Abstract
People with HIV (PWH) may be more susceptible to SARS-CoV-2 infection and worse clinical outcomes. We investigated the disparity in SARS-CoV-2 vaccination coverage between PWH and those without HIV (PWoH) in Catalonia, Spain, assessing primary and monovalent booster vaccination coverage from December 2021 to July 2022. The vaccines administered were BNT162, ChAdOx1-S, mRNA-127, and Ad26.COV2.S. Using a 1:10 ratio of PWH to PWoH based on sex, age, and socioeconomic deprivation, the analysis included 201,630 individuals (183,300 PWoH and 18,330 PWH). Despite a higher prevalence of comorbidities, PWH exhibited lower rates of complete primary vaccination (78.2% vs. 81.8%, p < 0.001) but surpassed PWoH in booster coverage (68.5% vs. 63.1%, p < 0.001). Notably, complete vaccination rates were lower among PWH with CD4 <200 cells/μL, detectable HIV viremia, and migrants compared to PWoH (p < 0.001, all). However, PWH with CD4 < 200 cells/μL received more boosters (p < 0.001). In multivariable logistic regression analysis of the overall population, a prior SARS-CoV-2 diagnosis, HIV status, migrants, and mild-to-severe socioeconomic deprivation were associated with lower primary vaccination coverage, reflecting barriers to healthcare and vaccine access. However, booster vaccination was higher among PWH. Targeted interventions are needed to improve vaccine coverage and address hesitancy in vulnerable populations.
Keywords: HIV, SARS-CoV-2, COVID-19, vaccination, booster doses
1. Introduction
Remarkable scientific and governmental investments have been made to develop multiple vaccine candidates against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an unprecedented time [1]. These vaccines have proven to be an effective and viable approach to combat the ongoing pandemic and mitigate its socioeconomic and health impacts. The European Medicines Agency (EMA) has authorized eight vaccines for use in the European Union [2]. As of 22 November 2023, over 13.5 billion SARS-CoV-2 vaccine doses had been administered across 184 countries, making it the largest vaccination campaign in human history [3].
People with HIV (PWH) may be more susceptible to SARS-CoV-2 infection and face worse outcomes. According to a report from the World Health Organization (WHO), PWH have a 30% higher risk of mortality from COVID-19 after hospital admission compared to people without HIV (PWoH) [4]. Additionally, PWH may face poorer COVID-19 outcomes due to other social determinants of health, chronic comorbid conditions, and poor HIV control [5,6]. As a result, many countries prioritized PWH for vaccine eligibility.
Existing evidence demonstrates that SARS-CoV-2 vaccines offer protection against COVID-19 by effectively reducing symptomatic infections and severe outcomes [7]. However, vaccine hesitancy among certain sub-populations [8,9] and the decline in IgG antibody levels after SARS-CoV-2 infection or vaccination [10] have hindered the full potential of vaccine protection. The emergence of SARS-CoV-2 variants with increased transmissibility and the ability to evade vaccine-induced immunity [11] is also a cause for concern. The uncertainties regarding the duration of vaccine protection and the impact of new variants underscored the importance of booster vaccinations [12]. Studies have shown that booster doses significantly decrease the risk of severe COVID-19 [13,14]. There are recommendations to administer booster doses to PWH with advanced immunosuppression or untreated HIV infection due to their increased risk of severe COVID-19 illness and potentially weaker immune response to SARS-CoV-2 vaccination [15]. In addition to their increased vulnerability, PWH may encounter barriers that limit their access to the crucial SARS-CoV-2 vaccinations [16].
Research on vaccination coverage among PWH is limited and lacks comparison with a matched sample from the general population [17,18]. Since vaccination strategies in many countries prioritize the public based on factors such as the nature of their jobs, age, presence of comorbidities, and other risk factors for adverse COVID-19 outcomes, matched studies are essential to assess the equity and effectiveness of current vaccination strategies, identify under-vaccinated groups, and provide valuable insights for future pandemics. The objective of this report is to compare primary and booster monovalent vaccination coverage among PWH with a well-matched representative sample of PWoH in Catalonia, Spain, and to identify subpopulations with low vaccination uptake to inform public health policies on ongoing vaccination strategies and future vaccination campaigns.
2. Materials and Methods
2.1. Study Design and Population
We conducted a retrospective cohort study using data from the prospective PISCIS cohort linked with integrated healthcare, clinical, and surveillance registries through the Data Analysis Program for Research and Innovation in Health (PADRIS) [19] to obtain information on vaccination. PISCIS is an ongoing, population-based, longitudinal, systematic, prospective, and multicentre HIV cohort study of individuals receiving care in Catalonia and the Balearic Islands, Spain. Details of the cohort have been described elsewhere [20]. For the purposes of this study, we used participants receiving care in the 16 PISCIS hospitals in Catalonia, representing approximately 84% of all PLWH in the region.
People with HIV were matched 1:10 to HIV-negative individuals from the general population in Catalonia for sex at birth, 5-year age group, and socioeconomic deprivation using exact matching. The socioeconomic index is generated by the Catalan government based on the basic health area of residence (ABS, abbreviation in Catalan) to determine the socioeconomic levels of Catalonia residents [21] and takes into account the following indicators: the proportion of manual workers, the proportion of residents with low education levels, the proportion with low incomes, the rate of premature mortality, and the rate of avoidable hospitalization [21].
We excluded PWH who were not alive as of 27 December 2020, the day the vaccination campaign began in Spain, as well as those not in active clinical follow-up (those who have not used healthcare services for at least 12 months) to ensure the accurate estimation of vaccine coverage. HIV-negative individuals were classified as such if there was no record of HIV infection based on the absence of HIV International Classification of Diseases (ICD) codes. The study period was from 27 December 2020 to 19 July 2022.
2.2. Outcomes
We defined complete primary vaccination according to the criteria set by the WHO: (a) two doses of the BNT162 (Pfizer), mRNA-1273 (Moderna), or ChAdOx1-S (Oxford/AstraZeneca) vaccines; or (b) a single dose of Janssen Ad26.COV2.S [22]. Incomplete vaccination was defined as receiving only a single dose of the BNT162 (Pfizer), mRNA-1273 (Moderna), or ChAdOx1-S (Oxford/AstraZeneca) vaccines. Booster vaccinations were defined as any additional doses administered after completing the primary vaccination series [22].
2.3. Covariates
The sociodemographic covariates included age as of 1 January 2021, sex assigned at birth, country of origin classified as Spanish or non-Spanish, socioeconomic deprivation grouped into least deprivation, mild deprivation, or moderate-to-severe deprivation. The COVID-19-associated variables included are: history of SARS-CoV-2 diagnosis defined as a positive nucleic acid amplification test (NAAT) and/or antigen detection from respiratory samples [23]. Comorbidity covariates included the most prevalent conditions in the PWH population cohort: chronic respiratory disease, cardiovascular disease, chronic kidney or liver disease, neuropsychiatric conditions, diabetes, cancer, hypertension, obesity, and autoimmune disease. Comorbidity data were extracted using the ICD-10 codes (Appendix A). Among PWH, additional data were collected on years since HIV diagnosis, HIV transmission risk group (people who inject drugs [PWID], men who have sex with men [MSM], male heterosexual, female sexual transmission, and others), antiretroviral therapy (ART) reception, most recent CD4 cell count (categorized as <200 cells/μL, 200–499 cells/μL, and ≥500 cells/μL), and HIV plasma RNA viral load categorized as detectable and undetectable (<50 copies/mL).
2.4. Statistical Analysis
We described the distribution of sociodemographic and clinical variables between PWH and PWoH to determine differences between the two populations. We used multivariable logistic regression models to assess the factors associated with complete vaccine reception and booster vaccinations, providing adjusted odds ratios (aOR) with 95% confidence intervals (95% CIs). The models were adjusted for age, sex, country of origin, socioeconomic deprivation, prior SARS-CoV-2 diagnosis, number of comorbidities, and HIV status. We calculated the cumulative incidence of complete vaccine reception and booster doses using Kaplan–Meier techniques from January 2021 to April 2022. We stratified the vaccine coverage analysis by HIV status, and among PWH, by country of origin (Spanish and non-Spanish), CD4 cell count categories, and HIV plasma RNA viral load. Log-rank tests were calculated to estimate the differences in cumulative vaccination coverage. We conducted subgroup analysis to investigate the factors associated with complete vaccine reception and booster vaccinations in both groups (Supplementary Material). We performed all analyses with R version 4.1.3 (R Project for Statistical Computing). A 2-sided p-value of <0.05 was considered statistically significant.
2.5. Ethics
The Institutional Review Board of Germans Trias i Pujol Hospital in Badalona, Spain approved the PISCIS cohort study. Patient-level information obtained from PADRIS was anonymized and deidentified before analysis. This study followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
3. Results
3.1. Baseline Characteristics of Study Population
A total of 18,330 PWH were matched to 183,300 PWoH in a ratio of 1:10. There were no differences in sex, age, and socioeconomic deprivation between the two groups. However, significant differences were observed regarding country of origin (p < 0.001), number of comorbidities (p < 0.001), and previous SARS-CoV-2 diagnosis (p < 0.001) between the two groups (Table 1).
Table 1.
Baseline characteristics of study participants according to HIV status.
| Total, n = 201,630 |
PWH, n = 18,330 |
PwoH, n = 183,300 |
p-Value | |
|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | |
| Sex a | >0.99 | |||
| Male | 165,682 (82.2) | 15,062 (82.2) | 150,620 (82.2) | |
| Female | 35,948 (17.8) | 3268 (17.8) | 32,680 (17.8) | |
| Age category, y b | >0.99 | |||
| 16–30 | 17,985 (8.9) | 1635 (8.9) | 16,350 (8.9) | |
| 31–40 | 48,081 (23.8) | 4371 (23.8) | 43,710 (23.8) | |
| 41–50 | 62,909 (31.2) | 5719 (31.2) | 57,190 (31.2) | |
| 51–60 | 52,602 (26.1) | 4782 (26.1) | 47,820 (26.1) | |
| 61–70 | 15,004 (7.4) | 1364 (7.4) | 13,640 (7.4) | |
| >70 | 5049 (2.5) | 459 (2.5) | 4590 (2.5) | |
| Country of origin c | <0.001 | |||
| Spain | 145,670 (72.2) | 10,666 (58.2) | 135,004 (73.7) | |
| Outside Spain | 55,841 (27.7) | 7662 (41.8) | 48,179 (26.3) | |
| Missing | 119 (0.1) | 2 (0) | 117 (0.1) | |
| Socioeconomic deprivation * | >0.99 | |||
| Least deprived | 99,836 (49.5) | 9076 (49.5) | 90,760 (49.5) | |
| Mildly deprived | 38,544 (19.1) | 3504 (19.1) | 35,040 (19.1) | |
| Moderately/severely deprived | 58,652 (29.1) | 5332 (29.1) | 53,320 (29.1) | |
| Missing | 4598 (2.3) | 418 (2.3) | 4180 (2.3) | |
| Number of comorbidities | <0.001 | |||
| 0 | 87,154 (43.2) | 5024 (27.4) | 82,130 (44.8) | |
| 1 | 46,042 (22.8) | 4083 (22.3) | 41,959 (22.9) | |
| 2 | 29,102 (14.4) | 3299 (18) | 25,803 (14.1) | |
| 3 | 18,348 (9.1) | 2387 (13) | 15,961 (8.7) | |
| ≥4 | 20,984 (10.4) | 3537 (19.3) | 17,447 (9.5) | |
| Type of comorbidities | ||||
| Respiratory disease | 18,472 (9.2) | 3852 (21) | 14,620 (8) | <0.001 |
| Cardiovascular disease | 20,974 (10.4) | 2925 (16) | 18,049 (9.8) | <0.001 |
| Autoimmune disease | 17,120 (8.5) | 2019 (11) | 15,101 (8.2) | <0.001 |
| Chronic kidney disease | 11,360 (5.6) | 1622 (8.8) | 9738 (5.3) | <0.001 |
| Chronic liver disease | 6764 (3.4) | 3530 (19.3) | 3234 (1.8) | <0.001 |
| Neuropsychiatric conditions | 59,822 (29.7) | 9107 (49.7) | 50,715 (27.7) | <0.001 |
| Diabetes (type I and II) | 10,949 (5.4) | 1043 (5.7) | 9906 (5.4) | 0.1 |
| Metabolic disease | 40,981 (20.3) | 4221 (23) | 36,760 (20.1) | <0.001 |
| Cancer | 9004 (4.5) | 1821 (9.9) | 7183 (3.9) | <0.001 |
| Hypertension | 36,362 (18) | 3688 (20.1) | 32,674 (17.8) | <0.001 |
| Obesity | 28,874 (14.3) | 1801 (9.8) | 27,073 (14.8) | <0.001 |
| Previous SARS-CoV-2 diagnosis | <0.001 | |||
| Yes | 25,093 (12.4) | 2454 (13.4) | 22,639 (12.4) | |
| No | 176,537 (87.6) | 15,876 (86.6) | 160,661 (87.6) | |
| HIV acquisition risk group | ||||
| PWID | 2360 (12.9) | |||
| MSM | 9761 (53.3) | |||
| Male heterosexual | 2443 (13.3) | |||
| Female sexual tranmission | 2419 (13.2) | |||
| Other | 519 (2.8) | |||
| Missing | 828 (4.5) | |||
| Years since HIV diagnosis, median (IQR) | 11.57 (5.91–18.57) | |||
| CD4 count (cells/μL) category | ||||
| <200 | 627 (3.4) | |||
| 200–499 | 3665 (20) | |||
| ≥500 | 11,928 (65.1) | |||
| Missing | 2110 (11.5) | |||
| CD4 count (cells/μL), median (IQR) | 680 (486–908) | |||
| CD4/CD8 ratio, median (IQR) | 0.85 (0.57–1.2) | |||
| Plasma HIV-RNA | ||||
| Detectable | 1749 (9.5) | |||
| Undetectable | 14,404 (78.6) | |||
| Missing | 2177 (11.9) | |||
| Years on ART, median (IQR) d | 8.75 (4.16–14.41) | |||
| On Treatment | ||||
| Yes | 14,685 (80.1) | |||
| No | 3645 (19.9) |
Abbreviations: PWH, people with HIV; PWoH, people without HIV; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IQR, interquartile range; PWID, people who inject drugs; MSM, men who have sex with men; ART, antiretroviral therapy. a Sex as assigned birth. b Age for all patients was as of 1 January 2021. c Country of origin was as indicated by the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS), recorded as Spanish or Non-Spanish. d Years on ART was defined as the difference in time between the first treatment administration date to the latest treatment date or the latest hospital visit if the last treatment date is missing. * Socioeconomic deprivation is based on the index of the Catalan government according to the basic health area (ABS) of residence. This index is based on five indicators which are: proportion of manual workers, proportion of residents with low education level, proportion with low income, rate of premature mortality, and rate of avoidable hospitalization.
Among the PWH, 15,062 individuals (82.2%) were male, and the majority (81.1%) fell within the age range of 31–60 years. The most common HIV acquisition risk group was MSM, accounting for 53.3%. The median (interquartile range [IQR]) CD4 cell count was 680 (486–908) cells/μL, with 627 PWH (3.4%) having a CD4 cell count below 200 cells/μL. The median (IQR) CD4/CD8 ratio was 0.85 (0.57–1.2), and 14,404 PWH (78.6%) had undetectable HIV RNA viremia (Table 1).
3.2. Vaccination Coverage
Among the 201,630 individuals included in the study, 81.4% had received complete primary vaccination, while 63.5% had received booster doses. People with HIV had lower rates of complete vaccination compared to those without HIV (78.2% vs. 81.8%, p < 0.001). However, PWH had higher coverage of booster doses compared to the non-HIV group (68.5% vs. 63.1%, p < 0.001). The median duration in months between the primary vaccination series and the reception of a booster was similar in both groups at 6.4 months (IQR 6.0–7.1). Regarding the types of vaccines administered, the majority of study participants received the BNT162 BioNTech/Pfizer vaccine for the primary vaccination series (61.5%). However, for booster doses, the mRNA-1273 Moderna vaccine was more commonly administered (86.5%). The general HIV-negative population was more frequently vaccinated with BNT162 BioNTech/Pfizer; however, PWH vaccinated at hospitals received the mRNA-1273 Moderna as their primary dose (Table 2).
Table 2.
SARS-CoV-2 vaccination coverage between people with and without HIV.
| Total | PWH | PWoH | p-Value | |
|---|---|---|---|---|
| Primary vaccination | n (%) | n (%) | n (%) | <0.001 |
| Unvaccinated | 29,606 (14.7) | 3343 (18.2) | 26,263 (14.3) | |
| Incomplete | 7835 (3.9) | 652 (3.6) | 7183 (3.9) | |
| Complete | 164,189 (81.4) | 14,335 (78.2) | 149,854 (81.8) | |
| Primary vaccination type | <0.001 | |||
| BNT162 | 105,743 (61.5) | 7924 (52.9) | 97,819 (62.3) | |
| ChAdOx1-S | 16,668 (9.7) | 1436 (9.6) | 15,232 (9.7) | |
| mRNA-1273 | 29,505 (17.2) | 3737 (24.9) | 25,768 (16.4) | |
| Ad26.COV2.S | 13,346 (7.8) | 1321 (8.8) | 12,025 (7.7) | |
| Combined | 6762 (3.9) | 569 (3.8) | 6193 (3.9) | |
| Booster doses | <0.001 | |||
| Yes | 104,332 (63.5) | 9823 (68.5) | 94,509 (63.1) | |
| No | 59,857 (36.5) | 4512 (31.5) | 55,345 (36.9) | |
| Booster doses type | <0.001 | |||
| BNT162 | 13,973 (13.4) | 1413 (14.4) | 12,560 (13.3) | |
| ChAdOx1-S | 26 (0) | 4 (0) | 22 (0) | |
| mRNA-1273 | 90,250 (86.5) | 8372 (85.2) | 81,878 (86.6) | |
| Ad26.COV2.S | 10 (0) | 2 (0) | 8 (0) | |
| Combined | 40 (0) | 17 (0.2) | 23 (0) | |
| Other | 33 (0) | 15 (0.2) | 18 (0) | |
| Median time between primary and booster dose, months (IQR) | 6.44 (5.98–7.1) | 6.44 (5.92–7.13) | 6.44 (6.02–7.1) | <0.001 |
Abbreviations: PWH, people with HIV; PWoH, people without HIV; IQR, interquaartile range.
3.3. Factors Associated with Vaccine Coverage
In the overall population, a multivariable logistic regression analysis, adjusted for all potential confounders, revealed that PWH were less likely to receive the complete primary vaccine compared to PWoH (aOR 0.86; 95% CI 0.82–0.89). Other factors associated with lower odds of receiving the complete primary vaccine included non-Spanish origin (aOR 0.39; 95% CI 0.38–0.40), mild socioeconomic deprivation (aOR 0.87; 95% CI 0.84–0.90), moderate-to-severe socioeconomic deprivation (aOR 0.87; 95% CI 0.85–0.90), and a previous SARS-CoV-2 diagnosis (aOR 0.20; 95% CI 0.19–0.20) (Table 3).
Table 3.
Factors associated with (a) complete and (b) booster vaccine reception in logistic regression analysis.
| Complete Primary Vaccination | Booster Vaccination | |||
|---|---|---|---|---|
| aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| HIV Status | ||||
| Negative | 1 (ref) | 1 (ref) | ||
| Positive | 0.86 (0.82, 0.89) | <0.001 | 1.41 (1.36, 1.47) | <0.001 |
| Sex | ||||
| Male | 1 (ref) | 1 (ref) | ||
| Female | 1.1 (1.07, 1.14) | <0.001 | 1.03 (1, 1.06) | 0.091 |
| Age category, y | ||||
| 16–30 | 1 (ref) | 1 (ref) | ||
| 31–40 | 1.3 (1.25, 1.36) | <0.001 | 1.61 (1.54, 1.68) | <0.001 |
| 41–50 | 1.79 (1.72, 1.87) | <0.001 | 2.76 (2.65, 2.88) | <0.001 |
| 51–60 | 2.21 (2.11, 2.31) | <0.001 | 4.67 (4.46, 4.89) | <0.001 |
| 61–70 | 2.12 (1.99, 2.27) | <0.001 | 9.88 (9.26, 10.55) | <0.001 |
| >70 | 2.73 (2.42, 3.07) | <0.001 | 17.48 (15.53, 19.67) | <0.001 |
| Place of Birth | ||||
| Spain | 1 (ref) | 1 (ref) | ||
| Outside Spain | 0.39 (0.38, 0.4) | <0.001 | 0.75 (0.73, 0.77) | <0.001 |
| Missing | ||||
| Socioeconomic deprivation * | ||||
| Least deprived | 1 (ref) | 1 (ref) | ||
| Mildly deprived | 0.87 (0.84, 0.9) | <0.001 | 0.8 (0.78, 0.83) | <0.001 |
| Moderately/severely deprived | 0.87 (0.85, 0.9) | <0.001 | 0.77 (0.75, 0.79) | <0.001 |
| Number of comorbidities | ||||
| 0 | 1 (ref) | 1 (ref) | ||
| 1 | 1.26 (1.22, 1.3) | <0.001 | 1.01 (0.98, 1.04) | 0.599 |
| 2 | 1.45 (1.39, 1.51) | <0.001 | 1.07 (1.04, 1.11) | <0.001 |
| 3 | 1.64 (1.56, 1.73) | <0.001 | 1.13 (1.08, 1.18) | <0.001 |
| ≥4 | 1.78 (1.69, 1.88) | <0.001 | 1.27 (1.21, 1.32) | <0.001 |
| Previous SARS-CoV-2 diagnosis | ||||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.2 (0.19, 0.2) | <0.001 | 0.24 (0.23, 0.25) | <0.001 |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Models adjusted for age, sex, country of origin, socioeconomic deprivation, prior SARS-CoV-2 diagnosis, number of comorbidities, and HIV status. * Socioeconomic deprivation is based on the index of the Catalan government according to the basic health area (ABS) of residence. This index is based on five indicators which are: proportion of manual workers, proportion of residents with low education level, proportion with low income, rate of premature mortality, and rate of avoidable hospitalization.
Regarding booster vaccination, similar associations were observed. Non-Spanish origin (aOR 0.75; 95% CI 0.73–0.77), mild socioeconomic deprivation (aOR 0.80; 95% CI 0.78–0.83), moderate-to-severe socioeconomic deprivation (aOR 0.77; 95% CI 0.75–0.79), and a previous SARS-CoV-2 diagnosis (aOR 0.24; 95% CI 0.23–0.25) were all associated with lower odds of receiving booster monovalent vaccines. Increasing age was associated with increasing odds of receiving boosters (Table 3).
3.4. Comparing Primary Complete Vaccination and Boosters between Key HIV Groups and the HIV-Negative Population
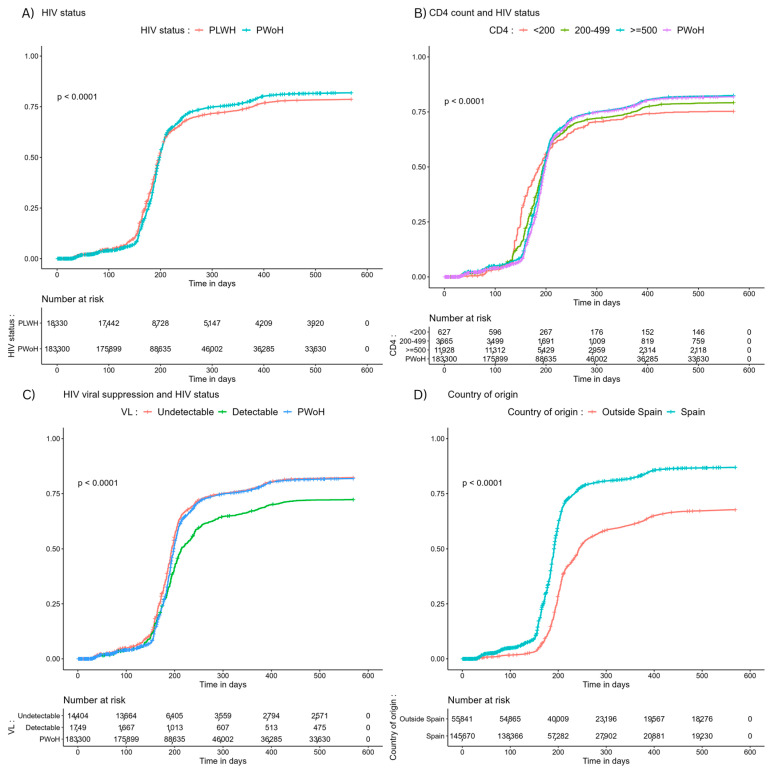
Compared to PWoH, individuals living with HIV had higher vaccination rates against SARS-CoV-2 in the first 200 days of the vaccination campaign. However, after 16 months, complete vaccination coverage was significantly lower among PWH (p < 0.001). We observed a similar vaccination coverage between PWoH and PWH with CD4 counts >500 cells/μL. However, among PWH with CD4 counts <200 cells/μL, complete primary vaccination coverage was significantly lower (p < 0.001). Similarly, primary vaccination coverage was similar between PWoH and PWH with undetectable HIV viral loads but was significantly lower among PWH with detectable viral loads (p < 0.001). Significant differences were also observed in primary vaccination coverage between the host Spanish population and individuals of non-Spanish origin (p < 0.001) (Figure 1).
Figure 1.
Cumulative incidence of complete primary SARS-CoV-2 vaccination stratified by (A) HIV status, (B) CD4 count and HIV status, (C) HIV viral suppression and HIV status, and (D) country of origin and HIV status. Abbreviations: PWH, people with HIV; PWoH, people without HIV; VL, HIV RNA viral load.
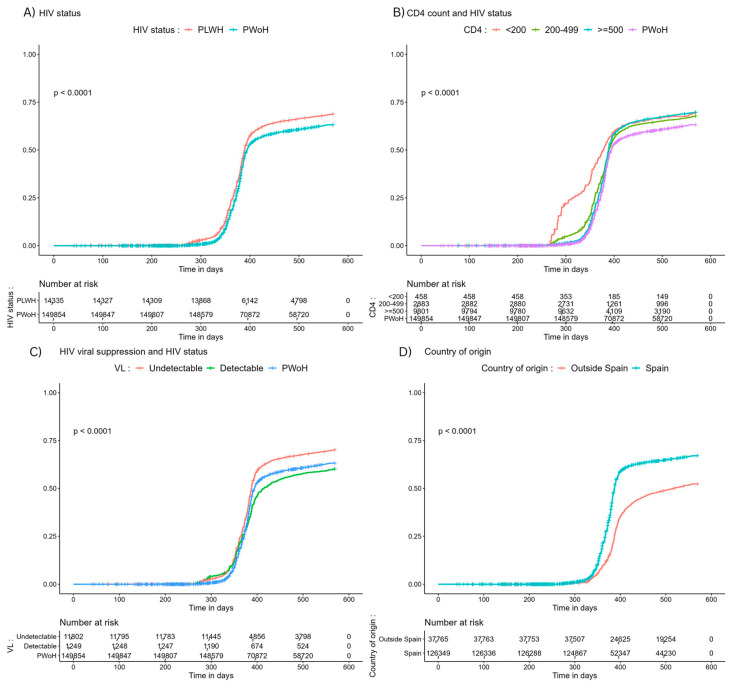
Regarding booster vaccinations, the coverage was higher among PWH compared to PWoH (p < 0.001). PWH with CD4 < 200 cells/μL received more boosters compared to PWoH (p < 0.001). Booster reception among PWH with undetectable viral loads; however, remained significantly lower compared to PWoH and PWH with undetectable HIV viral loads. In terms of country of origin, booster reception was lower among individuals of non-Spanish origin (Figure 2).
Figure 2.
Cumulative incidence of booster SARS-CoV-2 vaccinations stratified by (A) HIV status, (B) CD4 count and HIV status, (C) HIV viral suppression and HIV status, and (D) country of origin and HIV status. Abbreviations: PWH, people with HIV; PWoH, people without HIV; VL, HIV RNA viral load.
4. Discussion
PWH may be more susceptible to severe COVID-19 outcomes [4]. Therefore, ensuring equitable access to SARS-CoV-2 vaccines for this vulnerable population is vital [24]. Furthermore, specific sub-populations, such as older individuals, those with lower CD4 cell counts, detectable HIV viremia, and chronic comorbidities, face elevated risks and potentially worse clinical outcomes from HIV/COVID-19 co-infection [6]. Researchers recommend that prevention strategies should target these particular sub-groups [6,25].
In our study, the primary vaccination coverage in the overall population (PWH and PWoH) was 81.4%, surpassing the regional average of 75.1% reported by the European Centre for Disease Prevention and Control (ECDC) [26]. Similarly, the observed coverage of booster vaccinations in our cohort was 65.3%, also exceeding the reported regional average of 54.8% [26]. These findings underscore the significant efforts made by the Government of Spain and the Spanish Agency for Medicines and Healthcare Products (AEMPS) to implement nationwide vaccination strategies, particularly emphasizing access for the most vulnerable groups. They also underline historical vaccine acceptance and favourable willingness to be vaccinated in Spain [27]. However, it is worth noting that while our observed primary vaccination rate exceeds the regional average, it falls slightly below the reported 84.9% for the same period in Spain [28]. This suggests that further work is needed to achieve optimal vaccination coverage in the general population of Catalonia.
The observed lower SARS-CoV-2 primary vaccination rates among PWH compared to PWoH in Catalonia are concerning. This trend aligns with a global HIV cohort [17] and a study conducted in New York [29], indicating a consistent pattern of reduced vaccination coverage among PWH. The observed disparities in SARS-CoV-2 vaccination rates among PWH could be attributed to various factors, including potential barriers and hesitancy toward vaccination [18]. Even before the pandemic, vaccine hesitancy was recognized as a significant global health concern by the World Health Organization [30]. Concerns about the safety of the new SARS-CoV-2 vaccines have been a primary reason for vaccine refusal, as highlighted in reports [31]. Furthermore, access barriers, including limited availability or insufficient information tailored to the needs of PWH, could contribute to lower vaccination rates within this population [31].
Multiple unmeasured factors, including differences in the nature of jobs, might influence this reduced vaccination rate. The study also identified sociodemographic factors such as migration status and socio-economic deprivation as predictors of lower vaccine uptake, mirroring the findings of a New York-based report [29]. Despite the Catalan Healthcare system offering universal and cost-free access to all citizens, regardless of administrative status, studies in Catalonia have shown a correlation between migrants, socio-economic deprivation, and limited healthcare service utilization [32]. Additionally, non-Spanish origin encompasses a wide range of factors including cultural disparities, language barriers, and specific community beliefs and practices which might significantly influence perceptions and access to vaccination services. Of particular concern is the lower coverage of complete primary vaccination among PWH with CD4 counts below 200 cells/μL and those with detectable HIV viral load, which mirrors findings from previous US studies [17,33]. These individuals are likely to be at higher risk of severe COVID-19 clinical outcomes due to their compromised immune status [6]. The presence of detectable viral loads has been associated with a younger age, a higher likelihood of missing medical appointments, and a lack of treatment adherence [34]. These factors could also partially explain why this important sub-population is under-vaccinated and underscores the necessity for comprehensive, patient-centered approaches to support PWH in achieving optimal health outcomes. Historically, PWH have shown hesitancy toward vaccinations compared to their HIV-negative counterparts, and understanding this reluctance in future studies could be crucial to tailoring effective interventions.
Consistent with an earlier study conducted in Catalonia [35], individuals previously infected with SARS-CoV-2 showed lower vaccine uptake. This can be linked to Catalonia’s vaccination strategy, which delayed schedules for individuals with prior infections for their subsequent vaccination until six months after a confirmed SARS-CoV-2 diagnosis [36], presuming some level of immunity from their past exposure. Evaluating both natural and vaccine-induced immunity is crucial in understanding COVID-19 risk, especially in high-transmission risk settings.
Recommendations for vaccinating PWH favored mRNA vaccines over Ad5 vector SARS-CoV-2 ones [37] due to concerns arising from the Step [38] and Phambili [39] studies, revealing increased HIV-1 acquisition risk in Ad5-vaccinated men. In our study, 75.8% of PWH initially received mRNA vaccines. This could result from using public spaces for vaccinations in the early phase of the pandemic to improve accessibility and coverage without compulsory HIV-status disclosure. However, during booster doses, 99.6% of PWH received mRNA vaccines, likely because booster vaccinations were handled by HIV units and vaccines without adenoviral vectors were prioritized.
Despite the lower overall primary vaccination coverage, PWH showed higher rates of booster dose uptake compared to PWoH. This finding suggests that PWH and their healthcare providers may proactively seek additional doses to bolster their immune response, particularly following reports indicating inadequate immunogenicity and severe clinical outcomes from HIV/SARS-CoV-2 co-infection. It could also imply reluctance among the general population to receive booster shots, as reported in other settings [40,41]. Studies in the general population have linked this reluctance to perceived or reported side effects from the primary vaccination series, perceived (in)effectiveness of booster doses, low perception of COVID-19 risk, safety concerns, and lower education levels [40,41].
The higher odds of booster dose reception among PWH with CD4 levels below 200 cells/μL, aligning with public health recommendations [15], is encouraging due to their increased susceptibility to severe COVID-19 outcomes. However, the lower booster coverage among PWH with detectable viral loads requires attention. PWH with detectable viral loads might face challenges such as non-adherence to antiretrovirals, missing appointments, or engaging in risky behaviors, all of which could predict lower reception of booster doses. During the surge of the Omicron variant in Catalonia, the monovalent SARS-CoV-2 vaccine was introduced. Studies have demonstrated higher immunogenicity of monovalent booster doses against Omicron compared to a two-dose regimen [42,43]. Developing strategies to enhance booster vaccinations in vulnerable populations, like PWH, during this period was pertinent. These individuals stand to benefit greatly from booster doses due to their increased risk of breakthrough infections [44].
Our study has some notable strengths. To our knowledge, this is the first comprehensive evaluation of SARS-CoV-2 booster vaccination coverage among matched people with and without HIV. Additionally, the study used adequate matching of key sociodemographic factors to address potential differences between PWH and PWoH, enhancing the validity.
The study has some limitations as well. Firstly, the socioeconomic deprivation measure is an ecological variable based on an individual’s place of residence. A person’s place of residence may indeed not necessarily reflect their socioeconomic deprivation. Secondly, we did not report data regarding the post-vaccination experiences of participants, particularly side effects from the primary vaccination doses, which might influence participants’ willingness to receive booster vaccinations. Thirdly, due to the nature of our study design, there might be residual confounding as certain variables, such as religion and occupation, factors that could impact vaccine reception, are not reported in our databases. Additionally, self-made home SARS-CoV-2 antigen test results are not available in our database. We are not able to report if this influenced vaccine reception.
5. Conclusions
In conclusion, the study highlighted concerning discrepancies in SARS-CoV-2 vaccination rates between PWH and those without HIV in Catalonia, Spain. We observed lower primary vaccination rates among PWH compared to PWoH, even though PWH tended to have a higher prevalence of comorbidities. This indicates potential barriers to vaccination access or healthcare linkage among this group, especially for migrants, individuals experiencing socioeconomic deprivation, those with lower CD4 counts, and detectable HIV viral loads. However, the study uncovered a contrasting trend concerning booster vaccinations. PWH, particularly those with lower CD4 counts, were more likely to receive booster doses compared to PWoH. This is a positive finding suggesting increased awareness of booster shots among treating HIV physicians and PWH, particularly those with immunosuppression. Overall, the study underscores the need for targeted interventions to address the disparities in vaccination coverage among vulnerable populations. Improving access to primary vaccinations for PWH, especially those with lower CD4 counts and detectable viral loads, is crucial. Additionally, efforts to ensure equitable access to vaccines among migrants and socioeconomically deprived individuals are imperative. Improving HIV vaccination requires the involvement of HIV physicians, units, and effective communication emphasizing vaccine safety and efficacy.
Acknowledgments
The authors are grateful to the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS), the Sub-direction of Drug Dependencies, HIV, Sexually Transmitted Diseases, and Viral Hepatitis within the Public Health Secretary of the Catalan Health Department for their valuable support, and all staff of PISCIS collaborating hospitals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12010044/s1, Table S1: Factors associated with complete vaccine reception among HIV-negative participants in logistic regression analysis; Table S2: Factors associated with booster vaccine reception among HIV-negative participants in logistic regression analysis; Table S3: Factors associated with complete vaccine reception among PWH in logistic regression analysis; Table S4: Factors associated with booster vaccine reception among PWH in logistic regression analysis.
Appendix A
Eleven groups of the most prevalent chronic comorbidities among people living with and without HIV in Catalonia with corresponding International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9/10-CM) codes.
| Disease Groups | ICD-10-CM | Description (ICD-10) | ICD-9-CM | Description (ICD-9) |
|---|---|---|---|---|
| AUTOIMMUNE | I731 | Thromboangiitis obliterans [Buerger] | 443.1 | Obliterating thromboangeitis (Buerger’s disease) |
| AUTOIMMUNE | L10 | Pemphigus | ||
| AUTOIMMUNE | L12 | Pemphigoid | ||
| AUTOIMMUNE | L40 | Psoriasis | ||
| AUTOIMMUNE | L41 | Parapsoriasis | ||
| AUTOIMMUNE | L93 | Lupus erythematosus | ||
| AUTOIMMUNE | L94 | Other localized connective tissue disorders | ||
| AUTOIMMUNE | L95 | Vasculitis limited to skin, not elsewhere classified | ||
| AUTOIMMUNE | M30 | Polyarteritis nodosa and related conditions | ||
| AUTOIMMUNE | M31 | Other necrotizing vasculopathies | ||
| AUTOIMMUNE | M32 | Systemic lupus erythematosus | ||
| AUTOIMMUNE | M33 | Dermatopolymyositis | ||
| AUTOIMMUNE | M34 | Systemic sclerosis | ||
| AUTOIMMUNE | M35 | Other systemic involvement of connective tissue | ||
| AUTOIMMUNE | M36 | Systemic disorders of connective tissue in diseases classified elsewhere | ||
| AUTOIMMUNE | M023 | Reiter disease | ||
| AUTOIMMUNE | M05 | Seropositive rheumatoid arthritis | ||
| AUTOIMMUNE | M06 | Other rheumatoid arthritis | ||
| AUTOIMMUNE | M07 | Psoriatic and enteropathic arthropathies | ||
| AUTOIMMUNE | M08 | Juvenile arthritis | ||
| AUTOIMMUNE | M09 | Juvenile arthritis in diseases classified elsewhere | ||
| AUTOIMMUNE | M10 | Gout | ||
| AUTOIMMUNE | M11 | Other crystal arthropathies | ||
| AUTOIMMUNE | M12 | Other specific arthropathies | ||
| AUTOIMMUNE | M13 | Other arthritis | ||
| AUTOIMMUNE | M14 | Arthropathies in other diseases classified elsewhere | ||
| AUTOIMMUNE | M45 | Ankylosing spondylitis | ||
| AUTOIMMUNE | M460 | Spinal enthesopathy | ||
| AUTOIMMUNE | M461 | Sacroiliitis, not elsewhere classified | 720.2 | Sacroiliitis, not elsewhere classified |
| AUTOIMMUNE | M468 | Other specified inflammatory spondylopathies | ||
| AUTOIMMUNE | M469 | Inflammatory spondylopathy, unspecified | ||
| AUTOIMMUNE | K50 | Crohn disease [regional enteritis] | ||
| AUTOIMMUNE | K51 | Ulcerative colitis | ||
| AUTOIMMUNE | G35 | Multiple sclerosis | 340 | Multiple sclerosis |
| CANCER | C81 | Hodgkin lymphoma | ||
| CANCER | C82 | Follicular lymphoma | ||
| CANCER | C83 | Non-follicular lymphoma | ||
| CANCER | C84 | Mature T/NK-cell lymphomas | ||
| CANCER | C85 | Other and unspecified types of non-Hodgkin lymphoma | ||
| CANCER | C86 | Other specified types of T/NK-cell lymphoma | ||
| CANCER | C88 | Malignant immunoproliferative diseases | ||
| CANCER | C90 | Multiple myeloma and malignant plasma cell neoplasms | ||
| CANCER | C91 | Lymphoid leukaemia | ||
| CANCER | C92 | Myeloid leukaemia | ||
| CANCER | C93 | Monocytic leukaemia | ||
| CANCER | C94 | Other leukaemias of specified cell type | ||
| CANCER | C95 | Leukaemia of unspecified cell type | ||
| CANCER | C96 | Other and unspecified malignant neoplasms of lymphoid, haematopoietic and related tissue | ||
| CANCER | C | Malignant neoplasms | ||
| CANCER | D00 | Carcinoma in situ of oral cavity, oesophagus and stomach | ||
| CANCER | D01 | Carcinoma in situ of other and unspecified digestive organs | ||
| CANCER | D02 | Carcinoma in situ of middle ear and respiratory system | ||
| CANCER | D03 | Melanoma in situ | ||
| CANCER | D04 | Carcinoma in situ of skin | ||
| CANCER | D05 | Carcinoma in situ of breast | ||
| CANCER | D06 | Carcinoma in situ of cervix uteri | ||
| CANCER | D07 | Carcinoma in situ of other and unspecified genital organs | ||
| CANCER | D09 | Carcinoma in situ of other and unspecified sites | ||
| CANCER | D320 | Benign neoplasm: Cerebral meninges | 225.2 | Benign neoplasm of cerebral meninges |
| CANCER | D321 | Benign neoplasm: Spinal meninges | 225.4 | Benign neoplasm of spinal meninges |
| CANCER | D329 | Benign neoplasm: Meninges, unspecified | ||
| CANCER | D330 | Benign neoplasm: Brain, supratentorial | 225.0 | Benign neoplasm of brain and other parts of nervous system |
| CANCER | D331 | Benign neoplasm: Brain, infratentorial | ||
| CANCER | D332 | Benign neoplasm: Brain, unspecified | ||
| CANCER | D333 | Benign neoplasm: Cranial nerves | 225.1 | Benign neoplasm of cranial nerves convert |
| CANCER | D334 | Benign neoplasm: Spinal cord | 225.3 | Benign neoplasm of spinal cord |
| CANCER | Q85 | Phakomatoses, not elsewhere classified | ||
| CANCER | C81 | Hodgkin lymphoma | ||
| CANCER | C82 | Follicular lymphoma | ||
| CANCER | C83 | Non-follicular lymphoma | ||
| CANCER | C84 | Mature T/NK-cell lymphomas | ||
| CANCER | C85 | Other and unspecified types of non-Hodgkin lymphoma | ||
| CANCER | C86 | Other specified types of T/NK-cell lymphoma | ||
| CANCER | C88 | Malignant immunoproliferative diseases | ||
| CANCER | C90 | Multiple myeloma and malignant plasma cell neoplasms | ||
| CANCER | C91 | Lymphoid leukaemia | ||
| CANCER | C92 | Myeloid leukaemia | ||
| CANCER | C93 | Monocytic leukaemia | ||
| CANCER | C94 | Other leukaemias of specified cell type | ||
| CANCER | C95 | Leukaemia of unspecified cell type | ||
| CANCER | C96 | Other and unspecified malignant neoplasms of lymphoid, haematopoietic and related tissue | ||
| CARDIOVASCULAR | I48 | Atrial fibrillation and flutter | ||
| CARDIOVASCULAR | I441 | Atrioventricular block, second degree | 426.12 | Mobitz (type) II atrioventricular block |
| CARDIOVASCULAR | I441 | Atrioventricular block, second degree | 426.13 | Other second degree atrioventricular block |
| CARDIOVASCULAR | I442 | Atrioventricular block, complete | 426.0 | Atrioventricular block, complete |
| CARDIOVASCULAR | I443 | Other and unspecified atrioventricular block | ||
| CARDIOVASCULAR | I453 | Trifascicular block | 426.54 | Trifascicular block |
| CARDIOVASCULAR | I455 | Other specified heart block | 426.6 | Other heart block |
| CARDIOVASCULAR | Z950 | Presence of cardiac pacemaker | V45.01 | Status cardiac pacemaker |
| CARDIOVASCULAR | I05 | Rheumatic mitral valve diseases | ||
| CARDIOVASCULAR | I06 | Rheumatic aortic valve diseases | ||
| CARDIOVASCULAR | I07 | Rheumatic tricuspid valve diseases | ||
| CARDIOVASCULAR | I08 | Multiple valve diseases | ||
| CARDIOVASCULAR | I091 | Rheumatic diseases of endocardium, valve unspecified | 397.9 | Rheumatic diseases of endocardium, valve unspecified. |
| CARDIOVASCULAR | I098 | Other specified rheumatic heart diseases | ||
| CARDIOVASCULAR | I34 | Nonrheumatic mitral valve disorders | ||
| CARDIOVASCULAR | I35 | Nonrheumatic aortic valve disorders | ||
| CARDIOVASCULAR | I36 | Nonrheumatic tricuspid valve disorders | ||
| CARDIOVASCULAR | I37 | Pulmonary valve disorders | ||
| CARDIOVASCULAR | I38 | Endocarditis, valve unspecified | 424.90 | Endocarditis, valve unspecified, unspecified cause. |
| CARDIOVASCULAR | I38 | Endocarditis, valve unspecified | 424.99 | Endocarditis, valve unspecified. |
| CARDIOVASCULAR | I390 | Mitral valve disorders in diseases classified elsewhere | ||
| CARDIOVASCULAR | I391 | Aortic valve disorders in diseases classified elsewhere | ||
| CARDIOVASCULAR | I392 | Tricuspid valve disorders in diseases classified elsewhere | ||
| CARDIOVASCULAR | I393 | Pulmonary valve disorders in diseases classified elsewhere | ||
| CARDIOVASCULAR | I394 | Multiple valve disorders in diseases classified elsewhere | ||
| CARDIOVASCULAR | Q22 | Congenital malformations of pulmonary and tricuspid valves | ||
| CARDIOVASCULAR | Q23 | Congenital malformations of aortic and mitral valves | ||
| CARDIOVASCULAR | Z952 | Presence of prosthetic heart valve | V43.3 | Heart valve replaced by other means |
| CARDIOVASCULAR | Z953 | Presence of xenogenic heart valve | V42.2 | Heart valve replaced by transplant |
| CARDIOVASCULAR | Z954 | Presence of other heart-valve replacement | V42.2 | |
| CARDIOVASCULAR | G45 | Transient cerebral ischaemic attacks and related syndromes | ||
| CARDIOVASCULAR | G46 | Vascular syndromes of brain in cerebrovascular diseases | ||
| CARDIOVASCULAR | I60 | Subarachnoid haemorrhage | ||
| CARDIOVASCULAR | I61 | Intracerebral haemorrhage | ||
| CARDIOVASCULAR | I62 | Other nontraumatic intracranial haemorrhage | ||
| CARDIOVASCULAR | I63 | Cerebral infarction | ||
| CARDIOVASCULAR | I64 | Stroke, not specified as haemorrhage or infarction | ||
| CARDIOVASCULAR | I67 | Other cerebrovascular diseases | ||
| CARDIOVASCULAR | I69 | Sequelae of cerebrovascular disease | ||
| CARDIOVASCULAR | I110 | Hypertensive heart disease with (congestive) heart failure | 402.01 | Malignant hypertensive heart disease with heart failure |
| CARDIOVASCULAR | I110 | Hypertensive heart disease with (congestive) heart failure | 402.11 | Malignant hypertensive heart disease with heart failure |
| CARDIOVASCULAR | I110 | Hypertensive heart disease with (congestive) heart failure | 402.91 | Unspecified hypertensive heart disease |
| CARDIOVASCULAR | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.01 | Hypertensive heart and renal disease, with congestive heart failure, malignant |
| CARDIOVASCULAR | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.11 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease. |
| CARDIOVASCULAR | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.91 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease |
| CARDIOVASCULAR | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.03 | Hypertensive HF and CKD- Kidney Failure |
| CARDIOVASCULAR | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.13 | Hypertensive heart and chronic kidney disease with heart failure and with stage 5 chronic kidney disease |
| CARDIOVASCULAR | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.93 | Unspecified w/chf and renal failure |
| CARDIOVASCULAR | I27 | Other pulmonary heart diseases | ||
| CARDIOVASCULAR | I280 | Arteriovenous fistula of pulmonary vessels | 417.0 | Arteriovenous fistula of pulmonary vessels |
| CARDIOVASCULAR | I42 | Cardiomyopathy | ||
| CARDIOVASCULAR | I43 | Cardiomyopathy in diseases classified elsewhere | 425.8 | Cardiomyopathy in other diseases classified elsewhere |
| CARDIOVASCULAR | I50 | Heart failure | ||
| CARDIOVASCULAR | I515 | Myocardial degeneration | 429.1 | Myocardial degeneration |
| CARDIOVASCULAR | I517 | Cardiomegaly | 429.3 | Cardiomegaly |
| CARDIOVASCULAR | I528 | Other heart disorders in other diseases classified elsewhere | ||
| CARDIOVASCULAR | Z941 | Heart transplant status | V42.1 | Heart transplant status |
| CARDIOVASCULAR | Z943 | Heart and lungs transplant status | V42.1 | |
| CARDIOVASCULAR | Z943 | Heart and lungs transplant status | V42.6 | Lung transplant status |
| CARDIOVASCULAR | I20 | Angina pectoris | ||
| CARDIOVASCULAR | I21 | Acute myocardial infarction | ||
| CARDIOVASCULAR | I22 | Subsequent myocardial infarction | ||
| CARDIOVASCULAR | I24 | Other acute ischaemic heart diseases | ||
| CARDIOVASCULAR | I25 | Chronic ischaemic heart disease | ||
| CARDIOVASCULAR | Z951 | Presence of aortocoronary bypass graft | V45.81 | Aortocoronary bypass status |
| CARDIOVASCULAR | Z955 | Presence of coronary angioplasty implant and graft | V45.82 | Ercutaneous transluminal coronary angioplasty status |
| CARDIOVASCULAR | I09 | Other rheumatic heart diseases | ||
| CARDIOVASCULAR | I281 | Aneurysm of pulmonary artery | 417.1 | Aneurysm of pulmonary artery |
| CARDIOVASCULAR | I310 | Chronic adhesive pericarditis | 423.1 | Adhesive pericarditis |
| CARDIOVASCULAR | I311 | Chronic constrictive pericarditis | 423.2 | Constrictive pericarditis. |
| CARDIOVASCULAR | I456 | Pre-excitation syndrome | 426.7 | Anomalous atrioventricular excitation. |
| CARDIOVASCULAR | I456 | Pre-excitation syndrome | 426.81 | Lown-Ganong-Levine syndrome. |
| CARDIOVASCULAR | I495 | Sick sinus syndrome | 427.81 | Sinoatrial node dysfunction |
| CARDIOVASCULAR | I498 | Other specified cardiac arrhythmias | 427.89 | Other specified cardiac dysrhythmias |
| CARDIOVASCULAR | I70 | Atherosclerosis | ||
| CARDIOVASCULAR | I71 | Aortic aneurysm and dissection | ||
| CARDIOVASCULAR | I72 | Other aneurysm and dissection | ||
| CARDIOVASCULAR | I790 | Aneurysm of aorta in diseases classified elsewhere | 441.9 | Aortic aneurysm of unspecified site without mention of rupture |
| CARDIOVASCULAR | I791 | Aortitis in diseases classified elsewhere | 443.81 | Peripheral angiopathy in diseases classified elsewhere |
| CARDIOVASCULAR | I950 | Idiopathic hypotension | 458.1 | Chronic hypotension |
| CARDIOVASCULAR | I951 | Orthostatic hypotension | 458.0 | Orthostatic hypotension |
| CARDIOVASCULAR | I958 | Other hypotension | ||
| CARDIOVASCULAR | Q20 | Congenital malformations of cardiac chambers and connections | ||
| CARDIOVASCULAR | Q21 | Congenital malformations of cardiac septa | ||
| CARDIOVASCULAR | Q24 | Other congenital malformations of heart | ||
| CARDIOVASCULAR | Q25 | Congenital malformations of great arteries | ||
| CARDIOVASCULAR | Q26 | Congenital malformations of great veins | ||
| CARDIOVASCULAR | Q27 | Other congenital malformations of peripheral vascular system | ||
| CARDIOVASCULAR | Q28 | Other congenital malformations of circulatory system | ||
| CARDIOVASCULAR | Z958 | Presence of other cardiac and vascular implants and grafts | ||
| CARDIOVASCULAR | Z959 | Presence of cardiac and vascular implant and graft, unspecified | V45.00 | Cardiac device in situ |
| CARDIOVASCULAR | I091 | Rheumatic diseases of endocardium, valve unspecified | 397.9 | Rheumatic diseases of endocardium, valve unspecified |
| CARDIOVASCULAR | I098 | Other specified rheumatic heart diseases | ||
| CARDIOVASCULAR | I702 | Atherosclerosis of arteries of extremities | ||
| CARDIOVASCULAR | I780 | Hereditary haemorrhagic telangiectasia | 448.0 | Hereditary hemorrhagic telangiectasia. |
| CARDIOVASCULAR | I83 | Varicose veins of lower extremities | ||
| CARDIOVASCULAR | I87 | Other disorders of veins | ||
| CARDIOVASCULAR | I89 | Other noninfective disorders of lymphatic vessels and lymph nodes | ||
| CARDIOVASCULAR | I972 | Postmastectomy lymphoedema syndrome | 457.0 | Postmastectomy lymphedema syndrome |
| CARDIOVASCULAR | Q820 | Hereditary lymphoedema | 757.0 | Hereditary edema of legs |
| HYPERTENSION | I10 | Essential (primary) hypertension | 401.0 | Malignant essential hypertension |
| HYPERTENSION | I10 | Essential (primary) hypertension | 401.1 | Benign essential hypertension |
| HYPERTENSION | I10 | Essential (primary) hypertension | 401.9 | Unspecified essential hypertension |
| HYPERTENSION | I11 | Hypertensive heart disease | ||
| HYPERTENSION | I12 | Hypertensive renal disease | ||
| HYPERTENSION | I13 | Hypertensive heart and renal disease | ||
| HYPERTENSION | I15 | Secondary hypertension | ||
| CHRONIC KIDNEY DISEASES | I120 | Hypertensive renal disease with renal failure | 403.01 | Hypertensive chronic kidney disease, malignant, with chronic kidney disease stage V or end stage renal disease |
| CHRONIC KIDNEY DISEASES | I120 | Hypertensive renal disease with renal failure | 403.11 | Hypertensive chronic kidney disease, benign, with chronic kidney disease stage v or end stage renal disease |
| CHRONIC KIDNEY DISEASES | I120 | Hypertensive renal disease with renal failure | 403.91 | Hypertensive chronic kidney disease, unspecified, with chronic kidney disease stage V or end stage renal disease |
| CHRONIC KIDNEY DISEASES | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.01 | Hypertensive heart and renal disease, with congestive heart failure, malignant |
| CHRONIC KIDNEY DISEASES | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.11 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease, or unspecified chronic kidney disease |
| CHRONIC KIDNEY DISEASES | I130 | Hypertensive heart and renal disease with (congestive) heart failure | 404.91 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease, or unspecified chronic kidney disease |
| CHRONIC KIDNEY DISEASES | I131 | Hypertensive heart and renal disease with renal failure | ||
| CHRONIC KIDNEY DISEASES | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.03 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease, or unspecified chronic kidney disease |
| CHRONIC KIDNEY DISEASES | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.13 | Hypertensive HF and CKD–Kidney Failure |
| CHRONIC KIDNEY DISEASES | I132 | Hypertensive heart and renal disease with both (congestive) heart failure and renal failure | 404.93 | Hypertensive HF and CKD–Kidney Failure |
| CHRONIC KIDNEY DISEASES | I139 | Hypertensive heart and renal disease, unspecified | ||
| CHRONIC KIDNEY DISEASES | N01 | Rapidly progressive nephritic syndrome | ||
| CHRONIC KIDNEY DISEASES | N03 | Chronic nephritic syndrome | ||
| CHRONIC KIDNEY DISEASES | N04 | Nephrotic syndrome | ||
| CHRONIC KIDNEY DISEASES | N05 | Unspecified nephritic syndrome | ||
| CHRONIC KIDNEY DISEASES | N07 | Hereditary nephropathy, not elsewhere classified | ||
| CHRONIC KIDNEY DISEASES | N08 | Glomerular disorders in diseases classified elsewhere | 583.81 | Nephritis and nephropathy, not specified as acute or chronic, in diseases classified elsewhere |
| CHRONIC KIDNEY DISEASES | N11 | Chronic tubulo-interstitial nephritis | ||
| CHRONIC KIDNEY DISEASES | N183 | Chronic kidney disease, stage 3 | 585.3 | Chronic kidney disease, stage 3 (moderate) |
| CHRONIC KIDNEY DISEASES | N184 | Chronic kidney disease, stage 4 | 585.4 | Chronic kidney disease, Stage IV (severe) |
| CHRONIC KIDNEY DISEASES | N185 | Chronic kidney disease, stage 5 | 585.5 | Chronic kidney disease, stage v |
| CHRONIC KIDNEY DISEASES | N189 | Chronic kidney disease, unspecified | 585.9 | Chronic kidney disease, unspecified |
| CHRONIC KIDNEY DISEASES | Q60 | Renal agenesis and other reduction defects of kidney | ||
| CHRONIC KIDNEY DISEASES | Q611 | Polycystic kidney, autosomal recessive | ||
| CHRONIC KIDNEY DISEASES | Q612 | Polycystic kidney, autosomal dominant | 753.13 | Polycystic kidney, autosomal dominant |
| CHRONIC KIDNEY DISEASES | Q613 | Polycystic kidney, unspecified | 753.12 | Polycystic kidney, unspecified type |
| CHRONIC KIDNEY DISEASES | Q614 | Renal dysplasia | 753.15 | Renal dysplasia |
| CHRONIC KIDNEY DISEASES | Q615 | Medullary cystic kidney | 753.16 | Medullary cystic kidney |
| CHRONIC KIDNEY DISEASES | Q615 | Medullary cystic kidney | 753.17 | Medullary sponge kidney |
| CHRONIC KIDNEY DISEASES | Q618 | Other cystic kidney diseases | 753.19 | Other specified cystic kidney disease |
| CHRONIC KIDNEY DISEASES | Q619 | Cystic kidney disease, unspecified | 753.10 | Cystic kidney disease, unspecified |
| CHRONIC KIDNEY DISEASES | Z905 | Acquired absence of kidney | V45.73 | Acquired absence kidney |
| CHRONIC KIDNEY DISEASES | Z940 | Kidney transplant status | V42.0 | Organ or tissue replaced by transplant |
| CHRONIC LIVER DISEASES | B18 | Chronic viral hepatitis | ||
| CHRONIC LIVER DISEASES | K70 | Alcoholic liver disease | ||
| CHRONIC LIVER DISEASES | K713 | Toxic liver disease with chronic persistent hepatitis | 573.3 | Hepatitis, unspecified |
| CHRONIC LIVER DISEASES | K714 | Toxic liver disease with chronic lobular hepatitis | 573.3 | Hepatitis, unspecified |
| CHRONIC LIVER DISEASES | K715 | Toxic liver disease with chronic active hepatitis | ||
| CHRONIC LIVER DISEASES | K717 | Toxic liver disease with fibrosis and cirrhosis of liver | 573.3 | Hepatitis, unspecified |
| CHRONIC LIVER DISEASES | K721 | Chronic hepatic failure | ||
| CHRONIC LIVER DISEASES | K73 | Chronic hepatitis, not elsewhere classified | ||
| CHRONIC LIVER DISEASES | K74 | Fibrosis and cirrhosis of liver | ||
| CHRONIC LIVER DISEASES | K753 | Granulomatous hepatitis, not elsewhere classified | 573.3 | Hepatitis, unspecified |
| CHRONIC LIVER DISEASES | K754 | Autoimmune hepatitis | 571.42 | Chronic persistent hepatitis |
| CHRONIC LIVER DISEASES | K758 | Other specified inflammatory liver diseases | ||
| CHRONIC LIVER DISEASES | K761 | Chronic passive congestion of liver | 573.0 | Chronic passive congestion of liver |
| CHRONIC LIVER DISEASES | K761 | Chronic passive congestion of liver | 573.8 | Other disorders of liver |
| CHRONIC LIVER DISEASES | K766 | Portal hypertension | 572.3 | Portal hypertension |
| CHRONIC LIVER DISEASES | K767 | Hepatorenal syndrome | 572.4 | Hepatorenal syndrome |
| CHRONIC LIVER DISEASES | K778 | Liver disorders in other diseases classified elsewhere | ||
| CHRONIC LIVER DISEASES | Q446 | Cystic disease of liver | 751.62 | Congenital cystic disease of liver |
| CHRONIC LIVER DISEASES | Z944 | Liver transplant status | V42.7 | Liver transplant status |
| CHRONIC LIVER DISEASES | K700 | Alcoholic fatty liver | 571.0 | Alcoholic fatty liver |
| CHRONIC LIVER DISEASES | K701 | Alcoholic hepatitis | ||
| METABOLIC | E78 | Disorders of lipoprotein metabolism and other lipidaemias | ||
| METABOLIC | E20 | Hypoparathyroidism | ||
| METABOLIC | E21 | Hyperparathyroidism and other disorders of parathyroid gland | ||
| METABOLIC | E22 | Hyperfunction of pituitary gland | ||
| METABOLIC | E23 | Hypofunction and other disorders of pituitary gland | ||
| METABOLIC | E24 | Cushing syndrome | ||
| METABOLIC | E25 | Adrenogenital disorders | ||
| METABOLIC | E26 | Hyperaldosteronism | ||
| METABOLIC | E27 | Other disorders of adrenal gland | ||
| METABOLIC | E28 | Ovarian dysfunction | ||
| METABOLIC | E29 | Testicular dysfunction | ||
| METABOLIC | E31 | Polyglandular dysfunction | ||
| METABOLIC | E34 | Other endocrine disorders | ||
| METABOLIC | E35 | Disorders of endocrine glands in diseases classified elsewhere | 246.8 | Other specified disorders of thyroid |
| METABOLIC | E35 | Disorders of endocrine glands in diseases classified elsewhere | 255.8 | Other specified disorders of adrenal glands |
| METABOLIC | E35 | Disorders of endocrine glands in diseases classified elsewhere | 259.8 | Other specified endocrine disorders |
| METABOLIC | E40 | Kwashiorkor | 260 | Kwashiorkor |
| METABOLIC | E41 | Nutritional marasmus | 261 | Nutritional marasmus |
| METABOLIC | E42 | Marasmic kwashiorkor | 260 | Kwashiorkor |
| METABOLIC | E43 | Unspecified severe protein-energy malnutrition | 262 | Other severe protein-calorie malnutrition |
| METABOLIC | E44 | Protein-energy malnutrition of moderate and mild degree | ||
| METABOLIC | E45 | Retarded development following protein-energy malnutrition | 263.2 | Arrested development following protein-calorie malnutrition |
| METABOLIC | E46 | Unspecified protein-energy malnutrition | 263.8 | Unspecified protein-calorie malnutrition |
| METABOLIC | E46 | Unspecified protein-energy malnutrition | 263.9 | Unspecified protein-calorie malnutrition |
| METABOLIC | E64 | Sequelae of malnutrition and other nutritional deficiencies | ||
| METABOLIC | E70 | Disorders of aromatic amino-acid metabolism | ||
| METABOLIC | E71 | Disorders of branched-chain amino-acid metabolism and fatty-acid metabolism | ||
| METABOLIC | E72 | Other disorders of amino-acid metabolism | ||
| METABOLIC | E74 | Other disorders of carbohydrate metabolism | ||
| METABOLIC | E75 | Disorders of sphingolipid metabolism and other lipid storage disorders | ||
| METABOLIC | E76 | Disorders of glycosaminoglycan metabolism | ||
| METABOLIC | E77 | Disorders of glycoprotein metabolism | ||
| METABOLIC | E79 | Disorders of purine and pyrimidine metabolism | ||
| METABOLIC | E80 | Disorders of porphyrin and bilirubin metabolism | ||
| METABOLIC | E83 | Disorders of mineral metabolism | ||
| METABOLIC | E84 | Cystic fibrosis | ||
| METABOLIC | E85 | Amyloidosis | ||
| METABOLIC | E88 | Other metabolic disorders | ||
| METABOLIC | E89 | Postprocedural endocrine and metabolic disorders, not elsewhere classified | ||
| METABOLIC | E891 | Postprocedural hypoinsulinaemia | 251.3 | Postsurgical hypoinsulinemia |
| METABOLIC | K903 | Pancreatic steatorrhoea | 579.4 | Pancreatic steatorrhea |
| METABOLIC | K904 | Malabsorption due to intolerance, not elsewhere classified | ||
| METABOLIC | K908 | Other intestinal malabsorption | ||
| METABOLIC | K909 | Intestinal malabsorption, unspecified | 579.9 | Unspecified intestinal malabsorption |
| METABOLIC | K912 | Postsurgical malabsorption, not elsewhere classified | 579.3 | Postsurgical malabsorption, not elsewhere classified |
| METABOLIC | M83 | Adult osteomalacia | ||
| METABOLIC | M88 | Paget disease of bone [osteitis deformans] | ||
| METABOLIC | N25 | Disorders resulting from impaired renal tubular function | ||
| DIABETES | E10 | Insulin-dependent diabetes mellitus | 250.XX | Diabetes |
| DIABETES | E11 | Non-insulin-dependent diabetes mellitus | ||
| DIABETES | E13 | Other specified diabetes mellitus | ||
| DIABETES | E14 | Unspecified diabetes mellitus | ||
| OBERSITY | E66 | Obesity | ||
| NEUROPSYCHIATRIC | F00 | Dementia in Alzheimer disease | ||
| NEUROPSYCHIATRIC | F01 | Vascular dementia | ||
| NEUROPSYCHIATRIC | F02 | Dementia in other diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | F03 | Unspecified dementia | ||
| NEUROPSYCHIATRIC | F051 | Delirium superimposed on dementia | ||
| NEUROPSYCHIATRIC | G30 | Alzheimer disease | ||
| NEUROPSYCHIATRIC | G31 | Other degenerative diseases of nervous system, not elsewhere classified | ||
| NEUROPSYCHIATRIC | F30 | Manic episode | ||
| NEUROPSYCHIATRIC | F31 | Bipolar affective disorder | ||
| NEUROPSYCHIATRIC | F32 | Depressive episode | ||
| NEUROPSYCHIATRIC | F33 | Recurrent depressive disorder | ||
| NEUROPSYCHIATRIC | F34 | Persistent mood [affective] disorders | ||
| NEUROPSYCHIATRIC | F38 | Other mood [affective] disorders | ||
| NEUROPSYCHIATRIC | F39 | Unspecified mood [affective] disorder | 296.90 | Unspecified episodic mood disorder |
| NEUROPSYCHIATRIC | F412 | Mixed anxiety and depressive disorder | ||
| NEUROPSYCHIATRIC | G40 | Epilepsy | ||
| NEUROPSYCHIATRIC | B900 | Sequelae of central nervous system tuberculosis | 137.1 | Late effects of central nervous system tuberculosis |
| NEUROPSYCHIATRIC | D482 | Neoplasm of uncertain or unknown behaviour: Peripheral nerves and autonomic nervous system | 238.1 | Neoplasm of uncertain behavior of connective and other soft tissue |
| NEUROPSYCHIATRIC | G041 | Tropical spastic paraplegia | 344.1 | Paraplegia |
| NEUROPSYCHIATRIC | G09 | Sequelae of inflammatory diseases of central nervous system | 326 | Late effects of intracranial abscess or pyogenic infection |
| NEUROPSYCHIATRIC | G10 | Huntington disease | 333.4 | Huntington’s chorea |
| NEUROPSYCHIATRIC | G11 | Hereditary ataxia | ||
| NEUROPSYCHIATRIC | G12 | Spinal muscular atrophy and related syndromes | ||
| NEUROPSYCHIATRIC | G13 | Systemic atrophies primarily affecting central nervous system in diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | G24 | Dystonia | ||
| NEUROPSYCHIATRIC | G25 | Other extrapyramidal and movement disorders | ||
| NEUROPSYCHIATRIC | G26 | Extrapyramidal and movement disorders in diseases classified elsewhere | 333.99 | Other extrapyramidal diseases and abnormal movement disorders |
| NEUROPSYCHIATRIC | G32 | Other degenerative disorders of nervous system in diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | G37 | Other demyelinating diseases of central nervous system | ||
| NEUROPSYCHIATRIC | G51 | Facial nerve disorders | ||
| NEUROPSYCHIATRIC | G52 | Disorders of other cranial nerves | ||
| NEUROPSYCHIATRIC | G53 | Cranial nerve disorders in diseases classified elsewhere | 352.9 | Unspecified disorder of cranial nerves |
| NEUROPSYCHIATRIC | G70 | Myasthenia gravis and other myoneural disorders | ||
| NEUROPSYCHIATRIC | G71 | Primary disorders of muscles | ||
| NEUROPSYCHIATRIC | G723 | Periodic paralysis | 359.3 | Periodic paralysis |
| NEUROPSYCHIATRIC | G724 | Inflammatory myopathy, not elsewhere classified | ||
| NEUROPSYCHIATRIC | G728 | Other specified myopathies | ||
| NEUROPSYCHIATRIC | G729 | Myopathy, unspecified | 359.9 | Myopathy, unspecified |
| NEUROPSYCHIATRIC | G73 | Disorders of myoneural junction and muscle in diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | G80 | Cerebral palsy | ||
| NEUROPSYCHIATRIC | G81 | Hemiplegia | ||
| NEUROPSYCHIATRIC | G82 | Paraplegia and tetraplegia | ||
| NEUROPSYCHIATRIC | G83 | Other paralytic syndromes | ||
| NEUROPSYCHIATRIC | G90 | Disorders of autonomic nervous system | ||
| NEUROPSYCHIATRIC | G91 | Hydrocephalus | ||
| NEUROPSYCHIATRIC | G938 | Other specified disorders of brain | ||
| NEUROPSYCHIATRIC | G939 | Disorder of brain, unspecified | 348.9 | Unspecified condition of brain |
| NEUROPSYCHIATRIC | G95 | Other diseases of spinal cord | ||
| NEUROPSYCHIATRIC | G99 | Other disorders of nervous system in diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | M471 | Other spondylosis with myelopathy | ||
| NEUROPSYCHIATRIC | Q00 | Anencephaly and similar malformations | ||
| NEUROPSYCHIATRIC | Q01 | Encephalocele | ||
| NEUROPSYCHIATRIC | Q02 | Microcephaly | 742.1 | Microcephalus |
| NEUROPSYCHIATRIC | Q03 | Congenital hydrocephalus | ||
| NEUROPSYCHIATRIC | Q04 | Other congenital malformations of brain | ||
| NEUROPSYCHIATRIC | Q05 | Spina bifida | ||
| NEUROPSYCHIATRIC | Q06 | Other congenital malformations of spinal cord | ||
| NEUROPSYCHIATRIC | Q07 | Other congenital malformations of nervous system | ||
| NEUROPSYCHIATRIC | Q760 | Spina bifida occulta | 756.17 | Spina bifida occulta |
| NEUROPSYCHIATRIC | F04 | Organic amnesic syndrome, not induced by alcohol and other psychoactive substances | 294.0 | Amnestic disorder in conditions classified elsewhere |
| NEUROPSYCHIATRIC | F06 | Other mental disorders due to brain damage and dysfunction and to physical disease | ||
| NEUROPSYCHIATRIC | F07 | Personality and behavioural disorders due to brain disease, damage and dysfunction | ||
| NEUROPSYCHIATRIC | F09 | Unspecified organic or symptomatic mental disorder | 310.9 | Unspecified nonpsychotic mental disorder following organic brain damage |
| NEUROPSYCHIATRIC | F102 | Mental and behavioural disorders due to use of alcohol: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F106 | Mental and behavioural disorders due to use of alcohol: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F107 | Mental and behavioural disorders due to use of alcohol: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F112 | Mental and behavioural disorders due to use of opioids: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F116 | Mental and behavioural disorders due to use of opioids: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F117 | Mental and behavioural disorders due to use of opioids: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F122 | Mental and behavioural disorders due to use of cannabinoids: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F126 | Mental and behavioural disorders due to use of cannabinoids: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F127 | Mental and behavioural disorders due to use of cannabinoids: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F132 | Mental and behavioural disorders due to use of sedatives or hypnotics: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F136 | Mental and behavioural disorders due to use of sedatives or hypnotics: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F137 | Mental and behavioural disorders due to use of sedatives or hypnotics: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F142 | Mental and behavioural disorders due to use of cocaine: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F146 | Mental and behavioural disorders due to use of cocaine: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F147 | Mental and behavioural disorders due to use of cocaine: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F152 | Mental and behavioural disorders due to use of other stimulants, including caffeine: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F156 | Mental and behavioural disorders due to use of other stimulants, including caffeine: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F157 | Mental and behavioural disorders due to use of other stimulants, including caffeine: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F162 | Mental and behavioural disorders due to use of hallucinogens: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F166 | Mental and behavioural disorders due to use of hallucinogens: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F167 | Mental and behavioural disorders due to use of hallucinogens: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F172 | Mental and behavioural disorders due to use of tobacco: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F176 | Mental and behavioural disorders due to use of tobacco: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F177 | Mental and behavioural disorders due to use of tobacco: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F182 | Mental and behavioural disorders due to use of volatile solvents: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F186 | Mental and behavioural disorders due to use of volatile solvents: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F187 | Mental and behavioural disorders due to use of volatile solvents: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F192 | Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances: Dependence syndrome | ||
| NEUROPSYCHIATRIC | F196 | Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances: Amnesic syndrome | ||
| NEUROPSYCHIATRIC | F197 | Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances: Residual and late-onset psychotic disorder | ||
| NEUROPSYCHIATRIC | F50 | Eating disorders | ||
| NEUROPSYCHIATRIC | F52 | Sexual dysfunction, not caused by organic disorder or disease | ||
| NEUROPSYCHIATRIC | F60 | Specific personality disorders | ||
| NEUROPSYCHIATRIC | F61 | Mixed and other personality disorders | ||
| NEUROPSYCHIATRIC | F62 | Enduring personality changes, not attributable to brain damage and disease | ||
| NEUROPSYCHIATRIC | F63 | Habit and impulse disorders | ||
| NEUROPSYCHIATRIC | F68 | Other disorders of adult personality and behaviour | ||
| NEUROPSYCHIATRIC | F70 | Mild mental retardation | 317 | Mild intellectual disabilities |
| NEUROPSYCHIATRIC | F71 | Moderate mental retardation | 318.0 | Moderate intellectual disabilities |
| NEUROPSYCHIATRIC | F72 | Severe mental retardation | 318.1 | Severe intellectual disabilities |
| NEUROPSYCHIATRIC | F73 | Profound mental retardation | 318.2 | Profound intellectual disabilities |
| NEUROPSYCHIATRIC | F78 | Other mental retardation | 319 | Unspecified intellectual disabilities |
| NEUROPSYCHIATRIC | F79 | Unspecified mental retardation | 319 | Unspecified intellectual disabilities |
| NEUROPSYCHIATRIC | F80 | Specific developmental disorders of speech and language | ||
| NEUROPSYCHIATRIC | F81 | Specific developmental disorders of scholastic skills | ||
| NEUROPSYCHIATRIC | F82 | Specific developmental disorder of motor function | 315.4 | Developmental coordination disorder |
| NEUROPSYCHIATRIC | F83 | Mixed specific developmental disorders | ||
| NEUROPSYCHIATRIC | F84 | Pervasive developmental disorders | ||
| NEUROPSYCHIATRIC | F88 | Other disorders of psychological development | 315.8 | Other specified delays in development |
| NEUROPSYCHIATRIC | F89 | Unspecified disorder of psychological development | 315.9 | Unspecified delay in development |
| NEUROPSYCHIATRIC | F95 | Tic disorders | ||
| NEUROPSYCHIATRIC | F99 | Mental disorder, not otherwise specified | 300.9 | Unspecified nonpsychotic mental disorder |
| NEUROPSYCHIATRIC | G20 | Parkinson disease | 332.0 | Paralysis agitans |
| NEUROPSYCHIATRIC | G21 | Secondary parkinsonism | ||
| NEUROPSYCHIATRIC | G22 | Parkinsonism in diseases classified elsewhere | ||
| NEUROPSYCHIATRIC | G23 | Other degenerative diseases of basal ganglia | ||
| NEUROPSYCHIATRIC | F20 | Schizophrenia | ||
| NEUROPSYCHIATRIC | F22 | Persistent delusional disorders | 297.0 | Paranoid state, simple |
| NEUROPSYCHIATRIC | F22 | Persistent delusional disorders | 297.1 | Delusional disorder |
| NEUROPSYCHIATRIC | F22 | Persistent delusional disorders | 297.2 | Paraphrenia |
| NEUROPSYCHIATRIC | F24 | Induced delusional disorder | 297.3 | Shared psychotic disorder |
| NEUROPSYCHIATRIC | F25 | Schizoaffective disorders | ||
| NEUROPSYCHIATRIC | F28 | Other nonorganic psychotic disorders | 298.9 | Unspecified psychosis |
| RESPIRATORY | J45 | Asthma | ||
| RESPIRATORY | J41 | Simple and mucopurulent chronic bronchitis | ||
| RESPIRATORY | J42 | Unspecified chronic bronchitis | 491.9 | Unspecified chronic bronchitis |
| RESPIRATORY | J43 | Emphysema | ||
| RESPIRATORY | J44 | Other chronic obstructive pulmonary disease | ||
| RESPIRATORY | J47 | Bronchiectasis | ||
| RESPIRATORY | B909 | Sequelae of respiratory and unspecified tuberculosis | 137.0 | Late effects of respiratory or unspecified tuberculosis |
| RESPIRATORY | E662 | Extreme obesity with alveolar hypoventilation | 278.03 | Obesity hypoventilation syndrome |
| RESPIRATORY | J60 | Coalworker pneumoconiosis | 500 | Coal workers’ pneumoconiosis |
| RESPIRATORY | J61 | Pneumoconiosis due to asbestos and other mineral fibres | 501 | Asbestosis |
| RESPIRATORY | J62 | Pneumoconiosis due to dust containing silica | ||
| RESPIRATORY | J63 | Pneumoconiosis due to other inorganic dusts | ||
| RESPIRATORY | J64 | Unspecified pneumoconiosis | 505 | Pneumoconiosis, unspecified |
| RESPIRATORY | J65 | Pneumoconiosis associated with tuberculosis | 505 | Pneumoconiosis, unspecified |
| RESPIRATORY | J66 | Airway disease due to specific organic dust | ||
| RESPIRATORY | J67 | Hypersensitivity pneumonitis due to organic dust | ||
| RESPIRATORY | J684 | Chronic respiratory conditions due to chemicals, gases, fumes and vapours | 506.4 | Chronic respiratory conditions due to fumes and vapors |
| RESPIRATORY | J701 | Chronic and other pulmonary manifestations due to radiation | 508.1 | Chronic and other pulmonary manifestations due to radiation |
| RESPIRATORY | J703 | Chronic drug-induced interstitial lung disorders | 508.8 | Respiratory conditions due to other specified external agents |
| RESPIRATORY | J704 | Drug-induced interstitial lung disorders, unspecified | 508.8 | Respiratory conditions due to other specified external agents |
| RESPIRATORY | J84 | Other interstitial pulmonary diseases | ||
| RESPIRATORY | J92 | Pleural plaque | ||
| RESPIRATORY | J941 | Fibrothorax | 511.0 | Pleurisy without mention of effusion or current tuberculosis |
| RESPIRATORY | J953 | Chronic pulmonary insufficiency following surgery | 518.52 | Other pulmonary insufficiency, not elsewhere classified, following trauma and surgery |
| RESPIRATORY | J955 | Postprocedural subglottic stenosis | 997.39 | Other respiratory complications |
| RESPIRATORY | J961 | Chronic respiratory failure | ||
| RESPIRATORY | J98 | Other respiratory disorders | ||
| RESPIRATORY | Q33 | Congenital malformations of lung | ||
| RESPIRATORY | Q34 | Other congenital malformations of respiratory system | ||
| RESPIRATORY | Z902 | Acquired absence of lung [part of] | V45.76 | Acquired absence of organ, lung |
| RESPIRATORY | Z942 | Lung transplant status | V42.6 | Postsurgical states following surgery of eye and adnexa |
| RESPIRATORY | Z943 | Heart and lungs transplant status | V42.1 | Postsurgical renal dialysis status |
| RESPIRATORY | Z943 | Heart and lungs transplant status | V42.6 | Postsurgical states following surgery of eye and adnexa |
| RESPIRATORY | Z963 | Presence of artificial larynx | V43.81 | Larynx replacement |
Appendix B
Collaborators of the PISCIS Cohort Study Group.
Author Contributions
Conceptualization, D.K.N., J.R.-U., J.M.M. and J.M.L.; Data curation, D.K.N., L.A., Y.D., S.M.-F., J.A. (Jordi Aceiton), R.W.R. and the PISCIS Study Group; Formal analysis, D.K.N., J.R.-U., L.A., Y.D., S.M.-F. and J.M.L.; Funding acquisition, J.R.-U. and J.M.L.; Investigation, D.K.N., A.B., R.M.-I., A.I., M.d.M.G., P.S., J.A. (Juan Ambrosioni), J.C., J.M.M. and J.M.L.; Methodology, D.K.N., J.R.-U., L.A., Y.D. and S.M.-F.; Project administration, D.K.N., J.R.-U., A.B., J.C. and J.M.L.; Resources, D.K.N., J.R.-U., J.C. and J.M.L.; Supervision, J.R.-U., J.M.M. and J.M.L.; Validation, J.R.-U. and S.M.-F.; Visualization, L.A., Y.D. and J.A. (Jordi Aceiton); Writing—original draft, D.K.N.; Writing—review & editing, D.K.N., J.R.-U., L.A., Y.D., S.M.-F., J.A. (Jordi Aceiton), A.B., R.M.-I., A.I., M.d.M.G., R.W.R., P.S., J.A. (Juan Ambrosioni), J.C., J.M.M. and J.M.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Germans Trias i Pujol Hospital in Badalona, Spain (protocol code EO-11-108). Patient-level information obtained from PADRIS was anonymized and deidentified before analysis.
Informed Consent Statement
The PISCIS cohort has been approved by the ethics committee of the coordinating center, and Catalan patient data extraction is allowed by of the 203/2015 Decree, of the 15th of September, from the Catalan Health Department. Informed consent is therefore not applicable. PISCIS data is owned by each individual patient, and data is eliminated if requested by the hospital or by a patient. Data is pseudo-anonymized before arriving at the coordinating center, and confidentiality is guaranteed in accordance with the provisions of the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regarding the processing of personal data and on the free movement of such data and the new national Organic Law of Protection of Personal Data (3/2018), 5th of December, Data Protection and Digital Rights Act.
Data Availability Statement
The study protocol and statical code are available from the corresponding author upon request. The data for this study accessed on 1 October 2022 are available at the Centre for Epidemiological Studies of Sexually Transmitted Diseases and HIV/AIDS in Catalonia (CEEISCAT), the coordinating centre of the PISCIS cohort, the PADRIS, and from each of the collaborating hospitals upon request via https://pisciscohort.org/contacte/.
Conflicts of Interest
JMM reported receiving a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–24 and consulting honoraria and/or research grants from Angelini, Contrafect, Genentech, Gilead Sciences, Jansen, Lysovant, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work. DKN reported receiving consultation fees from OPIS outside the submitted work. JA has received personal fees from ViiV, Gilead, Janssen and MSD, has participated in Advisory Boards for ViiV, Gilead, Janssen and MSD, received funding for research from ViiV, Gilead and MSD, has been member of Data Safety Monitoring Boards for HIPRA and Grifols, all outside the current work. AI reported that his institution received research grants from Gilead Sciences, MSD, Janssen and ViiV and he personally received consultation fees from Gilead Sciences, Janssen, ViiV Healthcare, and Thera Technologies; honoraria for lectures and presentations from Gilead Sciences, MSD, Jansen, and ViiV Healthcare; travel support for attending meetings from Gilead Sciences, Jansen, and ViiV Healthcare, all outside the submitted work. JML has received consulting honoraria from Gilead Sciences, Janssen-Cilag, and ViiV Healthcare, all of them outside of the present work. RMI has received consulting honoraria from ViiV Healthcare and MSD outside the submitted work. PS has received honoraria and/or speaking fees from Gilead, Janssen-Cilag, Merck Sharp & Dome, Pfizer, and ViiV Healthcare, and has received a research grant from ViiV Healthcare, all outside of the submitted work. For the remaining authors, no conflicts of interest were declared. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by Fundació La Marató de TV3, grant number 202117-30-31. The Fundació La Marató de TV3 had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Callaway E. The Race for Coronavirus Vaccines: A Graphical Guide. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency COVID-19 Vaccines. [(accessed on 14 June 2023)]. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines.
- 3.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 22 November 2023)]. Available online: https://covid19.who.int/
- 4.Bertagnolio S., Thwin S., Silva R., Ford N., Baggaley R., Vitoria M., Jassat W., Doherty M. Clinical Characteristics and Prognostic Factors in People Living with HIV Hospitalized with COVID-19: Findings from the WHO Global Clinical Platform; Proceedings of the 11th International AIDS Society Conference on HIV Science; Berlin, Germany. 18–21 July 2021; PEBLB20. [Google Scholar]
- 5.Tesoriero J.M., Swain C.-A.E., Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., Gonzalez C.J., Udo T., Morne J.E., Hart-Malloy R., et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open. 2021;4:e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomah D., Reyes-Urueña J., Díaz Y., Moreno S., Aceiton J., Bruguera A., Vivanco-Hidalgo R. Sociodemographic, Clinical, and Immunological Factors Associated with SARS-CoV-2 Diagnosis and Severe COVID-19 Outcomes in People Living with HIV: A Retrospective Cohort Study. Lancet HIV. 2021;8:e701–e710. doi: 10.1016/S2352-3018(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N. Engl. J. Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troiano G., Nardi A. Vaccine Hesitancy in the Era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallam M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines. 2021;9:160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaway E. Omicron Likely to Weaken COVID Vaccine Protection. Nature. 2021;600:367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- 12.Chenchula S., Karunakaran P., Sharma S., Chavan M. Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review. J. Med. Virol. 2022;94:2969–2976. doi: 10.1002/jmv.27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patalon T., Gazit S., Pitzer V.E., Prunas O., Warren J.L., Weinberger D.M. Odds of Testing Positive for SARS-CoV-2 Following Receipt of 3 vs. 2 Doses of the BNT162b2 MRNA Vaccine. JAMA Intern. Med. 2022;182:179–184. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Centers for Disease Control and Prevention COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised. [(accessed on 2 September 2023)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
- 16.Sprague C., Simon S.E. Ending HIV in the USA: Integrating Social Determinants of Health. Lancet. 2021;398:742–743. doi: 10.1016/S0140-6736(21)01236-8. [DOI] [PubMed] [Google Scholar]
- 17.Fulda E.S., Fitch K.V., Overton E.T., Zanni M.V., Aberg J.A., Currier J.S., Lu M.T., Malvestutto C., Fichtenbaum C.J., Martinez E., et al. COVID-19 Vaccination Rates in a Global HIV Cohort. J. Infect. Dis. 2022;225:603–607. doi: 10.1093/infdis/jiab575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Han J., Li X., Chen D., Zhao X., Qiu Y., Zhang L., Xiao J., Li B., Zhao H. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines. 2021;9:1458. doi: 10.3390/vaccines9121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AquAS Programa Públic d’analítica de Dades per a La Recerca i La Innovació En Salut a Catalunya. 2017. [(accessed on 28 December 2023)]. pp. 1–38. Available online: https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2017/Programa_analitica_dades_PADRIS_aquas2017.pdf.
- 20.Bruguera A., Nomah D., Moreno-Fornés S., Díaz Y., Aceitón J., Reyes-Urueña J., Ambrosioni J., Llibre J.M., Falcó V., Imaz A. Cohort Profile: PISCIS, a Population-Based Cohort of People Living with HIV in Catalonia and Balearic Islands. Int. J. Epidemiol. 2023;52:e241–e252. doi: 10.1093/ije/dyad083. [DOI] [PubMed] [Google Scholar]
- 21.García-Altés A., Ruiz-Muñoz D., Colls C., Mias M., Bassols N.M. Socioeconomic Inequalities in Health and the Use of Healthcare Services in Catalonia: Analysis of the Individual Data of 7.5 Million Residents. J. Epidemiol. Community Health. 2018;72:871–879. doi: 10.1136/jech-2018-210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Interim Statement on Booster Doses for COVID-19 Vaccination. [(accessed on 1 July 2022)]. Available online: https://www.who.int/news/item/04-10-2021-interim-statement-on-booster-doses-for-covid-19-vaccination.
- 23.Ministerio de Sanidad del gobierno de España Instituto de Salud Carlos III (ISCIII). Estrategia de Detección Precoz, Vigilancia y Control de COVID-19. [(accessed on 1 October 2023)]. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf.
- 24.World Health Organization (WHO) Coronavirus Disease (COVID-19): COVID-19 Vaccines and People Living with HIV. [(accessed on 2 September 2023)]. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-covid-19-vaccines-and-people-living-with-hiv.
- 25.Boffito M., Waters L. More Evidence for Worse COVID-19 Outcomes in People with HIV. Lancet HIV. 2021;8:e661–e662. doi: 10.1016/S2352-3018(21)00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker. [(accessed on 2 September 2023)]. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab.
- 27.Falcon M., Rodríguez-Blázquez C., Romay-Barja M., Ayala A., Burgos A., Tena-Dávila D., José M., Forjaz M.J. COVID-19 Vaccine Hesitancy in Spain and Associated Factors. Front. Public Health. 2023;11:1129079. doi: 10.3389/fpubh.2023.1129079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statista Share of the Population Vaccinated against Coronavirus (COVID-19) in Spain from 4 January 2021 to 24 May 2023. [(accessed on 2 September 2023)]. Available online: https://www.statista.com/statistics/1218713/covid-19-share-of-people-vaccinated-in-spain/#:~:text=As%20of%20May%2024%2C%202023,one%20dose%20of%20the%20vaccine.
- 29.Tesoriero J.M., Patterson W., Daskalakis D., Chicoine J., Morne J., Braunstein S., Rajulu D.T., Rosenberg E. Notes from the Field: COVID-19 Vaccination Among Persons Living with Diagnosed HIV Infection—New York, October 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:182–184. doi: 10.15585/mmwr.mm7105a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO) Ten Threats to Global Health in 2019. [(accessed on 13 December 2023)]. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 31.Lin C., Tu P., Beitsch L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines. 2020;9:16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalmau-Bueno A., García-Altés A., Vela E., Clèries M., Pérez C.V., Argimon J.M. Frequency of Health-Care Service Use and Severity of Illness in Undocumented Migrants in Catalonia, Spain: A Population-Based, Cross-Sectional Study. Lancet Planet. Health. 2021;5:e286–e296. doi: 10.1016/S2542-5196(21)00036-X. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Patel R.C., Zheng Q., Madhira V., Olex A.L., Islam J.Y., French E., Chiang T.P.-Y., Akselrod H., Moffitt R. COVID-19 Disease Severity among People with HIV Infection or Solid Organ Transplant in the United States: A Nationally-Representative, Multicenter, Observational Cohort Study. Medrxiv. 2021:Preprint. doi: 10.2139/ssrn.3893539. [DOI] [Google Scholar]
- 34.SeyedAlinaghi S., Afsahi A.M., Moradi A., Parmoon Z., Habibi P., Mirzapour P., Dashti M., Ghasemzadeh A., Karimi E., Sanaati F. Current ART, Determinants for Virologic Failure and Implications for HIV Drug Resistance: An Umbrella Review. AIDS Res. Ther. 2023;20:74. doi: 10.1186/s12981-023-00572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomah D.K., Llibre J.M., Díaz Y., Moreno S., Aceiton J., Bruguera A., Gutiérrez-Macià M., Imaz A., Suanzes P., Navarro G., et al. SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV. Microorganisms. 2022;10:1666. doi: 10.3390/microorganisms10081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consejo International . Estrategia de Vacunación Frente a COVID-19 en España. Consejo International, Sistema Nacional de Salud; Madrid, Spain: 2021. Sistema Nacional de Salud. [Google Scholar]
- 37.Buchbinder S.P., McElrath M.J., Dieffenbach C., Corey L. Use of Adenovirus Type-5 Vectored Vaccines: A Cautionary Tale. Lancet. 2020;396:e68–e69. doi: 10.1016/S0140-6736(20)32156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C. Efficacy Assessment of a Cell-Mediated Immunity HIV-1 Vaccine (the Step Study): A Double-Blind, Randomised, Placebo-Controlled, Test-of-Concept Trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray G.E., Allen M., Moodie Z., Churchyard G., Bekker L.-G., Nchabeleng M., Mlisana K., Metch B., De Bruyn G., Latka M.H. Safety and Efficacy of the HVTN 503/Phambili Study of a Clade-B-Based HIV-1 Vaccine in South Africa: A Double-Blind, Randomised, Placebo-Controlled Test-of-Concept Phase 2b Study. Lancet Infect. Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rzymski P., Poniedziałek B., Fal A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines. 2021;9:1286. doi: 10.3390/vaccines9111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achrekar G.C., Batra K., Urankar Y., Batra R., Iqbal N., Choudhury S.A., Hooda D., Khan R., Arora S., Singh A., et al. Assessing COVID-19 Booster Hesitancy and Its Correlates: An Early Evidence from India. Vaccines. 2022;10:1048. doi: 10.3390/vaccines10071048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., Kreiss Y., Alroy-Preis S., Regev-Yochay G., Mendelson E. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Beltran W.F., Denis K.J.S., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M. MRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity against SARS-CoV-2 Omicron Variant. Cell. 2022;185:457–466. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coburn S.B., Humes E., Lang R., Stewart C., Hogan B.C., Gebo K.A., Napravnik S., Edwards J.K., Browne L.E., Park L.S. Analysis of Postvaccination Breakthrough COVID-19 Infections among Adults with HIV in the United States. JAMA Netw. Open. 2022;5:e2215934. doi: 10.1001/jamanetworkopen.2022.15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and statical code are available from the corresponding author upon request. The data for this study accessed on 1 October 2022 are available at the Centre for Epidemiological Studies of Sexually Transmitted Diseases and HIV/AIDS in Catalonia (CEEISCAT), the coordinating centre of the PISCIS cohort, the PADRIS, and from each of the collaborating hospitals upon request via https://pisciscohort.org/contacte/.