Abstract
The effects of gamma interferon (IFN-γ) on Chlamydia trachomatis growth in polarized epithelial cells were examined. The range of IFN-γ concentrations causing aberrant chlamydial growth was wider in polarized than in nonpolarized cultures. Results indicate that chlamydial growth modulation in polarized cells readily leads to persistence and better reflects in vivo conditions.
Chlamydia trachomatis is an obligate intracellular bacterial pathogen causing human ocular and genital tract disease. Chlamydial development is characterized by the alternation of infectious elementary bodies (EBs) and replicative reticulate bodies (9). Chlamydial persistence is characterized by aberrant morphology, loss of infectivity, and differential expression of key chlamydial antigens after gamma interferon (IFN-γ) treatment (2, 3, 5). In contrast, a recent study examining several other serovars failed to demonstrate persistence (10), suggesting distinctions between chlamydial strains. In this study, the archetypical strain for studying persistence, serovar A, was used.
C. trachomatis grows in columnar epithelial cells in vivo. These cells are polarized, with distinct apical and basolateral domains separated by tight junctions (11). A polarized culture system more closely resembles the environment encountered by chlamydiae in vivo and has been developed for studying chlamydial infections (16). The purpose of this study was to determine if host cell polarization affects IFN-γ-mediated chlamydial persistence or growth inhibition.
Cell lines were grown in appropriately supplemented minimal essential medium (15). C. trachomatis serovar A/HAR-13 was grown in HeLa 229 cells, and EBs were purified over discontinuous Renografin gradients (Squibb Diagnostics, New Brunswick, N.J.) (7). Polarized cultures were obtained by seeding Me180 cells at 5 × 104 cells per well onto collagen-coated 6.5-mm-diameter polycarbonate Transwell filter inserts (Costar, Cambridge, Mass.) (16).
Cells were infected with 100 μl of a C. trachomatis A/HAR-13 suspension at a multiplicity of infection of 0.5 and were treated with various doses of recombinant human IFN-γ (10 U/ng; Genzyme Diagnostics, Cambridge, Mass.) as previously described (3). Infectivity assays were performed essentially as described previously (3) except that filters were excised with a scalpel before sonication. Inclusions were visualized by indirect immunofluorescence with antilipopolysaccharide monoclonal antibodies (14M-3-B9; a generous gift from Harlan Caldwell, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health). Indoleamine 2,3-dioxygenase (IDO) assays (2) and high-performance liquid chromatography (18) were performed as described previously. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed as described previously (3), and the blots were visualized by chemiluminescence (ECL; Amersham, Arlington Heights, Ill.).
Preliminary evidence indicated host cell line variability in modulating chlamydial growth after IFN-γ treatment. Therefore, the IDO activities of several genital tract cell lines were assessed (Table 1). Both HeLa and Me180 cells catabolized tryptophan after 48 h of IFN-γ treatment, indicating IFN-γ-inducible IDO activity. Additionally, IDO activity of polarized Me180 cells was comparable to the activities of both nonpolarized Me180 and HeLa cells. This activity was IFN-γ dose dependent for all responsive cell types (data not shown). In contrast, Hec1B and Ishikawa cells neither exhibited IDO activity nor inhibited chlamydial growth after IFN-γ treatment; these findings are similar to results described by Thomas et al. (13) for an IDO-deficient Me180 mutant cell line. Therefore, Me180 cells were used for subsequent polarization studies.
TABLE 1.
Tryptophan catabolism in four genital tract cell lines
| Cell line | Tissue origin | IFN-γ treatmenta | IDO activityb (% specific catabolism ± SD)
|
|
|---|---|---|---|---|
| Nonpolarized | Polarized | |||
| Me180 | Cervical epithelium | − | 0 | 0 |
| + | 65.2 ± 13.0 | 58.2 ± 3.2 | ||
| HeLa | Cervical epithelium | − | 0 | ND |
| + | 61.0 ± 12.1 | ND | ||
| Ishikawa | Endometrial epitheliumc | − | 1.2 ± 1.8 | ND |
| + | 1.3 ± 1.5 | ND | ||
| Hec1B | Endometrial epithelium | − | 0 | ND |
| + | 0 | ND | ||
+, treated cells; −, untreated cells. Cells were treated with 10 ng of IFN-γ/ml for 48 h before being pulsed with [3H]tryptophan in Hanks balanced salt solution for 4 h.
Values are the mean results for at least two independent experiments in which samples were assayed in duplicate or triplicate. Me180 was the only cell line polarized in this study. ND, not determined.
Hormone responsive.
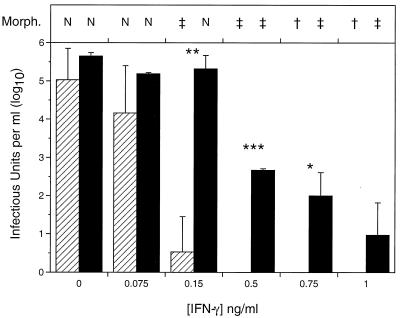
The levels of recovery of infectious chlamydiae from IFN-γ-treated polarized and nonpolarized genital tract cells were compared (Fig. 1). In the absence of IFN-γ treatment more organisms were recovered from polarized Me180 cells than from cells grown on plastic. This observation agrees with those made by Wyrick and colleagues (12, 16, 17). When IFN-γ was added to the cultures shortly after infection, a dose-dependent decrease in the number of recoverable organisms was observed for both polarized and nonpolarized cultures, and a significant difference in the number of chlamydiae recovered from each was observed starting at 0.15 ng of IFN-γ/ml until inhibitory doses of IFN-γ were reached. The infectivity in nonpolarized cells decreased significantly after treatment with 0.15 ng of IFN-γ/ml (P < 0.005; two-tailed Student’s t test) and was completely inhibited at IFN-γ concentrations of 0.2 ng/ml or more. In contrast, higher concentrations of IFN-γ were required to affect the recovery of infectious chlamydiae from the polarized cultures. There was little difference between polarized treated and untreated cultures until treatment with at least 0.5 ng of IFN-γ/ml (P < 0.0001). Complete inhibition of chlamydial growth in the polarized cultures was not seen until treatment with 1 ng of IFN-γ/ml or more was used, consistent with IFN-γ levels demonstrated locally in chlamydia culture-positive women (1).
FIG. 1.
Recovery of infectious chlamydiae from nonpolarized (▨) and polarized (▪) Me180 cells after IFN-γ treatment. Cells were infected, treated with IFN-γ, and harvested for infectivity at 48 h postinfection. The data are representative of several experiments and denote the means ± standard deviations for triplicate wells. Asterisks indicate statistically significant differences between polarized and nonpolarized cells by a two-tailed Welch’s t test: ∗, P < 0.05; ∗∗, P = 0.01; ∗∗∗, P < 0.0001. Compared to that in untreated samples, the infectivity decreased significantly after treatment with 0.15 ng of IFN-γ/ml in nonpolarized cells (P < 0.005 by two-tailed Student’s t test) and with 0.5 ng of IFN-γ/ml in polarized cells (P < 0.0001). Symbols above the graph indicate the morphology of the chlamydiae as follows: N, normal; ‡, many aberrant (persistent) forms; and †, scarce aberrant (persistent) forms.
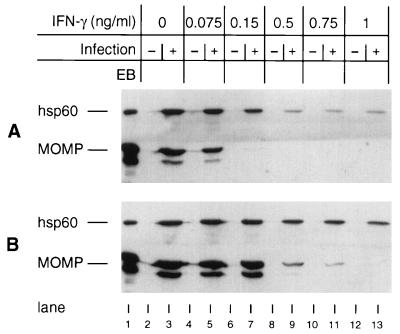
As one measure of persistence, chlamydial steady-state protein levels were examined after IFN-γ treatment. When nonpolarized cells were treated with 0.075 ng of IFN-γ/ml, both the chlamydial 57-kDa heat shock protein (hsp60) and major outer membrane protein (MOMP) levels were found to be similar to those seen for untreated cells (Fig. 2). But treatment with 0.15 ng of IFN-γ/ml resulted in a dramatic decrease in the amount of detectable MOMP without a concomitant decrease in hsp60 levels. This is consistent with the chlamydial protein profiles demonstrated for persistent organisms (3, 4). MOMP was undetectable after treatment with 0.5 ng of IFN-γ/ml or more, while hsp60 was detected in all samples tested. A similar pattern was observed for chlamydiae grown in polarized cells, although more IFN-γ was required to see this effect and the persistence protein profiles were seen over a broader range of IFN-γ treatment levels (0.5 to 0.75 ng/ml). The chlamydial proteins present during an infection may influence pathogenesis, since MOMP immune responses are believed to be protective, while hsp60 elicits delayed hypersensitivity (8) and is serologically associated with chronic sequelae of chlamydial genital tract diseases (6, 14).
FIG. 2.
Immunoblot analysis of nonpolarized (A) and polarized (B) Me180 cells with anti-hsp60 and anti-MOMP antibodies. Lane 1, C. trachomatis serovar A EBs; lanes 2, 4, 6, 8, 10, and 12, uninfected cells treated with 0, 0.075, 0.15, 0.5, 0.75, and 1 ng of IFN-γ per ml, respectively; lanes 3, 5, 7, 9, 11, and 13, identically treated C. trachomatis serovar A-infected cells. Autoradiographs were scanned on an Apple Color OneScanner with Photoshop version 3.0 software and assembled with Canvas version 3.5.4 software.
Chlamydial morphology, as determined by immunofluorescence staining, also was affected by IFN-γ treatment. Large, aberrant organisms were observed when cells were treated with 0.15 ng (nonpolarized) or 0.5 ng (polarized) of IFN-γ/ml (Fig. 1). Interestingly, intact aberrant forms were visible in polarized cells after treatment with as much as 5 ng of IFN-γ/ml (five times the inhibitory concentration). No inclusions were visible in nonpolarized cells treated similarly. These data demonstrate a much broader range of persistence-inducing IFN-γ levels in polarized cells, which may more accurately represent in vivo conditions, than in nonpolarized cells.
The polarization state, IDO levels, and the chlamydial serovar all influence the likelihood that chlamydiae will become persistent. Clearly there are some differences between cells grown by standard cell culture methods and those grown in a polarized orientation. In this study we have shown that cells grown in a polarized state respond differently to the effects of IFN-γ than nonpolarized cells. These differences were most obvious in observations which showed that larger doses of IFN-γ were required to reduce or inhibit the growth of chlamydiae and regulate heat shock protein stress responses in polarized cells. Furthermore, chlamydial antigens persist to a greater extent in polarized cultures as demonstrated by the presence of intact inclusions after treatment with levels of IFN-γ sufficient to completely inhibit growth in nonpolarized cells.
It is not known why more IFN-γ is required to affect C. trachomatis growth in polarized cells than in nonpolarized cells, nor is it clear why persistence in polarized cells can be maintained over a range of IFN-γ concentrations much broader than that for nonpolarized cells. Wyrick et al. (17) demonstrated both accelerated growth of chlamydiae and higher chlamydial titers in polarized versus nonpolarized cells. Polarized cells may provide a more nutrient-replete environment for chlamydial growth than nonpolarized cells, and thus the availability of metabolites important to the effects of IFN-γ on chlamydial growth may be modulated differently based on polarization status. These considerations are currently under investigation.
Acknowledgments
This work was supported by Public Health Service grant AI 34617 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Arno J N, Ricker V A, Batteiger B E, Katz B P, Caine V A, Jones R B. Interferon-γ in endocervical secretions of women infected with Chlamydia trachomatis. J Infect Dis. 1990;162:1385–1389. doi: 10.1093/infdis/162.6.1385. [DOI] [PubMed] [Google Scholar]
- 2.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty W L, Morrison R P, Byrne G I. Immunoelectron-microscopic quantitation of differential levels of chlamydial proteins in a cell culture model of persistent Chlamydia trachomatis infection. Infect Immun. 1994;62:4059–4062. doi: 10.1128/iai.62.9.4059-4062.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty W L, Morrison R P, Byrne G I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunham R C, Maclean I W, Binns B, Peeling R W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985;152:1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder J W. Characteristics of chlamydiae. In: Barron A L, editor. Microbiology of Chlamydia. Boca Raton, Fla: CRC Press; 1988. pp. 4–19. [Google Scholar]
- 10.Rasmussen S J, Timms P, Beatty P R, Stephens R S. Cytotoxic-T-lymphocyte-mediated cytolysis of L cells persistently infected with Chlamydia spp. Infect Immun. 1996;64:1944–1949. doi: 10.1128/iai.64.6.1944-1949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons K, Fuller S D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- 12.Tam J E, Knight S T, Davis C H, Wyrick P B. Eukaryotic cells grown on microcarrier beads offer a cost-efficient way to propagate Chlamydia trachomatis. BioTechniques. 1992;13:374–378. [PubMed] [Google Scholar]
- 13.Thomas S M, Garrity L F, Brandt C R, Schobert C S, Feng G-S, Taylor M W, Carlin J M, Byrne G I. IFN-γ-mediated antimicrobial response: indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol. 1993;150:5529–5534. [PubMed] [Google Scholar]
- 14.Wagar E A, Schachter J, Bavoil P, Stephens R S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990;162:922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe J, Holinka C F, Anzai Y, Kuramoto H, Gurpide E. Effects of serine and glycine on proliferation of an Ishikawa cell variant. J Steroid Biochem. 1989;34:165–168. doi: 10.1016/0022-4731(89)90078-2. [DOI] [PubMed] [Google Scholar]
- 16.Wyrick P B, Choong J, Davis C H, Knight S T, Royal M O, Maslow A S, Bagnell C R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989;57:2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyrick P B, Gerbig D G, Jr, Knight S T, Raulston J E. Accelerated development of genital Chlamydia trachomatis serovar E in McCoy cells grown on microcarrier beads. Microb Pathog. 1996;20:31–40. doi: 10.1006/mpat.1996.0003. [DOI] [PubMed] [Google Scholar]
- 18.Yong S, Lau S. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr. 1979;175:343–346. doi: 10.1016/s0021-9673(00)89443-1. [DOI] [PubMed] [Google Scholar]