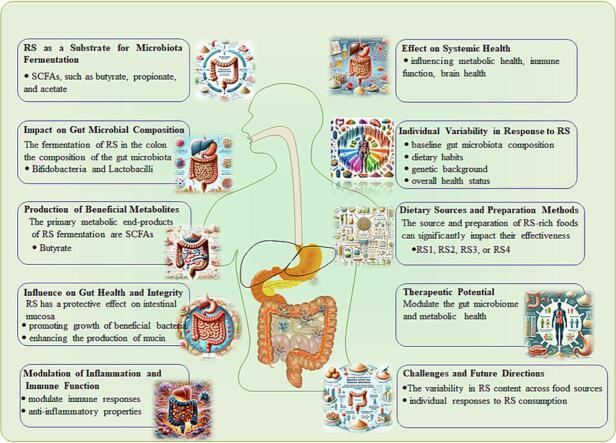
Graphical abstract
Keywords: Resistant starch, Gut microbiome, Fermentation, Volatile fatty acids, Dietary impacts, Gut health, Metabolic outcomes, Colon fermentation, Butyrate, Inflammation modulation
Highlights
-
•
Resistant starch (RS) modulates gut microbiome for health benefits.
-
•
RS fermentation produces vital volatile fatty acids for gut health.
-
•
RS source and processing impact microbiome interaction and VFAs.
-
•
RS-based diets show promise against chronic diseases via microbiome.
-
•
RS and gut microbiome link emphasizes integrated nutrition approaches.
Abstract
The intricate relationship between resistant starch (RS) and the gut microbiome presents a dynamic frontier in nutrition science. This review synthesizes current understandings of how RS, an indigestible form of starch found naturally in certain foods and also enhanced through various modification methods, interacts with the gut microbiome. We particularly focus on how RS fermentation in the colon contributes to the production of beneficial volatile fatty acids (VFAs) such as butyrate, acetate, and propionate. These VFAs have been recognized for their vital roles in maintaining gut barrier integrity, modulating inflammation, and potentially influencing systemic health. Additionally, we discuss the dietary implications of consuming foods rich in RS, both in terms of gut health and broader metabolic outcomes. By consolidating these insights, we emphasize the significance of RS in the context of dietary strategies aimed at harnessing the gut microbiome's potential to impact human health.
1. Introduction
The human gastrointestinal system, notably the large intestine, is hosting to trillions of microorganisms known collectively as the gut microbiome. This complex community of bacteria, viruses, fungi, and other microorganisms plays a pivotal role in nutrient absorption, immune function modulation, and protection against harmful pathogens (Clemente et al., 2012). Over the past decade, the profound influence of the gut microbiome on overall human health has gained widespread attention in both the scientific community and popular health discussions (Lynch & Pedersen, 2016). At the crossroads of nutrition science and microbial ecology is the exploration of dietary components that can beneficially modulate the gut microbiome. One such dietary component, resistant starch (RS), has emerged as a key player in this arena (Bindels et al., 2015).
Starches are long chains of glucose molecules, serving as primary energy storage in many plants (A. M. Smith, 2001). However, not all starches are equal regarding their digestibility in the human gut. While most starches are quickly hydrolyzed by enzymes and absorbed in the small intestine, RS evades this early digestion, proceeding undigested to the large intestine where it undergoes fermentation by the indigenous microbiota (Birt et al., 2013). This distinctive characteristic of RS has sparked interest in its potential health benefits, especially concerning its interaction with the gut microbiome.
The fermentation of RS by the gut microbiota results in the production of volatile fatty acids (VFAs) - mainly acetate, propionate, and butyrate. Among these, butyrate is particularly intriguing for its protective effects on the intestinal mucosa and potential anti-inflammatory properties (Louis & Flint, 2017). As these VFAs are absorbed into the bloodstream, they have the capability to produce systemic effects, influencing metabolic health, immune function, and potentially even brain health (Koh et al., 2016).
The source and type of RS can influence its fermentation pattern and the consequent production of VFAs. RS is naturally present in foods such as legumes, certain grains, and raw potatoes. Additionally, various food processing and cooking methods can enhance the RS content of foods, further diversifying the potential dietary sources of this starch (Furrer et al., 2018, Walsh et al., 2022). With the rise of diet-related chronic diseases, there's an increasing focus on understanding and leveraging the dietary strategies that modulate gut health, wherein RS presents promising potential (Everard & Cani, 2013).
In this review, we explore the complex interplay between RS and the gut microbiome. We aim to illuminate the mechanisms by which RS influences microbial communities, the health implications of these interactions, and the wider dietary scenarios in which RS can be incorporated for maximum health benefit. Through this, we highlight the growing significance of concurrently addressing both diet and the microbiome to develop effective strategies for health promotion and disease prevention (De Vadder et al., 2014).
2. Fundamentals of resistant starch (RS)
2.1. Biochemical structure and classification of RS
Resistant starch (RS) has increasingly been recognized for its unique chemical configuration and consequential physiological functions. Despite its name, RS is not a singular entity but represents a collection of starches that resist digestion in the small intestine, culminating in fermentation in the colon (Brown et al., 1997).
To begin, the fundamental structure of starches is based on amylose and amylopectin, two distinct polymers of glucose. Amylose, primarily a linear molecule comprised of α-1,4-linked glucose units, contrasts with the branched structure of amylopectin, which additionally contains α-1,6 linkages (Jane, 2009). The proportion of these two components and their organization within a starch granule significantly affects the starch's digestibility. Notably, the more denser and more tightly packed of these molecules, the less accessible they are to digestive enzymes (Sajilata et al., 2006).
Dietary sources of resistant starch
Resistant starch (RS) is a unique type of dietary fiber that is not digested in the small intestine but rather fermented in the large intestine. The content of RS in foods varies significantly across different food categories, including grains, legumes, tubers, and certain processed foods (Table 1). This section elaborates on the composition of RS in these food sources, offering a comprehensive understanding of where RS can be found in the diet.
Table 1.
Resistant Starch Content in Various Food Sources.
| Food Category | Food Items | Average RS Content (g/100 g) |
|---|---|---|
| Grains | Barley, Oats, Whole Wheat | 3–7 |
| Legumes | Lentils, Chickpeas, Beans | 4–10 |
| Tubers | Potatoes, Yams | 2–5 |
| Processed Foods | Whole Grain Breads, Pasta | 2–6 |
Grains, especially whole grains like barley, oats, and whole wheat, are significant sources of RS, particularly when they are minimally processed (Shu et al., 2014). Cooking and cooling rice, for example, increases its RS content due to the retrogradation of amylose (Harris, 2019, He et al., 2018, Zheng et al., 2020).
Legumes such as lentils, chickpeas, and beans are also rich in RS (de Almeida Costa et al., 2006). Their RS content is attributed to their high amylose and amylopectin ratio, which contributes to their slow digestibility (Brummer et al., 2015, Sajilata et al., 2006). Cooking and cooling processes further increase their RS content (Aguilera et al., 2009).
Tubers, including potatoes and yams, contain RS, especially when cooked and cooled, a process that induces starch retrogradation (Lal et al., 2021, Yadav et al., 2009, Zhao et al., 2018). This makes dishes like cold potato salad good RS sources (Raigond et al., 2015).
Certain processed foods, particularly those made from whole grains or incorporating RS as an ingredient, can be significant RS sources (Fuentes-Zaragoza et al., 2010). Whole grain breads and pasta that have undergone processes like extrusion cooking have been found to retain considerable amounts of RS (Alsaffar, 2011).
The inclusion of RS-rich foods like grains, legumes, tubers, and certain processed foods contributes to dietary fiber intake and offers various health benefits (Birt et al., 2013). Understanding these sources and their RS content is essential for dietary planning and nutrition optimization.
Expanding on this, RS can be broadly categorized into four main types based on their origins and properties:
RS Type 1 (RS1): This form of RS is physically inaccessible to enzymes due to the protective barrier formed by the food matrix and protein encasements. Common sources include whole grains and seeds (McCleary & Monaghan, 2002).
RS Type 2 (RS2): RS2 is characterized by its native granular form, predominantly found in certain raw foods. Examples include raw potatoes and green bananas. The high amylose content of these sources results in a tightly packed granular structure, limiting enzymatic access (Englyst & Hudson, 1996).
RS Type 3 (RS3): Also known as retrograded starch, RS3 forms when certain foods are cooked and then cooled. This cooling process leads to realignment and recrystallization of the starch molecules, further rendering them resistant to enzymatic breakdown. Foods such as cooked and cooled potatoes, pasta, and rice are primary sources of RS3 (Birt et al., 2013).
RS Type 4 (RS4): This type comprises chemically modified starches not naturally found in foods. Various industrial processes introduce cross-linking or substitution in starch molecules to enhance their resistance to digestion (Raigond et al., 2015).
It's crucial to understand that while these categories facilitate discussion and research, many real-world foods contain a mixture of RS types. Moreover, factors such as food processing methods, storage conditions, and the presence of other food components can significantly modulate the RS content in these foods (Zhang & Hamaker, 2009).
The potential health benefits and physiological implications of RS arise mainly from its fermentation in the large intestine. Yet, the extent and specificity of these benefits can vary depending on the RS type. For instance, different RS types may preferentially foster the growth of specific microbial species or lead to differential production rates of volatile fatty acids (Keenan et al., 2006).
In conclusion, the biochemical structure and classification of RS are crucial in determining its interaction with the gut microbiome and subsequent health outcomes. A comprehensive understanding of these foundational aspects is vital for both research and application in dietary interventions aimed at harnessing RS's potential benefits (J. L. Sonnenburg & Bäckhed, 2016).
2.2. Natural sources and dietary occurrence
The remarkable relationship between resistant starch (RS) and the gut microbiome draws not only from the intricate biochemical composition of RS but also from the variety and abundance of its natural sources (Fig. 1). Gaining a holistic understanding of these sources is essential, as it provides insights into potential dietary patterns conducive to harnessing RS's health benefits.
Fig. 1.

Diversifying sources of resistant starch.
Resistant starch, given its diverse classification, is derived from in a plethora of natural foods. Predominantly, whole plant foods are considered rich sources of RS. Legumes, for instance, are not only protein-packed but also abound with RS, especially when cooked and then cooled (Brouns et al., 2012). Beans, lentils, chickpeas, and green peas are particularly notable in this category. Similarly, grains, both whole and refined, can be significant sources of RS. Oats, barley, and whole wheat have all contain impressive amounts of this beneficial starch, especially in their less processed forms (Nugent, 2005).
Root vegetables, a staple in many diets worldwide, offer another avenue for RS intake. Raw potatoes, a prime example of RS Type 2, transform into a source of RS Type 3 when cooked and then cooled, showcasing the dynamic nature of RS to cooking methods. Moreover, green bananas and plantains, often overlooked in western diets, are rich in RS, particularly when consumed in their unripe state. Other natural sources include high-amylose corn, which has been bred to enhance its RS content, and certain nuts and seeds. Beyond these direct sources, certain traditional dishes and food preparations from diverse cultures naturally augment the RS content, emphasizing the global significance of this nutrient (Thynn et al., 2020).
The dietary occurrence of RS isn't merely about direct consumption of these foods. The manner in which they are incorporated into daily diets, combined with other foods, and subjected to various culinary processes can influence the RS content. For instance, cooling a dish containing potatoes or pasta after cooking can increase its RS content. Additionally, the fermentation process, an age-old culinary practice in many cultures, can also augment the RS content of foods. Examples include fermented rice dishes in Asia or sourdough breads in Europe (Singh et al., 2010).
In today's global nutritional landscape, understanding the natural sources and dietary occurrence of RS becomes even more pertinent. With the increasing burden of metabolic diseases and the search for dietary interventions that can modulate the gut microbiome for better health outcomes, RS stands out. It offers a confluence of tradition and science, urging both researchers and consumers to revisit traditional dietary practices and recognize their potential health implications. As more is understood about the gut microbiome's role in health and disease, the emphasis on dietary RS from natural sources is poised to grow, offering new avenues for nutrition-based interventions (Bindels et al., 2015).
2.3. Enhanced RS through food processing and cooking methods
Beyond the naturally occurring resistant starch (RS) in foods, there's a compelling domain where RS concentrations can be intentionally enhanced: food processing and cooking methods. These methods, often traditional and sometimes innovative, are paramount to our understanding, as they provide potential avenues to increase dietary intake of RS, thereby influencing gut health.
The behavior of starch during cooking and cooling is fascinating. One prevalent phenomenon is retrogradation. When certain starchy foods, like potatoes or rice, are cooked and then cooled, the amylose and amylopectin chains in the starch realign. This realignment increases the amount of RS, especially RS Type 3, in the food. For instance, cooking pasta and then cooling it can elevate its RS content. Such a transformation isn't merely limited to refrigeration but can also be observed when foods are left to cool at room temperature (Hu et al., 2011, Zhang et al., 2014).
Similarly, food processing methods are key players. Extrusion cooking, widely used for producing ready-to-eat cereals and snacks, can increase the RS content of the end product, subject to the applied conditions. Parameters such as moisture content, screw speed, and temperature can be adjusted to optimize the RS formation. Additionally, the annealing process, which involves hydrating the starch granules without gelatinizing them. This process has been found to increase the RS content in some grains (Wang & Copeland, 2013).
Moreover, fermentation, a time-honored culinary and preservation method across various cultures, has an inherent capacity to raise RS levels. The process often involves beneficial bacteria or yeasts breaking down and fermenting sugars, which can alter the starch structure, rendering more of it resistant to digestion. For example, the fermentation of grains to produce sourdough bread or certain traditional African dishes not only imparts unique flavors but also increases their RS concentration. It's a testament to the synergy of taste, tradition, and health (Gänzle, 2015).
While the exploration and application of these methods can significantly enhance RS content, it's crucial to consider the wider nutritional consequences. Not all methods that increases RS is universally beneficial. Some processing methods can strip foods of their vital nutrients or introduce undesirable compounds. Achieving a careful equilibrium between enhancing RS for gut health benefits and ensuring the overall nutritional value of the food remains intact. Hence, while these methods open exciting possibilities for harnessing the potential of RS, they highlight the need for a comprehensive approach in nutrition science, which weighs the benefits and trade-offs in a broader dietary context (Ashwar et al., 2016, Bojarczuk et al., 2022, Haralampu, 2000; Louis et al., 2017; Perera et al., 2010).
2.4. Detection methods for resistant Starch: Advancements and applications
The precise detection and quantification of resistant starch (RS) in various food matrices are crucial in advancing nutritional research and optimizing dietary formulations. This subsection delves deeper into the analytical methodologies used to detect and quantify RS, highlighting the latest advancements and their applications in current research. A detailed table is provided to encapsulate the essence of each method (Table 2).
Table 2.
Advanced Methods for Detection and Quantification of Resistant Starch.
| Method Type | Technique | Principle and Application |
|---|---|---|
| Enzymatic | Megazyme Resistant Starch Assay | Employs a series of specific enzymes to simulate human digestion, followed by spectrophotometric quantification of the remaining RS. Widely used for its accuracy and reproducibility in diverse food matrices. |
| Chromatographic | High-Performance Liquid Chromatography (HPLC) | Involves enzymatic hydrolysis followed by HPLC analysis. This method distinguishes RS from other dietary fibers based on molecular size and structure, offering high precision and sensitivity. |
| Spectroscopic | Nuclear Magnetic Resonance (NMR) Spectroscopy | Utilizes advanced NMR techniques to provide molecular-level insights into RS structures. This method is instrumental in differentiating between RS types and understanding their digestion-resistant features. |
Enzymatic Methods: The Megazyme Resistant Starch Assay represents a gold standard in RS quantification. By mimicking the human digestive process through specific enzymes and quantifying RS through spectrophotometry, this method offers high accuracy and reproducibility. Its application extends from basic research to industrial settings, providing essential data for nutritional labeling and dietary analysis (Krishnan et al., 2020, Raatz et al., 2016).
Chromatographic Techniques: High-Performance Liquid Chromatography (HPLC) has become an indispensable tool in RS analysis, particularly for its ability to handle complex food matrices. This technique separates RS based on molecular size and structure, following enzymatic hydrolysis. The precision and sensitivity of HPLC make it a preferred method for detailed RS profiling, critical for understanding its nutritional impact (Barros et al., 2014, Mangala et al., 1999).
Spectroscopic Techniques: Nuclear Magnetic Resonance (NMR) Spectroscopy offers unparalleled insights into the molecular structure of RS. By analyzing the molecular arrangement, NMR helps in distinguishing between different types of RS and provides a deeper understanding of their resistance to digestion. This method is particularly valuable in advanced RS research, exploring novel RS forms and their potential health benefits (Cao et al., 2021, Sang et al., 2007).
The advancement in RS detection techniques, from enzymatic assays to sophisticated chromatographic and spectroscopic methods, has significantly contributed to the expanding knowledge of RS and its health implications. These methods not only enable precise quantification but also offer a deeper understanding of RS types, their structural properties, and their functional roles in diet and health. This comprehensive approach to RS analysis is pivotal in driving forward nutritional science and developing targeted dietary strategies.
3. RS interaction with the gut microbiome
3.1. Mechanism of RS fermentation in the colon
The intricate dance between resistant starch (RS) and the gut microbiome unfolds primarily in the colon, the site where most undigested carbohydrates reach their metabolic fate. The human colon houses a dense microbial ecosystem, rich in diversity and complexity, which plays a vital role in fermenting undigested dietary components, particularly RS. This relationship between RS and gut microbes is not just of academic interest; it bears profound implications for human health.
Upon reaching the colon, RS is subject to anaerobic fermentation by the resident gut microbiota. This fermentation process results in the production of short-chain fatty acids (SCFAs) – predominantly acetate, propionate, and butyrate – along with gases like hydrogen, methane, and carbon dioxide (Hamer et al., 2008). Among the SCFAs, butyrate is particularly noteworthy for its crucial role in colon health. Serving as a primary energy source for colonocytes, butyrate also exhibits anti-inflammatory properties, reinforces the colonic defense barrier, and potentially reduces the risk of colon cancer (Canani et al., 2011). Additionally, the modulation of gut pH by SCFAs is another advantage, favoring the growth of beneficial bacteria while inhibiting the proliferation of pathogenic strains (Fig. 2).
Fig. 2.
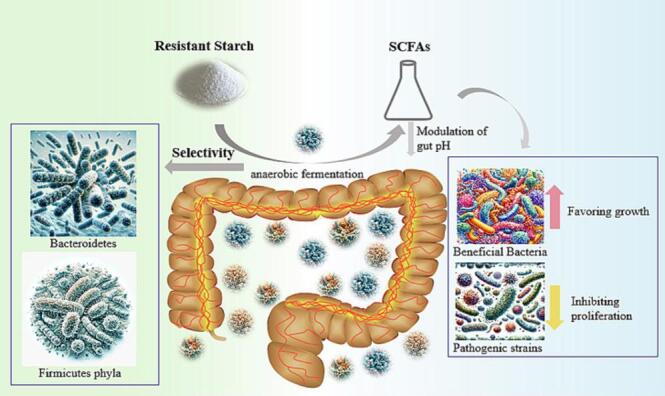
Mechanism of RS Fermentation in the Colon.
Another intriguing aspect of RS fermentation is its selectivity. Not all gut microbes are capable of effectively fermenting RS; specific bacterial groups, especially those from the Bacteroidetes and Firmicutes phyla, have been identified as primary RS fermenters (Walker et al., 2011). This selective fermentation can lead to shifts in the gut microbial composition. Consistent RS intake can promote the proliferation of these RS-fermenting bacteria, enriching the gut with beneficial microbes that further enhance the fermentation efficiency and SCFA production. The dynamic interplay between RS and gut microbiota, thus, holds promise for targeted interventions, potentially enabling the modulation of gut microbial composition and activity through dietary strategies (Fig. 2).
In conclusion, the mechanism of RS fermentation in the colon highlights the symbiotic relationship between dietary fibers and gut microbiota. While RS offers a substrate for microbial fermentation, the gut microbes, in return, produce metabolites that benefit host's health. The understanding of this mechanism is continually developing, with newer insights being added to the repertoire. Future research, building upon this foundational knowledge, may pave the way for innovative therapeutic strategies targeting the gut microbiome, employing RS as a potent tool.
3.2. Key microbial taxa involved in RS fermentation
The relationship between the human gut microbiota and resistant starch (RS) exemplifies the the intricate balance between diet and the gut environment. While the mechanism of RS fermentation in the colon is crucial, it becomes imperative to understand the key microbial players in this process. Recognizing these primary fermenters of RS not only enriches our comprehension of the gut microbial ecosystem but also unveils potential targets for both dietary and therapeutic interventions.
A principal group that actively participates in RS fermentation belongs to the Bacteroidetes phylum, with the genus Bacteroides being particularly notable (Ze et al., 2012). The metabolic prowess of Bacteroides allows them to thrive on a variety of complex carbohydrates, including RS. Their enzymatic arsenal facilitates the breakdown of RS into simpler units, which are then fermented to produce short-chain fatty acids (SCFAs). The contribution of Bacteroides to gut health extends beyond RS metabolism; they play crucial roles in maintaining gut barrier integrity, modulating immune responses, and even synthesizing essential vitamins for the host.
Another vital contributor to RS fermentation is the Firmicutes phylum, especially the Ruminococcus genus (Qin et al., 2010). Ruminococcus bromii is often recognized as a keystone species in this context, given its unparalleled efficiency in initiating RS degradation. The preliminary degradation carried out by R. bromii makes RS more accessible for further fermentation by other microbial taxa. Its significance becomes evident when considering its dominance in individuals consuming diets rich in RS. Besides, a higher abundance of Ruminococcus is associated with an improved gut health profile, indicating its potential protective roles.
While Bacteroidetes and Firmicutes stand out, Actinobacteria, another phylum, contributes to RS fermentation through the Bifidobacterium genus (Turroni et al., 2012). Bifidobacteria are well-regarded probiotics, known to confer a myriad of health benefits. In the context of RS, they ferment it to produce SCFAs, which consequently lowers the gut pH, creating an environment unfavorable for pathogenic bacteria. Moreover, bifidogenic effects of RS, where RS supplementation leads to increased Bifidobacterial counts, have been well-documented in various studies.
Archaea, specifically the methanogenic Methanobrevibacter smithii, also engage in the RS fermentation landscape (Samuel & Gordon, 2006). M. smithii consumes the hydrogen produced during RS fermentation by other microbes, converting it to methane. This hydrogen removal is crucial as it prevents the accumulation of hydrogen in the colon, which could otherwise hinder the fermentation process. Thus, M. smithii indirectly supports the RS fermentation process by maintaining a conducive environment for other fermenters.
To summarize, the RS fermentation in the colon is not an isolated process attributed to a single microbial taxon. It is a synergistic collaboration involving multiple microbial groups, each contributing uniquely to the process and the ensuing health benefits. Their collective actions underscore the concept of the gut being a metabolic 'organ,' where dietary components, primarily RS, are metabolized in a concerted manner. Unraveling the roles of these microbial groups offers an exciting potential for tailored dietary strategies to modulate the gut microbiota, with RS serving as a promising tool.
3.3. Factors influencing RS-Microbiome interaction
The realm of the gut microbiome and its intricate interactions with resistant starch (RS) is vast and multifaceted. As much as it is clear that RS can influence the gut microbiome composition and function, it's equally evident that the extent and nature of these effects are modulated by various factors. Unraveling these factors can enhance our understanding of the gut health intricacies and help tailor dietary and therapeutic interventions more efficiently.
One of the major determinants is the baseline composition of an individual’s gut microbiota. Every individual has a unique gut microbiota signature, influenced by genetics, early life exposures, antibiotics, and dietary patterns (Davenport et al., 2015). When introduced to the diet, RS may have varying effects across individuals based on their gut's microbial starting point. For instance, individuals with a lower baseline level of Bacteroides might experience a more pronounced increase in these bacteria upon RS consumption than those who already have a higher abundance.
The type of RS consumed is another significant factor. There are multiple types of RS, classified based on their physicochemical properties and sources: RS1, RS2, RS3, and RS4 (Birt et al., 2013). Each type might be preferentially metabolized by specific microbial taxa. For instance, Ruminococcus bromii shows a pronounced predilection for RS2 from high-amylose maize, while certain Bacteroides species might favor RS3 from retrograded starches. Therefore, the type of RS incorporated in the diet can guide the trajectory of microbiota changes.
Dietary context in which RS is consumed cannot be understated. The presence of other dietary fibers, proteins, fats, and micronutrients can influence the accessibility and fermentability of RS (Macfarlane & Macfarlane, 2003). For example, a diet rich in soluble fibers might amplify the prebiotic effects of RS by promoting the growth of beneficial bacteria, such as Lactobacilli. Conversely, a diet rich in proteins might divert some colonic bacteria towards protein fermentation, producing potentially harmful compounds like ammonia.
Duration of RS consumption also plays a pivotal role. Initial introduction of RS might cause rapid shifts in the microbiota composition. However, with prolonged intake, the microbiota might stabilize, indicating adaptation. Long-term RS intake can lead to a more resilient and diversified microbiota, which can be more resistant to perturbations and potential dysbiosis (Martínez et al., 2010).
Finally, host-related factors such as age, health status, and genetics modulate RS-microbiome interactions. Age-related shifts in microbiota, differences in gut transit time, and enzymatic activity can influence how RS is fermented in the gut (O’Toole & Jeffery, 2015). Similarly, individuals with gastrointestinal disorders like irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD) may have distinct responses to RS, given the altered gut environment and microbiota composition in these conditions.
In summation, the interaction between RS and the gut microbiota is a dynamic process influenced by a myriad of factors. Recognizing and understanding these factors are fundamental for individualized nutritional strategies aiming to harness the benefits of RS for gut health. Such insights beckon a more personalized approach in the realm of nutrition and gut health, moving beyond one-size-fits-all recommendations.
4. Volatile fatty acids (VFAs): Production, Roles, and benefits
4.1. VFA synthesis from RS fermentation
Resistant starch (RS) is a quintessential substrate for colonic fermentation, chiefly carried out by the resident microbial community. This fermentation process culminates in the production of volatile fatty acids (VFAs), mainly acetate, propionate, and butyrate, which are vital players in the sustenance of gut health and broader metabolic functions (Louis et al., 2017).
Upon reaching the colon, RS is acted upon by saccharolytic bacteria, which preferentially metabolize complex carbohydrates over proteins. During fermentation, glucose and other monosaccharides are generated from the breakdown of RS and subsequently undergo a series of bio-transformations to yield VFAs. Acetate is typically the most abundantly produced, followed by propionate and butyrate. The synthesis process is a microbial testament to resourcefulness. The Bacteroidetes and Firmicutes phyla, two groups of bacteria in the human gut, orchestrate these transformations. For instance, while butyrate is predominantly produced by Firmicutes, propionate synthesis is a stronghold of the Bacteroidetes phylum (Morrison and Preston, 2016, Ríos-Covián et al., 2016).
The production of acetate generally involves the Embden-Meyerhof-Parnas (EMP) pathway where pyruvate, a glucose fermentation product, is transformed into acetyl-CoA and then to acetate, releasing energy in the process. Conversely, propionate is primarily synthesized via the succinate or the acrylate pathways, depending on the bacterial species. The succinate pathway, employed by many Bacteroidetes, involves the conversion of pyruvate to succinate, which is then transformed to propionate. The acrylate pathway is an alternative route where lactate, produced from pyruvate, serves as a precursor. Butyrate synthesis is a multi-step process that sees acetyl-CoA, derived from pyruvate, being transformed into butyryl-CoA and subsequently to butyrate, mainly facilitated by the Firmicutes members like Faecalibacterium prausnitzii and Eubacterium rectale (Morrison et al., 2016).
Once produced, these volatile fatty acids (VFAs) are not confined to the colon. A majority of them are swiftly absorbed by the colonic epithelial cells. Butyrate, in particular, is a primary energy source for these cells, supporting their health and structural integrity. Moreover, these VFAs play pivotal roles beyond the gut. Acetate, the most predominant VFA, circulates systemically, impacting various organs, including the brain and muscles. Propionate predominantly influences liver functions, playing roles in gluconeogenesis and lipid metabolism. The benefits of VFAs are manifold and extend to anti-inflammatory effects, immune modulation, and potential anti-cancer properties, thereby underscoring the significance of RS fermentation in the gut (P. M. Smith et al., 2013).
In conclusion, the fermentation of RS in the colon, orchestrated by a symphony of microbiota, yields VFAs, molecules of profound physiological importance. These metabolites are central to gut health and echo the intricate interplay between diet, gut microbiota, and human health. Unraveling the nuances of VFA synthesis and functions promises more targeted dietary interventions to harness the benefits of RS and maintain optimal gut health.
4.2. Significance of individual VFAs: Acetate, Propionate, and butyrate
Volatile Fatty Acids (VFAs), primarily acetate, propionate, and butyrate, serve as the primary end products of resistant starch fermentation in the gut. Each of these VFAs possesses distinct physiological roles and potential benefits that are vital not only for the gut health but also for the holistic metabolic functioning of the human body.
Acetate, as the most abundantly produced volatile fatty acid (VFA), has diverse roles in the human body. Besides serving as an energy substrate, it plays a crucial role in cholesterol synthesis and lipid metabolism. Acetate has been recognized as a significant precursor for biosynthesis of long-chain fatty acids in adipose tissues, promoting energy storage (Perry et al., 2016). Additionally, circulating acetate can cross the blood–brain barrier and influence brain functions, especially in regulating appetite. Its anti-inflammatory properties, attributed to its influence on the production of cytokines like IL-10, underline its potential in modulating inflammatory responses and, by extension, chronic inflammatory disorders (LeBlanc et al., 2017).
Propionate, though synthesized in lesser quantities than acetate, is no less significant. One of its salient roles involves serving as a substrate for gluconeogenesis in the liver. This function makes propionate a pivotal player in maintaining glucose homeostasis, thereby impacting systemic energy metabolism. Furthermore, propionate has been associated with the inhibition of cholesterol synthesis, indicating potential cardioprotective benefits. Another intriguing aspect of propionate is its capacity to influence the gut-brain axis. Research suggests that it can signal the brain to produce appetite-regulating hormones, potentially influencing satiety and energy intake (Canfora et al., 2015). In terms of immune regulation, propionate, akin to acetate, exerts anti-inflammatory effects, primarily by inhibiting the NF-kB pathway and enhancing the release of anti-inflammatory cytokines (Arpaia et al., 2013).
Butyrate is often hailed as the VFA with the most profound implications for colonic health. As a primary energy source for the colonic epithelial cells, it underpins cellular integrity and functions. Its role in maintaining the gut barrier function is vital in preventing unwanted substances from entering the bloodstream, thereby averting potential inflammatory responses. Additionally, butyrate is a significant regulator of gene expression through its histone deacetylase inhibitor activity. This function implies that butyrate can influence various cellular processes, from cell differentiation to apoptosis, emphasizing its potential role in cancer prevention, particularly colorectal cancer. Studies have also highlighted butyrate’s anti-inflammatory properties, underlined by its capability to inhibit pro-inflammatory mediators and promote the differentiation of regulatory T cells, positioning it as a key molecule in inflammatory bowel disease research (Hamer et al., 2008, Leonel and Alvarez-Leite, 2012; H. Liu et al., 2018).
In summary, while these VFAs collectively contribute to gut health and systemic metabolism, each has unique roles and potentials. Acetate, propionate, and butyrate, through their individual pathways and interactions, impact a gamut of physiological processes, from energy metabolism and appetite regulation to immune modulation and cellular differentiation. Their production of these VFAs through the fermentation of resistant starch (RS) highlights the significant impact of diet on health. It illustrates a complex interplay between the food we consume, the gut microbiota, and our body's metabolic machinery.
4.3. Systemic impacts of VFAs on human health
Volatile Fatty Acids (VFAs) are not merely end-products of microbial fermentation but influential signaling molecules orchestrating a myriad of physiological processes. Their systemic effects stretch beyond the confines of the gut, influencing metabolic, cardiovascular, immune, and neurological systems.
The metabolic implications of VFAs are profound. Their role as energy substrates is a pivotal aspect, with acetate, propionate, and butyrate contributing to lipid synthesis, gluconeogenesis, and colonic epithelial energy respectively (Louis et al., 2014). Moreover, these fatty acids play a notable part in regulating glucose homeostasis and insulin sensitivity. VFAs stimulate the secretion of gut hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), which modulate insulin release and appetite regulation. The enhancement in GLP-1, specifically, improves insulin sensitivity, representing a potential therapeutic avenue for managing type 2 diabetes (Tolhurst et al., 2012).
The cardiovascular system also remains under the influence of VFAs. There's a demonstrated inverse correlation between dietary fiber (a precursor for VFA production) intake and cardiovascular disease risks. Acetate and propionate, in particular, possess cholesterol-lowering properties. They downregulate hepatic cholesterol synthesis, bolstering their potential role in cardioprotection. Furthermore, these fatty acids can modulate blood pressure via interaction with G-protein-coupled receptors, elucidating yet another mechanism through which they might confer cardiovascular benefits (Pluznick et al., 2013).
Finally, the neurological and immune systems emerge as unexpected beneficiaries of VFA actions. Butyrate has been observed to possess neuroprotective attributes, with potential to slow the progression of neurodegenerative diseases..VFAs also regulate the blood–brain barrier's integrity, highlighting their importance in neurological health. In terms of immunity, VFAs, especially butyrate, are instrumental in promoting the differentiation of regulatory T cells in the colon. This modulation is essential for maintaining gut immune homeostasis and offers a protective effect against inflammatory disorders such as inflammatory bowel disease (P. M. Smith et al., 2013).
In essence, the role of VFAs in human health is both multifaceted and indispensable. Emerging as key intermediaries linking diet, gut microbiota, and host health, they exemplify the quintessence of intricate physiological networking. Further research into harnessing their potential might pave the way for novel therapeutic interventions against a spectrum of diseases.
5. Impacts of RS on gut health and integrity
5.1. RS and gut barrier function
Resistant starch (RS) has emerged as a keystone dietary component, with implications far beyond its nutritional value. One of the most significant roles of RS resides in its capacity to uphold the gut's barrier function. This intricate lattice of mucosal cells and intercellular junctions holds the key to our systemic health, guarding against pathogenic invasion and maintaining metabolic equilibrium. Given the escalating burden of gut-related disorders, understanding how RS influences this barrier could offer pivotal insights into disease prevention and therapeutic intervention.
The gut barrier is it's a dynamic and responsive system, rather than a static entity. At its core, epithelial cells form the frontline, serving as the primary defense against the luminal environment (Turner, 2009). RS enhances the epithelial barrier by supporting cell turnover and promoting the secretion of mucin, a glycoprotein that lubricates and shields the epithelial surface from potential pathogens and abrasive food particles. The significance of the mucin layer is profound. It not only forms a protective blanket but also provides a habitat for commensal bacteria, aiding in the bi-directional relationship between host and microbiota.
Tight junction proteins, microscopic structures binding epithelial cells, are essential for maintaining barrier integrity (Suzuki, 2013). These proteins determine the permeability of the barrier, determining what substances are allowed to pass through and which remains excluded. In scenarios of “leaky gut,” these proteins become compromised, leading to increased gut permeability. Such a condition can allow unwanted substances, including pathogens and toxins, to enter the bloodstream, triggering systemic inflammation. Research indicates that RS positively modulates these proteins. The fermentation of RS to produce short-chain fatty acids, particularly butyrate, plays a role in upregulating the expression of tight junction proteins, fortifying the gut barrier.
The immune cells resident within the gut mucosa add another layer to the barrier's defense mechanism. Here, RS demonstrates its capacity for immunomodulation. By altering the gut microbiota composition, RS indirectly influences the local immune response. It promotes the growth of beneficial bacteria that, in turn, interact with immune cells, guiding their function. This crosstalk ensures the swift elimination of potential pathogens while preserving tolerance to dietary antigens and commensal microbes (González-Bosch et al., 2021; Morrison et al., 2016; Nogal et al., 2021, Ríos-Covián et al., 2016).
Apart from these direct impacts, RS-induced changes in the gut microbiota also influence the gut-brain axis. This bi-directional communication channel between the gut and the central nervous system is pivotal for overall health. Disruptions in the gut barrier function have been linked to neurological conditions, underscoring the importance of dietary components like RS in neuroprotection (Braniste et al., 2014).
Furthermore, the vascular structures within the gut, including blood and lymphatic vessels, play a role in barrier function. They ensure nutrient absorption and immune cell trafficking. RS, through its metabolites, modulates the vascular endothelial barrier, optimizing nutrient uptake and ensuring efficient immune surveillance.
In conclusion, RS stands as a multifaceted ally in the quest for optimal gut health. Its intricate interplay with the gut barrier's cellular, immunological, and vascular components offers a robust defense against environmental challenges. Embracing the therapeutic potential of RS could redefine strategies geared towards gastrointestinal health and beyond.
5.2. RS modulation of gut inflammation
Inflammation, a protective response initiated by the immune system against pathogens, injuries, or harmful stimuli, can become a double-edged sword when deregulated. Particularly within the gut, persistent inflammation can pave the way for numerous diseases ranging from inflammatory bowel disease (IBD) to colorectal cancer. With the rising understanding of gut health's paramount importance, the focus has been directed towards the dietary components that can modulate inflammation, and among them, resistant starch (RS) has emerged as a vital player.
RS, unlike other starches, resists digestion in the small intestine, reaching the colon largely intact (Birt et al., 2013). Once in the colon, RS acts as a substrate for certain beneficial gut bacteria, leading to the production of short-chain fatty acids (SCFAs), primarily acetate, propionate, and butyrate. Notably, butyrate is recognized for its significant anti-inflammatory effects (Hamer et al., 2008; P. Liu et al., 2021). Butyrate exerts its role by inhibiting the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which play central roles in propagating inflammation (Segain et al., 2000).
Moreover, RS fermentation and subsequent SCFA production have been shown to influence immune cell differentiation, especially regulatory T cells (Tregs). These cells play an indispensable role in maintaining immune homeostasis in the gut. Elevated numbers of Tregs have been associated with reduced inflammation, a testament to their ability to suppress aberrant immune responses (Furusawa et al., 2013). The SCFAs, particularly propionate, influence the differentiation of naïve T cells into Tregs, ensuring a balanced immune response within the gut.
The enteric nervous system (ENS) functions subtly, ensuring gut motility and secretion while interacting closely with the immune system. Disruptions in the ENS can cause gut dysmotility, creating an environment conducive for bacterial overgrowth and inflammation. RS, through its metabolites, especially butyrate, influences ENS functionality. It aids in maintaining the health and function of enteric neurons, subsequently promoting regular muscular contractions of the bowel, minimizing the chances of bacterial stasis and inflammation (Rao & Gershon, 2016).
Additionally, RS can influence gut inflammation by adjusting the gut's pH. The production of short-chain fatty acids (SCFAs) from RS fermentation leads to a slightly acidic environment in the colon. This acidity discourages the growth of pathogenic bacteria while fostering the proliferation of beneficial commensal bacteria.. The balance between these two bacterial groups is pivotal for maintaining gut health, and any shift towards pathogenic dominance, termed as dysbiosis, can incite inflammation. By maintaining an acidic pH, RS indirectly thwarts the initiation and progression of inflammation.
In summary, the intricate role of RS in modulating gut inflammation sheds light on its potential therapeutic applications. Its ability to alter the microbial composition, foster the production of anti-inflammatory SCFAs, influence immune cell differentiation, and maintain a balanced gut pH demonstrates its multifaceted approach in ensuring gut homeostasis. With the rising prevalence of gut-related inflammatory conditions, harnessing the benefits of RS could pave the way for novel dietary interventions that offer both preventive and therapeutic potential.
5.3. RS and immune system interactions
The interface between the gut and the immune system represents one of the most dynamic interactions within the human body. Ensconced within the gut, nearly 70 % of our entire immune system is ready to respond to various antigens from food and pathogenic organisms. It is within this context that dietary components like resistant starch (RS) take center stage. Not merely a bystander in the digestive process, RS shapes and influences the gut's immune responses in a myriad of ways.
RS, inherently resistant to digestion in the upper gastrointestinal tract, reaching the colon mostly unchanged (Bindels et al., 2015). In the colon, RS is fermented by specific bacterial populations, leading to an increase in the production of short-chain fatty acids (SCFAs), predominantly acetate, propionate, and butyrate. Beyond their role as energy substrates for colonocytes, these SCFAs modulate various immune cell functions. For instance, SCFAs can decrease the expression of inflammatory cytokines and increase anti-inflammatory mediators, effectively damping excessive immune responses. Butyrate, in particular, has been shown to have profound effects on neutrophil function and reduce the production of inflammatory mediators such as TNF-α and IL-6 (Vinolo, Rodrigues, Hatanaka, et al., 2011).
The gut-associated lymphoid tissue (GALT) is an integral part of the immune system and plays a crucial role in maintaining gut homeostasis. Within the GALT, dendritic cells continually sample the gut's luminal contents. These cells, upon encountering bacterial metabolites such as SCFAs produced from RS fermentation, their activity is modulated, leading to an increased production of regulatory T cells, which play a pivotal role in controlling inflammation and autoimmunity (P. M. Smith et al., 2013). Moreover, the direct impact of SCFAs on macrophages has been noted, with increased anti-inflammatory cytokine production and decreased pro-inflammatory cytokine production being observed.
Furthermore, RS fermentation products can influence the integrity of the gut barrier. Tight junction proteins, which maintain the continuity of the intestinal epithelial layer, are upregulated by SCFAs, thereby enhancing barrier function and reducing the translocation of bacterial endotoxins like lipopolysaccharide (LPS) into systemic circulation (Peng et al., 2009). Reduced LPS translocation leads to a decrease in endotoxemia-associated immune activation, benefiting overall systemic health.
The symbiotic relationship between gut bacteria and the host has been emphasized in numerous studies. RS acts as a prebiotic, selectively nourishing beneficial bacterial populations, which in turn positively modulate immune responses. For instance, the enrichment of beneficial bacteria like Bifidobacteria and Lactobacilli, commonly associated with RS consumption, is linked to enhanced production of immunoglobulin A (IgA), the primary antibody in mucosal defense. Elevated IgA levels play a pivotal role in neutralizing pathogens and maintaining mucosal homeostasis (Nayak, 2010, Yan and Polk, 2011, Zoumpopoulou et al., 2017).
In essence, the interaction between RS and the immune system underscores the complex interplay between diet, microbiota, and immunity. Through its fermentation products and the modulation of gut microbiota, RS holds the potential to be a significant dietary component in regulating immune responses and maintaining gut health. Its influence extends beyond the gut, providing systemic benefits and opening avenues for dietary strategies in immune modulation.
6. Dietary implications of RS consumption
6.1. Evaluating foods rich in RS: Benefits and precautions
The dietary inclusion of foods rich in resistant starch (RS) has garnered increasing attention in recent years, due to its multifaceted health benefits ranging from gut health enhancement to modulating systemic metabolism. As an essential dietary component, RS behaves differently than typical starch, primarily due to its resistance to digestion in the small intestine. Instead, it reaches the large intestine largely intact, where it serves as a substrate for microbial fermentation, producing beneficial metabolites like short-chain fatty acids (SCFAs) (Martínez et al., 2010).
Foods naturally abundant in RS include green bananas, legumes, whole grains, and certain types of cooked-then-cooled foods, such as potatoes and rice. Among the benefits of consuming such foods is their potential to enhance glycemic control. RS-rich foods have a lower glycemic index, translating into slower postprandial blood glucose increases (Nugent, 2005). This characteristic is particularly beneficial for individuals with metabolic disorders like diabetes. Furthermore, the SCFAs produced from RS fermentation, particularly butyrate, contribute significantly to gut health. Butyrate serves as a primary energy source for colonocytes and exhibits anti-inflammatory properties, making it indispensable for colonic health (Canani et al., 2011).
However, despite these advantages, some precautions are warranted. A rapid and substantial increase in RS intake can lead to gastrointestinal discomfort, including bloating, gas, and changes in bowel habits. It is generally advised to gradually introduce RS-rich foods into the diet to allow the gut microbiota time to adapt (Birt et al., 2013). Additionally, individuals with certain health conditions, such as those with irritable bowel syndrome (IBS) or specific carbohydrate intolerance, should approach RS-rich foods with caution and under professional guidance. The fermentation of RS can sometimes exacerbate symptoms in these individuals.
In conclusion, while foods rich in RS offer numerous health benefits, especially concerning gut health and metabolic regulation, individuals should be mindful of the sources and amounts of RS they incorporate into their diets. It's essential to strike a balance: optimizing health benefits while minimizing potential adverse effects. As research continues to shed light on the nuanced interactions between diet, gut health, and systemic health, RS-rich foods are poised to play a central role in future dietary recommendations.
6.2. RS in the context of dietary patterns and regimes
The role of resistant starch (RS) transcends its individual benefits, establishing it as a key component in various dietary patterns and regimes. For instance, when we consider the Mediterranean diet, hailed for its heart-protective benefits, we find legumes – a natural source of RS – as a cornerstone of its composition. The regular consumption of legumes, with their rich RS content, can contribute not only to enhanced gut health but also to the cardioprotective effects associated with this dietary pattern, given RS's potential to modulate postprandial blood glucose responses (Davis et al., 2015).
The paleolithic diet, commonly known as the paleo diet, is another intriguing realm wherein RS finds relevance. Contemporary interpretations of the paleo diet focus on the consumption of tubers and certain roots, which become rich in RS when prepared through specific methods, such as cooling after cooking. This gives credence to the notion that our ancestors might have consumed significant quantities of RS, providing a fermentable substrate for their gut microbiota. It is theorized that the symbiotic relationship between gut microbes and their host is believed to have co-evolved over millennia, and RS might have been a crucial dietary element driving this evolutionary journey (Spreadbury, 2012).
Furthermore, low-carb and ketogenic diets, popular for weight loss and metabolic health, typically limit starch consumption. However, integrating RS into these diets can offer a distinct advantage. Since RS doesn't exhibit the same digestibility as regular starch, its inclusion does not significantly raise blood sugar levels. This means individuals on such diets can reap the benefits of RS, like enhanced gut health and satiety, without compromising the state of ketosis or low-carb regimen (Kirkpatrick et al., 2019, Paoli et al., 2013). Fundamentally, RS allows for a symbiotic relationship between contemporary dietary approaches focused on weight loss or metabolic benefits and the ancient evolutionary importance of nourishing the gut microbiota.
6.3. Practical recommendations for RS intake and dietary integration
Incorporating resistant starch (RS) into one's diet is not just about acknowledging its physiological benefits, but also understanding its optimal intake and integration within diverse diets to maximize its potential. As the growing body of evidence continues to delineate the multifaceted advantages of RS, from modulating the gut microbiota to regulating blood glucose levels, it becomes crucial to provide actionable guidelines to the wider public.
To begin, it's essential to recognize that not all sources of RS are created equal. While legumes, whole grains, and certain tubers are naturally rich in RS, cooking method can further modulate their RS content. For instance, cooking and then cooling starchy foods like potatoes or rice can increase their RS content, offering a straightforward tactic to boost dietary RS levels without any drastic changes. The recommended daily intake of RS is typically between 15 and 30 g. This can be achieved through a varied diet, including foods like cold pasta salads, overnight oats, or legume-based dishes.
Moreover, it's equally pivotal to consider the individual's digestive tolerance. A sudden surge in RS intake can lead to gastrointestinal discomfort for some. Therefore, it's prudent to gradually enhance RS consumption over several weeks, allowing the gut to adjust (Birt et al., 2013). Furthermore, combining RS-rich foods with probiotic foods, such as yogurt or kefir, can create a synergistic effect, fostering a hospitable environment for beneficial gut bacteria to thrive. As with any dietary change, incorporating RS should be customized to suit individual tastes, health conditions, and eating habits, ensuring the approach is both balanced and sustainable.
7. Broader metabolic outcomes of RS consumption
7.1. RS, VFAs, and metabolic syndrome
Resistant starch (RS) is increasingly recognized as a crucial dietary component, not just for its immediate impact on gut health, but also for its wider metabolic implications, particularly in relation to metabolic syndrome. Metabolic syndrome, a cluster of conditions including increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels, heightens the risk of heart disease, stroke, and diabetes. The potential of RS to mitigate aspects of metabolic syndrome is largely attributed to its fermentation by gut microbiota into volatile fatty acids (VFAs) like acetate, propionate, and butyrate (Den Besten et al., 2013, Felizardo et al., 2019; Morrison et al., 2016; Nogal et al., 2021).
VFAs, especially butyrate, play a significant role in maintaining gut barrier integrity and possess anti-inflammatory properties, essential for combating the inflammatory processes associated with metabolic syndrome (Morrison et al., 2016). Butyrate, in particular, is associated with improved insulin sensitivity, a key factor in metabolic syndrome, by enhancing energy expenditure and fat oxidation in the colon. Additionally, propionate has a gluconeogenic effect, which has the potential to regulate blood sugar levels, crucial for those with or at risk of type 2 diabetes (Canfora et al., 2015).
Furthermore, the role of RS in appetite regulation deserves mention. As VFAs are produced, they stimulate the release of anorexigenic hormones like peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), leading to increased satiety and reduced calorie intake. This appetite-regulating effect, combined with the potential benefits on lipid profiles and blood pressure, makes RS consumption a promising strategy for preventing or managing metabolic syndrome (Chambers et al., 2015).
7.2. Implications for weight management and obesity
Weight management and the global challenge of obesity are intrinsically linked to dietary components and their metabolic outcomes. The rise in obesity is paralleled by increased risks of type 2 diabetes, cardiovascular diseases, and several types of cancer, making it an issue of paramount health concern (Swinburn et al., 2011). In this light, resistant starch (RS) stands out not just as a dietary fiber but as a potentially transformative dietary component in the fight against obesity.
Several studies have indicated that RS may have a direct influence on weight management. A primary mechanism is the thermic effect of food, a measure of energy expended while digesting and processing food. RS, being resistant to immediate digestion, tends to increase this thermic effect, leading to higher energy expenditure during its fermentation in the large intestine. This not only contributes to a negative energy balance but also impacts fat storage and enhances fat oxidation, crucial for weight management (Johnston et al., 2010). Additionally, as highlighted in previous sections, RS fermentation leads to the production of short-chain fatty acids (SCFAs), which play an active role in controlling appetite through the release of hormones like PYY and GLP-1. A regulated appetite equates to a reduced caloric intake, an essential aspect of weight management (J. Zhou et al., 2008).
Moreover, RS has been linked with improved gut health, which has a consequential effect on obesity. A healthy gut biome is associated with a leaner phenotype. When the gut microbiota ferments RS, it leads to a shift in the microbial composition favoring beneficial bacteria that have been inversely associated with obesity. As such, consistent consumption of RS-rich foods may lead to a gut environment less predisposed to weight gain and obesity (Martínez et al., 2013).
7.3. Rs's role in diabetes and glycemic control
Diabetes, a metabolic disorder characterized by chronic hyperglycemia, is an escalating global health concern with multi-faceted implications ranging from individual health deterioration to national economic burdens (Cho et al., 2018). Given the mounting incidence, there's an emergent need for dietary interventions that can alleviate or possibly reverse the progression of this disorder. Resistant starch (RS) emerges as a compelling dietary component with its multifarious metabolic impacts that bear relevance to diabetes management and glycemic control.
Central to the management of diabetes is the regulation of postprandial glucose and insulin responses. The ingestion of RS appears to modulate these responses advantageously. Unlike rapidly digestible starch, RS does not contribute directly to postprandial glucose spikes due to its bypass of digestion in the small intestine (Robertson, 2012). Instead, its fermentation in the large intestine produces short-chain fatty acids (SCFAs) that exert systemic effects. Particularly, propionate, one of the SCFAs, has been observed to promote hepatic glucose production regulation, reducing the risk of post-meal glucose excursions (Den Besten et al., 2015). Further, the SCFA butyrate has recognized for its role in boosting the secretion of glucagon-like peptide-1 (GLP-1), a hormone that enhances insulin secretion and reduces glucagon release, harmonizing blood glucose levels (Canfora et al., 2015).
Moreover, long-term RS consumption has been associated with improved insulin sensitivity, a critical factor in the pathogenesis of type 2 diabetes. Studies have shown that individuals with insulin resistance who consumed RS-enriched diets manifested marked improvements in insulin sensitivity (Belobrajdic et al., 2012, Keenan et al., 2015, Maki et al., 2012). This improvement is thought to be connected to the anti-inflammatory properties of SCFAs, especially butyrate, and its role in reducing oxidative stress, which contributes to insulin resistance (Vinolo, Rodrigues, Nachbar, et al., 2011). Additionally, RS's ability to foster a beneficial gut microbiota composition has indirect effects on metabolic health, further emphasizing its potential role in diabetes management.
8. Future directions and challenges
8.1. Challenges in RS Research: Varied RS sources and individual responses
Resistant starch (RS) has gained notoriety in the scientific community due to its potential health benefits. Yet, the dynamic nature of RS research presents a set of challenges that need thoughtful consideration to refine our understanding and application.
A primary challenge in RS research is the inherent variability in RS sources. While it's known that RS can be found in foods such as legumes, grains, and certain tubers, the precise RS content can vary significantly depending on factors such as the type, ripeness, processing, and cooking methods (Birt et al., 2013). For instance, the amount of RS in a banana changes with ripeness; unripe bananas contain higher levels of RS compared to their ripe counterparts (Moongngarm et al., 2014). Similarly, the method of cooking and cooling starchy foods like potatoes or rice can alter their RS content (Z. Zhou et al., 2002). This variability poses challenges when designing dietary interventions or generalizing results from one study to a broader context.
Furthermore, individual responses to resistant starch (RS) can vary significantly. Factors such as age, genetics, gut microbiota composition, and overall health status can influence how one processes and benefits from RS intake (E. D. Sonnenburg & Sonnenburg, 2014). For example, two individuals consuming the same amount of RS might show different postprandial glucose responses or different profiles of short-chain fatty acid production in the colon. Additionally, the capacity of the gut microbiota to ferment RS into beneficial metabolites can differ between individuals, particularly between those with a diverse microbiota and those with a less varied microbial community (De Filippis et al., 2016).
This individual variability underscores the necessity for personalized nutrition approaches. Rather than a one-size-fits-all recommendation for RS intake, there might be a need to customize dietary advice based on an individual's unique metabolic and microbial signatures. As we delve deeper into the era of personalized medicine, integrating genomic and metagenomic data might provide more precise insights into optimizing RS intake for individual health benefits (Zhernakova et al., 2016).
In the panorama of RS research, addressing these challenges requires rigorous experimental designs, more extensive cohort studies, and interdisciplinary collaborations. This would encompass not just nutritionists and dietitians, but also microbiologists, geneticists, and data scientists to harness the full potential of RS in human health.
8.2. Potential of RS in therapeutic interventions
The potential of resistant starch (RS) in therapeutic applications has piqued the interest of researchers worldwide. Beyond its nutritional attributes, the myriad of physiological benefits exhibited by RS offers intriguing possibilities for harnessing its therapeutic properties.
An intriguing avenue is the use of RS as a prebiotic. The ability of RS to modulate the gut microbiota composition has garnered attention, especially concerning gastrointestinal disorders (Gibson & Roberfroid, 1995). Dysbiosis, an imbalance in the gut microbiota, has been implicated in various diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer (Guarner and Malagelada, 2003, Sekirov et al., 2010). RS has shown promise in restoring gut microbial balance, providing a basis for its potential use as an adjunct treatment for these conditions. For instance, dietary interventions that include RS have led to a notable increase in beneficial Bifidobacteria and a reduction in pathogenic bacteria in IBD patients (Benjamin et al., 2011).
Another therapeutic implication of RS revolves around its anti-inflammatory properties. Chronic inflammation plays a pivotal role in the onset and progression of numerous diseases, such as cardiovascular disease, type 2 diabetes, and certain cancers (Chávez-Talavera et al., 2017). The fermentation of RS by the gut microbiota produces short-chain fatty acids (SCFAs), notably butyrate, which has anti-inflammatory effects (Vinolo, Rodrigues, Nachbar, et al., 2011). Butyrate can inhibit pro-inflammatory cytokines, making RS a potential candidate for mitigating inflammation-driven diseases.
In the realm of metabolic disorders, RS holds promise as a dietary strategy to combat obesity and associated complications. Given its ability to modulate satiety, improve insulin sensitivity, and regulate lipid metabolism, RS may serve as an adjunct or preventive measure for metabolic syndrome and related conditions (Keenan et al., 2006). For instance, incorporating RS into the diet has led to improved postprandial glucose responses in individuals with impaired glucose tolerance, underlining its potential therapeutic relevance (Robertson et al., 2005).
Cancer prevention and treatment is yet another domain where RS exhibits potential. Specifically, RS’s role in reducing the risk of colorectal cancer has been of particular focus (Bingham et al., 2003). It’s hypothesized that the protective effect stems from the production of SCFAs during RS fermentation, which can induce apoptosis in cancerous cells. Moreover, RS can influence gene expression in colon cells, potentially providing a mechanistic link to its anticancer effects.
While these therapeutic implications of RS offer immense promise, it's essential to approach with caution. Dose, duration, and individual variability play critical roles in determining the efficacy of RS in any therapeutic application. Moreover, clinical trials with rigorous designs are imperative to solidify the evidence base and ensure the safety and effectiveness of RS-based interventions.
In conclusion, RS is not just another dietary component; its therapeutic potential spans across a range of clinical scenarios. From modulating the gut microbiota and reducing inflammation to mitigating metabolic disorders and potential anticancer properties, RS stands out as a compelling candidate for future therapeutic interventions. However, further research and well-structured clinical trials are crucial to translate this potential into tangible health benefits.
8.3. Avenues for future investigations and Emerging RS technologies
The multifaceted nature of resistant starch (RS) and its implications in health and disease suggest a vast and promising landscape for future research. As science continues to uncover the manifold benefits of RS, it becomes crucial to identify the gaps and direct efforts towards innovative investigations and emerging technologies that could further elucidate the full spectrum of RS's potential.
A promising area of research is the deeper exploration of the symbiotic relationship between RS and gut microbiota. Recent studies have highlighted the critical role of microbiota in human health, from metabolic processes to immune responses and even mental health (Lynch et al., 2016). Unraveling the particular microbial species influenced by different RS types and their subsequent metabolic outputs would offer more precise dietary recommendations. Furthermore, utilizing advanced metagenomic and metabolomic techniques can provide deeper insights into how RS modulates gut microbial ecology and its systemic implications.
Emerging technologies are also set to revolutionize the way RS is incorporated into the diet. Nanotechnology, for instance, offers the potential to design and engineer RS at a molecular level, enhancing its therapeutic potential (M. Zhang & Merlin, 2018). With advancements in encapsulation techniques, it's possible to deliver RS more effectively to specific regions of the gastrointestinal tract, maximizing its fermentative benefits. Furthermore, these technological innovations can aid in creating RS-enhanced foods that are not only effective but also more appealing in taste and texture, thereby boosting consumer acceptability and adherence to RS-rich dietary interventions.
Additionally, the potential of RS in personalized nutrition cannot be overlooked. Given the variability in individual responses to RS, owing to genetic, microbial, and metabolic differences, tailoring RS interventions based on individual profiles can optimize benefits (Zeevi et al., 2015). Advancements in genomics and metabolomics could pave the way for such personalized approaches, ensuring that RS interventions are not only effective but also tailored to individual needs and preferences.
In summary, while the current understanding of RS is substantial, the horizon is ripe with opportunities for novel investigations and technological innovations. Embracing these avenues will not only deepen our knowledge of RS but also harness its potential in novel and impactful ways.
9. Conclusion
The multifaceted role of resistant starch (RS) in human health has emerged as a focal point of recent nutritional research. As elucidated, RS plays a significant role in modulating gut microbiota, leading to beneficial metabolic outcomes, notably in metabolic syndrome, weight management, obesity, diabetes, and glycemic control. Challenges in RS research, stemming from varied sources and individual responses, are matched by its therapeutic potential and the innovation in RS-related technologies. The increasing focus on RS emphasizes its centrality in the nexus between diet, health, and disease, promising tailored nutritional interventions and a holistic approach to health in the future. The journey through RS is not just academic; it underscores a shift towards recognizing the intricate interplay of dietary components in human well-being.
CRediT authorship contribution statement
Zhao Chena: Conceptualization, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. Ning Liang: Investigation, Methodology, Writing – original draft. Haili Zhang: . Huizhen Li: Investigation, Methodology, Writing – original draft, Writing – review & editing. Jing Guo: Investigation, Methodology, Writing – review & editing. Yujing Zhang: Investigation, Methodology, Writing – review & editing. Yaxin Chen: Investigation, Methodology, Writing – review & editing. Yanping Wang: Supervision. Nannan Shi: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thanks anonymous reviewers for their valuable comments and improvement suggestions that further lead us to improve this paper. We thanked the China Academy of Chinese Medical Sciences’ Independent Selection Project (Z0830) and Institute of Basic Research in Clinical Medicine’s Independent Selection Project, China Academy of Chinese Medical Sciences (Z0830-1) for their support for this work.
Contributor Information
Yanping Wang, Email: wangyanping4816@163.com.
Nannan Shi, Email: 13811839164@vip.126.com.
Data availability
No data was used for the research described in the article.
References
- Aguilera Y., Esteban R.M., Benitez V., Molla E., Martin-Cabrejas M.A. Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. Journal of agricultural and food chemistry. 2009;57(22):10682–10688. doi: 10.1021/jf902042r. [DOI] [PubMed] [Google Scholar]
- Alsaffar A.A. Effect of food processing on the resistant starch content of cereals and cereal products–a review. International Journal of Food Science & Technology. 2011;46(3):455–462. doi: 10.1111/j.1365-2621.2010.02529.x. [DOI] [Google Scholar]
- Arpaia N., Campbell C., Fan X., Dikiy S., Van Der Veeken J., Deroos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwar B.A., Gani A., Shah A., Wani I.A., Masoodi F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke. 2016;68(3–4):287–301. doi: 10.1002/star.201500064. [DOI] [Google Scholar]
- Barros F., Awika J., Rooney L.W. Effect of molecular weight profile of sorghum proanthocyanidins on resistant starch formation. Journal of the Science of Food and Agriculture. 2014;94(6):1212–1217. doi: 10.1002/jsfa.6400. [DOI] [PubMed] [Google Scholar]
- Belobrajdic D.P., King R.A., Christophersen C.T., Bird A.R. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutrition & Metabolism. 2012;9:1–10. doi: 10.1186/1743-7075-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin J.L., Hedin C.R., Koutsoumpas A., Ng S.C., McCarthy N.E., Hart A.L., Kamm M.A., Sanderson J.D., Knight S.C., Forbes A. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60(7):923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- Bindels L.B., Walter J., Ramer-Tait A.E. Resistant starches for the management of metabolic diseases. Current Opinion in Clinical Nutrition and Metabolic Care. 2015;18(6):559. doi: 10.1097/MCO.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham S.A., Day N.E., Luben R., Ferrari P., Slimani N., Norat T., Clavel-Chapelon F., Kesse E., Nieters A., Boeing H. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. The Lancet. 2003;361(9368):1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- Birt D.F., Boylston T., Hendrich S., Jane J.-L., Hollis J., Li L., McClelland J., Moore S., Phillips G.J., Rowling M. Resistant starch: Promise for improving human health. Advances in Nutrition. 2013;4(6):587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarczuk A., Skąpska S., Khaneghah A.M., Marszałek K. Health benefits of resistant starch: A review of the literature. Journal of Functional Foods. 2022;93 doi: 10.1016/j.jff.2022.105094. [DOI] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns F., Hemery Y., Price R., Anson N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Critical reviews in food science and nutrition. 2012;52(6):553–568. doi: 10.1080/10408398.2011.589540. [DOI] [PubMed] [Google Scholar]
- Brown I., Warhurst M., Arcot J., Playne M., Illman R.J., Topping D.L. Fecal numbers of bifidobacteria are higher in pigs fed Bifidobacterium longum with a high amylose cornstarch than with a low amylose cornstarch. The Journal of nutrition. 1997;127(9):1822–1827. doi: 10.1093/jn/127.9.1822. [DOI] [PubMed] [Google Scholar]
- Brummer Y., Kaviani M., Tosh S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Research International. 2015;67:117–125. doi: 10.1016/j.foodres.2014.11.009. [DOI] [Google Scholar]
- Canani R.B., Di Costanzo M., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World journal of gastroenterology: WJG. 2011;17(12):1519. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature Reviews Endocrinology. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- Cao R., Liu X., Liu Y., Zhai X., Cao T., Wang A., Qiu J. Applications of nuclear magnetic resonance spectroscopy to the evaluation of complex food constituents. Food chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128258. [DOI] [PubMed] [Google Scholar]
- Chambers E.S., Morrison D.J., Frost G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proceedings of the Nutrition Society. 2015;74(3):328–336. doi: 10.1017/s0029665114001657. [DOI] [PubMed] [Google Scholar]
- Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7) doi: 10.1053/j.gastro.2017.01.055. 1679–1694. e1673. [DOI] [PubMed] [Google Scholar]
- Cho N.H., Shaw J., Karuranga S., Huang Y., da Rocha Fernandes J., Ohlrogge A., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.R., Cusanovich D.A., Michelini K., Barreiro L.B., Ober C., Gilad Y. Genome-wide association studies of the human gut microbiota. PloS One. 2015;10(11):e0140301. doi: 10.1371/journal.pone.0140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Bryan J., Hodgson J., Murphy K. Definition of the Mediterranean diet: A literature review. Nutrients. 2015;7(11):9139–9153. doi: 10.3390/nu7115459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Costa G.E., da Silva Queiroz-Monici K., Reis S.M.P.M., de Oliveira A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food chemistry. 2006;94(3):327–330. doi: 10.1016/j.foodchem.2004.11.020. [DOI] [Google Scholar]
- De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H., Oosterveer M.H., Jonker J.W., Groen A.K., Reijngoud D.-J. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- Den Besten G., Van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englyst H.N., Hudson G.J. The classification and measurement of dietary carbohydrates. Food chemistry. 1996;57(1):15–21. doi: 10.1016/0308-8146(96)00056-8. [DOI] [Google Scholar]
- Everard A., Cani P.D. Diabetes, obesity and gut microbiota. Best practice & research Clinical gastroenterology. 2013;27(1):73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Felizardo R.J.F., Watanabe I.M., Dardi P., Rossoni L.V., Câmara N.O.S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacological research. 2019;141:366–377. doi: 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Fuentes-Zaragoza E., Riquelme-Navarrete M., Sánchez-Zapata E., Pérez-Álvarez J. Resistant starch as functional ingredient: A review. Food Research International. 2010;43(4):931–942. doi: 10.1016/j.foodres.2010.02.004. [DOI] [Google Scholar]
- Furrer A.N., Chegeni M., Ferruzzi M.G. Impact of potato processing on nutrients, phytochemicals, and human health. Critical reviews in food science and nutrition. 2018;58(1):146–168. doi: 10.1080/10408398.2016.1139542. [DOI] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gänzle M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Current Opinion in Food Science. 2015;2:106–117. doi: 10.1016/j.cofs.2015.03.001. [DOI] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. The Journal of nutrition. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- González-Bosch C., Boorman E., Zunszain P.A., Mann G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox biology. 2021;47 doi: 10.1016/j.redox.2021.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F., Malagelada J.-R. Gut flora in health and disease. The Lancet. 2003;361(9356):512–519. doi: 10.1016/s0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Troost F., Brummer R.J. The role of butyrate on colonic function. Alimentary Pharmacology and Therapeutics. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Haralampu S. Resistant starch—a review of the physical properties and biological impact of RS3. Carbohydrate Polymers. 2000;41(3):285–292. doi: 10.1016/S0144-8617(99)00147-2. [DOI] [Google Scholar]
- Harris K.F. An introductory review of resistant starch type 2 from high-amylose cereal grains and its effect on glucose and insulin homeostasis. Nutrition Reviews. 2019;77(11):748–764. doi: 10.1093/nutrit/nuz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Qiu C., Liao Z., Sui Z., Corke H. Impact of cooking conditions on the properties of rice: Combined temperature and cooking time. International Journal of Biological Macromolecules. 2018;117:87–94. doi: 10.1016/j.ijbiomac.2018.05.139. [DOI] [PubMed] [Google Scholar]
- Hu X., Xu X., Jin Z., Tian Y., Bai Y., Xie Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP) Journal of Food Engineering. 2011;106(3):262–266. doi: 10.1016/j.jfoodeng.2011.05.021. [DOI] [Google Scholar]
- Jane J.-L. Structural features of starch granules II. Starch. 2009;193–236 doi: 10.1016/b978-0-12-746275-2.00006-9. [DOI] [Google Scholar]
- Johnston K., Thomas E.L., Bell J.D., Frost G., Robertson M.D. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabetic Medicine. 2010;27(4):391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- Keenan M.J., Zhou J., Hegsted M., Pelkman C., Durham H.A., Coulon D.B., Martin R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Advances in Nutrition. 2015;6(2):198–205. doi: 10.3945/an.114.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan M.J., Zhou J., McCutcheon K.L., Raggio A.M., Bateman H.G., Todd E., Jones C.K., Tulley R.T., Melton S., Martin R.J. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14(9):1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, C. F., Bolick, J. P., Kris-Etherton, P. M., Sikand, G., Aspry, K. E., Soffer, D. E., Willard, K.-E., & Maki, K. C. (2019). Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. Journal of Clinical Lipidology, 13(5), 689-711. e681. https://doi.org/10.1016/j.jacl.2019.08.003. [DOI] [PubMed]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Awana M., Samota M.K., Warwate S.I., Kulshreshtha A., Ray M., Bollinedi H., Singh A.K., Thandapilly S.J., Praveen S. Pullulanase activity: A novel indicator of inherent resistant starch in rice (Oryza sativa. L) International Journal of Biological Macromolecules. 2020;152:1213–1223. doi: 10.1016/j.ijbiomac.2019.10.218. [DOI] [PubMed] [Google Scholar]
- Lal M.K., Tiwari R.K., Kumar R., Naga K.C., Kumar A., Singh B., Raigond P., Dutt S., Chourasia K.N., Kumar D. Effect of potato apical leaf curl disease on glycemic index and resistant starch of potato (Solanum tuberosum L.) tubers. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129939. [DOI] [PubMed] [Google Scholar]
- LeBlanc J.G., Chain F., Martín R., Bermúdez-Humarán L.G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microbial cell factories. 2017;16(1):1–10. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonel A.J., Alvarez-Leite J.I. Butyrate: Implications for intestinal function. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15(5):474–479. doi: 10.1097/MCO.0b013e32835665fa. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: A double-edged sword for health? Advances in Nutrition. 2018;9(1):21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Wang Y., Yang G., Zhang Q., Meng L., Xin Y., Jiang X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacological research. 2021;165 doi: 10.1016/j.phrs.2021.105420. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology. 2017;19(1):29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. New England Journal of Medicine. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proceedings of the Nutrition Society. 2003;62(1):67–72. doi: 10.1079/pns2002207. [DOI] [PubMed] [Google Scholar]
- Maki K.C., Pelkman C.L., Finocchiaro E.T., Kelley K.M., Lawless A.L., Schild A.L., Rains T.M. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. The Journal of nutrition. 2012;142(4):717–723. doi: 10.3945/jn.111.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangala S., Ramesh H., Udayasankar K., Tharanathan R. Resistant starch derived from processed ragi (finger millet, Eleusine coracana) flour: Structural characterization. Food chemistry. 1999;64(4):475–479. doi: 10.1016/s0308-8146(98)00129-0. [DOI] [Google Scholar]
- Martínez I., Kim J., Duffy P.R., Schlegel V.L., Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PloS One. 2010;5(11):e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G., Louk J.A., Rose D.J., Kyureghian G., Peterson D.A. Gut microbiome composition is linked to whole grain-induced immunological improvements. The ISME journal. 2013;7(2):269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary B.V., Monaghan D.A. Measurement of resistant starch. Journal of AOAC International. 2002;85(3):665–675. doi: 10.1093/jaoac/85.3.665. [DOI] [PubMed] [Google Scholar]
- Moongngarm A., Tiboonbun W., Sanpong M., Sriwong P., Phiewtong L., Prakitrum R., Huychan N. Resistant starch and bioactive contents of unripe banana flour as influenced by harvesting periods and its application. American Journal of Agricultural and Biological Sciences. 2014;9(3):457–465. doi: 10.3844/ajabssp.2014.457.465. [DOI] [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut microbes. 2016;7(3):189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S.K. Probiotics and immunity: A fish perspective. Fish & Shellfish Immunology. 2010;29(1):2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut microbes. 2021;13(1):1897212. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A.P. Health properties of resistant starch. Nutrition Bulletin. 2005;30(1):27–54. doi: 10.1111/j.1467-3010.2005.00481.x. [DOI] [Google Scholar]
- O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- Paoli A., Rubini A., Volek J., Grimaldi K. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition. 2013;67(8):789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Li Z.-R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition. 2009;139(9):1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera A., Meda V., Tyler R. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Research International. 2010;43(8):1959–1974. doi: 10.1016/j.foodres.2010.06.003. [DOI] [Google Scholar]
- Perry R.J., Peng L., Barry N.A., Cline G.W., Zhang D., Cardone R.L., Petersen K.F., Kibbey R.G., Goodman A.L., Shulman G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.-X., Rey F., Wang T. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatz S.K., Idso L., Johnson L.K., Jackson M.I., Combs G.F., Jr Resistant starch analysis of commonly consumed potatoes: Content varies by cooking method and service temperature but not by variety. Food Chemistry. 2016;208:297–300. doi: 10.1016/j.foodchem.2016.03.120. [DOI] [PubMed] [Google Scholar]
- Raigond P., Ezekiel R., Raigond B. Resistant starch in food: A review. Journal of the Science of Food and Agriculture. 2015;95(10):1968–1978. doi: 10.1002/jsfa.6966. [DOI] [PubMed] [Google Scholar]
- Rao M., Gershon M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nature reviews Gastroenterology & hepatology. 2016;13(9):517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., De Los Reyes-gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Frontiers in Microbiology. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M.D. Dietary-resistant starch and glucose metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15(4):362–367. doi: 10.1097/MCO.0b013e3283536931. [DOI] [PubMed] [Google Scholar]
- Robertson M.D., Bickerton A.S., Dennis A.L., Vidal H., Frayn K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism–. The American journal of clinical nutrition. 2005;82(3):559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- Sajilata M.G., Singhal R.S., Kulkarni P.R. Resistant starch–a review. Comprehensive reviews in food science and food safety. 2006;5(1):1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Samuel B.S., Gordon J.I. A humanized gnotobiotic mouse model of host–archaeal–bacterial mutualism. Proceedings of the National Academy of Sciences. 2006;103(26):10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y., Prakash O., Seib P.A. Characterization of phosphorylated cross-linked resistant starch by 31P nuclear magnetic resonance (31P NMR) spectroscopy. Carbohydrate Polymers. 2007;67(2):201–212. doi: 10.1016/j.carbpol.2006.05.009. [DOI] [Google Scholar]
- Segain J., De La Blétiere D.R., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., Blottiere H., Galmiche J. Butyrate inhibits inflammatory responses through NFκB inhibition: Implications for Crohn's disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiological Reviews. 2010 doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Shu X., Sun J., Wu D. Effects of grain development on formation of resistant starch in rice. Food chemistry. 2014;164:89–97. doi: 10.1016/j.foodchem.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Singh J., Dartois A., Kaur L. Starch digestibility in food matrix: A review. Trends in Food Science & Technology. 2010;21(4):168–180. doi: 10.1016/j.tifs.2009.12.001. [DOI] [Google Scholar]
- Smith A.M. The biosynthesis of starch granules. Biomacromolecules. 2001;2(2):335–341. doi: 10.1021/bm000133c. [DOI] [PubMed] [Google Scholar]
- Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-y M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg E.D., Sonnenburg J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metabolism. 2014;20(5):779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg J.L., Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes, metabolic syndrome and obesity: targets and therapy. 2012;175–189 doi: 10.2147/dmso.s33473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cellular and molecular life sciences. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L., Gortmaker S.L. The global obesity pandemic: Shaped by global drivers and local environments. The Lancet. 2011;378(9793):804–814. doi: 10.1016/s0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Thynn H.N., Chen X.-F., Hu W.-X., Duan Y.-Y., Zhu D.-L., Chen H., Wang N.-N., Chen H.-H., Rong Y., Lu B.-J. An allele-specific functional SNP associated with two systemic autoimmune diseases modulates IRF5 expression by long-range chromatin loop formation. Journal of Investigative Dermatology. 2020;140(2) doi: 10.1016/j.jid.2019.06.147. 348–360. e311. [DOI] [PubMed] [Google Scholar]
- Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nature reviews immunology. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Turroni F., Peano C., Pass D.A., Foroni E., Severgnini M., Claesson M.J., Kerr C., Hourihane J., Murray D., Fuligni F. Diversity of bifidobacteria within the infant gut microbiota. PloS One. 2012;7(5):e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Hatanaka E., Sato F.T., Sampaio S.C., Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. The Journal of Nutritional Biochemistry. 2011;22(9):849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The ISME journal. 2011;5(2):220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S.K., Lucey A., Walter J., Zannini E., Arendt E.K. Resistant starch—An accessible fiber ingredient acceptable to the Western palate. Comprehensive reviews in food science and food safety. 2022;21(3):2930–2955. doi: 10.1111/1541-4337.12955. [DOI] [PubMed] [Google Scholar]
- Wang S., Copeland L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food & Function. 2013;4(11):1564–1580. doi: 10.1039/C3FO60258C. [DOI] [PubMed] [Google Scholar]
- Yadav B.S., Sharma A., Yadav R.B. Studies on effect of multiple heating/cooling cycles on the resistant starch formation in cereals, legumes and tubers. International journal of food sciences and nutrition. 2009;60(sup4):258–272. doi: 10.1080/09637480902970975. [DOI] [PubMed] [Google Scholar]
- Yan F., Polk D. Probiotics and immune health. Current Opinion in Gastroenterology. 2011;27(6):496. doi: 10.1097/mog.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze X., Duncan S.H., Louis P., Flint H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. The ISME journal. 2012;6(8):1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang G., Hamaker B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Critical reviews in food science and nutrition. 2009;49(10):852–867. doi: 10.1080/10408390903372466. [DOI] [PubMed] [Google Scholar]
- Zhang M., Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflammatory Bowel Diseases. 2018;24(7):1401–1415. doi: 10.1093/ibd/izy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu W., Liu C., Luo S., Li T., Liu Y., Wu D., Zuo Y. Retrogradation behaviour of high-amylose rice starch prepared by improved extrusion cooking technology. Food Chemistry. 2014;158:255–261. doi: 10.1016/j.foodchem.2014.02.072. [DOI] [PubMed] [Google Scholar]
- Zhao X., Andersson M., Andersson R. Resistant starch and other dietary fiber components in tubers from a high-amylose potato. Food chemistry. 2018;251:58–63. doi: 10.1016/j.foodchem.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wei Z., Zhang R., Deng Y., Tang X., Zhang Y., Liu G., Liu L., Wang J., Liao N. Optimization of the autoclave preparation process for improving resistant starch content in rice grains. Food Science & Nutrition. 2020;8(5):2383–2394. doi: 10.1002/fsn3.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A., Kurilshikov A., Bonder M.J., Tigchelaar E.F., Schirmer M., Vatanen T., Mujagic Z., Vila A.V., Falony G., Vieira-Silva S. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Martin R.J., Tulley R.T., Raggio A.M., McCutcheon K.L., Shen L., Danna S.C., Tripathy S., Hegsted M., Keenan M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. American Journal of Physiology-Endocrinology and Metabolism. 2008;295(5):E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Robards K., Helliwell S., Blanchard C. Ageing of stored rice: Changes in chemical and physical attributes. Journal of Cereal Science. 2002;35(1):65–78. doi: 10.1006/jcrs.2001.0418. [DOI] [Google Scholar]
- Zoumpopoulou G., Pot B., Tsakalidou E., Papadimitriou K. Dairy probiotics: Beyond the role of promoting gut and immune health. International Dairy Journal. 2017;67:46–60. doi: 10.1016/j.idairyj.2016.09.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.