Abstract
Background
Scabies, caused by Sarcoptes scabiei variety hominis or the human itch mite, is a common parasitic infection. While anyone can become infected, it causes significant morbidity in immunocompromised hosts and it spreads easily between human hosts where there is overcrowding or poor sanitation. The most common symptom reported is itch which is worse at night. As the symptoms are attributed to an allergic reaction to the mite, symptoms usually develop between four to six weeks after primary infection. Therefore, people may be infected for some time prior to developing symptoms. During this time, while asymptomatic, they may spread infection to others they are in close contact with. Consequently, it is usually recommended that when an index case is being treated, others who have been in close contact with the index case should also be provided with treatment.
Objectives
To assess the effects of prophylactic interventions for contacts of people with scabies to prevent infestation in the contacts.
Search methods
We searched electronic databases (Cochrane Occupational Safety and Health Review Group Specialised Register, CENTRAL (The Cochrane Library), MEDLINE (Ovid), Pubmed, EMBASE, LILACS, CINAHL, OpenGrey and WHO ICTRP) up to November 2013.
Selection criteria
Randomised controlled trials (RCTs) or cluster RCTs which compared prophylactic interventions which were given to contacts of index cases with scabies infestation. Interventions could be compared to each other, or to placebo or to no treatment. Both drug treatments and non‐drug treatments were acceptable.
Data collection and analysis
Two authors intended to extract dichotomous data (developed infection or did not develop infection) for the effects of interventions and report this as risk ratios with 95% confidence intervals. We intended to report any adverse outcomes similarly.
Main results
We did not include any trials in this review. Out of 29 potentially‐relevant studies, we excluded 16 RCTs as the data for the contacts were either not reported or were reported only in combination with the outcomes for the index cases. We excluded a further 11 studies as they were not RCTs. We also excluded one study as not all subjects were examined at baseline and follow‐up, and another as it was a case study.
Authors' conclusions
The effects of providing prophylactic treatments for contacts of people with scabies to prevent infestation are unknown. We need well‐designed RCTs of the use of prophylactic measures to prevent the transmission of scabies conducted with people who had the opportunity for prolonged skin contact with an index case, such as family members, healthcare workers or residential care personnel, within the previous six weeks.
Keywords: Humans, Scabies, Scabies/prevention & control, Scabies/transmission
Plain language summary
Interventions for preventing the spread of infestation in close contacts of people with scabies
Background
Scabies is a common parasitic infection. It is caused by a mite, Sarcoptes scabiei variety hominis, also known as the human itch mite, which depends on humans to survive. Crusted scabies (or Norwegian scabies) is caused by the same mite, but tends to occur in people whose immune system is not working so well, such as transplant patients on immunosuppressive therapy, people who misuse alcohol, or other debilitated people. Scabies infection spreads from person to person by skin contact. This is why it is more prevalent in areas with poor sanitation or overcrowding. In high‐income countries it tends to spread between family contacts, between people in residential care, or between patients and staff in hospitals. People may be infected with these mites for several weeks before developing symptoms. During this time it is possible to spread the infection to other people. Consequently people who are in contact with suspected cases of scabies infection are often given preventative treatments in an attempt to stop the development of symptoms. Preventive treatment also aims to prevent further spread of the infection and to prevent the person who was the source of infection from getting reinfected. This review is important, as before conducting this review we were unable to say if using preventive treatment helps or not.
What does the research say?
We searched for studies in which people who had been in contact with scabies‐infected people had been given medical treatment, or had been advised about personal hygiene to prevent the scabies infection from spreading. We also wanted studies to have been designed so that the treatment received by participants (either medication or advice) was determined by chance. We did not find any studies fulfilling these criteria.
Conclusions
There is currently no evidence to say if treating or advising people who have been in contact with scabies‐infected people is effective in preventing the spread of scabies infection. We need researchers to conduct studies with people who may have been in skin contact with a person who has been diagnosed with a scabies infection within the previous six weeks. Half of these people should be given preventive treatment and the other half something else. Who gets what should be determined by chance so that the two groups are truly similar in every respect except the treatment they receive.
Background
Description of the condition
Scabies is a common parasitic infection. It is present worldwide and up to 300 million cases are thought to occur each year (Chosidow 2006). It is caused by a mite, Sarcoptes scabiei variety hominis (Green 1989), also known as the human itch mite. Crusted scabies (or Norwegian scabies) is caused by the same mite, but is associated with infestation with a very large number of mites and tends to occur in immunocompromised people, e.g. transplant patients on immunosuppressive therapy, people who misuse alcohol, or other debilitated people.
The Sarcoptes scabiei mite depends on humans for survival, and infection occurs by human to human spread (Hengge 2006). Thus, it tends to be more prevalent in areas with poor sanitation or in circumstances in which there is frequent, close person to person contact, as in overcrowding. In developed countries where sanitation problems and overcrowding are not as prevalent, it tends to spread between family contacts, between people in residential care, or between patients and staff in hospitals.
Mites crawl on the skin surface at approximately 2.5 cm per minute (Hengge 2006) but can neither fly nor jump. They can survive three to four days off the host, depending on environmental conditions (Green 1989). Mating occurs on the skin surface (Chouela 2002), before the gravid female mites burrow into the skin (Chosidow 2006), where eggs are laid. Several days later, the eggs hatch and nymphs emerge. Three moults are required before the mite reaches maturity (Chouela 2002).
Direct skin to skin contact is required for transmission to occur. Whether transmission can occur via fomites (object or substance capable of carrying infectious organisms, for example, clothing, bedding, etc.) is uncertain and conflicting opinions exist (Arlian 1988; Blumenthal 1976; Chosidow 2006; Chouela 2002; Orion 2006). Individuals with classical scabies are typically infected with up to 50 mites (Orion 2006). In immunocompromised hosts, however, crusted scabies can develop and the person is likely to be infected with a minimum of several thousand mites. Crusted scabies is thought to be more infective than classical scabies. This higher infectivity is attributed to the higher 'volume' of infection, and the increased shedding of skin scales which carry the mites, which some believe could facilitate the spread of infection via fomites (Arlian 1989; Chosidow 2006).
Itch is the most prominent symptom of scabies, although this is often limited in immunocompromised hosts with crusted scabies (Scheinfeld 2004). The itch tends to be worse at night (Green 1989). Those affected can develop a cutaneous eruption, consisting of a variety of lesions, over most of the body. Some patients may develop secondary bacterial infections, such as impetigo, as a consequence of the disrupted skin barrier from scratching due to the profound itch. In crusted scabies, patients typically develop a psoriatic‐type eruption, which can be present on the hands, feet, trunk and face (Orion 2006).
The symptoms experienced are generally attributed to the development of an allergic reaction to the mite or its excreta. Consequently, symptoms are not likely to develop until four to six weeks after primary infection (Green 1989). On subsequent infections, symptoms generally develop within hours to days (Chouela 2002). Additionally, successful treatment does not always result in elimination of symptoms until several weeks later, as the hypersensitive response to the mite or its products (i.e. post‐scabetic itch) can persist, even though neither the mite nor its products remain.
On examination, lesions may be noted in particular in the finger web spaces, on the elbows, in the axilla, on breasts and on the buttocks and genitalia. Burrows, nodules and vesicles may be seen. In adults, lesions do not generally occur above the neck. In young children and in vulnerable populations, lesions can occur above the neck, and mites can occasionally be observed in the retroauricular fold (Chouela 2002). Skin scales are commonly associated with crusted scabies.
Skin scrapings may facilitate direct observation of mites, eggs, or mite faeces pellets (Chosidow 2006; Hengge 2006). This is achieved by applying a drop of mineral oil to the suspected lesion, then using a scalpel blade to scrape away the oil and the entire lesion, which are transferred onto a slide for microscopic examination (Chouela 2002). Alternatively, a shave biopsy can be performed, whereby the top of the papule is removed and placed on a microscopic slide for further examination (Chouela 2002). Another option is the 'burrow ink test' which depends on the burrows absorbing ink (Hengge 2006).
There are several recommended treatments for scabies. These have been extensively discussed in another Cochrane review (Strong 2007). Both oral (e.g. ivermectin, thiabendazole, flubendazole) and topical therapies (e.g. lindane, permethrin, sulphur‐containing products, crotamiton, malathion, benzyl benzoate) are available (Chouela 2002; Scheinfeld 2004). Oral ivermectin (Guay 2004) is not widely available and in some jurisdictions it has not been approved for the treatment of scabies (Bouvresse 2010). The usual treatments are topical, and typically require application over all of the body for many hours duration. There is no international consensus on the appropriate schedule of treatment, and recommendations in one jurisdiction may not be appropriate in others (Bouvresse 2010). Multiple treatment doses are often recommended over days to weeks. Some patients require symptomatic treatment for the itch, including post‐scabetic itch or itch caused by medication. Antihistamines and emollients have been recommended in this regard (Chouela 2002).Topical or systemic antibiotics may be required if secondary skin infection has developed.
It is also advised that close contacts of people with scabies should be treated simultaneously (Chouela 2002; Paasch 2000; Scheinfeld 2004), as they may be infected without yet manifesting symptoms, and so act as a reservoir of infection. Treating the contacts may prevent reinfection of the index case following treatment. Although the treatments used are generally safe, allergies to treatment are possible, and adverse events including death have been reported (Nolan 2011). The logistics required to treat all contacts simultaneously are considerable (Scheinfeld 2004; Stoevesandt 2012). For example, this would be very difficult to co‐ordinate in an institutional setting where, along with the index case, other patients, family members and all staff who had contact with the index case are all advised to also have treatment.
There has been some success with the provision of scabies treatment for the whole community in settings where there is a high prevalence of scabies (Carapetis 1997). Such community initiatives have assisted in the eradication of scabies, but require screening, health education regarding the risk of scabies infestation, provision of drug treatments, and advice and support with non‐drug treatments (Kanaaneh 1976). Consequently, guidelines have been developed in some areas that recommend community‐wide treatment to control scabies (Currie 2000).
Description of the intervention
Following contact with an index case, where the contact has not been infected with scabies previously, symptoms often take up to four to six weeks to develop. During this long incubation period, the contact may act as a reservoir for onward infection to their contacts (Green 1989), or may cause re‐infection in the index case (Buehlmann 2009).
It is generally recommended that along with the index case, contacts of the index case should be considered for prophylactic treatment (Chouela 2002; Scheinfeld 2004). This recommendation is made for several reasons:
infection may have been transmitted to contacts who may remain asymptomatic but develop symptoms at a later stage,
untreated contacts may act as a reservoir of infection and may re‐infect the index case, and
untreated contacts may be a source of onward transmission of infection to others.
Onward transmission to others would be particularly problematic in healthcare or residential settings, where infection may be spread to vulnerable patients. Additionally, where employees are infected, they may require restriction from work until treatment has been initiated to limit the chance of onward transmission. This has implications for staffing levels and the workforce in general (Bouvresse 2010).
Generally, contacts of the index case are prescribed treatment (either the same treatment as the index case, or a shorter regimen, or a different treatment), and are provided with advice regarding washing of clothes and bedding (Buehlmann 2009).
How the intervention might work
Treating the contacts of the index case potentially limits the development of infection (both asymptomatic and symptomatic) in the contacts of the index case, restricts onward transmission of infection to others and prevents re‐infection of the index case (Chouela 2002). This is particularly important in settings where there are a large number of people in close proximity to each other or in settings where there are vulnerable populations, such as nursing homes, residential care homes, or other healthcare settings.
Why it is important to do this review
Prophylactic interventions continue to be recommended for contacts of index cases, including family contacts, residential or institutional contacts, and healthcare exposures, where skin to skin contact or contact with fomites may facilitate the transmission of the infection. The level of exposure of the contact to an index case in these settings, however, is subject to considerable variation: for example, shaking hands; cuddling a baby for a prolonged period; assisting a nursing home resident with bathing and dressing; sexual contact; holding hands; and children playing sports together.
It is not clear whether prophylaxis is more appropriate than a 'wait and see' approach (Chouela 2002), whereby contacts are educated regarding the possibility of infection and advised to seek medical attention should they develop symptoms suggestive of infection.
Concerns regarding prophylaxis include:
considerable commitment on the part of the exposed contacts of index cases of scabies and their required willingness to undergo treatment (Buehlmann 2009),
recommending prophylaxis where the contact may not be able to describe the level of contact they had with the index case, or may not be able to consent to treatment (Ejidokun 2007),
side‐effects associated with some of the treatments recommended, some of which are serious (Bouvresse 2010),
the possibility of resistance to anti‐scabietic treatments (Chouela 2002; Currie 2004),
the stigma associated with a diagnosis of scabies, which may lead to non‐compliance and a reluctance to disclose the diagnosis to close contacts (Heukelbach 2006), (as society frequently associates scabies with poor hygiene and poverty),
considerable cost associated with providing medical treatment to contacts (e.g. a whole family, other patients and staff in a residential care setting) (Vorou 2007), and
logistical difficulties when trying to identify all contacts of an index case (e.g. a child with scabies infestation may attend school, avail of after‐school care and be involved in other recreational activities) (Buehlmann 2009).
The results of this review were expected to influence occupational health policy and practice in particular, in the treatment of contacts of scabies in healthcare and residential care settings, and possibly be wider reaching, for example, school and prison workers.
This review aimed to summarise the effectiveness and safety of prophylactic treatment in various settings and will be updated as further new evidence becomes available.
Objectives
To assess the effects of using prophylactic interventions for contacts of people with scabies, in order to prevent the development of symptoms of infestation in the contacts.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include in this review all randomised controlled trials (RCTs) in which prophylactic interventions (both drug treatments and non‐drug treatments) were compared with another treatment or with no treatment or placebo treatment. In addition, we also planned to include any cluster‐RCTs, where groups of individuals were randomised to receive treatment.
Types of participants
We intended to include trials involving people who had had contact with an index case with scabies infestation within the previous six weeks. Index case infestation may have occurred either sporadically or in an outbreak setting.
Most people who develop scabies infestation develop the symptoms within six weeks of exposure to an index case. Therefore there is likely to be minimal benefit in treating contacts of index cases who were exposed more than six weeks previously but who had not yet developed symptoms.
The type of contact we were interested in was that where scabies transmission could have occurred, i.e. where there was the potential for prolonged skin to skin contact between the index case and the 'contact' person. This would include (but not necessarily be limited to) contact between family members residing together, other household contacts, contact between children and other children or workers in childcare centres, contact between patients and other patients or workers in hospitals, or contact between residents and employees in residential care settings. Casual interactions, such as when shaking hands, where prolonged skin to skin contact would not be expected, would not be considered to pose a risk of scabies transmission and therefore would not be considered relevant.
Diagnosis of the index case must have been made by a physician, or other suitably‐qualified healthcare professional, in those with symptoms suggestive of infection (e.g. itch that is worse at night), and either a positive dermatological examination (burrows, papules, vesicles), or a positive microscopic parasitological examination.
The population of interest was people of all ages, both male and female.
We excluded studies where:
greater than 10% of the study population had symptoms suggestive of scabies infestation prior to the administration of prophylaxis, or
study participants were diagnosed with and treated for scabies within three months prior to the reported exposure (to limit the potential of including people with possible treatment failure).
Types of interventions
We intended to include all studies where a prophylactic intervention was recommended for people (contacts) who were exposed to an index case of scabies.
Prophylactic interventions could have included medical treatment (medication which would generally be prescribed as a treatment for scabies) and non‐medical recommendations.
We planned to group the non‐medical interventions according to their working mechanism, for example:
barrier precautions (including patient isolation, patient cohorting, etc.),
personal hygiene measures (including hand washing), and
environmental decontamination (including advice to wash clothing and bedding).
Trials could have included one treatment or combinations of treatments versus placebo, no treatment, or other treatments.
Types of outcome measures
We were interested in the following outcome measures:
Primary outcomes
The primary outcome was the proportion of contacts (of index cases) who were diagnosed with scabies where the contacts of the index cases were advised to use some prophylactic treatment. The contacts of the index cases should have been examined for scabies within eight weeks of being recommended to use the prophylactic treatment. If contacts were examined more than eight weeks after using the prophylactic treatment, any positive diagnosis could have been due to an exposure to an index case during the intervening period; such diagnoses would bias the results. Diagnosis of scabies in contacts was based on the clinical opinion of a physician or other suitably‐qualified health professional, on the basis of the development of clinical symptoms suggestive of scabies and either positive physical examination findings or positive microscopy.
Secondary outcomes
Secondary outcomes consisted of reported adverse events in people treated with prophylaxis which is attributed to the prophylactic treatment. These included:
serious adverse events e.g. toxicity, hospital admission, fatality,
minor adverse events e.g. transient skin irritation.
Other secondary outcomes of interest in this review included:
patient acceptability e.g. complaints regarding application, and
compliance.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from the first day of entries to November 2013:
Cochrane Occupational Safety and Health Review Group Specialised Register
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library)
MEDLINE (Ovid);
Pubmed;
EMBASE (embase.com);
LILACS (Latin‐American and Caribbean Center on Health Sciences Information, via http://lilacs.bvsalud.org/en/);
CINAHL (Cumulative Index to Nursing and Allied Health Literature via EBSCO);
OpenGrey (System for Information on Grey Literature in Europe via www.opengrey.eu), and
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform via http://www.who.int/ictrp/en/).
The MEDLINE search strategy presented in Appendix 1 used the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy (Higgins 2011). We modified this strategy for use in the other databases (Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9). We did not limit the search by language.
Searching other resources
We scanned the reference lists of all potentially‐relevant studies for possible additional studies. We did not undertake any additional handsearching of journals.
Data collection and analysis
Selection of studies
Two review authors (DF and RG) were involved in the study selection. Where it was clear from the title or abstract that a study report did not include details of a study that involved treating asymptomatic contacts of an index patient, we excluded the study. The two review authors formed their opinions as to whether the study met the pre‐defined criteria independently. Where any differences of opinions arose, a third review author (AR) made the final decision. With non‐English language articles, we would have had enough details translated into English to determine eligibility for inclusion.
We excluded articles where it was clear from the title or abstract that:
the study was not an RCT, or
the trial did not provide treatment to contacts of an index case of appropriately diagnosed scabies infection, or
the trial did not investigate the incidence of scabies in those contacts who were treated with prophylactic treatment.
Data extraction and management
Two review authors (DF and RG) intended to independently extract the required data from the selected studies, and intended to record these data on an agreed data collection form. One review author (DF) generated the data collection form based on a template Cochrane Data Collection Form. Two review authors (DF and RG) would have piloted the data collection form regarding its' applicability and ease of use for three studies, before proceeding to extract the data from the remaining studies. Where there was a difference of opinion regarding the extracted data, we intended to seek further advice from a third review author (AR). We intended to compile data from multiple articles regarding the same study (if required) to ensure complete data extraction. One review author (DF) intended to enter numerical results data into Review Manager 5.2 software (RevMan 2012) and it was planned that a second review author (RG) would check the data.
Assessment of risk of bias in included studies
Two review authors (DF and RG) intended to independently assess the risk of bias of included studies.
We intended to judge the risk of bias of the included RCTs using the Cochrane Collaboration’s tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to grade each study for risk of bias in each of the following seven domains, with ratings of low risk of bias, high risk of bias or uncertain risk of bias.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other sources of bias
A third review author (AR) would have resolved any disagreements.
We acknowledged that it may be difficult to blind participants for some prophylactic treatments (both drug treatments and non‐drug treatments). We planned on discussing the blinding applied in the included studies in our review, including details of who of the participants or personnel were blinded, and if we thought it had a significant effect on the risk of bias.
Measures of treatment effect
In order to assess the effectiveness of the different prophylactic interventions, we planned to extract dichotomous data from the included studies (i.e. did or did not develop infection). We intended then to undertake a meta‐analysis of the dichotomous data and express the results as risk ratios (RRs) with 95% confidence intervals (CIs).
We would have also recorded the occurrence of adverse events as dichotomous data, and would then have calculated RRs (with 95% CIs) for each adverse outcome associated with each prophylactic treatment used.
Unit of analysis issues
We anticipated that some of the studies may have used a cluster‐RCT study design. Statistical errors can occur in cluster‐RCTs when the clustering has not been taken into account in the analysis (Eldridge 2004). Therefore, if any cluster‐RCTs met our inclusion criteria, we intended to assess the trials for unit of analysis error; if necessary we would have re‐analysed outcomes as per the methods outlined in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011), and reported this in our review. Where clustering had not been considered, and it was not possible to re‐analyse the results, we expected to also report this in our review.
Dealing with missing data
If data appeared to be missing, we would have sought to obtain the missing data through correspondence with the study authors. If the authors could not provide the missing data we intended to try to calculate the necessary values ourselves, such as standard deviations (SDs) from P values or CIs, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If this was not possible, we expected to use imputation methods to find the data required for meta‐analysis. For example we would have used Last Observation Carried Forward (LOCF), where the most recently observed outcome measure would have been assumed to hold for all subsequent assessment times (where the study design included more than one observation time). Alternatively, we would have taken values from other studies. We would have considered the impact of these decisions on the results of the meta‐analysis in a sensitivity analysis.
Assessment of heterogeneity
Two review authors (DF and RG) intended to assess studies for clinical heterogeneity, based on the interventions, control interventions, and outcomes. We would only have conducted meta‐analysis where the study population and interventions were sufficiently similar. Furthermore, heterogeneity would have been assessed using the I2 statistic. If the I2 statistic had been < 50%, we would have used a fixed‐effect model. If the I2 had been > 50% we would have used a random‐effects model.
Assessment of reporting biases
We intended to reduce the effect of reporting bias by including studies and not publications, in order to avoid the introduction of duplicated data (i.e. two articles could have represented duplicate publications of the same study). Following the Cho 2000 statement on redundant publications, we would have attempted to detect duplicate studies and, if more articles had reported on the same study, we would have extracted data only once (using all available data following consideration of all available reports). We attempted to prevent location bias by searching across multiple databases. We attempted to prevent language bias by not excluding any article based on language. If sufficient data had been available, we would have assessed whether there was a potential for publication bias by using a funnel plot.
Data synthesis
We intended to pool data from studies judged to be clinically homogeneous using Review Manager 5.2 software (RevMan 2012). If sufficient data had been available, we would have performed meta‐analyses. If studies had been statistically heterogeneous, we would have used a random‐effects model; otherwise we would have used a fixed‐effect model. When using the random‐effects model, we would have conducted a sensitivity check by using the fixed‐effect model to reveal differences in results. We intended to include a 95% CI for all estimates.
We planned to use the GRADE approach as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and as implemented in the GRADEPro 3.2 software (GRADEpro 2008) to present the quality of evidence and ‘Summary of findings’ tables.
The downgrading of the quality of a body of evidence for a specific outcome would have been based on five factors:
Limitations of study.
Indirectness of evidence.
Inconsistency of results.
Imprecision of results.
Publication bias.
The GRADE approach specifies four levels of quality (high, moderate, low and very low).
Subgroup analysis and investigation of heterogeneity
If there had been sufficient data we would have performed subgroup analysis to investigate the outcome in various defined populations (household, residential, nosocomial, non‐healthcare workers). Similarly, given sufficient data, we would have performed subgroup analysis of the treatment of contacts that were part of either an outbreak of one sporadic case or an outbreak with multiple cases. In addition, if possible, we would have performed subgroup analysis on groups exposed to crusted (Norwegian) scabies, as opposed to classical scabies.
Sensitivity analysis
We intended to use sensitivity analysis to assess the impact of the following:
decisions regarding the prophylactic dose of drug treatments chosen,
duration of study follow‐up, i.e. the impact of excluding studies that followed‐up contacts for more than eight weeks,
loss to follow‐up data, i.e. the impact of excluding studies with an attrition rate greater than or equal to 20%,
where data were imputed we would have assessed the impact this had on the results, and
blinding status of participants and personnel.
Results
Description of studies
Results of the search
After completing the systematic searches as described in Appendices, we had a total of 2860 references. We found an additional 8 references by scanning the reference lists of potentially‐relevant studies. After removal of duplicates there were 2034 references that we screened for inclusion in duplicate. Based on the title we selected 138 abstracts to be screened again. Of these, we selected 29 articles for full text assessment (Figure 1). The authors of four of these studies (Alrawashdeh 2013; Henderson 1992; Saqib 2012; Zargari 2006) provided additional information to enable us to decide whether to include them.
1.
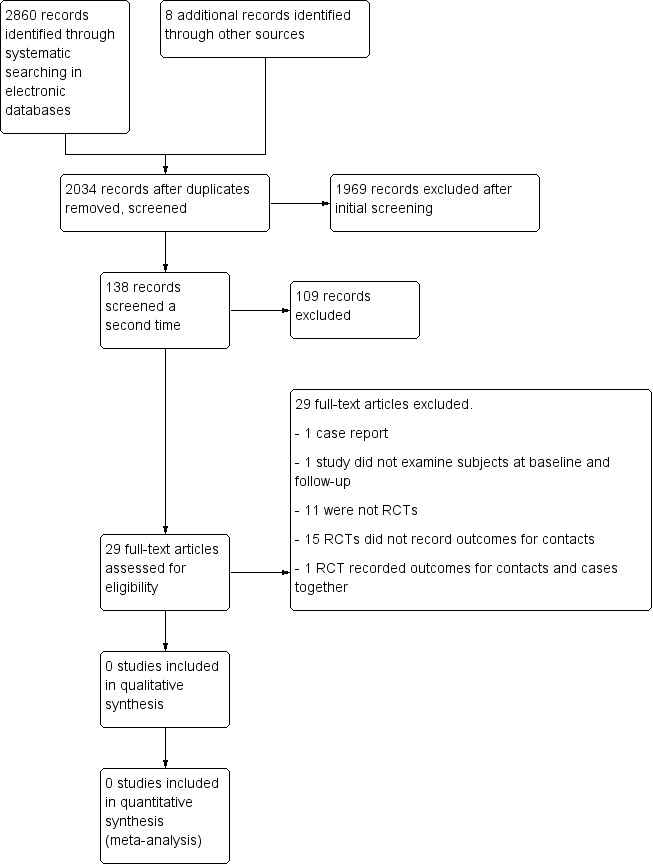
Study flow diagram.
Included studies
None of the 29 studies we considered for inclusion met the review's inclusion criteria. In other words, we did not find any reports of studies involving either medical or non‐medical interventions to prevent the transmission of scabies infestation from index cases to contacts.
Excluded studies
We excluded 16 randomised controlled trials (RCTs) that provided treatment for cases with scabies and also reported providing prophylaxis for their contacts (Alrawashdeh 2013; Avila‐Romay 1991; Bachewar 2008; Burgess 1986; Goldust 2012a; Goldust 2012b; Goldust 2013a; Goldust 2013b; Goldust 2013c; Goldust 2013d; Goldust 2013e; Mohebbipour 2012; Mohebbipour 2013; Saqib 2012; Usha 2000; Zargari 2006). The study by Avila‐Romay 1991 reported on the control of an outbreak but reported the outcomes for cases in combination with the outcomes for contacts so it was not possible to extract any data relating to the contacts alone. The other 15 of these studies (Alrawashdeh 2013; Bachewar 2008; Burgess 1986; Goldust 2012a; Goldust 2012b; Goldust 2013a; Goldust 2013b; Goldust 2013c; Goldust 2013d; Goldust 2013e; Mohebbipour 2012; Mohebbipour 2013; Saqib 2012; Usha 2000; Zargari 2006) provided prophylaxis for family contacts of cases, but did not report the outcomes in the contacts.
We excluded 11 studies as they were not RCTs (Abedin 2007; Bockarie 2000; Ejidokun 2007; Haar 2013; Hench 1994; Henderson 1992; Heukelbach 2004; Paasch 2000; Taplin 1983; Wong 2001; Yonkosky 1990). We also excluded one study (Talukder 2013) as not all of those treated were examined at baseline and follow‐up, and another study (Woltman 1994) because it was a case report.
We present descriptions of all 29 studies with the reasons for exclusion in the Characteristics of excluded studies.
Risk of bias in included studies
We did not conduct 'Risk of bias' assessment as this review contains no included studies.
Effects of interventions
As there were no studies to include there are no effects of interventions to report.
Discussion
Summary of main results
Our systematic search did not identify any randomised controlled trials (RCTs) on the use of prophylactic measures to limit the transmission of scabies in close contacts of people with scabies.
The effects of treatments for close contacts of people infected with scabies for preventing the spread of infestation in contacts is not known.
Overall completeness and applicability of evidence
Currently, there is no evidence of the effects of prophylaxis, either beneficial or adverse, when used for contacts of people with scabies.
Quality of the evidence
We found 15 RCTs (Alrawashdeh 2013; Bachewar 2008; Burgess 1986; Goldust 2012a; Goldust 2012b; Goldust 2013a; Goldust 2013b; Goldust 2013c; Goldust 2013d; Goldust 2013e; Mohebbipour 2012;Mohebbipour 2013; Saqib 2012; Usha 2000; Zargari 2006) in which investigators provided treatment for contacts of cases of scabies but they did not record outcomes in these contacts and one study (Avila‐Romay 1991) that recorded the outcomes for both the cases and their contacts together, so it is not possible to distinguish between the two groups. The existence of RCTs where contacts of index cases with scabies were provided with prophylaxis shows that it is possible to conduct randomised studies in this field. However, as none of the identified RCTs fulfilled the rest of our inclusion criteria we did not conduct a 'Risk of bias' assessment on them.
Potential biases in the review process
We conducted this systematic review closely following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As none of the identified studies fulfilled our inclusion criteria we did not have any decisions to take that might have introduced bias into the review process.
Agreements and disagreements with other studies or reviews
We did not find any other reviews on this topic so we can not compare our findings to previous reviews.
Authors' conclusions
Implications for practice.
Based on the currently available data, this systematic review can provide no recommendations about the use of treatment for close contacts of people with scabies to prevent either infestation, reinfection in the index case or onward transmission to other contacts.
Implications for research.
There is a need for well‐designed randomised controlled trials (RCTs) to provide conclusive evidence on the use of prophylactic measures to prevent the transmission of scabies.
Studies should recruit people who had been in contact with an index case with scabies infestation within the previous six weeks. All people who had substantial opportunity for prolonged skin to skin contact with the index case should be included, e.g. family members, healthcare workers or residential care personnel responsible for personal care of the index case, close friends where the index case is a child, other residents in nursing homes or residential care environments. Given that symptoms of infestation generally develop within the first six weeks after transmission of scabies, there is likely to be little clinical benefit in recommending prophylaxis for contacts of index cases who have not developed symptoms at six weeks after the exposure; therefore contacts who were exposed to an index case greater than six weeks previously should not be included in studies designed to examine any benefit of prophylaxis.
The diagnosis of the index case should be made by a physician, or other suitably‐qualified healthcare professional, in those with symptoms suggestive of infection (e.g. itch that is worse at night), and either a positive dermatological examination (burrows, papules, vesicles), or a positive microscopic parasitological examination. The contacts of the index case should also be examined similarly to exclude the presence of undiagnosed infection in the study participants at the start of the study period. Study participants who have been diagnosed with scabies or who have been treated for scabies infection within the previous three months should be excluded, as it would not be possible to distinguish between a new transmission and treatment failure.
The extent and type of contact between the index case and the study participants should be defined in advance of the start of the study and clearly described in the study protocol. For all study participants, the exposure type and duration should be accurately recorded. Potential study participants who do not meet these pre‐defined exposure criteria should be excluded from the study. Given that durations and types of exposure are likely to vary considerably in different populations (i.e. between family members, in hospitals or residential care facilities, between colleagues), separate studies will be required to determine the implications for prophylaxis accordingly.
Studies should randomise participants, either individually or in clusters, to receive either prophylactic intervention, alternative intervention or placebo. In settings where contacts could have close contact with each other (and therefore provide an opportunity for transmission between contacts), e.g. contacts of index cases in family settings, a cluster randomised controlled design would be more appropriate than randomising individual contacts to different interventions.
Prophylactic interventions should consist of one or more of the following components: medical treatment (with specified type, dose, and regimen); barrier precautions (including patient isolation, patient cohorting, etc.); personal hygiene measures (including hand washing), or environmental decontamination (including advice to wash clothing and bedding).
The effect of the intervention should be measured as the incidence of scabies within eight weeks of being recommended to use the prophylactic treatment. Diagnosis of scabies in the contacts should be based on the clinical opinion of a physician or other suitably‐qualified health professional, on the basis of the development of clinical symptoms suggestive of scabies and either positive physical examination findings or positive microscopy. If contacts were examined more than eight weeks after using the prophylactic intervention, there is an increased chance that other exposures may have occurred in the intervening period and it would not be possible to distinguish between transmission from the first exposure (of interest) and transmission any subsequent exposures. Outcomes in index cases should be reported separately from outcomes in the contacts of the index cases. Adverse outcomes associated with the intervention, such as side‐effects attributed to medication, should be recorded, as should compliance with the recommended treatment.
Acknowledgements
The authors are grateful for the support provided by the Cochrane Occupational Safety and Health Review Group's Co‐ordinating Editor Jos Verbeek, and Managing Editor Jani Ruotsalainen. We are indebted to Ms Leena Isotalo, the Trials Search Co‐ordinator for the Cochrane Occupational Safety and Health Group for her assistance with the searches. We thank Dr Basel Alrawashedh, Dr Catriona Henderson, Dr Munazza Saqib and Dr Omid Zargari for providing additional information about their studies. We also thank the following people for their constructive feedback when we were developing the protocol: Prof Hywel Williams, Dr Mark Strong, Dr Ira Madan, Mr Wim van Veelen, Ms Anneli Ojajärvi and Ms Sharea Ijaz. Constructive feedback from Prof Hywel Williams, Dr Mark Strong, Cindy Stern, Dr Anneli Ojajärvi, Ms Sharea Ijaz, and Dr Ira Madan during the peer review process was also most gratefully received. We thank Heather Maxwell, Megan Prictor and Jani Ruotsalainen for copy editing the text.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. randomised controlled trial.pt
2. controlled clinical trial.pt
3. randomized.ab
4. placebo.ab
5. drug therapy.mp
6. randomly.ab
7. trial.ab
8. groups.ab
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. animals.sh
11. humans.sh
12. 10 and 11
13. 10 not 12
14. 9 not 13
15. Scabies.mp
16. Sarcoptes scabiei.mp
17. Human itch mite.mp
18. (crusted adj6 scabies)
19. (Norwegian adj6 scabies)
20. 15 or 16 or 17 or 18 or 19
21. Prophyla*.mp
22. Prevent*.mp
23. Control*.mp
24. Treat*mp
25. Decontaminat*.mp
26. Therap*.mp
27. Lindane OR hexachlorocyclohexane.mp
28. Permethrin.mp
29. Ivermectin.mp
30. Tetmosol.mp
31. Crotamiton.mp
32. Sulphur.mp
33. Malathion.mp
34. Benzylbenzoate.mp
35. 21 or 22 or …..or 34
36. 14 AND 20 AND 35
Appendix 2. OSH Databases search strategy (International bibliographic, CISDOC, HSELINE, NIOSHTIC, NIOSHTIC‐2, RILOSH archive) (OSH UPDATE)
1. GW{scabies}
2. GW{Sarcoptes scabiei}
3. GW{itch mite*}
4. #1 OR #2 OR #3
5. GW{prophyla* OR prevent* OR control* OR treat* OR decontaminat* OR therap*}
6. GW{lindane OR hexachlorocyclohexane OR permethrin OR ivermectin OR crotamiton OR sulphur OR sulfur OR benzylbenzoate OR benzyl benzoate OR malation OR malathion}
7. #4 AND #5
8. #4 AND #6
9. #7 OR #8
10. DC{OUBIB OR OUCISD OR OUHSEL OR OUNIOC OR OUNIOS OR OURILO}
11. #9 AND #10
Appendix 3. CENTRAL (The Cochrane Library) search strategy
1. (Sarcoptes scabiei)
2. scabies
3. itch mite*
4. (#1 OR #2 OR #3)
5. prophyla*
6. prevent*
7. control*
8. treat*
9. decontaminat*
10. therap*
11. lindane
12. hexachlorohexane
13. permethrin
14. ivermectin
15. crotamiton
16. sulphur OR sulfur
17. malathion OR malation
18. benzylbenzoate
19. #5 OR #6 OR......OR #17 OR #18
20. #4 AND #19
Appendix 4. EMBASE search strategy
1. "clinical trial"/exp
2. "randomisation"/de
3. "single blind procedure"/de
4. "double blind procedure"/de
5. "crossover procedure"/de
6. "placebo"/de
7. "randomized clinical" NEXT/1 trial*
8. Randomised clinical" NEXT/1 trial*
9. rct
10. "random allocation"
11. "randomly allocated"
12. "allocated randomly"
13. allocated NEAR/2 random
14. single NEXT/1 blind*
15. double NEXT/1 blind*
16. (treble OR triple) NEAR/1 blind*
17. placebo*
18. "prospective study"/de
19. 1 OR 2 OR 3 OR...........OR 18
20. "case study"/de
21. "abstract study"/de
22. "letter"/de
23. "case report"
24. 20 OR 21 OR 22 OR 23
25. 19 NOT 24
26. "scabies"/de OR scabies
27. "sarcoptes scabiei"/de OR "sarcoptes scabiei"
28. human AND itch NEXT/1 mite*
29. 26 OR 27 OR 28
30. prophyla*
31. prevent*
32. control*
33. decontaminat*
34. therap*
35. lindane OR "hexachlorocyclohexane"/syn
36. "permethrin"/syn
37. "ivermectin"/syn
38. crotamiton
39. "sulphur"/syn
40. "malathion"/syn
41. "benzylbenzoate"/syn
42. 30 OR 31 OR 32 OR.......OR 41
43. 25 AND 29
44. 42 AND 43
45. 44 AND [embase]/lim
Appendix 5. PubMed search strategy
1. (randomized controlled trial[pt OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT (animals [mh] NOT humans[mh]))
2. search
3. sarcoptes scabiei
4. itch mite* [Text Word]
5. prophyla*[Text Word]
6. prevent*[Text Word]
7. control OR controls* OR controla* OR controle* OR controli* OR controll*[Text Word]
8. (treat*[Title]) OR treatm* OR treati* OR treate* OR treat OR treats[Text Word]
9. decontaminat*[Text Word]
10. therapy OR therape* OR therapi* [Text Word]
11. lindane OR hexachlorocyclohexane [Text Word]
12. permethrin [Text Word]
13. ivermectin[Text Word]
14. crotamiton [Text Word]
15. sulphur OR sulfur [Text Word]
16. malathion OR malation [Text Word]
17. benzylbenzoate[Text Word]
18. #2 OR #3 OR #4
19. #5 OR #6 OR......OR #16 OR #17
20. #1 AND #18
21. #19 AND #20
Appendix 6. LILACS search strategy
Search (All Fields) :
(MH:scabies OR sarcoptes scabiei OR "crusted scabies" OR sarna or escabiosis OR "sarna crostosa" OR "sarna costrosa" OR "human itch mite" OR "acaro de la sarna humano") AND
(treament OR tratamento OR tratmiento OR prophyla$ OR profila$ OR control OR controle OR prevent$ OR tetmosol ORlindane OR hexachlorocyclohexane OR hexachloride OR lindano OR hexachlorociclohexano OR hexachloruro OR hexachlorocicloexano OR hexacloreto OR permethrin OR permetrina OR ivermectin OR ivermectina OR crotamiton OR sulphur$ OR sulfurados OR malathion OR malatión OR malation OR benzylbenzoate) (Controlled clinical trials)
Appendix 7. CINAHL search strategy
1. PT clinical trial
2. AB controlled
3. AB trial
4. AB placebo
5. TX drug therapy
6. TX random*
7. AB groups
8. 1 OR 2 OR.....OR 7
9. MH animals NOT humans
10. 8 NOT 9
11. TX sarcoptes scabiei
12. TX human itch mite
13. TX scabies
14. 11 OR 12 OR 13
15. TX prophyla*
16. TX prevent*
17. TX control*
18. TX treat*
19. TX decontaminat*
20. TX therap*
21. TX lindane
22. TX hexachlorocyclohexane
23. TX permethrin
24. TX ivermectin
25. TX crotamiton
26. TX sulphur
27. TX malathion
28. TX benzylbenzoate
29. TX tetmosol
30. 15 OR 16 OR........29
31. 10 AND 14 AND 30
Appendix 8. OpenGrey search strategy
scabies OR sarcoptes scabiei OR Norwegian scabies OR crusted scabies OR human itch mite
Appendix 9. WHO ICTRP search strategy
Title: scabies OR sarcoptes scabiei OR crusted scabies OR norwegian scabies OR human itch mite
Condition: scabies OR sarcoptes scabiei OR crusted scabies OR norwegian scabies OR human itch mite
Intervention: prophyla* OR treatment OR control OR prevent* or lindane OR malathion OR benzylbenzoate OR hexachlorocyclohexane OR crotamiton OR permethrin OR ivermectin OR sulphur
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedin 2007 | Not a randomised controlled trial. |
| Alrawashdeh 2013 | We contacted the authors. They confirmed that follow‐up information on contacts was not recorded as part of their study. |
| Avila‐Romay 1991 | Outcomes for contacts not distinguished from cases. We attempted to contact authors but did not receive a reply. |
| Bachewar 2008 | Did not report outcome data on contacts of cases. |
| Bockarie 2000 | Not a randomised controlled trial. |
| Burgess 1986 | Did not report outcome data on contacts of cases. |
| Ejidokun 2007 | Not a randomised controlled trial. |
| Goldust 2012a | Did not report outcome data on contacts of cases. We attempted to contact authors but did not receive a reply. |
| Goldust 2012b | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Goldust 2013a | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Goldust 2013b | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Goldust 2013c | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Goldust 2013d | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Goldust 2013e | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Haar 2013 | Not a randomised controlled trial. |
| Hench 1994 | Not a randomised controlled trial. |
| Henderson 1992 | Not a randomised controlled trial. We contacted the authors. They confirmed that follow‐up information on contacts was not recorded as part of their study. |
| Heukelbach 2004 | Not a randomised controlled trial. |
| Mohebbipour 2012 | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Mohebbipour 2013 | Did not report outcome data on contacts of cases reported. We attempted to contact authors but did not receive a reply. |
| Paasch 2000 | Not a randomised controlled trial. |
| Saqib 2012 | We contacted the authors. They confirmed that follow‐up information on contacts was not recorded as part of their study. |
| Talukder 2013 | Not all subjects were examined at baseline and follow‐up. |
| Taplin 1983 | Not a randomised controlled trial. |
| Usha 2000 | Did not report outcome data on contacts of cases. |
| Woltman 1994 | A case report. |
| Wong 2001 | Not a randomised controlled trial. |
| Yonkosky 1990 | Not a randomised controlled trial. |
| Zargari 2006 | Did not report outcome data on contacts of cases. We contacted the authors. They confirmed that follow‐up information on contacts was not recorded as part of their study. |
Differences between protocol and review
Rachel Grainger replaced Fiona Kevitt as a co‐author.
We added 'Tetmosol' to the MEDLINE search strategy, as it was mentioned in an article found on a preliminary search after the protocol had been published.
At the protocol stage (Fitzgerald 2012), the title of this review was "Treatment of close contacts of people with scabies for preventing re‐infestation or spread of infestation in contacts". At the peer‐review stage it was highlighted that this review does not examine the implications of using prophylaxis for close contacts on the rate of re‐infestation. Therefore, after careful consideration and advice from the Occupational Safety and Health Review Group editorial team, the title has been changed. We could, alternatively, have changed the review to try to include searching for evidence regarding the impact of contacts using prophylaxis on the rate of re‐infestation in the index case, but on consideration acknowledged that this would be difficult, as it would not be possible for researchers to distinguish treatment failure from re‐infestation.
We also elaborated on the description of the types of participants we expected to have been subjects in the studies we would have included in this review.
Contributions of authors
Deirdre FitzGerald ‐ protocol development, searched literature, reference screening, data extraction, review writing.
Rachel Grainger ‐ searched literature, reference screening, data extraction, review editing.
Alex Reid ‐ expert advice, protocol development, review editing.
Sources of support
Internal sources
-
Occupational Health Department, Tallaght Hospital, Ireland.
IT access
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies excluded from this review
Abedin 2007 {published data only}
- Abedin S, Narang M, Gandhi V, Narang S. Efficacy of permethrin cream and oral ivermectin in treatment of scabies. Indian Journal of Pediatrics 2007;74(10):915‐6. [PUBMED: 17978449] [DOI] [PubMed] [Google Scholar]
Alrawashdeh 2013 {published and unpublished data}
- Alrawashdeh BT, Alazab K. Comparison between 25% benzyl benzoate, 5% permethrin and 10% crotamiton in the treatment of scabies in Gaza. Rawal Medical Journal 2013;38(2):125‐6. [Google Scholar]
Avila‐Romay 1991 {published data only}
- Avila‐Romay A, Alvarez‐Franco M, Ruiz‐Maldonado R. Therapeutic efficacy, secondary effects, and patient acceptability on 10% sulfur in either pork fat or cold cream for the treatment of scabies. Pediatric Dermatology 1991;8(1):64‐6. [PUBMED: 1862029] [DOI] [PubMed] [Google Scholar]
Bachewar 2008 {published data only}
- Bachewar NP, Thawani VR, Mali SN, Gharpure KJ, Shingade VP, Dakhale GN. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian Journal of Pharmacology 2009;41(1):9‐14. [DOI: 10.4103/0253-7613.48882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bockarie 2000 {published data only}
- Bockarie MJ, Alexander NDE, Kazura JW, Bockarie F, Griffin L, Alpers MP. Treatment with ivermectin reduces the high prevalence of scabies in a village in Papua New Guinea. Acta Tropica 2000;75(1):127‐30. [PUBMED: 10708015] [DOI] [PubMed] [Google Scholar]
Burgess 1986 {published data only}
- Burgess I, Robinson RJF, Robinson J, Maunder JW, Hassan Z. Aqueous malathion 0.5% as a scabicide: clinical trial. British Medical Journal (Clinical Research Edition) 1986;292(6529):1172. [PUBMED: 3085770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ejidokun 2007 {published data only}
- Ejidokun OO, Aruna OS, O'Neill B. A scabies outbreak in a further education college in Gloucestershire. Epidemiology and Infection 2007;135(3):455‐7. [DOI: 10.1017/S0950268806007072; PUBMED: 16948878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldust 2012a {published data only}
- Goldust M, Rezaee E, Hemayat S. Treatment of scabies: comparison of permethrin 5% versus ivermectin. Journal of Dermatology 2012;39(6):1‐3. [DOI: 10.1111/j.1346-8138.2011.01481.x] [DOI] [PubMed] [Google Scholar]
Goldust 2012b {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E, Hemayat S. Treatment of scabies: comparison of permethrin 5% versus ivermectin. Journal of Dermatology 2012;39(6):1‐3. [DOI: 10.1111/j.1346-8138.2011.01481.x] [DOI] [PubMed] [Google Scholar]
Goldust 2013a {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E. Comparative trial of oral ivermectin versus sulfur 8% ointment for the treatment of scabies. Journal of Cutaneous Medicine and Surgery 2013;17(5):299‐300. [PUBMED: PMID: 24067847] [DOI] [PubMed] [Google Scholar]
Goldust 2013b {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E. The efficacy of topical ivermectin versus malathion 0.5% lotion for the treatment of scabies. The Journal of Dermatological Treatment 2013 May 6;e‐pub ahead of print. [PUBMED: PMID: 23472617] [DOI] [PubMed] [Google Scholar]
Goldust 2013c {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E, Raghifar R. Comparison of oral ivermectin versus crotamiton 10% cream in the treatment of scabies. Cutaneous and Ocular Toxicology 2013 Feb 25;e‐pub ahead of print. [PUBMED: PMID: 23431958] [DOI] [PubMed] [Google Scholar]
Goldust 2013d {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E, Raghifar R, Naghavi‐Behzad M. Comparison of permethrin 2.5% cream vs. Tenutex emulsion for the treatment of scabies. Annals of Parasitology 2013;59(1):31‐5. [PUBMED: PMID: 23829056] [PubMed] [Google Scholar]
Goldust 2013e {published data only (unpublished sought but not used)}
- Goldust M, Rezaee E, Raghifar R, Naghavi‐Behzad M. Ivermectin vs. lindane in the treatment of scabies. Annals of Parasitology 2013;59(1):37‐41. [PUBMED: PMID: 23829057] [PubMed] [Google Scholar]
Haar 2013 {published data only}
- Haar K, Romani L, Filmone R, Kishore K, Tuicakau M, Koroivueta J, et al. Scabies community prevalence and mass drug administration in two Fijian villages. International Journal of Dermatology 2013 Oct 29;E‐pub ahead of print. [DOI: 10.1111/ijd.12353; PUBMED: PMID: 24168177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hench 1994 {published data only}
- Hench C, Paulson SS, Stevens DA, Thompson JD. Scabies outbreak on a spinal cord injury unit. Rehabilitation Nursing 1994;19(1):21‐3. [PUBMED: PMID: 8159860] [DOI] [PubMed] [Google Scholar]
Henderson 1992 {published and unpublished data}
- Henderson CA, Nykia M. Treatment of scabies in rural East Africa: a comparative study of two regimens. Tropical Doctor 1992;22(4):165‐7. [PUBMED: 1440885] [DOI] [PubMed] [Google Scholar]
Heukelbach 2004 {published data only}
- Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, Oliveira FA, et al. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bulletin of the World Health Organization 2004;82(8):563‐71. [PUBMED: 15375445] [PMC free article] [PubMed] [Google Scholar]
Mohebbipour 2012 {published data only (unpublished sought but not used)}
- Mehebbipour A, Saleh P, Goldust M, Amimia M, Zadeh YJ, Mohamadi RM, et al. Treatment of scabies: comparison of ivermectin vs. lindane lotion 1%. Acta Dermatovenerologica Crotica 2012;20(4):251‐5. [PUBMED: PMID: 23317486] [PubMed] [Google Scholar]
Mohebbipour 2013 {published data only (unpublished sought but not used)}
- Mohebbipour A, Saleh P, Goldust M, Amimia M, Zadeh YJ, Mohamad RM, et al. Comparison of oral ivermectin vs. lindane lotion 1% for the treatment of scabies. Clinical and Experimental Dermatology 2013;38(7):719‐23. [DOI: 10.1111/ced.12079; PUBMED: PMID: 23772999] [DOI] [PubMed] [Google Scholar]
Paasch 2000 {published data only}
- Paasch U, Haustein UF. Management of endemic outbreaks of scabies with allethrin, permethrin, and ivermectin. International Journal of Dermatology 2000;39(6):463‐70. [PUBMED: 10944095] [DOI] [PubMed] [Google Scholar]
Saqib 2012 {published data only (unpublished sought but not used)}
- Saqib M, Malik LM, Jahangir M. A comparison of efficacy of single topical permethrin and single oral ivermectin in the treatment of scabies. Journal of Pakistan Association of Dermatologists 2012;22:45‐9. [Google Scholar]
Talukder 2013 {published data only}
- Talukder K, Talukder MQ, Farooque MG, Khairul M, Sharmin F, Jerin I, et al. Controlling scabies in madrasahs (Islamic religious schools) in Bangladesh. Public Health 2013;127(1):83‐91. [DOI: 10.1016/j.puhe.2012.09.004; PUBMED: 23062631] [DOI] [PubMed] [Google Scholar]
Taplin 1983 {published data only}
- Taplin D, Rivera A, Walker JG, Roth WI, Reno D, Meinking T. A comparative trial of three treatment schedules for the eradication of scabies. Journal of the American Academy of Dermatology 1983;9(4):550‐4. [PUBMED: 6195201] [DOI] [PubMed] [Google Scholar]
Usha 2000 {published data only}
- Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. Journal of the American Academy of Dermatology 2000;42(2):236‐40. [PUBMED: 10642678] [DOI] [PubMed] [Google Scholar]
Woltman 1994 {published data only}
- Woltman L. Scabies: treatment failures and hope for new success. The Australian Journal of Rural Health 1994;2(4):13‐6. [DOI: 10.1111/j.1440-1584.1994.tb00126.x] [DOI] [Google Scholar]
Wong 2001 {published data only}
- Wong LC, Amega B, Connors C, Barker R, Dulla ME, Ninnal A, et al. Outcome of an interventional program for scabies in an Indigenous community. The Medical Journal of Australia 2001;175(7):367‐70. [PUBMED: 11700814] [DOI] [PubMed] [Google Scholar]
Yonkosky 1990 {published data only}
- Yonkosky D, Ladia L, Gackenheimer RN, Schultz MW. Scabies in nursing homes: an eradication program with permethrin 5% cream. Journal of the American Academy of Dermatology 1990;23(6):1133‐6. [PUBMED: 2273114] [DOI] [PubMed] [Google Scholar]
Zargari 2006 {published and unpublished data}
- Zargari O, Golchai J, Sobhani A, Dehpour AR, Sadr‐Ashkevari A, Alizadeh N, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: a randomized, double‐blind study. Indian Journal of Dermatology, Venereology and Leprology 2006;72(1):33‐6. [DOI: 10.4103/0378-6323.19715] [DOI] [PubMed] [Google Scholar]
Additional references
Arlian 1988
- Arlian LG, Estes SA, Vyszenski‐Moher DL. Prevalence of Sarcoptes scabiei in the homes and nursing homes of scabietic patients. Journal of the American Academy of Dermatology 1998;19:806‐11. [PUBMED: PMID: 3142938] [DOI] [PubMed] [Google Scholar]
Arlian 1989
- Arlian LG. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annual Review of Entomology 1989;34:139‐51. [PUBMED: 2494934] [DOI] [PubMed] [Google Scholar]
Blumenthal 1976
- Blumenthal DS, Taplin D, Schultz MG. A community outbreak of scabies. American Journal of Epidemiology 1976;104(6):667‐72. [PUBMED: 998612] [DOI] [PubMed] [Google Scholar]
Bouvresse 2010
- Bouvresse S, Chosidow O. Scabies in healthcare settings. Current Opinion in Infectious Diseases 2010;23(2):111‐8. [PUBMED: 20075729] [DOI] [PubMed] [Google Scholar]
Buehlmann 2009
- Buehlmann M, Beltraminelli H, Strub C, Bircher A, Jordan X, Battegay M, et al. Scabies outbreak in an intensive care unit with 1,659 exposed individuals ‐ key factors for controlling the outbreak. Infection Control and Hospital Epidemiology 2009;30:354‐60. [DOI: 10.1086/596113] [DOI] [PubMed] [Google Scholar]
Carapetis 1997
- Carapetis JR, Connors C, Yarmirr D, Krause V, Currie BJ. Success of a scabies control program in an Australian Aboriginal community. The Pediatric Infectious Disease Journal 1997;16(5):494‐9. [PUBMED: 9154544] [DOI] [PubMed] [Google Scholar]
Cho 2000
- Cho BK, Rosenfeldt F, Turina MI, Karp RB, Ferguson TB, Bodnar E, et al. Joint statement on redundant (duplicate) publication by the editors of the undersigned cardiothoracic journals. Annals of Thoracic Surgery 2000;69(2):663. [PUBMED: 10735731] [DOI] [PubMed] [Google Scholar]
Chosidow 2006
- Chosidow O. Scabies. The New England Journal of Medicine 2006;354:1718‐27. [PUBMED: 16625010] [DOI] [PubMed] [Google Scholar]
Chouela 2002
- Chouela E, Abeldano A, Pellerano G, Hernandez MI. Diagnosis and treatment of scabies. American Journal of Clinical Dermatology 2002;3(1):9‐18. [DOI: ] [DOI] [PubMed] [Google Scholar]
Currie 2000
- Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australasian Journal of Dermatology 2000;41:139‐45. [PUBMED: 10954983] [DOI] [PubMed] [Google Scholar]
Currie 2004
- Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clinical Infectious Diseases 2004;39(1):e8‐12. [PUBMED: 15206075] [DOI] [PubMed] [Google Scholar]
Eldridge 2004
- Eldridge SM, Ashby D, Feder GS, Rudnicka AR, Ukoumunne OC. Lessons for cluster randomized trials in the twenty‐first century: a systematic review of trials in primary care. Clinical Trials 2004 February;1(1):80‐90. [PUBMED: 16281464] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE working group, 2008.
Green 1989
- Green MS. Epidemiology of scabies. Epidemiology Reviews 1989;11:126‐50. [DOI] [PubMed] [Google Scholar]
Guay 2004
- Guay DR. The scourge of sarcoptes: oral ivermectin for scabies. The Consultant Pharmacist: the Journal of the American Society of Consultant Pharmacists 2004;19(3):222‐35. [PUBMED: 16553478] [DOI] [PubMed] [Google Scholar]
Hengge 2006
- Hengge UR, Currie BJ, Jager G, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. The Lancet Infectious Diseases 2006;6:769‐79. [PUBMED: 17123897] [DOI] [PubMed] [Google Scholar]
Heukelbach 2006
- Heukelbach J, Feldmeier H. Scabies. Lancet 2006;367:1767‐74. [PUBMED: 16731272] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kanaaneh 1976
- Kanaaneh HAK, Rabi SA, Bacarneh SM. The eradication of a large scabies outbreak using community‐wide health education. American Journal of Public Health 1976;66(6):564‐7. [PUBMED: 937602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolan 2011
- Nolan K, Kamrath J, Levitt J. Lindane toxicity: a comprehensive review of the medical literature. Pediatric Dermatology 2011;2:1‐6. [DOI: 10.1111/j.1525-1470.2011.01519.x] [DOI] [PubMed] [Google Scholar]
Orion 2006
- Orion E, Marcos B, Davidovici B, Wolf R. Itch and scratch: scabies and pediculosis. Clinics in Dermatology 2006;24:168‐75. [DOI: 10.1016/j.clindermatol.2005.11.001] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Scheinfeld 2004
- Scheinfeld N. Controlling scabies in institutional settings: a review of medication, treatment models, and implementation. American Journal of Clinical Dermatology 2004;5(1):31‐7. [PUBMED: 14979741] [DOI] [PubMed] [Google Scholar]
Stoevesandt 2012
Strong 2007
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000320.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vorou 2007
- Vorou R, Remoudaki HD, Maltezou HC. Nosocomial scabies. Journal of Hospital Infection 2007;65:9‐14. [DOI: 10.1016/j.jhin.2006.08.012] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fitzgerald 2012
- Fitzgerald D, Kevitt FA, Reid A. Treatment of close contacts of people with scabies for preventing re‐infestation or spread of infestation in contacts. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009943] [DOI] [PMC free article] [PubMed] [Google Scholar]


