Abstract
Rickettsia rickettsii infection of endothelial cells is manifested in very distinctive changes in cell morphology, consisting of extensive dilatation of the membranes of the endoplasmic reticulum and outer nuclear envelope and blebbing of the plasma membrane, as seen by transmission electron microscopy (D. J. Silverman, Infect. Immun. 44:545–553, 1984). These changes in cellular architecture are thought to be due to oxidant-mediated cell injury, since their occurrence correlates with dramatic alterations in cellular metabolism, particularly with regard to antioxidant systems. In this study, it was shown that R. rickettsii infection of human umbilical vein endothelial cells resulted in a significant depletion of intracellular reduced glutathione (thiol) content at 72 and 96 h and decreased glutathione peroxidase activity at 72 h postinfection. Infected cells displayed a dramatic increase in the concentration of intracellular peroxides by 72 h. Supplementation of the cell culture medium with 100, 200, or 500 μM α-lipoic acid, a metabolic antioxidant, after inoculation with R. rickettsii restored the intracellular levels of thiols and glutathione peroxidase and reduced the intracellular peroxide levels in infected cells. These effects were dose dependent. Treated infected monolayers maintained better viability at 96 h after inoculation with R. rickettsii than did untreated infected cells. Moreover, supplementation of the cell culture medium with 100 μM α-lipoic acid for 72 h after infection prevented the occurrence of morphological changes in the infected cells. The presence of 100 or 200 μM α-lipoic acid did not influence rickettsial growth in endothelial cells, nor did it affect the ability of R. rickettsii to form lytic plaques in Vero cells. Treatment with 500 μM α-lipoic acid decreased by 50% both the number and size of lytic plaques in Vero cells, and it also decreased the recovery of viable rickettsiae from endothelial cells. However, under all treatment conditions, a significant number of rickettsiae could be detected microscopically. Furthermore, the rickettsiae apparently retained their capacity for intracellular movement, since they possessed long polymerized actin tails after 72 and 96 h of treatment regardless of the concentration of α-lipoic acid used. Since α-lipoic acid does not seem to exhibit direct antirickettsial activity except with long-term exposure at very high concentrations, the mechanism of its protective activity for endothelial cells infected with rickettsiae may involve complex changes in cellular metabolism that only indirectly affect rickettsiae.
Rickettsia rickettsii is an obligate intracellular bacterium which causes Rocky Mountain spotted fever in humans. In vivo, rickettsiae initially invade vascular endothelial cells, where they replicate in the cytoplasm without being surrounded by a phagolysosomal membrane (34, 36). R. rickettsii has a great capacity for intracellular movement, penetrating the nucleus, and spreading to adjacent cells, capabilities associated with the formation of an actin tail (15). Rickettsial multiplication eventually causes lethal injury of infected cells, which is manifested as peripheral vasculitis, microhemorrhage, and thrombosis (34).
At the ultrastructural level, R. rickettsii infection of human endothelial cells is evidenced by very distinctive changes in host cell morphology, consisting of extensive dilatation of the membranes of the endoplasmic reticulum and outer nuclear membrane and blebbing of the plasma membrane (23). These changes in cellular architecture are thought to be due to oxidant-mediated cell injury since their appearance correlates with dramatic alterations in cellular metabolism, particularly in the host cell antioxidant system, during intracellular multiplication of rickettsiae (9, 19, 24, 25). This effect is probably mediated by accumulation of oxidative radicals, which may cause peroxidation of internal membrane lipids (24). Experimental in vitro infection of human umbilical vein endothelial cells (HUVEC) with R. rickettsii causes the generation of large amounts of extracellular superoxide, which can be detected 1 h after inoculation with rickettsiae (19). Superoxide dismutase expression increases in response to superoxide influx; it reaches a stable level which is maintained from 6 to 48 h postinfection (19). Peroxides produced by the dismutase reaction are primarily scavenged by catalase (peroxide) and glutathione peroxidase, which simultaneously catalyze the oxidation of reduced glutathione and the conversion of toxic peroxides to innocuous by-products. Under conditions of normal cellular metabolism, balanced interactions of these three enzymes are necessary to protect the cellular environment against oxidative injury, but their levels are significantly altered in HUVEC infected with R. rickettsii (9). Two other antioxidant enzymes—glucose-6-phosphate dehydrogenase, which plays a key role in the reduction of glutathione through NADPH produced by the hexose monophosphate shunt, and glutathione reductase, which catalyzes the reduction of oxidized glutathione to reduced glutathione—also have reduced activities in HUVEC infected with R. rickettsii (9, 27). The mechanisms causing the alterations in the antioxidant cellular systems and/or the sequential order of these events following infection with R. rickettsii are not well understood.
α-Lipoic acid, a lipoamide, is a constituent of biological membranes and an important cofactor of mitochondrial dehydrogenases. Multiple functions of α-lipoic acid have been recently reviewed (17, 18). α-Lipoic acid is easily absorbed from the diet, and in mammalian cells it is readily converted to its reduced form, dihydrolipoic acid (DHLA). Both α-lipoic acid and DHLA act as antioxidants in vitro and in vivo. The specific antioxidant effects of α-lipoic acid and DHLA include quenching of reactive oxygen species such as superoxide radicals, hydroxyl radicals, peroxyl radicals, singlet oxygen, and hypochlorous acid; chelation of copper, zinc, and iron; and intracellular recycling of vitamin E through interaction with vitamin C and glutathione. α-Lipoic acid also acts as a redox regulator of thiol-containing proteins, including transcription factor NF-κB, and it affects signal transduction events and gene expression under both normal and abnormal conditions. α-Lipoic acid has been shown to be very effective in the treatment of several conditions in which oxidative injury is thought to be very important, including ischemia-reperfusion, diabetes, cataract formation, human immunodeficiency virus activation, neurodegeneration, and radiation injury (17, 18).
The objective of this study was to determine whether α-lipoic acid can protect human endothelial cells against oxidative injury caused by infection with R. rickettsii.
MATERIALS AND METHODS
Rickettsiae.
R. rickettsii strain Sheila Smith (strain VR-149) was obtained from the American Type Culture Collection (Rockville, Md.). Rickettsiae were propagated in Vero cell monolayers (green monkey kidney cells; American Type Culture Collection) in Dulbecco’s minimal essential medium (Gibco Laboratories, Grand Island, N.Y.) supplemented with 4% fetal bovine serum (Gibco BRL Life Technologies) and 1 mM l-glutamine (Gibco BRL) in a 5% CO2 atmosphere as described elsewhere (19, 24). Five to 6 days after inoculation, the intensity of infection was examined in smears stained by the method of Gimenez (12), and heavily infected cells were harvested by using 3-mm-diameter glass beads. Rickettsiae were purified by sonication of the cells, multiple passages of the suspension through an 18-gauge needle, and then differential centrifugation (1, 10). The final suspension of purified rickettsiae was prepared in SRM buffer (0.218 M sucrose–5 mM potassium glutamate buffer, pH 7.0, supplemented with 1% Renografin-76 [E. R. Squibb & Sons, Inc., Princeton, N.J.] and 5 mM MgCl2), aliquoted, and frozen at −83°C. The viable titer of purified rickettsiae was determined by plaque titration on Vero cells as previously described (35).
Isolation, cultivation, and infection of endothelial cells.
Human endothelial cells were isolated from veins of freshly acquired umbilical cords according to the method of Gimbrone (11). Isolated cells were maintained in McCoy’s 5A medium supplemented with 20% fetal bovine serum, 30 μg of endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, N.Y.) per ml, and 50 μg of sodium heparin (Sigma, St. Louis, Mo.) per ml in 35- or 60-mm-diameter Nunc tissue culture dishes precoated with 0.2% gelatin (Difco, Detroit, Mich.) prepared in Dulbecco’s phosphate-buffered saline (PBS; Gibco BRL). Identification of endothelial cells was performed by immunofluorescence detection of endothelium-specific von Willebrand protein, using goat anti-human von Willebrand factor antibody (Biodesign International, Kennebunk, Maine) and rhodamine (tetramethyl rhodamine isothiocyanate)-conjugated rabbit anti-goat Fc fragment-specific immunoglobulin G (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) (33).
Rickettsial seeds were diluted in McCoy’s 5A medium containing 20% fetal bovine serum, and cells were inoculated at a ratio of 0.25 rickettsia per cell in 0.3 ml per 35-mm-diameter dish. After rickettsiae were allowed to adhere for 2 h at room temperature with rocking, the volume of the medium was adjusted to 2 ml per dish and the dishes were transferred to a 35°C incubator and maintained in a 5% CO2 atmosphere.
Treatment of HUVEC with α-lipoic acid.
To evaluate the effect of α-lipoic acid (dl-6,8-thioctic acid; Sigma), a 500 mM stock solution was prepared in dimethyl sulfoxide (Fisher Scientific, Fair Lawn, N.J.), and 1 to 500 μM was used for treatment of endothelial cells prior to or after inoculation with rickettsiae. Rickettsial infection and the effects of α-lipoic acid were examined at 24-h intervals. The cell medium was aspirated from each dish, the monolayers were rinsed with PBS, and the dishes of cells were processed for further analysis as described below.
Rickettsial infection was monitored microscopically after acridine orange staining of the monolayers (16) and by determination of plaque formation by infected cultures on Vero cells. Infected cells were harvested with sterile glass beads in medium, the beads and dishes were washed in 2× SRM, and the pooled medium and washings were frozen. On the day of assay, the cells were thawed and passaged through an 18-gauge needle. One milliliter each of 10−2 to 10−5 dilutions were used for plaque assays in triplicate in 12-well plates (Costar, Cambridge, Mass.). The plaques were counted 7 days after inoculation following staining with 0.3% neutral red (Gibco) in PBS for 4 h.
The viability of endothelial cells in monolayers during the experiment was determined by the trypan blue dye exclusion method as previously described (26). The cells were removed from the dishes by trypsinization, pelleted by centrifugation at 1,000 rpm for 10 min in a Beckman AccuSpin ER centrifuge, and resuspended in PBS.
Biochemical assays.
To examine the changes in infected endothelial cells and to evaluate the influence of α-lipoic acid, three parameters were estimated: the levels of intracellular reduced glutathione, glutathione peroxidase activity, and the levels of intracellular peroxide. The cell medium was aspirated from each dish, the monolayers were rinsed with PBS, and the dishes of cells were processed for further analysis with respect to requirements for each method. The protein concentration was measured by the method of Smith et al. (28), using the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.)
Determination of intracellular reduced glutathione levels.
Glutathione concentrations in infected and uninfected cells were determined by the method of Saville (20). One milliliter of 6.5% trichloroacetic acid in 0.5 mM EDTA was added to each dish, and then the cells were scraped with a rubber policeman, collected into 1.5-ml microcentrifuge tubes, and centrifuged at 13,000 rpm (Beckman microcentrifuge) for 5 min. The supernatants were transferred to new tubes, the protein pellets were dissolved in 0.5 ml of 0.1 M NaOH each, and the fractions were frozen for at least 24 h before performance of the assay. The thiol determination was carried out with freshly prepared reagents on supernatant fractions in 96-well microplates. Fifty microliters of thawed supernatant was mixed with 50 μl of 10 mM sodium nitrite solution prepared in 0.96% sulfuric acid, and the mixture was incubated for 5 min at room temperature. Ten microliters of 0.5% ammonium sulfamate was added, and the mixture was incubated for 5 min. Then 100 μl of a solution consisting of 1 part 0.5% mercuric chloride and 4 parts 3.4% sulfanilamide in 0.4 M hydrochloric acid was added, and the mixture was incubated for 5 min. The color reaction was developed after a 5-min incubation with 40 μl of substrate solution containing 0.2% N-(1-naphthyl)ethylenediamine dihydrochloride (ICN Biomedicals Inc., Aurora, Ohio) in distilled water. The absorbance at 540 nm was read in a Titertek Multiscan spectrophotometer; the blank solution consisted of 50 μl of 6.5% trichloroacetic acid processed as described for the supernatant fractions. The standard curve was generated from triplicate samples of serial dilutions of reduced glutathione (Sigma), and the results were expressed in nmoles of glutathione per milligram of protein.
Determination of glutathione peroxidase activity.
Glutathione peroxidase was measured by a modification of the method of Gunzler et al. (13). The monolayers were incubated with 0.5 ml of 1% Triton X-100 for 30 min at 4°C, and the cells were then scraped from the plate with a cold rubber policeman. The cell lysates were mixed with 0.5 ml of cold 10 mM sodium phosphate buffer, pH 7.0, and immediately frozen. The enzymatic reactions were performed in 2-ml cuvettes (Fisher). The reaction mixture was initially prepared by combining 200 μl of 50 mM sodium phosphate buffer (pH 7.0) containing 0.5 mM diethylenetriaminepentaacetic acid and 5 U of glutathione reductase (Sigma) per ml, 50 μl of 40 mM glutathione (pH 7.0), and 100 to 200 μl of thawed cell extract or blank buffer solution. The final volume was brought to 1 ml with distilled water, and the reaction mixture was equilibrated for 10 min at 37°C. Ten microliters of 20 μM NADPH (Sigma) was added to each cuvette, and the changes in absorbance at 340 nm were recorded for 2 min to estimate the levels of spontaneous oxidation of NADPH. The enzymatic reaction was initiated by addition of 20 μl of 15 mM t-butyl hydroperoxide (Sigma), and the linear decrease in the A340 was recorded for 10 min at 1-min intervals. Glutathione peroxidase activity was calculated by using the millimolar extinction coefficient of NADPH (6.22) and expressed as milliunits of enzyme per milligram of cell protein, assuming that 1 mU of glutathione peroxidase oxidizes 1 nmol of glutathione per min (9).
Determination of intracellular peroxide levels.
Endothelial cells were assayed for intracellular peroxide by a modification of the method of Cathcart et al. (6). Two milliliters of PBS containing 1 μM 5 (and 6)-carboxy-2′,7′-dichlorofluorescin diacetate (Molecular Probes, Eugene, Oreg.) was added to each dish, and the plates were incubated for 10 min at room temperature. The cells were then rinsed three times with 2 ml of PBS; after the final rinse, the PBS was aspirated and 2 ml of 0.05% Nonidet P-40 (Sigma) in distilled water was added to lyse the cells. The lysed cells were removed with a rubber policeman, and the contents were assayed in a Shimadzu model RF-5301PC fluorescence spectrophotometer with an emission wavelength of 535 nm and an excitation wavelength of 505 nm. The background fluorescence, determined in the absence of added cell samples, was subtracted. Peroxide levels were expressed as fluorescence units per milligram of protein.
Fluorescent staining of rickettsiae and actin.
Staining was performed at 72 h postinfection as previously described (15). The cell culture medium was aspirated, and the monolayers were washed three times for 5 min each with PBS. The infected monolayers were fixed with 3% paraformaldehyde solution prepared in PBS and permeabilized by treatment with 0.5% Triton X-100 as recommended by Clerc and Sansonetti (7). Rickettsiae were labeled by indirect immunofluorescence, using a mouse polyclonal serum prepared against Renografin-purified R. rickettsii (working dilution, 1:200; kindly prepared by G. A. Dasch, Naval Medical Research Institute, Bethesda, Md.) (1, 10) and a Texas red-labeled goat anti-mouse immunoglobulin G (heavy plus light chains) conjugate (10 μg/ml; Molecular Probes). Actin was stained with fluorescein-labeled phalloidin (Molecular Probes) at 10 U/ml. Slides were viewed and photographed with a Zeiss fluorescence microscope.
Transmission electron microscopy.
At 72 h postinfection, the culture medium was decanted and the cells were washed once with PBS. The monolayers were fixed overnight in situ at 4°C in a 2% glutaraldehyde solution prepared in 0.1 M sodium cacodylate buffer, pH 7.3, as previously described (23). Following fixation, the cells were washed three times in cacodylate buffer, postfixed in 1% osmium tetroxide for 1 h at room temperature, dehydrated in an ascending ethanol series, and embedded in PolyBed 812 (Polysciences, Inc., Warrington, Pa.). Ultrathin sections were collected on carbon-coated collodion copper grids, stained with uranyl acetate and lead citrate, and viewed in a JEOL 1200 EX electron microscope operating at 60 kV.
Statistical analysis.
Each experiment was performed two to five times, and two to six dishes were used for each time point and experimental variable. The mean, standard deviation, and standard error of the mean for each experimental condition were calculated. Statistical significance was assessed by Student’s t test (α = 0.05), using the computer program GB-Stat 6.01.
RESULTS
Influence of α-lipoic acid on the viability of uninfected and infected HUVEC.
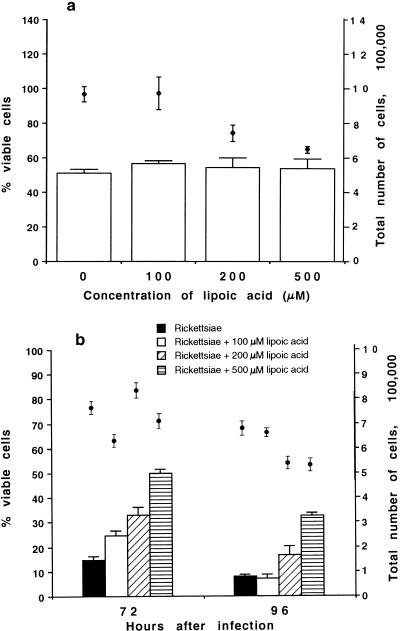
Treated and untreated uninfected monolayers contained about 50% viable cells after 96 h of cultivation, regardless of the concentration of α-lipoic acid used (Fig. 1a). The total number of cells at 96 h was about 106 per dish in untreated uninfected monolayers, as well as following treatment with 100 μM α-lipoic acid, but this number decreased by 23 and 33%, respectively, in monolayers maintained in the presence of 200 and 500 μM α-lipoic acid (P < 0.01). The cells cultivated in the presence of 500 μM α-lipoic acid looked atypical, as they were spindle shaped rather than having the characteristic endothelial cell morphology (not shown). However, uninfected cells maintained in medium with or without α-lipoic acid continuously displayed positive staining with an antiserum against endothelium-specific von Willebrand protein.
FIG. 1.
Influence of α-lipoic acid on the viability (open bars) and total number (closed circles) of human endothelial cells. (a) Uninfected endothelial cells at 96 h after infection; (b) endothelial cells infected with R. rickettsii. The number of viable cells was determined by the trypan blue dye exclusion method and expressed as a percentage of the total number of cells harvested per dish for each experimental variable. The data are given as means ± standard errors (for four and eight dishes, respectively, per experimental variable for uninfected and infected cells). The statistical significance of the variables compared is given in the text.
R. rickettsii infection typically causes a marked cytopathic effect in HUVEC. Only 15% of untreated infected cells were viable at 72 h after inoculation, and this value decreased furher to 8% (P < 0.01) by 96 h (Fig. 1b). Treatment of endothelial cells with 1, 10, 100, 200, or 500 μM α-lipoic acid overnight prior to infection with R. rickettsii did not change the resistance of HUVEC to rickettsiae, since pretreated infected cells were destroyed at about the same rate as untreated cells (data not shown). Similarly, inclusion of 1 or 10 μM α-lipoic acid in the medium after inoculation did not provide any protective effect for HUVEC. In contrast, supplementation of the medium with 100, 200, or 500 μM α-lipoic acid after inoculation protected HUVEC against rickettsial injury in a dose-dependent manner (Fig. 1b). At 72 h after inoculation, infected monolayers cultivated in the presence of 100, 200, and 500 μM α-lipoic acid contained 25, 33, and 50% viable cells, respectively (P < 0.01). At 96 h, while the viability of cells cultivated in medium with 200 and 500 μM was 17% (P < 0.025) and 33% (P < 0.01), respectively, two and four times higher than in untreated infected cells, the viability of the infected cells cultivated with 100 μM α-lipoic acid was the same as that of untreated infected cells.
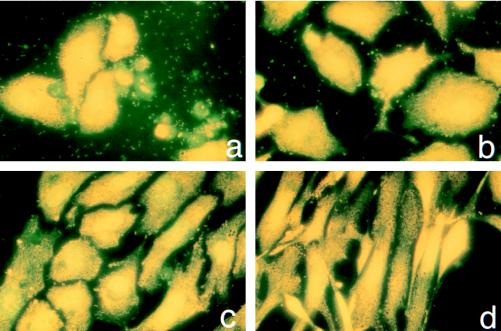
Light microscopy of acridine orange-stained cells demonstrated fewer cytopathic changes in infected HUVEC cultivated in the presence of 100 μM α-lipoic acid than in untreated infected cells (Fig. 2). While untreated infected cultures had only a few yellow-stained normal cells and a large amount of dead cells and cellular fragments stained in green (Fig. 2a), monolayers cultivated with 100 μM α-lipoic acid contained a significant number of normally shaped viable cells (Fig. 2b). Supplementation with 200 or 500 μM α-lipoic acid preserved the infected monolayer even better, since the detection of green-stained dead cells and membrane fragments was minimal (Fig. 2c and d). Infected monolayers maintained with 500 μM α-lipoic acid remained nearly confluent after 96 h of infection (Fig. 2d), but the cells became spindle shaped like those of uninfected cultures under the same conditions. Using this staining procedure, rickettsiae were detected in all slides of untreated and treated HUVEC; however, the best visualization was achieved for untreated cells. In all cases, significant numbers of rickettsiae were seen in the cytoplasm of infected cells, in association with membrane fragments from dead cells, and also extracellularly.
FIG. 2.
Light microscopic observations on the influence of α-lipoic acid on human endothelial cells infected with R. rickettsii for 96 h (acridine orange staining). (a) Infected endothelial cells grown without α-lipoic acid; (b) infected endothelial cells grown in the presence of 100 μM α-lipoic acid; (c) infected endothelial cells grown in the presence of 200 μM α-lipoic acid; (d) infected endothelial cells grown in the presence of 500 μM α-lipoic acid.
Transmission electron microscopy of human endothelial cells infected with R. rickettsii.
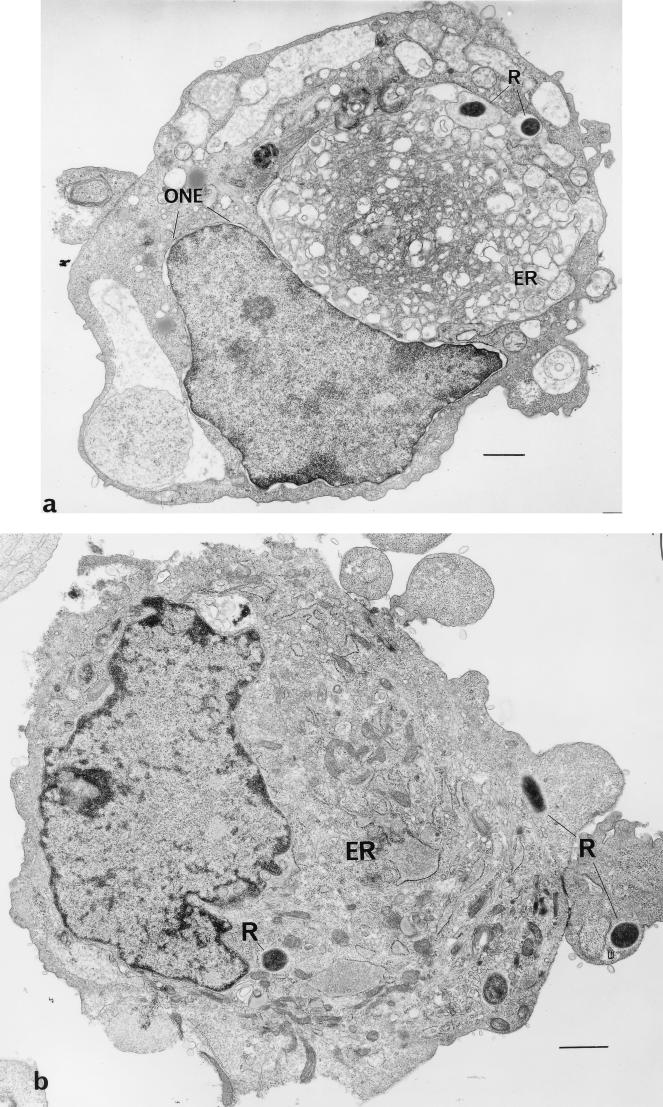
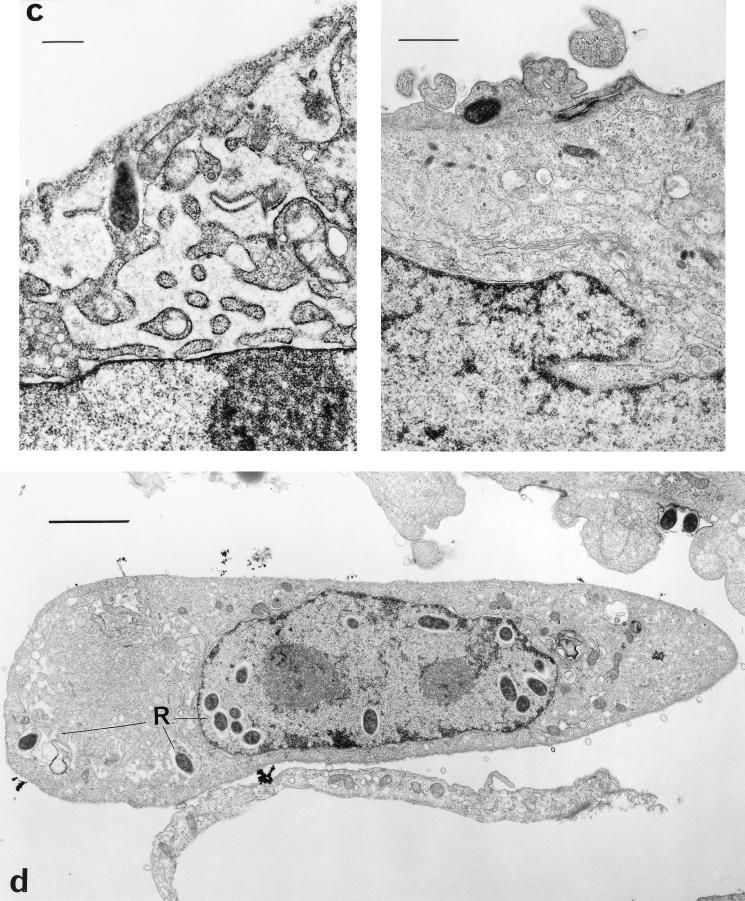
R. rickettsii infection of HUVEC causes ultrastructural morphological changes which are apparent as early as 48 h after inoculation (23). Extensive dilatation occurs in the membranes that constitute the complex of the endoplasmic reticulum and outer nuclear envelope (Fig. 3a). Very few rickettsiae are apparent in each cell. Supplementation of the growth medium with 100 μM α-lipoic acid for 72 h after infection of the cells prevented these morphological changes from occurring (Fig. 3b), as the endoplasmic reticulum of these cells is not distended and resembles the structure of uninfected cells (Fig. 3c). Rickettsiae were found in the cytoplasm and nuclei of treated cells in greater numbers (Fig. 3d) than in untreated infected cells (Fig. 3a).
FIG. 3.
Transmission electron micrographs of R. rickettsii-infected HUVEC from a culture at 72 h postinfection. (a) Cells in the absence of α-lipoic acid. Extensive dilatation of membranes of the endoplasmic reticulum and intracellular rickettsiae are demonstrated (bar, 2 μm). (b) Cells grown in medium supplemented with 100 μM α-lipoic acid (bar, 2 μm). (c) Demonstration of differences in membrane compartments of infected endothelial cells grown without α-lipoic acid (left; bar, 1 μm) and in the presence of 100 μM α-lipoic acid (right; bar, 0.5 μm). (d) Demonstration of intracytoplasmic and intranuclear R. rickettsii in an endothelial cell grown in the presence of 100 μM α-lipoic acid (bar, 5 μm). Abbreviations: R, rickettsiae; ER, endoplasmic reticulum; ONE, outer nuclear envelope.
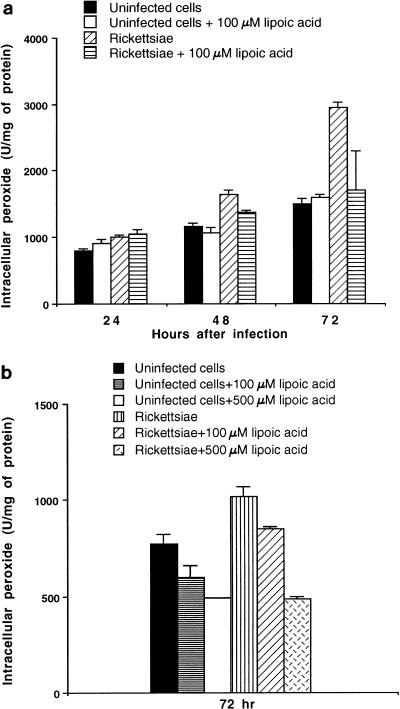
Influence of α-lipoic acid on levels of intracellular peroxides.
Cultivation of uninfected HUVEC, both untreated and treated with 100 μM α-lipoic acid for 72 h, resulted in a slight increase in intracellular peroxide levels compared to the 24-h levels (P < 0.05) (Fig. 4a). Since these changes occurred at similar rates in both untreated and treated cells, this probably reflects the changes in cellular metabolism as HUVEC monolayers age. Cultivation of rickettsia-infected HUVEC in the presence of 100 μM α-lipoic acid resulted in a significant reduction (P < 0.05) of intracellular peroxide levels at 48 and 72 h after inoculation compared with that which typically occurs following rickettsial multiplication in HUVEC (Fig. 4a). Changes in peroxide levels in samples infected for 96 h and maintained with or without α-lipoic acid could not be determined, since very few infected untreated cells remained on the dishes by this time point (Fig. 2b), and additional significant losses occurred during the wash steps employed in the procedure (see Materials and Methods).
FIG. 4.
Intracellular peroxide levels in human endothelial cells infected with R. rickettsii and cultivated in medium with or without supplementation with α-lipoic acid. The data are presented as means ± standard errors (for three dishes per experimental variable). (a) Time course of intracellular peroxide level changes in infected HUVEC; (b) effect of different concentrations of α-lipoic acid on intracellular peroxide levels at 72 h after infection. The statistical significance of the variables compared is given in the text.
In another experiment, a dose-dependent reduction of peroxide levels was observed at 72 h with 100 μM (P < 0.05) and 500 μM (P < 0.01) α-lipoic acid in both uninfected and infected HUVEC (Fig. 4b). α-Lipoic acid at 500 μM reduced the intracellular peroxide to the levels found in corresponding uninfected treated cells at 72 h of incubation. The responses of uninfected and infected endothelial cells to 100 μM α-lipoic acid treatment was somewhat different in the two experiments, probably due to the use of different pools of HUVEC. However, the decrease in peroxide levels elicited by α-lipoic acid treatment of infected cells was consistent.
Influence of α-lipoic acid on levels of reduced glutathione and glutathione peroxidase.
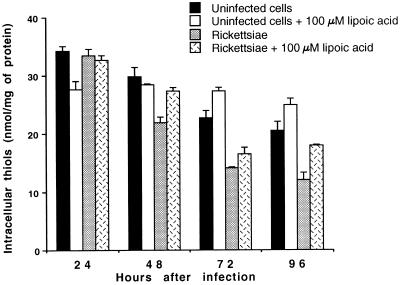
Uninfected untreated HUVEC cultivated in standard medium displayed decreasing glutathione (thiol) levels after 72 and 96 h of growth (P < 0.01) (Fig. 5). α-Lipoic acid supplementation at 100 μM resulted in significant increases (P < 0.025) in reduced glutathione levels in uninfected HUVEC at both time points (Fig. 5). R. rickettsii infection of untreated HUVEC caused the depletion of intracellular reduced glutathione by 48 h after inoculation (P < 0.025); this decline continued (P < 0.01) to 72 and 96 h (Fig. 5). In contrast, while infected cells cultured with 100 μM α-lipoic acid had increased levels of glutathione at 72 and 96 h compared to untreated infected cells (P < 0.1 and P < 0.05, respectively), they were lower than the levels found in treated uninfected HUVEC (P < 0.05) (Fig. 5). Supplementation of the culture medium with 100 or 500 μM α-lipoic acid resulted in dose-dependent increases in glutathione levels in both uninfected cells (P < 0.05 and P < 0.01, respectively) and infected cells (P < 0.01 and P < 0.01, respectively) at 72 h (data not shown).
FIG. 5.
Intracellular reduced glutathione levels in human endothelial cells infected with R. rickettsii and cultivated in medium with or without 100 μM α-lipoic acid. The data from a representative experiment are presented as means ± standard errors (for three dishes per experimental variable). The statistical significance of the variables compared is given in the text.
Changes in glutathione peroxidase activity determined at 72 h after infection were similar to those observed for levels of reduced glutathione (Fig. 6). The activity of this enzyme was significantly decreased in untreated infected cells compared with that in uninfected untreated or uninfected treated cells (P < 0.01). Supplementation of the culture medium with α-lipoic acid nearly restored the glutathione peroxidase activity of infected cells to the level of untreated uninfected cells. The protective effect was observed with both 100 and 500 μM α-lipoic acid, and the specific activity with the latter was significantly greater than that elicited by 100 μM α-lipoic acid treatment (P < 0.05).
FIG. 6.
Glutathione peroxidase activity in human endothelial cells infected with R. rickettsii for 72 h and cultivated in medium with or without supplementation with different concentrations of α-lipoic acid. The data are presented as means ± standard errors (for four dishes per experimental variable). The statistical significance of the variables compared is given in the text.
Influence of α-lipoic acid on the viability of R. rickettsii in HUVEC.
The viability of R. rickettsii recovered from HUVEC at different times after inoculation with and without supplementation with α-lipoic acid was determined by measuring the number of rickettsial PFU on Vero cells (Fig. 7). In both treated and untreated HUVEC, significant multiplication of rickettsiae (P < 0.01) occurred between 24 and 48 h after inoculation (data not shown). The number of viable rickettsiae in untreated HUVEC dishes reached its maximum by 72 h and then had decreased by 96 h (P < 0.1) (Fig. 7). The number of viable rickettsiae in infected HUVEC cultivated in the presence of 100 μM α-lipoic acid was similar to that of untreated infected cultures at 72 h, and the treated infected HUVEC continued to maintain the growth of rickettsiae at 96 h, resulting in about a twofold increase in rickettsial yield (P < 0.01) compared to untreated infected cells (Fig. 7). Although the infected cultures treated with 200 μM α-lipoic acid contained 33% fewer viable rickettsiae than untreated infected HUVEC at 72 h after inoculation (P < 0.1), the quantity of viable rickettsiae recovered at 96 h was significantly higher (P < 0.01) (Fig. 7). α-Lipoic acid at 500 μM, despite increasing HUVEC viability (as shown in Fig. 1a), significantly decreased the number of rickettsiae recovered at 72 h (P < 0.01) and 96 h (P < 0.01) after inoculation (Fig. 7).
FIG. 7.
Recovery of viable R. rickettsii from human endothelial cells treated with α-lipoic acid. Infected HUVEC were collected as described in Materials and Methods, and 1 ml of each 10−2 to 10−5 10-fold serial dilution was inoculated, in triplicate, in 22-mm-diameter wells. Plaques were counted on day 7 after inoculation following staining with 0.3% neutral red. The data are presented as means ± standard errors (for 15 to 18 wells for each treatment condition). The statistical significance of the variables compared is given in the text.
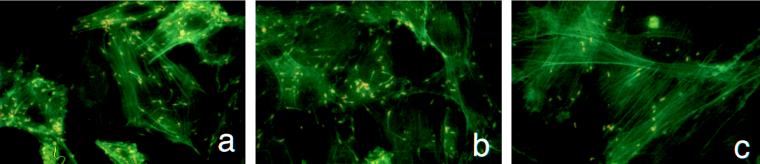
Under all treatment conditions, significant numbers of rickettsiae could be detected by indirect microimmunofluorescence assay (Fig. 8). Regardless of the concentration of α-lipoic acid used, rickettsiae apparently retained their capacity for intracellular movement, since they displayed actin tails after 72 and 96 h of treatment. At 72 h, we measured the lengths of actin tails on the rickettsiae detected in two independent experiments and found that the mean tail lengths of rickettsiae from control samples were 9.28 ± 0.46 μm and 11.61 ± 0.26 μm. In contrast, although there were a few rickettsiae with tails of this length in samples cultivated with α-lipoic acid, the median tail lengths of the rickettsiae increased to 15.45 ± 1.06 μm and 20.34 ± 1.01 μm at 100 μM α-lipoic acid (P < 0.01), and with 500 μM α-lipoic acid treatment, the majority were more than twice as long as the controls (P < 0.01).
FIG. 8.
Microimmunofluorescence detection of R. rickettsii (Texas red staining) and actin (fluorescein phalloidin staining) in infected human endothelial cells at 72 h after inoculation. (a) Without α-lipoic acid treatment; (b) treatment with 100 μM α-lipoic acid; (c) treatment with 500 μM α-lipoic acid.
Influence of α-lipoic acid on plaque formation by R. rickettsii on Vero cells.
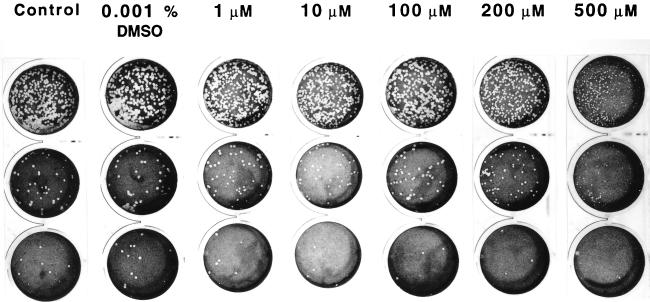
Addition of 1, 10, 100, or 200 μM α-lipoic acid to the agarose overlay did not affect the ability of R. rickettsii to form lytic plaques compared with untreated Vero cells (Fig. 9). Under these conditions, the titers of R. rickettsii ranged from 3.7 × 108 ± 0.9 × 108 to 5.4 × 108 ± 0.4 × 108, probably reflecting the intrinsic twofold limitations of plaque assay reproducibility. The observed plaques were morphologically similar to the plaques formed on untreated cells (5.3 × 108 ± 0.7 × 108) and to plaques formed in the presence of 0.001% dimethyl sulfoxide (6.8 × 108 ± 0.2 × 108). With 500 μM α-lipoic acid supplementation, a small but reproducible twofold decrease (P < 0.05) in plaque numbers (2.5 × 108 ± 0.7 × 108) and an obvious reduction in plaque size were observed (Fig. 9).
FIG. 9.
Influence of α-lipoic acid on the appearance of R. rickettsii plaques in Vero cells. One milliliter of each 10−5 to 10−8 10-fold serial dilution of a standard seed of R. rickettsii was inoculated, in triplicate, in 22-mm-diameter wells, with a subsequent 1-h incubation at 35°C. The inocula were aspirated, and the infected monolayers were overlaid with 0.5% agarose prepared in cell culture medium with or without α-lipoic acid supplementation. Plaques were counted on day 7 after inoculation, following staining with 0.3% neutral red. From the top: first row, 10−6 dilution; second row, 10−7 dilution; third row, 10−8 dilution.
DISCUSSION
High levels of toxic peroxides accumulate in the cytoplasm following infection of human endothelial cells with R. rickettsii (19). This peroxide level increase is often associated with lipid peroxidation, which may affect the structural integrity of cellular membranes (24). Growth of R. rickettsii also compromises the intracellular antioxidant system by reducing the concentration of glutathione and the activity of glutathione peroxidase (9, 25), two components which play a crucial role in protecting cells against oxidative injury by converting lipid peroxides and hydrogen peroxides to nontoxic by-products.
Here we have reported for the first time that treatment of R. rickettsii-infected endothelial cells with the metabolic antioxidant α-lipoic acid protects HUVEC against cellular injury caused by this microorganism. α-Lipoic acid at concentrations of 100 and 200 μM does not significantly influence the viability of either uninfected endothelial cells or rickettsiae, as determined, respectively, by the trypan blue dye exclusion test and rickettsial plaque assay on Vero cells. On the contrary, these concentrations of the antioxidant increased the viability of infected cells and also maintained rickettsial viability in the endothelial cells at 96 h after inoculation better than for untreated infected cells. Beginning at 72 h after inoculation, untreated infected cultures contained significant amounts of membrane fragments with associated rickettsiae, as well as numerous extracellular rickettsiae (Fig. 3a). Although spotted fever group rickettsiae are known for their ability to replicate even after the death of embryonated chicken eggs (37), their growth in injured or dead cells is probably quite limited, since we obtained a twofold decrease in viable rickettsiae in untreated infected samples between 72 and 96 h after infection and this paralleled the decline in HUVEC viability. In contrast, the enhancement of HUVEC viability by supplementation of the medium with 100 or 200 μM α-lipoic acid also resulted in better maintenance of rickettsial viability.
We characterized the recovery of rickettsiae by estimating the number of rickettsial PFU in suspensions of infected cells from individual culture dishes rather than by determining growth curves for the rickettsiae, since rickettsial growth curves were nearly impossible to obtain under our experimental conditions. Treated and untreated infected cells underwent different metabolic changes which appeared to influence the permeability of cellular membranes and resulted in differences in the retention of trypan blue dye (8). This was to be expected since treatment with α-lipoic acid indirectly influences membrane characteristics by preventing lipid peroxidation (2, 17). On the other hand, changes in membrane permeability and in cytoplasmic composition also affect the quality of rickettsia detection either by the method of Gimenez or by acridine orange staining (16). Each of these methods is based on the specific binding of a dye to nucleic acid, particularly RNA, whose density is considerably higher in rickettsiae than in the cytoplasm of infected cells. Treated and untreated cells stained with different degrees of basophilia, making consistent observations of the numbers of rickettsiae difficult.
Only long-term incubation with 500 μM α-lipoic acid seemed to have a marked effect on the rickettsiae in addition to its obvious effects on endothelial cells. A decline in the recovery of viable rickettsiae from endothelial cells incubated in medium with 500 μM α-lipoic acid occurred after 96 h of infection. However, significant numbers of rickettsiae were detected at 96 h by microimmunofluorescence despite the high concentration of antioxidant used. These rickettsiae had exceptionally large actin tails, suggesting that even under these treatment conditions, rickettsiae retain the capacity for intracellular movement and cell-to-cell spread. By analogy with findings on Listeria monocytogenes (31, 32), the increase in actin tail length can be associated with a higher rate of rickettsial intracellular movement and/or an increase in the length of time that the rickettsiae reside inside a given cell. Therefore, it appears that the primary protective effect of α-lipoic acid on infected cells is not directed toward inactivation of the rickettsiae but rather is mediated through changes in cellular metabolism, particularly the antioxidant system.
Effects of α-lipoic acid on the cellular physiology of HUVEC were profound, since the growth of uninfected cells maintained with 200 or 500 μM α-lipoic acid decreased at 96 h compared to that of cells supplemented with 0 or 100 μM α-lipoic acid. A significant inhibition of growth of murine tumor cells was obtained after 3 and 6 days of treatment with 10 or 100 μM of α-lipoic acid, but there were no significant changes in cell counts for normal mouse lung tissue treated similarly (5). Proliferation of the Jurkat and SupT1 T-cell lines was reduced up to 50% within 7 days in medium supplemented with ≥40 μg of α-lipoic acid per ml (3). Treatment of Jurkat T cells with 2 or 5 mM α-lipoic acid for 16 weeks resulted in cell shrinkage, thiol depletion, and DNA fragmentation (22). This apoptotic effect of α-lipoic acid was attributed to its fatty acid structure, since similar effects were observed with its nonthiolated homolog. Whether induction of apoptosis in HUVEC can be caused by long-term incubation with high dosages of α-lipoic acid is unknown. If apoptosis does occur, its effect on the clearance of rickettsial infection may be important.
α-Lipoic acid at 500 μM protected infected HUVEC significantly more at 96 h than did lower concentrations of this antioxidant. It also caused a reduction in lytic plaque numbers and a decrease in plaque size on Vero cells. The change in plaque formation on Vero cells could be due to a retardation of the rate of normal plaque development, since the antioxidant indirectly reduces the cytotoxic effect of rickettsiae for HUVEC. With additional time, normal plaques might be formed. The reduced plaque size could also be due to a decrease in the rate of cell-to-cell spread by the rickettsiae. To explore this possibility, additional studies to examine the effect of prolonged incubations with and without α-lipoic acid are needed.
Treatment of infected cells with α-lipoic acid significantly decreased the levels of intracellular peroxides and elevated the levels of intracellular reduced glutathione and glutathione peroxidase activity, in some cases to levels found in uninfected untreated cells. α-Lipoic acid treatment for 72 or 96 h caused an elevation of thiol levels in uninfected endothelial cells; peroxide levels were somewhat reduced, and the activity of glutathione peroxidase was increased. Accordingly, α-lipoic acid appears primarily to influence intracellular thiol levels, and this in turn may stabilize peroxide and peroxidase levels. The putative role of intracellular thiol pools in the protection of endothelial cells against injury caused by R. rickettsii has been previously suggested (25). Supplementation of the cell culture medium with 0.1 to 0.4 mM glutamylcysteine, a thiol dipeptide precursor, after inoculation with R. rickettsii resulted in dose-dependent increases in thiol levels in infected cells compared to those in untreated infected cells. Moreover, addition of 0.1 to 0.2 mM glutamylcysteine to the agarose overlay resulted in about a 60% reduction in plaque size on Vero cells compared to that of untreated samples (25). This effect was similar to that obtained with 500 μM α-lipoic acid in the present study. If treatment with glutamylcysteine can protect HUVEC against cell injury caused by R. rickettsii and its action is additive to effects mediated by α-lipoic acid, it may support the hypothesis that α-lipoic acid acts independently of the changes induced in glutathione pools.
The antioxidant effect of α-lipoic acid also could be mediated by its capacity for direct binding of oxygen species. The two major oxidant species thought to accumulate during R. rickettsii infection in endothelial cells, superoxide radical and hydrogen peroxide (19, 24), are not scavenged by α-lipoic acid itself but by its reduced form, DHLA (17). These functions could be accomplished in both the extracellular and intracellular environments (14). α-Lipoic acid itself is able to scavenge hydroxyl radicals and singlet oxygen in the intracellular milieu (27).
Finally, α-lipoic acid may mediate its effects by acting on the transcription factor NF-κB (21). The activation of NF-κB in R. rickettsii-infected endothelial cells has been recently demonstrated (29). It has a biphasic profile with an early peak at 3 h after infection, a return to baseline levels at 14 h, and a secondary activation at 24 h. Unfortunately, the multiplicity of infection we used was significantly lower than that employed in the activation study. Consequently, the kinetics of the resultant rickettsial infection and host responses will also differ, so it is difficult to directly correlate this profile of NF-κB activation with our studies. The precise mechanism of induction for R. rickettsii infection is not known, although NF-κB activation is stimulated by oxidants, particularly by hydrogen peroxide, in other model systems (21). Nevertheless, NF-κB activation probably still occurs to some degree in infected endothelial cell cultures maintained in the presence of α-lipoic acid, since the DNA binding activity of NF-κB was enhanced by DHLA and inhibited by α-lipoic acid in experiments using nuclear extracts from Jurkat T cells (30). To our knowledge, the effects of α-lipoic acid on NF-κB in vascular endothelial cells have been examined only in a bovine aorta model in a study of late diabetic complications (4). Administration of an antioxidant resulted in suppression of the NF-κB activation induced by advanced glycation end products that are thought to mediate the endothelial dysfunction commonly associated with diabetes. Hence, the effects of α-lipoic acid on NF-κB activation are very complex. The final interplay between α-lipoic acid/DHLA and NF-κB in endothelial cells infected with R. rickettsii is even more difficult to predict.
Our studies demonstrate the protective effects of α-lipoic acid for human endothelial cells infected with R. rickettsii in vitro. Further experiments are required to yield more information regarding the mechanism(s) of α-lipoic acid action in this model and to evaluate the antioxidant’s potential as an adjunct treatment for Rocky Mountain spotted fever.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 17416 from the National Institute of Allergy and Infectious Diseases.
We are grateful to Gregory A. Dasch for review of the manuscript and the gift of anti-R. rickettsii serum, to Lisa Santucci for assistance with biochemical procedures, to Xiaojiang Tian for preparation of primary cultures of HUVEC, and to Perry Comegys for excellent photographic work.
REFERENCES
- 1.Aniskovich L P, Eremeeva M E, Balayeva N M, Ignatovich V F, Artemiev M I, Emelianov V V, Smirnova N S. Methods for purification of Rickettsia prowazekii separated from host tissue: a step-by-step comparison. Acta Virol. 1989;33:361–370. [PubMed] [Google Scholar]
- 2.Bast A, Haenen G R M M. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta. 1988;963:558–561. doi: 10.1016/0005-2760(88)90326-8. [DOI] [PubMed] [Google Scholar]
- 3.Baur A, Harrer T, Peukert M, Jahn G, Kalden J R, Fleckenstein B. Alpha-lipoic acid is an effective inhibitor of human immuno-deficiency virus (HIV-1) replication. Klin Wochenschr. 1991;69:722–724. doi: 10.1007/BF01649442. [DOI] [PubMed] [Google Scholar]
- 4.Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, Luther T, Berentshtein E, Tritschler H, Muller M, Wahl P, Ziegler R, Nawroth P P. Advanced glycation end product-induced activation of NF-κB is suppressed by α-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–1490. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
- 5.Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of α-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneim-Forsch. 1992;42:829–831. [PubMed] [Google Scholar]
- 6.Cathcart R, Schwiers E, Ames B N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 7.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook J A, Mitchell J B. Viability measurements in mammalian cell systems. Anal Biochem. 1989;179:1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 9.Devamanoharan P S, Santucci L A, Hong J E, Tian X, Silverman D J. Infection of human endothelial cells by Rickettsia rickettsii causes a significant reduction in the levels of key enzymes involved in protection against oxidative injury. Infect Immun. 1994;62:2619–2621. doi: 10.1128/iai.62.6.2619-2621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eremeeva M E, Balayeva N M, Ignatovich V F, Raoult D. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J Clin Microbiol. 1993;31:2625–2633. doi: 10.1128/jcm.31.10.2625-2633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimbrone M A. Culture of vascular endothelium. Prog Hemostasis Thromb. 1976;3:2745–2756. [PubMed] [Google Scholar]
- 12.Gimenez D F. Staining rickettsiae in yolk-sac cultures. Stain Technol. 1964;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- 13.Gunzler W A, Kramers H, Flohe L. An improved coupled test procedure for glutathione peroxidase in blood. Z Klin Chem Klin Biochem. 1974;12:444–448. doi: 10.1515/cclm.1974.12.10.444. [DOI] [PubMed] [Google Scholar]
- 14.Handelman G J, Han D, Tritschler H, Packer L. α-Lipoic acid reduction by mammalian cells to the dithiol form, and release into the cell culture medium. Biochem Pharmacol. 1994;47:1725–1730. doi: 10.1016/0006-2952(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 15.Heinzen R A, Hayes S F, Peacock M G, Hackstadt T. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer B A, Reller L B, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J Clin Microbiol. 1981;14:201–205. doi: 10.1128/jcm.14.2.201-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer L, Witt E H, Tritschler H J. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 18.Packer L, Tritscher H J, Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 19.Santucci L A, Gutierrez P L, Silverman D J. Rickettsia rickettsii induces superoxide radical and superoxide dismutase in human endothelial cells. Infect Immun. 1992;60:5113–5118. doi: 10.1128/iai.60.12.5113-5118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst (London) 1958;83:670–672. [Google Scholar]
- 21.Sen C K, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 22.Sen C K, Roy S, Han D, Packer L. Regulation of cellular thiols in human lymphocytes by α-lipoic acid: a flow cytometric analysis. Free Radic Biol Med. 1997;22:1241–1257. doi: 10.1016/s0891-5849(96)00552-7. [DOI] [PubMed] [Google Scholar]
- 23.Silverman D J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984;44:545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman D J, Santucci L A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988;56:3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman D J, Santucci L A. A potential protective role for thiols against injury caused by Rickettsia rickettsii. Ann N Y Acad Sci. 1990;590:111–117. doi: 10.1111/j.1749-6632.1990.tb42213.x. [DOI] [PubMed] [Google Scholar]
- 26.Silverman D J, Santucci L A, Sekeyova Z. Heparin protects human endothelial cells infected by Rickettsia rickettsii. Infect Immun. 1991;59:4505–4510. doi: 10.1128/iai.59.12.4505-4510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman, D. J., and L. A. Santucci. Unpublished data.
- 28.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 29.Sporn L A, Sahni S K, Lerner N B, Marder V J, Silverman D J, Turpin L C, Schwab A L. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect Immun. 1997;65:2786–2791. doi: 10.1128/iai.65.7.2786-2791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y J, Mizuno M, Tritschler H J, Packer L. Redox regulation of NF-κB DNA binding activity by dihydrolipoate. Biochem Mol Biol Int. 1995;36:241–246. [PubMed] [Google Scholar]
- 31.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 32.Tilney L G, DeRosier D J, Tilney M S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992;118:71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner D D, Olmsted J B, Marder V J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker D H. Pathology and pathogenesis of the vasculotropic rickettsioses. In: Walker D H, editor. Biology of rickettsial diseases. I. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 115–138. [Google Scholar]
- 35.Wike D A, Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsii isolated directly from whole blood and tick hemolymph. Infect Immun. 1972;6:736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler H H, Turco J. Rickettsia prowazekii and the host cell: entry, growth and control of the parasite. Curr Top Microbiol Immunol. 1986;138:81–107. [PubMed] [Google Scholar]
- 37.Wisseman C L, Jr, Edlinger E A, Waddell A D, Jones M R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976;14:1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]