Abstract
The formaldehyde-killed, whole-spherule vaccine, which is protective against lethal challenge of laboratory animals with Coccidioides immitis, was fractionated. It yielded a soluble, multicomponent, subcellular fraction termed the 27K vaccine. This vaccine, when it was accompanied by adjuvant, protected mice against lethal intranasal and intravenous challenge with C. immitis.
Recovery from infection with Coccidioides immitis usually confers lifelong immunity to reinfection (33). Studies aimed at developing a protective anticoccidioidal vaccine indicated that the most efficacious nonviable vaccine was the formaldehyde-killed, whole-spherule (FKS) vaccine (12, 13, 20–25, 28, 29, 37). This vaccine protected against potentially lethal challenges with arthroconidia by the intranasal or intravenous routes in mice and by the respiratory route in monkeys. In humans, however, the maximum tolerable dose of the FKS was less than 1000th of the dose per kilogram of body weight that was protective in mice (27, 35). The low dose tolerated by humans may explain the lack of observed protective effect in a phase III field trial in humans (27). The present study was carried out to seek a less-irritating, yet protective, vaccine.
Kong et al. (18) found that the protective components were located primarily in the walls of mature spherules. An alkali-soluble, water-soluble extract of C. immitis mycelium prepared by Ward et al. (34), when it was administered with Freund’s complete adjuvant, provided a significant level of protection in mice against intraperitoneal challenge and some measure of protection against intranasal challenge (20). Pappagianis et al. (28) mechanically extracted washed spherule walls and obtained a water-soluble extract which, when it was administered with Freund’s or aluminum hydroxide (alum) adjuvants, provided a level of protection close to that afforded by FKS against a lethal intranasal challenge. More recently, Zimmer et al. (37) also extracted immunogens from the FKS vaccine. The results showed that immunizations with certain extracts and alum adjuvant were as protective as the FKS vaccine when mice were challenged intravenously with a lethal dose of arthroconidia.
Following the approach of Zimmer et al. (37), we have again shown that when a soluble, aqueous fraction (27K vaccine) prepared by mechanical disruption of the FKS vaccine was used with an alum adjuvant, it was nearly as protective as the parent FKS vaccine. The 27K vaccine was prepared from C. immitis Silveira (ATCC 28868). When suspended in sterile saline to a concentration of 3.5 mg/ml, the preparation was colorless, slightly opalescent, and virtually devoid of intact microscopically visible fragments. Both protein (26) and carbohydrate (presumably polysaccharides) (32) were present in the 27K vaccine.
Groups of seven 16- to 20-g Swiss Webster mice were injected with 0.2 to 0.4 ml of 27K vaccine, with or without alum adjuvant (Cutter Laboratories, Berkeley, Calif.) or with alum alone. At 1-week intervals, we administered subcutaneously three doses consisting of either 1 mg of an individual vaccine alone, 1 mg of an individual vaccine with 4 mg of alum, or 4 mg of alum alone. Four weeks after the third dose, the mice were challenged with C. immitis arthroconidia (Silveira strain; ATCC 28868) intravenously in a tail vein or intranasally. The experiment was terminated 13 weeks after the challenge; the survivors were sacrificed, and the whole lungs, liver, and spleen of each survivor were cultured on Mycobiotic agar (Difco, Detroit, Mich.). C. immitis was recovered from at least one of the organs cultured from each surviving mouse. Survival differences between groups of mice receiving different vaccines and alum were compared by the Mantel-Haenszel log rank test of significance. A P of <0.05 was considered significant.
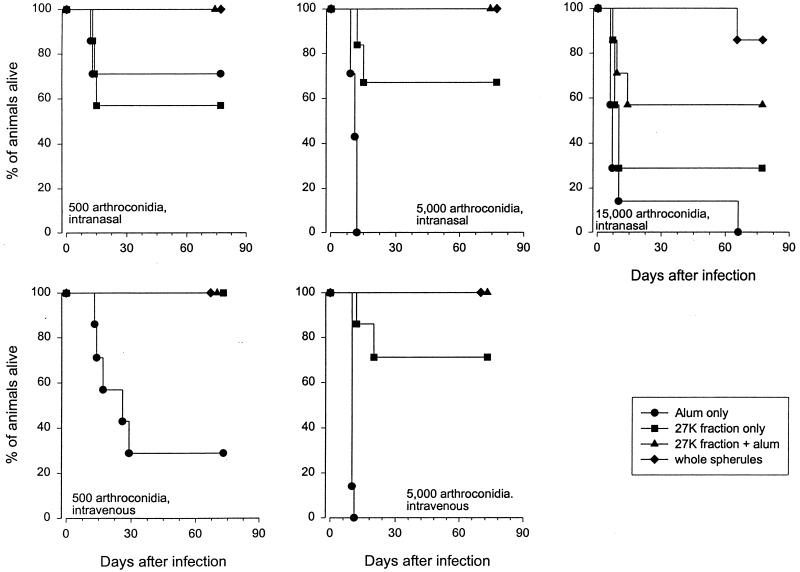
Mouse survival in the protection experiments is shown in Fig. 1. The intravenous challenge was virtually as rigorous as the intranasal challenge. When analyzed statistically, the survival of mice immunized with the 27K vaccine with alum was significantly different from that of mice injected with alum alone when they were challenged intravenously with 500 (P = 0.007) and 5,000 (P = 0.0002) arthroconidia and intranasally with 5,000 (P = 0.003) and 15,000 (P = 0.04) arthroconidia. Similar results were obtained when the survival of the groups of mice immunized with the 27K vaccine alone was compared to the survival of the groups of mice injected with alum alone (5,000 arthroconidia intranasally, P = 0.002; 500 and 5,000 arthroconidia intravenously, P = 0.007 and 0.0002, respectively). There was no significant difference between results with the 27K vaccine alone and those with alum alone in mice challenged with 15,000 arthroconidia intranasally (P = 0.17). Also, with the 500-arthroconidia intranasal challenge, there were no significant differences in the levels of protection given by the three vaccines versus those given by alum alone (FKS and 27K with alum, P = 0.14; 27K alone, P = 0.73).
FIG. 1.
Survival of vaccinated mice following intranasal or intravenous challenge by arthroconidia. Groups of seven mice were immunized and challenged with 500, 5,000, or 15,000 arthroconidia.
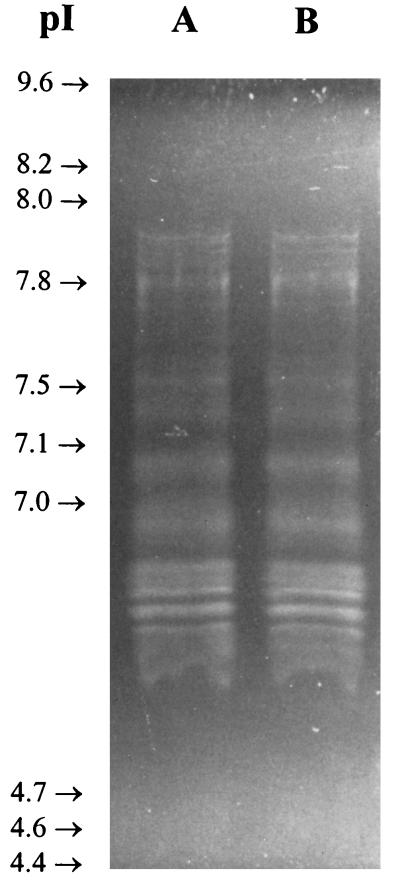
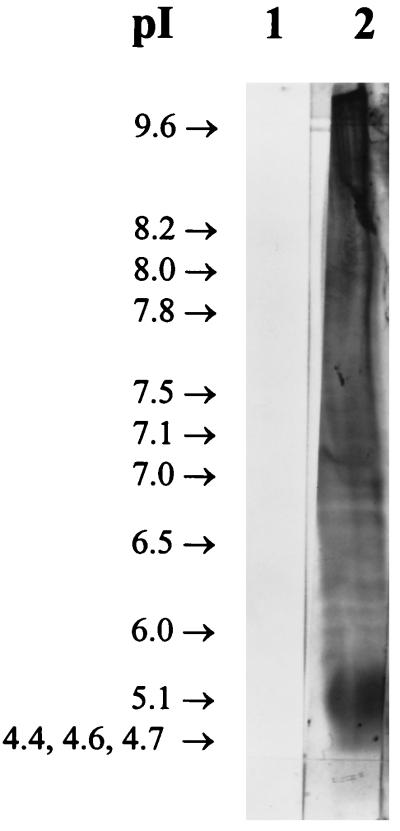
In order to resolve the components of the 27K vaccine, 50-μg aliquots of the 27K vaccine were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions with a 12% gel and accompanying molecular weight standards (Bio-Rad, Hercules, Calif.) (19). As shown in Fig. 2, the Coomassie blue-stained material was not separated into a pattern of discrete bands but rather took the form of a continuous smear extending the length of the gel. Interestingly, when similar gels were blotted onto nitrocellulose (14) and reacted with rabbit serum and an anti-27K vaccine rabbit serum, a few bands were resolved from the gel-long smear of reacting antigens (Fig. 3, lane 2). In Fig. 4 to 6, the 27K vaccine was fractionated by isoelectric focusing (IEF) in a 5.0% polyacrylamide gel containing 2.4% ampholytes (pH 3.5 to 10; Bio-Rad) at 200 V for 2 h with accompanying molecular pI standards (Bio-Rad) (6). The precipitated bands of two identical lanes were photographed with oblique light without additional staining (Fig. 4). Both unstained (Fig. 4) and Coomassie blue-stained (Fig. 5, lane 2) IEF gels of the 27K vaccine yielded discrete bands. In addition, when replicate gels were blotted onto nitrocellulose (14) and reacted with rabbit serum and an anti-27K vaccine rabbit serum, discrete bands were resolved (Fig. 6, lane 2).
FIG. 2.
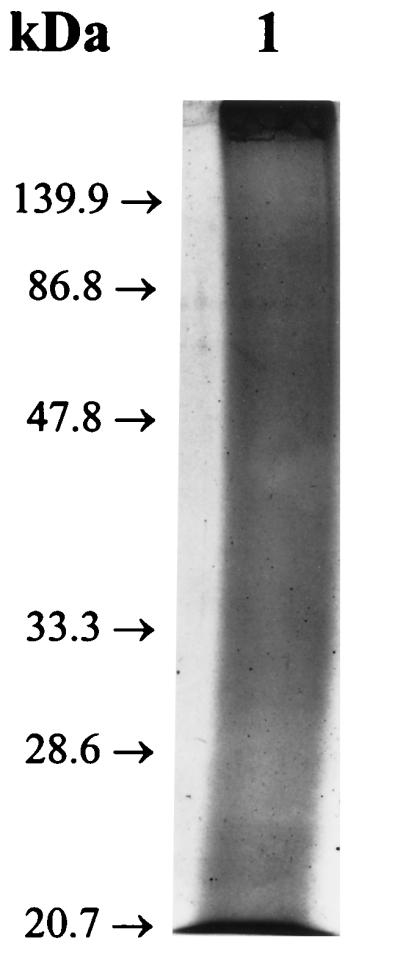
Reduced sodium dodecyl sulfate–12% polyacrylamide gel with the 27K vaccine after Coomassie blue staining.
FIG. 3.
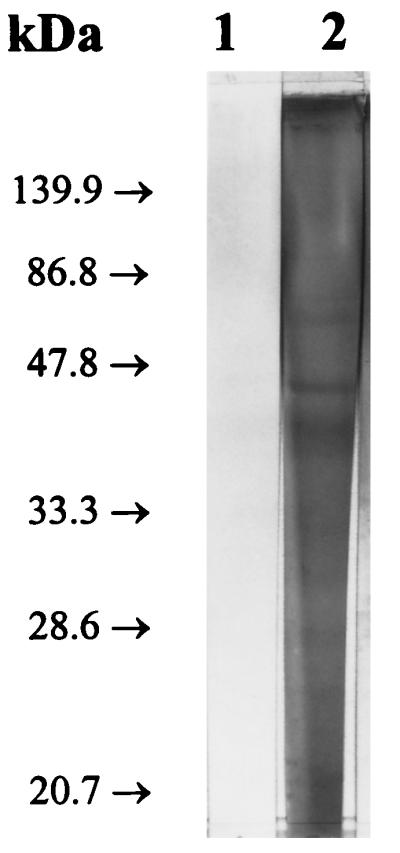
Immunoblots of gel lanes identical to that shown in Fig. 2 after being reacted with normal, prevaccination serum (lane 1) and anti-27K vaccine serum (lane 2).
FIG. 4.
Unstained 5% IEF gel (pH 3.5 to 10) of the 27K vaccine.
FIG. 6.
Immunoblots of gel lanes identical to those shown in Fig. 5 after being reacted with normal, prevaccination serum (lane 1) and anti-27K vaccine serum (lane 2).
FIG. 5.
Coomassie blue-stained 5% IEF gel (pH 3.5 to 10) of the 27K vaccine. Lane 1, molecular pI standards; lane 2, 27K vaccine.
In the experiments presented here, the 27K vaccine mixed with alum provided protection equal to that of the FKS vaccine against lethal challenge in mice. Alum was used in this study because it had shown enhancement of the protection of the earlier soluble vaccine (28) and, in an initial study, of the 27K vaccine (37). In addition, its use was based on the long-approved use of alum, still the only Food and Drug Administration-approved adjuvant in vaccines in clinical use for humans. There is now, however, a general impression that alum is a poor adjuvant for the induction of cell-mediated immunity, which appears central to resolution of Coccidioides infection and of immunity to C. immitis (1–5, 7–11, 17, 31). Although our results do not allow discrimination between antibody- and cell-mediated immunity, the ability of alum to enhance protection by the 27K vaccine raises the possibility that antibodies play a role in protection (27, 30, 36).
Optimal immunization by the 27K vaccine may require the addition of adjuvants that can enhance cell-mediated immunity, a function that may be inherent in the FKS vaccine. Future studies will make use of newer adjuvants, such as the RIBI (RIBI ImmunoChem Research, Hamilton, Mont.) preparations that favor Th1 responses, which may prove to enhance the immunogenicity of the 27K vaccine or its derivatives. Alternatively, the polysaccharides that are present in the 27K vaccine may themselves provide critical antigenic or adjuvant effects. Future studies will address the possibility that endogenous chitin is an important adjuvant for the protective immunity obtained with the FKS or 27K vaccine (15, 16). IEF gel fractionation of the 27K vaccine indicated the presence of several components. The protective components will have to be identified. Enhancement of the immunogenicity may require isolation of particularly active components and their synthesis by molecular genetic methods.
Acknowledgments
We thank Kris I. Orsborn and John N. Galgiani for all their help in preparing the manuscript. Statistical tests were kindly performed by Gretchen Cloud, NIAID Mycoses Study Group (contract NO1-AI-65296).
REFERENCES
- 1.Ampel N M, Bejarano G C, Salas S D, Galgiani J N. In vitro assessment of cellular immunity in human coccidioidomycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J Infect Dis. 1992;165:710–715. doi: 10.1093/infdis/165.4.710. [DOI] [PubMed] [Google Scholar]
- 2.Beaman L. Effects of recombinant gamma interferon and tumor necrosis factor on in vitro interactions of human mononuclear phagocytes with Coccidioides immitis. Infect Immun. 1991;59:4227–4229. doi: 10.1128/iai.59.11.4227-4229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaman L, Benjamini E, Pappagianis D. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun. 1983;39:1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman L, Pappagianis D, Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977;17:580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaman L, Pappagianis D, Benjamini E. Mechanisms of resistance to infection with Coccidioides immitis in mice. Infect Immun. 1979;23:681–685. doi: 10.1128/iai.23.3.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollag D M, Edelstein S J. Protein methods. New York, N.Y: Wiley-Liss; 1991. [Google Scholar]
- 7.Cole G T, Kirkland T N. Identification of antigens of Coccidioides immitis which stimulate immune T lymphocytes. Arch Med Res. 1993;24:281–291. [PubMed] [Google Scholar]
- 8.Corry D B, Ampel N M, Christian L, Locksley R M, Galgiani J N. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J Infect Dis. 1996;174:440–443. doi: 10.1093/infdis/174.2.440. [DOI] [PubMed] [Google Scholar]
- 9.Cox R A. Coccidioidomycosis. In: Murphy J W, Bendinelli M, Friedman H, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 173–211. [Google Scholar]
- 10.Cox R A, Kennell W, Boncyk L, Murphy J W. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988;56:13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox R A, Vivas J R. Spectrum of in vivo and in vitro immune responses in coccidioidomycosis. Cell Immunol. 1977;31:130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- 12.Eubanks E R, Leathers C R, Sanchez A, Todd C D, Katz G J. Abstracts of the Annual Meeting of the American Society for Microbiology 1979. Washington, D.C: American Society for Microbiology; 1979. Evaluation of subcellular fractions of Coccidioides immitis as protective immunogens, abstr. F 56; p. 372. [Google Scholar]
- 13.Friedman L, Smith C E. Vaccination of mice against Coccidioides immitis. Am Rev Tuberc Pulm Dis. 1956;74:245–248. doi: 10.1164/artpd.1956.74.2-1.245. [DOI] [PubMed] [Google Scholar]
- 14.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 15.Hector R F, Pappagianis D. Enzymatic degradation of the walls of spherules of Coccidioides immitis. Exp Mycol. 1982;6:136–152. [Google Scholar]
- 16.Hector R F, Zimmer B L, Pappagianis D. Abstracts of the Annual Meeting of the American Society for Microbiology 1985. Washington, D.C: American Society for Microbiology; 1985. The role of endogenous chitinase in the maturation of the spherule-endospore phase of Coccidioides immitis, abstr. F 61; p. 374. [Google Scholar]
- 17.Kirkland T N, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983;40:912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong Y C M, Levine H B, Smith C E. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia. 1963;2:131–142. [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lecara G, Cox R A, Simpson R B. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983;39:473–475. doi: 10.1128/iai.39.1.473-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine H B, Cobb J M, Smith C E. Immunity to coccidioidomycosis induced in mice by purified spherule, arthrospore and mycelial vaccines. Trans N Y Acad Sci. 1960;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine H B, Cobb J M, Smith C E. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J Immunol. 1961;87:218–227. [PubMed] [Google Scholar]
- 23.Levine H B, Kong Y C M, Smith C E. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J Immunol. 1965;94:132–142. [PubMed] [Google Scholar]
- 24.Levine H B, Miller R L, Smith C E. Influence of vaccination on respiratory coccidioidal disease in cynomolgous monkeys. J Immunol. 1962;89:242–251. [PubMed] [Google Scholar]
- 25.Levine H B, Smith C E. The reactions of eight volunteers injected with Coccidioides immitis spherule vaccine: first human trials. In: Ajello L, editor. Coccidioidomycosis. Tucson: University of Arizona Press; 1967. pp. 197–200. [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Pappagianis D the Valley Fever Vaccine Study Group. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis. 1993;148:656–660. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
- 28.Pappagianis D, Hector R, Levine H B, Collins M S. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect Immun. 1979;25:440–445. doi: 10.1128/iai.25.1.440-445.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappagianis D, Levine H B, Smith C E, Berman R J, Kobayashi G S. Immunization of mice with viable Coccidioides immitis. J Immunol. 1961;86:2834. [PubMed] [Google Scholar]
- 30.Pappagianis D, Zimmer B L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3:247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkus A F, Baum L L. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J Immunol. 1987;139:3107–3111. [PubMed] [Google Scholar]
- 32.Scott T A, Melvin E H. Determination of dextran with anthrone. Anal Chem. 1953;25:1656–1661. [Google Scholar]
- 33.Smith C E. Parallelism of coccidioidal and tuberculous infections. Radiology. 1942;38:643. [Google Scholar]
- 34.Ward E R, Cox R A, Smitt J A, Huppert M, Sun S H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975;12:1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams P L . the Valley Fever Vaccine Study Group. Further assessment of the morbidity associated with the killed Coccidioides immitis spherule vaccine in humans. In: Einstein H, Catanzaro A, editors. Coccidioidomycosis. Proceedings of the 4th International Conference on Coccidioidomycosis. Washington, D.C: National Foundation for Infectious Disease; 1985. pp. 339–346. [Google Scholar]
- 36.Zhu Y, Tyron V, Magee D M, Cox R A. Identification of a Coccidioides immitis antigen 2 domain that expresses B-cell-reactive epitopes. Infect Immun. 1997;65:3376–3380. doi: 10.1128/iai.65.8.3376-3380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmer B L, Johnson S M, Van Hoosear K, Pappagianis D. Abstracts of the 90th Annual Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1990. Immunization of mice with extracts of Coccidioides immitis, abstr. F-26; p. 413. [Google Scholar]