Abstract
Nanoparticle transport into plants is an evolving field of research with diverse applications in agriculture and biotechnology. This article provides an overview of the challenges and prospects associated with the transport of nanoparticles in plants, focusing on delivery methods and the detection of nanoparticles within plant tissues. Passive and assisted delivery methods, including the use of roots and leaves as introduction sites, are discussed, along with their respective advantages and limitations. The barriers encountered in nanoparticle delivery to plants are highlighted, emphasizing the need for innovative approaches (e.g., the stem as a new recognition site) to optimize transport efficiency. In recent years, research efforts have intensified, leading to an evendeeper understanding of the intricate mechanisms governing the interaction of nanomaterials with plant tissues and cells. Investigations into the uptake pathways and translocation mechanisms within plants have revealed nuanced responses to different types of nanoparticles. Additionally, this article delves into the importance of detection methods for studying nanoparticle localization and quantification within plant tissues. Various techniques are presented as valuable tools for comprehensively understanding nanoparticle–plant interactions. The reliance on multiple detection methods for data validation is emphasized to enhance the reliability of the research findings. The future outlooks of this field are explored, including the potential use of alternative introduction sites, such as stems, and the continued development of nanoparticle formulations that improve adhesion and penetration. By addressing these challenges and fostering multidisciplinary research, the field of nanoparticle transport in plants is poised to make significant contributions to sustainable agriculture and environmental management.
Keywords: assisted delivery, passive delivery, vascular bundles, localization, quantification, nanoparticle characterization, detection strategies
1. Introduction
The transport of matter into plants, also known as plant uptake, is a crucial process for the growth and development of plants. Plants require various essential nutrients, water, and gases for their survival and growth. The primary processes facilitating the transport of matter into plants encompass water uptake and the assimilation of nutrients. Water is essential for plants as it serves as a solvent for nutrients and is involved in various physiological processes [1]. Water is absorbed by the roots of plants through a process called osmosis [2]. The root hairs, tiny hair-like structures on the roots, play a critical role in water uptake [3]. Additionally, Choi and Cho discovered that root hairs significantly enhance the soil retention capacity of seedling roots in Arabidopsis thaliana [3]. Plants rely on essential nutrients like macronutrients (e.g., nitrogen, phosphorus, and potassium) and micronutrients (e.g., iron, zinc, and copper) [4], which they absorb through their roots from the soil. Nutrient uptake is a complex process utilizing both passive and active transport mechanisms. Smaller ions typically enter root cells through passive diffusion driven by concentration gradients [5,6]. However, for nutrients found in lower soil concentrations, plants employ active transport, an energy-consuming process facilitated by specific root cell transport proteins. Examples of these proteins include K+ transporters and channels like OsGORK, OsAKT1, OsHAK1, OsHAK5, and OsHAK21 [7]. Nutrients travel through two main pathways in plants. The symplastic pathway involves nutrient movement through the plant cells via plasmodesmata [8], while the apoplastic pathway involves movement through the cell walls and intercellular spaces [9]. After the roots absorb nutrients, the plant’s vascular system, composed of xylem and phloem, transports them to various tissues. Xylem carries water and nutrients from the roots to aerial parts, while phloem transports sugars and organic compounds throughout the plant [10]. In Zea mays, Wang et al. demonstrated varied localization and translocation patterns within the xylem and phloem [11]. Transpiration, the loss of water vapor through the stomata [12], creates tension in the plant’s vascular system, facilitating the upward pull of water and nutrients from the roots, known as transpiration pull [13]. Additionally, plants absorb carbon dioxide (CO2) through the stomata, mainly in the leaves, for photosynthesis, converting light energy into chemical energy and producing oxygen (O2) as a byproduct [14].
The transport in plants extends beyond water, nutrients, and gases, encompassing the movement of various particles or nanoparticles, as well as other exogenous materials. This includes genetic material such as double-stranded RNA (dsRNA). Nanoparticles can be transported into plants for several reasons, often as a result of environmental exposure or as a part of research and development efforts. The transport of nanoparticles into plants can have various implications, both beneficial and potentially concerning, depending on the type of nanoparticles and the intended purpose. Nanoparticles can be classified based on both their chemistry and size [15]. The chemical composition of nanoparticles dictates their inherent properties, such as optical, electrical, magnetic, and catalytic characteristics [16,17]. Different materials exhibit unique behaviors and functions. Additionally, particle size also directly influences their physical and chemical properties due to quantum effects and an increased surface-to-volume ratio [18]. In many cases, a combination of both chemistry and size is considered to tailor nanoparticles for specific applications. Nanoparticles have dual applications in delivering nutrients, fertilizers, and genetic materials to plants, offering significant benefits in agriculture and biotechnology [19,20]. Designed for controlled nutrient release, nanoparticles, like the nanoU-NPK fertilizer (Ca 23.3%, P 10.1%, K 1.5%, NO3 2.9%, urea 4.8%), minimize over-fertilization and enhance nutrient use efficiency [21]. This controlled release also mitigates environmental impacts related to nutrient leaching and runoff. Additionally, nanoparticles act as carriers for genetic materials, facilitating genetic modification, gene silencing, or gene editing [20,22]. They deliver transgenes or genetic constructs into plant cells for trait enhancement or modification [22]. Small RNA molecules, like small interfering RNA (siRNA), delivered by nanoparticles, initiate RNA interference (RNAi) for targeted gene regulation or virus resistance [23]. Combining nutrient and genetic material delivery through nanoparticles is particularly valuable in modern agriculture, addressing challenges and contributing to crop improvement.
In this review, we will provide an overview of the transport of nanoparticles in plants and the methods employed for their detection. Additionally, we will outline the challenges associated with current methodologies and explore potential future directions.
2. Transport of Nanoparticles into Plants
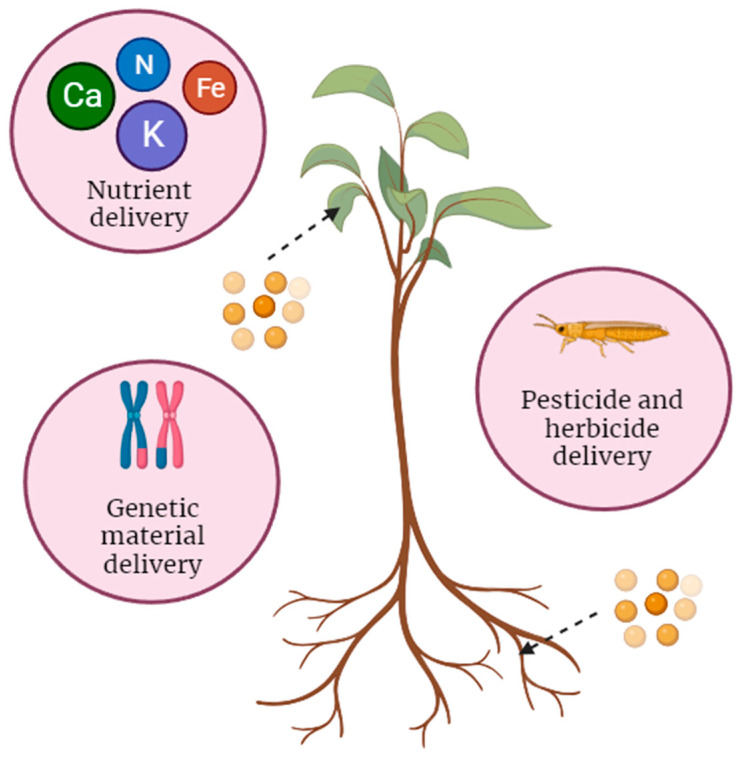
Understanding the transport of nanoparticles into plants is crucial for harnessing their potential in agriculture and biotechnology. The journey of nanoparticles from external environments to internal plant tissues involves intricate processes influenced by both the properties of the nanoparticles and the characteristics of plant structures. Nanoparticles can be designed to transport specific materials, compounds, or genetic information within plants, acting as carriers to deliver these payloads to target locations. The controlled delivery of nanoparticles can be advantageous for precise and efficient plant applications. Nanoparticles can be used for fertilizer or nutrient delivery, genetic material delivery, and pesticide or herbicide delivery (Figure 1). Nanoparticles can be used to transport essential nutrients, including micronutrients such as iron, zinc, and other trace elements, into plant cells [21,24,25]. Tombuloglu et al. successfully synthesized composites of micronutrient nanoparticles (NPs) and applied them to Hordeum vulgare L. [25]. They demonstrated the effective incorporation of these micronutrients into plant tissue. The transportation of these NPs significantly increased the quantity of elements in both the root and leaf tissues. Specifically, the contents of Fe, Zn, and Cu were raised to approximately 5, 3, and 18 times higher than the control, respectively [25]. This approach is known as nanoparticle-mediated nutrient delivery and is aimed at enhancing the nutrient uptake and utilization of plants. Nanoparticles, due to their small size, can be taken up by plant roots or leaves. The nanoparticles may enter plant cells through various mechanisms, such as diffusion, endocytosis, or direct uptake through ion channels and transporters [7,26]. Once inside the plant cells, the nanoparticles release the encapsulated nutrients. This controlled release ensures that the nutrients are made available within the plant at a rate that matches the plant’s needs [25]. By improving nutrient uptake and utilization, nanoparticle-mediated nutrient delivery can reduce the number of traditional fertilizers needed, minimizing the environmental impact associated with excess nutrient runoff and leaching.
Figure 1.
Nanoparticles used as carriers for nutrient (fertilizer), genetic material, and pesticide delivery into plants.
Nanoparticles can also serve as vehicles for delivering genetic material, such as DNA, RNA, or small interfering RNA (siRNA), into plant cells [22,23]. This technology, often referred to as “nanoparticle-mediated gene delivery”, has several applications in plant biotechnology and agriculture. Nanoparticles are engineered to encapsulate, bind, or complex with genetic material. These nanoparticles are designed to protect the genetic material from degradation and facilitate its delivery into plant cells [27]. Wang et al. used several types of NPs (CS, PEI, protamine, CQD, PAMAM, and CSC) to deliver dsRNA against rice sheath blight (Rhizoctonia solani) in Oryza sativa L. [28]. These nanoparticles could protect dsRNA from degradation by nucleases. Nanoparticles are introduced to plant tissues through various methods, such as direct injection into plant cells, root soaking, or foliar spray. Once inside the plant cells, the nanoparticles release the encapsulated genetic material. This can be achieved through controlled release mechanisms or by breaking down the nanoparticle complexes under specific conditions. Nanoparticles can be used to transport pesticides or herbicides to specific target areas within plants [29]. This approach can enhance the precision and efficiency of pesticide application and reduce the environmental impact associated with conventional spray applications. Nanoparticles can be designed to target specific plant tissues or cell types, ensuring that the pesticides or herbicides are delivered directly to the intended areas, such as the leaves, stem, or root system [29,30]. A water-soluble chitosan (CS) derivative (N-(2-hydroxyl)propyl-3-trimethyl ammonium CS chloride, HTCC) was successfully capped on the surface of pyraclostrobin-loaded MSNs by Cao et al. [31]. This particle directly targeted Phomopsis asparagi (Sacc.) and had fungicidal activity against it. By improving the targeted delivery of pesticides and herbicides, nanoparticle-mediated delivery can reduce the amount of chemicals needed, minimize off-target effects, and reduce environmental pollution and contamination.
Nanoparticles have also shown potential impacts on plant development and growth, and their applications in agriculture are an active area of research. Nanoparticles can influence seed germination rates and early plant growth [32,33]. They may enhance seedling vigor and promote healthier plant establishment. Our previous study showed that the use of SiO2 NPs could improve tomato (Solanum lycopersicum var. Momotaro) seed germination [33]. Some nanoparticles have been studied for their potential to enhance plant stress tolerance, such as resistance to drought, salinity, or heavy metal toxicity [34]. These nanoparticles may act as stress mitigators and improve overall plant health. The presence of nanoparticles in soil can also influence microbial communities and soil health [35]. The impact depends on the type of nanoparticles used and their interactions with soil microorganisms. Some nanoparticles may exhibit phytotoxic effects. This can result in stunted growth, reduced photosynthesis, or other negative impacts on plant health [36]. The fate of nanoparticles in the environment, including their persistence and potential for leaching into water sources, is an important consideration for sustainable agricultural practices. Balancing the potential benefits with environmental and human health considerations is crucial for the sustainable integration of nanotechnology in agriculture.
2.1. Types of Nanoparticles Used for Transport
Various types of nanoparticles are used for transport in plants, depending on the specific application and the payload (substance being transported). Different nanoparticles possess unique properties that make them suitable for diverse purposes in plant biology and agriculture. The choice of nanoparticle type in plant-related applications depends on several critical factors, as listed in Table 1. The careful consideration of these factors is essential when choosing the appropriate nanoparticle type for specific plant-related applications. The goal is to ensure that the selected nanoparticles align with the objectives of the application, provide benefits, and minimize potential risks to plants and the environment.
Nanoparticles can be categorized into commercial (engineered) and biogenic (naturally occurring) based on their origin [37]. Commercial nanoparticles, also referred to as engineered nanoparticles, are particles that are intentionally designed, synthesized, and produced with specific characteristics for various industrial, technological, agricultural, or consumer applications such as metal nanoparticles, metal oxide nanoparticles, carbon nanotubes, quantum dots, nanocomposites, and polymeric nanoparticles [37,38]. These nanoparticles are created through engineering processes to achieve desired properties at the nanoscale, typically ranging from 1 to 100 nanometers. The intentional manipulation of the size, shape, surface properties, and composition distinguishes engineered nanoparticles from naturally occurring nanoparticles. On the other hand, biogenic nanoparticles refer to nanoparticles that are naturally formed or synthesized by living organisms or natural processes without human intervention. Unlike engineered nanoparticles, which are intentionally produced by humans for specific purposes, biogenic nanoparticles occur naturally because of biological and environmental processes [39]. These nanoparticles can be found in various natural sources and are often associated with specific organisms or geological phenomena such as bacterial nanoparticles, fungal nanoparticles, volcanic ash, clay minerals, and wildfire ash [39].
Table 1.
Factors influencing nanoparticle selection for plant transport-related applications.
| Factor | Description |
|---|---|
| Desired application | Different applications, such as nutrient delivery, genetic material transfer, or pesticide transport, require specific nanoparticle types with suitable properties [25,28,31]. |
| Payload type | The nature of the payload, whether it is nutrients, genetic material (DNA or RNA), pesticides, or other substances, influences the selection of the appropriate nanoparticle [23,24,29]. |
| Payload size and solubility | The size and solubility of the payload may determine the choice of nanoparticle, as some nanoparticles are better suited for carrying particular types of cargo [40]. |
| Targeted delivery | If precise delivery to specific plant tissues or cells is required, the nanoparticle type should allow for targeted delivery [41]. |
| Biocompatibility | Some applications, such as those involving genetic material delivery or interactions with living organisms, necessitate biocompatible nanoparticles [42]. |
| Environmental considerations | The environmental impact of nanoparticle use, including factors like biodegradability and safety, is crucial in agriculture and ecological applications [43]. In addition to these considerations, environmental conditions such as soil pH, temperature, and relative humidity play pivotal roles in determining the fate and impact of nanoparticles [44]. |
| Size and shape | The size and shape of nanoparticles can influence their ability to enter plant cells or tissues. In some cases, specific shapes or sizes may be more effective [29,45]. |
| Crop or plant type | Different plant species or crops may have varying requirements and responses to nanoparticle-based applications, affecting the selection of nanoparticle types [46]. |
There are numerous types of nanoparticles, and the field of nanotechnology continues to evolve, leading to the development of new types and functionalities. Examples of nanoparticles used for transport in plants are presented in Table 2 along with their introduction site. Metals and metal oxides are types of nanoparticles that are commonly used in various plant transportation applications.
Table 2.
Types of nanoparticles used for transport in plants and their places of introduction.
| Type | NPs | Size (nm) | Plants | Introduction Site | Ref. |
|---|---|---|---|---|---|
| Metal or metalloid | Ag | 1–10 | Tomato | Roots | [47] |
| Ag | 10 | Triticum aestivum | Roots | [48] | |
| Ag | 20 ± 3 | Linum usitatissimum, Lolium perenne, Hordeum vulgare | Roots | [49] | |
| Ag | 20 | Arabidopsis thaliana | Roots | [50] | |
| Ag | 10 | Phaseolus radiatus, Sorghum bicolor | Roots | [51] | |
| Ag | 10–15 | Lycopersicum esculentum | Roots | [52] | |
| Ag | 27.3 ± 6 | Populus deltoides, Arabidopsis thaliana | Roots | [53] | |
| Al | 18 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Au | 1–3 | Oryza sativa | Roots | [55] | |
| Au | 3.5 and 18 | Nicotiana xanthi | Roots | [56] | |
| Au | 6–10 | Oryza sativa, Lolium perenne, Raphanus sativus, Cucurbita mixta | Roots | [57] | |
| Co | 28 | Tomato | Roots | [47] | |
| Ni | 28 | Tomato | Roots | [47] | |
| Si | 14 | Arabidopsis thaliana | Roots | [58] | |
| Zn | 35 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Metal or metalloid oxide | CeO2 | 25 | Holcus lanatus, Diplotaxis tenuifolia | Roots | [59] |
| CeO2 | 8 | Glycine max | Roots | [60] | |
| CeO2 | 8 ± 1 | Oryza sativa | Roots | [61] | |
| CeO2 | 20 ± 2 | Solanum lycopersicum | Roots | [62] | |
| CeO2 | 6.6 ± 1; 25.2 ± 2 |
Cucumis sativus | Roots | [63] | |
| Fe3O4 | 20–30 | Tomato | Roots | [47] | |
| Fe3O4 | 8 | Cucurbita maxima | Roots | [64] | |
| SiO2 | 10–20 | Chelidonium majus | Leaves | [65] | |
| SiO2 | 20 | Cucumis sativus | Leaves | [66] | |
| TiO2 | 20 | Tomato | Roots | [47] | |
| TiO2 | 20 ± 5 | Triticum aestivum | Roots | [67] | |
| TiO2 | 27 ± 4 | Cucumis sativus | Roots | [68] | |
| TiO2 | 27 | Lycopersicum esculentum | Roots | [52] | |
| TiO2 | 2.8 ± 1 | Arabidopsis thaliana | Roots | [69] | |
| ZnO | 10 | Glycine max | Roots | [60] | |
| ZnO | 20 ± 5 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Carbon-based | C | 20 | Zea mays | Roots | [70] |
| Carbon nanotubes (CNT) | 10–30 | Cicer arietinum | Roots | [71] | |
| Multi-walled carbon nanotubes (MWCNT) | 10–20 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| MWCNT | 6–9 | Zea mays | Roots | [72] | |
| MWCNT | 4–13 | Lactuca sativa, Oryza sativa, Cucumis sativus, Amaranthus tricolor, Abelmoschus esculentus, Capsicum annuum, Glycine max | Roots | [73] | |
| MWCNT | 30 | Brassica juncea | Roots | [74] | |
| MWCNT | 6–13 | Triticum aestivum | Roots | [75] | |
| Single-walled carbon nanotubes (SWCNT) | 20 | Nicotiana benthamiana | Leaves | [76] | |
| Semiconductor | 3-mercaptopropionic acid (MPA) quantum dots (QDs) | 4–5.4 | Lemna minor | Leaves | [77] |
| Cd-based QDs | 1.9 and 2.4 | Allium cepa | Roots | [78] | |
| CdSe/CdZnS QDs | 19.5 ± 7 | Populus deltoides | Roots | [79] | |
| CdTe QDs | 4 | Oryza sativa | Roots | ||
| Glutathione (GSH) QDs | 4–4.4 | Lemna minor | Leaves | [77] | |
| Polymeric | Chitosan | 19–21 | Oryza sativa | Roots | [80] |
| Thiamine loaded chitosan | 10 | Cicer arietinum | Roots | [81] | |
| Magnetic | Superparamagnetic iron oxide (SPION) | 9 | Glycine max | Roots | [82] |
Research on the interaction of nanomaterials with plants has mainly focused on phytotoxicology. At the same time, less research has been conducted on positive effects such as increasing crop productivity and enhancing plant resistance, and research on beneficial effects on plants is still incomplete. The entry of nanoparticles into plants, especially at high concentrations, can lead to phytotoxicity. Geisler-Lee et al. showed that Ag NPs could exert detrimental effects on A. thaliana, but the phytotoxic effect of Ag NPs could not be fully explained by the released silver ions [50]. Another study also indicated that the internalization and upward translocation of ZnO NPs by Lolium perenne could significantly reduce the biomass and cause damage to root epidermal and cortical cells [54]. Some nanoparticles, such as certain metal and metal oxide nanoparticles, can generate reactive oxygen species (ROS) when they enter plant cells [83]. Elevated ROS levels can damage cell structures, disrupt cellular functions, and cause oxidative stress, leading to plant injury or even cell death [83,84]. High nanoparticle concentrations can disrupt the integrity of plant cell membranes [85]. This can lead to the leakage of cellular contents and negatively impact cell viability. Rossi et al. stated that NPs could also influence plant symplastic pathways by altering ion transport activity or root cell membrane integrity [86]. Excessive nanoparticle accumulation can interfere with the uptake and distribution of essential nutrients, disrupting nutrient balance in plants and causing nutrient deficiencies or toxicities. High nanoparticle concentrations may also physically obstruct the plant’s nutrient and water transport systems, impeding the movement of substances throughout the plant [26]. Plants exposed to high nanoparticle concentrations may activate stress responses, diverting resources away from growth and development and reducing overall plant health [46,87]. The phytotoxicity of nanoparticles can vary depending on factors such as nanoparticle type, size, shape, surface properties, and the plant species involved. Therefore, selecting the appropriate concentration of nanoparticles is a critical decision, as it can significantly impact the performance and effectiveness of nanoparticle transport.
Nanoparticles, whether naturally occurring or engineered, can have various negative effects on plants. The impact of nanoparticles on plants depends on factors such as the type of nanoparticles, their concentration, exposure duration, and the specific plant species [88]. Nanoparticles can exhibit phytotoxic effects, leading to damage being caused to the plant cells, tissues, and overall plant structure. This can result in stunted growth, reduced biomass, and compromised plant health [36]. Exposure to certain nanoparticles can alter the root morphology and function. This includes changes in the root length, surface area, and the structure of root hairs, which can impact nutrient and water uptake [89]. Nanoparticles can induce oxidative stress in plants by generating reactive oxygen species (ROS). Elevated ROS levels can lead to the damage of cellular components, such as membranes, proteins, and DNA, affecting plant health [90]. Some nanoparticles may persist in the environment, leading to long-term exposure for plants. Additionally, there is a concern about the potential bioaccumulation of nanoparticles in plant tissues, which can have implications for organisms higher up the food chain [91].
2.2. Modes of Transport of Nanoparticles into Plants
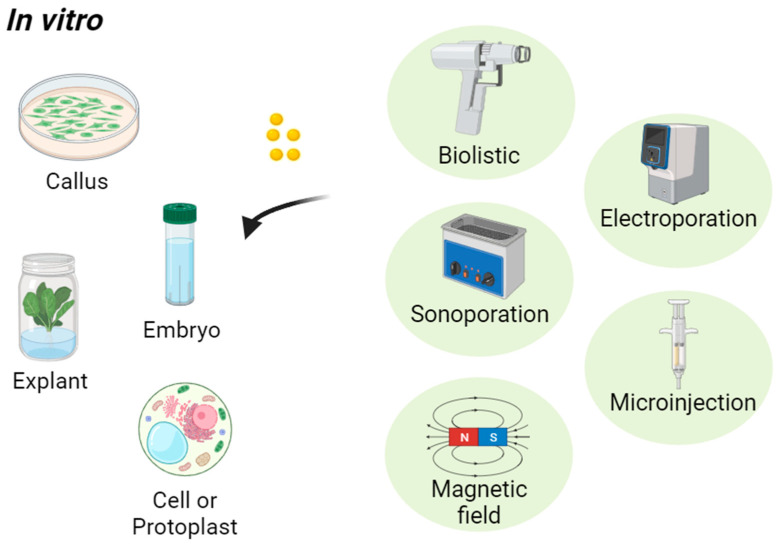
Nanoparticle transport in plants can be categorized based on two main mechanisms, i.e., assisted delivery and passive delivery. When referring to assisted delivery in the context of transporting nanoparticles to the plant body, it means that external power or forces are applied to facilitate the transport of nanoparticles into the plant tissues [20]. This external power or force assists in the delivery process, often overcoming barriers that would hinder passive transport [92]. Examples of assisted delivery techniques in plant nanoparticle applications are shown in Figure 2 and described briefly in Table 3. The biolistic (gene gun) is a device that uses an external force, such as compressed gas or helium, to propel nanoparticles [93,94]. Rajkumari et al. reported the use of Ag NPs as gene carriers, replacing Au microcarriers for biolistic gene delivery in Nicotiana tabacum L., and showed that the transformation efficiency was significantly higher with Ag NPs than Au microparticles as carriers [94]. Sonoporation involves the application of ultrasound waves to create temporary pores in plant cell membranes, allowing nanoparticles to enter the cells [95]. The external force of ultrasound assists in the delivery process. Zolghadrnasab et al. showed that ultrasonic treatment provides an economical and straightforward approach for poly-ethyleneimine (PEI)-coated mesoporous silica nanoparticles (MSNs) transferring into plant cells without any need for complicated devices and without concerns about safety issues [96]. Magnetic nanoparticles can be guided to specific plant tissues using external magnetic fields, effectively assisting in the targeted delivery of these nanoparticles [97]. Characterizing the intrinsic magnetic properties of nanoparticles involves understanding their behavior at the bulk level as well as at the level of individual molecules. Various techniques are employed for both bulk and single-molecule investigations. Bulk techniques include vibrating sample magnetometry (VSM), superconducting quantum interference device (SQUID), magnetic resonance imaging (MRI), Mössbauer spectroscopy, and X-ray magnetic circular dichroism (XMCD) [98,99]. Single-molecule techniques include magnetic force microscopy (MFM), scanning tunneling microscopy (STM), electron magnetic circular dichroism (EMCD), single-particle magnetometry, and fluorescence-based techniques [100,101]. Electroporation involves applying external electric fields to plant cells, creating temporary pores in the cell membranes [102]. This method uses an external electrical power source to facilitate nanoparticle entry. Microinjection, combined with external manipulation, can be used to introduce nanoparticles into specific plant cells [103]. Most of the assisted delivery methods are used for transporting nanoparticles in vitro (outside living organisms, typically in a controlled laboratory setting) as indicated in Figure 2. Nanoparticles can be introduced to plant embryos cultivated in a controlled laboratory setting [104]. Plant embryos, which are the earliest stages of plant development, offer a convenient point of entry for introducing nanoparticles that can influence the growth and characteristics of the resulting plant. Nanoparticles can also be introduced to plant callus cultures, which are undifferentiated masses of plant cells grown in vitro [105]. Plant callus cultures are typically initiated from explants, such as leaf pieces, stem segments, or immature embryos. These explants are sterilized and placed on a suitable culture medium containing plant growth regulators to induce callus formation. Introducing nanoparticles to plant protoplasts is also a technique that is commonly used in plant biotechnology. Protoplasts are plant cells that have had their cell walls enzymatically removed, leaving behind the cell membrane and cytoplasm [106].
Figure 2.
Assisted delivery techniques for introducing nanoparticles into plants in vitro.
Assisted delivery methods can facilitate the delivery of relatively larger particles due to the external forces involved, as indicated in Table 3. These methods are valuable for overcoming natural barriers and transporting particles efficiently. However, there are still many big issues related to this transport method, especially regarding their scalability to larger scales. Therefore, passive delivery still has advantages over it. In a controlled laboratory setting, it is easier to maintain uniform conditions for assisted delivery, ensuring consistency in nanoparticle uptake [107]. On larger scales, achieving uniformity across a field or agricultural area becomes more challenging. Variability in environmental conditions, soil composition, and plant physiology can affect the effectiveness of delivery methods [108]. Assisted delivery in vitro also allows for precise control over factors such as temperature, humidity, and nutrient levels. However, real-world conditions vary widely in agricultural settings. Adapting in vitro strategies to diverse environments becomes complex, and factors like wind, rainfall, and temperature fluctuations can impact the efficacy of nanoparticle delivery [109]. Implementing these assisted delivery methods on a large scale also requires significant resources and may not be economically feasible. Factors such as the cost of materials, equipment, and labor must be considered for practical application in agriculture. The large-scale implementation of assisted delivery may face regulatory challenges related to environmental impact, safety, and ethical considerations [110]. Demonstrating the safety and environmental compatibility of nanoparticle delivery systems becomes essential.
Table 3.
Examples of assisted delivery techniques for introducing nanoparticles into plants.
| Method | NPs | Size (nm) | Plants | Target | Ref. |
|---|---|---|---|---|---|
| Biolistic (gene gun) | Ag | 100 | Nicotiana tabacum | Leaf explants | [94] |
| Au | 712 ± 95 | Nicotiana benthamiana | Leaf explants | [106] | |
| Au-MSN | 600 | Nicotiana tabacum, Teosinte | Leaf explants | [111] | |
| Au-MSN | 600 | Zea mays | Embryos | [103] | |
| Fe | 255 ± 170 | Nicotiana benthamiana | Leaf explants | [106] | |
| Sonoporation | hPAMAM-G2 | 123 ± 21 | Medicago sativa | Cells | [112] |
| PEI-MSN | 100 ± 87 | Nicotiana tabacum | Cells | [96] | |
| Magnetic field | γ-Fe2O3 | 21.2 ± 3 | Zea mays | Roots | [113] |
| Carbon-coated iron | 10–50 | Cucurbita pepo | Roots | [114] | |
| Fe2O3 | 10 | Solanum lycopersicum | Roots | [115] | |
| Fe3O4 | 13 | Hordeum vulgare | Roots | [116] | |
| Electroporation | CPNs | 60–80 | Tobacco | Protoplasts | [105] |
| Microinjection | mGNPs | 20–30 | Brassica napus | Cells | [117] |
| SWNTs | 500 | Nicotiana tabacum | Cells | [118] |
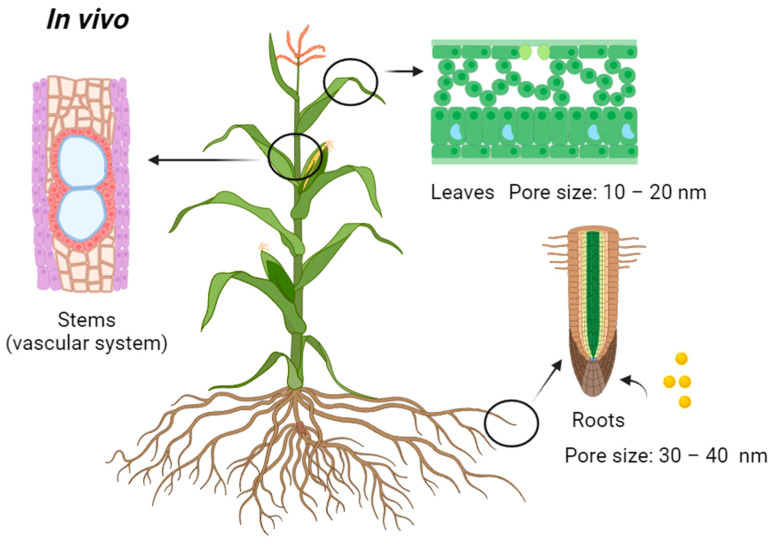
In contrast to assisted delivery, passive delivery in the context of nanoparticle transport in plants typically refers to the movement of nanoparticles through natural routes without the assistance of external forces or active mechanisms. It encompasses various methods through which nanoparticles enter the plant body, often via sites such as roots and leaves. In summary, assisted delivery and passive delivery are different concepts. Assisted delivery involves an intervention to enhance nanoparticle uptake, while passive delivery relies on natural processes, without external assistance. These approaches cater to different research goals and applications in the field of nanotechnology for plant science. These passive delivery routes include root uptake and foliar uptake [29]. Plants naturally absorb water and dissolved substances, and nanoparticles can enter the plant through the roots as they take up water and nutrients [26,29]. This is a common method for delivering nanoparticles to plants in vivo. When discussing in vivo delivery in the context of plants, it typically involves the intact, living plant. In vivo experiments aim to study the interaction between nanoparticles and the entire living organism, considering the complexities of the plant’s biological systems [119]. Nanoparticles can also be sprayed or applied to the leaves of plants [26,120]. Lian et al. showed that the response of Zea mays L. to Cd and TiO2 NPs was highly dependent on the exposure mode. They reported that leaf exposure provided more benefits than root exposure [120]. Some nanoparticles, like those used in foliar fertilizers or pesticides, can be taken up by the plant through the stomata (small openings on leaf surfaces) or through the leaf cuticle [29]. Examples of these two methods are shown in Table 1 above, which use the in vivo nanoparticle transport route (Figure 3). After nanoparticles enter the roots or leaves of a plant, they can be transported throughout the plant’s vascular system, which includes the xylem and phloem in the stem [121]. The vascular system plays a crucial role in the distribution of water, nutrients, and various substances, including nanoparticles, to different parts of the plant.
Figure 3.
Passive delivery introduction sites for in vivo transport of nanoparticles into plants.
2.3. Challenges during Nanoparticle Transport
One of the primary challenges of using assisted delivery techniques is the potential for plant cell damage [122]. Assisted delivery methods, especially those involving physical forces or electrical pulses, can cause physical stress or damage to the target plant cells [123]. This may result in cell death, reduced viability, or altered cell function. Each assisted delivery method often requires the careful optimization of parameters. Finding the optimal parameters for different applications can be labor-intensive and may involve trial and error. Some assisted delivery methods may raise safety concerns due to potential unintended biological effects. Some assisted delivery methods may also be less suitable for in vivo applications due to challenges related to safety, depth of penetration, and the potential for systemic effects. A description of the advantages and disadvantages of each assisted delivery method can be seen in Table 4. Some of the disadvantages associated with assisted delivery methods are precisely what make passive delivery methods preferable in certain situations. Passive delivery methods are often favored when conducting studies in living plants, particularly when the goal is to minimize risks to plant health and create conditions that more closely resemble real-world scenarios.
Table 4.
Advantages and disadvantages of using assisted delivery methods to introduce nanoparticles into plants.
| Method of Assisted Delivery | Advantages | Disadvantages |
|---|---|---|
| Biolistic (gene gun) |
|
|
| Sonoporation |
|
|
| Magnetic field |
|
|
| Electroporation |
|
|
| Microinjection |
|
|
Passive delivery methods in plants have several advantages over assisted delivery methods, especially in certain applications and contexts. Passive delivery methods do not require the application of external forces, such as mechanical or electrical forces, which can potentially damage plant cells or tissues [27,122]. This results in less invasive effects on the plant, reducing the risk of cell damage or stress. Characterizing the mechanical and electrical properties of nanoparticles is essential for understanding their behavior and performance, especially when they interact with plant tissues [135]. Mechanical properties at the bulk level are characterized by atomic force microscopy (AFM) and dynamic mechanical analysis (DMA) [136]. In contrast, AFM-based nanoindentation and scanning probe microscopy (SPM) are the techniques commonly used to characterize at the nanoscale level [137], and techniques used to characterize electrical properties at the bulk level are impedance spectroscopy and four-point probe measurement [138]. On the other hand, techniques used to characterize at the nanoscale level are conductive AFM (cAFM), Kelvin probe force microscopy (KPFM), and transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDS) [139]. Passive delivery methods are often simpler and more straightforward to implement. They do not require specialized equipment or complex optimization processes, making them accessible to a wider range of researchers and practitioners. Passive delivery methods are generally more cost-effective because they do not necessitate the use of expensive equipment or consumables. However, there are several challenges, particularly when it comes to particle size limitations. Passive delivery methods are often constrained by the physical characteristics of the nanoparticles being transported [140]. In the case of roots and leaves, the size of particles that can be transported is limited by the size of pores, channels, or structures within the plant tissue [141]. Large particles may encounter limitations in passing through these structures, thereby constraining the efficacy of passive transport. The examples presented in Table 1 indicate that the majority of the particle sizes utilized are below 40 nm. Particles with sizes above 40 nm may not be accommodated through passive delivery via roots and leaves. Plant cells have rigid cell walls, and the pore size diameter ranges from less than 10 nm in most pores to a rare maximum size of 40 nm [142,143,144]. McCann et al. observed that intact cell walls of Allium cepa var. Jumbo generally measure 10–20 nm [142]. The average cell wall size was 30 nm in width, which was visualized using spectroscopic methods [145]. The same is true for foliar uptake, where a waxy cuticle in leaves acts as a barrier for particles entering the plant [146]. Many particles, particularly larger ones, will not be able to pass through the stomata (microscopic openings) on the leaf surface, as these typically have diameters of less than 10 nm to 20 nm [147]. Passive delivery methods often involve incorporating nanoparticles into the growth medium, soil, or other components of the plant’s environment during specific developmental stages, such as seed germination or seedling growth, as indicated in Table 5. Passive delivery via root uptake and foliar uptake has its own set of advantages and disadvantages, as also listed in Table 5. Exploring alternative introduction sites for passive transport in plants is a worthwhile direction for future research and development. It allows for the customization of delivery methods and the potential to overcome limitations associated with roots and stems.
Table 5.
Advantages and disadvantages of using existing passive delivery methods and plant developmental stages to introduce nanoparticles into plants.
| Passive Delivery | Advantages | Disadvantages |
|---|---|---|
| Methodology | ||
| Root uptake |
|
|
| Foliar uptake |
|
|
| Plant developmental stages | ||
| Seed germination |
|
|
| Seedling growth |
|
|
3. Detection of Nanoparticles in Plants
Selecting the appropriate detection methods for nanoparticles in plants is crucial for various applications, including understanding nanoparticle uptake, distribution, and potential impacts on plant health and the environment [125,157]. The choice of detection method should depend on the specific research objectives, the characteristics of the nanoparticles, and the resources available. Often, a combination of techniques is employed to gain a comprehensive understanding of nanoparticle transport in plants [158,159]. Deng et al. studied the uptake of titanium dioxide NPs in Oryza sativa L. tissues using multiple orthogonal techniques: electron microscopy, single-particle inductively coupled plasma mass spectroscopy, and total elemental analysis using ICP optical emission spectroscopy [159]. It is also essential to consider the potential impact of the detection method on the integrity of the plant samples and the interpretation of the results. Some considerations for selecting detection methods are listed in Table 6. These considerations involve both the characteristics of the particles being studied and the context in which the research is conducted. The choice between in vivo, in vitro, and in silico approaches is an important decision that influences the type of detection methods that can be applied.
Table 6.
Considerations for selecting detection methods for studying particle transport in plants.
| Factors | Description |
|---|---|
| Methodological considerations |
Considerations: May be challenging to observe and quantify; has potential effects on plant health [122].
Considerations: May not fully represent the complexity of the in vivo environment [108].
Considerations: Requires accurate input parameters; simplifications may limit realism [161]. |
| Particle properties | The size and composition of nanoparticles may influence the choice of detection method. Some methods are better suited for specific sizes or materials [162]. |
| Objectives | |
| Non-invasive vs. invasive | Some methods are non-invasive and allow real-time monitoring, while others require destructive sampling [164]. |
| Sensitivity and precision | Considers the required sensitivity to detect low concentrations of nanoparticles [165]. |
| Sample preparation | Evaluates the ease and compatibility of sample preparation with the chosen method. Some methods may require complex sample processing [166]. |
3.1. Type of Detection Methods
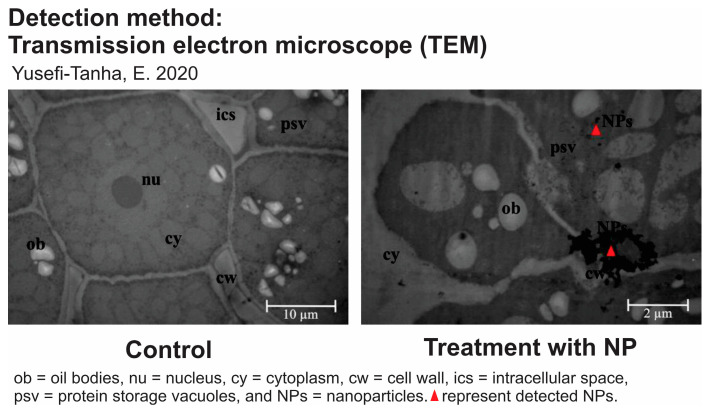
Detecting the transport of nanoparticles in plants is an important area of research with several potential applications. There are various techniques and methods for detecting and studying nanoparticle transport in plants, as listed in Table 7. All of these methods play crucial roles in understanding how nanoparticles are taken up and distributed and how they interact with plant tissues. Zhang et al. reported the detection of 7 nm and 25 nm CeO2 NPs in the roots of Cucumis sativus by TEM [63]. They observed the adsorption and aggregation of the particles on the root surface, but not inside the cells [63]. Similarly, TEM was used to monitor the distribution of 25 nm CuO NPs in the roots of Glycine max, as shown in Figure 4 [167]. SEM could also be used to see the presence of particles in plant tissue. α-Fe2O3 NPs led to alterations in the root morphology of Hordeum vulgare and induced cell membrane injury and, hence, root damage, as indicated by SEM observations [168]. The use of several detection methods can mutually validate each other, thereby increasing the confidence level regarding particle transport. Bao et al. have shown that TEM analysis agreed well with the SP-ICP-MS results [169]. They also demonstrated that NPs accumulated in the leaf tissues showed large variations in size and relatively few readings compared to the NPs in the root tissues. Other examples of the application of detection methods for particle transport studies are shown in Table 8.
Table 7.
Techniques and methods used to detect nanoparticles in plants.
| Methods | Description |
|---|---|
| Transmission electron microscopy (TEM) | TEM allows us to visualize nanoparticles at the nanoscale within plant tissues. By preparing ultrathin sections of plant material and using TEM, the internal distribution and movement of nanoparticles can be observed in different plant structures [167]. |
| Scanning electron microscopy (SEM) | SEM is another microscopy technique that can be used to study the surface morphology of plant tissues and detect the presence of nanoparticles. It provides high-resolution images and can help identify the localization of nanoparticles on the plant’s surface [168]. |
| Inductively coupled plasma mass spectrometry (ICP-MS) | ICP-MS is a highly sensitive analytical technique used to quantify the elemental composition of samples. By digesting plant tissues and then subjecting them to ICP-MS analysis, the presence and concentration of specific nanoparticles can later be determined [169]. |
| Confocal laser scanning microscopy (CLSM) | CLSM is a non-destructive imaging technique that can be used to track the movement of fluorescently labeled nanoparticles within plant tissues over time. This method is particularly useful for studying the dynamics of nanoparticle transport [163]. |
| X-ray fluorescence (XRF) | XRF can be employed to analyze the elemental composition of plant tissues and identify the presence of nanoparticles. It can provide information about the distribution of specific elements, including those contained in nanoparticles [33]. |
| Fluorescence and luminescence techniques (Flo-Lum) | Utilizing fluorescent or luminescent tags on nanoparticles, researchers can track the movement of nanoparticles through plants using fluorescence microscopy or other imaging techniques [170]. |
Figure 4.
Transmission electron microscopy used to monitor the presence of NPs in plant tissue (adapted from [167]).
Table 8.
Application of several detection methods for particle transport studies.
| Detection Method | Detected NPs | Size (nm) | Plants | Introduction Site | Ref. |
|---|---|---|---|---|---|
| TEM | Ag | 7 and 18 | Medicago sativa | Roots | [171] |
| Ag | 10 | Arabidopsis thaliana | Roots | [169] | |
| Au | 3.5 and 18 | Nicotiana xanthi | Roots | [56] | |
| CeO2 | 6.6 ± 1; 25.2 ± 2 | Cucumis sativus | Roots | [63] | |
| MgO | 27.7 | Watermelon | Leaves | [172] | |
| SiO2 | 30 | Cotton | Roots | [173] | |
| TiO2 | 24.5 | Watermelon | Leaves | [172] | |
| TiO2 | 27 ± 4 | Cucumis sativus | Roots | [68] | |
| ZnO | 20 | Ryegrass | Roots | [174] | |
| ZnO | 30 ± 5 | Zea mays | Roots | [175] | |
| SEM | α-Fe2O3 | 14 | Hordeum vulgare | Roots | [168] |
| Ag@CoFe2O4 | 10 | Triticum aestivum | Roots | [176] | |
| Ag2S | 35 | Cucumis sativus, Triticum aestivum | Roots | [177] | |
| CeO2 | 15.5 | Raphanus sativus | Roots | [178] | |
| Fe3O4 | 12–20 | Lactuca sativa | Roots | [179] | |
| MgO | 15–20 | Arachis hypogaea | Roots | [180] | |
| TiO2 | 19 | Oryza sativa | Roots | [159] | |
| TiO2 | 12–20 | Lactuca sativa | Roots | [179] | |
| ICP-MS | Ag | 10 | Arabidopsis thaliana | Roots | [169] |
| Ag | 7.6 ± 2 | Oryza sativa | Roots | [181] | |
| Au | 2 | Oryza sativa | Roots | [55] | |
| CeO2 | 30 | Solanum lycopersicum, Cucumis sativus, Cucurbita pepo, Glycine max | Roots | [182] | |
| CeO2 | 30 | Raphanus sativus | Roots | [183] | |
| CuO | 25 | Raphanus sativus | Roots | [183] | |
| La2O3 | 20–30 | Pfaffia glomerata | Roots | [184] | |
| TiO2 | 30 | Raphanus sativus | Roots | [185] | |
| CLSM | CeO2 | 1.7–18 | Gossypium hirsutum, Zea mays | Leaves | [186] |
| MSNs | 20 | Lupin, wheat, maize | Roots | [163] | |
| QD | 10 | Arabidopsis thaliana | Leaves | [187] | |
| SiO2 | 1.7–18 | Gossypium hirsutum, Zea mays | Leaves | [186] | |
| ZnO | 30 | Triticum aestivum | Leaves | [188] | |
| XRF | Au | 13.4 ± 1 | Arabidopsis thaliana | Roots | [189] |
| CeO2 | 12 ± 3 | Triticum aestivum | Roots | [190] | |
| CeO2 | 4 | Zea mays, Lactuca sativa, Solanum lycopersicum, Oryza sativa | Roots | [190] | |
| CuO | 30.7 ± 4 | Oryza sativa | Roots | [191] | |
| SiO2 | 10 | Solanum lycopersicum | Roots | [33] | |
| TiO2 | 14 and 25 | Brassica napus, Triticum aestivum | Roots | [192] | |
| TiO2 | 5–10 | Salvinia natans | Roots | [193] | |
| TiO2 | 30 | Pisum sativum | Roots | [194] | |
| TiO2 | 14 | Oryza sativa | Roots | [195] | |
| ZnO | 24.5 ± 4 | Oryza sativa | Roots | [191] | |
| Flo-Lum | Ag | 35 ± 15 | Stevia rebaudiana | Roots | [170] |
| CdSe/ZnS QD | 6.3 ± 1 | Arabidopsis thaliana | Roots | [196] | |
| CeO2 | 8 ± 1 | Zea mays | Roots | [197] | |
| TiO2 | 8 | Spirodela polyrrhiza | Roots | [198] | |
| TiO2 | 2.8 ± 1 | Arabidopsis thaliana | Roots | [199] |
3.2. Challenges in Using Selected Detection Methods
Detecting nanoparticles in plants can be challenging due to the complex biological matrix and the small size of nanoparticles. Plants naturally contain a variety of elements and compounds that can interfere with the detection of nanoparticles, making it difficult to distinguish the nanoparticles from background signals [162,200]. The size of nanoparticles can be similar to or smaller than the cellular structures in plants [201]. This can make it challenging to visualize and accurately quantify nanoparticle uptake and distribution. Some nanoparticles may be present at low concentrations in plant tissues, requiring highly sensitive detection methods to accurately measure their presence [202]. Achieving high spatial resolution to precisely locate nanoparticles within plant tissues can be difficult, especially for in vivo imaging techniques. Some detection methods are destructive, requiring the disintegration of plant samples for analysis, which may limit the ability to conduct further studies on the same samples [203]. A summary of the advantages and disadvantages of each detection method can be seen in Table 9. The use of multiple detection methods, often referred to as a multi-method or multi-technique approach, can significantly enhance the robustness and reliability of research findings related to particle transport. Each detection method has its strengths and limitations, and combining several methods provides complementary information, cross-validation, and a more comprehensive understanding [184]. In a study conducted by Neves et al., the uptake and translocation of La2O3 NPs to the stems and leaves of Pfaffia glomerata (Spreng.) Pedersen were demonstrated after in vitro cultivation in the presence of 400 mg/L of NPs. Various detection methods, including laser ablation-ICP-MS (LA-ICP-MS) and μ-XRF, were employed. Both techniques proved to be effective for detecting nanoparticles in plants, yet LA-ICP-MS exhibited higher sensitivity than μ-XRF, enabling the improved detection and visualization of La distribution throughout the entire leaf [184].
Table 9.
Advantages and disadvantages of detection methods for localizing or quantifying nanoparticles in plants.
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| TEM | ||
| SEM | ||
| ICP-MS |
|
|
| CLSM | ||
| XRF | ||
| Flo-Lum |
4. Plant Stems as Future Recognition Sites for Transport
Introducing particles through the stem of a plant, as opposed to the roots or leaves, can offer several advantages. The vascular system in plant stems is better equipped to transport larger particles, such as nanoparticles, microspheres, or even macro-sized particles, compared to the relatively finer structures in roots or leaves. This means that when specific substances or materials need to be delivered in a particulate form, stem application allows for larger and more complex particles to be used. When larger particles are introduced through roots or leaves, there is a higher risk of blockage in the smaller vessels or stomata, potentially hampering nutrient and substance transport [219,220]. The stem’s larger vascular system can handle larger particles more efficiently, reducing the risk of clogs [221].
The vascular system in the stem, comprising the xylem and phloem, possesses specific characteristics that make it suitable for the introduction of larger particles compared to the roots or leaves [10,121]. The xylem and phloem vessels in the stem are larger in diameter compared to the corresponding structures in roots or leaves, as indicated in Table 10. This increased diameter allows for the accommodation of larger particles without clogging or obstructing the vascular pathways. The primary function of the vascular system is to transport water, nutrients, and other essential substances throughout the plant [29,222]. This natural transport system within the stem is well adapted to carry and distribute larger particles efficiently. The stem’s vascular system is responsible for the long-distance transport of water and nutrients from the roots to the leaves and other plant parts [223]. It is capable of maintaining the flow of materials over significant distances, making it an ideal route for distributing larger particles throughout the plant. The xylem and phloem vessels provide a continuous pathway throughout the stem, ensuring that introduced particles can be transported uniformly to different parts of the plant. The stem serves as a structural support for the plant, and this mechanical strength helps ensure that the vascular system remains intact even when larger particles are introduced, minimizing the risk of damage to the plant [224]. When particles are introduced through the roots or leaves, there is a greater risk of surface deposition, where the particles may remain on the outer surfaces and not penetrate the plant’s interior. In contrast, introducing particles through the stem offers a more direct and internal pathway for distribution. The vascular system in the stem typically transports materials in a unidirectional manner, following the natural flow of water and nutrients from the roots upward toward the leaves [225]. This consistent transport direction can be advantageous for certain applications.
These characteristics of the stem’s vascular system make it well suited for the introduction of larger particles, whether for the purpose of nutrient delivery, disease control, or other agricultural and horticultural practices. In our previous investigation, we successfully introduced 110 nm SiO2 nanoparticles into tomato seedlings using the stem cutting method [226]. However, it is essential to use appropriate techniques and precautions to ensure that the introduction of particles through the stem is carried out safely and effectively.
Table 10.
Vascular bundle size in the stem, comprising the xylem and phloem, of various plant species.
| Plants | Xylem Diameter (μm) | Phloem Diameter (μm) | Ref. |
|---|---|---|---|
| Arabidopsis thaliana | 16 | - | [227] |
| Cucurbita maxima | - | 5 | [228] |
| Ipomoea hederifolia | - | 323–358 | [229] |
| Larix sibirica | - | 24–29 | [230] |
| Phaseolus vulgaris | - | 1.5–20 | [228] |
| Populus trichocarpa | 29–104 | 16–43 | [231] |
| Portulaca grandiflora | 121.5 | - | [232] |
| Portulaca oleracea | 98 | - | [232] |
| Portulaca quadrifida | 101.9 | - | [232] |
| Quercus chapmanni | 333 | - | [233] |
| Quercus falcata | 492 | - | [233] |
| Quercus hemisphaerica | 474 | - | [233] |
| Quercus incana | 470 | - | [233] |
| Quercus laevis | 469 | - | [233] |
| Quercus margaretta | 345 | - | [233] |
| Quercus myrtifolia | 455 | - | [233] |
| Quercus nigra | 517 | - | [233] |
| Quercus pubescens | 202.4 | 35.8 | [234] |
| Quercus sessiliflora | 1837.6 | 286.8 | [235] |
| Quercus austrina | 340 | - | [233] |
| Quercus geminata | 273 | - | [233] |
| Quercus michauxii | 332 | - | [233] |
| Quercus shumardii | 569 | - | [233] |
| Quercus virginiana | 286 | - | [233] |
| Ricinus communis | 300 | 100–150 | [236] |
| Rosmarinus officinalis | 467.3 | 7.2 | [237] |
| Solanum lycopersicum | - | 1.5–20 | [228] |
| Talinum fruticosum | 133.2 | - | [232] |
| Tectona grandis | 110.2–212.5 | - | [238] |
| Vitis vinifera | 33.6–35.8 | - | [239] |
5. Conclusions and Perspectives
The transport of nanoparticles into plants is indeed a growing area of research with a wide range of potential applications. Nanoparticles can be employed as delivery agents for a variety of substances, including nutrients, genetic material, or pesticides. Their small size, high surface area, and tunable properties make them useful for enhancing the targeted delivery of these materials to plants. However, it is important to consider factors such as nanoparticle toxicity, long-term effects, and environmental impact, as well to optimize the nanoparticles’ properties for specific delivery requirements. There are various methods for delivering nanoparticles to plants, and they can be broadly categorized into two main approaches: assisted delivery and passive delivery. Each method has its own set of advantages and disadvantages, depending on the specific application and goals of the research or practice.
Passive delivery methods are more appealing due to their simplicity and reduced reliance on external sources, making them environmentally friendly and cost-effective. Roots and leaves are the primary sites for the passive delivery of exogenous materials into plants. These natural entry points have evolved to allow plants to absorb water, nutrients, and gases from their environment. However, both have natural barriers that can inhibit the delivery of nanoparticles or other exogenous materials to plants. These barriers can pose challenges in achieving efficient and reliable passive delivery. Exploring new introduction sites for delivering nanoparticles to plants is an interesting avenue of research, and the stem is indeed a potential candidate. Stems are natural conduits for the transport of water and nutrients within plants, as they contain vascular systems with xylem and phloem tissues. The large diameter of these vascular tissues suggests that they could potentially carry larger particles, making stems intriguing alternative introduction sites for nanoparticle delivery. The particle delivery process is also closely tied to the choice of detection methods, especially when it comes to studying nanoparticle localization and quantifying the number of particles in plant tissues. Utilizing multiple detection methods can enhance the reliability of the data obtained, as it allows for cross-validation and a more comprehensive understanding of the interaction between nanoparticles and plants. The plant effects produced by different nanomaterials vary greatly, and focus should be placed on the intrinsic mechanism of the effects of nanomaterials on plants, which can be studied in depth from the genetic and molecular levels to investigate the mechanism of the effects of nanomaterials on plant growth and development, as well as the plant’s response mechanism. The problem of considering stems as alternative introduction sites remains to be further investigated.
Acknowledgments
The authors thank Toshiyuki Fukuhara (Tokyo University of Agriculture and Technology), Takeshi Suzuki (Tokyo University of Agriculture and Technology), Shinya Maki (Nagaoka University of Technology), and Ahmad Faizal (Bandung Institute of Technology ITB) for their support in fostering and providing a lot of valuable knowledge to the first author, especially in the fields of plant science and agricultural biotechnology. The Japanese government MEXT scholarship awarded to the first author is also gratefully acknowledged.
Author Contributions
Conceptualization, A.A.S. and I.W.L.; investigation, A.A.S. and I.W.L.; resources, A.A.S.; data curation, A.A.S.; writing—original draft preparation, A.A.S.; writing—review and editing, A.A.S. and I.W.L.; visualization, A.A.S.; supervision, I.W.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data generated or analyzed during this work are included in this published article.
Conflicts of Interest
There are no conflicts of interest to declare.
Funding Statement
This work was partially supported by JSPS KAKENHI (Grants 20K05188, 21H02193, and 23K04470), and the Cabinet Office, Japan’s Moonshot R&D Program for Agriculture, Forestry, and Fisheries (Bio-oriented Technology Research Advancement Institution).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ahanger M.A., Tomar N.S., Tittal M., Argal S., Agarwal R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants. 2017;23:731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwiczak A., Osiak M., Cárdenas-Pérez S., Lubińska-Mielińska S., Piernik A. Osmotic stress or ionic composition: Which affects the early growth of crop species more? Agronomy. 2021;11:435. doi: 10.3390/agronomy11030435. [DOI] [Google Scholar]
- 3.Choi H.S., Cho H.T. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019;9:11181. doi: 10.1038/s41598-019-47733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S., Kumar S., Mohapatra T. Interaction between macro-and micro-nutrients in plants. Front. Plant Sci. 2021;12:665583. doi: 10.3389/fpls.2021.665583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canarini A., Kaiser C., Merchant A., Richter A., Wanek W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019;10:157. doi: 10.3389/fpls.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muratore C., Espen L., Prinsi B. Nitrogen uptake in plants: The plasma membrane root transport systems from a physiological and proteomic perspective. Plants. 2021;10:681. doi: 10.3390/plants10040681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Shabala S., Zhang J., Ma G., Chen D., Shabala L., Zeng F., Chen Z.H., Zhou M., Venkataraman G., et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020;43:2591–2605. doi: 10.1111/pce.13759. [DOI] [PubMed] [Google Scholar]
- 8.Kurotani K.I., Notaguchi M. Cell-to-cell connection in plant grafting—Molecular insights into symplasmic reconstruction. Plant Cell Physiol. 2021;62:1362–1371. doi: 10.1093/pcp/pcab109. [DOI] [PubMed] [Google Scholar]
- 9.Farvardin A., González-Hernández A.I., Llorens E., García-Agustín P., Scalschi L., Vicedo B. The apoplast: A key player in plant survival. Antioxidants. 2020;9:604. doi: 10.3390/antiox9070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bel A.J. The plant axis as the command centre for (re) distribution of sucrose and amino acids. J. Plant Physiol. 2021;265:153488. doi: 10.1016/j.jplph.2021.153488. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Dinh Q.T., Qi M., Wang M., Yang W., Zhou F., Liang D. Radicular and foliar uptake, and xylem-and phloem-mediated transport of selenium in maize (Zea mays L.): A comparison of five Se exogenous species. Plant Soil. 2020;446:111–123. doi: 10.1007/s11104-019-04346-w. [DOI] [Google Scholar]
- 12.Brewer K., Clulow A., Sibanda M., Gokool S., Odindi J., Mutanga O., Naiken V., Chimonyo V.G.P., Mabhaudhi T. Estimation of maize foliar temperature and stomatal conductance as indicators of water stress based on optical and thermal imagery acquired using an unmanned aerial vehicle (UAV) platform. Drones. 2022;6:169. doi: 10.3390/drones6070169. [DOI] [Google Scholar]
- 13.Wheeler T.D., Stroock A.D. The transpiration of water at negative pressures in a synthetic tree. Nature. 2008;455:208–212. doi: 10.1038/nature07226. [DOI] [PubMed] [Google Scholar]
- 14.Driesen E., Van den Ende W., De Proft M., Saeys W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy. 2020;10:1975. doi: 10.3390/agronomy10121975. [DOI] [Google Scholar]
- 15.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 16.Dontsova T.A., Nahirniak S.V., Astrelin I.M. Metaloxide nanomaterials and nanocomposites of ecological purpose. J. Nanomater. 2019;2019:5942194. doi: 10.1155/2019/5942194. [DOI] [Google Scholar]
- 17.Handy R.D., Von der Kammer F., Lead J.R., Hassellöv M., Owen R., Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- 18.Dolai J., Mandal K., Jana N.R. Nanoparticle size effects in biomedical applications. ACS Appl. Nano Mater. 2021;4:6471–6496. doi: 10.1021/acsanm.1c00987. [DOI] [Google Scholar]
- 19.Xin X., Judy J.D., Sumerlin B.B., He Z. Nano-enabled agriculture: From nanoparticles to smart nanodelivery systems. Environ. Chem. 2020;17:413–425. doi: 10.1071/EN19254. [DOI] [Google Scholar]
- 20.Yan Y., Zhu X., Yu Y., Li C., Zhang Z., Wang F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022;34:2106945. doi: 10.1002/adma.202106945. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez-Rodríguez G.B., Dal Sasso G., Carmona F.J., Miguel-Rojas C., Pérez-de-Luque A., Masciocchi N., Guagliardi A., Delgado-López J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020;3:1344–1353. doi: 10.1021/acsabm.9b00937. [DOI] [PubMed] [Google Scholar]
- 22.Wang J.W., Grandio E.G., Newkirk G.M., Demirer G.S., Butrus S., Giraldo J.P., Landry M.P. Nanoparticle-mediated genetic engineering of plants. Mol. Plant. 2019;12:1037–1040. doi: 10.1016/j.molp.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mujtaba M., Wang D., Carvalho L.B., Oliveira J.L., Espirito Santo Pereira A.D., Sharif R., Jogaiah S., Paidi M.K., Wang L., Ali Q., et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021;1:417–435. doi: 10.1021/acsagscitech.1c00146. [DOI] [Google Scholar]
- 24.Monreal C.M., DeRosa M., Mallubhotla S.C., Bindraban P.S., Dimkpa C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils. 2016;52:423–437. doi: 10.1007/s00374-015-1073-5. [DOI] [Google Scholar]
- 25.Tombuloglu H., Ercan I., Alshammari T., Tombuloglu G., Slimani Y., Almessiere M., Baykal A. Incorporation of micro-nutrients (nickel, copper, zinc, and iron) into plant body through nanoparticles. J. Soil Sci. Plant Nutr. 2020;20:1872–1881. doi: 10.1007/s42729-020-00258-2. [DOI] [Google Scholar]
- 26.Hong J., Wang C., Wagner D.C., Gardea-Torresdey J.L., He F., Rico C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano. 2021;8:1196–1210. doi: 10.1039/D0EN01129K. [DOI] [Google Scholar]
- 27.Jat S.K., Bhattacharya J., Sharma M.K. Nanomaterial based gene delivery: A promising method for plant genome engineering. J. Mater. Chem. B. 2020;8:4165–4175. doi: 10.1039/D0TB00217H. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Yan Q., Lan C., Tang T., Wang K., Shen J., Niu D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023;5:2. doi: 10.1186/s42483-023-00157-1. [DOI] [Google Scholar]
- 29.Su Y., Ashworth V., Kim C., Adeleye A.S., Rolshausen P., Roper C., White J., Jassby D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano. 2019;6:2311–2331. doi: 10.1039/C9EN00461K. [DOI] [Google Scholar]
- 30.Singh R.P., Handa R., Manchanda G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release. 2021;329:1234–1248. doi: 10.1016/j.jconrel.2020.10.051. [DOI] [PubMed] [Google Scholar]
- 31.Cao L., Zhang H., Cao C., Zhang J., Li F., Huang Q. Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomaterials. 2016;6:126. doi: 10.3390/nano6070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sembada A.A., Lenggoro I.W. Comparative analysis of germination performance from several species of seeds under influence of silica nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 2023;1271:012085. doi: 10.1088/1755-1315/1271/1/012085. [DOI] [Google Scholar]
- 33.Sembada A.A., Maki S., Faizal A., Fukuhara T., Suzuki T., Lenggoro I.W. The role of silica nanoparticles in promoting the germination of tomato (Solanum lycopersicum) seeds. Nanomaterials. 2023;13:2110. doi: 10.3390/nano13142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faizan M., Yu F., Chen C., Faraz A., Hayat S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Protein Pept. Sci. 2021;22:362–375. doi: 10.2174/1389203721666201016144848. [DOI] [PubMed] [Google Scholar]
- 35.Asadishad B., Chahal S., Akbari A., Cianciarelli V., Azodi M., Ghoshal S., Tufenkji N. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environ. Sci. Technol. 2018;52:1908–1918. doi: 10.1021/acs.est.7b05389. [DOI] [PubMed] [Google Scholar]
- 36.Yan A., Chen Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019;20:1003. doi: 10.3390/ijms20051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barhoum A., García-Betancourt M.L., Jeevanandam J., Hussien E.A., Mekkawy S.A., Mostafa M., Omran M.M., Abdalla M.S., Bechelany M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials. 2022;12:177. doi: 10.3390/nano12020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil S., Chandrasekaran R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020;18:67. doi: 10.1186/s43141-020-00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vega-Vásquez P., Mosier N.S., Irudayaraj J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020;8:79. doi: 10.3389/fbioe.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santana I., Wu H., Hu P., Giraldo J.P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 2020;11:2045. doi: 10.1038/s41467-020-15731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert J., Chanana M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018;25:4553–4586. doi: 10.2174/0929867325666180601101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sampathkumar K., Tan K.X., Loo S.C.J. Developing nano-delivery systems for agriculture and food applications with nature-derived polymers. Iscience. 2020;23:101055. doi: 10.1016/j.isci.2020.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goswami L., Kim K.H., Deep A., Das P., Bhattacharya S.S., Kumar S., Adelodun A.A. Engineered nano particles: Nature, behavior, and effect on the environment. J. Environ. Manag. 2017;196:297–315. doi: 10.1016/j.jenvman.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H., Goh N.S., Wang J.W., Pinals R.L., González-Grandío E., Demirer G.S., Butrus S., Fakra S.C., Flores A.D.R., Zhai R., et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022;17:197–205. doi: 10.1038/s41565-021-01018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittal D., Kaur G., Singh P., Yadav K., Ali S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020;2:579954. doi: 10.3389/fnano.2020.579954. [DOI] [Google Scholar]
- 47.Antisari L.V., Carbone S., Gatti A., Vianello G., Nannipieri P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ. Sci. Pollut. Res. 2014;22:1841–1853. doi: 10.1007/s11356-014-3509-0. [DOI] [PubMed] [Google Scholar]
- 48.Dimkpa C.O., McLean J.E., Martineau N., Britt D.W., Haverkamp R., Anderson A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013;47:1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- 49.El-Temsah Y.S., Joner E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012;27:42–49. doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- 50.Geisler-Lee J., Wang Q., Yao Y., Zhang W., Geisler M., Li K., Huang Y., Chen Y., Kolmakov A., Ma X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology. 2013;7:323–337. doi: 10.3109/17435390.2012.658094. [DOI] [PubMed] [Google Scholar]
- 51.Lee W.M., Kwak J.I., An Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere. 2012;86:491–499. doi: 10.1016/j.chemosphere.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 52.Song U., Jun H., Waldman B., Roh J., Kim Y., Yi J., Lee E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum) Ecotoxicol. Environ. Saf. 2013;93:60–67. doi: 10.1016/j.ecoenv.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Wang J., Koo Y., Alexander A., Yang Y., Westerhof S., Zhang Q., Schnoor J.L., Colvin V.L., Braam J., Alvarez P.J.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013;47:5442–5449. doi: 10.1021/es4004334. [DOI] [PubMed] [Google Scholar]
- 54.Lin D., Xing B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Koelmel J., Leland T., Wang H., Amarasiriwardena D., Xing B. Investigation of gold nanoparticles uptake and their tissue level distribution in rice plants by laser ablation-inductively coupled-mass spectrometry. Environ. Pollut. 2013;174:222–228. doi: 10.1016/j.envpol.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Sabo-Attwood T., Unrine J.M., Stone J.W., Murphy C.J., Ghoshroy S., Blom D., Bertsch P.M., Newman L.A. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology. 2012;6:353–360. doi: 10.3109/17435390.2011.579631. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Z.J., Wang H., Yan B., Zheng H., Jiang Y., Miranda O.R., Rotello V.M., Xing B., Vachet R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012;46:12391–12398. doi: 10.1021/es301977w. [DOI] [PubMed] [Google Scholar]
- 58.Slomberg D.L., Schoenfisch M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012;46:10247–10254. doi: 10.1021/es300949f. [DOI] [PubMed] [Google Scholar]
- 59.Lizzi D., Mattiello A., Adamiano A., Fellet G., Gava E., Marchiol L. Influence of cerium oxide nanoparticles on two terrestrial wild plant species. Plants. 2021;10:335. doi: 10.3390/plants10020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez-Viezcas J.A., Castillo-Michel H., Andrews J.C., Cotte M., Rico C., Peralta-Videa J.R., Ge Y., Priester J.H., Holden P.A., Gardea-Torresdey J.L. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max) ACS Nano. 2013;7:1415–1423. doi: 10.1021/nn305196q. [DOI] [PubMed] [Google Scholar]
- 61.Rico C.M., Morales M.I., McCreary R., Castillo-Michel H., Barrios A.C., Hong J., Tafoya A., Lee W.Y., Varela-Ramirez A., Peralta-Videa J.R., et al. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ. Sci. Technol. 2013;47:14110–14118. doi: 10.1021/es4033887. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q., Ma X., Zhang W., Pei H., Chen Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics. 2012;4:1105–1112. doi: 10.1039/c2mt20149f. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z., He X., Zhang H., Ma Y., Zhang P., Ding Y., Zhao Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics. 2011;3:816–822. doi: 10.1039/c1mt00049g. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H., Han J., Xiao J.Q., Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 65.Lysenko V., Guo Y., Rajput V.D., Chalenko E., Yadronova O., Zaruba T., Varduny T., Kirichenko E. Changes of dorsoventral asymmetry and anoxygenic photosynthesis in response of Chelidonium majus leaves to the SiO2 nanoparticle treatment. Photosynthetica. 2023;61:275–284. doi: 10.32615/ps.2023.016. [DOI] [Google Scholar]
- 66.Li S., Liu J., Wang Y., Gao Y., Zhang Z., Xu J., Xing G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.) Food Energy Secur. 2021;10:e269. doi: 10.1002/fes3.269. [DOI] [Google Scholar]
- 67.Du W., Sun Y., Ji R., Zhu J., Wu J., Guo H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011;13:822–828. doi: 10.1039/c0em00611d. [DOI] [PubMed] [Google Scholar]
- 68.Servin A.D., Castillo-Michel H., Hernandez-Viezcas J.A., Diaz B.C., Peralta-Videa J.R., Gardea-Torresdey J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012;46:7637–7643. doi: 10.1021/es300955b. [DOI] [PubMed] [Google Scholar]
- 69.Wang S., Kurepa J., Smalle J.A. Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011;34:811–820. doi: 10.1111/j.1365-3040.2011.02284.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhao F., Xin X., Cao Y., Su D., Ji P., Zhu Z., He Z. Use of carbon nanoparticles to improve soil fertility, crop growth and nutrient uptake by corn (Zea mays L.) Nanomaterials. 2021;11:2717. doi: 10.3390/nano11102717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tripathi S., Sonkar S.K., Sarkar S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale. 2011;3:1176–1181. doi: 10.1039/c0nr00722f. [DOI] [PubMed] [Google Scholar]
- 72.Tiwari D.K., Dasgupta-Schubert N., Villaseñor Cendejas L.M., Villegas J., Carreto Montoya L., Borjas García S.E. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl. Nanosci. 2014;4:577–591. doi: 10.1007/s13204-013-0236-7. [DOI] [Google Scholar]
- 73.Begum P., Ikhtiari R., Fugetsu B., Matsuoka M., Akasaka T., Watari F. Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl. Surf. Sci. 2012;262:120–124. doi: 10.1016/j.apsusc.2012.03.028. [DOI] [Google Scholar]
- 74.Mondal A., Basu R., Das S., Nandy P. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect. J. Nanopart. Res. 2011;13:4519–4528. doi: 10.1007/s11051-011-0406-z. [DOI] [Google Scholar]
- 75.Wang X., Han H., Liu X., Gu X., Chen K., Lu D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012;14:841. doi: 10.1007/s11051-012-0841-5. [DOI] [Google Scholar]
- 76.Demirer G.S., Zhang H., Goh N.S., Pinals R.L., Chang R., Landry M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020;6:eaaz0495. doi: 10.1126/sciadv.aaz0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Modlitbová P., Novotný K., Pořízka P., Klus J., Lubal P., Zlámalová-Gargošová H., Kaiser J. Comparative investigation of toxicity and bioaccumulation of Cd-based quantum dots and Cd salt in freshwater plant Lemna minor L. Ecotoxicol. Environ. Saf. 2018;147:334–341. doi: 10.1016/j.ecoenv.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 78.Modlitbová P., Pořízka P., Novotný K., Drbohlavová J., Chamradová I., Farka Z., Zlámalová-Gargošová H., Romih T., Kaiser J. Short-term assessment of cadmium toxicity and uptake from different types of Cd-based Quantum Dots in the model plant Allium cepa L. Ecotoxicol. Environ. Saf. 2018;153:23–31. doi: 10.1016/j.ecoenv.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 79.Wang J., Yang Y., Zhu H., Braam J., Schnoor J.L., Alvarez P.J. Uptake, translocation, and transformation of quantum dots with cationic versus anionic coatings by Populus deltoides× nigra cuttings. Environ. Sci. Technol. 2014;48:6754–6762. doi: 10.1021/es501425r. [DOI] [PubMed] [Google Scholar]
- 80.Lian F., Wang C., Wang C., Gu S., Cao X. Variety-dependent responses of rice plants with differential cadmium accumulating capacity to cadmium telluride quantum dots (CdTe QDs): Cadmium uptake, antioxidative enzyme activity, and gene expression. Sci. Total Environ. 2019;697:134083. doi: 10.1016/j.scitotenv.2019.134083. [DOI] [PubMed] [Google Scholar]
- 81.Muthukrishnan S., Murugan I., Selvaraj M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019;212:169–177. doi: 10.1016/j.carbpol.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 82.Divya K., Vijayan S., Nair S.J., Jisha M.S. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019;124:1053–1059. doi: 10.1016/j.ijbiomac.2018.11.185. [DOI] [PubMed] [Google Scholar]
- 83.Rastogi A., Zivcak M., Sytar O., Kalaji H.M., He X., Mbarki S., Brestic M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017;5:78. doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nair P.M.G., Chung I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 85.Wang Q., Ebbs S.D., Chen Y., Ma X. Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics. 2013;5:753–759. doi: 10.1039/c3mt00033h. [DOI] [PubMed] [Google Scholar]
- 86.Rossi L., Sharifan H., Zhang W., Schwab A.P., Ma X. Mutual effects and in planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.) Environ. Sci. Nano. 2018;5:150–157. doi: 10.1039/C7EN00931C. [DOI] [Google Scholar]
- 87.Rani N., Kumari K., Sangwan P., Barala P., Yadav J., Vijeta, Rahul, Hooda V. Nano-iron and nano-zinc induced growth and metabolic changes in Vigna radiata. Sustainability. 2022;14:8251. doi: 10.3390/su14148251. [DOI] [Google Scholar]
- 88.Ali S., Mehmood A., Khan N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021;2021:6677616. doi: 10.1155/2021/6677616. [DOI] [Google Scholar]
- 89.Wang X., Xie H., Wang P., Yin H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials. 2023;16:3097. doi: 10.3390/ma16083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kralova K., Jampilek J. Responses of medicinal and aromatic plants to engineered nanoparticles. Appl. Sci. 2021;11:1813. doi: 10.3390/app11041813. [DOI] [Google Scholar]
- 91.Abbas Q., Yousaf B., Ullah H., Ali M.U., Ok Y.S., Rinklebe J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020;50:2523–2581. doi: 10.1080/10643389.2019.1705721. [DOI] [Google Scholar]
- 92.Ahmar S., Mahmood T., Fiaz S., Mora-Poblete F., Shafique M.S., Chattha M.S., Jung K.H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021;12:663849. doi: 10.3389/fpls.2021.663849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roychoudhury A. Nanobiolistics: New generation transfection system for animals and plants. J. Mol. Cell. Biol. Forecast. 2020;3:1020 [Google Scholar]
- 94.Rajkumari N., Alex S., Soni K.B., Anith K.N., Viji M.M., Kiran A.G. Silver nanoparticles for biolistic transformation in Nicotiana tabacum L. 3 Biotech. 2021;11:497. doi: 10.1007/s13205-021-03043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Das A.K., Gupta P., Chakraborty D. Physical methods of gene transfer: Kinetics of gene delivery into cells: A Review. Agric. Rev. 2015;36:61–66. doi: 10.5958/0976-0741.2015.00007.0. [DOI] [Google Scholar]
- 96.Zolghadrnasab M., Mousavi A., Farmany A., Arpanaei A. Ultrasound-mediated gene delivery into suspended plant cells using polyethyleneimine-coated mesoporous silica nanoparticles. Ultrason. Sonochem. 2021;73:105507. doi: 10.1016/j.ultsonch.2021.105507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.González-Melendi P., Fernández-Pacheco R., Coronado M.J., Corredor E., Testillano P.S., Risueño M.C., Marquina C., Ibarra M.R., Rubiales D., Pérez-de-Luque A. Nanoparticles as smart treatment-delivery systems in plants: Assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 2008;101:187–195. doi: 10.1093/aob/mcm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calvo R., Rodriguez Mariblanca I., Pini V., Dias M., Cebrian V., Thon A., Saad A., Salvador-Matar A., Ahumada Ó., Silván M.M., Saunders A.E., et al. Novel characterization techniques for multifunctional plasmonic–magnetic nanoparticles in biomedical applications. Nanomaterials. 2023;13:2929. doi: 10.3390/nano13222929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nisticò R., Cesano F., Garello F. Magnetic materials and systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics. 2020;8:6. doi: 10.3390/inorganics8010006. [DOI] [Google Scholar]
- 100.Winkler R., Ciria M., Ahmad M., Plank H., Marcuello C. A review of the current state of magnetic force microscopy to unravel the magnetic properties of nanomaterials applied in biological systems and future directions for quantum technologies. Nanomaterials. 2023;13:2585. doi: 10.3390/nano13182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adhikari S., Orrit M. Progress and perspectives in single-molecule optical spectroscopy. J. Chem. Phys. 2022;156:160903. doi: 10.1063/5.0087003. [DOI] [PubMed] [Google Scholar]
- 102.Eggenberger K., Frey N., Zienicke B., Siebenbrock J., Schunck T., Fischer R., Bräse S., Birtalan E., Nann T., Nick P. Use of nanoparticles to study and manipulate plant cells. Adv. Eng. Mater. 2010;12:B406–B412. doi: 10.1002/adem.201080009. [DOI] [Google Scholar]
- 103.Martin-Ortigosa S., Peterson D.J., Valenstein J.S., Lin V.S.Y., Trewyn B.G., Lyznik L.A., Wang K. Mesoporous silica nanoparticle-mediated intracellular Cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014;164:537–547. doi: 10.1104/pp.113.233650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Golkar P., Moradi M., Garousi G.A. Elicitation of stevia glycosides using salicylic acid and silver nanoparticles under callus culture. Sugar Tech. 2019;21:569–577. doi: 10.1007/s12355-018-0655-6. [DOI] [Google Scholar]
- 105.Silva A.T., Nguyen A., Ye C., Verchot J., Moon J.H. Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 2010;10:291. doi: 10.1186/1471-2229-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Makhotenko A.V., Snigir E.A., Kalinina N.O., Makarov V.V., Taliansky M.E. Data on a delivery of biomolecules into Nicothiana benthamiana leaves using different nanoparticles. Data Br. 2018;16:1034–1037. doi: 10.1016/j.dib.2017.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang T., Xu Q., Huang T., Ling D., Gao J. New insights into biocompatible iron oxide nanoparticles: A potential booster of gene delivery to stem cells. Small. 2020;16:2001588. doi: 10.1002/smll.202001588. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad A., Hashmi S.S., Palma J.M., Corpas F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere. 2022;290:133329. doi: 10.1016/j.chemosphere.2021.133329. [DOI] [PubMed] [Google Scholar]
- 109.Kjeldskov J., Skov M.B. Studying usability in sitro: Simulating real world phenomena in controlled environments. Int. J. Hum. Comput. Interact. 2007;22:7–36. doi: 10.1080/10447310709336953. [DOI] [Google Scholar]
- 110.Foulkes R., Man E., Thind J., Yeung S., Joy A., Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020;8:4653–4664. doi: 10.1039/D0BM00558D. [DOI] [PubMed] [Google Scholar]
- 111.Martin-Ortigosa S., Valenstein J.S., Lin V.S.Y., Trewyn B.G., Wang K. Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv. Funct. Mater. 2012;22:3576–3582. doi: 10.1002/adfm.201200359. [DOI] [Google Scholar]
- 112.Amani A., Zare N., Asadi A., Zakaria R.A. Ultrasound-enhanced gene delivery to alfalfa cells by hPAMAM dendrimer nanoparticles. Turk. J. Biol. 2018;42:63–75. doi: 10.3906/biy-1706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Hu J., Ma C., Wang Y., Wu C., Huang J., Xing B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.) Chemosphere. 2016;159:326–334. doi: 10.1016/j.chemosphere.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 114.Ozyigit I.I. Gene transfer to plants by electroporation: Methods and applications. Mol. Biol. Rep. 2020;47:3195–3210. doi: 10.1007/s11033-020-05343-4. [DOI] [PubMed] [Google Scholar]
- 115.Shankramma K., Yallappa S., Shivanna M.B., Manjanna J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 2016;6:983–990. doi: 10.1007/s13204-015-0510-y. [DOI] [Google Scholar]
- 116.Tombuloglu H., Slimani Y., Tombuloglu G., Almessiere M., Baykal A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.) Chemosphere. 2019;226:110–122. doi: 10.1016/j.chemosphere.2019.03.075. [DOI] [PubMed] [Google Scholar]
- 117.Hao Y., Yang X., Shi Y., Song S., Xing J., Marowitch J., Chen J., Chen J. Magnetic gold nanoparticles as a vehicle for fluorescein isothiocyanate and DNA delivery into plant cells. Botany. 2013;91:457–466. doi: 10.1139/cjb-2012-0281. [DOI] [Google Scholar]
- 118.Liu Q., Chen B., Wang Q., Shi X., Xiao Z., Lin J., Fang X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009;9:1007–1010. doi: 10.1021/nl803083u. [DOI] [PubMed] [Google Scholar]
- 119.Anjum N.A., Rodrigo M.A.M., Moulick A., Heger Z., Kopel P., Zítka O., Kizek R. Transport phenomena of nanoparticles in plants and animals/humans. Environ. Res. 2016;151:233–243. doi: 10.1016/j.envres.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 120.Lian J., Zhao L., Wu J., Xiong H., Bao Y., Zeb A., Tang J., Liu W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.) Chemosphere. 2020;239:124794. doi: 10.1016/j.chemosphere.2019.124794. [DOI] [PubMed] [Google Scholar]
- 121.Wang Z., Xie X., Zhao J., Liu X., Feng W., White J.C., Xing B. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.) Environ. Sci. Technol. 2012;46:4434–4441. doi: 10.1021/es204212z. [DOI] [PubMed] [Google Scholar]
- 122.Cunningham F.J., Goh N.S., Demirer G.S., Matos J.L., Landry M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36:882–897. doi: 10.1016/j.tibtech.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rivera A.L., Gómez-Lim M., Fernández F., Loske A.M. Physical methods for genetic plant transformation. Phys. Life Rev. 2012;9:308–345. doi: 10.1016/j.plrev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 124.O’brien J.A., Lummis S.C. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat. Protoc. 2006;1:977–981. doi: 10.1038/nprot.2006.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lv Z., Jiang R., Chen J., Chen W. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. Plant J. 2020;104:880–891. doi: 10.1111/tpj.14973. [DOI] [PubMed] [Google Scholar]
- 126.Du S., Kendall K., Toloueinia P., Mehrabadi Y., Gupta G., Newton J. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J. Nanopart. Res. 2012;14:758. doi: 10.1007/s11051-012-0758-z. [DOI] [Google Scholar]
- 127.Lentacker I., De Cock I., Deckers R., De Smedt S.C., Moonen C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Rich J., Tian Z., Huang T.J. Sonoporation: Past, present, and future. Adv. Mater. Technol. 2022;7:2100885. doi: 10.1002/admt.202100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snipstad S., Hanstad S., Bjørkøy A., Mørch Ý., de Lange Davies C. Sonoporation using nanoparticle-loaded microbubbles increases cellular uptake of nanoparticles compared to co-incubation of nanoparticles and microbubbles. Pharmaceutics. 2021;13:640. doi: 10.3390/pharmaceutics13050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cardoso V.F., Francesko A., Ribeiro C., Bañobre-López M., Martins P., Lanceros-Mendez S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018;7:1700845. doi: 10.1002/adhm.201700845. [DOI] [PubMed] [Google Scholar]
- 131.Young J.L., Dean D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015;89:49–88. doi: 10.1016/bs.adgen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Napotnik T.B., Polajžer T., Miklavčič D. Cell death due to electroporation—A review. Bioelectrochemistry. 2021;141:107871. doi: 10.1016/j.bioelechem.2021.107871. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y., Yu L.C. Microinjection as a tool of mechanical delivery. Curr. Opin. Biotechnol. 2008;19:506–510. doi: 10.1016/j.copbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 134.Noori A., Selvaganapathy P.R., Wilson J. Microinjection in a microfluidic format using flexible and compliant channels and electroosmotic dosage control. Lab Chip. 2009;9:3202–3211. doi: 10.1039/b909961a. [DOI] [PubMed] [Google Scholar]
- 135.Narita F., Wang Y., Kurita H., Suzuki M. Multi-scale analysis and testing of tensile behavior in polymers with randomly oriented and agglomerated cellulose nanofibers. Nanomaterials. 2020;10:700. doi: 10.3390/nano10040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magazzù A., Marcuello C. Investigation of soft matter nanomechanics by atomic force microscopy and optical tweezers: A comprehensive review. Nanomaterials. 2023;13:963. doi: 10.3390/nano13060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Angeloni L., Reggente M., Passeri D., Natali M., Rossi M. Identification of nanoparticles and nanosystems in biological matrices with scanning probe microscopy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018;10:e1521. doi: 10.1002/wnan.1521. [DOI] [PubMed] [Google Scholar]
- 138.Okpara E.C., Nde S.C., Fayemi O.E., Ebenso E.E. Electrochemical characterization and detection of lead in water using SPCE modified with BiONPs/PANI. Nanomaterials. 2021;11:1294. doi: 10.3390/nano11051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang K., Jariwala B., Li J., Briggs N.C., Wang B., Ruzmetov D., Burke R.A., Lerach J.O., Ivanov T.G., Haque M., et al. Large scale 2D/3D hybrids based on gallium nitride and transition metal dichalcogenides. Nanoscale. 2018;10:336–341. doi: 10.1039/C7NR07586C. [DOI] [PubMed] [Google Scholar]
- 140.Rico C.M., Majumdar S., Duarte-Gardea M., Peralta-Videa J.R., Gardea-Torresdey J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011;59:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A.J., Quigg A., Santschi P.H., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 142.McCann M.C., Wells B., Roberts K. Direct visualization of cross-links in the primary plant cell wall. J. Cell Sci. 1990;96:323–334. doi: 10.1242/jcs.96.2.323. [DOI] [Google Scholar]
- 143.Fujino T., Itoh T. Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for cell wall architecture. Plant Cell Physiol. 1998;39:1315–1323. doi: 10.1093/oxfordjournals.pcp.a029336. [DOI] [Google Scholar]
- 144.Rose J.K., Bennett A.B. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999;4:176–183. doi: 10.1016/S1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 145.Caffall K.H., Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 146.Eichert T., Kurtz A., Steiner U., Goldbach H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 147.Eichert T., Goldbach H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces–further evidence for a stomatal pathway. Physiol. Plant. 2008;132:491–502. doi: 10.1111/j.1399-3054.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 148.Malejko J., Godlewska-Żyłkiewicz B., Vanek T., Landa P., Nath J., Dror I., Berkowitz B. Uptake, translocation, weathering and speciation of gold nanoparticles in potato, radish, carrot and lettuce crops. J. Hazard. Mater. 2021;418:126219. doi: 10.1016/j.jhazmat.2021.126219. [DOI] [PubMed] [Google Scholar]
- 149.Avellan A., Yun J., Morais B.P., Clement E.T., Rodrigues S.M., Lowry G.V. Critical review: Role of inorganic nanoparticle properties on their foliar uptake and in planta translocation. Environ. Sci. Technol. 2021;55:13417–13431. doi: 10.1021/acs.est.1c00178. [DOI] [PubMed] [Google Scholar]
- 150.Deans R.M., Brodribb T.J., Busch F.A., Farquhar G.D. Optimization can provide the fundamental link between leaf photosynthesis, gas exchange and water relations. Nat. Plants. 2020;6:1116–1125. doi: 10.1038/s41477-020-00760-6. [DOI] [PubMed] [Google Scholar]
- 151.García-Gómez C., Pérez R.A., Albero B., Obrador A., Almendros P., Fernández M.D. Interaction of ZnO nanoparticles with metribuzin in a soil–plant system: Ecotoxicological effects and changes in the distribution pattern of Zn and metribuzin. Agronomy. 2023;13:2004. doi: 10.3390/agronomy13082004. [DOI] [Google Scholar]
- 152.Avellan A., Yun J., Zhang Y., Spielman-Sun E., Unrine J.M., Thieme J., Li J., Lombi E., Bland G., Lowry G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano. 2019;13:5291–5305. doi: 10.1021/acsnano.8b09781. [DOI] [PubMed] [Google Scholar]
- 153.Stanton M.L. Seed variation in wild radish: Effect of seed size on components of seedling and adult fitness. Ecology. 1984;65:1105–1112. doi: 10.2307/1938318. [DOI] [Google Scholar]
- 154.Lamichhane J.R., Debaeke P., Steinberg C., You M.P., Barbetti M.J., Aubertot J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil. 2018;432:1–28. doi: 10.1007/s11104-018-3780-9. [DOI] [Google Scholar]
- 155.Ibrahim E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 156.Chen Y., Palta J., Prasad P.V., Siddique K.H. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020;20:185. doi: 10.1186/s12870-020-02390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rajput V., Minkina T., Mazarji M., Shende S., Sushkova S., Mandzhieva S., Burachevskaya M., Chaplygin V., Singh A., Jatav H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020;65:137–143. doi: 10.1016/j.aoas.2020.08.001. [DOI] [Google Scholar]
- 158.Lombi E., Susini J. Synchrotron-based techniques for plant and soil science: Opportunities, challenges and future perspectives. Plant Soil. 2009;320:1–35. doi: 10.1007/s11104-008-9876-x. [DOI] [Google Scholar]
- 159.Deng Y., Petersen E.J., Challis K.E., Rabb S.A., Holbrook R.D., Ranville J.F., Nelson B.C., Xing B. Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 2017;51:10615–10623. doi: 10.1021/acs.est.7b01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fouad A., Hegazy A.E., Azab E., Khojah E., Kapiel T. Boosting of antioxidants and alkaloids in Catharanthus roseus suspension cultures using silver nanoparticles with expression of CrMPK3 and STR genes. Plants. 2021;10:2202. doi: 10.3390/plants10102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pilalis E., Chatziioannou A., Thomasset B., Kolisis F. An in silico compartmentalized metabolic model of Brassica napus enables the systemic study of regulatory aspects of plant central metabolism. Biotechnol. Bioeng. 2011;108:1673–1682. doi: 10.1002/bit.23107. [DOI] [PubMed] [Google Scholar]
- 162.Laborda F., Bolea E., Cepriá G., Gómez M.T., Jiménez M.S., Pérez-Arantegui J., Castillo J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta. 2016;904:10–32. doi: 10.1016/j.aca.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 163.Sun D., Hussain H.I., Yi Z., Siegele R., Cresswell T., Kong L., Cahill D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014;33:1389–1402. doi: 10.1007/s00299-014-1624-5. [DOI] [PubMed] [Google Scholar]
- 164.Chaturvedi P., Taguchi M., Burrs S.L., Hauser B.A., Salim W.W.A.W., Claussen J.C., McLamore E.S. Emerging technologies for non-invasive quantification of physiological oxygen transport in plants. Planta. 2013;238:599–614. doi: 10.1007/s00425-013-1926-9. [DOI] [PubMed] [Google Scholar]
- 165.Arruda S.C.C., Silva A.L.D., Galazzi R.M., Azevedo R.A., Arruda M.A.Z. Nanoparticles applied to plant science: A review. Talanta. 2015;131:693–705. doi: 10.1016/j.talanta.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 166.De la Calle I., Menta M., Séby F. Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim. Acta B At. Spectrosc. 2016;125:66–96. doi: 10.1016/j.sab.2016.09.007. [DOI] [Google Scholar]
- 167.Yusefi-Tanha E., Fallah S., Rostamnejadi A., Pokhrel L.R. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants. 2020;9:1326. doi: 10.3390/plants9101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tombuloglu H., Slimani Y., AlShammari T.M., Bargouti M., Ozdemir M., Tombuloglu G., Akhtar S., Sabit H., Hakeem K.R., Almessiere M., et al. Uptake, translocation, and physiological effects of hematite (α-Fe2O3) nanoparticles in barley (Hordeum vulgare L.) Environ. Pollut. 2020;266:115391. doi: 10.1016/j.envpol.2020.115391. [DOI] [PubMed] [Google Scholar]
- 169.Bao D., Oh Z.G., Chen Z. Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front. Plant Sci. 2016;7:32. doi: 10.3389/fpls.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Castro-González C.G., Sánchez-Segura L., Gómez-Merino F.C., Bello-Bello J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019;9:10372. doi: 10.1038/s41598-019-46828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Taylor A.F., Rylott E.L., Anderson C.W., Bruce N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE. 2014;9:e93793. doi: 10.1371/journal.pone.0093793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang W.N., Tarafdar J.C., Biswas P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013;15:1417. doi: 10.1007/s11051-013-1417-8. [DOI] [Google Scholar]
- 173.Le V.N., Rui Y., Gui X., Li X., Liu S., Han Y. Uptake, transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnology. 2014;12:50. doi: 10.1186/s12951-014-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin D., Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008;42:5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 175.Lv J., Zhang S., Luo L., Zhang J., Yang K., Christie P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano. 2015;2:68–77. doi: 10.1039/C4EN00064A. [DOI] [Google Scholar]
- 176.López-Luna J., Cruz-Fernández S., Mills D.S., Martínez-Enríquez A.I., Solís-Domínguez F.A., del Carmen Ángeles González-Chávez M., Carrillo-González R., Martinez-Vargas S., Mijangos-Ricardez O.F., del Carmen Cuevas-Díaz M. Phytotoxicity and upper localization of Ag@CoFe2O4 nanoparticles in wheat plants. Environ. Sci. Pollut. Res. 2020;27:1923–1940. doi: 10.1007/s11356-019-06668-9. [DOI] [PubMed] [Google Scholar]
- 177.Wang P., Lombi E., Sun S., Scheckel K.G., Malysheva A., McKenna B.A., Menzies N.W., Zhao F.J., Kopittke P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano. 2017;4:448–460. doi: 10.1039/C6EN00489J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang W., Dan Y., Shi H., Ma X. Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. J. Environ. Chem. Eng. 2017;5:572–577. doi: 10.1016/j.jece.2016.12.036. [DOI] [Google Scholar]
- 179.Zahra Z., Arshad M., Rafique R., Mahmood A., Habib A., Qazi I.A., Khan S.A. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J. Agric. Food Chem. 2015;63:6876–6882. doi: 10.1021/acs.jafc.5b01611. [DOI] [PubMed] [Google Scholar]
- 180.Jhansi K., Jayarambabu N., Reddy K.P., Reddy N.M., Suvarna R.P., Rao K.V., Kumar V.R., Rajendar V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech. 2017;7:263. doi: 10.1007/s13205-017-0894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang Q., Shan W., Hu L., Zhao Y., Hou Y., Yin Y., Liang Y., Wang F., Cai Y., Liu J., et al. Uptake and transformation of silver nanoparticles and ions by rice plants revealed by dual stable isotope tracing. Environ. Sci. Technol. 2018;53:625–633. doi: 10.1021/acs.est.8b02471. [DOI] [PubMed] [Google Scholar]
- 182.Dan Y., Ma X., Zhang W., Liu K., Stephan C., Shi H. Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles. Anal. Bioanal. Chem. 2016;408:5157–5167. doi: 10.1007/s00216-016-9565-1. [DOI] [PubMed] [Google Scholar]
- 183.Hayder M., Wojcieszek J., Asztemborska M., Zhou Y., Ruzik L. Analysis of cerium oxide and copper oxide nanoparticles bioaccessibility from radish using SP-ICP-MS. J. Sci. Food Agric. 2020;100:4950–4958. doi: 10.1002/jsfa.10558. [DOI] [PubMed] [Google Scholar]
- 184.Neves V.M., Heidrich G.M., Rodrigues E.S., Enders M.S., Muller E.I., Nicoloso F.T., Pereira de Carvalho H.W., Dressler V.L. La2O3 nanoparticles: Study of uptake and distribution in Pfaffia glomerata (spreng.) pedersen by LA-ICP-MS and μ-XRF. Environ. Sci. Technol. 2019;53:10827–10834. doi: 10.1021/acs.est.9b02868. [DOI] [PubMed] [Google Scholar]
- 185.Wojcieszek J., Jiménez-Lamana J., Ruzik L., Asztemborska M., Jarosz M., Szpunar J. Characterization of TiO2 NPs in radish (Raphanus sativus L.) by single-particle ICP-QQQ-MS. Front. Environ. Sci. 2020;8:100. doi: 10.3389/fenvs.2020.00100. [DOI] [PubMed] [Google Scholar]
- 186.Hu P., An J., Faulkner M.M., Wu H., Li Z., Tian X., Giraldo J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano. 2020;14:7970–7986. doi: 10.1021/acsnano.9b09178. [DOI] [PubMed] [Google Scholar]
- 187.Wu H., Santana I., Dansie J., Giraldo J.P. In vivo delivery of nanoparticles into plant leaves. Curr. Protoc. Chem. Biol. 2017;9:269–284. doi: 10.1002/cpch.29. [DOI] [PubMed] [Google Scholar]
- 188.Zhu J., Li J., Shen Y., Liu S., Zeng N., Zhan X., White J.C., Gardea-Torresdey J., Xing B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano. 2020;7:3901–3913. doi: 10.1039/D0EN00658K. [DOI] [Google Scholar]
- 189.Avellan A., Schwab F., Masion A., Chaurand P., Borschneck D., Vidal V., Rose J., Santaella C., Levard C. Nanoparticle uptake in plants: Gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 2017;51:8682–8691. doi: 10.1021/acs.est.7b01133. [DOI] [PubMed] [Google Scholar]
- 190.Spielman-Sun E., Lombi E., Donner E., Howard D., Unrine J.M., Lowry G.V. Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum) Environ. Sci. Technol. 2017;51:7361–7368. doi: 10.1021/acs.est.7b00813. [DOI] [PubMed] [Google Scholar]
- 191.Peng C., Tong H., Shen C., Sun L., Yuan P., He M., Shi J. Bioavailability and translocation of metal oxide nanoparticles in the soil-rice plant system. Sci. Total Environ. 2020;713:136662. doi: 10.1016/j.scitotenv.2020.136662. [DOI] [PubMed] [Google Scholar]
- 192.Larue C., Veronesi G., Flank A.M., Surble S., Herlin-Boime N., Carrière M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A. 2012;75:722–734. doi: 10.1080/15287394.2012.689800. [DOI] [PubMed] [Google Scholar]
- 193.Shan Q., Liu Y., Zhang X., Shao J., Hei D., Ling Y., Jia W. EDXRF analysis of TiO2 nanoparticles bioaccumulation in aquatic plant, Salvinia natans. Microchem. J. 2020;155:104784. doi: 10.1016/j.microc.2020.104784. [DOI] [Google Scholar]
- 194.Muccifora S., Castillo-Michel H., Barbieri F., Bellani L., Ruffini Castiglione M., Spanò C., Pradas del Real A.E., Giorgetti L., Tassi E.L. Synchrotron radiation spectroscopy and transmission electron microscopy techniques to evaluate TiO2 NPs incorporation, speciation, and impact on root cells ultrastructure of Pisum sativum L. plants. Nanomaterials. 2021;11:921. doi: 10.3390/nano11040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cai F., Wu X., Zhang H., Shen X., Zhang M., Chen W., Gao Q., White J.C., Tao S., Wang X. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.) Nanoimpact. 2017;5:101–108. doi: 10.1016/j.impact.2017.01.006. [DOI] [Google Scholar]
- 196.Navarro D.A., Bisson M.A., Aga D.S. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 2012;211:427–435. doi: 10.1016/j.jhazmat.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 197.Zhao L., Peralta-Videa J.R., Varela-Ramirez A., Castillo-Michel H., Li C., Zhang J., Aguilera R.J., Keller A.A., Gardea-Torresdey J.L. Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: Insight into the uptake mechanism. J. Hazard. Mater. 2012;225:131–138. doi: 10.1016/j.jhazmat.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Movafeghi A., Khataee A., Abedi M., Tarrahi R., Dadpour M., Vafaei F. Effects of TiO2 nanoparticles on the aquatic plant Spirodela polyrrhiza: Evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J. Environ. Sci. 2008;64:130–138. doi: 10.1016/j.jes.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 199.Kurepa J., Paunesku T., Vogt S., Arora H., Rabatic B.M., Lu J., Wanzer M.B., Woloschak G.E., Smalle J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010;10:2296–2302. doi: 10.1021/nl903518f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Praetorius A., Gundlach-Graham A., Goldberg E., Fabienke W., Navratilova J., Gondikas A., Kaegi R., Günther D., Hofmann T., von der Kammer F. Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ. Sci. Nano. 2017;4:307–314. doi: 10.1039/C6EN00455E. [DOI] [Google Scholar]
- 201.Ma X., Geiser-Lee J., Deng Y., Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 202.Nima Z.A., Lahiani M.H., Watanabe F., Xu Y., Khodakovskaya M.V., Biris A.S. Plasmonically active nanorods for delivery of bio-active agents and high-sensitivity SERS detection in planta. RSC Adv. 2014;4:64985–64993. doi: 10.1039/C4RA10358K. [DOI] [Google Scholar]
- 203.Nečemer M., Kump P., Ščančar J., Jaćimović R., Simčič J., Pelicon P., Budnar M., Jeran Z., Pongrac P., Regvar M., et al. Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim. Acta B At. Spectrosc. 2008;63:1240–1247. doi: 10.1016/j.sab.2008.07.006. [DOI] [Google Scholar]
- 204.Leppard G.G. Nanoparticles in the environment as revealed by transmission electron microscopy: Detection, characterisation and activities. Curr. Nanosci. 2008;4:278–301. doi: 10.2174/157341308785161109. [DOI] [Google Scholar]
- 205.Schrand A.M., Schlager J.J., Dai L., Hussain S.M. Preparation of cells for assessing ultrastructural localization of nanoparticles with transmission electron microscopy. Nat. Protoc. 2010;5:744–757. doi: 10.1038/nprot.2010.2. [DOI] [PubMed] [Google Scholar]
- 206.Denk W., Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Khwaja H.A., Parekh P.P., Khan A.R., Hershey D.L., Naqvi R.R., Malik A., Khan K. An in-depth characterization of urban aerosols using electron microscopy and energy dispersive x-ray analysis. Clean (Weinh) 2009;37:544–554. doi: 10.1002/clen.200900012. [DOI] [Google Scholar]
- 208.Jaques V.A.J., Zikmundová E., Holas J., Zikmund T., Kaiser J., Holcová K. Conductive cross-section preparation of non-conductive painting micro-samples for SEM analysis. Sci. Rep. 2022;12:19650. doi: 10.1038/s41598-022-21882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rodushkin I., Engström E., Baxter D.C. Isotopic analyses by ICP-MS in clinical samples. Anal. Bioanal. Chem. 2013;405:2785–2797. doi: 10.1007/s00216-012-6457-x. [DOI] [PubMed] [Google Scholar]
- 210.Fabricius A.L., Duester L., Meermann B., Ternes T.A. ICP-MS-based characterization of inorganic nanoparticles—Sample preparation and off-line fractionation strategies. Anal. Bioanal. Chem. 2014;406:467–479. doi: 10.1007/s00216-013-7480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang Y., Xu R., Luo G., Wu J. Three-dimensional reconstruction of light microscopy image sections: Present and future. Front. Med. 2015;9:30–45. doi: 10.1007/s11684-014-0337-z. [DOI] [PubMed] [Google Scholar]
- 212.Prikler S., Pick D., Einax J.W. Comparing different means of signal treatment for improving the detection power in HPLC-ICP-MS. Anal. Bioanal. Chem. 2012;403:1109–1116. doi: 10.1007/s00216-011-5571-5. [DOI] [PubMed] [Google Scholar]
- 213.Jin C., Liu J., Chen Y., Guan R., Ouyang C., Zhu Y., Ji L., Chao H. Cyclometalated iridium (III) complexes as AIE phosphorescent probes for real-time monitoring of mitophagy in living cells. Sci. Rep. 2016;6:22039. doi: 10.1038/srep22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klockenkämper R., Von Bohlen A., Moens L. Analysis of pigments and inks on oil paintings and historical manuscripts using total reflection x-ray fluorescence spectrometry. X-ray Spectrom. 2000;29:119–129. doi: 10.1002/(SICI)1097-4539(200001/02)29:1<119::AID-XRS400>3.0.CO;2-W. [DOI] [Google Scholar]
- 215.Zhu Y., Cai X., Li J., Zhong Z., Huang Q., Fan C. Synchrotron-based X-ray microscopic studies for bioeffects of nanomaterials. Nanomedicine. 2014;10:515–524. doi: 10.1016/j.nano.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 216.Lee S., Kang S.H. Selective fluorescence and fluorescence-free detection of single biomolecules on nanobiochips. J. Biomed. Nanotechnol. 2014;10:2620–2640. doi: 10.1166/jbn.2014.1883. [DOI] [PubMed] [Google Scholar]
- 217.Ozawa T., Yoshimura H., Kim S.B. Advances in fluorescence and bioluminescence imaging. Anal. Chem. 2013;85:590–609. doi: 10.1021/ac3031724. [DOI] [PubMed] [Google Scholar]
- 218.Jarvi M.T., Patterson M.S., Wilson B.C. Insights into photodynamic therapy dosimetry: Simultaneous singlet oxygen luminescence and photosensitizer photobleaching measurements. Biophys. J. 2012;102:661–671. doi: 10.1016/j.bpj.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yu Z., Xu X., Guo L., Jin R., Lu Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024;907:168155. doi: 10.1016/j.scitotenv.2023.168155. [DOI] [PubMed] [Google Scholar]
- 220.Martínez-Fernández D., Barroso D., Komárek M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut Res. 2016;23:1732–1741. doi: 10.1007/s11356-015-5423-5. [DOI] [PubMed] [Google Scholar]
- 221.Schwab F., Zhai G., Kern M., Turner A., Schnoor J.L., Wiesner M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology. 2016;10:257–278. doi: 10.3109/17435390.2015.1048326. [DOI] [PubMed] [Google Scholar]
- 222.Yadav B., Jogawat A., Lal S.K., Lakra N., Mehta S., Shabek N., Narayan O.P. Plant mineral transport systems and the potential for crop improvement. Planta. 2021;253:45. doi: 10.1007/s00425-020-03551-7. [DOI] [PubMed] [Google Scholar]
- 223.Zhang G., Kong G., Li Y. Long-distance communication through systemic macromolecular signaling mediates stress defense responses in plants. Physiol. Plant. 2021;173:1926–1934. doi: 10.1111/ppl.13535. [DOI] [PubMed] [Google Scholar]
- 224.Gigli-Bisceglia N., Engelsdorf T., Hamann T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020;77:2049–2077. doi: 10.1007/s00018-019-03388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Laskowski M., Grieneisen V.A., Hofhuis H., Hove C.A.T., Hogeweg P., Marée A.F.M., Scheres B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6:e307. doi: 10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sembada A.A., Fukuhara T., Suzuki T., Lenggoro I.W. Stem cutting: A novel introduction site for transporting water-insoluble particles into tomato (Solanum lycopersicum) seedlings. Plant Physiol. Biochem. 2024;206:108297. doi: 10.1016/j.plaphy.2023.108297. [DOI] [PubMed] [Google Scholar]
- 227.Tixier A., Cochard H., Badel E., Dusotoit-Coucaud A., Jansen S., Herbette S. Arabidopsis thaliana as a model species for xylem hydraulics: Does size matter? J. Exp. Bot. 2013;64:2295–2305. doi: 10.1093/jxb/ert087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mullendore D.L., Windt C.W., Van As H., Knoblauch M. Sieve tube geometry in relation to phloem flow. Plant Cell. 2010;22:579–593. doi: 10.1105/tpc.109.070094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rajput K.S., Patil V.S., Rao K.S. Wood anatomy and the development of interxylary phloem of Ipomoea hederifolia Linn. (Convolvulaceae) J. Plant Growth Regul. 2013;32:654–662. doi: 10.1007/s00344-013-9334-8. [DOI] [Google Scholar]
- 230.Antonova G.F., Stasova V.V. Seasonal development of phloem in Siberian larch stems. Russ. J. Dev. Biol. 2008;39:207–218. doi: 10.1134/S1062360408040024. [DOI] [PubMed] [Google Scholar]
- 231.Jacobsen A.L., Valdovinos-Ayala J., Rodriguez-Zaccaro F.D., Hill-Crim M.A., Percolla M.I., Venturas M.D. Intra-organismal variation in the structure of plant vascular transport tissues in poplar trees. Trees. 2018;32:1335–1346. doi: 10.1007/s00468-018-1714-z. [DOI] [Google Scholar]
- 232.Pal K., Rahaman C.H. Studies on foliar epidermal micromorphology, vegetative anatomy and xylem elements of four members of Protulacaceae. Int. J. Curr. Res. 2014;6:4968–4975. [Google Scholar]
- 233.Romero C., Bolker B.M., Edwards C.E. Stem responses to damage: The evolutionary ecology of Quercus species in contrasting fire regimes. New Phytol. 2009;182:261–271. doi: 10.1111/j.1469-8137.2008.02733.x. [DOI] [PubMed] [Google Scholar]
- 234.Gričar J., Lavrič M., Ferlan M., Vodnik D., Eler K. Intra-annual leaf phenology, radial growth and structure of xylem and phloem in different tree parts of Quercus pubescens. Eur. J. For. Res. 2017;136:625–637. doi: 10.1007/s10342-017-1060-5. [DOI] [Google Scholar]
- 235.Gričar J. Xylem and phloem formation in sessile oak from Slovenia in 2007. Wood Res. 2010;55:15–22. [Google Scholar]
- 236.Peuke A.D., Rokitta M., Zimmermann U., Schreiber L., Haase A. Simultaneous measurement of water flow velocity and solute transport in xylem and phloem of adult plants of Ricinus communis over a daily time course by nuclear magnetic resonance spectrometry. Plant Cell Environ. 2001;24:491–503. doi: 10.1046/j.1365-3040.2001.00704.x. [DOI] [Google Scholar]
- 237.Elsayed A.A., Ahmed E.G., Taha Z.K., Farag H.M., Hussein M.S., AbouAitah K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022;295:110851. doi: 10.1016/j.scienta.2021.110851. [DOI] [Google Scholar]
- 238.Dié A., Kitin P., Kouamé F.N.G., Van den Bulcke J., Van Acker J., Beeckman H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann. Bot. 2012;110:861–873. doi: 10.1093/aob/mcs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Santarosa E., de Souza P.V.D., de Araujo Mariath J.E., Lourosa G.V. Physiological interaction between rootstock-scion: Effects on xylem vessels in Cabernet Sauvignon and Merlot grapevines. Am. J. Enol. Vitic. 2016;67:65–76. doi: 10.5344/ajev.2015.15003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this work are included in this published article.