acute myocardial infarction ‐ a new IT‐based concept. Copenhagen: Danish Centre for Evaluation and Health Technology Assessment (DACEHTA) (formerly DIHTA) 2002.
Dedoncker 2012 {published data only}
- Dedoncker A, Lejeune C, Dupont C, Antoine Dl, Laurent Y, Casillas JM, et al. Nurse‐led educative consultation setting personalized tertiary prevention goals after cardiovascular rehabilitation: evaluation of patient satisfaction and long‐term effects (online www.rehabnurse.org). Rehabilitation Nursing 2012;37:105‐13. [DOI] [PubMed] [Google Scholar]
Deligiannis 2010 {published data only}
- Deligiannis P, Kouidis N, Farmakiotis A, Kouidi E, Deligiannis A. Efficacy of telemonitoring service in cardiac rehabilitation programs in public gyms. European Journal of Cardiovascular Prevention and Rehabilitation 2010;Conference:S106. [Google Scholar]
DeVon 2010 {published data only}
Di 2000 {published data only}
- Di MC, Moses JW, Anderson TJ, Bonan R, Muramatsu T, Jain AC, et al. Randomized comparison of elective stent implantation and coronary balloon angioplasty guided by online quantitative angiography and intracoronary Doppler. DESTINI Study Group (Doppler Endpoint STenting INternational Investigation). Circulation 2000; Vol. 102, issue 24:2938‐44. [DOI] [PubMed]
Eccles 2002 {published data only}
- Eccles M, McColl E, Steen N, Rousseau N, Grimshaw J, Parkin D, et al. Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ 2002;325(7370):941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 2005 {published data only}
- Feldman PH. Just‐in‐time evidence‐based e‐mail "Reminders" in home health care: impact on patient outcomes. Health Services Research 2005;40(3):885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1984 {published data only}
- Fletcher GF, Chiaramida AJ, LeMay MR, Johnston BL, Thiel JE, Spratlin MC, et al. Telephonically‐monitored home exercise early after coronary artery bypass surgery. Chest 1984;86(2):198‐202. [DOI] [PubMed] [Google Scholar]
Frederix 2011 {published data only}
- Frederix I, Dendale P, Berger J, Vandereyt F, Everts S, Hansen D. Comparison of two motion sensors for use in cardiac telerehabilitation. Journal of Telemedicine and Telecare 2011;17:231‐4. [DOI] [PubMed] [Google Scholar]
Giannuzzi 2006 {published data only}
- Giannuzzi P, Maggioni A, Ceci V, Chieffo C, Gattone M, Griffo R, et al. Global secondary prevention StrategiEs to limit event recurrence after myocardial infarction: The GOSPEL study. A trial from the Italian cardiac rehabilitation network: Final results. Circulation 2006;114(18):852. [DOI] [PubMed] [Google Scholar]
Giannuzzi 2008 {published data only}
- Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP, Balestroni G, Ceci V, et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: Results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Archives of Internal Medicine 2008;168(20):2194‐204. [DOI] [PubMed] [Google Scholar]
Gilutz 2009 {published data only}
- Gilutz H, Novack L, Shvartzman P, Zelingher J, Bonneh DY, Henkin Y, et al. Computerized community cholesterol control (4C): meeting the challenge of secondary prevention. Israel Medical Association Journal: IMAJ 2009;11(1):23‐9. [PubMed] [Google Scholar]
Goessens 2008 {published data only}
- Goessens BMB, Visseren FLJ, Nooijer J, Borne, Algra A, Wierdsma J, et al. A pilot‐study to identify the feasibility of an Internet‐based coaching programme for changing the vascular risk profile of high‐risk patients. Patient Education and Counseling 2008;73(1):67‐72. [DOI] [PubMed] [Google Scholar]
Goff 2002 {published data only}
- Goff DC, Gu L, Cantley LK, Parker DG, Cohen SJ. Enhancing the quality of care for patients with coronary heart disease: The design and baseline results of the Hastening the Effective Application of Research through Technology (HEART) trial. American Journal of Managed Care 2002;8(12):1069‐78. [PubMed] [Google Scholar]
Goff 2003 {published data only}
- Goff DC, Gu L, Cantley LK, Sheedy DJ, Cohen SJ. Quality of care for secondary prevention for patients with coronary heart disease: Results of the Hastening the Effective Application of Research through Technology (HEART) trial. American Heart Journal 2003;146(6):1045‐51. [DOI] [PubMed] [Google Scholar]
Guzik 2001 {published data only}
- Guzik P, Drozdz J, Rzetecka K, Kasztelowicz P, Rosiak T, Chrzanowski L, et al. Treatment, complications and rescue revascularization procedures in older patients in Poland ‐ POLish multicentre WEB‐based trial on acute myocardial infarction (POL‐WEB‐AMI). European Heart Journal 2001;22:461. [Google Scholar]
Hetlevik 1999 {published data only}
- Hetlevik I, Holmen J, Kruger O. Implementing clinical guidelines in the treatment of hypertension in general practice. Evaluation of patient outcome related to implementation of a computer‐based clinical decision support system. Scandinavian Journal of Primary Health Care 1999;17(1):35‐40. [DOI] [PubMed] [Google Scholar]
Janssen 2010 {published data only}
- Janssen H. Longterm cost‐effectiveness analysis of a telemedicine programme for patients with chronic heart failure. European Heart Journal 2010;Conference:226. [Google Scholar]
Jelinek 2009 {published data only}
- Jelinek M, Vale MJ, Liew D, Grigg L, Dart A, Hare DL, et al. The COACH program produces sustained improvements in cardiovascular risk factors and adherence to recommended medications ‐ two years follow‐up. Heart, Lung and Circulation 2009;18(6):388‐92. [DOI] [PubMed] [Google Scholar]
Jenny 2001 {published data only}
- Jenny NYY. Evaluating the effectiveness of an interactive multimedia computer‐based patient education program in cardiac rehabilitation. Occupational Therapy Journal of Research 2001;21(4):Fal‐275. [Google Scholar]
Kashem 2006 {published data only}
- Kashem A, Bove AA. Web‐based internet telemedicine management of patients with heart failure. Telemedicine and E‐Health 2006;12(4):447. [DOI] [PubMed] [Google Scholar]
Katalinic 2008 {published data only}
- Katalinic A, Waldmann A, Schwaab B, Richardt G, Sheikhzadeh A, Raspe H, et al. The TeleGuard trial of additional telemedicine care in CAD patients. 1 Utilization of the system. Journal of Telemedicine and Telecare 2008;14(1):17‐21. [DOI] [PubMed] [Google Scholar]
Keeping‐Burke 2011 {published data only}
- Keeping‐Burke L, Purden M, Frasure‐Smith N, Cossette S, McCarthy F, Amsel R. Evaluation of the psychosocial effects of a telehealth program for caregivers of coronary artery bypass graft (CABG) surgery patients. Canadian Journal of Cardiology 2011;1:S346. [Google Scholar]
Kerr 2008 {published data only}
- Kerr C, Murray E, Burns J, Turner I, Westwood M. Applying user‐generated quality criteria to develop an internet intervention for patients with heart disease. Journal of Telemedicine and Telecare 2008;14(3):2008‐127. [DOI] [PubMed] [Google Scholar]
Körtke 2005 {published data only}
- Körtke H, Zittermann A, El‐Arousy M, Zimmermann E, Wienecke E, Korfer R. New Eastern Westfalian Postoperative Therapeutic Concept (NOPT). A telemedically guided study for ambulatory rehabilitation of patients after cardiac surgery. Medizinische Klinik 2005;100(7):383‐9. [DOI] [PubMed] [Google Scholar]
Körtke 2006 {published data only}
- Körtke H, Stromeyer H, Zittermann A, Buhr N, Zimmermann E, Wienecke E, et al. New East‐Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemedicine and e‐Health 2006;12(4):475‐83. [DOI] [PubMed] [Google Scholar]
Kothe 2012 {published data only}
- Kothe EJ, Mullan BA, Butow P. Promoting fruit and vegetable consumption. Testing an intervention based on the theory of planned behaviour. Appetite 2012;58(3):997‐1004. [DOI] [PubMed] [Google Scholar]
Kukafka 2002 {published data only}
- Kukafka R, Lussier YA, Eng P, Patel VL, Cimino JJ, Kukafka R, et al. Web‐based tailoring and its effect on self‐efficacy: results from the MI‐HEART randomized controlled trial. Proceedings/AMIA 2002;Annual Symposium:410‐4. [PMC free article] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee TJ, Cameron LD. A randomized trial of computer‐based communications using imagery and text information to alter representations of heart disease risk and motivate protective behaviour. British Journal of Health Psychology 2011;16(1):72‐91. [DOI] [PubMed] [Google Scholar]
Lehmann 2011 {published data only}
- Lehmann N, Paul A, Moebus S, Budde T, Dobos GJ, Michalsen A. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients ‐ 3‐year results of the randomized SAFE‐LIFE trial. Atherosclerosis 2011;219:630‐6. [DOI] [PubMed] [Google Scholar]
Lester 2006 {published data only}
- Lester WT, Grant RW, Barnett GO, Chueh HC, Lester WT, Grant RW, et al. Randomized controlled trial of an informatics‐based intervention to increase statin prescription for secondary prevention of coronary disease. Journal of General Internal Medicine 2006;21(1):22‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2011 {published data only}
- Levine DA, Funkhouser EM, Houston TK, Gerald JK, Johnson‐Roe N, Allison JJ, et al. Improving care after myocardial infarction using a 2‐year internet‐delivered intervention: the Department of Veterans Affairs myocardial infarction‐plus cluster‐randomized trial. Archives of Internal Medicine 2011;171:1910‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu JJ. Pervasive telemonitoring for patients living with chronic heart failure: A quantitative study of telemedicine acceptance. Dissertation Abstracts International: Section B: The Sciences and Engineering 2010;70(9):2010. [Google Scholar]
Mattera 2012 {published data only}
- Mattera JA. Patients' adoption and adherence to a heart failure telemonitoring intervention. Dissertation Abstracts International: Section B: The Sciences and Engineering 2012;72:5252. [Google Scholar]
McGillion 2008 {published data only}
- McGillion MH, Watt‐Watson J, Stevens B, LeFort SM, Coyte P, Graham A. Randomized controlled trial of a psychoeducation program for the self‐management of chronic cardiac pain. Journal of Pain and Symptom Management 2008;36:126‐40. [DOI] [PubMed] [Google Scholar]
Michal 2013 {published data only}
- Michal M, Simon P, Gori T, König J, Wild PS, Wiltink J, et al. Psychodynamic Motivation and Training program (PMT) for the secondary prevention in patients with stable coronary heart disease: study protocol for a randomized controlled trial of feasibility and effects. Trials 2013;14(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammady 2011 {published data only}
- Mohammady M, Memari A, Shaban M, Mehran A, Yavari P, Salari Far M. Comparing computer‐assisted vs. face to face education on dietary adherence among patients with myocardial infarction. Journal of Hayat 2011;16:77‐85. [Google Scholar]
Moore 2001 {published data only}
- Moore SM, Dolansky MA. Randomized trial of a home recovery intervention following coronary artery bypass surgery. Research in Nursing & Health 2001;24(2):93‐104. [DOI] [PubMed] [Google Scholar]
Murtaugh 2005 {published data only}
- Murtaugh CM. Just‐in‐time evidence‐based e‐mail "reminders" in home health care: impact on nurse practices. Health Services Research 2005;40(3):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolan 2011 {published data only}
- Nolan RP, Upshur RE, Lynn H, Crichton T, Rukholm E, Stewart DE, et al. Therapeutic benefit of preventive telehealth counseling in the Community Outreach Heart Health and Risk Reduction Trial. American Journal of Cardiology 2011;107(5):690‐6. [DOI] [PubMed] [Google Scholar]
Nolan 2012 {published data only}
- Nolan RP, Liu S, Shoemaker JK, Hachinski V, Lynn H, Mikulis DJ, et al. Therapeutic benefit of internet‐based lifestyle counselling for hypertension. Canadian Journal of Cardiology 2012;28:390‐6. [DOI] [PubMed] [Google Scholar]
O'Neil 2011 {published data only}
- O'Neil A, Hawkes AL, Chan B, Sanderson K, Forbes A, Hollingsworth B, et al. A randomised, feasibility trial of a tele‐health intervention for acute coronary syndrome patients with depression ('MoodCare'): Study protocol. BMC Cardiovascular Disorders 2011;11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oranta 2011 {published data only}
- Oranta O, Luutonen S, Salokangas RKR, Vahlberg T, Leino‐Kilpi H. The effects of interpersonal counselling on health‐related quality of life after myocardial infarction. Journal of Clinical Nursing 2011;20:3373‐82. [DOI] [PubMed] [Google Scholar]
Oranta 2012 {published data only}
- Oranta O, Luutonen SR, Vahlberg T, Leino‐Kilpi H. Depression‐focused interpersonal counseling and the use of healthcare services after myocardial infarction. Perspectives in Psychiatric Care 2012;48:47‐55. [DOI] [PubMed] [Google Scholar]
Parekh 2012 {published data only}
- Parekh S, Vandelanotte C, King D, Boyle FM. Design and baseline characteristics of the 10 Small Steps Study: a randomised controlled trial of an intervention to promote healthy behaviour using a lifestyle score and personalised feedback. BMC Public Health 2012;12:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park LG, Howie‐Esquivel J, Chung ML, Dracup K. A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Education and Counseling 2014;94(2):261‐8. [DOI] [PubMed] [Google Scholar]
Pogosova 2008 {published data only}
- Pogosova GV, Kalinina AM, Spivak EI, Nazarkina VA, Pogosova GV, Kalinina AM, et al. Efficacy of an educational preventive technology in patients with stable angina in ambulatory conditions. Kardiologiia 2008;48(7):4‐9. [PubMed] [Google Scholar]
Richardson 2010 {published data only}
- Richardson CR, Buis LR, Janney AW, Goodrich DE, Sen A, Hess ML, et al. An online community improves adherence in an internet‐mediated walking program. Part 1: results of a randomized controlled trial. Journal of Medical Internet Research 2010;12(4):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollman 2009 {published data only}
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone‐delivered collaborative care for treating post‐CABG depression: a randomized controlled trial. JAMA 2009;302(19):2095‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2004 {published data only}
- Ross SE, Moore LA, Earnest MA, Wittevrongel L, Lin CT. Providing a web‐based online medical record with electronic communication capabilities to patients with congestive heart failure: randomized trial. Journal of Medical Internet Research 2004;6(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 1997 {published data only}
- Rossi RA, Every NR. A computerized intervention to decrease the use of calcium channel blockers in hypertension. Journal of General Internal Medicine 1997;12(11):672‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruffin 2011 {published data only}
- Ruffin MT, Nease DE Jr, Sen A, Pace WD, Wang C, Acheson LS, et al. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Annals of Family Medicine 2011;9(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saffi 2014 {published data only}
- Saffi MA, Polanczyk CA, Rabelo‐Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: A randomized clinical trial. European Journal of Cardiovascular Nursing 2014;13(5):436–43. [DOI] [PubMed] [Google Scholar]
Scalvini 2009 {published data only}
- Scalvini S, Zanelli E, Comini L, Dalla T, Scalvini S. Home‐based exercise rehabilitation with telemedicine following cardiac surgery. Journal of Telemedicine and Telecare 2009;15(6):297‐301. [DOI] [PubMed] [Google Scholar]
Schweier 2014 {published data only}
- Schweier R, Romppel M, Richter C, Hoberg E, Hahmann H, Scherwinski I, et al. A web‐based peer‐modeling intervention aimed at lifestyle changes in patients with coronary heart disease and chronic back pain: sequential controlled trial. Journal of Medical Internet Research 2014;16(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequist 2005 {published data only}
- Sequist TD, Gandhi TK, Karson AS, Fiskio JM, Bugbee D, Sperling M, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. Journal of the American Medical Informatics Association 2005;12(4):431‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2010 {published data only}
- Sheridan SL, Griffith JM, Behrend L, Gizlice Z, Jianwen C, Pignone MP. Effect of adding a values clarification exercise to a decision aid on heart disease prevention: a randomized trial. Medical Decision Making 2010:E28‐39. [DOI] [PMC free article] [PubMed]
Sheridan 2011 {published data only}
- Sheridan SL, Draeger LB, Pignone MP, Keyserling TC, Simpson RJ, Rimer B, et al. A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies. BMC Health Services Research 2011;11:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2011 {published data only}
- Stewart S, Carrington MJ, Marwick T, Davidson PM, MacDonald P, Horowitz J, et al. The WHICH? Trial: Rationale and design of a pragmatic randomized, multicentre comparison of home‐ vs. clinic‐based management of chronic heart failure patients. European Journal of Heart Failure 2011;13:909‐16. [DOI] [PubMed] [Google Scholar]
Thompson 2008 {published data only}
- Thompson D. Telehome monitoring reduced readmissions and improved quality of life in heart failure or angina. Evidence Based Nursing 2008;11(3):86. [DOI] [PubMed] [Google Scholar]
Thomsen 2001 {published data only}
- Thomsen TF, Davidsen M, Ibsen H, Jorgensen T, Jensen G, Borch‐Johnsen K, et al. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD and the Copenhagen Risk Score. [Erratum appears in J Cardiovasc Risk 2001 Dec;8(6):391]. Journal of Cardiovascular Risk 2001;8(5):291‐7. [DOI] [PubMed] [Google Scholar]
Vandelanotte 2010 {published data only}
- Vandelanotte C, Dwyer T, Itallie A, Hanley C, Mummery WK. The development of an internet‐based outpatient cardiac rehabilitation intervention: a Delphi study. BMC Cardiovascular Disorders 2010;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verheijden 2004 {published data only}
- Verheijden M, Bakx JC, Akkermans R, Hoogen, Godwin NM, Rosser W, et al. Web‐based targeted nutrition counselling and social support for patients at increased cardiovascular risk in general practice: Randomized controlled trial. Journal of Medical Internet Research 2004;6(4):31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakefield 2008 {published data only}
- Wakefield BJ. Nurse and patient communication profiles in a home‐based telehealth intervention for heart failure management. Patient Education and Counseling 2008;71(2):292. [DOI] [PubMed] [Google Scholar]
Waldmann 2008 {published data only}
- Waldmann A, Katalinic A, Schwaab B, Richardt G, Sheikhzadeh A, Raspe H, et al. The TeleGuard trial of additional telemedicine care in CAD patients. 2 Morbidity and mortality after 12 months. Journal of Telemedicine and Telecare 2008;14(1):22‐6. [DOI] [PubMed] [Google Scholar]
Waldron 2010 {published data only}
- Waldron CA, Gallacher J, Weijden, Newcombe R, Elwyn G. The effect of different cardiovascular risk presentation formats on intentions, understanding and emotional affect: a randomised controlled trial using a web‐based risk formatter (protocol). BMC Medical Informatics and Decision Making 2010:41. [DOI] [PMC free article] [PubMed]
Wister 2007 {published data only}
- Wister A, Loewen N, Kennedy‐Symonds H, McGowan B, McCoy B, Singer J, et al. One‐year follow‐up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ Canadian Medical Association Journal 2007;177(8):859‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodend 2008 {published data only}
- Woodend AK, Sherrard H, Fraser M, Stuewe L, Cheung T, Struthers C, et al. Telehome monitoring in patients with cardiac disease who are at high risk of readmission. Heart & Lung 2008;37(1):36‐45. [DOI] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu CJ, Chang AM, Courtney M, Ramis MA. Using user‐friendly telecommunications to improve cardiac and diabetes self‐management programme: A pilot study. Journal of Evaluation in Clinical Practice 2012;18:695‐7. [DOI] [PubMed] [Google Scholar]
Yehle 2012 {published data only}
- Yehle KS, Chen AM, Plake KS, Yi JS, Mobley AR. A qualitative analysis of coronary heart disease patient views of dietary adherence and web‐based and mobile‐based nutrition tools. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(4):203‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Dale 2014 {published data only}
- Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self‐management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials 2014;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN29243064 {unpublished data only}
- ISRCTN29243064. Effectiveness of a comprehensive telerehabilitation program for the heart. http://www.controlled‐trials.com/ISRCTN29243064 (accessed January 2015).
NCT02228603 {unpublished data only}
- NCT02228603. How to enhance physical activity after cardiac rehabilitation? A randomised controlled study comparing two follow‐up training exercise programs. https://clinicaltrials.gov/show/NCT02228603 (accessed January 2015).
NCT02350192 {unpublished data only}
- NCT02350192. Effectiveness of an e‐health educational intervention for cardiovascular disease adults in improving total exercise and outcomes. https://clinicaltrials.gov/ct2/show/NCT02350192 (accessed January 2015).
Redfern 2014 {published data only}
- Redfern J, Usherwood T, Harris MF, Rodgers A, Hayman N, Panaretto K, et al. A randomised controlled trial of a consumer‐focused e‐health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study protocol. BMJ Open 2014; Vol. 4, issue 2. [DOI: 10.1136/bmjopen-2013-004523] [DOI] [PMC free article] [PubMed]
Reinwand 2013 {published data only}
- Reinwand D, Kuhlmann T, Wienert J, Vries HD, Lippke S. Designing a theory‐ and evidence‐based tailored eHealth rehabilitation aftercare program in Germany and the Netherlands: study protocol. BMC Public Health 2013;13:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2011 {published data only}
- Shah B, Adams M, Peterson E, Powers B, Oddone E, Royal K, et al. Secondary Prevention Risk Interventions Via Telemedicine and Tailored Patient Education (SPRITE): A randomized trial to improve postmyocardial infarction management. Circulation: Cardiovascular Quality and Outcomes 2011;4(2):235‐42. [DOI] [PubMed] [Google Scholar]
Additional references
Beswick 2004
- Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under‐represented groups. Health Technology Assessment 2004;8(41). [DOI] [PubMed] [Google Scholar]
Ciccolo 2008
- Ciccolo JT, Lewis B, Marcus B. Internet‐based physical activity interventions. Current Cardiovascular Risk Reports 2008;2(4):299‐304. [Google Scholar]
Civljak 2010
- Civljak M, Sheikh A, Stead LF, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007078.pub3] [DOI] [PubMed] [Google Scholar]
Clark 2010
- Clark AM, Haykowsky M, Kryworuchko J, MacClure T, Scott J, DesMeules M, et al. A meta‐analysis of randomized control trials of home‐based secondary prevention programs for coronary artery disease. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17(3):261‐70. [DOI] [PubMed] [Google Scholar]
Cole 2011
- Cole JA, Smith SM, Hart N, Cupples ME. Systematic review of the effect of diet and exercise lifestyle interventions in the secondary prevention of coronary heart disease. Cardiology Research and Practice 2011;2011:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Critchley 2003
- Critchley JA, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003041.pub2] [DOI] [PubMed] [Google Scholar]
Davies 2012
- Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta‐analysis of internet‐delivered interventions to increase physical activity levels. International Journal of Behavioral Nutrition and Physical Activity 2012;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vos 2013
- Vos C, Li X, Vlaenderen I, Saka O, Dendale P, Eyssen M. Participating or not in a cardiac rehabilitation programme: factors influencing a patient’s decision. European Journal of Preventive Cardiology 2013;20(2):341‐8. [DOI] [PubMed] [Google Scholar]
Dutton 2013
- Dutton WH, Blank G, Groselj D. Cultures of the Internet: the Internet in Britain. Oxford Internet Survey 2013. http://oxis.oii.ox.ac.uk/wp‐content/uploads/sites/43/2014/11/OxIS‐2013.pdf (accessed May 2015).
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eng 1999
- Eng TR, Gustafson DH, Henderson J, Jimison H, Patrick K. Introduction to evaluation of interactive health communication applications. Science Panel on Interactive Communication and Health. American Journal of Preventive Medicine 1999;16(1):10‐5. [DOI] [PubMed] [Google Scholar]
European Society of Cardiology 2012
- European Society of Cardiology 2012. European cardiovascular disease statistics 2012 edition. http://www.escardio.org/static_file/Escardio/Press‐media/press‐releases/2013/EU‐cardiovascular‐disease‐statistics‐2012.pdf (accessed May 2015).
Eysenbach 2001
- Eysenbach G. What is e‐Health?. Journal of Medical Internet Research 2001;3(2):E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2006
- Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the Internet? A systematic review of the published literature. Journal of Medical Internet Research 2006;8(2):E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2011
- Heran BS, Chen JMH, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lavie 2009
- Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clinic Proceedings 2009;84:373‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liang 2006
- Liang H, Xue Y, Berger BA. Web‐based intervention support system for health promotion. Decision Support Systems 2006;42(1):435‐49. [Google Scholar]
McAlister 2001
- McAlister FA, Lawson FM, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. BMJ 2001;323(7319):957‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKee 2013
- McKee G, Biddle M, O'Donnell S, Mooney M, O'Brien F, Moser DK. Cardiac rehabilitation after myocardial infarction: What influences patients’ intentions to attend?. European Journal of Cardiovascular Nursing 2013. [DOI: 10.1177/1474515113496686] [DOI] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81‐95. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore TJ, Alsabeeh N, Apovian CM, Murphy MC, Coffman GA, Cullum‐Dugan D, et al. Weight, blood pressure, and dietary benefits after 12 months of a web‐based nutrition education program (DASH for Health): longitudinal observational study. Journal of Medical Internet Research 2008;10(4):E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 2013
- Munro J, Angus N, Leslie SJ. Patient focused Internet‐based approaches to cardiovascular rehabilitation – a systematic review. Journal of Telemedicine and Telecare 2013;19(6):347‐53. [DOI] [PubMed] [Google Scholar]
Murray 2008
- Murray E. Internet‐delivered treatments for long‐term conditions: strategies, efficiency and cost‐effectiveness. Expert Review of Pharmacoeconomics & Outcomes Research 2008;8(3):261‐72. [DOI] [PubMed] [Google Scholar]
NACR 2014
- British Heart Foundation. The national audit of cardiac rehabilitation: annual statistical report. http://www.cardiacrehabilitation.org.uk/docs/2014.pdf (accessed 7 April 2015).
Neubeck 2009
- Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. European Journal of Cardiovascular Prevention and Rehabilitation 2009;16(3):281‐9. [DOI] [PubMed] [Google Scholar]
Neubeck 2010
Neubeck 2012
- Neubeck L, Freedman SB, Clark AM, Briffa T, Bauman A, Redfern J. Participating in cardiac rehabilitation: a systematic review and meta‐synthesis of qualitative data. European Journal of Preventive Cardiology 2012;19(3):494‐503. [DOI] [PubMed] [Google Scholar]
Neville 2009
- Neville LM, O'Hara B, Milat AJ. Computer‐tailored dietary behaviour change interventions: a systematic review. Health Education Research 2009;24(4):699‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2004
- Nguyen HQ, Carrieri‐Kohlman V, Rankin SH, Slaughter R, Stulbarg MS. Internet‐based patient education and support interventions: a review of evaluation studies and directions for future research. Computers in Biology and Medicine 2004;34(2):95‐112. [DOI] [PubMed] [Google Scholar]
O'Connor 1989
- O'Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger R, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989;80(2):234‐44. [DOI] [PubMed] [Google Scholar]
O'Driscoll 2007
- O'Driscoll JM, Shave R, Cushion CJ. A National Health Service Hospital's cardiac rehabilitation programme: a qualitative analysis of provision. Journal of Clinical Nursing 2007;16(10):1908‐18. [DOI] [PubMed] [Google Scholar]
Oldridge 1988
- Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: combined experience of randomized clinical trials. JAMA 1988;260(7):945‐50. [PubMed] [Google Scholar]
Sternfeld 2009
- Sternfeld B, Block C, Quesenberry CP Jr, Block TJ, Husson G, Norris JC, et al. Improving diet and physical activity with ALIVE: a worksite randomized trial. American Journal of Preventive Medicine 2009;26(6):475‐83. [DOI] [PubMed] [Google Scholar]
Taylor 2010
- Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wantland 2004
- Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web‐based vs. non‐web‐based interventions: a meta‐analysis of behavioral change outcomes. Journal of Medical Internet Research 2004;6(4):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whalley 2011
- Whalley B, Rees K, Davies P, Bennett P, Ebrahim S, Liu Z, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD002902] [DOI] [PubMed] [Google Scholar]
Widmer 2015
- Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta‐analysis. Mayo Clinic Proceedings 2015;90(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


