Abstract
Enterohemorrhagic Escherichia coli (EHEC) produces Shiga-like toxins (SLT), potent protein synthesis inhibitors. To further dissect the role of SLT-II in the course of disease, we have constructed E. coli TUV86-2, an isogenic SLT-II-negative mutant of EHEC strain 86-24. The slt-ii gene was inactivated by suicide vector mutagenesis. We also isolated derivatives of strain 86-24 that were cured of the phage carrying the toxin genes.
Enteric infections of humans with enterohemorrhagic Escherichia coli (EHEC) have a wide spectrum of clinical symptoms, with intestinal as well as extraintestinal manifestations. Within the intestine the infection can vary from an inapparent carrier status to nonbloody or bloody diarrhea and hemorrhagic colitis as the most severe form (1, 21, 38). Systemically, an EHEC infection can lead to neurological symptoms and microangiophathic thrombocytobpenic disorders known as hemolytic uremic syndrome (HUS) (35) or thrombotic thrombocytopenic purpura. HUS affects mostly young children (31) and represents the major cause for acute kidney failure in childhood (35, 36). Thrombocytopenic purpura is more a disease of the elderly (6).
Isolates from outbreak cases usually have the serotype O157:H7 and produce Shiga-like toxins (SLTs) (2, 23, 38, 39). The SLTs are genetically and biochemically related to Shiga toxin produced by Shigella dysenteriae type I strains. In general there are two major subclasses of SLTs, SLT-I and SLT-II. These two classes of toxin are differentiated immunologically by their cross-reaction (SLT-I) or lack of cross-reaction (SLT-II) with antisera to Shiga toxin (1). Although at the molecular level the genetics and the mode of action of Shiga-like toxins have been very well dissected, the toxins’ role in pathogenesis in both the intestinal and extraintestinal manifestations is still not fully developed. In this study, using suicide vector mutagenesis, we have constructed E. coli TUV86-2, an isogenic, toxin-negative mutant of E. coli 86-24, an O157:H7 EHEC strain producing SLT-II.
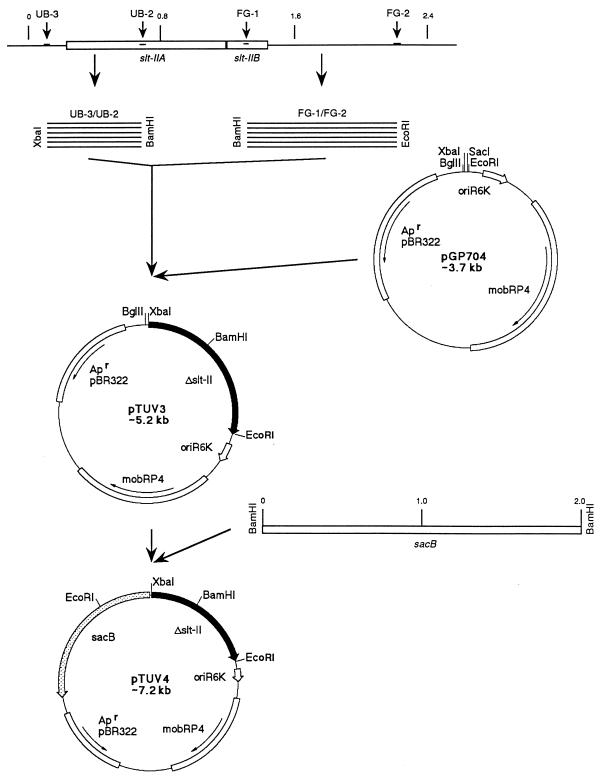
The suicide plasmid pTUV4 was constructed as outlined in Fig. 1. Using the primer pairs UB-3–UB-2 and FG-1–FG-2 (Table 1), two DNA fragments, 610 and 870 bp respectively, were amplified by PCR. They were cloned into pGP704 (25) (Fig. 1) linearized with XbaI and EcoRI and ligated together at their BamHI restriction site, generating an slt-ii operon, with an internal 589-bp deletion. The two PCR-derived DNA fragments covered the entire slt-ii toxin operon and flanking upstream and downstream sequences. The internal primers UB-2 and FG-1 were chosen to delete nucleotides 702 to 1290, based on the slt-ii gene sequence published by Jackson et al. (17). We deleted the codon for glutamic acid 166 (nucleotides 803 to 805), which is an active-site residue of SLT-II (16), as well as the entire leader sequence of the B-subunit. Furthermore the deletion was designed to result in an out-of-frame reading of the B-subunit. Thus, the product from this gene deletion would result in neither an enzymatic active A-subunit nor a mature B-subunit with the ability to induce apoptosis in certain cells (24). The sacB gene from Bacillus subtilis, which codes for the enzyme levansucrase, was used as a positive selection system, as described by other investigators (8, 19, 29). Ligation of the sacB gene from B. subtilis (12) into BglII (New England Biolabs)-digested pTUV3 yielded pTUV4 (Fig. 1). The plasmid was electroporated into E. coli SM10 λpir. E. coli SM10 λpir(pTUV4) and EHEC strain 86-24 were grown in Luria-Bertani (LB) media with E. coli SM10 λpir and without ampicillin (AMP) (strain 86-24) to an optical density at 600 nm (OD600) of 0.5. The donor strain was washed twice in sterile saline and mixed with the recipient (strain 86-24) in a donor-to-recipient ratio of 2 to 1. An aliquot of 1.5 ml of the bacterial mixture was spun down and resuspended in 100 μl of sterile saline and spread onto a sheep blood agar plate and incubated overnight at 37°C. The entire bacterial lawn from the plate was resuspended in 1 ml of sterile saline and diluted 10-fold to 10−5. A 100-μl aliquot of each dilution was spread onto MacConkey-AMP (200 μg/ml) agar plates. Lactose-positive Ampr colonies, representing potential E. coli 86-24 exconjugants, were picked and streaked on MacConkey-AMP agar plates for single-colony isolation. Bacterial colonies were then replica plated on LB-AMP agar and LB-AMP agar with 10% sucrose and grown overnight at 30°C. Colonies that showed growth on the LB-AMP agar and no growth or inhibited growth on the sucrose-containing plates were further tested in a PCR using the primers UB-3 and FG-2. Exconjugants were lactose positive, Ampr, and sucrose sensitive and in a PCR with primers UB-3 and FG-2 yielded two bands, 2,069 bp and 1,480 bp in size (slt-ii wild-type and mutated genes). An exconjugant was grown in LB medium without antibiotic stress to slight turbidity. Five 10-fold dilutions were spread out on plain LB and LB-sucrose agar plates in parallel. Colonies growing on sucrose plates were further tested for their sensitivity to ampicillin. Ampicillin-sensitive and sucrose-resistant colonies were likely to have undergone a second recombinational event, resulting in the loss of the suicide vector and leaving behind one copy of the toxin genes, either the wild-type or mutated genes. The ratio of sucrose-resistant to sucrose-sensitive colonies was about 10−4, reflecting the frequency of the loss of pTUV4. These numbers were in concordance with published data (3). The sucrose-resistant and ampicillin-sensitive colonies were inoculated into LB supplemented with mitomycin C (400 ng/ml; Sigma Chemical Company, St. Louis, Mo.) and grown overnight to induce phage lysis and toxin release. Of 150 colonies grown, 120 showed low turbidity, with an OD600 of ∼0.4, and the presence of flocculent debris, indicative of phage induction and cell lysis. Thirty of the isolates, however, showed good growth, with an OD600 of >2.5, and no sign of lysis. Similar growth was seen with the nonlysogenic C600 strain. Culture supernatants were tested in triplicate in a toxin capture enzyme-linked immunosorbent assay (ELISA) with the monoclonal antibody 4D1, as described previously (9). Supernatants from the thirty colonies which showed no growth inhibition in mitomycin C-containing LB were all negative by ELISA for SLT-II. Based on the lack of apparent phage induction and the lack of toxin production, we termed this class of mutants phage-cured derivatives of strain 86-24. Only one supernatant from 120 mitomycin C-sensitive cultures was negative in the toxin ELISA. This isolate was termed TUV86-2.
FIG. 1.
Construction of pTUV4. PCR fragments generated from E. coli 86-24 by utilizing the primer pairs UB-3–UB-2 (610 bp) and FG-1–FG-2 (870 bp) were eluted from agarose gels and digested with XbaI and BamHI or BamHI and EcoRI, respectively. Cloning of these fragments into pGP704, digested with XbaI and EcoRI, created a mutagenized slt-ii gene copy, harboring a 589-bp internal deletion. The resulting plasmid was named pTUV3. Introduction of the sacB selection system into BglII-digested pTUV3 yielded pTUV4. Δslt-ii, deletion mutation of the slt-ii gene; oriR6K, origin of replication from plasmid R6K; mobRP4, oriT from plasmid RP4 (allows mobilization of pTUV4 using the RP4 broad-host-range mobilization system); Apr, gene encoding ampicillin resistance from plasmid pBR322; sacB, gene from B. subtilis encoding the enzyme levansucrase (positive selection system).
TABLE 1.
List of oligonucleotide primers used in this study
| Primer | Sequence (5′ to 3′)a | Location (bp) | Reference |
|---|---|---|---|
| UB-3 | GC-TCT-AGA-CAG-AGC-AAT-TGC-CTT-CTG-AGC | 92–112 | 17 |
| Xbal | |||
| UB-2 | CG-GGA-TCC-GAC-GAC-TGA-TTT-GCA-TTC-CGG | 681–701 | 17 |
| BamHI | |||
| FG-1 | CG-GGA-TCC-GAG-TTT-TCC-AAG-TAT-AAT-GAG | 1,291–1,311 | 17 |
| BamHI | |||
| FG-2 | G-GAA-TTC-TTG-CCT-GGC-TCC-TCT-GGT-GAT | 870 downstream of FG-1 | This study |
| EcoRI | |||
| FG-5 | ATA-CCA-CTC-TGC-AAC-GTG | 645–662 | 17 |
| SLT-II-1 | CTT-CGG-TAT-CCT-ATT-CCC-GG | 288–307 | 27 |
| SLT-II-2 | GGA-TGC-ATC-TCT-GGT-CAT-TG | 747–766 | 27 |
Recognition sites for restriction endonucleases are underlined, and restriction endonucleases are indicated.
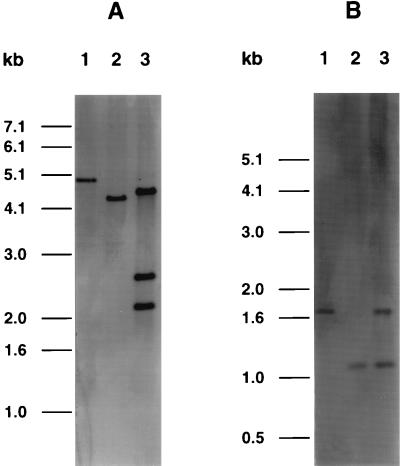
PCR with the primers UB-3 and FG-2 yielded single bands at about 2,100 bp for E. coli 86-24 and at about 1,500 bp for E. coli TUV86-2 (data not shown). The 600-bp difference reflects the deletion within the slt-ii A and B genes. The phage-cured derivatives of strain 86-24 yielded no PCR product when the primers UB-3 and FG-2 were used. Nucleotide sequence analysis of the PCR product from TUV86-2, using the primer FG-5, revealed that nucleotides 701 to 1291 were deleted and a BamHI restriction site had been introduced at the gap. Using primers SLT-II-1 and SLT-II-2 (27) and DNA from strain 86-24, a PCR product was obtained and nonradioactively labeled by using the digoxigenin kit Genius 1 from Boehringer Mannheim (Indianapolis, Ind.). The probe was used in Southern hybridizations to detect slt-ii gene sequences in chromosomal DNA from strain 86-24 and TUV86-2 digested with EcoRI (Fig. 2A) or DraI (Fig. 2B). EcoRI recognizes sites outside the toxin operon, and DraI recognizes sites within the toxin operon. With both restriction enzyme digests, the difference between the two strains showed a size shift of 600 bp, reflecting the DNA deletion. The exconjugant strain was also analyzed by Southern blotting. The presence of three probe-positive bands in the EcoRI digest suggests that the exconjugant possessed a double insert of the suicide vector. The fragment excised from the suicide vector was 2.1 kb, the size of the smaller band on the Southern blot. TUV86-2 gave a positive signal in a colony blot with an EHEC large plasmid probe (22).
FIG. 2.
Southern blot of total chromosomal DNAs from EHEC 86-24 (lanes 1), E. coli TUV86-2 (lanes 2), and the E. coli 86-24 exconjugant recombined with plasmid pTUV4 (lanes 3). The DNA was digested with either EcoRI (A) or DraI (B); a 1-kb DNA ladder served as the molecular size standard.
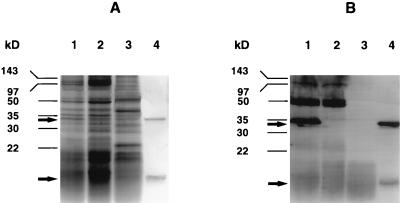
E. coli TUV86-2 was agglutinated with anti-E. coli O157 latex particles (Unipath Limited, Ogdensburg, N.Y.), identical to its parental strain. Biochemical profiles, obtained with the BBL CRYSTAL E/NF identification system (Becton Dickinson Microbiological Systems, Cockeysville, Md.), were identical for the two strains. No protein bands comparable in size to either the SLT-II A subunit or an individual SLT-II B subunit could be detected in either sodium dodecyl sulfate protein gel stained with Coomassie blue or Western blots using polyclonal anti-SLT-II serum with supernatants from a mitomycin C-induced bacterial culture of TUV86-2 (Fig. 3). There was no evidence of a new band representing a truncated form of the toxin, suggesting that the truncated A subunit may be rapidly degraded. The supernatants from both TUV86-2 and the phage-cured derivative of strain 86-24 showed no cytotoxicity in a [3H]leucine incorporation assay with HeLa cells (9). The in vivo toxicity of supernatants from the EHEC strain 86-24 wild type and TUV86-2 was examined by mouse lethality assays. Groups of five 2-month-old BALB/c mice received 1-ml intraperitoneal injections of dilutions of sterile filtered supernatant, containing 50 and 5 μg of total protein from EHEC 86-24 or E. coli TUV86-2, respectively. All 10 mice receiving injections of supernatants from EHEC strain 86-24 died within 48 h, and all 10 mice receiving injections of supernatants from TUV86-2 survived.
FIG. 3.
Proteins from supernatants of mitomycin C cultures and SLT-II holotoxin were separated on a sodium dodecyl sulfate-polyacrylamide gel (A) and exposed to a polyclonal pig anti-SLT-II serum in the corresponding Western blot (B). EHEC strain 86-24 is shown in lanes 1, E. coli TUV86-2 is shown in lanes 2, E. coli C600 is used as a negative control in lanes 3, and SLT-II holotoxin is used as a positive control in lanes 4. Molecular masses were estimated by utilizing a prestained marker from Bio-Rad. Arrows indicate the position of the SLT-II A- and B-subunits at 32 and 7 kDa, respectively.
The plaques of E. coli TUV86-2 were similar in numbers and shape to those of the E. coli 86-24 positive control. A representative from the phage-cured derivatives of strain 86-24 did not release any phage that were capable of forming plaques.
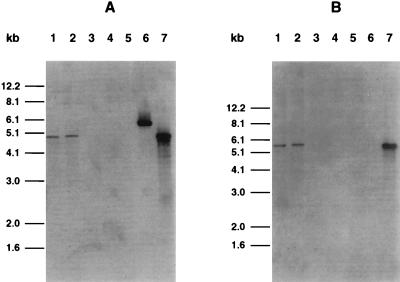
To further investigate the phage-cured nature of this isolate, its DNA was subjected to Southern blot analysis using two phage probes derived from the phage within strain 86-24. DNA from E. coli bacteriophage containing the slt-ii gene was prepared according to standard procedure (32). The DNA was digested with the enzyme EcoRI. Two resulting fragments, 4.9 and 5.5 kb, were cloned separately into pUC 18. Neither fragment contained toxin genes. They were labeled with digoxigenin and used as probes in Southern hybridizations with EcoRI-digested DNA from E. coli 86-24, TUV86-2, the phage-cured isolate, EHEC strain 87-23 (a natural toxin-negative strain), and phage DNA from both λ and the phage from strain 86-24. Both strain 86-24 and E. coli TUV86-2 gave positive hybridization bands to both probes (Fig. 4), and the phage-cured isolate showed no hybridization, consistent with the absence of the converting phage. The 4.9-kb but not the 5.5-kb fragment reacted with a fragment from λ DNA.
FIG. 4.
Southern blot of total chromosomal DNA extracted from various toxigenic and nontoxigenic E. coli, digested with EcoRI and hybridized with either a 4.9-kb (A) or a 5.5-kb (B) probe from the toxin-converting phage isolated from E. coli 86-24. Lanes 1, EHEC strain 86-24; lanes 2, E. coli TUV82-2; lanes 3, phage-cured isolate of EHEC strain 86-24; lanes 4, E. coli 87-23 (no SLT), lanes 5, E. coli C600; lanes 6, phage λ; lanes 7, the SLT-II-converting phage from EHEC strain 86-24. Fragment sizes were estimated by using a 1-kb DNA ladder.
The production of cytotoxins, termed SLT-I or SLT-II after their relatedness to the Shiga toxin of S. dysenteriae type I, was found to be one classical hallmark of EHEC. Molecular biological and biochemical research has produced a lot of information about the genetics of these toxins (4, 26) and their mode of action (10, 11, 13, 15, 18, 33, 34). However, despite all this knowledge, the role of the toxin in the course of the disease or in the development of HUS is still not fully understood.
An isogenic, toxin deletion mutant of an EHEC wild type would be a prerequisite to further dissecting the role of SLTs in suitable in vivo models. An E. coli strain, RDEC, that caused diarrhea in rabbits was converted into an SLT-producing strain by making the strain lysogenic with a phage carrying the SLT (37). The resulting mutant was called isogenic and compared in a rabbit model system to its parental strain to investigate the role of the toxin in the course of an infection. The introduction of toxin genes into an E. coli strain which causes attaching-and-effacing lesions is not the equivalent of creating an EHEC strain. Besides the site of colonization there may be multiple differences between EHEC and enteropathogenic E. coli or other attaching-and-effacing bacteria. Therefore we constructed E. coli TUV86-2, a toxin-negative mutant of E. coli 86-24, that was fully isogenic to its parental strain.
In the construction of E. coli TUV86-2, the majority of our toxin-negative mutants seemed to also be cured entirely of their toxin-converting phages. We tested them for the presence of the phage by PCRs, phage plaque assays, and Southern hybridizations. All tests showed no evidence for the presence of a converting phage. In general, phage curing can be difficult (5) and there are no protocols with guaranteed success available. To date not much is known about the toxin-converting phages except that they are λ-like (7, 14, 28) and about 60 kb in size (30, 41). It has been reported by Karch et al. (20) that SLT-converting phages can be lost upon subcultivation of EHEC strains. However, the rate of phage curing seen in this study with this particular EHEC strain does not explain the rapid loss seen by Karch et al.
Acknowledgments
This work was supported by Public Health Service grants AI-20325 (A.D.-R.), DK-52154 (A.D.-R.), and P30DK-34928 (from the Center for Gastroenterology Research on Absorptive and Secretory Processes) from the National Institutes of Health. F. Gunzer was supported by the Deutsche Forschungsgemeinschaft (grant Gu 387/1-1).
REFERENCES
- 1.Acheson D W K, Donohue-Rolfe A, Keusch G T. The family of Shiga and Shiga-like toxins. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press Ltd.; 1991. pp. 415–433. [Google Scholar]
- 2.itzan M, Ludwig K, Klemt M, Konig H, Buren J, Müller- Wiefel D E. The role of Escherichia coli O 157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a Central European, multicentre study. Epidemiol Infect. 1993;110:183–196. doi: 10.1017/s0950268800068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomfield I C, Vaughn V, Rest R F, Eisenstein B I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 4.Calderwood S B, Auclair F, Donohue-Rolfe A, Keusch G T, Mekalanos J J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcanti S M, Siqueira J P., Jr Cure of prophage in Staphylococcus aureus by furocoumarin photoadditions. Microbios. 1995;81:85–91. [PubMed] [Google Scholar]
- 6.Cleary T G. Escherichia coli that cause hemolytic uremic syndrome. Infect Dis Clin N Am. 1992;6:163–176. [PubMed] [Google Scholar]
- 7.Datz M, Janetzki-Mittmann C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene p and Shiga-like toxin structural genes. Appl Environ Microbiol. 1996;62:791–797. doi: 10.1128/aem.62.3.791-797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue-Rolfe A, Acheson D W K, Kane A V, Keusch G T. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donohue-Rolfe A, Jacewicz M, Keusch G T. Isolation and characterization of functional Shiga toxin subunits and renatured holotoxin. Mol Microbiol. 1989;3:1231–1236. doi: 10.1111/j.1365-2958.1989.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 11.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 12.Gay P, Le Coq D, Steinmetz M, Ferrari E, Hoch J A. Cloning structural gene sacB, which codes for exoenzyme levansucrase of Bacillus subtilis: expression of the gene in Escherichia coli. J Bacteriol. 1983;153:1424–1431. doi: 10.1128/jb.153.3.1424-1431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovde C J, Calderwood S B, Mekalanos J J, Collier R J. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci USA. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang A, Friesen J, Brunton J L. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin 1 in Escherichia coli. J Bacteriol. 1987;169:4308–4312. doi: 10.1128/jb.169.9.4308-4312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacewicz M, Clausen H, Nudelman E, Donohue-Rolfe A, Keusch G T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986;163:1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson M P, Deresiewicz R L, Calderwood S B. Mutational analysis of the Shiga toxin and Shiga-like toxin II enzymatic subunits. J Bacteriol. 1990;172:3346–3350. doi: 10.1128/jb.172.6.3346-3350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson M P, Neill R J, O’Brien A D, Holmes R K, Newland J W. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 18.Jackson M P, Wadolkowski E A, Weinstein D L, Holmes R K, O’Brien A D. Functional analysis of the Shiga toxin and Shiga-like toxin type II variant binding subunits by using site-directed mutagenesis. J Bacteriol. 1990;172:653–657. doi: 10.1128/jb.172.2.653-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 20.Karch H, Meyer T, Rüssmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992;60:3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine M M, Xu J G, Kaper J B, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig K, Bitzan M, Zimmermann S, Kloth M, Ruder H, Müller-Wiefel D E. Immune response to non-O157 Vero toxin-producing Escherichia coli in patients with hemolytic uremic syndrome. J Infect Dis. 1996;174:1028–1039. doi: 10.1093/infdis/174.5.1028. [DOI] [PubMed] [Google Scholar]
- 24.Mangeney M, Lingwood C A, Taga S, Caillou B, Tursz T, Wiels J. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993;53:5314–5319. [PubMed] [Google Scholar]
- 25.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 27.Olsvik Ø, Strockbine N A. PCR detection of heat-stable, heat-labile, and Shiga-like toxin genes in Escherichia coli. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. 1st ed. Washington, D.C: American Society for Microbiology; 1993. pp. 271–276. [Google Scholar]
- 28.Paton A W, Paton J C, Goldwater P N, Heuzenroeder M W, Manning P A. Sequence of a variant Shiga-like toxin type-I operon of Escherichia coli O111:H- Gene. 1993;129:87–92. doi: 10.1016/0378-1119(93)90700-d. [DOI] [PubMed] [Google Scholar]
- 29.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 30.Rietra P J, Willshaw G A, Smith H R, Field A M, Scotland S M, Rowe B. Comparison of Vero-cytotoxin-encoding phages from Escherichia coli of human and bovine origin. J Gen Microbiol. 1989;135:2307–2318. doi: 10.1099/00221287-135-8-2307. [DOI] [PubMed] [Google Scholar]
- 31.Rowe P C, Orrbine E, Lior H, Wells G A, McLaine P N. A prospective study of exposure to verotoxin-producing Escherichia coli among Canadian children with haemolytic uraemic syndrome. Epidemiol Infect. 1993;110:1–7. doi: 10.1017/s0950268800050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sandvig K, Garred O, Prydz K, Kozlov J V, Hansen S H, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- 34.Sandvig K, Olsnes S, Brown J E, Petersen O W, van Deurs B. Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J Cell Biol. 1989;108:1331–1343. doi: 10.1083/jcb.108.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegler R L. Hemolytic uremic syndrome in children. Curr Opin Pediatr. 1995;7:159–163. doi: 10.1097/00008480-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Siegler R L. The hemolytic uremic syndrome. Pediatr Clin N Am. 1995;42:1505–1529. doi: 10.1016/s0031-3955(16)40096-9. [DOI] [PubMed] [Google Scholar]
- 37.Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C, Fondacaro J, Gemski P, Boedeker E. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106:306–317. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 38.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–8. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Tarr P I, Neill M A, Clausen C R, Watkins S L, Christie D L, Hickman R O. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J Infect Dis. 1990;162:553–556. doi: 10.1093/infdis/162.2.553. [DOI] [PubMed] [Google Scholar]
- 40.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 41.Willshaw G A, Smith H R, Scotland S M, Field A M, Rowe B. Heterogeneity of Escherichia coli phages encoding Vero cytotoxins: comparison of cloned sequences determining VT1 and VT2 and development of specific gene probes. J Gen Microbiol. 1987;133:1309–1317. doi: 10.1099/00221287-133-5-1309. [DOI] [PubMed] [Google Scholar]