Abstract
Brazilian descendants of former Black-slave (quilombola) communities have been predisposed to several zoonotic diseases due to social vulnerability, characterized by subsistence and close contact with livestock and companion animals. Accordingly, the present study has assessed anti-Coxiella burnetii antibodies in 200 individuals and 20 dogs from four quilombola communities located in Paraná State, southern Brazil. Serum samples were tested by indirect immunofluorescence assay (IFA) using in-house and commercial diagnostic protocols, with analysis of seropositive titers and antibody type. Fisher’s exact test was used to compare seropositivity to C. burnetti with binary variables, with variables with three or more possible responses submitted to logistic regression. In total, 44/200 (22%; 95% CI 16.82–28.24) people tested positive, and 4.5% had titers higher than 128, indicating a recent onset of C. burnetii infection. Seropositive individuals were statistically associated with the Limitão community (p = 0.0013), urban workers as occupations (p = 0.0475), consumption of undercooked meat (p = 0.0159), and contact with animal abortion (p = 0.0276). No seropositivity association was found for age, sex, education, habit of entering forest areas, consumption of game meat, consumption of raw milk, flea and tick bites, dog contact, or history of female miscarriage. Only one of 20 dogs was seropositive with a titer of 128, probably related to an acute animal infection. Despite the prevalence here being higher than previous Brazilian reports, including with symptomatic populations, the results were within range for worldwide outbreaks and occupational risk populations. To the reader’s knowledge, this is the first human survey of Q fever in southern Brazil and should be considered a warning for C. burnetii in vulnerable populations, particularly Quilombola communities.
Keywords: Q fever, vulnerable populations, seroprevalence, indirect immunofluorescence assay
1. Introduction
Despite the fact that Coxiella burnetii, the causative agent of Q fever, has been mostly described as an asymptomatic infection, over time manifestation may lead to an acute self-limiting disease or a persistent focalized infection with fatal consequences [1]. Due to the bacterial ability to persist within the cell phagolysosomes for years, C. burnetii has been frequently reported in cardiac patients and with vascular infections [2,3,4,5,6]. Pathogen transmission may include contact with infected animals, inhalation of contaminated aerosols, and, to a lesser extent, consumption of contaminated raw milk, tick bites, and fomites [7]. The infection outcome depends on the individual susceptibility and the transmission route, resulting in a lower or higher infective dose [7].
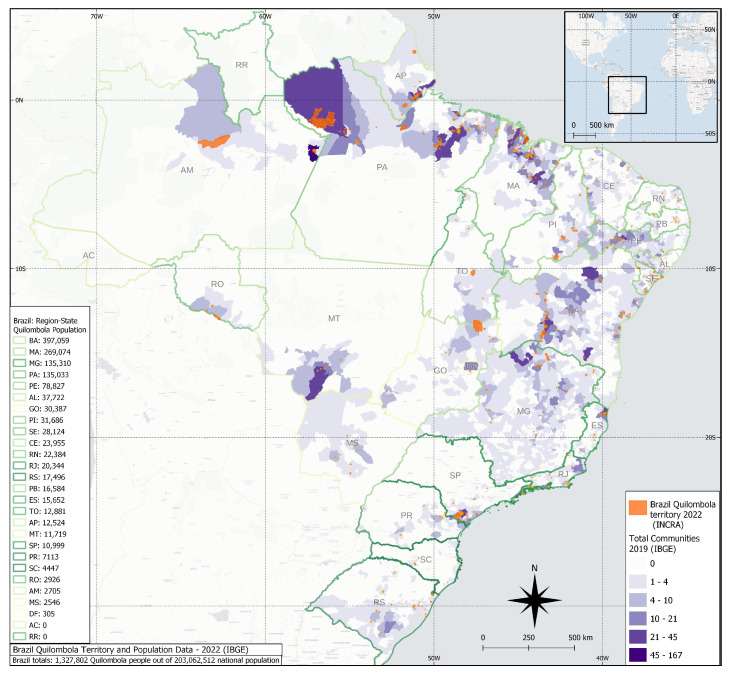
Brazil has been the home of the largest African-descendant population outside Africa, with a history of slavery resistance and establishing rural communities called “quilombos,” distributed mainly in remote and isolated areas, maintaining their ancestral culture, and living a life characterized by subsistence agriculture and livestock raising [8]. The latest Brazilian 2022 census has shown an overall population of 1.3 million quilombo individuals (0.65% of the total population), distributed in 1696/5568 (30.45%) Brazilian cities, with the highest population living in the northeastern (905,415; 68.2%) and the lowest in the southern region (29,056; 2.19%) (Figure 1) [9].
Figure 1.
Map of current quilombola communities in Brazil, with state distribution per population and density (purple), with the recent areas officially recognized in 2022 (orange) by INCRA (Brazilian Government).
Human infection has been associated with direct contact with domestic ruminants and living in rural areas, which may contribute to C. burnetti spreading [10]. Although quilombola individuals and their dogs may be exposed to C. burnetti, no study to date has assessed these Brazilian quilombola populations. Accordingly, the aim of the present study was to assess the presence of anti-Coxiella burnetii antibodies and associated risk factors in humans and dogs from quilombola communities in southern Brazil.
2. Materials and Methods
2.1. Study Design and Sample Collection
This study was conducted with humans (n = 200) and dogs (n = 20) belonging to four Brazilian quilombola communities located in rural regions of the state of Paraná, southern Brazil. The four quilombos (Limitão, Mamans, Serra do Apon, and Tronco) were established during the late 19th century by individuals fleeing and seeking refuge from Black slavery. Livelihoods primarily cultivated yerba mate plants and engaged in livestock-related activities, later through subsistence agriculture, rudimentary livestock, and handmade crafting [11]. These communities were located about 50 to 60 km (31 to 37 miles) from the main roads, solely connected by unmaintained gravel and sand roads at the time [11].
Samplings in this Q fever study were obtained by convenience and were part of a public policy project conducted by the same on-field group for COVID-19 screening, not in the context of comparing communities but rather targeting specific vulnerable populations in partnership with public healthcare institutions during pandemics. The quilombola population of Paraná was recently estimated at 7113 individuals, officially recognized as direct descendants of blacks from colonial times [9]. A representative sample size was proportionally calculated using open-source epidemiological statistics (Openepi) [12]. The calculation was made considering a regional population of 4000 quilombola individuals with a precision of 5%, a confidence interval of 95%, and a frequency of 12.68%, considering the average prevalence obtained in studies in nearby states [10].
The on-field samplings were performed in a series of six incursions from December 2021 to March 2022, preceded by door-to-door visits organized locally by community leaders and city healthcare workers. The four quilombola communities herein were part of the Rural Association of Castro County Quilombola Communities, established in 2005 for empowering member communities, preserving their African heritage, and advocating for public policies. The research taskforce team comprised certified nurses, pharmacists, veterinarians, and biologists, visiting the quilombola communities in a convoy of equipped vehicles with sampling tables, chairs, field tents, and blood sampling materials. As an immediate health approach, all dogs brought for sampling were vaccinated, dewormed, and given anti-flea drugs. All dogs have shown the presence of ticks and/or fleas at the moment of blood sampling. This study area included both natural and anthropized regions of the Atlantic Forest and Cerrado biomes, under a moist temperate climate with 17.5 °C of annual average temperature and 1495 mm3 of average precipitation. All individuals voluntarily participated, were sampled after signing a formal consent, and responded to an epidemiological questionnaire. A total of 10 mL of blood samples were collected from humans by cephalic puncture, performed by certified nurses, and from dogs by jugular venipuncture, after physical restraint and performed by certified veterinarians. All samples were collected in tubes without anticoagulant and centrifuged at 1500 revolutions per minute for five minutes, serum separated, and stored at −80 °C until processing.
2.2. Human Serological Testing
The human serum samples were tested by indirect immunofluorescence assay (IFA), performed at the Laboratory of Rickettsioses and Hantaviruses of the Octávio Magalhães Institute, Ezequiel Dias Foundation (Funed-MG). A previously validated in-house assay (IFA Kit, Belo Horizonte, Brazil) was used with an antigen produced from embryonated eggs of the Argentine strain At12 isolated from ticks [13] and tested using an anti-human IgG conjugate antibody (Bethyl Laboratories, Montgomery, TX, EUA). Positive and negative controls were obtained from a previously laboratory daily routine, with positive titers of 1:16.
In short, serum aliquots at a dilution of 1:16 in phosphate buffered saline (PBS, 0.1 M, pH 7.2) were placed on antigen-containing (30 µL) slides, incubated (37 °C for 30 min), washed with PBS, and then placed in a humidity chamber to dry. Following, 30 µL of fluorescein isothiocyanate (FITC)-anti-human IgG antibody was inserted into the concavities, with another incubation in a humid chamber for 30 min at 37 °C. After another washing and drying, slides were mounted with buffered glycerin and coverslips with immunofluorescence readings under a specific microscope (Olympus BX53, Tokyo, Japan) with a 40x optical objective. Positive and negative reaction controls were prepared for each slide reading, with positive samples submitted to serial dilutions of 1:16, 1:32, 1:64, and so on until the last titer was reached, as previously described [13].
2.3. Dog Serological Testing
Dog serum samples were submitted to the same protocol of indirect immunofluorescence assay (IFA) used in human samples. In addition, a commercially available kit (SCIMEDX Corporation, Denville, NJ, USA) containing phase I and phase II antigens of Coxiella burnetii Nine Mile was used. The procedure was the same as that used in human testing, except for the use of a fluorescein isothiocyanate (FITC) anti-dog antibody (Zoonosis Control Center, São Paulo, Brazil).
2.4. Data Analysis
Fisher’s exact test was used to compare the prevalence of C. burnetii seropositivity for the binary variables. The statistical level was considered significant at p ≤ 0.05. The analyses were performed using Statistical Analysis Software Studio 3.81 (SAS) (SAS Institute Inc., Cary, NC, USA).
3. Results
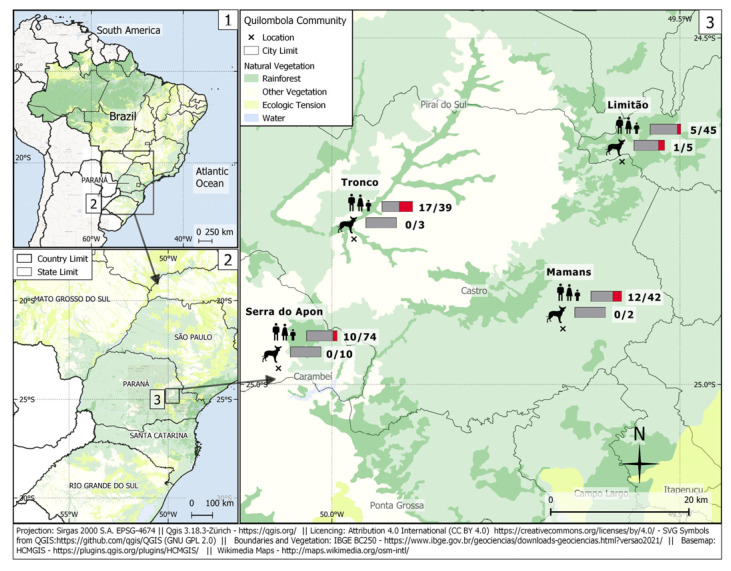
A total of 44/200 (22.0%; 95% CI 16.82–28.24) human samples were seropositive for C. burnetii. The titers of positive samples varied from 1:16 in 14/200 (7.0%), 1:64 in 21/200 (10.5%), and 1:128 or higher in 9/200 (4.5%) individuals. Overall, seropositivity was observed in only one/20 dogs from the Limitão community, with a 1:128 titer for the phase II antibody, suggesting an acute infection (Figure 2). This seropositive dog was 11 years old, male, clinically healthy, of mixed breed, and used to eating raw meat. This dog frequented forest areas and did not receive anti-parasite prophylaxis.
Figure 2.
1. Map of Brazil, with closeup location of Paraná state. 2. Map of Paraná State, with a closeup of the region in which this study was performed. 3. Map of Castro municipality, with the distribution of seropositive cases of anti-Coxiella burnetii antibodies in the human and dog quilombola populations studied. Accessed on: 20 January 2023.
Seropositivity to C. burnetti was statistically associated with farm workers’ occupation (p = 0.0475), consumption of undercooked meat (p = 0.0159), and contact with animal abortion (p = 0.0276) (Table 1). No association was found considering age (p = 0.5035), sex (0.7755), education level (p = 0.3826), access to forest areas (p = 0.4820), consumption of game meat (p = 0.0761), consumption of raw milk (p = 0.3513), flea bites (p = 0.5250), tick bites (p = 0.5768), history of miscarriage (p = 0.3529), and dog breeding (0.2033).
Table 1.
Sociodemographic characteristics of the quilombola population in the state of Paraná, Brazil (2023) and their respective seropositivity for Q fever.
| Variables | C. burnetii Seronegative | C. burnetii Seropositive | Total Population | ||
|---|---|---|---|---|---|
| N | % | N | % | N | |
| Quilombola community | |||||
| Limitão | 40 | 88.9 | 5 | 11.1 | 45 |
| Mamans | 30 | 71.4 | 12 | 28.6 | 42 |
| Serra do Apon | 64 | 86.5 | 10 | 13.5 | 74 |
| Tronco | 22 | 56.4 | 17 | 43.6 | 39 |
| Age | |||||
| Young (1 to 18) | 36 | 81.8 | 7 | 18.2 | 44 |
| Adults (19 to 59) | 96 | 75.6 | 31 | 24.4 | 127 |
| Elderly (≥60) | 24 | 82.8 | 5 | 17.2 | 29 |
| p value = 0.5035 | |||||
| Sex | |||||
| Female | 92 | 78.0 | 26 | 22.0 | 118 |
| Male | 65 | 79.3 | 17 | 20.7 | 82 |
| p value = 0.7755 | |||||
| Education | |||||
| Illiteracy | 29 | 80.6 | 7 | 19.4 | 36 |
| Elementary school | 103 | 77.4 | 30 | 22.6 | 133 |
| High school | 21 | 84.0 | 4 | 16.0 | 25 |
| Graduate | 3 | 50.0 | 3 | 50.0 | 6 |
| p value = 0.3826 | |||||
| Occupation | |||||
| Rural Workers | 33 | 67.3 | 16 | 32.7 | 49 |
| Urban Workers | 123 | 82.5 | 28 | 18.5 | 151 |
| p value = 0.0475 | |||||
| Access forest areas | |||||
| Yes | 134 | 78.8 | 36 | 21.2 | 170 |
| No | 24 | 80.0 | 6 | 20.0 | 30 |
| p value = 0.4820 | |||||
| Game meat consumption | |||||
| Yes | 23 | 92.0 | 2 | 8.0 | 25 |
| No | 134 | 76.6 | 41 | 23.4 | 175 |
| p value = 0.0761 | |||||
| Meat consumption | |||||
| Undercooked | 7 | 50.0 | 7 | 50.0 | 14 |
| Well-done | 149 | 80.1 | 37 | 19.9 | 186 |
| p value = 0.0159 | |||||
| Raw milk consumption | |||||
| Yes | 114 | 79.7 | 29 | 20.3 | 143 |
| No | 42 | 73.7 | 15 | 26.3 | 57 |
| p value = 0.3513 | |||||
| Flea bites | |||||
| Yes | 127 | 78.9 | 34 | 21.1 | 161 |
| No | 29 | 74.4 | 10 | 25.6 | 39 |
| p value = 0.5250 | |||||
| Tick bites | |||||
| Yes | 111 | 79.3 | 29 | 20.7 | 140 |
| No | 45 | 75.0 | 15 | 25.0 | 60 |
| p value = 0.5768 | |||||
| Miscarriage | |||||
| Yes | 11 | 68.7 | 5 | 31.3 | 16 |
| No | 81 | 79.4 | 21 | 20.6 | 102 |
| p value = 0.3529 | |||||
| Dog breeding | |||||
| Yes | 141 | 79.7 | 36 | 20.3 | 177 |
| No | 14 | 60.9 | 8 | 34.8 | 23 |
| p value = 0.2033 | |||||
| Animal abortion contact | |||||
| Yes | 24 | 63.2 | 14 | 36.8 | 38 |
| No | 132 | 81.5 | 30 | 18.5 | 162 |
| p value = 0.0276 | |||||
p values in bold indicate significant differences (p < 0.05) within the categories.
Seropositive quilombola individuals were more likely to consume rare meat to consume undercooked (50.0%) than well-cooked (19.9%) meat, to have contact with animal abortion and fomites (36.8%) than not (18.5%), and to work on farms (32.7%) than in urban areas (18.5%). Out of the titers ≥ 1:128, 5/9 (55.6%) persons lived in the Tronco community, 3/7 (42.9%) consumed undercooked meat, and 3/9 (33.3%) had contact with animal abortion.
4. Discussion
The human seropositivity of 44/200 (22.0%) individuals herein has been the second highest reported to date in Brazil, surpassed only by a series of reported Q fever cases with 10/16 (62.5%) in Minas Gerais state, Brazil [14]. All previous Brazilian serosurveys have reported lower seropositivity, including 1/61 (1.6%) patients with infective endocarditis in Sao Paulo state [15], 4/125 (3.2%) HIV-positive patients in Rio de Janeiro state [16], 17/437 (3.9%) in healthy individuals from Minas Gerais state, southeastern [17], 25/437 (5.72%) Dengue suspected patients in Minas Gerais state [18], 4/51 (7.8%) patients with culture-negative endocarditis in São Paulo state [19], 26/272 (10.0%) Dengue suspected patients in Rio de Janeiro state [20], and 129/604 (21.4%) Dengue suspected patients in São Paulo state [21]. If considering the Tronco community itself, with 17/39 (43.6%) seropositive individuals, the study herein had the highest reported frequency in healthy populations in Brazil.
Despite these few studies, Brazil still has the highest number of Q fever surveys in Latin America, followed by French Guyana, Colombia, Ecuador, and Argentina [22]. French Guiana presented an incidence rate (100,000 inhabitants/year) of 37 cases from 1990 to 2006, with 132 sampled patients [23], and of 27.4 cases from 2007 to 2017, with 695 sampled patients [24]. In addition, French Guiana reported Q fever in 32/131 (24.4%) patients with pneumonia and 25/275 (9.1%) with suspected Dengue [25,26]. A total of 83/153 (54.2%) cases were reported in slaughterhouse workers in Medellín, Colombia [27], 13/54 (24.1%) in veterinary professionals and students in Quito, Ecuador [28], and 1/99 (1.0%) in the healthy population of Buenos Aires, Argentina [29]. The study of vulnerable populations has brought a different perspective on how zoonotic diseases affect populations, alerting them to new associated risk factors and demanding a One Health approach to human, animal, and environmental health [30]. In the Brazilian Amazon, an indigenous population was investigated, which resulted in no (0/73) seropositive individuals [31]. In French Guyana, a cross-sectional study of illegal miners showed 11/380 (2.9%) seropositivity [32]. Based on such findings, the quilombola seropositivity (22.0%) herein becomes the highest in Latin America to date, highlighting the exposure of vulnerable Brazilian populations.
Although this is the highest Latin American prevalence in a healthy population, a study in Africa showed 86/137 (62.8%) seropositive pastoralists in Nigeria, likely due to livestock contact on rural farms [33]. Not surprisingly, large epidemics have occurred in farm populations in the Netherlands and Australia. Pathogen maintenance in such populations may become a serious human and animal health issue, impairing disease control and leading to social and economic losses [34,35].
Q fever has been indicated as an underestimated disease and an emerging health concern in Brazil [10]. Molecular and serological surveys have found, respectively, 44/360 (12.2%) positive bovines [36] and 129/604 (21.4%) positive humans [21] in São Paulo state, southeastern Brazil. The occurrence of Q fever has been evidenced since 2016 in Rio de Janeiro state [20,37].
The serological 1:128 cutoff point has been defined to determine acute disease in persons with Q fever [38]. Although the study herein has been designed for a survey of four clinically healthy quilombola populations, 9/200 (4.5%) of the total, or 9/44 (20.4%) of positive individuals, presented ≥1:128 serology, suggesting a recent infection. No titer ≥1:800 was found, considered a chronic disease characteristic [38]. Noteworthy, C. burnetii can remain in the body for years, even in asymptomatic cases, later developing serious complications such as endocarditis and hepatitis [4,5,39].
In the present study, we noted that individuals living in quilombola communities showed significant seropositivity for C. burnetii as a reflection of the sum of rural habits and characteristics, such as proximity to cattle. When eliminated by aerosols at the time of animal calving or as a result of abortions, C. burnetii spores attach themselves to dust and travel by wind, reaching up to 30 km from the aerosolization site [40]. Similarly, workers who spend most of the day on farms were more seropositive than those working in urban environments, probably due to their contact with cattle.
Consumption of undercooked meat was associated with C. burnetii seropositivity. Although the role of raw and undercooked meat in C. burnetii remains to be described, molecular studies have detected C. burnetii in meat samples of cattle, buffalo, goats, and sheep collected from the slaughterhouses of Pakistan [41] and in raw meat destined exclusively for companion animal consumption in Australia [42].
The epidemiology of Q fever remains to be clarified, demanding an investigation of all possible associated risk factors. Although milk contamination by C. burnetti has been reportedly observed [40,43,44], its presence was not statistically associated with population seropositivity, and raw milk consumption remains to be fully established as an important human transmission route. On the other hand, the consumption of undercooked meat was significantly associated with seropositivity. Contact with ectoparasites does not seem to be an important factor for human infection, although ticks may be commonly infected [45,46]. Despite contact with dogs not being a risk factor, contact with dog abortions and vaginal fluids has been significant for infection [45]. Although not statistically significant, abortion in women was highly referred to and may require more attention, since 5/16 (31.3%) of the women who mentioned abortion were seropositive. Data on human abortions resulting from Q fever has been scarce, and such a risk factor should be further investigated.
Dogs may become at risk of infection at the time of birth or in cases of abortion [47]. In other situations, dogs may not present a risk of transmission to humans or other animals [10]. The existence of only one seropositive dog in the present study and in the community with a lower percentage of seropositive human samples may indicate that dogs have distinct infection routes. Shedding patterns of C. burnetii in dogs have not been assessed, and the association between dogs and human infection is a query to be evaluated. Nevertheless, the serological outcome of our survey may suggest that dogs are not a potential source of C. burnetii for humans. Although there was low dog sampling, this is the first study that concomitantly accessed human and dog populations under socioeconomic vulnerability. In the present study, human seropositivity was associated with canine abortion contact. Thus, further studies should be conducted to clarify the role of dogs in C. burnetti transmission in such areas.
Despite small domestic ruminants being recognized as its main transmission source [48], C. burnetii was also reported in large ruminants and detected in bovine abortion remains in southeastern Brazil [36]. There were approximately 213,000 cattle farms in Paraná State at the time of the survey, with approximately 6.3 million cattle heads and dairy production accounting for 25% of the local economy [49]. Although C. burnetii in wildlife has been studied worldwide [48,50,51], few studies were conducted in Brazil, including detection in 6/131 (4.6%) of the wild rodents of southeastern Brazil [52], in 4/21 (19.0%) Artibeus spp. bats in northeastern Brazil [53], and in 9/169 (5.32%) deer in southeast and central-western Brazil [54]. In addition, future studies should also investigate the role of other livestock populations in C. burnetti transmission in such communities in Paraná State.
Our study presents some limitations regarding its results. First, we have not performed IgM detection. Although IgM antibodies may provide ancillary information to IgG titers in human serodiagnosis, IgM specificity has been much lower than IgG, with higher cross-reactivity, making it not ideally the best C. burnetii detection tool in exposed populations [38,55]. In addition, an in-house serological test for Q fever was used instead of commercially available kits, which may have generated differences in positivity and correspondent titers. However, the in-house assay produced for the study herein with the Argentine antigen has proven to be very efficient in detecting anti-phase II antibodies, i.e., IgG anti-C. burnetii antibodies, in patients with Q fever [13].
As this study was a convenience survey with no random sampling, biased outcomes may be a limitation, as statistical differences on associated risk factors among groups may not represent the actual possibilities. However, as Q fever epidemiology has been poorly understood, the inferences herein may still be valuable as potential insights and associated causes of exposure in quilombola populations. Bearing in mind such limitations, the data herein have indicated the habits of communities as potential associated risk factors, which may also impact other infectious diseases and therefore common prevention measures.
Despite the fact that the health impact of C. burnetii on the studied population could not be confirmed, as a healthy population was tested in a single sampling, the primary aim herein was to assess the C. burnetti exposure in this vulnerable population. As C. burnetti was detected in the quilombola population by sole sampling, further studies should screen and investigate febrile cases in quilombola communities locally, as fever may reappear further, being reactivated in seropositive individuals. Nonetheless, despite not characterizing active infections or differentiating acute from persistent infection, the evidence herein of anti-C. burnetti antibodies has indicated exposure in such a vulnerable population and should be further investigated.
5. Conclusions
To the author’s knowledge, this is the first survey of human and animal populations for C. burnetti in southern Brazil. This study has reported high C. burnetii seropositivity in the quilombola population, likely associated with close contact with livestock. Such results may provide important findings for future public health actions to prevent C. burnetii infection in overlapping areas of vulnerable human populations, livestock, and wildlife.
Acknowledgments
To the Ezequiel Dias Foundation (Funed-MG) for the collaboration with equipment for the research. To the communities quilombolas of Limitão, Mamans, Serra do Apon, and Tronco for supporting the research and teaching us so much. Finally, to João Henrique Farinhas and Fernando Rodrigo Doline for their valuable help with blood samplings and epidemiological questionnaires.
Author Contributions
Conceptualization, D.A.d.F.; methodology, D.A.d.F., F.P.d.S., A.Í.d.L.D., M.V.F.S. and M.M.D.; validation, L.B.K., G.A.K.P., O.J.D., G.M.F., A.W.B. and H.L. formal analysis, D.A.d.F., L.B.K., M.d.S.R.M. and F.S.P.; investigation, D.A.d.F., L.B.K., G.A.K.P. and A.W.B.; resources, A.W.B. and H.L.; data curation, D.A.d.F., L.B.K., L.M.B., M.d.S.R.M. and F.S.P.; writing—original draft preparation, D.A.d.F., L.B.K. and A.W.B.; writing—review and editing, D.A.d.F., L.B.K., A.W.B. and H.L.; visualization, D.A.d.F.; supervision, A.W.B. and H.L.; project administration, A.W.B. and H.L.; funding acquisition, A.W.B. and H.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The present study was approved by the Human Health Ethics Committee of the Brazilian Ministry of Health (protocol 53828121.1.0000.0105) and the Animal Ethics Committee of the State University of Ponta Grossa (protocol 22.000075139-9).
Informed Consent Statement
Formal, signed consent was obtained from all participants prior to blood sampling and the completion of an epidemiological questionnaire administered by certified healthcare professionals. Illiterate individuals were given detailed information by interview, and official consent was obtained by fingerprinting. Participants under the age of 18 were asked for signed consent from parents or legal guardians. All dogs living in the quilombola communities had unrestricted outdoor access and often changed households and ownership. Thus, formal, signed consent for dog sample collection and epidemiological questionnaire completion was obtained from one or more owners.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the São Paulo State Research Support Foundation (FAPESP) grant number 2022/07124-6.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kazar J. Coxiella burnetii infection. Ann. N. Y. Acad. Sci. 2005;1063:105–114. doi: 10.1196/annals.1355.018. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajjar S., Hussain S., Al-Sabban E., Jäger C. Coxiella burnetii endocarditis in a child. Pediatr. Infect. Dis. J. 1997;16:911–913. doi: 10.1097/00006454-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Recalde A., Maté I., López E., Yebra M., Merino J.L., Perea J. Coxiella burnetii endocarditis: Long-term clinical course in 20 patients. Rev. Esp. Cardiol. 2000;53:940–946. doi: 10.1016/S0300-8932(00)75179-7. [DOI] [PubMed] [Google Scholar]
- 4.Fenollar F., Fournier P.E., Raoult D. Molecular Detection of Coxiella burnetii in the Sera of Patients with Q Fever Endocarditis or Vascular Infection. J. Clin. Microbiol. 2004;42:4919–4924. doi: 10.1128/JCM.42.11.4919-4924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siciliano R., Strabelli T., Paddock C., Jones T., Zeigler R., Rodrigues C., Uip D.E., Castelli J.B., Sampaio R., Grinberg M., et al. Culture-negative endocarditis in Sao Paulo, Brazil. Serologic investigation of Coxiella burnetii and Bartonella spp. Clin. Res. Cardiol. 2007;96:411–412. [Google Scholar]
- 6.Lemos E.R., Rozental T., Mares-Guia M.A., Almeida D.N., Moreira N., Silva R.G., Barreira J.D., Lamas C.C., Favacho A.R., Damasco P.V. Q fever as a cause of fever of unknown origin and thrombocytosis: First molecular evidence of Coxiella burnetii in Brazil. Vector Borne Zoonotic Dis. 2011;11:85–87. doi: 10.1089/vbz.2009.0261. [DOI] [PubMed] [Google Scholar]
- 7.Toman R., Heinzen R.A., Samuel J.E., Mege J.L. Coxiella burnetii: Recent Advances and New Perspectives in Research of the Q Fever Bacterium. 1st ed. Springer; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 8.Leite I.B. Quilombos no Sul do Brasil: Perícias Antropológicas. Nuer; Santa Catarina, Brasil: 2006. [Google Scholar]
- 9.Instituto Brasileiro de Geografia e Estatística (IBGE) Censo Demográfico: Quilombolas—Primeiros Resultados do Universo. IBGE; Rio de Janeiro, Brasil: 2022. [Google Scholar]
- 10.França D.A., Mioni M.S.R., Fernandes J., Lemos E.R.S., Duré A.Í.L., Silva M.V.F., Langoni H., Megid J. Overview of Q fever in Brazil: An underestimated zoonosis. Rev. Inst. Med. Trop. São Paulo. 2023;65:e39. doi: 10.1590/s1678-9946202365039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos M.D.C., Gallinari T.S. Permanência e Resistência das Comunidades Remanescentes de Quilombos No Paraná. Geosaberes. 2017;8:131. doi: 10.26895/geosaberes.v8i15.576. [DOI] [Google Scholar]
- 12.Dean A.G., Sullivan K.M., Soe M.M. Open Source Epidemiologic Statistics for Public Health. 2013. [(accessed on 20 April 2022)]. Available online: https://www.openepi.com/SampleSize/SSPropor.htm.
- 13.França D.A., Mioni M.S.R., Fornazari F., Rodrigues N.J.L., Polido L.R.F., Appolinario C.M., Ribeiro B.L.D., de Lima Duré A.I., Ferreira Silva M.V., Richini-Pereira B.L., et al. Comparison of Three Serologic Tests for the Detection of Anti-Coxiella burnetii Antibodies in Patients with Q Fever. Pathogens. 2023;12:873. doi: 10.3390/pathogens12070873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa P.S., Brigatte M.E., Greco D.B. Questing one Brazilian query: Reporting 16 cases of Q fever from Minas Gerais, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2006;48:5–9. doi: 10.1590/S0036-46652006000100002. [DOI] [PubMed] [Google Scholar]
- 15.Siciliano R.F., Strabelli T.M., Zeigler R., Rodrigues C., Castelli J.B., Grinberg M., Colombo S., da Silva L.J., Nascimento E.M.M.D., Dos Santos F.C.P., et al. Infective endocarditis due to Bartonella spp. and Coxiella burnetii: Experience at a cardiology hospital in Sao Paulo, Brazil. Ann. N. Y. Acad. Sci. 2006;1078:215–222. doi: 10.1196/annals.1374.123. [DOI] [PubMed] [Google Scholar]
- 16.Lamas C.C., Rozental T., Bóia M.N., Favacho A.R., Kirsten A.H., da Silva A.P. Seroprevalence of Coxiella burnetii antibodies in human immunodeficiency virus-positive patients in Jacarepaguá, Rio de Janeiro, Brazil. Clin. Microbiol. Infect. 2009;15:140–141. doi: 10.1111/j.1469-0691.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 17.Costa P.S., Brigatte M.E., Greco D.B. Antibodies to Rickettsia rickettsii, Rickettsia typhi, Coxiella burnetii, Bartonella henselae, Bartonella quintana and Ehrlichia chaffeensis among healthy population in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2005;100:853–859. doi: 10.1590/S0074-02762005000800006. [DOI] [PubMed] [Google Scholar]
- 18.Meurer I.R., Silva M.R., Silva M.V., Duré A.I., Adelino T.E., Costa A.V., Pereira Vanelli C., Rozental T., Sampaio de Lemos E.G., do Amaral Corrêa J.O., et al. Soroprevalência de anticorpos anti-Coxiella burnetii em pacientes com suspeita de dengue no Estado de Minas Gerais, Brasil. Braz. J. Infect. Dis. 2021;25:169–170. doi: 10.1016/j.bjid.2020.101415. [DOI] [Google Scholar]
- 19.Siciliano R.F., Ribeiro H.B., Furtado R.H., Castelli J.B., Sampaio R.O., Santos F.C.P., Colombo S., Grinberg M., Strabelli T.M.V. Endocardite por Coxiella burnetii (febre Q): Doença rara ou pouco diagnosticada? Relato de caso. Rev. Soc. Bras. Med. Trop. 2008;41:409–412. doi: 10.1590/S0037-86822008000400017. [DOI] [PubMed] [Google Scholar]
- 20.Mares-Guia M.A., Rozental T., Guterres A., Ferreira M.S., Botticini R.G., Terra A.K., Guterres A. Molecular identification of Q fever in patients with a suspected diagnosis of dengue in Brazil in 2013–2014. Am. J. Trop. Med. Hyg. 2016;94:1090–1094. doi: 10.4269/ajtmh.15-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.França D.A., Mioni M.S.R., Fornazari F., Duré A.Í.L., Silva M.V.F., Possebon F.S., Richini-Pereira V.B., Langoni H., Megid J. Seropositivity for Coxiella burnetii in suspected patients with dengue in São Paulo state, Brazil. PLoS Negl. Trop. Dis. 2022;16:e0010392. doi: 10.1371/journal.pntd.0010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelboin L., Mioni M.S.R., Couesnon A. Infecção por Coxiella burnetii em gado, animais de estimação, vida selvagem e carrapatos na América Latina e no Caribe: Uma revisão abrangente da literatura. Curr. Trop. Med. Rep. 2023;10:94–137. doi: 10.1007/s40475-023-00288-7. [DOI] [Google Scholar]
- 23.Grangier C., Debin M., Ardillon V., Mahamat A., Fournier P.E., Simonnet C., Guillot G., Louvel D., Ravachol F., Perenhou V., et al. Epidémiologie de la fièvre Q en Guyane, 1990–2006. Bull. Veill. Sanitaire. CIRE Antill. Guyane. 2009;10:2–4. [Google Scholar]
- 24.Thill P., Eldin C., Dahuron L., Berlioz-Artaud A., Demar M., Nacher M., Beillard E., Djossou F., Epelboin L. High endemicity of Q fever in French Guiana: A cross sectional study (2007–2017) PLoS Negl. Trop. Dis. 2022;16:e0010349. doi: 10.1371/journal.pntd.0010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epelboin L., Chesnais C., Boullé C., Drogoul A.-S., Raoult D., Djossou F., Mahamat A. Q Fever Pneumonia in French Guiana: Prevalence, Risk Factors, and Prognostic Score. Clin. Infect. Dis. 2012;55:67–74. doi: 10.1093/cid/cis288. [DOI] [PubMed] [Google Scholar]
- 26.Epelboin L., Nacher M., Mahamat A., Pommier-de-Santi V., Berlioz-Arthaud A., Eldin C. Q Fever in French Guiana: Tip of the Iceberg or Epidemiological Exception? PLoS Negl. Trop. Dis. 2016;10:e0004598. doi: 10.1371/journal.pntd.0004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ruiz L. Q Fever in Colombia, S.A. A Serological Survey of Human and Bovine Populations. Zoonoses Public Health. 1977;24:287–292. doi: 10.1111/j.1439-0450.1977.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 28.Echeverría G., Reyna-Bello A., Minda-Aluisa E., Celi-Erazo M., Olmedo L., García H.A., Garcia-Bereguiain M.A., Waard J.H. Serological evidence of Coxiella burnetii infection in cattle and farm workers: Is Q fever an underreported zoonotic disease in Ecuador? Infect. Drug Resist. 2019;12:701–706. doi: 10.2147/IDR.S195940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cicuttin G.L., Degiuseppe J.I., Mamianetti A., Corin M.V., Linares M.C., Salvo M.N., Dohmen F.E. Serological evidence of Rickettsia and Coxiella burnetii in humans of Buenos Aires, Argentina. Comp. Immunol. Microbiol. Infect. Dis. 2015;43:57–60. doi: 10.1016/j.cimid.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Panazzolo G.K., Kmetiuk L.B., Domingues O.J., Farinhas J.H., Doline F.R., França D.A., Rodrigues N.J.L., Biondo L.M., Giuffrida R., Langoni H., et al. One Health Approach in Serosurvey of Toxoplasma gondii in Former Black Slave (Quilombola) Communities in Southern Brazil and Among Their Dogs. Trop. Med. Infect. Dis. 2023;8:377. doi: 10.3390/tropicalmed8070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira V.K., Rozental T., Lemos E.R.S., Pessoa A., Jr., Assis M., Espinosa M.M., Nascimento V.F., Terças-Trettel A.C.P., Atanaka M. Seroprevalence and risk factors of q fever in an indigenous community in the Brazilian Legal Amazonia. J. Vet. Sc. Public. Health. 2022;9:68–82. [Google Scholar]
- 32.Douine M., Bonifay T., Lambert Y., Mutricy L., Galindo M.S., Godin A., Bourhy P., Picardeau M., Saout M., Demar M., et al. Zoonoses and gold mining: A cross-sectional study to assess yellow fever immunization, Q fever, leptospirosis and leishmaniasis among the population working on illegal mining camps in French Guiana. PLoS Negl. Trop. Dis. 2022;16:e0010326. doi: 10.1371/journal.pntd.0010326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadmus S., Salam S.P., Adesokan H.K., Akporube K., Ola-Daniel F., Awosanya E.J. Seroprevalence of brucellosis and Q fever infections amongst pastoralists and their cattle herds in Sokoto State, Nigeria. PLoS ONE. 2021;16:e0254530. doi: 10.1371/journal.pone.0254530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Asseldonk M.A., Prins J., Bergevoet R.H. Economic assessment of Q fever in the Netherlands. Prev. Vet. Med. 2013;112:27–34. doi: 10.1016/j.prevetmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Sloan-Gardner T.S., Massey P.D., Hutchinson P., Knope K., Fearnley E. Trends and risk factors for human Q fever in Australia, 1991–2014. Epidemiol. Infect. 2017;145:787–795. doi: 10.1017/S0950268816002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mioni M.S.R., Costa F.B., Ribeiro B.L.D., Teixeira W.S.R., Pelicia V.C., Labruna M.B., Rousset E., Sidi-Boumedine K., Thiéry R., Megid J. Coxiella burnetii in slaughterhouses in Brazil: A public health concern. PLoS ONE. 2020;15:e0241246. doi: 10.1371/journal.pone.0241246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemos E.R.S., Rozental T., Siqueira B.N., Pessoa A.A., Joaquim T.E., Silva R.G., de Andrade Leite C., Alvarez Arantes A., Ferreira da Cunha M., Provençano Borghi D. Q fever in military firefighters during cadet training in Brazil. Am. J. Trop. Med. Hyg. 2018;99:303–305. doi: 10.4269/ajtmh.17-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson A., Bijlmer H., Fournier P.E., Graves S., Hartzell J., Kersh G.J., Limonard G., Marrie T.J., Massung R.F., McQuiston J.H., et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:730. [PubMed] [Google Scholar]
- 39.Harris R.J., Storm P.A., Lloyd A., Arens M., Marmion B.P. Long-term persistence of Coxiella burnetii in the host after primary Q fever. Epidemiol. Infect. 2000;124:543–549. doi: 10.1017/S0950268899003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tissot-Dupont H., Raoult D. Q fever. Infect. Dis. Clin. N. Am. 2008;22:505–514. doi: 10.1016/j.idc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Shujat S., Shehzad W., Anjum A.A., Hertl J.A., Zahoor M.Y., Gröhn Y.T. Molecular detection of Coxiella burnetii in raw meat samples collected from different abattoirs in districts Kasur and Lahore of Punjab, Pakistan. PLoS ONE. 2023;18:e0289944. doi: 10.1371/journal.pone.0289944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro A., Bosward K., Mathews K., Vincent G., Stenos J., Tadepalli M., Norris J. Molecular detection of Coxiella burnetii in raw meat intended for pet consumption. Zoonoses Public Health. 2020;67:443–452. doi: 10.1111/zph.12707. [DOI] [PubMed] [Google Scholar]
- 43.Pexara A., Solomakos N., Govaris A. Q fever and prevalence of Coxiella burnetii in milk. Trends Food Sci. Technol. 2018;71:65–72. doi: 10.1016/j.tifs.2017.11.004. [DOI] [Google Scholar]
- 44.de Souza Ribeiro Mioni M., Ribeiro B.L.D., Peres M.G., Teixeira W.S.R., Pelícia V.C., Motta R.G., Labruna M.B., Ribeiro M.G., Sidi-Boumedine K., Megid J. Real-time quantitative PCR-based detection of Coxiella burnetii in unpasteurized cow’s milk sold for human consumption. Zoonoses Public Health. 2019;66:695–700. doi: 10.1111/zph.12609. [DOI] [PubMed] [Google Scholar]
- 45.Maurin M., Raoult D. Q fever. Clin. Microbiol. Rev. 1999;12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenner A.E., Muñoz-Leal S., Sachan M., Labruna M.B., Raghavan R. Coxiella burnetii and Related Tick Endosymbionts Evolved from Pathogenic Ancestors. Genome Biol. Evol. 2021;13:evab108. doi: 10.1093/gbe/evab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro A.J., Norris J.M., Heller J., Brown G., Malik R., Bosward K.L. Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses Public Health. 2016;63:458–466. doi: 10.1111/zph.12250. [DOI] [PubMed] [Google Scholar]
- 48.Cumbassá A., Barahona M.J., Cunha M.V., Azórin B., Fonseca C., Rosalino L.M., Tilburg J., Hagen F., Santos A.S., Botelho A. Coxiella burnetii DNA detected in domestic ruminants and wildlife from Portugal. Vet. Microbiol. 2015;180:136–141. doi: 10.1016/j.vetmic.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Brazilian Institute of Geography and Statistics (IBGE) Censo Agropecuário. Pesquisa. Paraná. [(accessed on 18 September 2023)]; Available online: https://cidades.ibge.gov.br/brasil/pr/pesquisa/24/27745.
- 50.Enright J.B., Behymer D.E., Franti C.E., Dutson V.J., Longhurst W.M., Wright M.E., Goggin J.E. The behavior of Q fever rickettsiae isolated from wild animals in Northern California. J. Wildl. Dis. 1971;7:83–90. doi: 10.7589/0090-3558-7.2.83. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz-Fons F., Rodríguez O., Torina A., Naranjo V., Gortázar C., Fuente J. Prevalence of Coxiella burnetti infection in wild and farmed ungulates. Vet. Microbiol. 2008;126:282–286. doi: 10.1016/j.vetmic.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Rozental T., Ferreira M.S., Guterres A., Mares-Guia M.A., Teixeira B.R., Gonçalves J., Bonvicino C.R., D’andrea P.S., de Lemos E.R.S. Zoonotic pathogens in Atlantic Forest wild rodents in Brazil: Bartonella and Coxiella infections. Acta Trop. 2017;168:64–73. doi: 10.1016/j.actatropica.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira M.S., Guterres A., Rozental T., Novaes R.L.M., Vilar E.M., Oliveira R.C., Fernandes J., Forneas D., Junior A.A., Brandão M.L., et al. Coxiella and Bartonella spp. in bats (Chiroptera) captured in the Brazilian Atlantic Forest biome. BMC Vet. Res. 2018;14:279. doi: 10.1186/s12917-018-1603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanatto D.C.S., Duarte J.M.B., Labruna M.B., Tasso J.B., Calchi A.C., Machado R.Z., André M.R. Evidence of exposure to Coxiella burnetii in neotropical free-living cervids in South America. Acta Trop. 2019;197:105037. doi: 10.1016/j.actatropica.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 55.Musso D., Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Diagn. Lab. Immunol. 1997;4:208–212. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.