Abstract
Since they are difficult and sometimes impossible to treat, infections sustained by multidrug-resistant (MDR) pathogens, emerging especially in nosocomial environments, are an increasing global public health concern, translating into high mortality and healthcare costs. In addition to having acquired intrinsic abilities to resist available antibiotic treatments, MDR bacteria can transmit genetic material encoding for resistance to non-mutated bacteria, thus strongly decreasing the number of available effective antibiotics. Moreover, several pathogens develop resistance by forming biofilms (BFs), a safe and antibiotic-resistant home for microorganisms. BFs are made of well-organized bacterial communities, encased and protected in a self-produced extracellular polymeric matrix, which impedes antibiotics’ ability to reach bacteria, thus causing them to lose efficacy. By adhering to living or abiotic surfaces in healthcare settings, especially in intensive care units where immunocompromised older patients with several comorbidities are hospitalized BFs cause the onset of difficult-to-eradicate infections. In this context, recent studies have demonstrated that quaternary ammonium compounds (QACs), acting as membrane disruptors and initially with a low tendency to develop resistance, have demonstrated anti-BF potentialities. However, a paucity of innovation in this space has driven the emergence of QAC resistance. More recently, quaternary phosphonium salts (QPSs), including tri-phenyl alkyl phosphonium derivatives, achievable by easy one-step reactions and well known as intermediates of the Wittig reaction, have shown promising anti-BF effects in vitro. Here, after an overview of pathogen resistance, BFs, and QACs, we have reviewed the QPSs developed and assayed to this end, so far. Finally, the synthetic strategies used to prepare QPSs have also been provided and discussed to spur the synthesis of novel compounds of this class. We think that the extension of the knowledge about these materials by this review could be a successful approach to finding effective weapons for treating chronic infections and device-associated diseases sustained by BF-producing MDR bacteria.
Keywords: multidrug resistant (MDR) microorganisms, biofilm, biofilm life cycle, anti-biofilm agents, cationic antimicrobial agents, quaternary ammonium salts (QASs), quaternary phosphonium salts (QPSs)
1. Antimicrobial Resistance
Antibiotics have been an essential tool of modern medicine to fight illnesses, but antibiotic treatment is becoming increasingly unreliable as antimicrobial resistance (AMR) has evolved in many bacterial pathogens [1]. Antimicrobial resistance happens when germs like bacteria, fungi, and viruses develop the ability to defeat the drugs designed to kill them [2]. Despite the use of antibiotics initially functioning, germs that have developed resistance continue to grow, making infections increasingly difficult, and sometimes impossible, to treat [3]. Antimicrobial resistance is a naturally occurring process promoted mainly by the misuse and overuse of antimicrobials, thus causing the need for costly new treatments to be developed [4]. Antimicrobial-resistant organisms continue to take thousands of lives per year, including a wide variety of people, such as those within the medical community, public health officials, food producers, pharmaceutical developers, and the general public. Antimicrobial resistance is an urgent global public health threat, with the forecast of 10 million deaths per year globally by 2050 [4]. Gram-negative bacteria, such as Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Burkholderia cepacian, and Escherichia coli, are responsible for severe infections including pneumonia, bloodstream infections, wound or surgical site infections, and meningitis in healthcare settings and especially in intensive care units where immunocompromised older patients with several comorbidities are hospitalized [5]. Since antibiotics from the carbapenem family are now the last line of defense against antibiotic-resistant bacterial infections, carbapenem-resistant Enterobacteriaceae, extensively drug-resistant (XDR) P. aeruginosa and XDR A. baumannii are the most clinically relevant resistance phenotypes [5].
Antibiotic degradation, antibiotic target modification, modulation of membrane permeability, structural modifications of bacterial lipopolysaccharide, hyper-expression of efflux pumps, as well as the formation of biofilms (BFs) are some of the recognized mechanisms used by bacteria to develop resistance [6]. Table S1 in the Supplementary Materials reports some of the most clinically relevant MDR pathogens with their associated infections [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
To ameliorate this worrying scenario, where antibacterial resistance is incessantly increasing, the available effective antibiotics are decreasing dramatically, and infections sustained by BF-producing pathogens are practically untreatable, researchers have focused mainly on developing drugs acting on contact as membrane disruptors that therefore have a low tendency to develop resistance. In this context, quaternary ammonium salts (QASs) have demonstrated promising biocidal effects and anti-BF potentialities [39,40,41,42,43,44]. Unfortunately, their continuous use over more than 80 years has driven the emergence of QAS resistance [45]. In this regard, quaternary phosphonium salts (QPSs), including tri-phenyl alkyl phosphonium derivatives, achievable by easy, low-cost, one-step reactions and already known as intermediates of the Wittig reaction, have shown promising biocidal and anti-BF effects in vitro. In this paper, to draw more attention to QPSs, which are still too little studied by researchers, after having provided essential information on pathogen resistance and BFs and a discussion on QASs, we have reviewed the QPSs developed and assayed to this end in recent years. Finally, to spur the synthesis of novel compounds of this class, potentially active against BFs, the synthetic strategies used to prepare the antibacterial QPSs developed so far have been also provided and discussed.
2. Biofilms: The Antibiotic-Resistant Home Self-Produced by Microorganisms
A particularly worrying form of pathogen resistance is represented by biofilms (BFs), which are a safe and antibiotic-resistant home to microorganisms [46]. BFs are well-organized heterogeneous communities of self-immobilized microorganisms, attached to each other and to a surface, which are encased within self-produced extracellular polymeric substances (EPSs). EPSs protect them from environmental stressors, including extreme conditions of pH, oxygen, osmotic shock, heat, freezing, UV radiation, predators, disinfectants, and antibiotics [47]. In BFs, cell–cell interactions are facilitated, including those that allow the transfer of resistance genes, and microorganisms cooperate in a synchronized way to capture resources, such as nutrients [7,48]. BFs are a lifestyle for 40–80% of prokaryotic or unicellular eukaryotic cells [49], representing an incubator for genotypic and phenotypic variants [48]. In fact, BFs should be regarded as a multicellular superorganism where sessile cells, persistent cells, and dormant cells, with physiological characteristics differentiating them from planktonic cells, cohabit and communicate by the quorum sensing (QS) system [7,48]. The extracellular polymeric matrix in which BF-producing cells are encased consists of extracellular polymeric substances (EPSs), including a combination of enzymatic proteins, polysaccharides (cellulose, poly-glucosamine (PGA), and exopolysaccharides), extracellular DNA (eDNA), as well as cationic and anionic glycoproteins and glycolipids, which allow real communication between the bacteria and stabilize the three-dimensional structure of the BF itself [50,51]. Table S2 in the Supplementary Materials schematizes the general composition of BF biomass.
EPSs not only protect bacteria from external dangers but also provide them with the necessary nourishments and water, which is retained by means of H bonds with hydrophilic polysaccharides. Depending on the required nutrients, the microorganisms in a BF can change the composition of the EPSs by secreting proper signaling molecules [50,51]. Although beneficial microorganisms could form BFs associated with human, animal, and plant hosts as an essential part of the holobiont [52,53], BFs produced by pathogens are responsible for about 80% of bacterial infections [54], which are often extremely difficult to treat due to the specific protection mechanisms provided by the BF to bacterial cells, immobilized within the EPSs and not reachable by antibiotics [55]. Indeed, BF-producing cells are 10–1000-fold less susceptible to various antimicrobial agents than their planktonic forms. BF-related infections can be divided into device-associated infections and non-device-associated ones. Table 1 collects the most relevant BF-producing pathogens and their related infections.
Table 1.
Most clinically relevant BF-producing bacteria and related infections.
| Device-associated | Type of Device | Typical Bacterial Species | Infection | Ref. |
| Contact lenses |
E. coli P. aeruginosa S. aureus S. epidermidis Candida spp. Serratia spp. Proteus spp. |
Keratitis | [56,57,58] | |
| Central venous catheters |
P. aeruginosa Enterobacteriaceae Klebsiella spp. |
Bacteremia | [57,58,59,60] | |
| Mechanical heart valves |
Streptococci S. aureus S. epidermidis Bacillus spp. Enterococci Candida spp. |
PVE | [56,57,58] | |
| Peritoneal dialysis catheters |
Staphylococci
P. aeruginosa E. coli |
CESI TI PI |
[61] | |
| Prosthetic joints |
S. aureus CN Staphylococci Propionibacterium spp. Enterococcus spp. Gram-negative bacilli Beta-hemolytic streptococci |
PPI | [62] | |
| Pacemakers |
S. aureus CN S. aureus E. coli K. pneumoniae P. aeruginosa A. baumannii |
Endocarditis | [63] | |
| Urinary catheters |
E. coli
Enterococcus faecalis S. epidermidis P. aeruginosa Proteus mirabilis K. pneumoniae |
UTI | [57,58,64,65] | |
| Voice prostheses |
Streptococcus mitis
Streptococcus sobrinus S. aureus P. aeruginosa |
TTI | [66] | |
| Non-device-associated | N.E. |
P. aerobicus
Fusobacterium nucleatum |
Periodontitis | [67,68,69] |
| Different Bacteria Fungi |
Osteomyelitis | [70] | ||
|
Haemophilus influenzae
S. pneumoniae |
Otitis media | [71] | ||
|
Fusobacterium
Streptococcus Veillonella Actinomyces Prevotella Porphyromonas Neisseria Eubacteria Treponema Lactobacterium Haemophilus Eikenella Capnocytophaga Peptostreptococcus Leptotrichia Propionibacterium Staphylococcus |
Dental plaque | |||
| Uropathogenic E. coli (UPEC) K. pneumoniae E. faecalis |
UTI | |||
| P. aeruginosa | Cystic fibrosis | |||
|
Viridans group Streptococci Staphylococcus spp. |
Infective endocarditis |
|||
| Group A Streptococci | Tonsillitis | |||
| Group A Streptococci | Necrotizing fasciitis | |||
| Gram-negative bacilli | Infection kidney stone |
UTI = Urinary tract infections; PVE = Prosthetic valve endocarditis; CESI = catheter exit site infections; TI = tunnel infections; PI = peritoneum infections; PPI = peri-prosthetic infection; CN = Coagulase-negative; TTI = tracheoesophageal tract infection.
2.1. Biofilm Formation and Dispersal
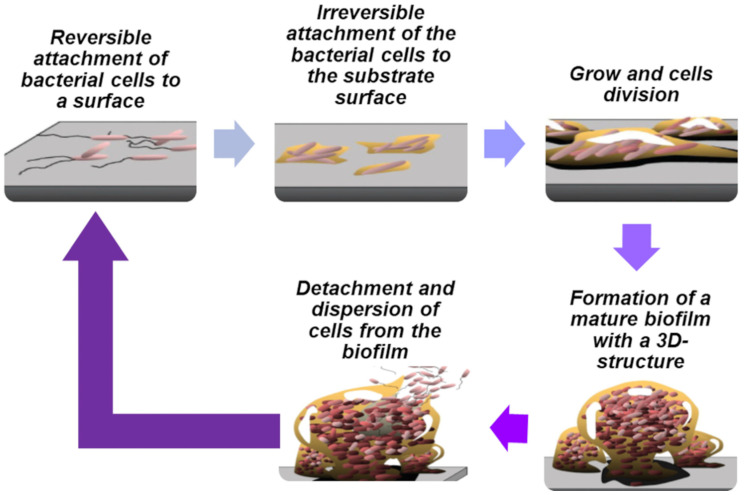
Bacteria can exhibit a free-living (mobile) planktonic state of living and a sessile (non-mobile) biofilm-living state when adhered to a substrate [72]. The in vitro BF life cycle involves the different stages reported in Table S3 in the Supplementary Materials [73] and visualized in Figure 1.
Figure 1.
Biofilm life cycle (reworked from the image reported in the work by Monroe et al. [74], an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium.
At stage 5 concerning the detachment and dispersion of cells from the BF, the initiation of new BF formation has been observed. Dispersed cells are morphologically more like planktonic cells than those of mature BFs and are crucial for developing into a new BF [61]. Reproducible periodic BF dispersal in bacterial species such as Actinobacillus spp., Actinomycetes comitans [75], P. aeruginosa [76], and Serratia marcescens [77] has been reported. The main factors, cues, and signals that induce BF detachment include temperature, pH, enzymatic degradation, starvation or excess of nutrients, presence of D-amino acids, nitric oxide, biosurfactants, and QS signaling molecules. In vitro and in vivo experiments have shown that the cells liberated by BFs are extremely cytotoxic to macrophages, more sensitive to iron diminution, and significantly more virulent to nematode hosts than planktonic bacteria. Additionally, it was reported that dispersed bacteria derived from BFs treated with glycoside hydrolase rapidly induced fatal septicemia in a mouse chronic wound infection model.
2.1.1. Quorum Sensing (QS) in Bacterial Biofilms
The formation of BF is governed by physiological processes and by the coordinated activities of the population of germs, which communicate with each other mainly through the quorum sensing (QS) machine [78]. Particularly, QS is a specific intercellular communication method based on the release of signal molecules that diffuse in BFs and are detected by other microorganisms, which in turn respond. QS machinery plays a vital role in BF formation, exopolysaccharide synthesis, motility, and chemotaxis, which are imperative for bacteria during the degradation or detoxification of any pollutant [78]. QS, first reported in the marine bioluminescent bacterium Vibrio fischeri [79], is currently the paramount target of strategies to control BF formation by disrupting cell-to-cell communication, conjugation, nutrient acquisition, and even motility and production of certain metabolites [78]. QS signaling molecules are different in Gram-positive and Gram-negative bacteria. There are at least three types of QS systems, including the acyl-homoserine lactone system (AHL) in Gram-negative bacteria, the autoinducing oligopeptide (API) QS system in Gram-positive bacteria, and the LuxS-encoded autoinducer-2 QS system (AI-2) in both species [80]. AHL and API QS systems, which are based on signaling molecules such as acyl-homoserine lactones and small peptides, respectively, are planned specifically for “intraspecies” signaling. An increase in AHL molecules corresponds to high bacterial growth [81], while high concentrations of APIs affect target gene expression, which are responsible for the production of numerous toxins and degradable exoenzymes [78]. The production of AI-2 signals, also called “universal language”, is catalyzed by LuxS synthase and allows communication between microorganisms of different species [82]. Moreover, LuxS is involved in the activation of the methylation cycle, which is involved in the control of the expression of hundreds of genes associated with the microbial processes of surface adhesion, detachment, and toxin production [82].
2.1.2. Cyclic Dimeric Guanosine Monophosphate (c-di-GMP) in Bacterial Biofilms
Bis-cyclic dimeric guanosine monophosphate (c-di-GMP) is a secondary messenger that mediates crucial cellular activities in bacteria, including growth, motility, virulence, BF formation, and cell cycle progression [83]. In particular, c-di-GMP is involved in the transformation of planktonic cells to sessile ones during BF formation, as well as in the passage from the sessile lifestyle to the planktonic one through the BF dispersal process [84]. The BF formation and dispersal processes are governed by c-di-GMP through several genetic interactions. In Gram-negative bacteria, low and high c-di-GMP levels correlate with the motile and sessile phenotypes, respectively [83], so high concentrations lead to BF formation, while low concentrations stimulate motility and thus dispersal of bacterial cells from BF [84]. Such a correlation has been demonstrated especially in E. coli, P. aeruginosa, and Salmonella typhimurium [85].
3. Current and New Therapeutic Approaches against BF
Infections caused by BFs are a worrying problem causing chronic diseases that are difficult to treat and require high-dose antibiotics according to the severity of infection. As previously reported, cells in BFs are up to 1000-fold more tolerant to antibiotics and disinfectants than planktonic ones [86]. Additionally, when infections are device-associated and antibiotics fail due to the increasing resistance of bacteria in BFs, surgical replacement of the infected devices is necessary [87]. In this regard, it has been reported that the best possible treatment for BF-based infections could be the possibility of an early diagnosis using biosensors, advanced imaging, or theranostic nanoparticles (NPs), as well as inhibiting the initial attachment stage, thus preventing the infection from starting [86]. Innovative and effective antibiotic strategies have been designed to counteract BF formation and promote its dispersal, such as treatments for dispersing BFs by dissolution of EPSs, associations of conventional antibiotics with QS inhibitors, and a mixture of these novel techniques. Further advanced treatments include antimicrobial photodynamic therapies, bioacoustics, as well as electric and magnetic fields [88]. However, this research area is still too little explored and far from undergoing clinical research and entering the commercial market [88]. Currently, there are a few molecules under clinical development that are active against MDR pathogens and/or BF producers. Among the molecules in Phase III clinical trials in 2020, only caspofungin, which is an antifungal drug, has been demonstrated to inhibit the synthesis of the polysaccharide components of the S. aureus BF [89]. In the field of NPs, polymeric lipid NPs deriving from the conjugation of rhamnolipids (biosurfactants secreted by P. aeruginosa) and polymer NPs made of clarithromycin encapsulated in a polymeric core of chitosan have been studied, while rhamnolipid-coated silver and iron oxide NPs have been developed, which were effective in eradicating S. aureus and P. aeruginosa BFs [89]. Naturally occurring cationic antimicrobial peptides (CAMPs), a class of non-β-lactam antimicrobial agents, acting on contact as membrane disruptors, have been demonstrated to be effective on a wide variety of Gram-positive and Gram-negative bacteria, fungi, protozoa, and yeast and to be active alone or in combination with conventional antibiotics against Staphylococci and P. aeruginosa BFs [90,91,92,93]. So, inspired by CAMPs, several cationic compounds have been developed that demonstrate similar mechanisms of action and effects. In this context, some antimicrobial cationic dendrimers have proven anti-BF effects and the capability to penetrate EPSs and prevent BF formation, thus representing novel promising substances to counteract this alarming form of bacterial resistance [7,88]. Quaternary ammonium salts (QASs) include the most recognizable commercial disinfectants and antiseptics, such as benzalkonium chloride [39], miramistin [40], di-decyl-dimethyl-ammonium chloride [41], cetyl-pyridinium chloride [42], and octenidine dichloride [43]. Moreover, studies reported that surfactants classified as gemini quaternary ammonium salts (g-QASs) have been inserted in biocidal preparations which were active against the biofilm created by P. aeruginosa by disrupting its functioning, and lysing BF cells upon electrostatic interactions [44]. In the case of BFs formed by Gram-positive bacteria, g-QASs such as alanine gemini surfactants manifested anti-BF activity against S. epidermidis [44]. With the aim of preventing the phenomenon of adhesion of microorganisms, surfaces modified with QASs and their gemini derivatives have been developed. Moreover, the ability of QASs to eradicate C. albicans and R. mucilaginosa BFs was observed [44].
3.1. Nitrogen-Based Quaternary Salts
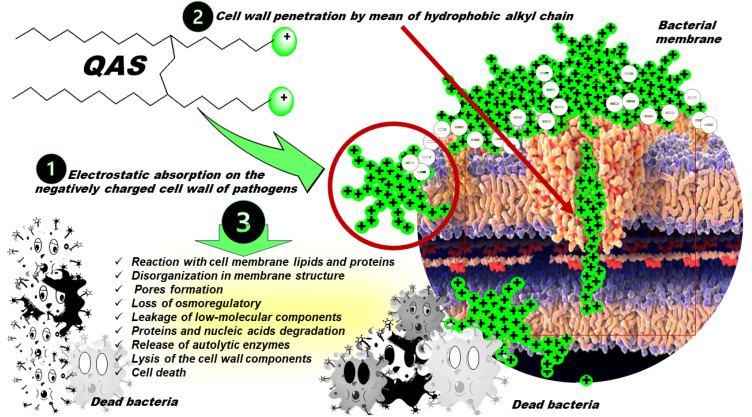
Permanently cationic nitrogen-based surfactants, such as quaternary ammonium salts (QASs) and derivatives are made of one or more polar cationic heads and differently structured hydrophobic carbon tails, often including long carbon chains. QASs possess algistatic, bacteriostatic, tuberculostatic, sporostatic, fungistatic, and antiviral activities [94,95,96,97]. Although their real mechanism of action has not been fully understood, as schematically shown in Figure 2, it has been reported that at a concentration close to the MIC, after the initial adsorption of QASs on the cell wall of pathogens by electrostatic interaction, they penetrate it, due to the presence of hydrophobic alkyl chains [98].
Figure 2.
Schematic representation of the most recognized mode of action of antibacterial QASs. Symbols (+) refer to the cationic QAS, while symbols (-) indicate the negatively charged constituents of bacterial membrane.
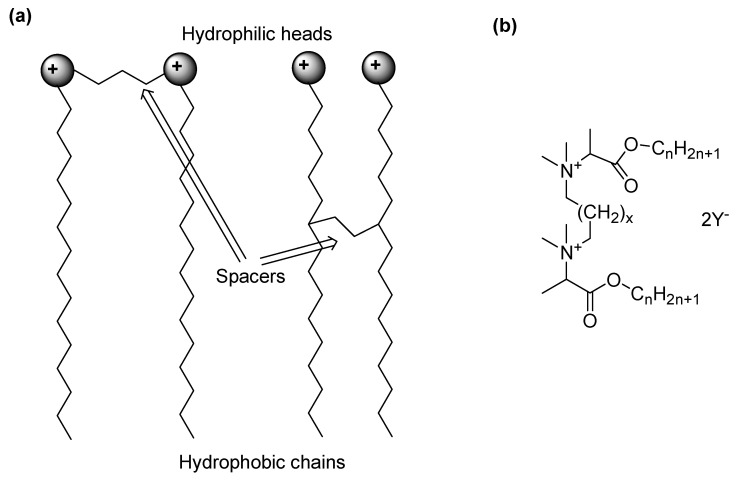
Here, QASs react with lipids and proteins of the cell membrane, thus causing disorganization in its structure, pore formation, loss of osmoregulatory and physiological functions, and leakage of low-molecular-weight components out of the cell [98]. Subsequently, proteins and nucleic acids degrade inside the cell, and the release of autolytic enzymes occurs, which leads to the lysis of the cell wall components and a complete loss of the structural organization of the cell [98]. At concentrations higher than the MIC, aggregates are formed that solubilize hydrophobic membrane elements [99]. It has been shown that the highest biocidal activity against Gram-positive bacteria and yeast was provided by QASs with C10–C14 carbon chains, while QASs with C14–C16 carbon chains were biocidal against Gram-negative bacteria [100]. However, to exert their biocidal effects, QASs need to pass through the outer envelope of the bacterial cells. In this regard, since that of Gram-negative species is composed of more layers than that of Gram-positive strains, it is not surprising that Gram-negative bacteria show more resistance to QASs than Gram-positive species [101]. Concerning BFs, the inhibition of microbial adhesion to surfaces seems to be a good target for their prevention, and numerous studies have focused on surface modification in order to impede the interaction of microbial cells with it. In this context, the immobilization of QASs on surfaces has been reported as a successful way of reducing bacterial cell attachment. In the past years, the groups of Tan and Peng have shown that chitosan derivatives of monomeric QASs deposited on bone cement and titanium inhibited the adhesion of Staphylococci [102,103], while their incorporation into silica NPs specifically reduced the adhesion of S. aureus [104]. Although the anti-adhesion effect of QASs also depends on the material properties of the surfaces (hydrophobicity, charge) and on the characteristics of the cell envelope, it has been demonstrated that the presence of the second QAS molecule in the gemini structures (Figure 3) enhances their surface activity, resulting in a stronger interaction with the material [105].
Figure 3.
Structure of gemini QASs. Two possible structures of a gemini QAS schematically represented (a); a possible general chemical structure of a gemini QAS (b).
Concerning this, polystyrene coatings decorated with glycine and alanine gemini QASs reduced the adhesion of S. epidermidis and C. albicans, with the best effects achieved for C12 and C14 compounds. A greater challenge is represented by eradicating mature BFs due to the presence of EPSs and sessile cells with low metabolic activity. Glycine- and alanine-derived gemini QASs with 12 carbons in the chains exhibited not only high antimicrobial effects against planktonic forms of bacteria and fungi but also impressive biofilm-eradicating properties. Specifically, gemini-like surfactants were able to penetrate the BF structure by extracting adherent cells or lowering the viability of cells within the BF. In 2013, Obłak et al. synthesized a series of cationic gemini chlorides and bromides, which poorly reduced the adhesion of microorganisms to the polystyrene plate. However, one compound eradicated the BFs of C. albicans and Rodotorula mucilaginosa, thus resulting in promise in overcoming catheter-associated infections [106]. Later, Tran et al. developed polyurethane (PU) foam wound dressings coated with poly diallyl-dimethylammonium chloride (pDADMAC–PU), which was able to inhibit the growth and development of BFs of S. aureus, P. aeruginosa, and A. baumannii within the wound dressing. A colony-forming unit assay revealed that the pDADMAC–PU dressing produced more than an eight-log reduction in the BF formation of each pathogen [107]. More recent examples of the nitrogen-based permanently cationic materials studied as anti-BF agents have been included in Table 2, which collects mainly the foremost compounds developed in the last five years.
Table 2.
Nitrogen-based cationic and quaternary compounds developed in the last fifteen years endowed with anti-biofilm effects.
| Mechanism of Action | Type of Compound | Target Pathogens | Effects on BFs | Contration | [Ref.] Year |
|---|---|---|---|---|---|
| Electrostatic interactions (EI) Membrane depolarization (MD) |
C12 di-cationic BODIPY-based fluorescent amphiphiles |
S. epidermidis 1 S. epidermidis 2 |
Prevention of BF formation Destruction of mature BF |
16 µg/mL | [108] 2023 |
| EI Membrane disruption (MDI) |
DMAHDM | Streptococcus mutans | ⇓ Colony-forming unit counts ⇓ Metabolic activity ⇓ Exopolysaccharide synthesis ⇓ Overall acid production ⇓ Tolerance to oxygen stress (OS) |
5 wt% # | [109] 2019 |
| EI, MDI | QASI |
S. mutans
Lactobacillus acidophilus |
Sortase-A protein conformational change ⇓ Carbohydrate intensities Absence of bacterial colonies ⇓ DAPI staining ⇑ Fatty acid compositions |
1–2 wt% # | [110] 2020 |
| MDI Cell lysis |
ATAB | S. mutans | ⇓ Viability of BF ⇓ Viability planktonic bacteria |
5 wt% # 10 wt% # |
[111] 2022 |
| EI Cell wall disintegration |
Amphiphilic quaternized chitosan |
S. mutans | 29% residual BF | 1100 µg/mL | [112] 2022 |
| Mixed antifouling + bactericidal actions | PSBMA/PQA4C-10%, PSBMA/PQA4C-30% PSBMA/PQA8C-10% |
S. aureus | Prevention of bacterial attachment and BF formation | N.A. | [113] 2019 |
| Contact inhibition | DMADDM | S. mutans | ⇓ Amount of BF ⇓ Metabolic activity ⇓ Lactic acid production ⇓ EPS ⇓ Viable S. mutans in BF |
2.5–10 wt% # | [114] 2020 |
| MDI Bind to DNA gyrase Bind to topoisomerase IV |
QA fluoroquinolones | S. aureus | ⇓Total biomass of P. aeruginosa ATCC 15442 BF | 5–10 µM | [115] 2023 |
| P. aeruginosa | 25 µM | ||||
| MDI | QASI (K21) | E. faecalis | Destruction of BF | 0.5 wt% # | [116] 2021 |
| Interference with adhesion Interference with QS |
QAL, QAH |
S. epidermidis
P. aeruginosa |
50% ⇓ in BF formation | 0.037–0.15 mg/mL | [117] 2019 |
| Contact-kill | C14-QAAM C14-QAMAM |
S. mutans | ⇓ 99% in BF formation No BF adhesion |
10 wt% # | [118] 2019 |
| MDI | [Ch] [Gly], [Ch] [Ala] |
Bacillus cereus
Pseudomonas fluorescens. |
56–83% removal of biomass | 70–100 mg/mL | [119] 2022 |
| ⇓ 1.3–2 log CFU/cm2 culturable population | |||||
| Multiple mechanisms |
Dequalinium chloride (DQC) |
Bacterial vaginosis Gardnerella spp. |
⇓ 80% BF biomass ⇓ 80% metabolic activity |
25.64 µg/mL | [120] 2021 |
| MD | DDAB * | S. aureus | Total removal of BF | 32 µg/mL | [121] 2022 |
| P. aeruginosa | Disruption of BF structure ⇓ BF polysaccharides, proteins, and phospholipids |
600 µg/mL | |||
| MD | DMAHDM |
S. mutans
S. sanguinis |
Suppression of cariogenic species in BF Modulation of BF equilibrium from cariogenic state to non-cariogenic state |
3% wt# | [122] 2023 |
| Formation of eDNA–PHMG-Cl complexes | PHMG-Cl |
P. aeruginosa ATCC
S. aureus ATCC |
Blockage of BF development | 0.1% and 0.5% | [123] 2022 |
| Interference with the ion transport Membrane lysis |
QASI |
S. mutans
L. acidophilus Actinomyces naeslundii S. sanguis |
Significant decrease in BF growth | 2% | [124] 2020 |
| EI, MDI, Cytoplasmic leakage | DMADDM | N.R. | ⇓ BF viability ⇓ BF formation |
1.1–2.2 wt% # | [125] 2023 |
| N.R. | DADMAC-based coatings | Methicillin-resistant S. aureus (MRSA) |
⇓ 99% adhesion and BF grow | N.R. | [126] 2018 |
| Vancomycin-resistant Enterococcus spp. (VRE) | ⇓ 94% adhesion and BF grow | ||||
| N.R. | Guanidinium anthranilamide compounds |
S. au reus | ⇓ 83–92%established BF | 62.4–64 µM | [127] 2018 |
| MDI | QADM | S. mutans | (1–3 days) Significant inhibition of BF formation | 10 wt% # | [128] 2022 |
| N.R. | Pyridine-based QASs | E. coli | BF eradication (MBEC) | 16 mg/L | [129] 2018 |
| S. aureus | 8–16 mg/L | ||||
| EI, MDI | GTMAC-modified PCL:PEG:GelMA | E. coli | Significant ⇓ in CFU | N.R. | [130] 2022 |
| S. aureus | |||||
| S. epidermidis | |||||
| EI, membrane infiltration MDI |
Metal-free quaternized carbon dots | S . aureus | ⇓ BF viability ⇓ BF formation |
1000 µg/mL | [131] 2019 |
| MDI DNA damage ROS generation |
MUTAB-based AuNCs |
B.subtilis
E. faecalis S. pneumoniae E. coli P. aeruginosa C. albicans |
No live bacteria in BF of E. coli | 200 µg/mL | [132] 2020 |
| Hydrophobic and EI MDI ROS production DNA damage |
Si-QAC/TEOS NPs | S . aureus | Eradication of mature BF Inhibition of BF formation |
100 µg/mL | [133] 2022 |
| Disrupt the charge balance of bacterial membranes ⇑ Membrane permeability ⇑ ROS by irradiation at 660 nm |
CP5/TFPP-QA |
E. coli MRSA |
70–80% BF dispersion | 100 µg/mL | [134] 2022 |
| N.R. | QA polyethyleneimine PEI1200-C6 |
E. coli
P. aeruginosa B. amyloliquefaciens S. aureus |
>80% BF dispersion | 1–8 mg/mL | [135] 2019 |
| MD MDI |
phenylglyoxamide-based QA iodide | S . aureus | 70% inhibition of BF formation 44% BF disruption |
16 µM 32 μM |
[136] 2021 |
| E. coli | 28% BF disruption | 64 μM | |||
| Contact Killing | DMAHDM |
Streptococci
S. mutans Lactobacilli |
⇓ 80% metabolic activity ⇓ 95% lactic acid production |
3–5 wt% # | [137] 2020 |
| Acidic release of AgNPs MDI by QAS DNA damage |
Ag@QAS@CM | S. aureus | ⇓ 80% BF mass | 6.25 μg/mL (Ag) | [138] 2020 |
| EPS penetration ability Acidic release of CuNPs MDI by QAS DNA damage |
Cu@QAS@CM | Sulfate-reducing bacteria (SRB) | ⇓ 75% BF mass | 250 μM | [139] 2022 |
| EI, MDI, cytoplasmic leakage | DMADDM |
S. mutans
S. sanguinis S. gordonii |
Significant ⇓ of multispecies BFs Significant ⇓ in metabolic activity of multispecies BFs |
40–200 μg/mL | [140] 2019 |
| Displacement of divalent cations from membrane Membrane destabilization Lysis |
QA carbosilane dendrimers and dendron |
S. aureus | BF inhibition | 16–64 μg/mL MBIC | [141] 2023 |
| BF eradication | 32–64 μg/mL MBEC | ||||
| MRSA | BF inhibition | 16–64 μg/mL MBIC | |||
| BF eradication | 16–64 μg/mL MBEC | ||||
| MDI ROS generation |
Cu2+/PDDA | S. aureus | ⇓ EPS Kill cells in BF |
144 μg/mL PDDA 26.5 μM Cu2+ |
[142] 2023 |
| EI, MDI, cytoplasmic leakage | N-alkylated pyridine-based QAS |
S.aureus
E. coli |
52–95% inhibition of BF formation | 50–250 μg/mL | [143] 2020 |
| MDI Pyridoxal-dependent enzyme targeting |
Pyridoxine-based QAS of terbinafine (KFU127) |
S. aureus (SA)
C. albicans (CA) E. coli (EC) S. epidermidis (SE) |
Full destruction of cells in BF | 400 μg/mL (CA) 400–800 μg/mL (CA+SA) |
[144] 2020 |
| ⇓ >3 LogCFU/cm2 | 128 μg/mL (SA) 128 μg/mL (SE) 256 μg/mL (EC) |
||||
| EI, MDI, PAI | N-Alkyl pyridinium QAS | E. faecalis | 85–100% killed cells in BF No BF removal |
250 µM (60′) | [145] 2020 |
| 65–95% killed cells in BF 15–20% BF removal |
250 µM 1′ + 10 s PAI |
||||
| EI, MDI, EPS permeation | Pyridine-based tris-QASs | E. coli | MBIC MBEC |
8–32 μg/mL 16–32 μg/mL |
[146] 2023 |
| K. pneumoniae | MBIC MBEC |
16–64 μg/mL 64–500 μg/mL |
|||
| S. aureus | MBIC MBEC |
8 μg/mL 16 μg/mL |
|||
| P. aeruginosa | MBIC MBEC |
64 μg/mL 250–500 μg/mL |
|||
| A. baumannii | MBIC MBEC |
32 μg/mL 250 μg/mL |
|||
| C. albicans | MBIC MBEC |
4–16 μg/mL 8–32 μg/mL |
|||
| EI, MDI, cell lysis | DTAB vs. C6 | P. aeruginosa | Full eradication of BF (C6, 4 h) | 0.29 mM | [147] 2018 |
| EI, MDI, cytoplasmic leakage | TMACS | E. coli | BF inhibition rate 57.6% | 156 µg/mL | [148] 2023 |
| BF removal rate 41.6% | 2.5 mg/mL | ||||
| S. aureus | BF inhibition rate 58.5% | 20 µg/mL | |||
| BF removal rate 59.01% | 2.5 mg/mL | ||||
| EI, MDI | Glut/ADBAC | P. aeruginosa | 5 log killing of sessile cells | 1000 ppm (2 h) 100 ppm (24 h) |
[149] 2010 |
| CDA | 100 ppm (2 h) 10 ppm (24 h) |
||||
| Glut/ADBAC | 30% (24 h)–35% (2 h) biomass residual | 100 ppm | |||
| 45% (24 h)–40% (2 h) biomass residual | 1000 ppm | ||||
| CDA | 30% (24 h)–30% (2 h) biomass residual | 100 ppm | |||
| 20% (24 h)–25% (2 h) biomass residual | 1000 ppm | ||||
| N.R. | Barquat® MB-50 Ucarcide® 42 |
P. aeruginosa | N.R. | N.R. | [150] 2010 |
| EI, MDI EPS penetration ability due to the C18 chain |
Pyridoxine-based QASs |
S. aureus
S. epidermidis |
QAS (1–3) killed BF-detached Staphylococci cells | 16–32 µg/mL | [151] 2015 |
| S. aureus | MBCadh (QAS 3) | 32 µg/mL | |||
| S. epidermidis | MBCadh (QAS 3) | 16 µg/mL | |||
| EI by well-accessible positive charges Host–guest properties of pillar[n]arenes |
Pillar[n]arene QASs and imidalonium salts |
S. aureus
E. faecalis S. epidermidis S. mutans |
Inhibition of BF formation (MBIC50) No biocidal, no hemolytic |
0.4–8.8 µM | [152] 2016 |
| Pillar [5]arene QASs |
S. aureus
E. faecalis |
Inhibition of BF formation (MBIC50) No biocidal, no hemolytic |
2–4 µg/mL | [153] 2016 |
BODIPY = boron dipyrromethene; 1 ATCC 12228; 2 YT-169a; DMAHDM = dimethylaminohexadecylmethacrylate; # in dental cement or resin; ⇓ = decrease, reduction; ⇑ = increase; QASI = quaternary ammonium silane; ATAB = alkyl trimethyl ammonium bromide; D-PQA = dopamine-terminated quaternary ammonium salt polymer; D-PSBMA = dopamine-terminated poly(sulfobetaine methacrylate); DMADDM = dimethylaminododecyl methacrylate; QAL = low-molecular-weight quaternized chitosan derivatives; QAH = high-molecular-weight quaternized chitosan derivatives; ROS = reactive oxygen species; CFU = colony-forming unit; N.A. = not applicable; C14-QAAM = quaternary ammonium acrylamides with a C14 chain; C14-QAMAM = quaternary ammonium methacrylamides with a C14 chain; [Ch] [Ala] = choline alaninate; [Ch] [Gly] = choline glycinate; DDAB = didecyldimethylammonium bromide; * combined with slightly acidic electrolyzed water (SAEW); DMAHDM = dimethylaminohexadecyl methacrylate; PHMG-Cl = polyhexamethylene guanidine hydrochloride; N.R. = not reported; DADMAC = diallyl dimethyl ammonium chloride; QADM = bis(2-methacryloyloxyethyl) dimethylammonium bromide; MBIC = minimum biofilm inhibitory concentration; MBEC = minimum biofilm eradication concentration; PCL = polycaprolactone; PEG = polyethylene glycol; GelMA = gelatin methacryloyl; GTMAC = glycidyl trimethyl ammonium chloride; MUTAB = (11-mercaptoundecyl)-N,N,N-trimethylammonium bromide; Si-QAC = dimethyloctadecyl [3-(trimethoxysilyl)propyl]ammonium chloride; TEOS = tetraethyl orthosilicate; CP5 = carboxylatopillar [5]arene; TFPP-QA = tetrafluorophenyl porphyrin functionalized with a quaternary ammonium group; Ag@QAS@CM = silver-containing quaternary ammonium salt nanocomposite; PDDA = poly(diallyl dimethyl ammonium chloride); PAI = Er:YAG photoacoustic irrigation; DTAB = dodecyltrimethylammonium bromide; C6 = hexamethylene-1,6-bis-(N,N-dimethyl-N-dodecylammonium bromide; TMACS = N-(4-N′, N′, N′-trimethylmethanaminium chloride) benzoyl chitosan; Glut = glutaraldehyde; ADBAC = alkyldimethylbenzyl ammonium chloride; CDA = cocodiamine; Barquat® MB-50: contains 50% alkyl (C14 50%, C12 40%, C16 10%) dimethyl benzyl ammonium chloride (DMBA); Ucarcide® 42: contains 42.5% glutaraldehyde plus 50% alkyl (C14 50%, C12 40%, C16 10%) DMBA; MBCadh = minimum biocidal concentration of cells in biofilm; MBIC50 = the lowest concentration at which at least 50% reduction in BF formation was measured compared to untreated cells; Barquat® MB-50 and Ucarcide® 42 are from Azelis Americas, LLC, Westport, CT, USA.
3.2. Quaternary Phosphonium Salts (QPS)
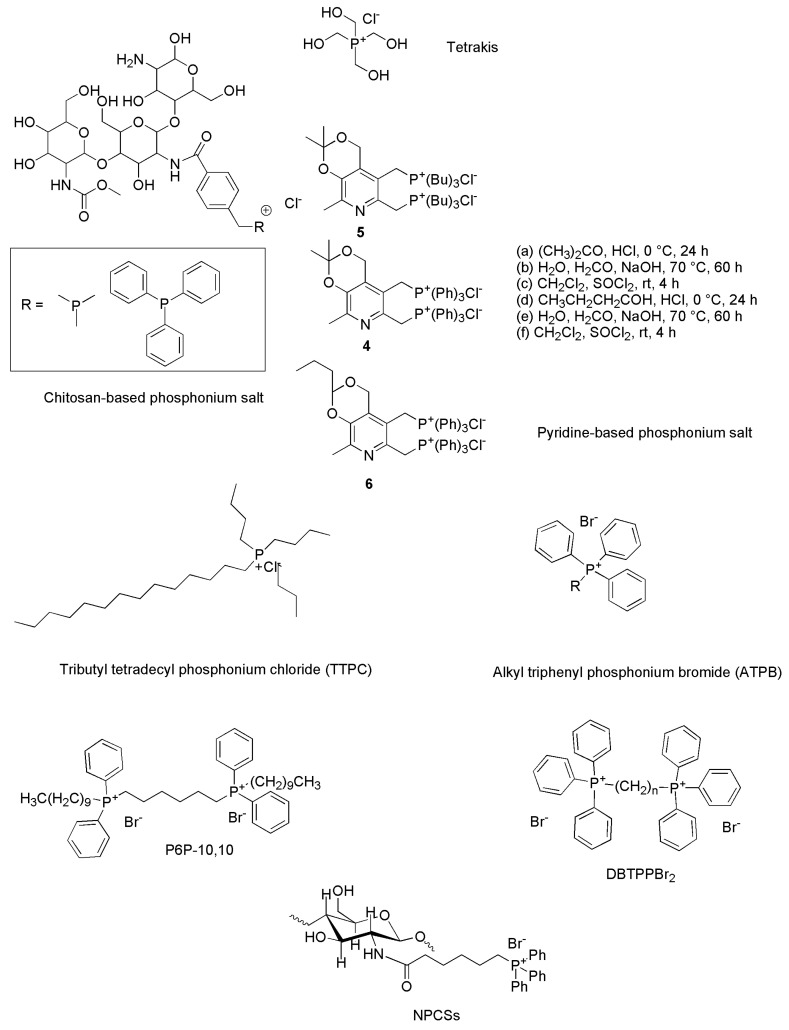
Although they are thermally and chemically more stable [154,155,156,157,158,159] and often display increased antimicrobial properties and lower toxicity than their nitrogen-based counterparts [155,160,161,162], phosphonium compounds as antibacterial agents are inexplicably lesser reported than ammonium ones. Chart 1 reports the chemical structures of some QPSs developed in recent years that have shown remarkable antibacterial and anti-biofilm effects.
Chart 1.
Examples of QPS chemical structures.
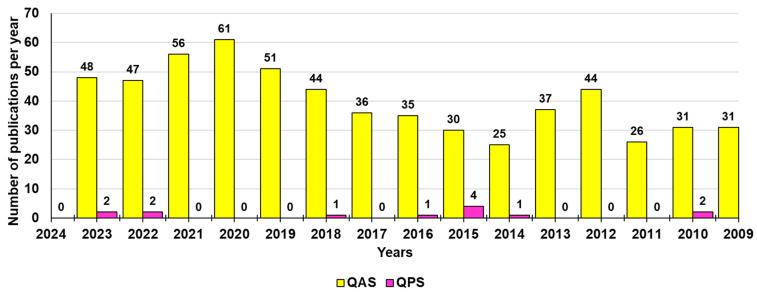
Nevertheless, compounds with phosphonium moieties have been reported and used in various biomedical applications, such as antibacterials [163], anticancer agents [164], and water treatment [165,166]. Concerning their mechanism of action, like CAMPs and QASs, QPSs also act on contact as membrane disruptors, following the phases reported in Figure 2. However, it has recently been demonstrated that some representatives of this class, including alkyl triphenyl phosphonium and alkyl tributyl phosphonium salts, are also endowed with remarkable antioxidant activity, thus being capable of reducing oxidative stress triggered by bacteria, helping the human body in fighting them and blocking the replication of microbial pathogens [158]. In the past, the antioxidant properties of tetrakys (THPS) and its biocidal activity by disrupting the disulfide bonds in bacterial cells were reported by Keasler et al. (2010) [149]. The remarkable antimicrobial effects of phosphonium ionic liquids (PILs) have been reported by Brunel et al. [167]. Benzyl triphenyl phosphonium salts exhibited anti-trypanosomal activity against Trypanosoma brucei, and it was established that the existence of bulky substituents (alkyl or aryl) surrounding the phosphonium ions resulted in a reduction in the MIC of these ILs [168]. Bis-phosphonium salt-derived benzophenone exhibited remarkable toxicity to the human protozoan parasite Leishmania [169]. More recently, Das et al. reported the development of mono- and bis-PILs through a purely ionic approach, and the resulting PILs demonstrated selective bacterial toxicity toward Gram-positive S. aureus and Gram-negative E. coli depending on the number of phosphonium ions present in the salt [154]. Phosphonium salts with single and double alkyl chains were investigated by Endo and coworkers [160]. Herein, the effect of the chain length (C10–C18) on antibacterial activity against a wide range of pathogens including MRSA was examined. The study brought forward a direct correlation between molecular structure and antibacterial activity. In particular, it was observed that higher alkyl chain lengths and the presence of aryl groups improved the antimicrobial activity of PILs. However, the aforementioned studies reported the specific use of phosphonium salts as biocides, while studies conducted on their potential activity against BFs are very limited. In this regard, Kim et al. reported on the antifouling properties of tributyl tetradecyl phosphonium chlorides in reverse osmosis processes, which were capable of reducing the thickness and volume of a BF of P. aeruginosa [170]. Among QPSs, triphenyl alkyl phosphonium compounds, known for years as chemical intermediates of the Wittig reaction, easily synthesizable by a one-step low-cost reaction and easily purifiable by crystallizations, have been re-evaluated as promising template molecules to prepare new antibacterial agents active against bacterial BFs. In this regard, a report by Fernández and co-workers described several bioeffects of numerous alkyl-triphenyl phosphonium salts, including the broad-spectrum biocidal activity of compounds with alkyl chains > C7, the antifouling properties of some of them, and the abilities of some others to act as non-biocidal, non-toxic QS disruptors, thus without tendency to develop resistance in microorganisms [171]. In this regard, Melander and co-workers pointed out that it would be extremely important and desirable to develop anti-biofilm agents operating via non-biocidal mechanisms for several reasons, the most important one being avoiding resistance development [172]. In this context, Joseph et al. found that while ammonium and methyl imidazolium cationic pillar[n]arenes were effective inhibitors of BF formation in several strains of Gram-positive bacteria, they were deprived of any antimicrobial activity, thus not causing damage to red blood cells or toxicity to human cells in culture [152]. In the same year, the same authors prepared both ammonium and phosphonium derivatives, which demonstrated similar anti-biofilm properties [153]. Collectively, to have an idea of the discrepancy that exists between the interest in QASs rather than QPSs as possible anti-biofilm agents, Figure 4 shows how many experimental publications have been developed in the last 15 years reporting on quaternary ammonium salts (602) vs. those reporting on quaternary phosphonium salts (13) that were demonstrated to be effective against BFs and BF-based biofouling according to Scopus and PubMed.
Figure 4.
Number of publications per year during the last fifteen years according to Scopus and PubMed, concerning the development of QASs and QPSs for use against BFs. The survey was made by crossing the information obtained by Scopus and PubMed using the keywords Quaternary AND ammonium AND compounds AND biofilm (yellow bars) and Quaternary AND phosphonium AND compounds AND biofilm (fuchsia bars).
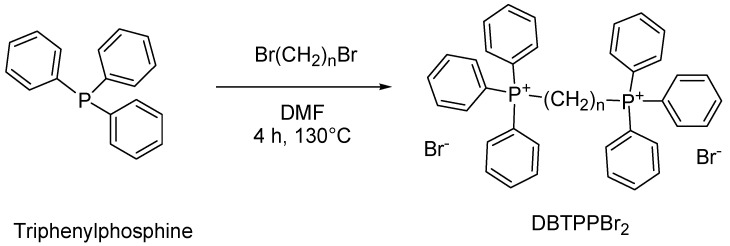
Unfortunately, the paucity of innovation evidenced in Figure 4 concerning “onium” compounds has driven the emergence of QAS resistance, and nowadays it is necessary to identify the next generation of disinfectant molecules with efficacy against highly resistant BF-producing clinical isolates [45]. In this regard, a more intensive study on phosphonium-containing compounds could be a rational route to achieve next-generation disinfectants. As confirmation of this thesis, Michaud et al. recently tested a series of quaternary ammonium and quaternary phosphonium compounds (QPCs) against a panel of 35 resistant A. baumannii clinical isolates. The authors found that in contrast to QASs, which developed resistance, a QPC, namely P6P-10,10, maintained efficacy against resistant strains with an IC90 of 3 µM and an MBEC as low as 32 µM against extensively drug-resistant clinical isolates [45]. Furthermore, this year, Shi et al. reported on the synthesis, characterization, and microbiological evaluation of alkyl-bis-(triphenyl) phosphonium bromides, namely (1,2-DBTPP)Br2, (1,4-DBTPP)Br2, and (1,6-DBTPP)Br2. The biological results revealed that the butyl derivative (1,4-DBTPP)Br2 exhibited low toxicity and low hemolytic activity on eukaryotic cells and the capability to inhibit bacterial growth and BF formation and to promote the recovery process of infected wounds in vivo [173].
In addition to those discussed here, the QPSs developed and studied in the last 15 years have been included in Table 3.
Table 3.
Anti-biofilm QPSs developed in the last fifteen years.
| Mechanism of Action | Type of Compound | Target Pathogens | Effects on BF | Concentration | [Ref.] Year |
|---|---|---|---|---|---|
| EI, MDI, cytoplasmic leakage | TMPCS TPPCS |
E. coli | BF inhibition rate 33.9% (TMPCS), 56.6% (TPPCS) | 156 µg/mL | [148] 2023 |
| BF removal rate 46.4% (TMPCS), 48.9% (TPPCS) | 2.5 mg/mL | ||||
| S. aureus | BF inhibition rate 53.8% (TMPCS), 62.2% (TPPCS) | 20 µg/mL | |||
| BF removal rate 60.4% (TMPCS), 69.9% V | 2.5 mg/mL | ||||
| Disrupts disulfide bonds on the cell surface |
THPS | P. aeruginosa | 5 log killing of sessile cells | 100 ppm (2 h) 100 ppm (24 h) |
[149] 2010 |
| 35% (24 h)–45% (2 h) biomass residual | 100 ppm | ||||
| 40% (24 h)–40% (2 h) biomass residual | 1000 ppm | ||||
| N.R. | Bellacide® 350 Tolcide® PS75 |
P. aeruginosa | N.R. | N.R. | [150] 2010 |
| EI, MDI Partial diffusion in EPS |
Pyridoxine-based QPSs |
S. aureus
S. epidermidis |
QPS (6) killed detached S. epidermidis cells 68–77% cells located in BF killed |
32 µg/mL 8 µg/mL |
[151] 2015 |
| EI by well-accessible positive charges on QPSs Host–guest properties of pillar[n]arenes |
Pillar [5]arene QPS |
S. aureus
E. faecalis |
Inhibition of BF formation (MBIC50) No biocidal, no hemolytic |
2–4 µg/mL | [153] 2016 |
| N.R. | TTPC | S. aureus | ⇓ BF (OD545 < 1) | 20 µg/mL | [170] 2015 |
| P. aeruginosa | ⇓ BF (OD545 < 1) | 40 µg/mL | |||
| ⇓ Biofilm thickness (73.9%) and volume (73.8%) | 40 µg/mL | ||||
| QS disruption | ATPB |
Chromobacterium
violaceum |
Inhibition of BF formation (violacein inhibition) |
52.9–142.2 μM * | [171] 2015 |
| Vibrio harveyi. | Inhibition of BF formation (bioluminescence inhibition) |
128.6–348.5 μM * | |||
| EI, MDI Cell lysis, cytoplasmic leakage |
TATPBP (P6P-10,10) | EDR A. baumannii | BF eradication (MBEC) | MBEC 32–63 µM | [45] 2022 |
| EI, MDI, MD ROS production |
(1,2-DBTPP)Br2 (1,4-DBTPP)Br2 (1,6-DBTPP)Br2 |
S. aureus | 100% inhibition of BF formation | 64–128 µg/mL ** | [173] 2023 |
| 80% ⇓ metabolic activity | 32–64 µg/mL ** | ||||
| MRSA | 100% inhibition of BF formation | 128–256 µg/mL ** | |||
| 80% ⇓ metabolic activity | 64–128 µg/mL ** | ||||
| EI with cell surface Cell wall penetration |
NCPS | S. aureus | Significant ⇓ of BF formation | 64–128 µg/mL | [174] 2014 |
| E. coli | 64–256 µg/mL | ||||
| CTPBs |
S. aureus
E. coli |
Not active | MIC > 1600 µg/mL | ||
| N.R. | THPS |
Desulfovibrio
Desulfomicrobium Desulfocurvus |
⇓ Microbiologically influenced corrosion | 17% | [175] 2018 |
| N.R. | THPS | SRB APB |
Significant ability to penetrate BF 100% inhibition of SRB >85% inhibition of APB |
0.6% | [176] 2015 |
| Antioxidant effects Hydroxyl radical scavenging |
ATBPB, ATPB | MDR A. baumannii | N.R. | 6.25–25.0 μM | [158] 2022 |
EI = electrostatic interactions; MD = membrane depolarization; MDI = membrane disruption; N.R. = not reported; TMPCS = N-(4-N′, N′, N′-trimethylphosphonium chloride) benzoyl chitosan); TPPCS = N-(4-N′, N′, N′-triphenylphosphonium chloride) benzoyl chitosan; THPS = tetrakis (hydroxymethyl) phosphonium sulfate; Bellacide® 350: contains 50% tributyl tetradecyl phosphonium chloride (TBTP); Tolcide® PS75: contains 75% THPS; TTPC = tributyl tetradecyl phosphonium chloride; ⇓ = reduction; NPCSs = N-phosphonium chitosans with different degrees of substitution (3%, 13%, and 21%); CTPB = (5-carboxypentyl)triphenylphosphonium bromide; ATPB = alkyl triphenyl phosphonium bromide; ATBPB = alkyl tributyl phosphonium bromide; * biocidal effects at concentrations ≥500 μM; EDR = extensively drug resistant; TATPBP = tetraalkyl tetraphenyl bis-phosphonium; (1,2-DBTPP)Br2 = ethyl-bis-(triphenyl)-phosphonium bromide; (1,4-DBTPP)Br2 = butyl-bis-(triphenyl)-phosphonium bromide; (1,6-DBTPP)Br2 = hexyl-bis-(triphenyl)-phosphonium bromide; SRB = sulfate-reducing bacteria; APB = acid-producing bacteria; ** refers to compound in bold; numbers in bold indicate the number of compound as reported in the study; Bellacide® 350 and Tolcide® PS75 are from Azelis Americas, LLC, Westport, CT, USA.
4. Synthetic Strategies Applied to Prepare the Aforementioned QPSs
In this last section, the synthetic procedures followed to synthesize the best-performing QPSs included in Table 3 have been reported, providing reaction Schemes, molecule structures, and some more detail.
4.1. Synthesis of Chitosan-Based QPSs
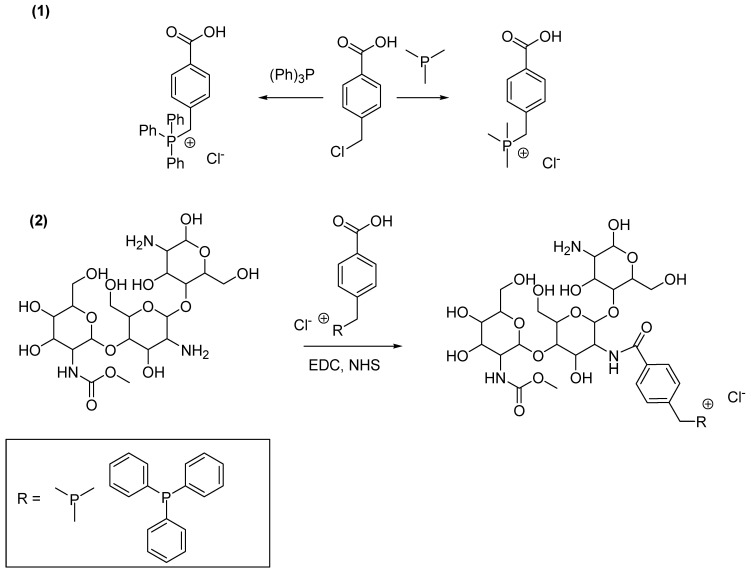
Chitosan QPSs were prepared by means of a two-step reaction. The first step consisted of the synthesis of quaternary benzyl phosphonium salts with a p-carboxylic acid group, which was used to graft them onto commercial chitosan, thus obtaining the related chitosan-based QPS derivatives (Scheme 1).
Scheme 1.
Synthesis of chitosan-based QPSs reported and prepared by Wang et al. [148]. Preparation of the quaternary phosphonium carboxylic acids (1); esterification of chitosan with the quaternary phosphonium carboxylic acids (2). EDC = 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; NHS = N-Hydroxy-succinimide.
Particularly, 4-(chloromethyl) benzoic acid was dissolved in ethyl acetate (EtOAc) and treated with trimethyl phosphine or triphenylphosphine under constant magnetic stirring. The mixture was heated to 85 °C for 10 h, then cooled to room temperature. The precipitate was collected by filtration and subjected to repeated washing with EtOAc to afford the desired products, which were dried in a vacuum at 60 °C for 24 h.
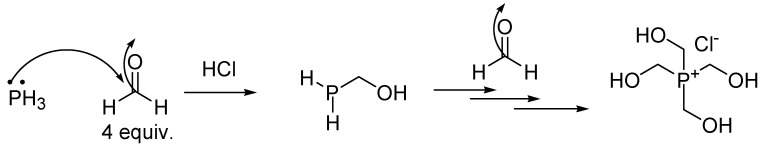
4.2. Synthesis of Tetrakis (Hydroxymethyl) Phosphonium Salt (THPS)
A very effective method of preparing tetrakis (hydroxymethyl) phosphonium salt (THPS) consists of reacting phosphine (PH3) with excess formaldehyde in the presence of hydrogen chloride, according to Scheme 2.
Scheme 2.
Synthesis of THPS according to Svara et al. [177].
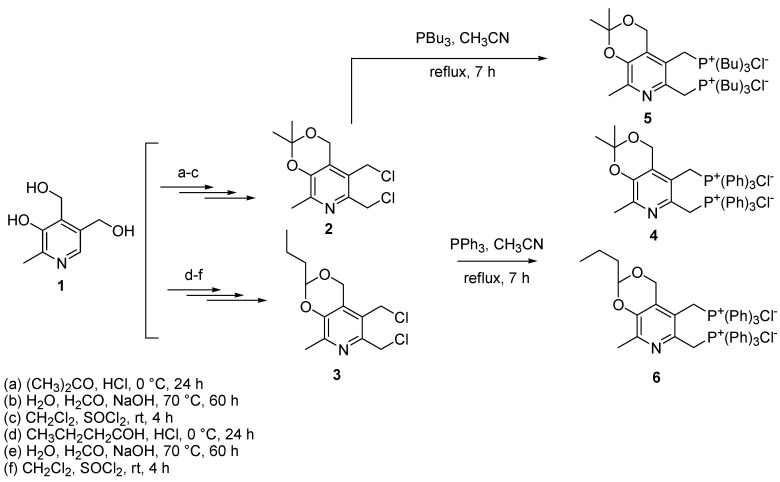
4.3. Synthesis of Bis-Phosphonium Salts of Pyridoxine
The bis-phosphonium salts reported by Kayumov et al. (namely compounds 4, 5, and 6 in their paper) can be prepared following the procedure described and then optimized by Pugachev et al. [178,179]. Briefly, the chlorine derivatives of pyridoxine 1 (compounds 2 and 3) were prepared by first introducing the ketal or acetal protections using acetone or butyl aldehyde, respectively, in acidic conditions. Then, an additional hydroxymethyl group was introduced into position 6 of the pyridine ring of pyridoxine by hydroxy methylation in an alkaline medium. Finally, by treating the hydroxyl derivatives with SOCl2 at room temperature, 2 and 3 were achieved, which were treated with a 2.0-fold molar excess of triphenyl or tributyl phosphine in CH3CN at reflux temperature for 7 h, achieving compounds 4, 6, and 5, respectively (Scheme 3).
Scheme 3.
Synthesis of bis-phosphonium salts of pyridoxine reported by Kayumov et al. (namely compounds 4, 5, and 6) [151], as prepared by Pugachev et al. [178] and subsequently optimized [179]. Bu = butyl.
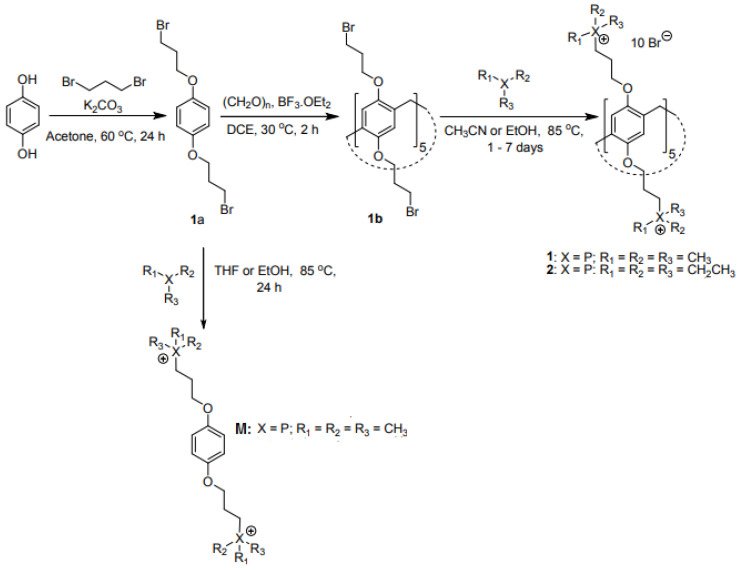
4.4. Synthesis of Pillar [5]Arene-Based QPSs
The pillar [5]arene-based QPSs 1 and 2 and monomer (M) reported by Joseph et al. [153] were prepared according to Scheme 4.
Scheme 4.
Synthesis of pillar [5] arene-based QPS 1 and 2 and the phosphonium monomer M reported by Joseph et al. [153]. THF = tetrahydrofuran.
Briefly, compound 1a was prepared by refluxing hydroquinone and a strong excess of 1,3-dibromopropane in the presence of potassium carbonate (K2CO3) in acetone for 24 h under an argon atmosphere. The reaction mixture was then cooled to 25 °C and filtered through celite, the solvent was evaporated under vacuum, and the residue was dissolved in dichloromethane, washed with water, 3 N HCl, and brine, dried with sodium sulphate, and concentrated in vacuo. After purification by column chromatography and recrystallization, the obtained white solid was converted into compound 1b by dissolution in 1,2-dichloroethane and treatment with paraformaldehyde followed by BF3·OEt2 at 30 °C for 30 min under an argon atmosphere. Compound 1b was obtained as a white solid after precipitation in methanol and purification by chromatography. Finally, by refluxing 1b in a pressure tube in acetonitrile with trimethyl phosphine or triethyl phosphine dissolved in tetrahydrofuran (THF) for 72–96 h, compound 1 or 2 was obtained as white solids in an 86% or 41% yield. Monomer M was prepared as compound 1 but starting from compound 1a rather than 1b.
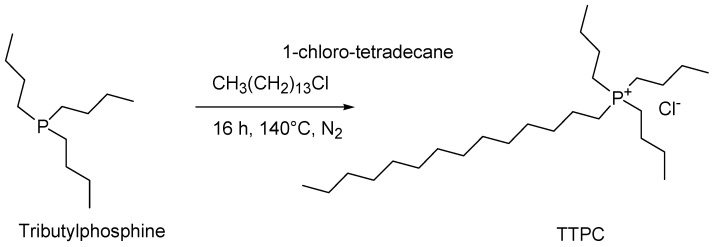
4.5. Synthesis of Tributyl Tetradecyl Phosphonium Chloride (TTPC)
Tributyl tetradecyl phosphonium chloride (TTPC) was easily synthesized following the procedure proposed by Swapnil Dharaskar and Mika Sillanpaa [180] (Scheme 5).
Scheme 5.
Synthesis of tributyl tetradecyl phosphonium chloride (TTPC) reported by Kim et al. [170].
Briefly, tri-butyl-phosphine was added to an equimolar amount of 1-chlorotetradecane at 140 °C under a nitrogen (N2) atmosphere and was vigorously stirred for 12–16 h. After reaction completion, the mixture was vacuum stripped to remove volatile organic components and excess 1-chlorotetradecane. TTPC was obtained as a clear, pale-yellow liquid.
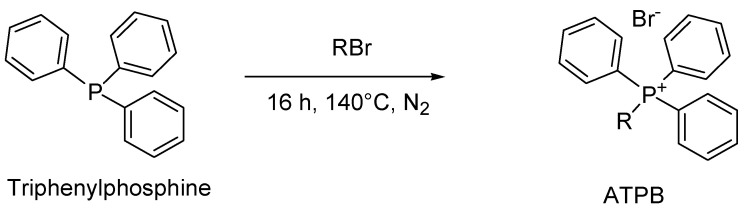
4.6. General Synthesis of Alkyl Triphenyl Phosphonium Bromide (ATPB)
The most common method to prepare the alkyl triphenyl phosphonium salts used by Martín-Rodríguez et al. in their study [171] involves the quaternization of triphenyl phosphine with the proper alkyl halides at 140 °C, according to Scheme 6 [181].
Scheme 6.
General procedure to prepare ATPB.
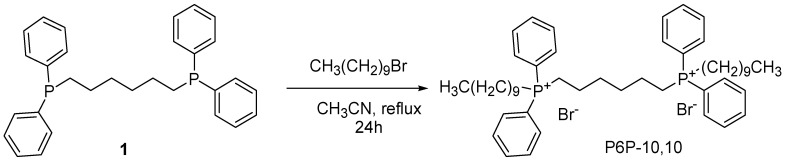
4.7. Synthesis of Tetra Alkyl Tetraphenyl Bis-Phosphonium Compound (TATPBP, Namely P6P-10,10)
The synthesis of the best-performing biocidal QPS reported by Michaud et al. [45], was previously reported by the same authors (Scheme 7) [182].
Scheme 7.
Synthesis of P6P-10,10 [182].
Briefly, to 1,6-bis (diphenyl phosphino) hexane (1) in acetonitrile, 1-bromodecane was added. The obtained solution was heated to reflux and stirred for 24 h. After cooling to room temperature and solvent removal, an oil was obtained, which was triturated with 1/1 ether/hexane and cooled at −25 °C overnight. The resulting precipitate was dissolved in dichloromethane and then concentrated to afford P6P-10,10 as a white crystalline powder, in a 94% yield.
4.8. Synthesis of Alkyl Triphenyl Bis-Phosphonium Bromides
A simple one-step reaction of triphenylphosphine with proper dibromo alkanes was carried out to obtain the alkyl-bis-(triphenyl) phosphonium bromides (1,2-DBTPP)Br2, (1,4-DBTPP)Br2, and (1,6-DBTPP)Br2 (Scheme 8).
Scheme 8.
Synthesis of alkyl-bis-(triphenyl) phosphonium bromides (1,2-DBTPP)Br2, (1,4-DBTPP)Br2, and (1,6-DBTPP)Br2 [173]. n = 2, 4, 6.
Briefly, 1,2-, 1,4-, and 1,6-haloalkanes and Ph3P were dissolved in DMF and were reacted at 130 °C for 4 h. The mixtures were then cooled to room temperature, and the solvent was removed by rotation volatilization to extract the raw products, which were washed with n-hexane and dried in an oven.
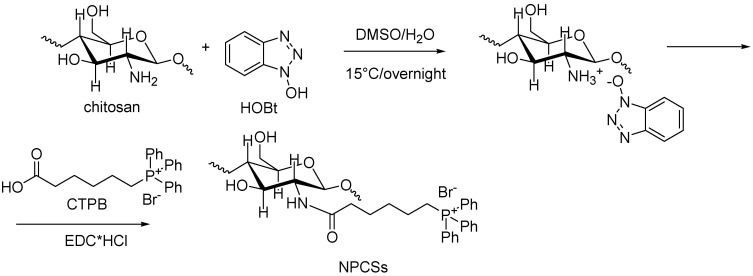
4.9. Synthesis of N-Phosphonium Chitosans (NPCSs) with Different Degrees of Substitution
N-phosphonium chitosans (NPCSs) with different degrees of substitution (3%, 13%, and 21%) were synthesized according to previous studies by the same authors [183,184] (Scheme 9).
Scheme 9.
Synthesis of N-phosphonium chitosans (NPCSs) with different degrees of substitution [183]. EDC HCl = N-(3-dimetilaminopropil)-N′-etilcarbodiimide hydrochloride; HOBt = hydroxybenzotriazole.
In brief, chitosan and hydroxybenzotriazole (HOBt) were stirred in an H2O/DMSO (v/v = 2/1) mixture overnight at 15 °C until the homogeneous solution was obtained. Then, commercial CTPB dissolved in H2O/DMSO (v/v = 2/1) was added to the solution, followed by the dropwise addition of a solution of N-(3-dimetilaminopropil)-N′-etilcarbodiimide hydrochloride (EDC·HCl) in DMSO. After a 24 h reaction at 15 °C, the mixture was precipitated in diethyl ether/acetone (v/v = 1/2). The product was further purified using a dialysis tube (molecular weight cut-off, MWCO 3500 Da) with distilled water. The final product was obtained by lyophilization.
5. Conclusions
The main scope of this review was to attract more attention to a class of quaternary “onium” salts, such as quaternary phosphonium salts (QPSs), extensively studied for other purposes but still little considered as new antibacterial agents in comparison to their nitrogen-based counterpart. In this context, the study of QPSs as possible next-generation weapons to prevent BF formation or eradicate mature BFs is even more limited. In fact, we have underlined that in the last 15 years, publications concerning the anti-biofilm effects of QPSs total only 13 vs. the 602 concerning quaternary ammonium salts (QASs). However, the paucity of innovation in this space has driven the emergence of QAC resistance by different mechanisms including the overexpression of efflux pumps. On the contrary, there could be several advantages of employing QPSs in place of QASs, including less tendency to develop resistance, low toxicity towards eukaryotic cells, higher antibacterial and biocidal effects, capacity of quorum sensing (QS) disruption, and higher thermal stability. Additionally, the synthetic procedures useful to prepare the most promising antibacterial QPSs developed so far have been reviewed, to stimulate scientists to synthesize new QPS derivatives and enlarge the research concerning these compounds, which are still too little studied, and their antimicrobial effects. We are confident that the extension of knowledge on these materials by this review could be a successful approach to finding effective new weapons for treating chronic infections sustained by biofilm-producing MDR pathogens and environmental issues associated with biofilm-sustained fouling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics16010080/s1, Table S1: Clinically relevant MDR pathogens with the associated infections [7]; Table S2: General composition of BFs; Table S3: Stages of the BF life cycle.
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Materials).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cook M.A., Wright G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022;14:eabo7793. doi: 10.1126/scitranslmed.abo7793. [DOI] [PubMed] [Google Scholar]
- 2.CDC Center for Disease Control and Prevention. About Antimicrobial Resistance. [(accessed on 23 November 2023)]; Available online: https://www.cdc.gov/drugresistance/about.html.
- 3.Chinemerem Nwobodo D., Ugwu M.C., Oliseloke Anie C., Al-Ouqaili M.T.S., Chinedu Ikem J., Victor Chigozie U., Saki M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022;36:e24655. doi: 10.1002/jcla.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang K.W.K., Millar B.C., Moore J.E. Antimicrobial Resistance (AMR) Br. J. Biomed. Sci. 2023;80:11387. doi: 10.3389/bjbs.2023.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karakonstantis S., Kritsotakis E.I., Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection. 2020;48:835–851. doi: 10.1007/s15010-020-01520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4:481–511. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alfei S., Caviglia D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics. 2022;14:2016. doi: 10.3390/pharmaceutics14102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang Z., Raudonis R., Glick B.R., Lin T.-J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Jia W., Li G., Wang W. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Hospital-Based Study in China. Int. J. Environ. Res. Public Health. 2014;11:3424–3442. doi: 10.3390/ijerph110303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassotti T.T., de Angelis Z.D., da Fontoura Xavier Costa L., de Araújo A.J.G., Pereira R.I., Soares R.O., Wagner P.G.C., Frazzon J., Frazzon A.P.G. Antimicrobial Resistance Profiles in Enterococcus spp. Isolates from Fecal Samples of Wild and Captive Black Capuchin Monkeys (Sapajus nigritus) in South Brazil. Front. Microbiol. 2018;9:2366. doi: 10.3389/fmicb.2018.02366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakidis I., Vasileiou E., Pana Z.D., Tragiannidis A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens. 2021;10:373. doi: 10.3390/pathogens10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galindo-Méndez M. Antimicrobial Resistance in Escherichia coli. In: Louis R., editor. E. Coli Infections-Importance of Early Diagnosis and Efficient Treatment. IntechOpen; London, UK: 2020. p. 234. [DOI] [Google Scholar]
- 13.Moya C., Maicas S. Antimicrobial Resistance in Klebsiella pneumoniae Strains: Mechanisms and Outbreaks. Proceedings. 2020;66:11. doi: 10.3390/proceedings2020066011. [DOI] [Google Scholar]
- 14.Foster T.J. Antibiotic Resistance in Staphylococcus aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017;41:430–449. doi: 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- 15.Cillóniz C., Garcia-Vidal C., Ceccato A., Torres A. Antimicrobial Resistance Among Streptococcus pneumoniae. In: Fong I.W., Shlaes D., Drlica K., editors. Antimicrobial Resistance in the 21st Century. Springer International Publishing; Cham, Switzerland: 2018. pp. 13–38. [Google Scholar]
- 16.Cuypers W.L., Jacobs J., Wong V., Klemm E.J., Deborggraeve S., Van Puyvelde S. Fluoroquinolone Resistance in Salmonella: Insights by Whole-Genome Sequencing. Microb. Genom. 2018;4:e000195. doi: 10.1099/mgen.0.000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampel K.A. Chapter 7—Antimicrobial Resistance in Shigella Species. In: Chen C.-Y., Yan X., Jackson C.R., editors. Antimicrobial Resistance and Food Safety. Academic Press; San Diego, CA, USA: 2015. pp. 119–135. [Google Scholar]
- 18.WHO Multi-Drug Resistant Gonorrhoea. [(accessed on 21 November 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea.
- 19.Li H., Yuan J., Duan S., Pang Y. Resistance and Tolerance of Mycobacterium tuberculosis to Antimicrobial Agents—How M. Tuberculosis Can Escape Antibiotics. WIREs Mech. Dis. 2022;14:e1573. doi: 10.1002/wsbm.1573. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler J., Stevens D.A. Antifungal Drug Resistance. Clin. Infect. Dis. 2003;36:S31–S41. doi: 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- 21.Rodero L., Mellado E., Rodriguez A.C., Salve A., Guelfand L., Cahn P., Cuenca-Estrella M., Davel G., Rodriguez-Tudela J.L. G484S Amino Acid Substitution in Lanosterol 14-α Demethylase (ERG11) Is Related to Fluconazole Resistance in a Recurrent Cryptococcus Neoformans Clinical Isolate. Antimicrob. Agents Chemother. 2003;47:3653–3656. doi: 10.1128/aac.47.11.3653-3656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard S.J., Arendrup M.C. Acquired Antifungal Drug Resistance in Aspergillus fumigatus: Epidemiology and Detection. Med. Mycol. 2011;49:S90–S95. doi: 10.3109/13693786.2010.508469. [DOI] [PubMed] [Google Scholar]
- 23.Cuenca-Estrella M., Gomez-Lopez A., Mellado E., Buitrago M.J., Monzón A., Rodriguez-Tudela J.L. Scopulariopsis brevicaulis, a Fungal Pathogen Resistant to Broad-Spectrum Antifungal Agents. Antimicrob. Agents Chemother. 2003;47:2339–2341. doi: 10.1128/AAC.47.7.2339-2341.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lurain N.S., Chou S. Antiviral Drug Resistance of Human Cytomegalovirus. Clin. Microbiol. Rev. 2010;23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wutzler P. Antiviral Therapy of Herpes Simplex and Varicella-Zoster Virus Infections. Intervirology. 2008;40:343–356. doi: 10.1159/000150567. [DOI] [PubMed] [Google Scholar]
- 26.Cortez K.J., Maldarelli F. Clinical Management of HIV Drug Resistance. Viruses. 2011;3:347–378. doi: 10.3390/v3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurt A.C. The Epidemiology and Spread of Drug Resistant Human Influenza Viruses. Curr. Opin. Virol. 2014;8:22–29. doi: 10.1016/j.coviro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Suppiah J., Mohd Zain R., Haji Nawi S., Bahari N., Saat Z. Drug-Resistance Associated Mutations in Polymerase (p) Gene of Hepatitis B Virus Isolated from Malaysian HBV Carriers. Hepat. Mon. 2014;14:e13173. doi: 10.5812/hepatmon.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloland P.B. Drug Resistance in Malaria. WHO; Geneva, Switzerland: 2001. A Background Document for the WHO Global Strategy for Containment of Antimicrobial Resistance. [Google Scholar]
- 30.Vanaerschot M., Dumetz F., Roy S., Ponte-Sucre A., Arevalo J., Dujardin J.-C. Treatment Failure in Leishmaniasis: Drug-Resistance or Another (Epi-) Phenotype? Expert Rev. Anti-Infect. Ther. 2014;12:937–946. doi: 10.1586/14787210.2014.916614. [DOI] [PubMed] [Google Scholar]
- 31.Mohapatra S. Drug Resistance in Leishmaniasis: Newer Developments. Trop. Parasitol. 2014;4:4–9. doi: 10.4103/2229-5070.129142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallon P.G., Doenhoff M.J. Drug-Resistant Schistosomiasis: Resistance to Praziquantel and Oxamniquine Induced in Schistosoma Mansoni in Mice Is Drug Specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 33.Qi L., Cui J. A Schistosomiasis Model with Praziquantel Resistance. Discret. Dyn. Nat. Soc. 2013;2013:945767. doi: 10.1155/2013/945767. [DOI] [Google Scholar]
- 34.Bansal D., Malla N., Mahajan R. Drug Resistance in Amoebiasis. Indian J. Med. Res. 2006;123:115–118. [PubMed] [Google Scholar]
- 35.Muzny C.A., Schwebke J.R. The Clinical Spectrum of Trichomonas Vaginalis Infection and Challenges to Management. Sex. Transm. Infect. 2013;89:423–425. doi: 10.1136/sextrans-2012-050893. [DOI] [PubMed] [Google Scholar]
- 36.McFadden D.C., Tomavo S., Berry E.A., Boothroyd J.C. Characterization of Cytochrome b from Toxoplasma Gondii and Qo Domain Mutations as a Mechanism of Atovaquone-Resistance. Mol. Biochem. Parasitol. 2000;108:1–12. doi: 10.1016/S0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 37.Nagamune K., Moreno S.N., Sibley L.D. Artemisinin-Resistant Mutants of Toxoplasma gondii Have Altered Calcium Homeostasis. Antimicrob. Agents Chemother. 2007;51:3816–3823. doi: 10.1128/AAC.00582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doliwa C., Escotte-Binet S., Aubert D., Sauvage V., Velard F., Schmid A., Villena I. Sulfadiazine Resistance in Toxoplasma gondii: No Involvement of Overexpression or Polymorphisms in Genes of Therapeutic Targets and ABC Transporters. Parasite. 2013;20:19. doi: 10.1051/parasite/2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merchel Piovesan Pereira B., Tagkopoulos I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019;85:e00377-19. doi: 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osmanov A., Farooq Z., Richardson M.D., Denning D.W. The antiseptic Miramistin: A review of its comparative in vitro and clinical activity. FEMS Microbiol. Rev. 2020;44:399–417. doi: 10.1093/femsre/fuaa012. [DOI] [PubMed] [Google Scholar]
- 41.Kampf G. Didecyldimethylammonium Chloride. In: Kampf G., editor. Antiseptic Stewardship: Biocide Resistance and Clinical Implications. Springer International Publishing; Cham, Switzerland: 2018. pp. 371–394. [Google Scholar]
- 42.Mao X., Auer David L., Buchalla W., Hiller K.-A., Maisch T., Hellwig E., Al-Ahmad A., Cieplik F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020;64:e00576-20. doi: 10.1128/AAC.00576-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assadian O. Octenidine dihydrochloride: Chemical characteristics and antimicrobial properties. J. Wound Care. 2016;25:S3–S6. doi: 10.12968/jowc.2016.25.Sup3.S3. [DOI] [PubMed] [Google Scholar]
- 44.Kwaśniewska D., Chen Y.-L., Wieczorek D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens. 2020;9:459. doi: 10.3390/pathogens9060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaud M.E., Allen R.A., Morrison-Lewis K.R., Sanchez C.A., Minbiole K.P.C., Post S.J., Wuest W.M. Quaternary Phosphonium Compound Unveiled as a Potent Disinfectant against Highly Resistant Acinetobacter baumannii Clinical Isolates. ACS Infect. Dis. 2022;8:2307–2314. doi: 10.1021/acsinfecdis.2c00382. [DOI] [PubMed] [Google Scholar]
- 46.Rather M.A., Gupta K., Mandal M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021;52:1701–1718. doi: 10.1007/s42770-021-00624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos A.L.S., dos Galdino A.C.M., Mello T.P., de Ramos L.D.S., Branquinha M.H., Bolognese A.M., Columbano Neto J., Roudbary M. What Are the Advantages of Living in a Community? A Microbial Biofilm Perspective! Memórias Do Inst. Oswaldo Cruz. 2018;113:e180212. doi: 10.1590/0074-02760180212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penesyan A., Paulsen I.T., Kjelleberg S., Gillings M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. NPJ Biofilms Microbiomes. 2021;7:80. doi: 10.1038/s41522-021-00251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flemming H.-C., Wuertz S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019;17:247–260. doi: 10.1038/s41579-019-0158-9. [DOI] [PubMed] [Google Scholar]
- 50.Dincer S., Özdenefe M.S., Arkut A. Bacterial Biofilms. IntechOpen; Rijeka, Yugoslavia: 2020. [Google Scholar]
- 51.Kostakioti M., Hadjifrangiskou M., Hultgren S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013;3:a010306. doi: 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Vos W.M. Microbial Biofilms and the Human Intestinal Microbiome. NPJ Biofilms Microbiomes. 2015;1:15005. doi: 10.1038/npjbiofilms.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carthey A.J.R., Blumstein D.T., Gallagher R.V., Tetu S.G., Gillings M.R. Conserving the Holobiont. Funct. Ecol. 2020;34:764–776. doi: 10.1111/1365-2435.13504. [DOI] [Google Scholar]
- 54.Jiang Y., Geng M., Bai L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms. 2020;8:1222. doi: 10.3390/microorganisms8081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penesyan A., Gillings M., Paulsen I.T. Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules. 2015;20:5286–5298. doi: 10.3390/molecules20045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donlan R.M., Costerton J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donlan R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Percival S.L., Kite P. Intravascular Catheters and Biofilm Control. J. Vasc. Access. 2007;8:69–80. doi: 10.1177/112972980700800202. [DOI] [PubMed] [Google Scholar]
- 59.Safdar N., Mermel L.A., Maki D.G. The epidemiology of catheter-related infection in the critically ill. In: O’Grady N., Pittet D., editors. Catheter-Related Infections in the Critically Ill. Kluwer; New York, NY, USA: 2004. pp. 1–23. [Google Scholar]
- 60.Donlan R., Murga R., Carson L. Growing biofilms in intravenous fluids. In: Wimpenny J., Gilbert P., Walker J., Brading M., Bayston R., editors. Biofilms, the Good, the Bad, and the Ugly. Biofilm Club; Powys, UK: 1999. pp. 23–29. [Google Scholar]
- 61.Chow K.M., Li P.K.-T., Cho Y., Abu-Alfa A., Bavanandan S., Brown E.A., Cullis B., Edwards D., Ethier I., Hurst H., et al. ISPD Catheter-Related Infection Recommendations: 2023 Update. Perit. Dial. Int. 2023;43:201–219. doi: 10.1177/08968608231172740. [DOI] [PubMed] [Google Scholar]
- 62.Zheng J.M.D., Romanelli A.M. An Overview of Prosthetic Joint Infection (PJI) Definition and Diagnosis. 13 July 2021. [(accessed on 21 November 2023)]. Available online: https://health.ucdavis.edu/blog/lab-best-practice/an-overview-of-prosthetic-joint-infection-pji-definition-and-diagnosis/2021/07#:~:text=Pathogens%20involved%20in%20Prosthetic%20joint%20infection%201%20Early,after%20surgery%29%20Staphylococcus%20aureus%20Gram-negative%20bacilli%20Beta-hemolytic%20streptococci.
- 63.Palmeri N.O., Kramer D.B., Karchmer A.W., Zimetbaum P.J. A Review of Cardiac Implantable Electronic Device Infections for the Practicing Electrophysiologist. JACC Clin. Electrophysiol. 2021;7:811–824. doi: 10.1016/j.jacep.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Kokare C.R., Chakraborty S., Khopade A., Mahadik K.R. Biofilm: Importance and Applications. Indian J. Biotechnol. 2009;8:159–168. [Google Scholar]
- 65.Stickler D.J. Bacterial Biofilms and the Encrustation of Urethral Catheters. Biofouling. 1996;9:293–305. doi: 10.1080/08927019609378311. [DOI] [Google Scholar]
- 66.Pentland D.R., Stevens S., Williams L., Baker M., McCall C., Makarovaite V., Balfour A., Mühlschlegel F.A., Gourlay C.W. Precision Antifungal Treatment Significantly Extends Voice Prosthesis Lifespan in Patients Following Total Laryngectomy. Front. Microbiol. 2020;11:975. doi: 10.3389/fmicb.2020.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kokare C.R., Kadam S.S., Mahadik K.R., Chopade B.A. Studies on bioemulsifier production from marine Streptomyces sp. S1. Ind. J. Biotechnol. 2007;6:78–84. [Google Scholar]
- 68.Lamont R.J., Jenkinson H.F. Life Below the Gum Line: Pathogenic Mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/MMBR.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Overman P.R. Biofilm: A New View of Plaque. J. Contemp. Dent. Pract. 2000;1:18–29. doi: 10.5005/jcdp-1-3-37. [DOI] [PubMed] [Google Scholar]
- 70.Nasser A., Azimi T., Ostadmohammadi S., Ostadmohammadi S. A comprehensive review of bacterial osteomyelitis with emphasis on Staphylococcus aureus. Microb. Pathog. 2020;148:104431. doi: 10.1016/j.micpath.2020.104431. [DOI] [PubMed] [Google Scholar]
- 71.Mirzaei R., Mohammadzadeh R., Alikhani M.Y., Shokri Moghadam M., Karampoor S., Kazemi S., Barfipoursalar A., Yousefimashouf R. The Biofilm-Associated Bacterial Infections Unrelated to Indwelling Devices. IUBMB Life. 2020;72:1271–1285. doi: 10.1002/iub.2266. [DOI] [PubMed] [Google Scholar]
- 72.Arunasri K., Mohan S.V. Chapter 2.3—Biofilms: Microbial Life on the Electrode Surface. In: Mohan S.V., Varjani S., Pandey A., editors. Microbial Electrochemical Technology. Elsevier; Amsterdam, The Netherlands: 2019. pp. 295–313. [Google Scholar]
- 73.Jamal M., Tasneem U., Hussain T., Andleeb S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. Res. Rev. J. Microbiol. Biotechnol. 2015;4:59 [Google Scholar]
- 74.Monroe D. Looking for Chinks in the Armor of Bacterial Biofilms. PLoS Biol. 2007;5:e307. doi: 10.1371/journal.pbio.0050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan J.B. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010;89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies D.G., Marques C.N.H. A Fatty Acid Messenger Is Responsible for Inducing Dispersion in Microbial Biofilms. J. Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rice S.A., Koh K.S., Queck S.Y., Labbate M., Lam K.W., Kjelleberg S. Biofilm Formation and Sloughing in Serratia marcescens Are Controlled by Quorum Sensing and Nutrient Cues. J. Bacteriol. 2005;187:3477–3485. doi: 10.1128/JB.187.10.3477-3485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Preda V., Sandulescu O. Communication Is the Key: Biofilms, Quorum Sensing, Formation and Prevention. Discoveries. 2019;7:e10. doi: 10.15190/d.2019.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma S.C., Miyashiro T. Quorum Sensing in the Squid-Vibrio Symbiosis. Int. J. Mol. Sci. 2013;14:16386–16401. doi: 10.3390/ijms140816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brackman G., Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015;21:5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 81.Li Z., Nair S.K. Quorum Sensing: How Bacteria Can Coordinate Activity and Synchronize Their Response to External Signals? Protein Sci. 2012;21:1403–1417. doi: 10.1002/pro.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Q., Chen J., Yang C., Yin Y., Yao K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. BioMed Res. Int. 2019;2019:2015978. doi: 10.1155/2019/2015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schirmer T. C-Di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation. J. Mol. Biol. 2016;428:3683–3701. doi: 10.1016/j.jmb.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 84.Lin Y., Mi D., Hou Y., Lin M., Xie Q., Niu X., Chen Y., He C., Tao J., Li C. Systematic Analysis of the Roles of C-Di-GMP Signaling in Xanthomonas oryzae Pv. Oryzae Virulence. FEMS Microbiol. Lett. 2022;369:fnac068. doi: 10.1093/femsle/fnac068. [DOI] [PubMed] [Google Scholar]
- 85.Valentini M., Filloux A. Biofilms and Cyclic Di-GMP (c-Di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria. J. Biol. Chem. 2016;291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dieltjens L., Appermans K., Lissens M., Lories B., Kim W., Van der Eycken E.V., Foster K.R., Steenackers H.P. Inhibiting Bacterial Cooperation Is an Evolutionarily Robust Anti-Biofilm Strategy. Nat. Commun. 2020;11:107. doi: 10.1038/s41467-019-13660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.-F., Alarcon E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alfei S., Schito A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials. 2020;10:2022. doi: 10.3390/nano10102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terreni M., Taccani M., Pregnolato M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules. 2021;26:2671. doi: 10.3390/molecules26092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mataraci E., Dosler S. In Vitro Activities of Antibiotics and Antimicrobial Cationic Peptides Alone and in Combination against Methicillin-Resistant Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2012;56:6366–6371. doi: 10.1128/AAC.01180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dosler S., Mataraci E. In Vitro Pharmacokinetics of Antimicrobial Cationic Peptides Alone and in Combination with Antibiotics against Methicillin Resistant Staphylococcus aureus Biofilms. Peptides. 2013;49:53–58. doi: 10.1016/j.peptides.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Dosler S., Karaaslan E. Inhibition and Destruction of Pseudomonas aeruginosa Biofilms by Antibiotics and Antimicrobial Peptides. Peptides. 2014;62:32–37. doi: 10.1016/j.peptides.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 93.Field D., O’ Connor R., Cotter P.D., Ross R.P., Hill C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus biofilms. Front. Microbiol. 2016;7:508. doi: 10.3389/fmicb.2016.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obłąk E., Gamian A. Biologiczna aktywność czwartorzędowych soli amoniowych (CSA) * The biological activity of quaternary ammonium salts (QASs) Postepy Hig. Med. Dosw. 2010;64:201–211. [PubMed] [Google Scholar]
- 95.Gerba C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015;81:464–469. doi: 10.1128/AEM.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cole E.C., Addison R.M., Rubino J.R., Leese K.E., Dulaney P.D., Newell M.S., Wilkins J., Gaber D.J., Wineinger T., Criger D.A. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J. Appl. Microbiol. 2003;95:664–676. doi: 10.1046/j.1365-2672.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 97.Mbithi J.N., Springthorpe V.S., Sattar S.A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl. Environ. Microbiol. 1990;56:3601–3604. doi: 10.1128/aem.56.11.3601-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tischer M., Pradel G., Ohlsen K., Holzgrabe U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? Chem. Med. Chem. 2012;7:22–31. doi: 10.1002/cmdc.201100404. [DOI] [PubMed] [Google Scholar]
- 99.Gilbert P., Moore L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 100.Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Bragg R., Jansen A., Coetzee M., van der Westhuizen W., Boucher C. Bacterial resistance to quaternary ammonium compounds (QAC) disinfectants. In: Adhikari R., Thapa S., editors. Infectious Diseases and Nanomedicine II. Volume 808. Springer; New Delhi, India: 2014. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 102.Peng Z.-X., Tu B., Shen Y., Du L., Wang L., Guo S.-R., Tang T.-T. Quaternized Chitosan Inhibits icaA Transcription and Biofilm Formation by Staphylococcus on a Titanium Surface. Antimicrob. Agents Chemother. 2011;55:860–866. doi: 10.1128/AAC.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan H., Peng Z., Li Q., Xu X., Guo S., Tang T. The Use of Quaternised Chitosan-Loaded PMMA to Inhibit Biofilm Formation and Downregulate the Virulence-Associated Gene Expression of Antibiotic-Resistant Staphylococcus. Biomaterials. 2012;33:365–377. doi: 10.1016/j.biomaterials.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 104.Song J., Kong H., Jang J. Bacterial Adhesion Inhibition of the Quaternary Ammonium Functionalized Silica Nanoparticles. Colloids Surf. B Biointerfaces. 2011;82:651–656. doi: 10.1016/j.colsurfb.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 105.Obłąk E., Piecuch A., Rewak-Soroczyńska J., Paluch E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019;103:625–632. doi: 10.1007/s00253-018-9523-2. [DOI] [PubMed] [Google Scholar]
- 106.Obłąk E., Piecuch A., Krasowska A., Łuczyński J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013;168:630–638. doi: 10.1016/j.micres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Tran P.L., Hamood A.N., de Souza A., Schultz G., Liesenfeld B., Mehta D., Reid T.W. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015;23:74–81. doi: 10.1111/wrr.12244. [DOI] [PubMed] [Google Scholar]
- 108.Kocak H.S., Bulut O., Yilmaz M.D. A Dicationic BODIPY-Based Fluorescent Bactericide to Combat Infectious Diseases and to Eradicate Bacterial Biofilms. ACS Appl. BioMaterials. 2023;6:1604–1610. doi: 10.1021/acsabm.3c00021. [DOI] [PubMed] [Google Scholar]
- 109.Ibrahim M.S., Ibrahim A.S., Balhaddad A.A., Weir M.D., Lin N.J., Tay F.R., Oates T.W., Xu H.H.K., Melo M.A.S. A Novel Dental Sealant Containing Dimethylaminohexadecyl Methacrylate Suppresses the Cariogenic Pathogenicity of Streptococcus Mutans Biofilms. Int. J. Mol. Sci. 2019;20:3491. doi: 10.3390/ijms20143491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daood U., Matinlinna J.P., Pichika M.R., Mak K.-K., Nagendrababu V., Fawzy A.S. A Quaternary Ammonium Silane Antimicrobial Triggers Bacterial Membrane and Biofilm Destruction. Sci. Rep. 2020;10:10970. doi: 10.1038/s41598-020-67616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balbinot G.S., Marcon N., Sauro S., Luxan S.A., Collares F.M. Alkyl Trimethyl Ammonium Bromide for the Formulation of Antibacterial Orthodontic Resins. Clin. Oral Investig. 2022;26:7011–7019. doi: 10.1007/s00784-022-04661-0. [DOI] [PubMed] [Google Scholar]
- 112.Phuangkaew T., Booranabunyat N., Kiatkamjornwong S., Thanyasrisung P., Hoven V.P. Amphiphilic Quaternized Chitosan: Synthesis, Characterization, and Anti-Cariogenic Biofilm Property. Carbohydr. Polym. 2022;277:118882. doi: 10.1016/j.carbpol.2021.118882. [DOI] [PubMed] [Google Scholar]
- 113.He Y., Wan X., Xiao K., Lin W., Li J., Li Z., Luo F., Tan H., Li J., Fu Q. Anti-Biofilm Surfaces from Mixed Dopamine-Modified Polymer Brushes: Synergistic Role of Cationic and Zwitterionic Chains to Resist: Staphyloccocus aureus. Biomater. Sci. 2019;7:5369–5382. doi: 10.1039/C9BM01275C. [DOI] [PubMed] [Google Scholar]
- 114.Yu J., Huang X., Zhou X., Han Q., Zhou W., Liang J., Xu H.H.K., Ren B., Peng X., Weir M.D., et al. Anti-Caries Effect of Resin Infiltrant Modified by Quaternary Ammonium Monomers. J. Dent. 2020;97:103355. doi: 10.1016/j.jdent.2020.103355. [DOI] [PubMed] [Google Scholar]
- 115.Fedorowicz J., Cruz C.D., Morawska M., Ciura K., Gilbert-Girard S., Mazur L., Mäkkylä H., Ilina P., Savijoki K., Fallarero A., et al. Antibacterial and Antibiofilm Activity of Permanently Ionized Quaternary Ammonium Fluoroquinolones. European J. Med. Chem. 2023;254:115373. doi: 10.1016/j.ejmech.2023.115373. [DOI] [PubMed] [Google Scholar]
- 116.Daood U., Bapat R.A., Sidhu P., Ilyas M.S., Khan A.S., Mak K.-K., Pichika M.R., Nagendrababu V., Peters O.A. Antibacterial and Antibiofilm Efficacy of K21-E in Root Canal Disinfection. Dent. Mater. 2021;37:1511–1528. doi: 10.1016/j.dental.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Piras A.M., Esin S., Benedetti A., Maisetta G., Fabiano A., Zambito Y., Batoni G. Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives. Int. J. Mol. Sci. 2019;20:6297. doi: 10.3390/ijms20246297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fugolin A.P., Dobson A., Huynh V., Mbiya W., Navarro O., Franca C.M., Logan M., Merritt J.L., Ferracane J.L., Pfeifer C.S. Antibacterial, Ester-Free Monomers: Polymerization Kinetics, Mechanical Properties, Biocompatibility and Anti-Biofilm Activity. Acta Biomater. 2019;100:132–141. doi: 10.1016/j.actbio.2019.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rita Pereira A., Gomes I.B., Simões M. Choline-Based Ionic Liquids for Planktonic and Biofilm Growth Control of Bacillus cereus and Pseudomonas fluorescens. J. Mol. Liq. 2022;346:117077. doi: 10.1016/j.molliq.2021.117077. [DOI] [Google Scholar]
- 120.Gaspar C., Rolo J., Cerca N., Palmeira de Oliveira R., Martinez-de-Oliveira J., Palmeira-de-Oliveira A. Dequalinium Chloride Effectively Disrupts Bacterial Vaginosis (BV) Gardnerella Spp. Biofilms. Pathogens. 2021;10:261. doi: 10.3390/pathogens10030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y., Wang H., Zheng X., Li Z., Wang M., Luo K., Zhang C., Xia X., Wang Y., Shi C. Didecyldimethylammonium Bromide: Application to Control Biofilms of Staphylococcus aureus and Pseudomonas aeruginosa Alone and in Combination with Slightly Acidic Electrolyzed Water. Food Res. Int. 2022;157:111236. doi: 10.1016/j.foodres.2022.111236. [DOI] [PubMed] [Google Scholar]
- 122.Yu W., Ren C., Zhang N., Cao L., Weir M.D., Yang K., Xu H.H.K., Bai Y. Dual Function of Anti-Biofilm and Modulating Biofilm Equilibrium of Orthodontic Cement Containing Quaternary Ammonium Salt. Dent. Mater. J. 2023;42:149–157. doi: 10.4012/dmj.2022-142. [DOI] [PubMed] [Google Scholar]
- 123.Moshynets O.V., Baranovskyi T.P., Iungin O.S., Kysil N.P., Metelytsia L.O., Pokholenko I., Potochilova V.V., Potters G., Rudnieva K.L., Rymar S.Y., et al. eDNA Inactivation and Biofilm Inhibition by the Polymeric Biocide Polyhexamethylene Guanidine Hydrochloride (PHMG-Cl) Int. J. Mol. Sci. 2022;23:731. doi: 10.3390/ijms23020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daood U., Burrow M.F., Yiu C.K.Y. Effect of a Novel Quaternary Ammonium Silane Cavity Disinfectant on Cariogenic Biofilm Formation. Clin. Oral Investig. 2020;24:649–661. doi: 10.1007/s00784-019-02928-7. [DOI] [PubMed] [Google Scholar]
- 125.Feng J., Cheng L., Zhou X., Xu H.H.K., Weir M.D., Li Q., Hannig M., Rupf S. Effects of Water Aging on the Mechanical and Anti-Biofilm Properties of Glass-Ionomer Cement Containing Dimethylaminododecyl Methacrylate. Dent. Mater. 2019;35:434–443. doi: 10.1016/j.dental.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 126.Zhou C., Song H., Loh J.L.C., She J., Deng L., Liu B. Grafting Antibiofilm Polymer Hydrogel Film onto Catheter by SARA SI-ATRP. J. Biomater. Sci. Polym. Ed. 2018;29:2106–2123. doi: 10.1080/09205063.2018.1507268. [DOI] [PubMed] [Google Scholar]
- 127.Kuppusamy R., Yasir M., Yee E., Willcox M., Black D.S.C., Kumar N. Guanidine Functionalized Anthranilamides as Effective Antibacterials with Biofilm Disruption Activity. Org. Biomol. Chem. 2018;16:5871–5888. doi: 10.1039/C8OB01699B. [DOI] [PubMed] [Google Scholar]
- 128.Melo M.A.S., Weir M.D., Passos V.F., Rolim J.P.M., Lynch C.D., Rodrigues L.K.A., Xu H.H.K. Human in Situ Study of the Effect of Bis(2-Methacryloyloxyethyl) Dimethylammonium Bromide Immobilized in Dental Composite on Controlling Mature Cariogenic Biofilm. Int. J. Mol. Sci. 2018;19:3443. doi: 10.3390/ijms19113443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frolov N., Detusheva E., Fursova N., Ostashevskaya I., Vereshchagin A. Microbiological Evaluation of Novel Bis-Quaternary Ammonium Compounds: Clinical Strains, Biofilms, and Resistance Study. Pharmaceuticals. 2022;15:514. doi: 10.3390/ph15050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soares N.S., Hollanda L.M., Elias C.M.V., Azevedo M.M.F., Santos F.E.P., Lobo A.O., Marciano F.R. Modified PCL/PEG/GelMA Electrospun Blends Reduced Biofilm Formation. Mater. Lett. 2022;320:132315. doi: 10.1016/j.matlet.2022.132315. [DOI] [Google Scholar]
- 131.Ran H.-H., Cheng X., Bao Y.-W., Hua X.-W., Gao G., Zhang X., Jiang Y.-W., Zhu Y.-X., Wu F.-G. Multifunctional Quaternized Carbon Dots with Enhanced Biofilm Penetration and Eradication Efficiencies. J. Mater. Chem. B. 2019;7:5104–5114. doi: 10.1039/C9TB00681H. [DOI] [PubMed] [Google Scholar]
- 132.Li Y., Zhen J., Tian Q., Shen C., Zhang L., Yang K., Shang L. One Step Synthesis of Positively Charged Gold Nanoclusters as Effective Antimicrobial Nanoagents against Multidrug-Resistant Bacteria and Biofilms. J. Colloid Interface Sci. 2020;569:235–243. doi: 10.1016/j.jcis.2020.02.084. [DOI] [PubMed] [Google Scholar]
- 133.Yang J., Zhu Y.-X., Lu P., Zhu B., Wu F.-G. One-Step Synthesis of Quaternized Silica Nanoparticles with Bacterial Adhesion and Aggregation Properties for Effective Antibacterial and Antibiofilm Treatments. J. Mater. Chem. B. 2022;10:3073–3082. doi: 10.1039/D1TB02830H. [DOI] [PubMed] [Google Scholar]
- 134.Xia L., Tian J., Yue T., Cao H., Chu J., Cai H., Zhang W. Pillar [5]Arene-Based Acid-Triggered Supramolecular Porphyrin Photosensitizer for Combating Bacterial Infections and Biofilm Dispersion. Adv. Healthc. Mater. 2022;11:2102015. doi: 10.1002/adhm.202102015. [DOI] [PubMed] [Google Scholar]
- 135.Lan T., Guo Q., Shen X. Polyethyleneimine and Quaternized Ammonium Polyethyleneimine: The Versatile Materials for Combating Bacteria and Biofilms. J. Biomater. Sci. Polym. Ed. 2019;30:1243–1259. doi: 10.1080/09205063.2019.1627650. [DOI] [PubMed] [Google Scholar]
- 136.Yu T.T., Kuppusamy R., Yasir M., Hassan M.M., Sara M., Ho J., Willcox M.D.P., Black D.S., Kumar N. Polyphenylglyoxamide-based Amphiphilic Small Molecular Peptidomimetics as Antibacterial Agents with Anti-biofilm Activity. Int. J. Mol. Sci. 2021;22:7344. doi: 10.3390/ijms22147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balhaddad A.A., Ibrahim M.S., Garcia I.M., Collares F.M., Weir M.D., Xu H.H., Melo M.A.S. Pronounced Effect of Antibacterial Bioactive Dental Composite on Microcosm Biofilms Derived from Patients with Root Carious Lesions. Front. Mater. 2020;7:583861. doi: 10.3389/fmats.2020.583861. [DOI] [Google Scholar]
- 138.He D., Yu Y., Liu F., Yao Y., Li P., Chen J., Ning N., Zhang S. Quaternary Ammonium Salt-Based Cross-Linked Micelle Templated Synthesis of Highly Active Silver Nanocomposite for Synergistic Anti-Biofilm Application. Chem. Eng. J. 2020;382:122976. doi: 10.1016/j.cej.2019.122976. [DOI] [Google Scholar]
- 139.Wu G.-Y., Yu L., Wang Y.-R., Yuan X., Tang Y.-F., Chen W., Zeng L.-Z. Quaternary Ammonium Salt-Based Cross-Linked Micelle with Copper Nanoparticles for Treatment of Sulfate Reducing Bacteria Biofilm. React. Funct. Polym. 2022;180:105405. doi: 10.1016/j.reactfunctpolym.2022.105405. [DOI] [Google Scholar]
- 140.Zhou Y., Wang S., Zhou X., Zou Y., Li M., Peng X., Ren B., Xu H.H.K., Weir M.D., Cheng L., et al. Short-Time Antibacterial Effects of Dimethylaminododecyl Methacrylate on Oral Multispecies Biofilm in Vitro. BioMed Res. Int. 2019;2019:6393470. doi: 10.1155/2019/6393470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hernando-Gozalo M., Aguilera-Correa J.J., Rescalvo-Casas C., Seijas-Pereda L., García-Bertolín C., de la Mata F.J., Sánchez-Nieves J., Cuadros J., Pérez-Tanoira R. Study of the Antimicrobial Activity of Cationic Carbosilane Dendrimers against Clinical Strains of Multidrug-Resistant Bacteria and Their Biofilms. Front. Cell. Infect. Microbiol. 2023;13:3991. doi: 10.3389/fcimb.2023.1203991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao S., Sun Y., Lu Z., Jiang N., Yao H. Synergistic Antibacterial and Biofilm Eradication Activity of Quaternary-Ammonium Compound with Copper Ion. J. Inorg. Biochem. 2023;243:112190. doi: 10.1016/j.jinorgbio.2023.112190. [DOI] [PubMed] [Google Scholar]
- 143.Ali I., Burki S., El-Haj B.M., Parveen S., Nadeem H.Ş., Nadeem S., Shah M.R. Synthesis and Characterization of Pyridine-Based Organic Salts: Their Antibacterial, Antibiofilm and Wound Healing Activities. Bioorganic Chem. 2020;100:103937. doi: 10.1016/j.bioorg.2020.103937. [DOI] [PubMed] [Google Scholar]
- 144.Garipov M.R., Sabirova A.E., Pavelyev R.S., Shtyrlin N.V., Lisovskaya S.A., Bondar O.V., Laikov A.V., Romanova J.G., Bogachev M.I., Kayumov A.R., et al. Targeting Pathogenic Fungi, Bacteria and Fungal-Bacterial Biofilms by Newly Synthesized Quaternary Ammonium Derivative of Pyridoxine and Terbinafine with Dual Action Profile. Bioorganic Chem. 2020;104:104306. doi: 10.1016/j.bioorg.2020.104306. [DOI] [PubMed] [Google Scholar]
- 145.Hympanova M., Terlep S., Markova A., Prchal L., Dogsa I., Pulkrabkova L., Benkova M., Marek J., Stopar D. The Antibacterial Effects of New N-Alkylpyridinium Salts on Planktonic and Biofilm Bacteria. Front. Microbiol. 2020;11:573951. doi: 10.3389/fmicb.2020.573951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frolov N.A., Seferyan M.A., Valeev A.B., Saverina E.A., Detusheva E.V., Vereshchagin A.N. The Antimicrobial and Antibiofilm Potential of New Water-Soluble Tris-Quaternary Ammonium Compounds. Int. J. Mol. Sci. 2023;24:10512. doi: 10.3390/ijms241310512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koziróg A., Otlewska A., Brycki B. Viability, Enzymatic and Protein Profiles of Pseudomonas aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules. 2018;23:1294. doi: 10.3390/molecules23061294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L., Xin M., Li M., Liu W., Mao Y. Effect of the Structure of Chitosan Quaternary Phosphonium Salt and Chitosan Quaternary Ammonium Salt on the Antibacterial and Antibiofilm Activity. Int. J. Biol. Macromol. 2023;242:124877. doi: 10.1016/j.ijbiomac.2023.124877. [DOI] [PubMed] [Google Scholar]
- 149.Keasler V., Bennett B., McGinley H. Analysis of Bacterial Kill Versus Corrosion from Use of Common Oilfield Biocides; Proceedings of the 2010 8th International Pipeline Conference; Calgary, AB, Canada. 27 September–1 October 2010; New York, NY, USA: ASME; pp. 935–944. [DOI] [Google Scholar]
- 150.Kramer J.F., Arpita S., Strba S.F. NACE CORROSION. NACE; San Antonio, TX, USA: 2010. Comparative Performance of Biocides Versus Corrosion Causing Biofilms. [Google Scholar]
- 151.Kayumov A.R., Nureeva A.A., Trizna E.Y., Gazizova G.R., Bogachev M.I., Shtyrlin N.V., Pugachev M.V., Sapozhnikov S.V., Shtyrlin Y.G. New Derivatives of Pyridoxine Exhibit High Antibacterial Activity against Biofilm-Embedded Staphylococcus Cells. BioMed Res. Int. 2015;2015:890968. doi: 10.1155/2015/890968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Joseph R., Naugolny A., Feldman M., Herzog I.M., Fridman M., Cohen Y. Cationic Pillararenes Potently Inhibit Biofilm Formation without Affecting Bacterial Growth and Viability. J. Am. Chem. Soc. 2016;138:754–757. doi: 10.1021/jacs.5b11834. [DOI] [PubMed] [Google Scholar]
- 153.Joseph R., Kaizerman D., Herzog I.M., Hadar M., Feldman M., Fridman M., Cohen Y. Phosphonium Pillar [5]Arenes as a New Class of Efficient Biofilm Inhibitors: Importance of Charge Cooperativity and the Pillar Platform. Chem. Commun. 2016;52:10656–10659. doi: 10.1039/C6CC05170G. [DOI] [PubMed] [Google Scholar]
- 154.Das S., Paul A., Bera D., Dey A., Roy A., Dutta A., Ganguly D. Design, Development and Mechanistic Insights into the Enhanced Antibacterial Activity of Mono and Bis-Phosphonium Fluoresceinate Ionic Liquids. Mater. Today Commun. 2021;28:102672. doi: 10.1016/j.mtcomm.2021.102672. [DOI] [Google Scholar]
- 155.Cieniecka-Rosłonkiewicz A., Pernak J., Kubis-Feder J., Ramani A., Robertson A.J., Seddon K.R. Synthesis, Anti-Microbial Activities and Anti-Electrostatic Properties of Phosphonium-Based Ionic Liquids. Green Chem. 2005;7:855–862. doi: 10.1039/b508499g. [DOI] [Google Scholar]
- 156.Shive P.N., Diehl J.F. Reduction of Hematite to Magnetite under Natural and Laboratory Conditions. J. Geomagn. Geoelectr. 1977;29:345–354. doi: 10.5636/jgg.29.345. [DOI] [Google Scholar]
- 157.Atefi F., Garcia M.T., Singer R.D., Scammells P.J. Phosphonium Ionic Liquids: Design, Synthesis and Evaluation of Biodegradability. Green Chem. 2009;11:1595–1604. doi: 10.1039/b913057h. [DOI] [Google Scholar]
- 158.Metelytsia L.O., Hodyna D.M., Semenyuta I.V., Kovalishyn V.V., Rogalsky S.P., Derevianko K.Y., Brovarets V.S., Tetko I.V. Theoretical and Experimental Studies of Phosphonium Ionic Liquids as Potential Antibacterials of MDR Acinetobacter baumannii. Antibiotics. 2022;11:491. doi: 10.3390/antibiotics11040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hemp S.T., Zhang M., Allen M.H., Cheng S., Moore R.B., Long T.E. Comparing Ammonium and Phosphonium Polymerized Ionic Liquids: Thermal Analysis, Conductivity, and Morphology. Macromol. Chem. Phys. 2013;214:2099–2107. doi: 10.1002/macp.201300322. [DOI] [Google Scholar]
- 160.Kanazawa A., Ikeda T., Endo T. Synthesis and Antimicrobial Activity of Dimethyl- and Trimethyl-Substituted Phosphonium Salts with Alkyl Chains of Various Lengths. Antimicrob. Agents Chemother. 1994;38:945–952. doi: 10.1128/AAC.38.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kurata S., Hamada N., Kanazawa A., Endo T. Study on Antibacterial Dental Resin Using Tri-n-Butyl(4-Vinylbenzyl)Phosphonium Chloride. Dent. Mater. J. 2011;30:960–966. doi: 10.4012/dmj.2011-157. [DOI] [PubMed] [Google Scholar]
- 162.Kanazawa A., Ikeda T., Endo T. Polymeric Phosphonium Salts as a Novel Class of Cationic Biocides. V. Synthesis and Antibacterial Activity of Polyesters Releasing Phosphonium Biocides. J. Polym. Sci. Part A Polym. Chem. 1993;31:3003–3011. doi: 10.1002/pola.1993.080311216. [DOI] [Google Scholar]
- 163.Chang H.-I., Yang M.-S., Liang M. The Synthesis, Characterization and Antibacterial Activity of Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)s Modified with Ammonium and Phosphonium Salts. React. Funct. Polym. 2010;70:944–950. doi: 10.1016/j.reactfunctpolym.2010.09.005. [DOI] [Google Scholar]
- 164.Kumar V., Malhotra S.V. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorganic Med. Chem. Lett. 2009;19:4643–4646. doi: 10.1016/j.bmcl.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 165.Hatakeyama E.S., Ju H., Gabriel C.J., Lohr J.L., Bara J.E., Noble R.D., Freeman B.D., Gin D.L. New Protein-Resistant Coatings for Water Filtration Membranes Based on Quaternary Ammonium and Phosphonium Polymers. J. Membr. Sci. 2009;330:104–116. doi: 10.1016/j.memsci.2008.12.049. [DOI] [Google Scholar]
- 166.Wang K., Zeng Y., He L., Yao J., Suresh A.K., Bellare J., Sridhar T., Wang H. Evaluation of Quaternary Phosphonium-Based Polymer Membranes for Desalination Application. Desalination. 2012;292:119–123. doi: 10.1016/j.desal.2012.02.016. [DOI] [Google Scholar]
- 167.Brunel F., Lautard C., Giorgio C.D., Garzino F., Raimundo J.-M., Bolla J.M., Camplo M. Antibacterial Activities of Mono-, Di- and Tri-Substituted Triphenylamine-Based Phosphonium Ionic Liquids. Bioorganic Med. Chem. Lett. 2018;28:926–929. doi: 10.1016/j.bmcl.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 168.Taladriz A., Healy A.R., Pérez E.J.F., García V.H., Martínez C.H.R., Alkhaldi A.A.M., Eze A.A., Kaiser M., Koning H.P., de Chana A., et al. Synthesis and Structure-Activity Analysis of New Phosphonium Salts with Potent Activity against African Trypanosomes. J. Med. Chem. 2012;55:2606–2622. doi: 10.1021/jm2014259. [DOI] [PubMed] [Google Scholar]
- 169.Luque-Ortega J.R., Reuther P., Rivas L., Dardonville C. New Benzophenone-Derived Bisphosphonium Salts as Leishmanicidal Leads Targeting Mitochondria through Inhibition of Respiratory Complex II. J. Med. Chem. 2010;53:1788–1798. doi: 10.1021/jm901677h. [DOI] [PubMed] [Google Scholar]
- 170.Kim T.-S., Park H.-D. Tributyl Tetradecyl Phosphonium Chloride for Biofouling Control in Reverse Osmosis Processes. Desalination. 2015;372:39–46. doi: 10.1016/j.desal.2015.06.019. [DOI] [Google Scholar]
- 171.Martín-Rodríguez A.J., Babarro J.M.F., Lahoz F., Sansón M., Martín V.S., Norte M., Fernández J.J. From Broad-Spectrum Biocides to Quorum Sensing Disruptors and Mussel Repellents: Antifouling Profile of Alkyl Triphenylphosphonium Salts. PLoS ONE. 2015;10:e0123652. doi: 10.1371/journal.pone.0123652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Worthington R.J., Richards J.J., Melander C. Small Molecule Control of Bacterial Biofilms. Org. Biomol. Chem. 2012;10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shi L.W., Zhuang Q.Q., Wang T.Q., Jiang X.D., Liu Y., Deng J.W., Sun H.H., Li Y., Li H.H., Liu T.B., et al. Synthetic Antibacterial Quaternary Phosphorus Salts Promote Methicillin-Resistant Staphylococcus aureus-Infected Wound Healing. Int. J. Nanomed. 2023;18:1145–1158. doi: 10.2147/IJN.S398748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo A., Wang F., Lin W., Xu X., Tang T., Shen Y., Guo S. Evaluation of Antibacterial Activity of N-Phosphonium Chitosan as a Novel Polymeric Antibacterial Agent. Int. J. Biol. Macromol. 2014;67:163–171. doi: 10.1016/j.ijbiomac.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 175.Pinnock T., Voordouw J., Voordouw G. Use of Carbon Steel Ball Bearings to Determine the Effect of Biocides and Corrosion Inhibitors on Microbiologically Influenced Corrosion under Flow Conditions. Appl. Microbiol. Biotechnol. 2018;102:5741–5751. doi: 10.1007/s00253-018-8974-9. [DOI] [PubMed] [Google Scholar]
- 176.Okoro C.C. The Biocidal Efficacy of Tetrakis-hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on Oil Pipeline PigRuns Liquid Biofilms. Pet. Sci. Technol. 2015;33:1366–1372. doi: 10.1080/10916466.2015.1062781. [DOI] [Google Scholar]
- 177.Svara J., Weferling N., Hofmann T. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley; Hoboken, NJ, USA: 2000. Phosphorus Compounds, Organic. [DOI] [Google Scholar]
- 178.Pugachev M.V., Shtyrlin N., Sysoeva L.P., Nikitina E.V., Abdullin T.I., Iksanova A.G., Ilaeva A.A., Musin R.Z., Berdnikov E.A., Shtyrlin Y.G. Synthesis and Antibacterial Activity of Novel Phosphonium Salts on the Basis of Pyridoxine. Bioorganic Med. Chem. 2013;21:4388–4395. doi: 10.1016/j.bmc.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 179.Pugachev M.V., Shtyrlin N.V., Sapozhnikov S.V., Sysoeva L.P., Iksanova A.G., Nikitina E.V., Musin R.Z., Lodochnikova O.A., Berdnikov E.A., Shtyrlin Y.G. Bis-Phosphonium Salts of Pyridoxine: The Relationship between Structure and Antibacterial Activity. Bioorganic Med. Chem. 2013;21:7330–7342. doi: 10.1016/j.bmc.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 180.Dharaskar S., Sillanpaa M. Synthesis, Characterization, and Application of Trihexyl(Tetradecyl)Phosphonium Chloride as Promising Solvent for Extractive Desulfurization of Liquid Fuel. Chem. Eng. Res. Des. 2018;133:388–397. doi: 10.1016/j.cherd.2018.03.016. [DOI] [Google Scholar]
- 181.Keglevich G., Grün A., Hermecz I., Odinets I.L. Quaternary Phosphonium Salt and 1,3-Dialkylimidazolium Hexafluorophosphate Ionic Liquids as Green Chemical Tools in Organic Syntheses. Curr. Org. Chem. 2011;15:3824–3848. doi: 10.2174/138527211797884557. [DOI] [Google Scholar]
- 182.Sommers K.J., Michaud M.E., Hogue C.E., Scharnow A.M., Amoo L.E., Petersen A.A., Carden R.G., Minbiole K.P.C., Wuest W.M. Quaternary Phosphonium Compounds: An Examination of Non-Nitrogenous Cationic Amphiphiles That Evade Disinfectant Resistance. ACS Infect. Dis. 2022;8:387–397. doi: 10.1021/acsinfecdis.1c00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang L., Xu X., Guo S., Peng Z., Tang T. Novel Water Soluble Phosphonium Chitosan Derivatives: Synthesis, Characterization and Cytotoxicity Studies. Int. J. Biol. Macromol. 2011;48:375–380. doi: 10.1016/j.ijbiomac.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 184.Qian C., Xu X., Shen Y., Li Y., Guo S. Synthesis and Preliminary Cellular Evaluation of Phosphonium Chitosan Derivatives as Novel Non-Viral Vector. Carbohydr. Polym. 2013;97:676–683. doi: 10.1016/j.carbpol.2013.05.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Materials).