Abstract
The neisserial fbpABC locus has been proposed to act as an iron-specific ABC transporter system. To confirm this assigned function, we constructed an fbpABC mutant in Neisseria meningitidis by insertional inactivation of fbpABC with a selectable antibiotic marker. The mutant was unable to use iron supplied from human transferrin, human lactoferrin, or iron chelates. However, the use of iron from heme and human hemoglobin was unimpaired. These results support the obligatory participation of fbpABC in neisserial periplasmic iron transport and do not indicate a role for this genetic locus in the heme iron pathway.
The evolutionary success of bacterial pathogens may be considered to reside in their extraordinary talent to adapt to the environmental rigors imposed by their human host. This characteristic is exemplified by the bacterial mechanisms used to acquire iron (9, 11, 16, 22). The extracellular secretion of siderophores, low-molecular-weight iron chelators, represents a common means of securing this scarce but critical element (11). It is also becoming apparent that direct binding of host iron-containing proteins to specific receptors located on the bacterial cell surface constitutes another significant bacterial strategy for the capture of iron (9, 16, 22).
The pathogenic Neisseria spp., Neisseria gonorrhoeae and Neisseria meningitidis, produce no siderophores (28, 40). These organisms express an array of outer membrane receptors that specifically interact with human iron-bearing proteins. Genetic studies of the gonococcus and the meningococcus have indicated that iron acquisition from human transferrin and human lactoferrin is initiated by the binding of these glycoproteins to their respective receptors (9, 16). Similarly, the recently described neisserial hemoglobin receptors, HmbR (35, 36) and HpuB (7, 24), mediate the uptake of heme iron from hemoglobin and haptoglobin-hemoglobin complexes.
The events occurring subsequent to receptor binding of the iron ligands are unclear. Iron is released from transferrin and lactoferrin by unknown mechanisms and is deposited in the periplasmic space by a tonB-mediated process (37), presumably by gated pore properties of the lactoferrin and transferrin receptors energized by TonB protein (29). Biochemical data indicates that iron is subsequently transferred to the periplasmic iron-binding protein, FbpA (8). Sequence analysis and genetic complementation studies demonstrate that the gene encoding this protein, fbpA, belongs to an operon termed fbpABC, whose gene products have been proposed to behave as an ATP-binding cassette (ABC) transporter (1). These unidirectional transporter systems are ubiquitous in bacteria and function to deliver scarce nutrients from the periplasmic space into the cytosolic compartment (3, 13, 20). These systems are minimally comprised of three polypeptides, a ligand-specific periplasmic binding protein, one or two proteins embedded in the cytoplasmic membrane, and a membrane-associated nucleotide-binding protein (3, 13, 20). This latter feature is a hallmark of these systems and is responsible for the designation ABC transporter (3, 13, 20).
Similar genetic loci have been described for Haemophilus influenzae (31) and Serratia marcescens (4, 42). Mutation analysis and comparisons of the open reading frames to those of fbpABC indicate that the H. influenzae hitABC and S. marcescens sfuABC operons are functional equivalents of the neisserial fbpABC locus.
Therefore, this investigation was undertaken to address the proposed role of the fbpABC locus as a periplasmic iron transporter by first constructing a meningococcal fbpABC mutant and subsequently determining the phenotype of the mutant with respect to iron acquisition.
Bacterial strains, plasmids, and growth conditions.
The bacteria and plasmids used in this study are listed in Table 1. Neisserial strains were grown on chocolate agar at 37°C in an atmosphere of 5% CO2. Escherichia coli was cultured on Luria-Bertani (LB) agar plates or in LB broth. Transformation of neisserial (35) and E. coli (30) strains was performed as previously described. Antibiotics, where appropriate, were added at the following concentrations: kanamycin, 40 μg/ml; ampicillin, 100 μg/ml; and chloramphenicol, 15 μg/ml (Neisseria spp.) or 25 μg/ml (E. coli).
TABLE 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Relevant characteristics | Source |
|---|---|---|
| Strains | ||
| Neisseria gonorrhoeae | ||
| PID543 | Clinical isolate from a patient with pelvic inflammatory disease | R. Brunham |
| FA19 | 25a | |
| F62 | 5 | |
| Neisseria meningitidis | ||
| B16B6 | Clinical isolate; serogroup B, serotype 2a | C. Frasch |
| N16δFBPA | B16B6 with the cat cassette inserted in the NotI site of the fbpA gene | This study |
| Escherichia coli DH5αF′ | F′ φ80 dlacZΔM15 Δ(lacZYA-argF) U169 deoR recA1 endA1 hsdR (rK− mK+) supE44 thi-1 gyrA96 relA1 | Gibco BRL |
| Plasmids | ||
| pTnMax4 | Plasmid containing the mini-transposon TnMax4 with the cat gene | 17 |
| pSBGL | pUC13 containing the fbpA gene from N. gonorrhoeae F62 cloned into the SmaI site | 5 |
| pCR2 | Cloning vector, ampicillin resistance | Invitrogen |
| pCR2CmOFDU | pCR2 containing the cat cartridge PCR amplified from pTnMax4 | R. Bonnah |
| pCR2NGFA | pCR2 containing the cloned fbpA gene | This study |
| pT7-7 | Cloning vector, ampicillin resistance | 38 |
| pT7-7NGFA | pT7-7 with the fbpA gene cloned into the EcoRI site | This study |
| pT7-7NGFAC | pT7-7NGFA containing the cat cassette in the NotI site of the fbpA gene | This study |
| Primers (5′ to 3′) | ||
| 5′fbpA | CAGCCGTCTGAAAGGAATACACTACACCCG; 5′ oligonucleotide containing the gonococcal uptake sequence (in boldface type), for the amplification of fbpAB, nucleotide positions 45 to 70a | This study |
| 3′fbpB | GCGGTGCTTCCTCAAGCTG; 3′ oligonucleotide for the amplification of fbpAB, nucleotide positions 2707 to 2725a | This study |
| 180 | ATGAAAACATCTATCCGATACGCACTG; 5′ oligonucleotide for the amplification of fbpA, nucleotide positions 120 to 146a | 5 |
| 181 | AGGCAGGGTAAGCGGCAGGGCGATCAG; 3′ oligonucleotide for the amplification of fbpA, nucleotide positions 1228 to 1254a | 5 |
| 394 | TCGATCCCTTTAGGGTTCCGATT; 5′ oligonucleotide for the amplification of the cat gene from pTnMax4, nucleotide positions 216 to 238b | R. Bonnah |
| 394B | GAACGTGGACTCCAACGTC; 3′ oligonucleotide for DNA sequencing, nucleotide positions 404 to 422b | This study |
| 395 | AGTGAATTTCGCTGCCGGGT; 3′ oligonucleotide for the amplification of the cat gene from pTnMax4, nucleotide positions 1472 to 1492b | R. Bonnah |
| 403 | ATTTAGGAGAAAATCGATATGAAAACATCTATC; 5′ oligonucleotide for the amplification of fbpA, nucleotide positions 102 to 134a | This study |
Growth assays were conducted to determine the ability of meningococci to use various iron-containing compounds as the sole exogenous source of iron. A single colony was selected from an overnight growth of meningococci on chocolate agar and was used to inoculate 5 ml of brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) containing 100 μM of the iron chelator EDDA [ethylenediamine-di(o-hydroxyphenylacetic acid)]. The culture was grown in a shaking incubator at 37°C in the presence of 5% CO2 until mid-logarithmic growth was achieved. Aliquots were removed to inoculate fresh iron-starved BHI broth containing 100 μM of EDDA to a starting A600 of 0.05, as measured with a Pye Unicam PU8800 spectrophotometer, to which was added either iron-loaded human transferrin (98% iron saturated; Sigma Chemical Co., St. Louis, Mo.), iron-loaded human lactoferrin (98% iron saturated; Sigma), ferric nitrate, ferric chloride, ferric citrate, hemin, human hemoglobin, or bovine catalase. Growth was monitored hourly at A600 for 8 h. Each experiment was performed in triplicate.
The minimum concentration of ferric nitrate, ferric chloride, or ferric citrate required to support the growth of the wild-type meningococcal strain B16B6 in iron-limited BHI broth was determined in a series of titration experiments in which growth of B16B6 in iron-limited BHI broth was assessed in response to the addition of increasing amounts of the iron salt. Therefore, the concentration of the iron compound used, i.e., 5 μM FeCl3 or FeNO3, represents the concentration of these iron compounds added in excess of the concentration of the chelator EDDA.
DNA preparation and manipulation.
Gonococcal and meningococcal genomic DNA were prepared by standard methods (30). Isolation and purification of plasmid DNA were performed with Qiagen (Clarita, Canada) columns as described in the manufacturer’s specifications. Restriction endonuclease digestions, ligation reactions, and agarose gel electrophoresis were performed as described previously (36). PCR products were recovered from low-melting-point agarose gels and purified by using NuSorb columns. Chromosomal DNA purified from wild-type and mutant N. meningitidis strains was digested to completion with the restriction enzyme XhoI and probed by high-stringency Southern blot analysis (36) employing the digoxigenin chemiluminescent system (Boehringer Mannheim Canada). The DNA probes included the 1.3-kb cat cartridge from TnMax4 and the 1.2-kb fbpA gene.
PCR.
PCR amplification of chromosomal DNA from Neisseria spp. was performed in a 25-μl reaction volume containing 1× Taq reaction buffer, 1 U of Taq polymerase (Gibco BRL), 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (Gibco BRL), oligonucleotide primers at 0.5 μM each, and 50 ng of genomic DNA as template. Thirty cycles of amplification were performed with a Perkin-Elmer model 480 DNA thermal cycler. The reaction profile consisted of a 30-s denaturation step at 94°C, a 30-s primer annealing step at 52°C, and a 3-min primer extension step at 72°C.
DNA sequencing.
DNA sequencing was performed on PCR fragments by the dideoxynucleotide chain termination method (32) using a PRISM Ready Reaction Dye Cycle Sequencing kit (Applied Biosystems) with fluorescence-labelled M13 primers or with synthetic oligonucleotide primers based on known fbpA and cat sequences. All sequence reactions were run and analyzed on an Applied Biosystems 373A automated DNA sequencer.
Western immunoblotting.
Whole-cell meningococcal and gonococcal lysates, prepared by using a previously described procedure (5) from cultures grown under iron-limited conditions, were separated on a sodium dodecyl sulfate–10% polyacrylamide gel and electroblotted at 10 V of constant voltage for 12 h at 4°C onto polyvinylidene difluoride membranes (Immobilon-P, 0.45-μm pore size; Millipore Canada) in 25 mM Tris–192 mM glycine–20% (vol/vol) methanol (pH 8.3) by the method of Towbin et al. (39) with a Bio-Rad MiniTransblot apparatus. After saturating for unspecific binding sites with a buffer containing 0.5% (wt/vol) skim milk in 50 mM Tris-HCl (pH 7.5)–1 M NaCl (TBS-M) for 3 h at 25°C, the membrane was probed with a 1:2,000 dilution of an affinity-purified polyclonal antiserum to the gonococcal FbpA (5) (kindly provided by T. Mietzner). After incubating at 35°C for 1 h, the immunoblot was rinsed with three 5-min washes of TBS-M. A 1:2,500 dilution of the second antibody (goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate; Pierce Chemical Co.) was allowed to bind for 1 h at 35°C, and this was followed by three 5-min washes with TBS-M. The membrane was developed with a chloronaphthol-hydrogen peroxide substrate mixture (horseradish peroxidase reagent; Bio-Rad) for 20 min. The paper was washed with water to stop the reaction.
Whole-cell transferrin and lactoferrin binding assays.
Whole-cell dot enzyme assays to detect binding to human transferrin and to human lactoferrin were performed as described previously (23).
Construction of the neisserial fbpABC mutant.
The wild-type fbpA gene was cloned by PCR amplification from the recombinant plasmid pSBGL, which contains the full-length fbpA gene from gonococcal strain F62 (5). Site-specific primers were designed to encompass the entire coding region of fbpA, including the native Shine-Dalgarno region (primer 403) and 140 bp downstream of the fbpA stop codon (primer 181, modelled on primer F5 [5]). The PCR-amplified fbpA gene was ligated into the TA cloning vector pCR2 (Invitrogen, San Diego, Calif.), creating pCR2NGFA. The fbpA gene in pCR2NGFA was subcloned into the EcoRI site in plasmid pT7-7 (38), taking advantage of the flanking EcoRI sites surrounding the fbpA gene provided by the multiple cloning site of the pCR2 vector. A clone which possessed the fbpA gene in the same orientation as the T7 promoter in pT7-7 was designated pT7-7NGFA. The absence of a NotI restriction site in pT7-7 facilitated the subsequent ligation of the cat gene, encoding chloramphenicol acetyltransferase, into fbpA.
The EagI fragment carrying the cat cassette was excised from pCR2CmOFDU, and the ends were filled in with the Klenow fragment of DNA polymerase I. The cat cassette was inserted into the unique NotI site that is present in the middle of fbpA by first digesting pT7-7NGFA with NotI, followed by blunt-ending the linearized plasmid with the Klenow fragment of DNA polymerase I and ligating the fragments with T4 DNA ligase. The resultant plasmid, designated pT7-7NGFAC, placed the cat cassette in the same transcriptional orientation as that of the fbpA gene. This plasmid was used to transform competent gonococcal and meningococcal strains.
Plasmid pCR2CmOFDU had been previously prepared by PCR amplification of the cat cartridge, using oligonucleotide primers 394 and 395 (R. Bonnah, University of Calgary, Calgary, Alberta, Canada), from pTnMax4 (17), a Tn1721-based minitransposon. The PCR-generated product is composed of a promoterless Tn9 cat gene placed under the transcriptional control of the opacity gene promoter of gonococcal strain MS11 (34), and an fd-terminator sequence. The antibiotic cassette is flanked by an orifd origin of replication at the 5′ end and by a 60-bp sequence carrying the 10-bp gonococcal DNA uptake signal (15) immediately downstream of the transcriptional terminator. The remaining elements of TnMax4, comprising the 38-bp inverted repeats of Tn1721, the res sequence, and the phoA gene, are not incorporated in this fragment. This cat cartridge was subsequently cloned into a pCR2 vector, creating plasmid pCR2CmOFDU. Flanking EagI restriction sites in pCR2CmOFDU facilitated the subcloning of this cat construct into pT7-7NGFA.
All intermediate plasmid constructs were transformed into E. coli DH5αF′ by established methods (25). The pCR2 and pT7-7 plasmid constructs were selected on ampicillin-containing LB agar plates. E. coli transformants containing plasmid pT7-7NGFAC were selected by using LB agar plates containing ampicillin and chloramphenicol.
Identification of neisserial fbpABC mutants.
Gonococcal strains PID543, F62, and FA19 and meningococcal strain B16B6 were transformed with plasmid pT7-7NGFAC that was linearized with BamHI. After 5 h of phenotypic expression, cells were plated on chocolate agar plates containing chloramphenicol. Three chloramphenicol-resistant colonies derived from meningococcal strain B16B6 were observed within 36 h. These colonies were transferred onto fresh chocolate agar-chloramphenicol plates, and one of these, designated N16δFBPA, was selected for further study. No gonococcal transformants were detected despite prolonged incubation of 50 h.
Three methods were used to confirm that the proper allelic exchange had occurred in the mutant strain. First, the chromosomal DNAs purified from wild-type strain B16B6 and from mutant strain N16δFBPA were digested to completion with the restriction enzyme XhoI. The DNA fragments were hybridized against either cat- or fbpA-specific gene probes by Southern blot analysis. The fbpA gene probe, a 1.2-kb fragment comprising the entire coding sequence of the fbpA gene, hybridized to an approximately 19-kb fragment derived from the wild-type strain, whereas the mutant strain displayed a hybridizing fragment of approximately 20.3 kb (data not shown). The increase in size of the 20.3-kb fragment corresponds to the presence of the cat cartridge in the fbpA locus of the mutant strain, as indicated by hybridization of this same fragment to the cat-specific probe (data not shown). As expected, the wild-type strain did not bind to this probe (data not shown).
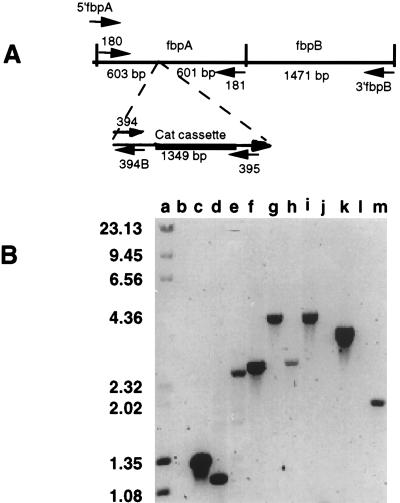
The second method of verifying that the appropriate gene replacement had occurred consisted of PCR amplification analysis using a variety of fbpA-, fbpAB-, and cat-specific primers (Table 1; Fig. 1A) and chromosomal preparations from the wild-type strain B16B6 and from the fbpA mutant as templates. Primers designed to anneal to sequences bracketing the cat cartridge would be predicted to generate a larger product from the fbpA mutant than from the wild-type strain. Primers engineered to the extreme ends of the fbpAB sequence amplified a product that was 1.35-kb larger in N16δFBPA than in B16B6 (Fig. 1B, lanes f and g, oligonucleotides 5′fbpA and 3′fbpB; Fig. 1B, lanes h and i, oligonucleotides 180 and 3′fbpB). Using primer pair 180/181, which amplifies the intact fbpA, PCR amplification demonstrated that the 1.35-kb fragment had inserted into the fbpA locus (Fig. 1B, lanes d and e).
FIG. 1.
PCR amplification based analysis of fbpA from the wild-type and mutant strains of N. meningitidis B16B6. (A) Schematic diagram of the neisserial fbpA and fbpB genes displaying the orientations and approximate positions of the oligonucleotide primers used in the PCR amplification assay. (B) Agarose gel demonstrating the PCR products amplified by the oligonucleotide primer pairs. Lanes: b, d, f, h, j, and l, B16B6; c, e, g, i, k, and m, N16δFBPA. Primer pairs: 394-395 (lanes b and c), 180-181 (5′fbpA-3′fbpB (lanes f and g), 180-3′fbpB (h and i), 394-3′fbpB (lanes j and k), 395-5′fbpA (lanes l and m). The sizes of standards in kilobases are shown on the left. Figures were imaged with a Hewlett-Packard ScanJet IIp and labelled by using Adobe Photoshop 3.0.
Reactions primed with the cat-specific oligonucleotide 395 and oligonucleotide 5′fbpA (Fig. 1B, lanes l and m) indicated that insertion of the cat cartridge at the NotI site of fbpA was responsible for the increase in size of the fbpA locus in N16δFBPA. The reciprocal reaction using the cat-specific oligonucleotide 394 and oligonucleotide 3′fbpB (Fig. 1B, lanes j and k) generated the expected 3.4-kb product from N16δFBPA, indicating that the cat cassette was inserted in the same orientation as the fbpA gene sequence. No PCR product was obtained when the wild-type chromosomal DNA was used as template (Fig. 1B, lanes j and l) because of the absence of the cat cassette in the wild-type strain. The presence of a 1.35-kb PCR product only in N16δFBPA in reactions using the cat-specific primers 394 and 395 confirms this conclusion (Fig. 1B, lanes b and c).
Finally, limited sequencing of the mutated fbpA gene from N16δFBPA was performed by using oligonucleotide primer 394B, which is complementary to the 5′ end of the cat cassette. The location of the fbpA-5′ cat junction was correctly identified by analysis of the nucleotide sequence (data not shown).
Therefore, from these data, we conclude that the fbpA::cat construct has recombined appropriately onto the chromosome of strain B16B6. Furthermore, a single insertion of the cat cassette into fbpA has occurred, and no gross rearrangements or deletions have resulted.
FbpA expression.
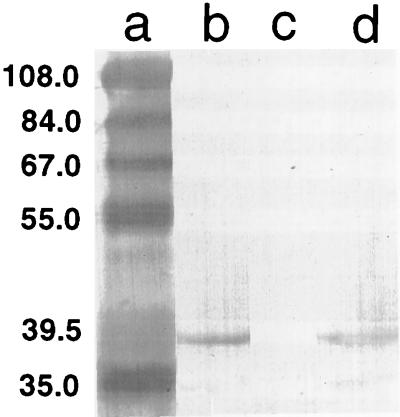
To confirm the absence of FbpA in the mutant strain, whole-cell lysates were reacted with FbpA-specific polyclonal antiserum in Western immunoblots. The antibody recognized a 37-kDa band corresponding to FbpA in the wild-type meningococcal strain B16B6 (Fig. 2, lane b) and in gonococcal strain PID543 (Fig. 2, lane d). In contrast, no immunoreactive band was observed in the mutant strain N16δFBPA (Fig. 2, lane c). These results indicate that FbpA expression was abolished in the mutant.
FIG. 2.
Identification of FbpA protein from the wild-type and mutant strains of N. meningitidis B16B6. Whole-cell extracts, prepared from the parental strain B16B6 (lane b), the fbpABC mutant strain N16δFBPA (lane c), and N. gonorrhoeae PID543 (lane d) that were grown under iron-restricted conditions, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. The blot was probed with a polyclonal antiserum directed against the gonococcal FbpA. Positions of protein standards in kilodaltons are indicated in lane a. Figures were imaged with a Hewlett-Packard ScanJet IIp and labelled by using Adobe Photoshop 3.0.
Growth assays.
Growth assays were conducted to determine the ability of the fbpABC mutant to use various iron compounds as the sole exogenous source of iron. All cultures were examined at 22 h of growth. Stationary phase was attained at this time in all cultures (data not shown). The kinetics of growth were identical to those observed at 8 h (data not shown).
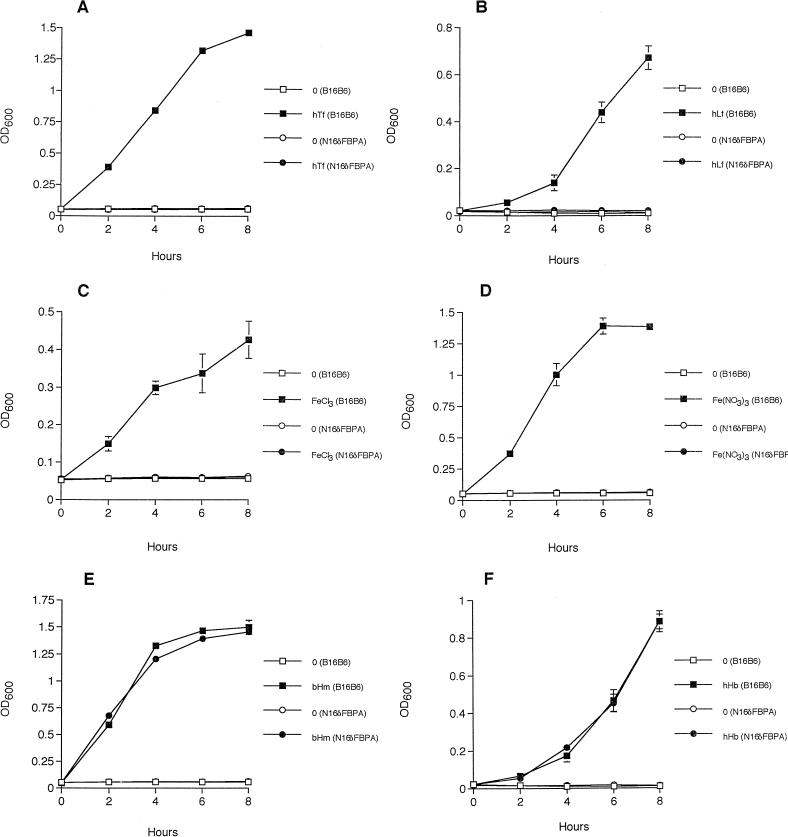
The fbpABC mutant was incapable of growth in iron-limited BHI broth when iron was supplied as human transferrin (Fig. 3A), human lactoferrin (Fig. 3B), or the iron salts ferric nitrate (Fig. 3C), ferric chloride (Fig. 3D), and ferric citrate (data not shown). The presence of both transferrin and lactoferrin binding activities in the fbpABC mutant at levels that were equivalent to those seen in the wild-type strain (data not shown) indicates that a functional loss of these receptors is not responsible for the inability of the mutant to use these glycoproteins as iron sources. No lactoferrin or transferrin binding activity was observed in the E. coli DH5αF′ control sample (data not shown).
FIG. 3.
Growth of the wild-type and mutant strains of N. meningitidis B16B6 in iron-limited BHI broth supplemented with various iron compounds. The parental isolate B16B6 and the fbpABC mutant strain N16δFBPA were grown in the presence of 8 μM iron-loaded human transferrin (hTf) (A), 8 μM iron-loaded human lactoferrin (hLf) (B), 5 μM ferric chloride (C), 5 μM ferric nitrate (D), 2 μM bovine hemin (bHm) (E), and 0.5 μM human hemoglobin (hHb) (F). Each value represents the mean of three replicate cultures. Standard deviations of the mean are shown by error bars. The absence of error bars indicates that the standard deviation of the three replicate cultures was too small to be represented on the graph.
In contrast, the ability of the fbpABC mutant to assimilate iron from heme (Fig. 3E), human hemoglobin (Fig. 3F), and bovine catalase (data not shown) was unimpaired when compared to that of the wild-type strain B16B6. The addition of 2 μM human serum albumin to the hemoglobin-containing cultures did not inhibit growth (data not shown). Since human serum albumin binds free hemin, the addition of this compound permitted the distinction of growth due to hemoglobin from growth due to hemin that had dissociated from hemoglobin. N. meningitidis is incapable of using hemin complexed to albumin as an iron source (14).
This study represents the first successful construction of a viable mutation in the neisserial fbpA gene. Such a claim is supported by three lines of evidence. First, limited nucleotide sequence analysis indicates that the cat cassette has been cloned into the wild-type fbpA gene. Second, Southern blot hybridization and PCR amplification-based analyses show that in the mutant, a single chromosomal copy of the cat cartridge has been inserted in fbpA, confirming the allelic replacement of the wild-type gene in the mutant strain. Finally, in a Western blot assay in which whole-cell lysates were probed with FbpA-specific polyclonal antiserum, no fbpA gene product was detected in the mutant.
The approach of using a gonococcal construct to mutagenize a meningococcal homolog derives from the demonstration that interspecies recombination occurs among Neisseria spp. (33, 41). The reverse strategy of using an insertionally inactivated meningococcal gene to construct a gonococcal mutant has been successfully applied to produce a gonococcal hemoglobin receptor mutant deficient in the expression of the HpuB protein (7).
In the pathogenic Neisseria spp., the accumulated evidence has led to the proposal that FbpA shuttles iron across the periplasm into the cytosol (1, 8). Since its iron transport function occurs independently of TonB-mediated iron translocation across the outer membrane (2), the fbpABC operon may serve a global role in the delivery of iron to the cytoplasmic compartment. This supposition predicts that the absence of a functional FbpA would severely restrict the ability of such a cell to assimilate iron. The fbpABC mutant exhibited such a phenotype since human transferrin, human lactoferrin, ferric nitrate, and ferric chloride were incapable of serving as iron sources for the organism. Particular attention was paid to limit the concentration of the supplied iron compounds to a level that would ensure that the iron requirements of the mutant would be satisfied only through a high-affinity pathway, such as would be provided by the fbpABC operon. This iron-deficient phenotype recapitulates the phenotype displayed by a hitA mutant in H. influenzae (21).
The presence of a transcriptional terminator at the 3′ end of the cat cartridge precludes a rigorous assignment of this phenotype solely to the FbpA protein because a polar effect on the transcription of the gene products from the remainder of the fbpABC operon is probable. However, the inability of either hitA (21) or hitC (31) mutants in H. influenzae to use iron suggests that the functional absence of any one of the components of the ABC transporter will abolish the function of the transporter. Therefore, these results strongly support the pivotal role of the fbpABC operon in neisserial periplasmic iron transport.
The mechanism of neisserial heme iron acquisition is postulated to entail a complex multistep receptor-mediated process (22, 35). Although the entire repertoire and specificity of these surface receptors remain to be delineated, it is believed that receptor-bound heme is released intact into the periplasmic space (22, 35) and subsequently conveyed to the cytosol by a dedicated ABC transporter (22, 35). Passage across the outer membrane does not appear to be an exclusively TonB-dependent process (37).
The results from a prior biochemical investigation, showing that 59Fe associates with FbpA subsequent to the binding of radiolabelled hemin to the gonococcal cell surface (12), have formed the basis for an alternative version of heme uptake. This model contends that iron is removed from the porphyrin ring of heme prior to entry into the periplasm. By ferrying the liberated iron into the cytosol, FbpA would play a role in the periplasmic transport of iron derived from heme.
However, our observation that the fbpABC mutant was unimpaired in its ability to access heme iron for growth unambiguously indicates that the fbpABC operon is not involved in heme iron uptake. This result provides a cogent genetic argument in favor of a heme periplasmic transporter that is functionally distinct from FbpABC. Indeed, functional disruption of the homologous hitABC operon by the insertional inactivation of the hitC locus resulted in a mutant that retained the ability to use heme as an iron source (31). Implicit in this model is that the entire heme molecule enters intact into the cell. This contention is supported by uptake studies using double-labelled heme in the heme-obligate bacterium H. influenzae (10) and by genetic complementation studies with Shigella dysenteriae (27) and Vibrio cholerae (18, 19).
The crystal structure of hFBP, the ferric-ion-binding protein of H. influenzae, has recently been determined (6a). The geometry of the iron binding site indicates that a molecule of the size of heme would not be accommodated. Since all of the iron-ligand residues of hFBP are conserved in the neisserial FbpA protein, and because both proteins have an amino acid sequence identity of 72% (6a), the iron-binding sites of these two proteins are likely similar. Therefore, structural considerations provide an additional strong argument precluding the involvement of fbpABC in heme acquisition.
In summary, we have constructed a meningococcal fbpABC mutant. By determining the iron utilization phenotype of the fbpABC mutant, we have established the crucial role of this locus in the periplasmic transport of iron derived from human transferrin, human lactoferrin, and iron chelates. Furthermore, the observation that the fbpABC mutant retained the ability to use heme as an iron source suggests the existence of an analogous heme transporter system. The genetic (6), structural, and immunological (26) conservation of FbpABC in the pathogenic Neisseria spp. suggests that the results from this investigation are applicable to N. gonorrhoeae.
Acknowledgments
This work was supported by a grant (MT-12670) from the Medical Research Council of Canada. S.D.K. is the recipient of a studentship from the Alberta Heritage Foundation for Medical Research.
We are grateful to R. Bonnah for the generous gift of the cat gene construct.
REFERENCES
- 1.Adhikari P, Berish S A, Nowalk A J, Veraldi K L, Morse S A, Mietzner T A. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol. 1996;178:2145–2149. doi: 10.1128/jb.178.7.2145-2149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari P, Kirby S D, Nowalk A J, Veraldi K L, Schryvers A B, Mietzner T A. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J Biol Chem. 1995;270:25142–25149. doi: 10.1074/jbc.270.42.25142. [DOI] [PubMed] [Google Scholar]
- 3.Ames G F-L. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- 4.Angerer A, Gaisser S, Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990;172:572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berish S A, Chen C-Y, Mietzner T A, Morse S A. Expression of a functional neisserial fbp gene in Escherichia coli. Mol Microbiol. 1992;6:2607–2615. doi: 10.1111/j.1365-2958.1992.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 6.Berish S A, Kapczunski D, Morse S A. Sequence of the meningococcal Fbp gene. Nucleic Acids Res. 1990;18:4596. doi: 10.1093/nar/18.15.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Bruns C M, Nowalk A J, Arvai A S, McTigue M A, Vaughan K G, Mietzner T A, McRee D E. Structure of Haemophilus influenzae Fe+3-binding protein reveals convergent evolution within a superfamily. Nat Struct Biol. 1997;4:919–924. doi: 10.1038/nsb1197-919. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-J, Sparling P F, Lewis L A, Dyer D W, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C-Y, Berish S A, Morse S A, Mietzner T A. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol. 1993;10:311–318. doi: 10.1111/j.1365-2958.1993.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 10.Coulton J W, Pang J C S. Transport of hemin by Haemophilus influenzae type b. Curr Microbiol. 1983;9:93–98. [Google Scholar]
- 11.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai P J, Nzeribe R, Genco C A. Binding and accumulation of hemin in Neisseria gonorrhoeae. Infect Immun. 1995;63:4634–4641. doi: 10.1128/iai.63.12.4634-4641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doige C A, Ames G F-L. ATP-dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Annu Rev Microbiol. 1993;47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- 14.Dyer D W, West E P, Sparling P F. Effects of serum carrier proteins on the growth of pathogenic neisseriae with heme-bound iron. Infect Immun. 1987;55:2171–2175. doi: 10.1128/iai.55.9.2171-2175.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–190. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 17.Haas R, Kahrs A F, Facius D, Allmeier H, Schmitt R, Meyer T F. TnMax: a versatile mini-transposon for the analysis of cloned genes and shuttle mutagenesis. Gene. 1993;130:23–31. doi: 10.1016/0378-1119(93)90342-z. [DOI] [PubMed] [Google Scholar]
- 18.Henderson D P, Payne S M. Cloning and characterization of the Vibrio cholerae genes encoding the utilization of iron from haemin and haemoglobin. Mol Microbiol. 1993;7:461–469. doi: 10.1111/j.1365-2958.1993.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 19.Henderson D P, Payne S M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994;176:3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 21.Kirby S D, Gray-Owen S D, Schryvers A B. Characterization of a ferric-binding-protein mutant in Haemophilus influenzae. Mol Microbiol. 1997;25:979–987. doi: 10.1111/j.1365-2958.1997.mmi535.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee B C. Quelling the red menace: heme capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee B C, Hill P. Identification of an outer membrane haemoglobin-binding protein in Neisseria meningitidis. J Gen Microbiol. 1992;138:2647–2656. doi: 10.1099/00221287-138-12-2647. [DOI] [PubMed] [Google Scholar]
- 24.Lewis L A, Dyer D W. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liss L R. New M13 host: DH5αF′ competent cells. BRL Focus. 1987;9:13. [Google Scholar]
- 25a.Mickelsen P A, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mietzner T A, Barnes R C, JeanLouis Y A, Shafer W M, Morse S A. Distribution of an antigenically related iron-regulated protein among the Neisseria spp. Infect Immun. 1986;51:60–68. doi: 10.1128/iai.51.1.60-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norrod E P, Williams R P. Growth of Neisseria gonorrhoeae in medium deficient in iron without detection of siderophores. Curr Microbiol. 1978;1:281–284. [Google Scholar]
- 29.Rutz J M, Liu J, Lyons J A, Goranson J, Armstrong S K, McIntosh M A, Feix J B, Klebba P E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sanders J D, Cope L D, Hansen E J. Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun. 1994;62:4515–4525. doi: 10.1128/iai.62.10.4515-4525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spratt B G, Bowler L D, Zhang Q-Y, Zhou J, Maynard Smith J. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 34.Stern A, Brown M, Nickel P, Meyer T F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 35.Stojiljkovic I, Hwa V, de Saint Martin L, O’Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 36.Stojiljkovic I, Larson J, Hwa V, Anjic S, So M. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stojiljkovic I, Srinivasan N. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J Bacteriol. 1997;179:805–812. doi: 10.1128/jb.179.3.805-812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabor S. Expression using the T7 RNA polymerase/promoter system. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. 6–10. [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West S E H, Sparling P F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J J, Spratt B G. Sequence diversity within the argF, fbp and recA genes of natural isolates of Neisseria meningitidis: interspecies recombination within the argF gene. Mol Microbiol. 1992;6:2135–2146. doi: 10.1111/j.1365-2958.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann L, Angerer A, Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989;171:238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]