Abstract
Background & Aims
Spontaneous portosystemic shunts (SPSS) develop frequently in cirrhosis. Changes over time and the effect of aetiological interventions on SPSS are unknown, so we aimed to explore the effect of these variables on SPSS evolution.
Methods
Patients with cirrhosis from the Baveno VI-SPSS cohort were selected provided a follow-up abdominal CT or MRI scan was available. Clinical and laboratory data were collected at baseline and follow-up. Imaging tests were reviewed to evaluate changes in the presence and size of SPSS (large (L)-SPSS was ≥8 mm) over time. Regarding alcohol- or HCV-related cirrhosis, two populations were defined: cured patients (abstinent from alcohol or successful HCV therapy), and non-cured patients.
Results
A total of 617 patients were included. At baseline SPSS distribution was 22% L-SPSS, 30% small (S)-SPSS, and 48% without (W)-SPSS. During follow-up (median follow-up of 63 months), SPSS distribution worsened: L-SPSS 26%, S-SPSS 32%, and W-SPSS 42% (p <0.001). Patients with worse liver function during follow-up showed a simultaneous aggravation in SPSS distribution. Non-cured patients (n = 191) experienced a significant worsening in liver function, more episodes of liver decompensation and lower transplant-free survival compared to cured patients (n = 191). However, no differences were observed regarding SPSS distribution at inclusion and at follow-up, with both groups showing a trend to worsening. Total shunt diameter increased more in non-cured (52%) than in cured patients (28%). However, total shunt area (TSA) significantly increased only in non-cured patients (74 to 122 mm2, p <0.001).
Conclusions
The presence of SPSS in cirrhosis increases over time and parallels liver function deterioration. Aetiological intervention in these patients reduces liver-related complications, but SPSS persist although progression is decreased.
Impact and implications
There is no information regarding the evolution of spontaneous portosystemic shunts (SPSS) during the course of cirrhosis, and especially after disease regression with aetiological interventions, such as HCV treatment with direct-acting antivirals or alcohol abstinence. These results are relevant for clinicians dealing with patients with cirrhosis and portal hypertension because they have important implications for the management of cirrhosis with SPSS after disease regression. From a practical point of view, physicians should be aware that in advanced cirrhosis with portal hypertension, after aetiological intervention, SPSS mostly persist despite liver function improvement, and complications related to SPSS may still develop.
Keywords: Collateral vessels, Portal hypertension, Advanced chronic liver disease, Computed tomography, Magnetic resonance imaging, Hepatitis C virus, Sustained virological response, Alcohol, Ascites, Hepatic encephalopathy
Graphical abstract
Highlights
-
•
In patients with cirrhosis, SPSS prevalence increases over time, along with deteriorating liver function.
-
•
HCV eradication and alcohol abstinence improve liver function, portal hypertension and clinical outcomes.
-
•
Amelioration of liver function does not correlate with changes in SPSS distribution.
-
•
SPSS persist but progression is decreased after HCV eradication or alcohol abstinence.
Introduction
Portal hypertension (PH) is the main consequence of cirrhosis and is responsible for its most severe complications, including ascites, variceal bleeding, and hepatic encephalopathy (HE).1 Another consequence of PH is the formation of spontaneous portosystemic shunts (SPSS), which are collateral blood vessels that form communications between the portal or splanchnic venous system and the systemic venous system, in an attempt to decompress the portal circulation.2 However, SPSS are insufficient to significantly lower portal pressure. Furthermore, they contribute to increase splanchnic blood flow, worsening PH, and decrease hepatocyte perfusion, contributing to liver insufficiency and liver function deterioration.
The previous multicentre study performed by the Baveno VI-SPSS group3 with data from 1,729 patients showed a high prevalence of SPSS in patients with cirrhosis. Large-SPSS (L-SPSS-diameter ≥8 mm) were identified in 28% of patients and small-SPSS (S-SPSS) in 32%. SPSS increased as liver function deteriorated and they were associated with PH-related complications. HE was more common in patients with SPSS irrespective of liver function. However, SPSS were related to other hepatic complications and transplant-free survival only in the subgroup of patients with low model for end-stage liver disease (MELD) scores or Child-Pugh class A.
In a subsequent study performed by Praktiknjo et al.,4 including patients with SPSS from the same cohort, a high total shunt area (TSA), defined as a TSA ≥83 mm2, corresponding to a single shunt of approximately 10 mm diameter, was associated with more complications and mortality. All these findings suggest that the identification and size of SPSS could be a prognostic marker of cirrhosis.
The Baveno VI-SPSS study reported only cross-sectional clinical and imaging data. Cirrhosis is a progressive disease in many patients, but different interventions might also change its course. Radiological changes and their relationship with the evolution of the disease have not been evaluated in a longitudinal study. The influence of alcohol abstinence and direct-acting antivirals (DAAs) against HCV has not been assessed either. Therefore, aetiological interventions provide a unique opportunity to study dynamic changes in liver function, portal hypertension and SPSS. Lens et al.5 found that after achieving sustained viral response (SVR) with DAAs, patients with HCV-related cirrhosis achieved a reduction in hepatic venous pressure gradient (HVPG), but clinically significant portal hypertension (CSPH, HVPG ≥10 mmHg) persisted in most patients. The effect that treatment has on SPSS is still to be determined.
The main objective of the study was to evaluate morphological changes in SPSS over time and their relationship with liver function, portal hypertension and clinical outcomes. Based on this objective, special attention was dedicated to elucidating the influence of aetiological interventions (alcohol abstinence and DAAs for HCV) on SPSS.
Patients and methods
A retrospective study, prolonging the follow-up of the Baveno VI-SPSS study, was performed, consisting of a second cross-sectional analysis of the cohort to collect new clinical and radiological information. From the initial group of 14 participating centres, 13 were able to collaborate in this follow-up study. Data on clinical outcomes were obtained from medical records in every centre. Information was anonymous for the entire length of the study. Data was collected in all centres from January 2020 to December 2021 and a RedCap database was used for data registration. The protocol conformed to the Declaration of Helsinki and was approved by the local ethics committee of each participating centre.
Study cohort and data collection
All patients with cirrhosis included in the previous study with Child-Pugh class A or B, or MELD score ≤14 if Child-Pugh was not available, were considered as candidates to participate. The imaging (contrast-enhanced abdominal computed tomography [CT] or MRI) reviewed in the previous study was considered as the basal test. Therefore, a second CT/MRI performed at least 12 months after the basal one was necessary for patient inclusion. Patients with worse liver function (Child-Pugh C and MELD >14) were excluded because of low expected 1-year survival. Patients included in the initial study who had died, received a liver transplant (LT), developed hepatocellular carcinoma (HCC) beyond Milan criteria or had undergone a transjugular intrahepatic portosystemic shunt (TIPS) procedure during the first 12 months after the baseline imaging test were excluded. If more than one CT/MRI scan was available during follow-up, the last CT/MRI was considered. Only CT-CT or MRI-MRI comparisons were analysed. Follow-up CT or MRI was compared with the test performed at inclusion: radiological changes over time were studied and correlated with liver function and complications of cirrhosis.
Radiological data and definitions
SPSS were considered as spontaneous communications between the portal venous system or splanchnic veins and the systemic venous system, excluding gastroesophageal varices. SPSS were divided between large and small according to its maximum diameter, with a cut-off of 8 mm. This cut-off was already chosen for the Baveno VI-SPSS study, because it was the size of the smallest symptomatic shunt embolized reported in the literature.6 A minimum diameter of >5 mm was considered to provide accurate SPSS size. Moreover, in patients with more than one shunt, total shunt diameter was calculated summing up the diameter of every single shunt. In order to compare total shunt diameter at baseline and at follow-up, those patients who developed a collateral during follow-up but presented no SPSS (W-SPSS group) initially were granted a 0 value at baseline. Similarly, patients in whom the shunt disappeared during the study were given a 0 value at follow-up. Shunt area was calculated by the formula πr2, as done in the study mentioned before.4 In patients with more than one shunt, all SPSS areas were summed up to calculate the TSA for each patient.
Aetiological intervention
The two aetiological interventions that were evaluated were HCV treatment and alcohol abstinence. Variables related to HCV DAA treatment (start date and SVR) were collected. Regarding alcohol, the presence of alcohol consumption/abstinence was specifically queried, and responses were collected by the different investigators based on medical records information. Thus, two special populations were defined. We defined a subgroup of cured patients, including patients with HCV-related cirrhosis who had achieved SVR, alcohol-related cirrhosis who were abstinent during follow-up, and patients with mixed HCV and alcohol cirrhosis with both SVR and abstinence. In patients with HCV, we established a minimum time of 6 months between initiation of treatment and the follow-up imaging test to be considered as cured. In alcohol-related cirrhosis, we assumed as cured only patients who had been abstinent during the entire follow-up. However, inclusion of patients with alcohol abstinence among cured patients might be a potential bias. Therefore, we performed a subanalysis including, in the cured group, only patients with HCV-related cirrhosis and SVR (HCV cured). Simultaneously, we described another subgroup of non-cured patients, including patients with HCV-related cirrhosis who were not treated or did not achieve SVR and/or alcohol-related cirrhosis who did not stop alcohol consumption, independently of the quantity of alcohol. Patients with cirrhosis from mixed aetiologies different from alcohol or HCV were not considered for this subanalysis. Patients with no information regarding hepatitis C treatment or alcohol consumption were also excluded from this analysis. Imaging tests after HCV treatment and/or alcohol abstinence were examined and compared to baseline.
Statistical analysis
Statistical analysis was performed using Stata Statistical Software (version 16, StataCorp, College Station, TX, 2019). Continuous variables are reported as mean (SD) or median (IQR), and categorical variables are reported as absolute number and percentage. For unpaired data, quantitative variables among groups were compared using the analysis of variance, or Kruskal-Wallis’ test when appropriate. Student’s t test, or Mann-Whitney’s U test when appropriate, were used when comparing data between two groups. Categorical variables were compared using Pearson’s Chi-square test, or Fisher’s exact test when appropriate. For paired variables, comparisons between baseline and follow-up data of the same patients were performed by paired t test, or Wilcoxon signed-rank test when appropriate, for continuous variables, and McNemar-Bowker’s test for categorical variables with more than two categories. For statistical analysis of survival, transplantation-free survival was considered. To assess the impact of aetiological intervention on survival, we used the Kaplan-Meier survival curve modified according to the Simon-Makuch method with log-rank test, thus including aetiological intervention as a time-dependent covariate to avoid immortal bias.7,8 Cox regression was applied to test the relationship between aetiological treatment and transplantation-free survival, including it as a time-dependent covariate as well.8 A p value less than 0.05 was considered statistically significant. All p values were two-sided.
Results
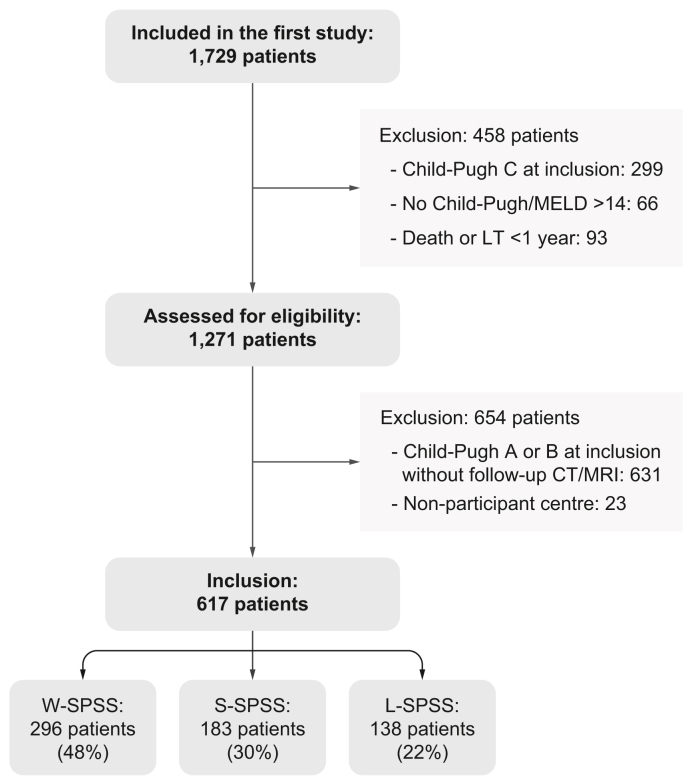
From the initial cohort of 1,729 patients included in the Baveno VI-SPSS study, 1,271 were assessed for eligibility, after excluding patients with Child-Pugh C cirrhosis at inclusion, those who had died or undergone a LT less than 1 year after inclusion, and patients in whom Child-Pugh score could not be calculated but who had a baseline MELD score >14 (Fig. 1). From these 1,271 patients, 631 patients could not be included because no follow-up imaging test was available, and 23 patients because one centre could not participate in the study. Finally, 617 patients were included in the study: L-SPSS were identified in 138 (22%) patients, S-SPSS in 183 (30%) patients, while no shunt was identified in 296 patients (W-SPSS: 48%) (Fig. 1, Fig. 2 and Table 1). Table S1 compares included patients with patients who could not be included.
Fig. 1.
Flow diagram of participants included the study.
CT, contrast-enhanced abdominal computed tomography; LT, liver transplant; MELD, model for end-stage liver disease; L-SPSS, large-SPSS; SPSS, spontaneous portosystemic shunts; S-SPSS, small-SPSS; W-SPSS, without SPSS.
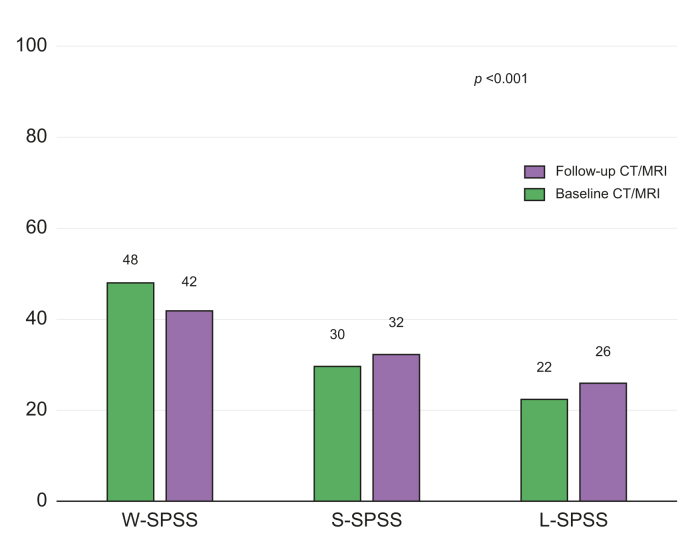
Fig. 2.
Radiological changes between baseline and follow-up imaging tests in SPSS distribution.
Data are shown as percentages. p <0.001 (McNemar-Bowker’s test). CT, contrast-enhanced abdominal computed tomography; L-SPSS, large-SPSS; SPSS, spontaneous portosystemic shunts; S-SPSS, small-SPSS; W-SPSS, without SPSS.
Table 1.
Baseline and follow-up characteristics of patients included distributed according to the type of SPSS.
| Characteristic | Total, N = 617 | W-SPSS, n = 296 | S-SPSS, n = 183 | L-SPSS, n = 138 | p value |
|---|---|---|---|---|---|
| Age, yr, mean (SD) | 60 (12) | 61 (12) | 60 (11) | 58 (12) | 0.0194 |
| Sex, male, n (%) | 416 (67.8) | 209 (70.9) | 128 (70.3) | 79 (57.7) | 0.016 |
| Etiology, n (%) | |||||
| Alcohol | 168 (27.2) | 67 (22.6) | 62 (33.9) | 39 (28.3) | 0.026 |
| HCV | 225 (36.5) | 126 (42.6) | 59 (32.2) | 40 (29.0) | 0.009 |
| Alcohol + HCV | 28 (4.5) | 14 (4.7) | 8 (4.4) | 6 (4.3) | 0.976 |
| HBV | 32 (5.2) | 18 (6.1) | 6 (3.3) | 8 (5.8) | 0.379 |
| MAFLD | 38 (6.2) | 20 (6.8) | 9 (4.9) | 9 (6.5) | 0.704 |
| Othera | 104 (16.9) | 42 (14.2) | 31 (16.9) | 31 (22.5) | 0.100 |
| Time between CT/MRIb, months, median (IQR) | 45 (49) | 48 (45) | 40 (49) | 45 (50) | 0.2855 |
| Time of follow-upc, months, median (IQR) | 63 (53) | 69 (51) | 56 (56) | 54 (53) | <0.001 |
| Baseline parameters | |||||
| MELD, median (IQR) | 10 (4) | 9 (4) | 10 (4) | 11 (4) | <0.001 |
| MELD-Na, median (IQR) | 11 (6) | 10 (5) | 11 (5) | 12 (5) | <0.001 |
| Child-Pugh, median (IQR) | 6 (2) | 5 (1) | 6 (2) | 7 (2) | <0.001 |
| Child-Pugh, n (%) | |||||
| A | 387 (67) | 210 (76) | 113 (65) | 64 (49) | <0.001 |
| B | 191 (33) | 65 (24) | 60 (35) | 66 (51) | |
| Prior decompensations, n (%) | |||||
| GIB | 90 (14.7) | 22 (7.5) | 40 (22.0) | 28 (20.4) | <0.001 |
| HE | 73 (11.9) | 19 (6.4) | 22 (12.2) | 32 (23.4) | <0.001 |
| Ascites | 189 (31.0) | 69 (23.3) | 67 (37.2) | 53 (39.8) | <0.001 |
| HCC | 76 (12.4) | 38 (12.8) | 20 (11.0) | 18 (13.2) | 0.789 |
| Follow-up parametersd | |||||
| MELD, median (IQR) | 11 (6) | 9 (6) | 11 (5) | 13 (8) | <0.001 |
| MELD-Na, median (IQR) | 11 (8) | 10 (7) | 11 (8) | 14 (10) | <0.001 |
| Child-Pugh, median (IQR) | 6 (3) | 6 (2) | 6 (3) | 8 (3) | <0.001 |
| Child-Pugh, n (%) | |||||
| A | 311 (55) | 181 (67) | 87 (52) | 43 (35) | <0.001 |
| B | 177 (32) | 66 (24) | 61 (36) | 50 (41) | |
| C | 74 (13) | 24 (9) | 20 (12) | 30 (24) | |
| Decompensations during all follow-upe, n (%) | |||||
| GIB | 84 (13.7) | 34 (11.5) | 33 (18.0) | 17 (12.3) | 0.112 |
| HE | 125 (20.2) | 41 (13.9) | 40 (21.9) | 44 (31.9) | <0.001 |
| Ascites | 126 (20.4) | 50 (16.9) | 42 (23.0) | 34 (24.6) | 0.105 |
| Infectionsf | 104 (16.9) | 47 (15.9) | 29 (15.9) | 28 (20.3) | 0.214 |
| HCC | 22 (3.6) | 8 (2.7) | 8 (4.4) | 6 (4.3) | 0.540 |
Continuous variables are reported as mean (SD) or as median (IQR), and categorical variables are reported as absolute number (percentage). Level of significance: p <0.05 (analysis of variance or Kruskal-Wallis’ test for quantitative variables; Pearson’s Chi-square test for categorical variables). p values report the statistical differences between the three groups.
CT, contrast-enhanced abdominal computed tomography; GIB, gastrointestinal bleeding; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; L-SPSS, large-SPSS; MAFLD, metabolic dysfunction-associated fatty liver disease; MELD, model for end-stage liver disease; MELD-Na, model for end-stage liver disease-sodium; SPSS, spontaneous portosystemic shunts; S-SPSS, small-SPSS; W-SPSS, without SPSS.
Includes cholestatic, autoimmune, cryptogenic, other aetiologies, and patients with more than one aetiology (excluding alcohol + HCV). In 22 patients there was no information regarding aetiology.
Median time between the basal and the follow-up imaging test.
Median time between the basal imaging test and the date of last follow-up.
MELD, MELD-Na and Child-Pugh score and distribution were calculated according to clinical situation and blood test closest to follow-up CT or MRI.
Decompensations were recorded until end of follow-up.
Includes spontaneous bacterial peritonitis and other infections.
Baseline and follow-up clinical and radiological characteristics
Characteristics and decompensating events of all included patients at baseline and during follow-up are shown in Table 1 and Table S2. Alcohol was the main aetiology in the L-SPSS group, while HCV infection was predominantly found in the W-SPSS group. Time between both imaging tests was similar between groups, but total follow-up was longer for the W-SPSS group. As expected, liver function was worse in patients with SPSS at baseline and follow-up. Regarding complications of cirrhosis, patients with both types of SPSS had previously experienced more complications than those from the W-SPSS group. However, during follow-up, these differences disappeared, except for HE. Nevertheless, after adjusting for basal liver function, both baseline and follow-up differences between patients with and without SPSS regarding complications were only observed in patients with low Child-Pugh and MELD scores (Table S3).
In the follow-up imaging test, SPSS were identified in 359 (58%) patients: 160 (26%) patients with L-SPSS and 199 (32%) with S-SPSS, and no shunt was identified in 258 patients (W-SPSS: 42%); this was significantly different from the baseline distribution of SPSS (Fig. 2). However, when stratifying patients according to time between imaging tests, only those with a follow-up CT or MRI performed at least 3 years after the basal one showed significant differences from baseline (Fig. S1). Regarding total shunt diameter and TSA, a total of 260 patients had a >5 mm diameter shunt at inclusion and/or at follow-up. Both shunt diameter and mean TSA significantly increased between baseline and follow-up imaging tests (Table S4).
Radiological changes based on liver function changes
The distribution of SPSS groups at baseline and follow-up in relation to changes in liver function was evaluated. Patients in whom MELD score worsened (defined as an increase of ≥2 points compared to baseline MELD score) showed a simultaneous aggravation in SPSS distribution. As shown in Table 2, at baseline, 55% of patients belonged to the SPSS group (28% L-SPSS and 27% S-SPSS) and 45% to the W-SPSS group; at follow-up, no shunt was found in 32% patients while 68% presented one (34% in each group) (p <0.001). No differences were found in those patients in whom MELD score improved or was stable. Similar results were found when comparing Child-Pugh score changes.
Table 2.
Radiological changes based on liver function changes.
| Baseline CT/MRI | Follow-up CT/MRI | p value | |
|---|---|---|---|
| Radiological changes if MELD worsens (n = 240), n (%) | |||
| W-SPSS | 109 (45) | 77 (32) | <0.001 |
| S-SPSS | 65 (27) | 81 (34) | |
| L-SPSS |
66 (28) |
82 (34) |
|
|
Radiological changes if MELD improves or remains the same (n = 340), n (%) | |||
| W-SPSS | 167 (49) | 162 (48) | 0.1005 |
| S-SPSS | 108 (32) | 101(30) | |
| L-SPSS |
65 (19) |
77 (22) |
|
| Radiological changes if Child-Pugh worsens (n = 159), n (%) | |||
| W-SPSS | 59 (37) | 40 (25) | <0.001 |
| S-SPSS | 52 (33) | 58 (37) | |
| L-SPSS |
48 (30) |
61 (38) |
|
| Radiological changes if Child-Pugh improves or remains the same (n = 367), n (%) | |||
| W-SPSS, n (%) | 194 (53) | 177 (48) | 0.0608 |
| S-SPSS, n (%) | 106 (29) | 112 (31) | |
| L-SPSS, n (%) | 67 (18) | 78 (21) | |
MELD or Child-Pugh scores worsening is defined as an increase of 2 points or more compared to baseline MELD or Child-Pugh scores. Variables are reported as absolute number (percentage). Level of significance: p <0.05 (McNemar-Bowker’s test).
CT, contrast-enhanced abdominal computed tomography; L-SPSS, large-SPSS; MELD, model for end-stage liver disease; SPSS, spontaneous portosystemic shunts; S-SPSS, small-SPSS; W-SPSS, without SPSS.
Radiological and clinical changes based on aetiological intervention
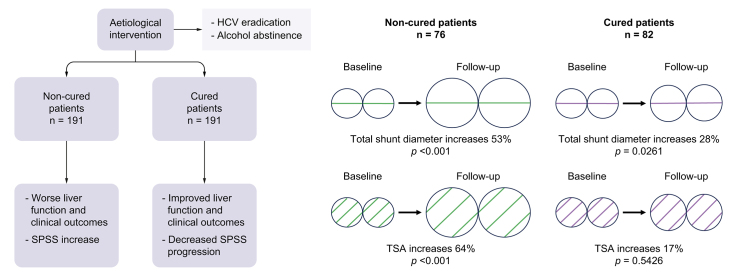
Among all the patients with HCV- and alcohol-related cirrhosis, 191 were considered cured (95 [50%] patients with SVR, 90 [47%] abstinent, and 6 [3%] with SVR and abstinent) and 191 non-cured (123 [64%] patients without SVR, 50 [26%] non-abstinent, and 18 [10%] without SVR and/or non-abstinent).
SPSS distribution
No significant radiological changes in SPSS distribution were observed when comparing patients with HCV or alcohol-related cirrhosis after aetiological intervention (Table 3). At inclusion, 20% of cured patients had L-SPSS, 30% S-SPSS, and 50% W-SPSS; at follow-up the distribution was 24%, 32% and 44%, respectively (p = 0.0518). Among non-cured patients, the differences were similar. The distribution was also similar after adjusting for a median time between imaging tests of at least 36 months (Table S5). No differences were found when separately comparing patients with HCV-related cirrhosis and patients with alcohol-related cirrhosis (Table S6).
Table 3.
Radiological changes between baseline and follow-up imaging tests in SPSS distribution in cured and non-cured patients.
| Baseline CT/MRI | Follow-up CT/MRI | p value | |
|---|---|---|---|
| Cured patients (n = 191), n (%) | |||
| W-SPSS | 96 (50) | 83 (44) | 0.0518 |
| S-SPSS | 57 (30) | 62 (32) | |
| L-SPSS | 38 (20) | 46 (24) | |
| Non-cured patients (n = 191), n (%) | |||
| W-SPSS | 102 (53) | 92 (48) | 0.1767 |
| S-SPSS | 47 (25) | 51 (27) | |
| L-SPSS | 42 (22) | 48 (25) | |
Cured patients are defined as those who are abstinent for alcohol-related cirrhosis and/or those who have received successful HCV therapy for HCV-related cirrhosis. Non-cured patients are defined as patients with alcohol-related cirrhosis without abstinence and/or HCV-related cirrhosis without treatment or without sustained viral response. Variables are reported as absolute number (percentage). Level of significance: p <0.05 (McNemar-Bowker’s test).
CT, contrast-enhanced abdominal computed tomography; L-SPSS, large-SPSS; SPSS, spontaneous portosystemic shunts; S-SPSS, small-SPSS; W-SPSS, without SPSS.
Total shunt diameter and TSA
Total shunt diameter significantly increased in both cured and non-cured patients, comparing baseline and follow-up measurements (Table 4). However, the enlargement was more significant in non-cured (7.9 [7.1] mm vs. 12.1 [7.5] mm, 53% increase, p <0.001) than in cured patients (8.6 [8.8] mm vs. 11 [7.7] mm, 28% increase, p = 0.0261). Furthermore, we evaluated TSA differences in the same patients. Mean TSA was significantly larger in non-cured patients comparing baseline and follow-up imaging tests (74.3 [88.7] mm2 vs. 122.1 [116.4] mm, 64% increase, p <0.001), whereas cured patients experienced a slight, non-significant growth (102.5 [252.0] mm2 vs. 119.8 [142.1] mm2, 17% increase, p = 0.5426) (Table 4). Similar results were obtained when comparing only HCV-cured patients (Table S7).
Table 4.
Radiological changes between baseline and follow-up imaging tests in total shunt diameter and total shunt area in cured and non-cured patients.
| Total shunt diameter, mm, mean (SD) | p value | TSA, mm2, mean (SD) | p value | |
|---|---|---|---|---|
| Cured patients (n = 82) | ||||
| SPSS inclusion | 8.6 (8.8) | 0.0261 | 102.5 (252.0) | 0.5426 |
| SPSS follow-up |
11.0 (7.7) |
119.8 (142.1) |
||
| Non-cured patients (n = 76) | ||||
| SPSS inclusion | 7.9 (7.1) | <0.001 | 74.3 (88.7) | <0.001 |
| SPSS follow-up | 12.1 (7.5) | 122.1 (116.4) | ||
Cured patients are defined as those who are abstinent for alcohol-related cirrhosis and/or those who have received successful HCV therapy for HCV-related cirrhosis. Non-cured patients are defined as patients with alcohol-related cirrhosis without abstinence and/or HCV-related cirrhosis without treatment or without sustained viral response. A minimum diameter of >5 mm was considered to provide accurate SPSS size. Variables are reported as mean (SD). Level of significance: p <0.05 (paired t test).
SPSS, spontaneous portosystemic shunts; TSA, total shunt area.
Liver function, analytical and clinical changes
Liver function and metabolic parameters were not significantly different between cured and non-cured patients at baseline. However, at follow-up, non-cured patients had a higher Child-Pugh score and worse distribution, with more patients belonging to the Child-Pugh C group (Table 5). No differences in MELD and MELD sodium (MELD-Na) scores were observed. At follow-up, non-cured patients presented higher bilirubin levels and lower albumin levels. Regarding complications of cirrhosis, at baseline both groups had experienced similar rates of liver-related events apart from HCC, which was more frequent in non-cured patients. However, during follow-up, aetiological intervention was associated with a lower risk of decompensation, whereas no significant differences were observed regarding HCC (Table 5). Similar results were obtained when comparing only HCV-cured patients (Table S8).
Table 5.
Liver function, analytical and clinical changes between cured and non-cured patients.
| Characteristic | Total, n = 382 | Cured, n = 191 | Non-cured, n = 191 | p value |
|---|---|---|---|---|
| Age, years, mean (SD) | 60 (11) | 59 (11) | 62 (12) | 0.177 |
| Sex, male, n (%) | 268 (70.3) | 143 (75.3) | 125 (65.4) | 0.036 |
| Metabolic factors, n (%) | ||||
| Diabetes | 105 (27.5) | 54 (28.3) | 51 (26.7) | 0.731 |
| Hypertension | 132 (34.6) | 71 (37.2) | 61 (31.9) | 0.282 |
| Overweight | 198 (68.0) | 101 (69.2) | 97 (66.9) | 0.676 |
| Obesity |
71 (24.4) |
40 (27.4) |
31 (21.4) |
0.232 |
| Baseline parameters | ||||
| MELD, median (IQR) | 10 (4) | 10 (4) | 10 (5) | 0.6083 |
| MELD-Na, median (IQR) | 11 (5) | 11 (5) | 11 (6) | 0.5513 |
| Child-Pugh, median (IQR) | 6 (2) | 6 (2) | 6 (2) | 0.5652 |
| Child-Pugh, n (%) | ||||
| A | 239 (66) | 123 (68) | 116 (65) | 0.527 |
| B | 121 (34) | 58 (32) | 63 (35) | |
| Prior decompensations, n (%) | ||||
| GIB | 49 (12.9) | 29 (15.3) | 20 (10.5) | 0.168 |
| HE | 45 (11.8) | 26 (13.6) | 19 (10.0) | 0.275 |
| Ascites | 120 (31.6) | 64 (33.5) | 56 (29.6) | 0.416 |
| HCC |
53 (13.9) |
19 (10.0) |
34 (17.9) |
0.025 |
| Follow-up parametersa | ||||
| MELD, median (IQR) | 10 (6) | 10 (6) | 10 (7) | 0.4531 |
| MELD-Na, median (IQR) | 11 (8) | 10 (6) | 11 (11) | 0.1772 |
| Child-Pugh, median (IQR) | 6 (3) | 6 (2) | 6 (4) | <0.001 |
| Child-Pugh, n (%) | ||||
| A | 205 (59) | 116 (66) | 89 (51) | 0.003 |
| B | 102 (29) | 47 (27) | 55 (32) | |
| C | 42 (12) | 12 (7) | 30 (17) | |
| Decompensations during all follow-upb, n (%) | ||||
| GIB | 50 (13.1) | 23 (12.0) | 27 (14.1) | 0.544 |
| HE | 70 (18.3) | 27 (14.1) | 43 (22.5) | 0.034 |
| Ascites | 78 (20.4) | 27 (14.1) | 51 (26.7) | 0.002 |
| Infectionsc | 68 (17.8) | 24 (12.6) | 44 (23.0) | 0.007 |
| HCC | 9 (2.4) | 6 (3.1) | 3 (1.6) | 0.312 |
| Analytical parametersa, mean (SD) | ||||
| Haemoglobin, g/dl | 12.6 (2.5) | 12.7 (2.5) | 12.5 (2.6) | 0.3342 |
| Platelets, x1,000/mm3 | 116 (65) | 116 (67) | 115 (62) | 0.8516 |
| Creatinine, mg/dl | 1.05 (0.80) | 1.08 (0.89) | 1.02 (0.70) | 0.5187 |
| Bilirubin, mg/dl | 2.04 (3.26) | 1.67 (2.59) | 2.41 (3.79) | 0.0275 |
| INR | 1.27 (0.37) | 1.25 (0.38) | 1.30 (0.37) | 0.1835 |
| Albumin, g/dl | 3.6 (0.7) | 3.7 (0.7) | 3.5 (0.8) | <0.001 |
Cured patients are defined as those who are abstinent for alcohol-related cirrhosis and/or those who have received successful HCV therapy for HCV-related cirrhosis. Non-cured patients are defined as patients with alcohol-related cirrhosis without abstinence and/or HCV-related cirrhosis without treatment or without sustained viral response. Overweight is defined as a BMI ≥25.0 and obesity as BMI ≥30.0.
Continuous variables are reported as mean (SD) or as median (IQR), and categorical variables are reported as absolute number (percentage). Level of significance: p <0.05 (Student’s t test or Mann-Whitney’s U test for quantitative variables; Pearson’s Chi-square test or Fisher’s exact test for categorical variables).
GIB, gastrointestinal bleeding; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, model for end-stage liver disease-sodium.
MELD, MELD-Na, Child-Pugh score and Child-Pugh distribution, and analytical parameters, were calculated according to clinical situation and blood test closest to follow-up CT or MRI.
Decompensations were recorded until end of follow-up.
Includes spontaneous bacterial peritonitis and other infections.
We also compared the intragroup differences concerning liver function between baseline and follow-up. Cured patients remained stable or even experienced an improvement, although non-significant, whereas non-cured patients experienced a significant worsening in MELD, MELD-Na and Child-Pugh scores (Table S9). Regarding Child-Pugh score distribution, there were patients who progressed to Child-Pugh C stage in both subgroups; however, this was markedly higher in non-cured patients. Results were similar when comparing only HCV-cured patients (Table S10).
Survival analysis in cured and non-cured patients
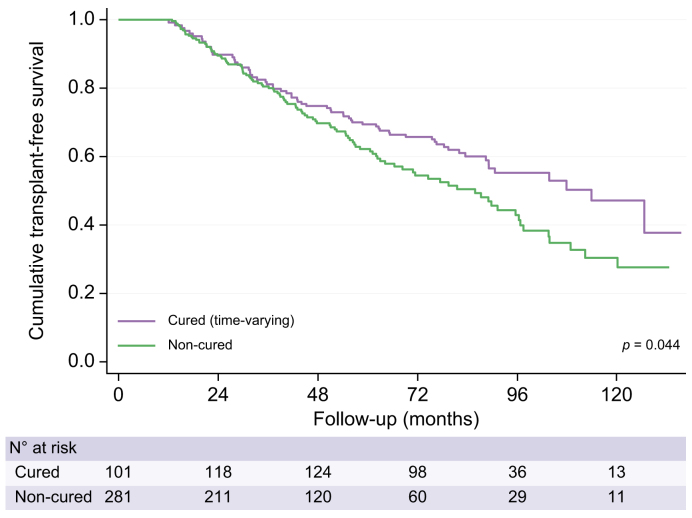
Transplant-free survival was significantly higher in cured patients compared to the non-cured subgroup (Fig. 3). At the end of the follow-up period, 128 patients had died (34% cured patients and 66% non-cured) and 43 patients had received an LT (cured 53% and non-cured 47%). Adjusting for aetiological intervention as a time-dependent covariate, HR for death/LT was 1.37 (95% CI 1.01-1.88, p = 0.0432) in non-cured patients with respect to cured patients. Table S11 shows the clinical situation at last follow-up in cured and non-cured patients. Similar results were observed when comparing only HCV-cured patients (Fig. S2 and Table S12).
Fig. 3.
Probability of transplant-free survival in cured and non-cured patients using Kaplan-Meier survival curve modified according to the Simon-Makuch method.
Log-rank test: p = 0.044. The Simon-Makuch method accounts for a change in exposure status over time. Therefore, the number of patients in the cured category increased and the number in the non-cured group decreased, as patients with HCV-related cirrhosis received successful DAA therapy. In this time-varying approach, 101 patients already started as cured patients (90 patients with alcohol-related cirrhosis assumed to be abstinent from inclusion; 10 patients with HCV-related cirrhosis who had already received treatment at inclusion; and one patient with both).
Discussion
In the previous study performed by the Baveno VI-SPSS group,3 results highlighted a relationship between the presence of SPSS and higher risk of worse outcomes in patients with cirrhosis, especially in those with preserved liver function. The present study, being a subset of the original population with an extended follow-up, reveals the same tendency to radiological progression paralleled by a worsening liver function. As an important novelty, this study provides evidence for the first time that an aetiological intervention, defined as DAA treatment in HCV-associated cirrhosis and alcohol abstinence in alcohol-related cirrhosis, is not translated into significant changes in portosystemic shunting.
The presence of a second cross-sectional evaluation of patients in the present study allowed us to evaluate radiological changes in relation to clinical and liver function changes. It is well known that the presence of portosystemic collaterals increases in patients with worse liver function.3,4,[9], [10], [11], [12], [13] Since liver function worsened in our cohort, the presence of SPSS consequently increased. This SPSS increase was obviously more remarkable in the subgroup of patients whose MELD or Child-Pugh scores worsened.
The population defined as cured after aetiological intervention (HCV with SVR and abstinent from alcohol) experienced less liver-related events, such as HE and ascites, during follow-up, and presented better liver synthesis parameters. Moreover, even though both groups had similar Child-Pugh, MELD and MELD-Na scores at baseline before treatment, non-cured patients experienced a significant worsening in liver function during follow-up. Even more importantly, transplant-free survival was significantly better in cured patients than in the non-cured subgroup. However, no radiological amelioration regarding SPSS distribution was observed in cured patients. In fact, both cured and non-cured patients showed a tendency to a worsening in SPSS distribution. Since SPSS distribution only considers the single largest shunt, and the prevalence of more than one collateral among patients with cirrhosis is high,3,4 the mean progression in total shunt diameter was assessed. Mean diameter increased in both cured and non-cured patients, although the enlargement was more significant in the latter. Finally, we compared mean TSA between cured and non-cured patients. In the study performed by Praktiknjo et al.,4 results showed that TSA was a better predictor of mortality and risk of decompensation, outperforming single SPSS classification. In our study, TSA increased significantly in the subgroup of non-cured patients. Importantly, in cured patients, there was only a small, non-significant increase in TSA.
Regarding the impact of DAAs on HCV-related cirrhosis, after SVR is achieved, liver function improvement and PH amelioration have been widely demonstrated,[14], [15], [16], [17] preventing hepatic decompensation and leading to improved outcomes.18 Carrat et al.19 showed that exposure to DAAs was associated with a decrease in all-cause mortality and HCC. In a study performed by Pons et al.,20 70% of patients with Child-Pugh A compensated advanced chronic liver disease experienced a liver stiffness improvement (defined as a decrease of ≥20% from baseline), with a mean decrease of nearly 30%. However, other research has also highlighted that in patients with cirrhosis and pre-treatment CSPH, although successful antiviral therapy is associated with a decrease in HVPG, CSPH persists in many cases.5,21,22 This has also been demonstrated in other studies where gastroesophageal varices did not improve after DAA treatment23 and in patients treated with interferon-based regimens, showing no decrease in the size of pre-existing varices in patients who achieved SVR, despite a lower incidence of de novo oesophageal varices.24,25 In another study by Mandorfer et al.26 in patients who achieved SVR after DAA therapy and had CSPH pre-treatment, a high variability in HVPG reduction or even worsening after successful HCV eradication was shown, especially in those with HVPG ≥15 mmHg. Thus, if high portal pressure persists after successful DAA therapy, it might be expected that portosystemic collaterals shall persist as well. In patients with CSPH, regression of cirrhosis and PH is less frequent, highlighting the presence of a point of no return.16
Evolution of portosystemic shunts has already been assessed following HCV eradication. Kotanki et al.27 evaluated 17 patients who achieved SVR after DAA therapy with a pre-treatment CT to evaluate the presence of SPSS. After treatment, no differences were observed in shunt diameter even though there was an improvement in Child-Pugh score and a decrease in HVPG. On the other hand, two previous studies highlighted an association between presence of SPSS and impaired improvement in liver function after DAA therapy.28,29 Another study with 51 patients treated successfully with interferon, also identified portosystemic collaterals as a risk factor for progression of gastroesophageal varices and incidence of HE after treatment.30
Alcohol abstinence has also been associated with a lower risk of hepatic decompensation and mortality,31,32 as well as a decrease in portal pressure,[33], [34], [35], [36] both in compensated and decompensated patients.37 In a recent study, abstinent patients with CSPH and high-risk PH (HVPG ≥20 mmHg) showed a significant reduction of hepatic decompensation.38 In another study which evaluated patients with alcohol-related cirrhosis and/or hepatitis B- or hepatitis C-related cirrhosis, patients with both alcohol abstinence and antiviral treatment had the lowest risk of HCC and variceal bleeding.39 No studies regarding the effect of alcohol withdrawal in SPSS have been published so far.
In our daily practice, we have found other situations wherein patients with cirrhosis experience an improvement in or even resolution of PH without regression or disappearance of portosystemic collaterals. After TIPS placement it has been described that SPSS might remain unchanged in up to one-third of patients,40,41 pointing to the need to evaluate a prophylactic embolization of L-SPSS before TIPS placement to diminish the risk of HE and worse outcomes after TIPS.42 SPSS may also persist after LT.43,44 Current recommendations suggest considering intra-operative management of L-SPSS to avoid graft dysfunction, portal vein thrombosis or reappearance of HE.45,46 However, in small-sized grafts, authors advocate that SPSS should not be ligated to avoid graft hyperperfusion.46 During follow-up, in case of symptomatic L-SPSS, embolization has been considered,44,47 although the presence of SPSS after LT is more often related to development of graft cirrhosis and PH rather than persistence of pre-transplant collaterals.
Our results have several limitations. First, this is a retrospective study, which may lead to potential bias in data reporting. Furthermore, a systematic protocol for imaging analysis was not defined in the previous study performed by the Baveno VI-SPSS group,3 nor in the present study. However, CT and MRI were reviewed by expert radiologists in hepatic diseases (12 of 13 participant centres), or an hepatologist trained by a radiologist in one centre. Regarding alcohol consumption, abstinence was based on data reported in clinical history, and assumed to be constant throughout study follow-up. No other test was performed to ensure patients were truly abstinent. Concerning HCV, treatment might occur years after enrolment, and the baseline imaging test may not accurately represent the basal patient condition. Another limitation is that median time between baseline and follow-up imaging tests was longer in cured patients because of higher transplant-free survival. Finally, only patients who underwent a second CT or MRI were included. This might have excluded asymptomatic patients who had only abdominal ultrasound every 6 months for HCC surveillance, as well as patients with impaired kidney function.
The strengths of the study are the size of the cohort and the extended follow-up. All participating centres were tertiary-care hospitals with a protocolized management of liver diseases. This is the largest cohort reported with longitudinal data regarding SPSS and their impact on patients with cirrhosis, with data from 13 different centres, allowing for a generalization of the results.
In conclusion, SPSS are frequent in patients with cirrhosis, and they are associated with portal hypertension-related complications. Furthermore, their prevalence increases over time, along with liver function deterioration. Patients with cirrhosis undergoing an aetiological intervention, such as HCV treatment with DAA and/or alcohol abstinence, experience lower risk of decompensation and liver function deterioration, and higher transplant-free survival. However, SPSS persist in the majority of patients, although progression seems to be decreased. Therefore, despite SVR achievement or alcohol abstinence, complications related to SPSS may still develop.
Financial support
Judit Vidal-González is a recipient of the PFIS grant FI19/00330 from Instituto de Salud Carlos III, Spain. The work was partially funded by grant PI21/00312 and PI21/00691 from Instituto de Salud Carlos III and co-funded by the European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”). CIBERehd is supported by Instituto de Salud Carlos III.
Authors’ contributions
JV-G, MS-T and JG contributed to the study concept and design. All authors contributed to the acquisition of data. JV-G, MS-T and JG contributed to the analysis and interpretation of data and drafted the manuscript. All authors contributed to the critical revision of the manuscript. All authors approved the final manuscript prior to submission.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest
JG has received consulting fees from Boehringer Ingelheim and speaking fees from Echosens. MS-T has received consulting fees from Grifols. AK has served as speaker for Novo Nordisk, Norgine, Siemens and Nordic Bioscience and participated in advisory boards for Norgine, Siemens, Resalis Therapeutics, Boehringer Ingelheim and Novo Nordisk, all outside the submitted work. Research support Norgine, Siemens, Nordic Bioscience, Astra, Echosense. Consulting Takeda, Resalis Therapeutics, Zealand Pharma, Novo Nordisk, Boehringer Ingelheim. Board member and co-founder Evido.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100977.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.de Franchis R., Baveno VII Faculty Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillaume M., Bureau C. Should the presence of spontaneous portosystemic shunts Be implemented to the model for end-stage liver disease score for a better prediction of outcome? Gastroenterology. 2018 May;154(6):1569–1571. doi: 10.1053/j.gastro.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Simón-Talero M., Roccarina D., Martínez J., et al. Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis. Gastroenterology. 2018 May;154(6):1694–1705.e4. doi: 10.1053/j.gastro.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Praktiknjo M., Simón-Talero M., Römer J., et al. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J Hepatol. 2020 Jun;72(6):1140–1150. doi: 10.1016/j.jhep.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Lens S., Alvarado-Tapias E., Mariño Z., et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017 Nov;153(5):1273–1283.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Sakurabayashi S., Sezai S., Yamamoto Y., et al. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997 Mar-Apr;20(2):120–124. doi: 10.1007/s002709900118. [DOI] [PubMed] [Google Scholar]
- 7.Simon R., Makuch R.W. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984 Jan-Mar;3(1):35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 8.Shintani A.K., Girard T.D., Eden S.K., et al. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009 Nov;37(11):2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipinski M., Saborowski M., Heidrich B., et al. Clinical characteristics of patients with liver cirrhosis and spontaneous portosystemic shunts detected by ultrasound in a tertiary care and transplantation centre. Scand J Gastroenterol. 2018 Sep;53(9):1107–1113. doi: 10.1080/00365521.2018.1498913. [DOI] [PubMed] [Google Scholar]
- 10.Nardelli S., Gioia S., Ridola L., et al. Radiological intervention for shunt related encephalopathy. J Clin Exp Hepatol. 2018 Dec;8(4):452–459. doi: 10.1016/j.jceh.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zardi E.M., Uwechie V., Caccavo D., et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44(1):76–83. doi: 10.1007/s00535-008-2279-1. [DOI] [PubMed] [Google Scholar]
- 12.Vidal-González J., Quiroga S., Simón-Talero M., et al. Spontaneous portosystemic shunts in liver cirrhosis: new approaches to an old problem. Therap Adv Gastroenterol. 2020 Sep 29;13 doi: 10.1177/1756284820961287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama H., Shiina S. Collaterals in portal hypertension: anatomy and clinical relevance. Quant Imaging Med Surg. 2021 Aug;11(8):3867–3881. doi: 10.21037/qims-20-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung M.C.M., Walker A.J., Hudson B.E., et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016 Oct;65(4):741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Nahon P., Bourcier V., Layese R., et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017 Jan;152(1):142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Calvaruso V., Craxì A. Hepatic benefits of HCV cure. J Hepatol. 2020 Dec;73(6):1548–1556. doi: 10.1016/j.jhep.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Mandorfer M., Kozbial K., Schwabl P., et al. Changes in hepatic venous pressure gradient predict hepatic decompensation in patients who achieved sustained virologic response to interferon-free therapy. Hepatology. 2020 Mar;71(3):1023–1036. doi: 10.1002/hep.30885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons B., Saleem J., Heath K., et al. Long-Term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. 2015 Sep 1;61(5):730–740. doi: 10.1093/cid/civ396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrat F., Fontaine H., Dorival C., et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019 Apr 6;393(10179):1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 20.Pons M., Rodríguez-Tajes S., Esteban J.I., et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020 Mar;72(3):472–480. doi: 10.1016/j.jhep.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Lens S., Baiges A., Alvarado-Tapias E., et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol. 2020 Dec;73(6):1415–1424. doi: 10.1016/j.jhep.2020.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Díez C., Berenguer J., Ibañez-Samaniego L., et al. Persistence of clinically significant portal hypertension after eradication of hepatitis C virus in patients with advanced cirrhosis. Clin Infect Dis. 2020 Dec 17;71(10):2726–2729. doi: 10.1093/cid/ciaa502. [DOI] [PubMed] [Google Scholar]
- 23.Hisanaga H., Takedatsu H., Emori K., et al. Effect of direct-acting antiviral agents on gastroesophageal varices in patients with hepatitis C virus-related cirrhosis. Medicina (Kaunas) 2022 Aug 10;58(8):1077. doi: 10.3390/medicina58081077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Marco V., Calvaruso V., Ferraro D., et al. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016 Jul;151(1):130–139.e2. doi: 10.1053/j.gastro.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 25.D'Ambrosio R., Aghemo A., Rumi M.G., et al. The course of esophageal varices in patients with hepatitis C cirrhosis responding to interferon/ribavirin therapy. Antivir Ther. 2011;16(5):677–684. doi: 10.3851/IMP1807. [DOI] [PubMed] [Google Scholar]
- 26.Mandorfer M., Kozbial K., Schwabl P., et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016 Oct;65(4):692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Kotani K., Enomoto M., Uchida-Kobayashi S., et al. Short-term hepatocyte function and portal hypertension outcomes of sofosbuvir/velpatasvir for decompensated hepatitis C-related cirrhosis. J Gastroenterol. 2023 Apr;58(4):394–404. doi: 10.1007/s00535-023-01963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji S., Uchida Y., Uemura H., et al. Involvement of portosystemic shunts in impaired improvement of liver function after direct-acting antiviral therapies in cirrhotic patients with hepatitis C virus. Hepatol Res. 2020 Apr;50(4):512–523. doi: 10.1111/hepr.13471. [DOI] [PubMed] [Google Scholar]
- 29.Takaoka Y., Miura K., Morimoto N., et al. Real-world efficacy and safety of 12-week sofosbuvir/velpatasvir treatment for patients with decompensated liver cirrhosis caused by hepatitis C virus infection. Hepatol Res. 2021 Jan;51(1):51–61. doi: 10.1111/hepr.13576. [DOI] [PubMed] [Google Scholar]
- 30.Nagaoki Y., Aikata H., Kobayashi T., et al. Risk factors for the exacerbation of esophageal varices or portosystemic encephalopathy after sustained virological response with IFN therapy for HCV-related compensated cirrhosis. J Gastroenterol. 2013 Jul;48(7):847–855. doi: 10.1007/s00535-012-0679-8. [DOI] [PubMed] [Google Scholar]
- 31.Pearson M.M., Kim N.J., Berry K., et al. Associations between alcohol use and liver-related outcomes in a large national cohort of patients with cirrhosis. Hepatol Commun. 2021 Dec;5(12):2080–2095. doi: 10.1002/hep4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toshikuni N., Izumi A., Nishino K., et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009 Jul;24(7):1276–1283. doi: 10.1111/j.1440-1746.2009.05851.x. [DOI] [PubMed] [Google Scholar]
- 33.Muntaner L., Altamirano J.T., Augustin S., et al. High doses of beta-blockers and alcohol abstinence improve long-term rebleeding and mortality in cirrhotic patients after an acute variceal bleeding. Liver Int. 2010 Sep;30(8):1123–1130. doi: 10.1111/j.1478-3231.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 34.Spahr L., Goossens N., Furrer F., et al. A return to harmful alcohol consumption impacts on portal hemodynamic changes following alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2018 Aug;30(8):967–974. doi: 10.1097/MEG.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 35.Vorobioff J., Groszmann R.J., Picabea E., et al. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology. 1996 Sep;111(3):701–709. doi: 10.1053/gast.1996.v111.pm8780575. [DOI] [PubMed] [Google Scholar]
- 36.Villanueva C., López-Balaguer J.M., Aracil C., et al. Maintenance of hemodynamic response to treatment for portal hypertension and influence on complications of cirrhosis. J Hepatol. 2004 May;40(5):757–765. doi: 10.1016/j.jhep.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Lackner C., Spindelboeck W., Haybaeck J., et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017 Mar;66(3):610–618. doi: 10.1016/j.jhep.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hofer B.S., Simbrunner B., Hartl L., et al. Alcohol abstinence improves prognosis across all stages of portal hypertension in alcohol-related cirrhosis. Clin Gastroenterol Hepatol. 2022 Aug;21(9):2308–2317.e7. doi: 10.1016/j.cgh.2022.11.033. S1542-3565(22)1113-2. [DOI] [PubMed] [Google Scholar]
- 39.Abassa K.K., Wu X.Y., Xiao X.P., et al. Effect of alcohol on clinical complications of hepatitis virus-induced liver cirrhosis: a consecutive ten-year study. BMC Gastroenterol. 2022 Mar 19;22(1):130. doi: 10.1186/s12876-022-02198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borentain P., Soussan J., Resseguier N., et al. The presence of spontaneous portosystemic shunts increases the risk of complications after transjugular intrahepatic portosystemic shunt (TIPS) placement. Diagn Interv Imaging. 2016 Jun;97(6):643–650. doi: 10.1016/j.diii.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 41.He C., Lv Y., Wang Z., et al. Association between non-variceal spontaneous portosystemic shunt and outcomes after TIPS in cirrhosis. Dig Liver Dis. 2018 Dec;50(12):1315–1323. doi: 10.1016/j.dld.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Vidal-González J., Simón-Talero M., Genescà J. Should prophylactic embolization of spontaneous portosystemic shunts be routinely performed during transjugular intrahepatic portosystemic shunt placement? Dig Liver Dis. 2018 Dec;50(12):1324–1326. doi: 10.1016/j.dld.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 43.De Carlis L., Del Favero E., Rondinara G., et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl Int. 1992 Mar;5(1):9–14. doi: 10.1007/BF00337182. PMID: 1580990. [DOI] [PubMed] [Google Scholar]
- 44.Saks K., Jensen K.K., McLouth J., et al. Influence of spontaneous splenorenal shunts on clinical outcomes in decompensated cirrhosis and after liver transplantation. Hepatol Commun. 2018 Feb 9;2(4):437–444. doi: 10.1002/hep4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golse N., Mohkam K., Rode A., et al. Surgical management of large spontaneous portosystemic splenorenal shunts during liver transplantation: splenectomy or left renal vein ligation? Transpl Proc. 2015 Jul-Aug;47(6):1866–1876. doi: 10.1016/j.transproceed.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Gomez Gavara C., Bhangui P., Salloum C., et al. Ligation versus no ligation of spontaneous portosystemic shunts during liver transplantation: audit of a prospective series of 66 consecutive patients. Liver Transpl. 2018 Apr;24(4):505–515. doi: 10.1002/lt.24999. [DOI] [PubMed] [Google Scholar]
- 47.Álvarez-López P., Campos-Varela I., Quiroga S., et al. Spontaneous portosystemic shunt embolization in liver transplant recipients with recurrent hepatic encephalopathy. Ann Hepatol. 2022 May-Jun;27(3) doi: 10.1016/j.aohep.2022.100687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.