Abstract
To investigate the role of monocyte chemoattractant protein 1 (MCP-1) in the immune response to Mycobacterium tuberculosis, we studied MCP-1 production in tuberculosis patients. CD14+ blood monocytes from tuberculosis patients spontaneously expressed higher levels of MCP-1 mRNA and protein than CD14+ monocytes from healthy tuberculin reactors. MCP-1 production in lymph nodes from tuberculosis patients was also markedly increased. These findings suggest that MCP-1 can contribute to the antimycobacterial inflammatory response by attracting monocytes and T lymphocytes.
During the immune response to Mycobacterium tuberculosis infection, T cells and macrophages are recruited to the site of infection, resulting in tissue inflammation and granuloma formation. The mechanism for recruitment of these cells is likely to involve chemokines, which are a large family of proteins that include chemoattractants for neutrophils, lymphocytes, and monocytes. Among the chemokines, monocyte chemoattractant protein 1 (MCP-1) is likely to play an important role in the pathogenesis of tuberculosis because it is a chemoattractant for T lymphocytes and monocytes (8), which are central components of the granulomatous response. To investigate the potential role of MCP-1 in the human immune response to M. tuberculosis, we studied MCP-1 production in blood and tissue of patients with tuberculosis.
Patient population.
Blood was obtained from 8 healthy tuberculin reactors and 12 patients with culture-proven pulmonary tuberculosis who had received <2 weeks of therapy. All patients had positive sputum acid-fast smears and were human immunodeficiency virus seronegative. All subjects gave informed consent to participate in the study. Frozen lymph node tissue was obtained from six human immunodeficiency virus-negative patients with tuberculous lymphadenitis who had received <4 weeks of therapy and from six healthy adults whose lymph nodes showed benign follicular hyperplasia.
MCP-1 production.
Peripheral blood mononuclear cells (PBMCs) were centrifuged on a Percoll (Pharmacia, Uppsala, Sweden) gradient, and purified CD14+ cells (>95% CD14+ by cytofluorometric analysis) were isolated from the monocyte fraction by positive selection with magnetic beads conjugated to anti-CD14 antibody (Miltenyi Biotech, Bergisch-Gladbach, Germany). CD14− cells were approximately 40% T cells, 30% NK cells, and 10% B cells (by cytofluorometric analysis) and approximately 20% monocytes (by Giemsa staining). In some experiments, PBMCs were separated into adherent and nonadherent cells by standard techniques (9). Adherent cells were 85 to 90% monocytes, as judged by nonspecific esterase staining.
PBMC or cell subpopulations were plated at 1.5 × 105 cells/200-μl well in medium in the presence or absence of heat-killed M. tuberculosis Erdman (1 μg/ml) for 24 to 96 h. Preliminary experiments revealed that maximal MCP-1 concentrations were measured after 24 h, so supernatants were harvested at this time point and frozen at −70°C. MCP-1 concentrations were measured by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, Minn. [sensitivity, 5 pg/ml]).
Immunohistochemistry.
Cryostat sections of lymph nodes were fixed in acetone by standard methods (7) prior to immunostaining with the primary antibodies mouse anti-human MCP-1 (5D3-F7, immunoglobulin G1 [IgG1] subtype; Pharmingen, San Diego, Calif.) and Ber-MAC3 (IgG1; Dako, Carpinteria, Calif.), which recognizes a 140-kDa protein in human tissue macrophages (2). Irrelevant isotype-matched monoclonal antibodies (Zymed Laboratories, Inc., South San Francisco, Calif.) were used as controls for nonspecific staining.
For single staining, endogenous peroxidase and nonspecific binding were blocked by standard methods (7). Sections were then incubated with anti-MCP-1 (20 μg/ml) and then biotinylated horse anti-mouse IgG, followed by avidin-biotinylated horseradish peroxidase complex (ABC-HRP reagent; Vector Laboratories, Burlingame, Calif.). Slides were then developed (AEC substrate kit; Vector Laboratories) and counterstained with hematoxylin. For double staining, the slides were incubated with 1:100 Ber-MAC3 and then biotinylated horse anti-mouse IgG. Avidin-biotinylated alkaline phosphatase complex (Vector Laboratories) was added, and slides were developed with Vector-blue (alkaline phosphate substrate kit III; Vector Laboratories). Staining for MCP-1 was then performed as outlined above, except that no counterstaining with hematoxylin was done.
Measurement of MCP-1 mRNA.
A total of 106 CD14+ cells, cultured in 2-ml wells in medium alone, were harvested after 24 h, lysed with 4 M guanidinium isothiocyanate, and stored at −20°C. Total cellular RNA was extracted and cDNA was synthesized, as described previously. MCP-1 mRNA was quantitated by competitive reverse transcription-PCR, as previously described (7, 11). Briefly, we normalized samples for β-actin cDNA content by competitive PCR by using the 5′ and 3′ primers GAGCGGGAAATCGTGCGTGACATT and GATGGAGTTGAAGGTAGTTTCGTG, respectively, with 27 cycles of denaturation (94°C for 1 min) and annealing plus extension (65°C for 2 min). Aliquots containing equivalent amounts of β-actin cDNA were amplified by PCR with the 5′ and 3′ MCP-1-specific primers CAAACTGAAGCTCGCACTCTCGCC and ATTCTTGGGTTGTGGAGTGAGTGTTCA, respectively. Thirty cycles of amplification were used, with the same reaction conditions as for β-actin.
Cellular source of MCP-1 in PBMCs stimulated with M. tuberculosis.
MCP-1 is produced by human monocytes in response to M. tuberculosis (5). To determine if monocytes were the predominant source of MCP-1 in PBMCs upon exposure to M. tuberculosis, we separated PBMCs into adherent and nonadherent cell populations. In addition, we separated the monocyte fraction from Percoll gradients into CD14+ and CD14− subpopulations. PBMCs and the cellular subpopulations were cocultured with heat-killed M. tuberculosis, and MCP-1 concentrations were measured in supernatants (Table 1). MCP-1 concentrations produced by adherent cells were three- to sevenfold higher than those produced by nonadherent cells, suggesting that monocytes were the predominant source of MCP-1. MCP-1 concentrations produced by CD14+ cells were approximately 100-fold higher than those produced by CD14− cells. This indicates that the predominant source of MCP-1 was CD14+ cells, which were used for all further experiments.
TABLE 1.
Production of MCP-1 by cellular subpopulations in response to M. tuberculosis
| Cell typea | MCP-1 concn (pg/ml)
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| PBMCs | 7,541 | 10,650 |
| Adherent | 48,120 | 29,520 |
| Nonadherent | 6,902 | 9,068 |
| CD14+ | 37,380 | 55,850 |
| CD14− | 472 | 407 |
CD14+ and CD14− cells were prepared from the monocyte fraction on a Percoll gradient. CD14− cells were composed of a mixture of T cells, NK cells, B cells, and monocytes.
Production of MCP-1 by CD14+ cells.
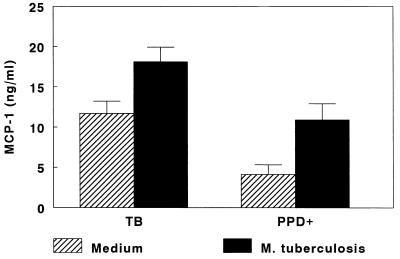
To determine if MCP-1 production was increased in human tuberculosis, we measured MCP-1 concentrations in M. tuberculosis-stimulated CD14+ cells isolated from PBMCs of 12 tuberculosis patients and 8 healthy tuberculin reactors (Fig. 1). Mean MCP-1 concentrations were significantly higher in CD14+ cells from tuberculosis patients than in those from healthy tuberculin reactors (18.1 ± 1.8 versus 10.9 ± 2.0 ng/ml [P = 0.02]). This difference reflected the fact that CD14+ cells from tuberculosis patients produced substantially more MCP-1 spontaneously when cultured in medium alone (11.7 ± 1.5 versus 4.1 ± 1.2 ng/ml [P = 0.001]). The increases in MCP-1 concentrations induced by M. tuberculosis were similar in both groups (6.4 ± 1.5 versus 6.8 ± 2.2 ng/ml [P, not significant]).
FIG. 1.
Production of MCP-1 by CD14+ cells isolated from PBMCs of tuberculosis patients (TB [n = 12]) and healthy tuberculin reactors (PPD+ [n = 8]). CD14+ cells were isolated from PBMCs and cultured for 24 h in the presence of medium alone or with 1 μg of heat-killed M. tuberculosis per ml. MCP-1 concentrations were measured by enzyme-linked immunosorbent assay. Mean values (± standard error) are shown.
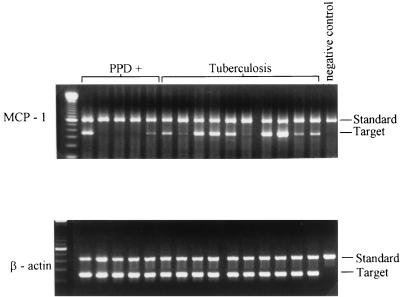
Expression of MCP-1 mRNA by CD14+ cells.
To determine if the increased MCP-1 production by CD14+ cells from tuberculosis patients was due to increased transcription of MCP-1 mRNA, we compared levels of MCP-1 mRNA expression by CD14+ cells from 5 healthy tuberculin reactors and 10 tuberculosis patients. CD14+ cells were cultured in medium alone for 24 h, and RNA was prepared from cell lysates, reverse transcribed into cDNA, and amplified by competitive PCR. After normalization for β-actin cDNA content, MCP-1 mRNA expression was greater in most tuberculosis patients than in healthy tuberculin reactors (Fig. 2).
FIG. 2.
Expression of MCP-1 mRNA in CD14+ cells from tuberculosis patients and healthy tuberculin reactors (PPD+). CD14+ cells were isolated from PBMCs of 5 healthy tuberculin reactors and 10 tuberculosis patients and then were cultured for 18 h in medium alone. mRNA expression for MCP-1 and β-actin was determined by competitive reverse transcription-PCR. In each panel, the far left lane shows molecular weight markers, and the far right lane contains no cDNA.
MCP-1 production at the site of disease.
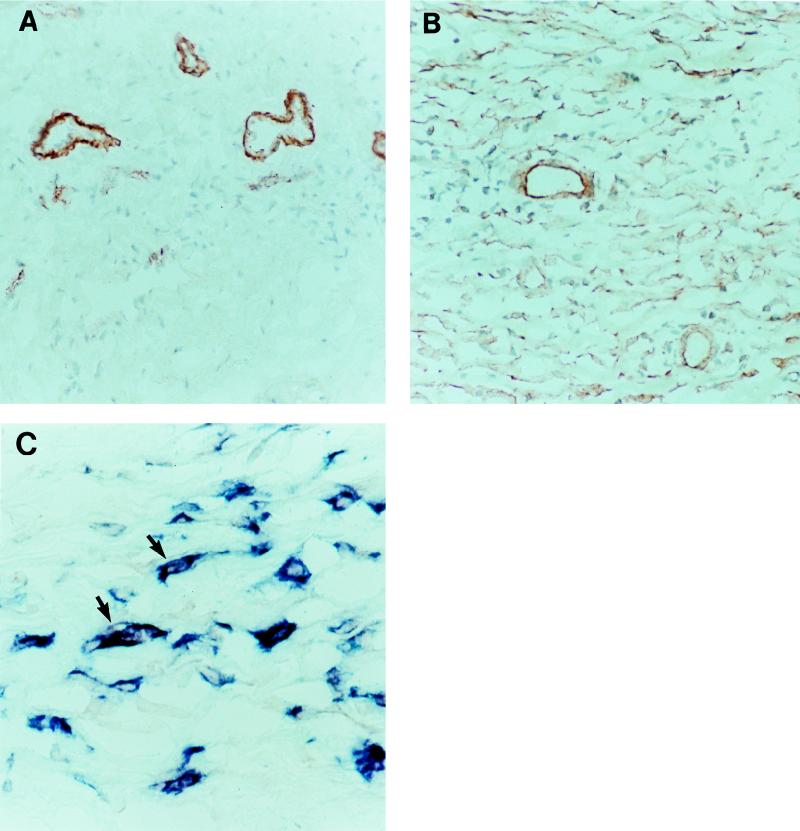
To determine if MCP-1 production was increased at the site of disease, we studied the distribution of MCP-1 in lymph nodes from patients with or without tuberculosis. In lymph nodes from patients with nonspecific inflammation from benign follicular hyperplasia, red MCP-1-expressing cells were clustered around blood vessels and probably represent endothelial cells (Fig. 3A). No staining was observed with isotype-matched irrelevant antibodies (data not shown). In tuberculosis patients, the number of MCP-1-expressing cells was strikingly increased (Fig. 3B), including endothelial cells and cells that were distributed in granulomas and throughout the lymph node. Double immunolabeling with anti-MCP-1 (red) and Ber-MAC3 antibodies, which stained macrophages blue, revealed that these MCP-1-expressing cells were macrophages. Figure 3C shows purple double-stained cells in a lymph node from a tuberculosis patient.
FIG. 3.
Immunolabeling with anti-MCP-1 and antimacrophage antibodies in lymph nodes from a patient with benign follicular hyperplasia (A) and from a patient with tuberculosis (B and C). (A and B) Single staining with anti-MCP-1 antibodies (red). Magnification, ×200. (C) Staining with anti-MCP-1 (red) and anti-Ber-MAC3 (blue) antibodies. Arrows show examples of double-stained purple cells. Magnification, ×1,000.
Recruitment of inflammatory cells is a critical component of the host immune response to microbial pathogens, and an increasing body of evidence suggests that chemokines play a central role in this process. In the current study, we found that CD14+ monocytes from PBMCs of tuberculosis patients expressed higher levels of MCP-1 mRNA and spontaneously produced more MCP-1 than CD14+ monocytes from healthy tuberculin reactors. Furthermore, in lymph nodes from tuberculosis patients, there was a striking increase in MCP-1 production by macrophages. These findings suggest that in human tuberculosis, there is enhanced local and systemic production of MCP-1, which can contribute to the inflammatory response by attracting monocytes and CD4+ and CD8+ T cells (8).
The role of MCP-1 in the granulomatous response to microbial pathogens is controversial, because animal models have yielded conflicting results. In a murine model of Cryptococcus neoformans pneumonia, depletion of MCP-1 markedly reduces mononuclear cell recruitment and clearance of organisms (4). In contrast, anti-MCP-1 antibodies had no effect on granuloma formation in mice exposed to beads coupled to purified protein derivative of M. tuberculosis (3). In humans, MCP-1 concentrations are elevated in pleural fluid of patients with tuberculous pleuritis (1) and in bronchoalveolar lavage fluid of patients with pulmonary tuberculosis (6). During convalescence from pulmonary tuberculosis, MCP-1 concentrations in bronchoalveolar lavage fluid decline, confirming the association of active tuberculosis with enhanced MCP-1 production (6). Our findings confirm and extend these observations, demonstrating that MCP-1 production is enhanced in blood and at the site of disease in human tuberculosis and that macrophages are the predominant source of MCP-1. Further studies are needed to determine if MCP-1 contributes to the immune response in vivo in mycobacterial disease.
Acknowledgments
This work was supported by the National Institutes of Health (AI 27285 and AI36069). Peter F. Barnes holds the Margaret E. Byers Cain Chair for Tuberculosis Research. Mycobacterial products were provided through contract AI05074 from the National Institute of Allergy and Infectious Diseases.
We thank Bharat Nathwani for providing lymph node specimens.
REFERENCES
- 1.Antony V B, Godbey S W, Kunkel S L, Hott J W, Hartman D L, Burdick M D, Strieter R M. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151:7216–7223. [PubMed] [Google Scholar]
- 2.Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. Ber-MAC3: new monoclonal antibody that defines a human monocyte/macrophage differentiation antigen. J Clin Pathol. 1991;44:936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chensue S W, Warmington K S, Ruth J H, Sanghi P S, Lincoln P, Kunkel S L. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation. Relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 4.Huffnagle G B, Strieter R M, Standiford T J, McDonald R A, Burdick M D, Kunkel S L, Toews G B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 5.Kasahara K, Tobe T, Tomita M, Mukaida N, Shao-Bo S, Matsushima K, Yoshida T, Sugihara S, Kobayashi K. Selective expression of monocyte chemotactic and activating factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–1247. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 6.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T, Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–1477. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Zhang M, Hofman F M, Gong J, Barnes P F. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taub D D, Proost P, Murphy W J, Anver M, Longo D L, Damme J V, Oppenheim J J. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95:1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, M., M. K. Gately, E. Wang, S. F. Wolf, S. Lu, R. L. Modlin, and P. F. Barnes. Interleukin-12 at the site of disease in tuberculosis. J. Clin. Invest. 93:1733–1739. [DOI] [PMC free article] [PubMed]
- 10.Zhang M, Gong J, Iyer D V, Jones B E, Modlin R L, Barnes P F. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]