Abstract
Despite considerable progress, peritonitis and sepsis remain life-threatening conditions. To improve the understanding of the pathophysiology encountered in sepsis, a new standardized and highly reproducible murine model of abdominal sepsis termed colon ascendens stent peritonitis (CASP) was developed. In CASP, a stent is inserted into the ascending colon, which generates a septic focus. CASP employing a stent of 14-gauge diameter (14G stent) results in a mortality of 100% within 18 to 48 h after surgery. By inserting stents of small diameters, mortality can be exactly controlled. Thus, CASP surgery with insertion of a 22G or 18G stent (22G or 18G CASP surgery) results in 38 or 68% mortality, respectively. 14G CASP surgery leads to a rapid invasion of bacteria into the peritoneum and the blood. As a consequence, endotoxemia occurs, inflammatory cells are recruited, and a systemic inflammatory response syndrome develops. Interestingly, the most pronounced upregulation of inflammatory cytokines (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α] and interleukin-12) is observed in spleen and lungs. CASP surgery followed by stent removal at specific time intervals revealed that all animals survived if intervention was performed after 3 h, whereas removal of the septic focus after 9 h did not prevent death, suggesting induction of autonomous mechanisms of a lethal inflammatory response syndrome. 18G CASP surgery in IFN-γ receptor-deficient (IFNγR−/−) mice revealed an essential role of IFN-γ in survival of sepsis, whereas TNF receptor p55-deficient (TNFRp55−/−) mice did not show altered survival rates. In summary, this study describes a novel animal model that closely mimics human sepsis and appears to be highly suitable for the study of the pathophysiology of abdominal sepsis. Importantly, this model demonstrates a protective role of IFN-γ in survival of bacterial sepsis.
Bacterial invasion of body cavities often leads to organ failure, septic shock, and death despite aggressive surgical intervention, adequate antibiotic therapy, and intensive life support (2, 11, 58). Two related but distinct mechanisms of dysregulation of the immune system have been considered to cause this fatal process. On the one hand, it is assumed that an exuberant infection results in a decreased ability of the immune response to mount an antimicrobial defense, finally leading to immune paralysis (57, 58). On the other hand, the hypothesis has been put forward that in sepsis, microbial components activate a strong immune response resulting in an overproduction of harmful immune mediators (6, 10). Insights into these complicated pathophysiological processes have, at least in part, been gained from animal studies. Generally, two types of experimental settings can be distinguished: (i) bolus injections of bacteria, microbial components (endotoxin, lipoteichoic acid, and mannans) or toxins (superantigens) (34, 38, 46), and (ii) injury models with the consecutive liberation of endogenous microbial flora from a septic focus (18). Both types of models attempt to mimic distinct aspects of the pathological changes typically encountered in sepsis as observed in human patients, such as hypo- or hyperthermia, tachycardia, tachypnea, organ failure, and lethal outcome (2, 10). Most of the current experimental treatment strategies have been derived from results gained by bolus injection-type experiments (30, 34, 38, 46, 51, 55). Thus, numerous studies identified cytokines as crucially involved in the pathogenesis of sepsis, and blockade of these cytokines was shown to ameliorate the challenge with bacterial endo- or exotoxins (41, 46, 47, 52, 55). The prototype of a host-damaging cytokine is considered to be tumor necrosis factor (TNF-α) (6, 56). Anti-TNF-α antibodies protect from lipopolysaccharide (LPS) or superantigen-induced shock (7, 38), TNF-α injection leads to a septic shock-like syndrome (51), and infusion of anti-TNF antibodies into baboons protects from septic shock triggered by Escherichia coli infusion (52). Furthermore, TNFRp55−/− mice are protected from bolus shock induced by LPS–d-GalN and Staphylococcus aureus superantigen–d-GalN (41). A harmful role in sepsis was also assigned to gamma interferon (IFN-γ) since it was observed that IFN-γ or IFN-γ receptor (IFN-γR)-deficient mice show decreased susceptibility to high-dose-bolus LPS injection (14, 30).
Surprisingly, recent results of clinical studies have not provided clear evidence that systemic anti-inflammatory therapies with corticosteroids (13), anti-LPS treatment (29, 61), or the neutralization of host mediators such as TNF-α or interleukin-1 (IL-1) (1, 24–26, 48) are improving the clinical course of sepsis. In a subgroup of patients suffering from gram-positive sepsis, it was even suggested that anti-TNF-α treatment is harmful (48). With regard to the human sepsis syndrome, injury-type models such as cecal ligation and puncture (CLP) aim to more closely resemble the course of sepsis as observed in patients with an early hyperdynamic, hypermetabolic state, followed by a pronounced hypodynamic, hypometabolic state (2, 18). Interestingly, in accordance with clinical studies, animal models of the injury type performed in LPS-resistant mice (37) or using antagonism of host mediators such as TNF-α (anti-TNF-α, TNF receptor p55 [TNFRp55]-immunoglobulin Fc protein), or IL-1 receptor antagonist (4, 17, 22, 23, 36) could not provide clear evidence for an improved survival from sepsis. Some studies even indicated that TNF-α is required for survival after CLP (17, 19).
TNF-α binds to two distinct cell surface receptors, TNFRp55 and TNFRp75 (50). It has been shown that TNFRp55-deficient animals are highly susceptible to infection with intracellular bacteria such as listeria and mycobacteria (28, 41), whereas TNFRp75-deficient animals appear not be substantially impaired in their host defense (20). IFN-γ is a potent inducer of macrophage activity and may have dichotomous functions in sepsis (3, 54). IFN-γ has been shown to be of relevance as a mediator of septic shock (14), but recent clinical data suggest that IFN-γ administration may have a beneficial effect on the outcome of sepsis (16). IFN-γ increases the antigen-presenting capacity of mononuclear phagocytes and enhances the production of proinflammatory cytokines by monocytes and macrophages triggered by LPS (3, 9, 54). IFN-γ is induced synergistically by IL-12 and TNF-α and appears to be negatively regulated by IL-10 (35, 45, 53). Animal studies investigating the therapeutic application of IFN-γ in injury-type models revealed an increased mortality (39).
Present knowledge regarding the pathophysiology of sepsis, and especially the study of supportive and causal therapies, has to be reevaluated by using more relevant animal models that are designed to reliably mimic human sepsis (42, 44). The prime aim of this study was to establish a novel surgical animal model closely resembling the pathophysiology found in human postoperative abdominal sepsis and reevaluate the roles played by cytokines in sepsis. To investigate the regulation of these cytokines in sepsis, the kinetics of IL-10, IL-12, TNF-α, and IFN-γ induction were investigated in various tissues in a novel injury-type animal model of bacterial sepsis (colon ascendens stent peritonitis [CASP]). Furthermore, mice deficient in TNFRp55 or IFN-γR were subjected to CASP to address the role of TNF-α and IFN-γ on a molecular level in sepsis.
MATERIALS AND METHODS
Mice.
For all experiments, 8- to 12-week-old female mice (weight 20 to 25 g) were used. C57BL/6 mice were purchased from Charles River, Sulzfeld, Germany. TNFRp55-deficient (C57/BL6 background) (41) and IFN-γR-deficient (C57BL/6 × 129/SvJ background) mice (32) as well as control mice were bred in a conventional animal facility. Prior to surgery, mice were kept for at least 1 week in the animal facility to recover after shipment. All experimental procedures were performed according to German animal safety regulations.
CASP CASPI, and sham surgery.
The surgical procedure of CASP was performed as described recently (60). For anesthesia, ether (Hoechst, Frankfurt, Germany) or narketan-xylopan (WDT, Garbsen, Germany) was used. Prior to surgery, a venous catheter (14, 18, or 22 gauge, as indicated; Venflon; BOC, Ohmeda AB, Sweden) was prepared by creating a notch at a distance of 3 mm from the orifice; 1 mm beyond, the catheter was circumferentially incised with a scalpel, sparing only a slim bar. In complete anesthesia and after desinfection of the abdomen, the abdominal wall was opened through a 1-cm midline incision. After exposure of the ascending colon, the prepared catheter was stitched through the antimesenteric wall into the lumen of the ascending colon and then fixed with two stitches (7/0 Ethilon thread; Ethicon, Norderstedt, Germany) placed approximately 10 mm from the ileocecal valve. Consecutively, the inner needle of the stent was removed and the stent was cut at the prepared site. To ensure proper intraluminal positioning of the stent, stool was milked from the cecum into the ascending colon and the stent until a small drop of stool appeared. Fluid resuscitation of animals was performed by flushing 0.5 ml of sterile saline solution into the peritoneal cavity before closure of the abdominal walls (two layers, muscle and skin; 4/0 Ethilon thread). Surgical intervention after CASP surgery (CASPI) was performed at specified intervals after CASP (3, 5, and 9 h). The abdomen was reopened by a 3-cm midline laparatomy. The fixing suture of the stent was cut, and the stent was removed. The defect in the colonic wall was closed by a transversal sinking suture (7/0 Ethilon thread) as described by Lembert (34a). Thereafter, an intensive peritoneal lavage using 5 ml of sterile saline solution was performed. The abdomen was closed as described above. For control purposes, two different types of sham operations were performed. As a control monitoring CASP surgery, sham surgery was performed according to the CASP procedure except that the stent was fixed outside the ascending colon without puncturing the colonic wall (sham CASP). For a control after CASPI surgery, a 3-cm midline laparatomy was performed. Then a small patch of the antimesenteric wall of the ascending colon (diameter of about 2 mm) was excised. The resulting transmural defect was immediately closed by a standard transversal sinking suture as described by Lembert (34a). After fluid resuscitation using 0.5 ml of sterile saline solution, closure of the abdomen was performed as described above (sham CASPI).
Survival after surgery was assessed every 4 h within the first 48 h and then every 8 h for 14 days. In accordance with animal care guidelines, a sepsis score was developed for the assessment of severity of sepsis, including clinical symptoms such as heart rate, breath frequency, change of weight, and activity. Scoring points were assigned to each item and then added up. Lethal outcome was assumed when mice showed a sepsis score of more than 7 points on a scale ranging from 0 to 12. Mice scoring 8 or more points were sacrificed.
Bacterial culture.
At given time points (3, 5, 12, and 24 h after CASP surgery), animals were sacrificed. For performance of peritoneal lavages, the skin of the abdomen was cut open in the midline after thorough disinfection and without injury of the muscle. Sterile saline solution (5 ml) was injected into and aspirated out of the peritoneal cavity twice, using a sterile syringe and needle, to rinse out bacteria from the peritoneal cavity. Aliquots of a serial log dilution of this peritoneal lavage fluid were plated on Columbia blood agar and MacConkey plates (Becton Dickinson, Heidelberg, Germany); CFU per entire peritoneal lavage were counted after overnight incubation at 37°C. For identification of numbers and bacteria species of bacteria in blood and solid organs, mice were sacrificed 12 h after CASP surgery using a 14-gauge catheter (14G CASP surgery) or sham surgery. Blood was collected and lungs, liver, and spleen were harvested under sterile conditions. Organs were homogenized in 4 ml of sterile phosphate-buffered saline (PBS) buffer. Ten microliters of 10-fold dilutions of blood and organ suspensions were plated on Colombia blood agar and MacConkey plates (Becton Dickinson). Bacterial counts are given as number of bacteria per whole organ, entire volume of peritoneal lavage, and milliliter of blood (sample means ± standard deviation, n = 7 mice for peritoneal lavage and peripheral blood, n = 3 mice for liver, lungs, and spleen). Bacteria were grown and identification tests were conducted in accordance with routine bacteriological methods.
Endotoxin measurements.
Endotoxin (LPS) was determined in the plasma of mice by conducting a kinetic quantitative chromogenic Limulus amebocyte assay (KQCL test; Serva, Heidelberg, Germany). Animals were sacrificed at indicated time points, and blood was collected by sterile puncture of the caudal caval vein, using heparinized syringes (sodium heparinate; Ratiopharm, Ulm, Germany). Following centrifugation (7,000 × g, 10 min, 4°C), the plasma was removed and incubated for 10 min at 75°C to inactivate LPS binding proteins. The plasma samples were diluted 1:2 with LPS-free water (BioWhittaker, Heidelberg, Germany). The LPS content of each sample was determined in duplicate, and control duplicates were spiked with a given amount of standard E. coli LPS (0.05 endotoxin units [EU]/ml) to control the inhibitory activity of the samples. For each LPS determination, a standard curve, determined by dilution of standard E. coli LPS (0.005, 0.05, 0.5, 5, and 25 EU/ml) in heat-inactivated plasma from naive control mice, was established. LPS measurements were performed on an enzyme-linked immunosorbent assay (ELISA) reader (BioWhittaker) at 37°C. The results were evaluated by using BioWhittaker computer software.
Measurement of TNF-α.
Animals were sacrificed at indicated time points, and blood was collected by sterile puncture of the posterior caval vein, using heparinized syringes (sodium heparinate; Ratiopharm). Plasma was removed after centrifugation of blood samples (7,000 × g, 10 min, 4°C). The amount of TNF-α in the plasma was determined according to the protocol of the manufacturer with a murine TNF-α ELISA kit purchased from Genzyme, Rüsselsheim, Germany.
Immunohistochemistry of organ cryosections.
Tissue samples were snap-frozen in 2-methylbutane (Merck, Darmstadt, Germany) prechilled in liquid nitrogen. Cryostat sections (8 μm; Leica, Nussdorf, Germany) were fixed for 10 min in ice-cold acetone (Merck) and air dried at room temperature. For reduction of nonspecific staining and inactivation of endogenous peroxidase, sections were preincubated for 30 min with 100 μl of PBS containing 1% (wt/vol) bovine serum albumin (Sigma Chemical, Eggenstein, Germany), 5% (vol/vol) normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, Pa.) 0.015% (vol/vol) hydrogen peroxide (Merck), and 10% (vol/vol) avidin D solution (Vector Laboratories, Burlingame, Calif.). For immunohistology of the colon, 0.5% (vol/vol) Fc receptor block (rat anti-mouse CD16/CD32 FcγIII/II receptor; Pharmingen, Homburg, Germany) was included in addition. Consecutively, avidin was blocked by 10% (vol/vol) biotin solution (Vector Laboratories) in PBS containing 1% (wt/vol) bovine serum albumin. After three washes with PBS, the sections were incubated for 30 min with 100 μl of biotin-conjugated primary antibody as indicated (CD11b, Mac-1αM chain; all from Pharmingen). After three washes with PBS, slides were incubated with ExtrAvidin-horseradish peroxidase conjugate (Sigma). The sections were washed with PBS and stained for 10 min with 5% (vol/vol) 3-aminoethylcarbazole (5 mg/ml; Sigma) in N,N′-dimethylformamide (Merck) and 0.015% (vol/vol) hydrogen peroxide in 50 mM acetate buffer. After three washes with PBS, the sections were counterstained with Mayer’s hematoxylin (Sigma) for 10 min, washed in PBS, and mounted with glycerol-gelatin (Sigma). The stained sections were photographed with a Leica DMBRE photomicroscope (Leica).
Purification of RNA, cDNA synthesis, and internal competitive semiquantitative RT-PCR.
Mice were sacrificed at indicated time points. Organs or tissues from experimental mice were immediately removed and snap-frozen in liquid nitrogen. Approximately 100 mg of tissue was homogenized in 3 ml of lysis buffer consisting of 4 M guanidinium thiocyanate (Merck), 0.5% (wt/vol) N-lauroylsarcosine (Serva), 15 mM sodium citrate (pH 7.0; Merck), and 100 mM 2-mercaptoethanol (Sigma). Total RNA was prepared as described previously (15). RNA precipitates were washed with 75% ethanol, repelleted by centrifugation, and dissolved in 100 μl of diethylpyrocarbonate (Merck)-treated double-distilled H2O. Four micrograms of total RNA was added to 10 mM oligo(dT) primer (12- to 18-mer; BRL-Gibco, Eggenstein, Germany) and 10 mM random hexamer primer (BRL-Gibco) in a 10-μl reaction mix at 65°C for 10 min. The reaction mix was cooled on ice, and cDNA synthesis was carried out by adding 4 μl of 5× transcription buffer (250 mM Tris-HCl, 375 mM KCl, 15 mM MgCl2 [pH 8.3]; BRL-Gibco), 2 μl of 0.1 M dithiothreitol (BRL-Gibco), 2 μl of deoxynucleoside triphosphate (dNTP) mix (final concentration, 1 mM each dNTP), 0.5 μl of RNAsin (15 U; Promega, Madison, Wis.), and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/ml; Superscript; BRL-Gibco). The mixture was incubated for 60 min at 37°C and then heated to 95°C for 5 min. After cooling on ice, 20 μl of reaction mix was diluted with double-distilled H2O to 60 μl, and a twofold serial dilution row was prepared. For internal competitive semiquantitative reverse transcription-PCR (RT-PCR), 10 μl from each cDNA dilution was amplified in PCR plates (Corning-Costar, Corning, N.Y.) containing a known amount of control fragment DNA, specific 5′ and 3′ primers (Table 1), at a final concentration of 200 nM, 200 mM dNTP, 5 U of Taq polymerase (BRL-Gibco), and 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl [pH 8.3], 25 mM MgCl2, 1% [wt/vol] gelatin) (Sigma). The PCR cycling was performed after hot start in a 96-well thermal cycler (Biometra, Göttingen, Germany) for 30 cycles with a 1-min denaturation step (94°C), a 30-s annealing step (63°C), and a 1.5-min extension step (72°C). Linear internal control fragments were designed to be identical to the specific cDNA sequence amplified except for the insertion of a 125-bp insert of murine β-actin cDNA inside the control fragment (39a). The amount of β-actin cDNA was estimated from the dilution at which ethidium bromide-stained bands of coamplified cDNA and control fragment showed equal density after agarose gel electrophoresis. The specific cytokine amounts were determined accordingly.
TABLE 1.
Oligonucleotide primers used for internal competitive semiquantitative RT-PCR
| Gene encoding: | Orientation | Primer sequence | Size (bp) of amplified product |
|---|---|---|---|
| β-actin | Sense | 5′-ATG GAT GAC GAT ATC GCT-3′ | 569 |
| Antisense | 5′-ATG AGG GTA GTC TGT CAG GT-3′ | ||
| TNF-α | Sense | 5′-GAC AAG CCT GTA GCC CAC GTC GTA G-3′ | 456 |
| Antisense | 5′-ACA CCC ATT CCC TTC ACA GAG CAA-3′ | ||
| IL-12p40 | Sense | 5′-CGT GCT CAT GGC TGG TGC AAA G-3′ | 576 |
| Antisense | 5′-GAA CAC ATG CCC ACT TGC TG-3′ | ||
| IFN-γ | Sense | 5′-GAA AGC CTA GAA AGT CTG AAT AAC T-3′ | 388 |
| Antisense | 5′-ATC AGC AGC GAC TCC TTT TCC GCT T-3′ | ||
| IL-10 | Sense | 5′-ACC TGG TAG AAG TGA TGC CCC AGG CA-3′ | 237 |
| Antisense | 5′-CTA TGC AGT TGA TGA AGA TGT CAA A-3′ |
Statistical analysis.
For statistical analysis of survival data after CASP surgery, the Mann-Whitney rank sum test was performed, using the Jandel Scientific software Sigma Stat 2.0. Statistical significance was assumed if the P value was P <0.05.
RESULTS
CASP surgery as a murine model for peritonitis and sepsis.
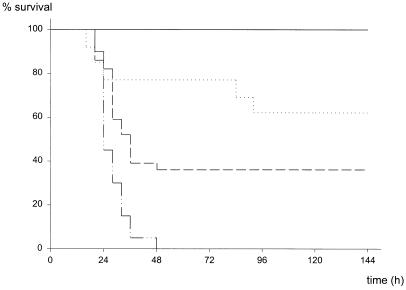
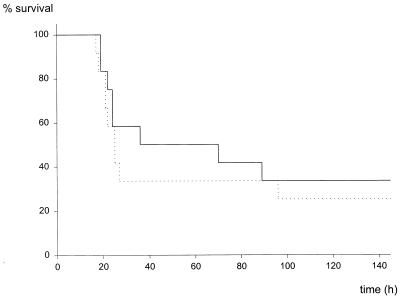
CASP surgery was developed as an easily reproducible and highly standardized method for the investigation of sepsis (60). For the induction of peritonitis, a stent of a given diameter is punctured through the ascending colon thus allowing intraluminal bacteria to transmigrate and invade the peritoneal cavity (Fig. 1). Depending on the diameter of the stent inserted, the mortality rate of mice after CASP surgery varied (Fig. 2). CASP surgery with a 22-gauge-diameter stent (22G stent) led to a mortality rate of 38% (5 of 13 mice); insertion of a 18G or 14G stent resulted in 64% (28 of 44 mice) or 100% mortality, respectively (n = 20; P < 0.001 versus 18G stent, P < 0.003 versus 22G stent). No significant statistical difference was found between the two groups operated with 18G and 22G stent needles (P = 0.135). However, the results clearly demonstrate that the CASP procedure using different stent sizes allows the generation of both lethal and sublethal experimental groups. In the 14G CASP group, all mice reliably developed clinical signs of severe sepsis between 24 and 48 h after surgery. In contrast, after sham CASP (laparatomy and extraluminal fixation of the stent; see Materials and Methods) surgery, all mice survived without clinical signs of sepsis (Fig. 2 and data not given). In summary, CASP surgery provides a highly standardized and homogeneous animal model designed for the study of bacterial peritonitis and sepsis.
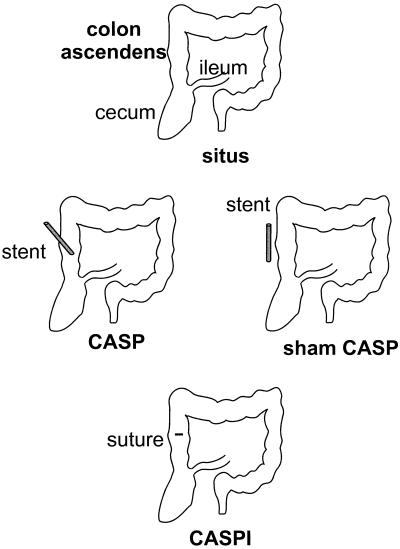
FIG. 1.
Schematic principle of CASP, sham CASP, and CASPI surgery. In CASP surgery, a stent of a defined diameter is introduced into the ascending colon by puncture and fixed with a suture to the colonic wall. Insertion of the stent allows transmigration of colonic flora from the gut into the peritoneal cavity. In sham CASP surgery, the stent is fixed to the colonic wall with a suture without puncturing the colonic lumen. In CASPI, the stent is surgically removed and the defect of the colonic wall is closed by a sinking suture as described by Lembert (34a). In sham CASPI, a defect measuring the diameter of the stent is cut into the wall of the ascending colon and immediately closed by surgery as in CASPI.
FIG. 2.
Mortality after CASP surgery using different stent diameters. After insertion of stents of different diameters (22, 18, or 14 gauge), survival of mice was closely monitored. Mice scoring more than 8 points on the sepsis score (from 0 [healthy] to 12 [comatose]) were sacrificed. ——, sham CASP (n = 9); ·····, 22G CASP (n = 13); — —, 18-G CASP (n = 44); —···, 14-G CASP (n = 20).
Bacterial spread after CASP surgery.
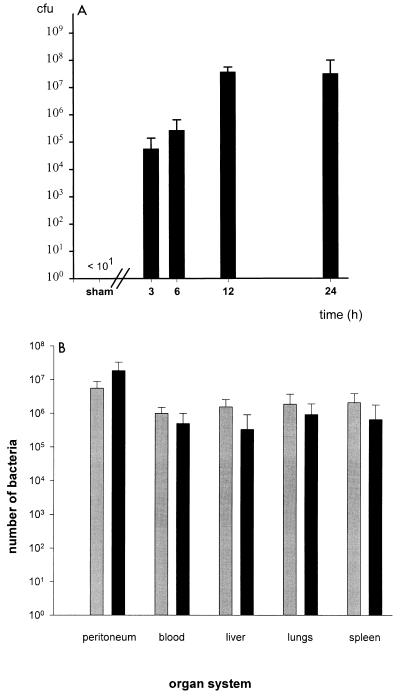
In human patients, systemic spread of bacteria from a septic focus during sepsis is a well-known phenomenon. To examine whether implantation of a stent into the colon ascendens mimics a septic focus, the spread of intestinal bacteria into different anatomical compartments of sham- and CASP-operated mice was monitored. Bacterial counts were determined by plating serial dilutions of peritoneal lavage fluid, suspensions of homogenized organs (liver, lungs, and spleen), and blood specimens on solid bacterial growth medium. Between 3 and 12 h after 14G CASP surgery, an exponential increase of bacterial CFU was observed in the peritoneal cavity; thereafter a plateau was reached (Fig. 3A). In sham-operated mice, no bacteria were detected in any specimen. Furthermore, 12 h after 14G CASP surgery, blood and organs were readily invaded by bacteria of the endogenous murine intestinal flora (enterococci, Bacillus spp., and enterobacteriaceae such as E. coli, Proteus spp., or Enterobacter spp.) (Fig. 3B).
FIG. 3.
(A) Bacterial counts in the peritoneal cavity. The peritoneal cavity was flushed with sterile saline solution 3, 6, 12, and 24 h after CASP surgery. Titrated serial dilutions of the lavage fluid were plated on selective agar plates, and bacterial CFU were calculated. After sham CASP surgery, no bacteria were detected in peritoneal lavage fluid. (B) Bacteria counts and species in different organ systems. Mice were sacrificed 12 h after 14G CASP surgery or sham surgery. Peritoneal lavage was performed, blood was collected, and lungs, liver, and spleen were harvested under sterile conditions as described in the text. Organs were homogenized in 4 ml of sterile PBS buffer. Then 10 μl-aliquots of 10-fold dilutions of blood, peritoneal lavage fluid, or organ suspensions were plated on blood agar and MacConkey plates and incubated under both aerobic and anaerobic conditions. Bacterial counts are given as number of bacteria per whole organ, entire volume of peritoneal lavage, or milliliter of blood (sample means ± standard deviation, n = 7 mice for peritoneal lavage and peripheral blood, n = 3 mice for liver, lungs, and spleen). All specimens of sham-operated control mice were sterile (data not shown). ░⃞, gram-negative bacteria; ▪, gram-positive bacteria.
CASPI.
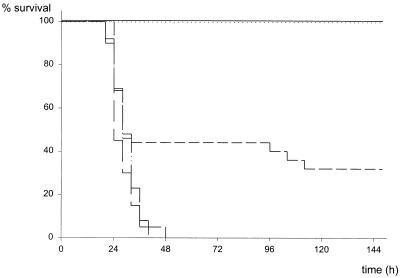
CASPI surgery was performed to determine whether surgical removal of the septic focus would prevent a lethal outcome after induction of peritonitis (14G CASP). In CASPI, the stent was surgically removed and the defect in the ascending colon was closed (Fig. 1). As shown in Fig. 4, removal of the implanted stent after 3 h rescued all mice, whereas CASPI after 9 h could not revert the lethal course of peritonitis. Accordingly, surgical intervention after 5 h led to intermediate mortality rates. These findings clearly demonstrate that between 3 and 9 h after sepsis induction, critical pathophysiological events are initiated, developing independently of continued bacterial invasion. After 9 h (point of no return), sole sanitation of the septic focus cannot prevent detrimental host mechanisms triggered after CASP surgery. The next set of experiments was aimed to characterize the immunological mechanisms triggered after CASP surgery.
FIG. 4.
Survival after stent removal (CASPI). CASP surgery employing a 14G stent was performed at a time point defined as 0 h. After 3, 5, or 9 h, the stent was surgically removed and the colonic wall was closed. Sham CASPI was performed as described in Materials and Methods and the legend to Fig. 1. ——, sham CASPI (n = 11); ·····, 14G CASPI, stent removal after 3 h (n = 13); ——, 14G CASPI, stent removal after 5 h (n = 25); —···, 14G CASPI, stent removal after 9 h (n = 13); —, 14G CASP (n = 20).
Recruitment of inflammatory cells after CASP.
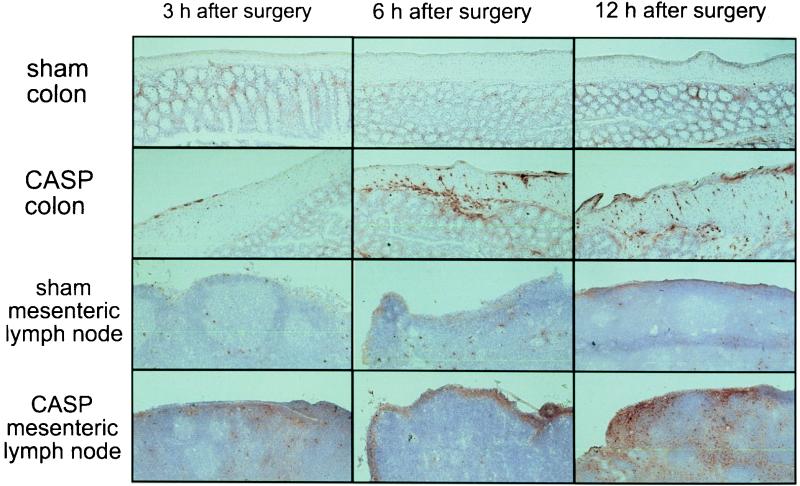
Inspection of the peritoneal cavity after CASP surgery revealed typical hallmarks of inflammation such as redness, swelling of the bowel, and fibrinous deposits. To scrutinize the inflammatory reaction, infiltration of granulocytes and macrophages into the colonic wall and the mesenteric lymph nodes was monitored at given points in time after CASP surgery. Ascending colon and mesenteric lymph nodes were removed from CASP- and sham-operated mice after 3, 6, and 12 h. Tissue sections were stained with a monoclonal antibody to anti-Mac-1α, an antigen that is expressed on granulocytes, macrophages, and natural killer cells (49). As early as 3 h after 14G CASP, infiltration of Mac-1α-positive cells could be observed in the wall of the colon and in the subcapsular sinus of the mesenteric lymph node (Fig. 5). After 6 and 12 h, a massive infiltration was present in the subserosa, muscle, and submucosal parts of the colonic wall. In the mesenteric lymph nodes, the accumulation of Mac-1α-positive cells increased after 6 h; after 12 h, Mac-1α-positive cells were numerous in the interfollicular sinuses (Fig. 5). In sham-operated mice, significant infiltration of inflammatory cells was not detectable at any time. Thus, bacterial invasion occurring after CASP surgery acts as a massive stimulus for the recruitment of inflammatory cells in the local tissue and draining lymph nodes.
FIG. 5.
Infiltration of tissues with inflammatory cells. Recruitment of inflammatory cells was examined by immunohistochemical staining of colon and mesenteric lymph node cryosections of sham- or 14G CASP-operated mice, which were sacrificed 3, 6, or 12 h after surgery. Ascending colon and mesenteric lymph nodes were removed at given points in time, sectioned, and stained with biotinylated CD11b (Mac-1α) monoclonal antibody followed by streptavidin-peroxidase. The stain was reacted with 3-aminoethylcarbazole (see Materials and Methods). CD11b-positive cells (granulocytes and monocytes/macrophages) are labeled in red.
Endotoxinemia and systemic inflammatory response after CASP surgery.
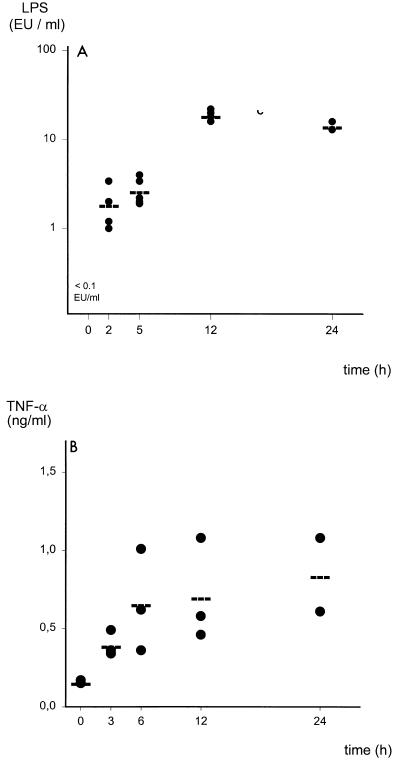
Endotoxin or LPS contained in the cell wall of gram-negative bacteria is a potent inducer of inflammatory cytokines (46). Mice that underwent 14G CASP surgery were sacrificed, and blood was collected to measure the amounts of LPS in the systemic circulation. LPS amounts in nontreated mice and sham-operated mice were below the detection limit of 0.1 EU/ml at all time points (Fig. 6A and data not shown). In contrast, significant amounts of LPS could be detected as early as 2 h after CASP (1.9 EU/ml), increased after 5 h (2.7 EU/ml) and 12 h (19 EU/ml), and remained elevated until death (14.5 EU/ml) (Fig. 6A).
FIG. 6.
(A) LPS amounts in the bloodstream of mice that underwent CASP surgery. Plasma amounts of LPS (endotoxin) were determined in 14G CASP-operated mice at given points in time by conducting a KQCL test (see Materials and Methods). LPS amounts in healthy mice (0 h) and sham-operated mice (data not shown) were below the detection limit of the LPS assay of 0.005 EU/ml. As early as 2 h after induction of sepsis, substantial amounts of LPS could be detected. (B) Kinetics of TNF serum amounts after CASP surgery. TNF-α content in the plasma of mice after 14G CASP surgery was determined by ELISA.
As an indicator of the systemic response to endotoxin, TNF-α and IFN-γ serum levels were determined by ELISA. In parallel to the increase in LPS, an induction of TNF-α was found in mice that underwent 14G CASP (Fig. 6B). Serum TNF-α amounts were below 0.1 ng/ml in naive and sham-operated mice 0, 3, 6, 12, and 24 h after surgery. However, 3 h after CASP surgery, operated mice had detectable serum TNF-α amounts that increased up to 24 h after CASP. In contrast, IFN-γ could not be detected in the serum in CASP or sham-CASP-operated mice at any time (data not shown).
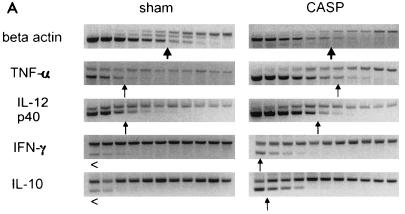
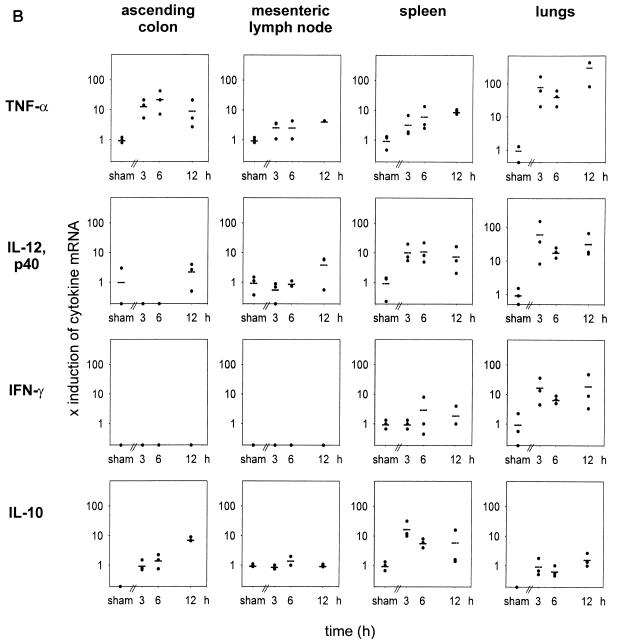
To investigate the response of other cytokines, the regulation of a set of inflammatory cytokines was examined by a sensitive semiquantitative internal competitive RT-PCR approach. In the first set of experiments, TNF-α, IL-12p40, IFN-γ, and IL-10 transcription in the spleens of CASP mice was compared to that in spleens of sham-operated mice. Equilibration of cDNAs was performed by β-actin PCR (Fig. 7A). After 6 h, a significant upregulation of TNF-α, IL-12p40, IFN-γ, and IL-10 could be observed in the spleens of mice that underwent 14G CASP surgery compared to sham-operated or naive mice (Fig. 7A and data not shown). To examine the production of cytokines in a systematic approach, the kinetic of the cytokine response of different tissues and organs was investigated. For this purpose, ascending colon, mesenteric lymph nodes, spleens, and lungs were removed from 14G CASP-operated mice after 3, 6, and 12 h after surgery. The means of mRNA amounts detected in sham-operated mice were arbitrarily defined as 1 and taken as basis for the calculation of the induction of the respective cytokine mRNAs in CASP-operated mice. For those cases where we could not detect in undiluted cDNA a PCR product for a given cytokine in sham-operated mice (i.e., basal transcription was below the detection limit of the RT-PCR method), mRNA amounts 3 h after CASP surgery were assigned a value of 1. As depicted in Fig. 7B, TNF-α mRNA was induced locally in the colonic wall as well as systemically in the mesenteric lymph nodes and spleen. The most prominent induction, however, occurred in the lungs. Interestingly, induction of IL-12p40 was pronounced in the spleen and lungs but not in the ascending colon and the mesenteric lymph nodes. IFN-γ was produced mainly in the lungs and to a lesser extent in the spleen, whereas no IFN-γ mRNA was detected in the colon and mesenteric lymph nodes. The mRNA for the immunosuppressive cytokine IL-10 was slowly upregulated in the colon and lungs, whereas a massive induction was detected in the spleen. In summary, these data clearly indicate that after CASP surgery, a rapid increase of endotoxin followed by a systemic inflammatory response syndrome occurs. Surprisingly, the strongest upregulation (Fig. 7 and data not shown) of TNF-α and IFN-γ was observed in lungs.
FIG. 7.
Detection of cytokine mRNA in organs after CASP surgery. (A) Internal competitive semiquantitative cytokine RT-PCR from spleens harvested 6 h after 14G CASP or sham surgery. mRNA was extracted from spleens of mice after sham or 14G CASP surgery, and cDNA was transcribed. cDNAs were serially diluted, and the content of cDNA was estimated by internal competitive semiquantitative RT-PCR with β-actin-specific primers in the presence of known amounts of β-actin control fragment. TNF-α, IL-12p40, IFN-γ, and IL-10 cDNA amounts were determined by PCR amplification of serial dilutions from equilibrated cDNA amounts in the presence of the relevant PCR primers (Table 1) and the corresponding control fragment. The upper band represents the amplified control fragment of a known constant concentration, whereas the lower band shows the signal obtained after amplification of each titrated cytokine cDNA. The arrows indicate the concentrations of equal amounts of control fragment and cytokine cDNA. Upregulation of mRNAs for TNF-α, IL-12p40, and, to a smaller extent, IFN-γ can be observed. (B) Kinetics of the induction of cytokine mRNA transcription in colon, mesenteric lymph nodes, spleen, and lungs after CASP and sham surgery. Upregulation of TNF-α, IL-12p40, IFN-γ, and IL-10 was analyzed 3, 6, and 12 h after 14G CASP or sham surgery in various organs of mice; ascending colon, mesenteric lymph nodes, spleen, and lungs were removed, RNA was extracted, and cDNA was prepared. Semiquantitative PCR was performed as described for panel A. Induction of cytokine mRNA was calculated as fold induction over basal levels as determined after sham surgery. For explanation of calculations, see text.
Survival of CASP in mice deficient in TNFRp55 and IFN-γR.
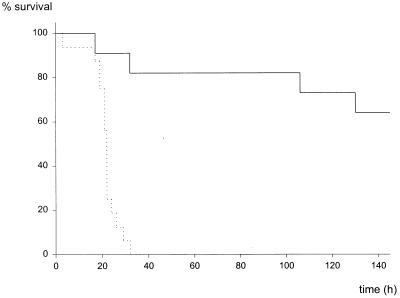
TNF-α and IFN-γ are considered harmful mediators of septic shock. In bolus shock models, TNFRp55-deficient (LPS–d-GalN) and IFN-γR-deficient (high-dose LPS) mice are highly resistant (14, 41, 47). The course of sublethal 18G CASP was therefore investigated in TNFRp55−/− (C57BL/6 inbred background) and IFN-γR−/− mice (129/SvJ × C57BL/6 mixed background). In contrast to results observed after bolus injection of bacterial toxins, the mortality rates of TNFRp55−/− animals (9 of 12 [75%]) after CASP surgery were not significantly different from those of control mice (8 of 12 [67%]; P = 0.488) (Fig. 8). However, the IFN-γR conferred protective functions after bacterial invasion since IFN-γR-deficient mice rapidly succumbed after 18G CASP surgery (100% [15 of 15]), whereas 36% (4 of 11; P < 0.001) of the control littermates died (Fig. 9). The differences between survival rates of TNFRp55+/+ mice (C57BL/6 inbred) and IFNγR+/+ mice (129J/SvJ × C57BL/6 mixed background) are not statistically significant (P = 0.131). However, the small differences in mortality observed in these groups may be attributable to the phenomenon of hybrid resistance to infection. These findings clearly indicate that gene-deficient mice are valuable tools for dissection of cytokine functions in vivo, that sterile toxic shock models do not mimic pathophysiological responses in sepsis initiated by replicating pathogens, and, moreover, that IFN-γ is required for survival of abdominal sepsis.
FIG. 8.
Mortality of TNFRp55−/− mice after CASP surgery. TNFRp55+/+ (——) and TNFRp55−/− (·····) (41) animals (C57BL/6 background) were subjected to 18G CASP surgery. Survival was monitored. Four of 12 mice in the control group and 3 of 12 TNFRp55−/− mice survived 18G CASP.
FIG. 9.
Mortality of IFNγR−/− mice after CASP surgery. IFNγR−/− (32) (·····) and IFNγR+/+ (——) control littermates (C57BL/6 × 129/Sv background) were analyzed after 18G CASP surgery. Seven of 11 IFNγR+/+ mice survived, whereas all of the 15 mice with an inactivated receptor for IFN-γ died.
DISCUSSION
The current view regarding the pathogenesis of sepsis is based on the concept that bacteria, bacterial toxins, and/or virulence factors trigger inflammatory host responses culminating in a systemic inflammatory response syndrome characterized by the overproduction of host mediators such as TNF-α, IL-1, and IL-6 (12, 21). These host mediators finally cause multiorgan damage resulting in death. However, this concept is now being challenged, most probably because it has been derived mainly from animal studies in which bolus injections of bacteria or toxins were used and where the occurrence of septic shock was prevented by early interference with TNF-α bioactivities (30, 34, 38, 46, 51, 55). However, these models do not necessarily mirror the various conditions leading to sepsis in human patients (mechanical trauma, burn injury, surgery, gastrointestinal tract infection, etc.) and thus neither distinguish between distinct entities of patients nor take into account the invasion of the host by live replicating bacteria (11). It is conceivable that these problems account for the indecisive outcome of clinical trials based on bolus shock models.
The aim of this study was to develop a model for postoperative abdominal sepsis and to carefully dissect the immune pathophysiology during the course of sepsis (60). In CASP, a physical connection from the ascending colon into the peritoneal cavity is established. The surgical techniques involved readily allow the reproducible generation of a septic focus leading to the immediate onset of generalized peritonitis. Moreover, CASPI provides a novel model for the study of mechanisms that may affect the efficacy of common surgical treatment regimens, addressing the removal of the septic focus combined with supportive therapies. The data presented here provide clear evidence that CASP and CASPI are indeed well suited for the reliable and highly standardized investigation of sepsis. The prime objective of this model that resembles human conditions much more closely than bolus sepsis models is to elucidate the pathophysiology of sepsis encountered in a defined group of patients and evaluate supportive surgical and nonsurgical therapy methods.
The clinical course of abdominal sepsis is characterized by a continuous or intermittent release of bacteria or bacterial toxins from a septic focus that induces a variety of inflammatory host mediators (12, 21). In CLP, a surgical peritonitis model described by Wichtermann et al. (59), different numbers of holes with various diameters are punctured into the ligated cecum. In our hands, ligation of the entire cecum led to 100% lethality independent of the size of the puncture holes (data not shown), probably due to necrosis of the entire cecum with fulminant release of bacteria. To obtain a sublethal experimental group, ligation of a small portion of the cecum was tried (data not shown). However, because it is obviously impossible to ligate always exactly the same volume of the cecum, we reasoned that the insertion of a stent with a defined diameter would provide a more standardized way to produce sublethal experimental groups. In CASP, we observed exponentially increasing bacterial numbers in the peritoneal cavity after surgery, whereas in CLP there was a short initial peak, followed by a period of low bacterial counts culminating in a fulminant increase of bacteria in the peritoneal cavity (data not shown). As in CASPI, excision of the ligated cecum can be performed at different time points after primary surgery to eliminate the septic focus (5). However, since there is no standardized leakage of bacteria in CLP, the CASP and CASPI model is clearly favored for the investigation of experimental sepsis in the mouse.
Regulation of cytokines during sepsis and their tissue-specific expression patterns are widely unknown. Furthermore, it is not clear whether in a septic host hyperinflammatory and immunosuppressive conditions can coexist in distinct organs or tissues. It appears that determination of the relative contribution of local versus systemic cytokine production could be important for understanding the pathophysiology of sepsis, especially since numerous clinical studies have attempted to systemically neutralize LPS, TNF-α, or IL-1 and could not provide evidence for a beneficial effect of these treatment strategies (25, 29, 48, 61).
With use of CASP surgery, these questions can readily be addressed. As shown in this study, an increase of bacteria numbers in the host is rapidly encountered. As soon as 3 h after CASP surgery, LPS is detectable in the blood. Also, cultures from peripheral blood showed growth of enterobacteriaceae and enterococci as soon as 3 h after CASP surgery (59b). In the peritoneal cavity, in the peripheral blood, and in organs such as liver, lungs, and spleen, rapid invasion of bacteria occurs. As in the clinical situation, the host reacts by a systemic inflammatory response syndrome that is experimentally verified by the highly elevated amount of TNFα found in the systemic circulation. Interestingly, comparable levels of LPS and TNF-α were observed in patients suffering from severe secondary peritonitis (31). Additionally, IFN-γ could not be detected in septic CASP-operated mice in the circulation, consistent with findings in septic patients, who did not show a significant increase of systemic IFN-γ compared to baseline levels (59a).
Interestingly, the upregulation of cytokines such as IL-10, IL-12, IFN-γ, or TNF-α is highly site specific within the host after CASP surgery. While the local response in the colon is characterized by an induction of TNF-α, and interestingly of IL-10, the recruitment of inflammatory cells into the colonic wall is not accompanied by IL-12p40 or IFN-γ production. In the mesenteric lymph nodes, only a relatively weak induction of TNF-α and no upregulation of IL-12 and IFN-γ could be found. These data may indicate that the local reaction of the peritoneal environment is biased to evoke protective TNF-α actions such as procoagulatant activity (8). Local TNF-α activity might be beneficial, since TNF-α appears to be required for closure of the septic focus by local abscess formation and for clearance of bacteria. This assumption is based on results from experiments of the CLP type (19) and on our own studies using the CLP model (59c). Here, neutralization of TNF-α leads to increased mortality, whereas treatment with TNF-α increases survival (17, 19). In marked contrast, if local abscess formation, to encapsulate the septic focus, is not effective, which is the case in 14G CASP (59c), the early and constant bacterial invasion of the body leads to a generalized reaction. Subsequently, a rapid induction of TNF-α, IL-12, and IL-10 is detected in the spleen. Interestingly, in the spleen, IFN-γ was only weakly upregulated, possibly because the simultaneous expression of IL-10 and IL-12 is counteractive with respect to IFN-γ production. In contrast, the most dramatic upregulation of IL-12, IFN-γ, and TNF-α was observed in the lungs, where the induction of IL-10 was almost absent. Thus, a differential pattern of local cytokine induction can clearly be established. These findings indicate that organ damage of the lungs resulting in a respiratory distress syndrome, as is frequently encountered in sepsis patients, might result from the concerted and synergistic actions of TNF-α and IFN-γ and lack of the counterregulatory cytokine IL-10. Furthermore, compartmentalized neutralization of one or both of these cytokines might reduce organ damage and could be beneficial for the outcome of sepsis.
To further substantiate this notion, the roles of TNF-α and IFN-γ in CASP were verified on a molecular level. To this end, TNFRp55- and IFN-γR-deficient mice (32, 41) were subjected to 18G CASP surgery. In accordance with previous clinical studies (1, 27) and murine experiments (4, 22, 37) where TNF-α was systemically neutralized, the mortality rate of TNFRp55−/− mice did not differ substantially from that of control mice. This might be interpreted to mean that TNF-α plays no decisive role in abdominal sepsis, or, more likely, that by neutralization of TNF-α, both harmful and protective effects of this cytokine are lost, which results in an equivocal outcome.
Based on the expression pattern of IFN-γ described in this study, it might have been assumed that in the absence of IFN-γ signaling, an improved survival after CASP surgery would ensue, especially since the exuberant inflammatory reaction in the lung might be attenuated or absent. This is clearly not the case, since IFN-γR−/− mice readily succumb to 18G CASP, indicating that IFN-γ is of critical importance for coping with the invading bacteria. The experimental data obtained for IFNγR−/− mice support the results from a recent animal study where survival of peritonitis after burn injury was improved by IL-12 therapy (40) and are in agreement with a recent clinical study where IFN-γ-treated septic patients showed an improved clinical course (33). However, it also should be noted that in CLP experiments using mice, adverse reactions of IFN-γ therapy were observed (39). Thus, it is possible that in the spleen, where the primary clearance site for bacteria after systemic invasion is located, IFN-γ effects are essential for activation of monocytes and macrophages to destroy bacteria, but that IFN-γ actions in the lungs (and other tissues) cause harmful effects. Thus, it is unclear how systemic administration of IFN-γ acts to improve the clinical situation and whether targeting of IFN-γ or IL-12 bioactivity to certain organs can improve sepsis therapy. Further studies dissecting the local roles of IFN-γ in mice harboring conditional deletions (43) of the IFN-γR gene are required to clarify the situation.
In summary, CASP and CASPI may provide a very useful experimental model of bacterial sepsis in which basic pathophysiological events can be elucidated and in which surgical, immunological, and supportive treatment protocols can be thoroughly investigated.
ACKNOWLEDGMENTS
We thank K. Mink, E. Schaller, and A. Fütterer for expert technical help. For suggestions, helpful scientific discussion, and critical reading of the manuscript, we thank H. Neubauer, T. Plitz, R. Endres, and G. Häcker.
This work was supported by Klinische Forschergruppe Si208/5-1 “Immunsuppression und postoperative Sepsis,” DFG grant Pf259/2, and SFB391 project B3 to K.P.
REFERENCES
- 1.Abraham E, Glauser M P, Butler T, Garbino J, Gelmont D, Laterre P F, Kudsk K, Bruining H A, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA. 1997;277:1531–1538. [PubMed] [Google Scholar]
- 2.Anonymous. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 3.Bach E A, Aguet M, Schreiber R D. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 4.Bagby G J, Plessala K J, Wilson L A, Thompson J J, Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991;163:83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Baker C C, Chaudry I H, Gaines H O, Baue A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 6.Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 8.Beutler B. Tumor necrosis factors. New York, N.Y: Raven Press Ltd.; 1992. [Google Scholar]
- 9.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 10.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 11.Bone R C. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Bone R C. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17:175–181. doi: 10.1016/s0272-5231(05)70307-5. [DOI] [PubMed] [Google Scholar]
- 13.Bone R C, Fisher C J, Jr, Clemmer T P, Slotman G J, Metz C A, Balk R A. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 14.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Docke W D, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk H D, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 17.Echtenacher B, Falk W, Mannel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 18.Echtenacher B, Hultner L, Mannel D N. Cellular and molecular mechanisms of TNF protection in septic peritonitis. J Inflamm. 1995;47:85–89. [PubMed] [Google Scholar]
- 19.Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 20.Erickson S L, de Sauvage F J, Kikly K, Carver Moore K, Pitts Meek S, Gillett N, Sheehan K C, Schreiber R D, Goeddel D V, Moore M W. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 21.Ertel W, Morrison M H, Wang P, Ba Z F, Ayala A, Chaudry I H. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskandari M K, Bolgos G, Miller C, Nguyen D T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 23.Fischer E, Marano M A, Van Zee K J, Rock C S, Hawes A S, Thompson W A, DeForge L, Kenney J S, Remick D G, Bloedow D C. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992;89:1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher C J, Jr, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 25.Fisher C J, Jr, Dhainaut J F, Opal S M, Pribble J P, Balk R A, Slotman G J, Iberti T J, Rackow E C, Shapiro M J, Greenman R L. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 26.Fisher C J, Jr, Slotman G J, Opal S M, Pribble J P, Bone R C, Emmanuel G, Ng D, Bloedow D C, Catalano M A. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. The IL-1RA Sepsis Syndrome Study Group. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Fisher C J J, Jr, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M H, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 28.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 29.Greenman R L, Schein R M, Martin M A, Wenzel R P, MacIntyre N R, Emmanuel G, Chmel H, Kohler R B, McCarthy M, Plouffe J. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991;266:1097–1102. [PubMed] [Google Scholar]
- 30.Heremans H, Van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990;171:1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzheimer R G, Schein M, Wittmann D H. Inflammatory response in peritoneal exudate and plasma of patients undergoing planned relaparotomy for severe secondary peritonitis. Arch Surg. 1995;130:1314–1319. doi: 10.1001/archsurg.1995.01430120068010. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 33.Kox W J, Bone R C, Krausch D, Docke W D, Kox S N, Wauer H, Egerer K, Querner S, Asadullah K, von Baehr R, Volk H D. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med. 1997;157:389–393. [PubMed] [Google Scholar]
- 34.Lehmann V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Lembert A. Mémoire sur l’enterorrhaphie avec description d’un procède nouveau pour pratiquer cette opération chirurgicale. Répertoire général d’anatomie et de physiologie pathologique et de clinique. Chirurgicale. 1826;2:104. [Google Scholar]
- 35.Li L, Elliott J F, Mosmann T R. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–3978. [PubMed] [Google Scholar]
- 36.Mancilla J, Garcia P, Dinarello C A. The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniaesepsis in newborn rats. Infect Immun. 1993;61:926–932. doi: 10.1128/iai.61.3.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMasters K M, Peyton J C, Hadjiminas D J, Cheadle W G. Endotoxin and tumour necrosis factor do not cause mortality from caecal ligation and puncture. Cytokine. 1994;6:530–536. doi: 10.1016/1043-4666(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles R H, Paxton T P, Dries D J, Gamelli R L. Interferon-gamma increases mortality following cecal ligation and puncture. J Trauma. 1994;36:607–611. doi: 10.1097/00005373-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 39a.Neubauer, H., and K. Pfeffer. Unpublished data.
- 40.O’Suilleabhain C, O’Sullivan S T, Kelly J L, Lederer J, Mannick J A, Rodrick M L. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery. 1996;120:290–296. doi: 10.1016/s0039-6060(96)80300-x. [DOI] [PubMed] [Google Scholar]
- 41.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 42.Piper R D, Cook D J, Bone R C, Sibbald W J. Introducing critical appraisal to studies of animal models investigating novel therapies in sepsis. Crit Care Med. 1996;24:2059–2070. doi: 10.1097/00003246-199612000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Rajewsky K, Gu H, Kuhn R, Betz U A, Muller W, Roes J, Schwenk F. Conditional gene targeting. J Clin Invest. 1996;98:600–603. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remick D G. Applied molecular biology of sepsis. J Crit Care. 1995;10:198–212. doi: 10.1016/0883-9441(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 45.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukocyte Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 46.Rietschel E T, Brade H, Holst O, Brade L, Muller-Loennies S, Mamat U, Zahringer U, Beckmann F, Seydel U, Brandenburg K, Ulmer A J, Mattern T, Heine H, Schletter J, Loppnow H, Schonbeck U, Flad H D, Hauschildt S, Schade U F, di Padova F, et al. Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- 47.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 48.Russell D A, Thompson R C. Targets for sepsis therapies: tumor necrosis factor versus interleukin- 1. Curr Opin Biotechnol. 1993;4:714–721. doi: 10.1016/0958-1669(93)90055-2. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Madrid F, Simon P, Thompson S, Springer T A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 51.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J, Zentella A, Albert J D, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 52.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 54.van den Broek M F, Muller U, Huang S, Zinkernagel R M, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 55.Van Zee K J, Moldawer L L, Oldenburg H S, Thompson W A, Stackpole S A, Montegut W J, Rogy M A, Meschter C, Gallati H, Schiller C D, Richter W F, Loetscher H, Ashkenazi A, Chamow S M, Wurm F, Calvano S E, Lowry S F, Lesslauer W. Protection against lethal Escherichia coli bacteremia in baboons (Papio anubis) by pretreatment with a 55-kDa TNF receptor (CD120a)-Ig fusion protein, Ro 45-2081. J Immunol. 1996;156:2221–2230. [PubMed] [Google Scholar]
- 56.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 57.Volk, H. D., P. Reinke, D. Krausch, H. Zuckermann, K. Asadullah, J. M. Muller, W. D. Docke, and W. J. Kox. 1996. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 22(Suppl. 4):474–481. [DOI] [PubMed]
- 58.Warren H S. Strategies for the treatment of sepsis. N Engl J Med. 1997;336:952–953. doi: 10.1056/NEJM199703273361311. [DOI] [PubMed] [Google Scholar]
- 59.Wichtermann K A, Baue A E, Chaudry I H. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 59a.Zantl, N., and B. Holzmann. Unpublished data.
- 59b.Zantl, N., and K. Pfeffer. Unpublished data.
- 59c.Zantl, N., B. Holzmann, C.-D. Heidecke, and K. Pfeffer. Unpublished data.
- 60.Zantl N, Holzmann B, Pfeffer K, Heidecke C-D. Colon ascendens stent peritonitis (CASP): a novel surgical model for the induction of bacterial peritonitis/sepsis in mice. In: Faist E, editor. The immune consequences of trauma, shock and sepsis. Bologna, Italy: Monduzzi Editore; 1997. pp. 467–471. [Google Scholar]
- 61.Ziegler E J, Fisher C J, Jr, Sprung C L, Straube R C, Sadoff J C, Foulke G E, Wortel C H, Fink M P, Dellinger R P, Teng N N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]