Abstract
In this study we demonstrate the capability of Chlamydia trachomatis to infect cultured human mesothelial cell (MC) monolayers and to induce the production of the proinflammatory cytokines interleukin 1β (IL-1β) and IL-8. Seventy-two hours after initial infection, both the procoagulant activity of MC and the activity of the fibrinolytic inhibitor (plasminogen activator inhibitor 1) in the supernatants were enhanced. These findings support the hypothesis that provoked proinflammatory responses contribute to the development of complications after chlamydial infection.
Chlamydial infections of the urogenital tract are frequently asymptomatic but can result in severe complications, particularly in women. Although Chlamydia trachomatis preferentially infects the columnar or transitional epithelium, this microorganism can also induce more invasive infections causing Fitz-Hugh-Curtis syndrome (8). Progression and complications induced by ascending C. trachomatis infections may depend on local immunologically mediated inflammation and fibrosis (6, 9, 11, 18). However, little is known about the pathogenesis of this infection. We initiated infection of human mesothelial cells (MC) with C. trachomatis and studied proinflammatory, fibrinolytic, and procoagulant responses during one life cycle (24- to 72-h period).
MC were obtained from the omental tissue during a patient’s elective abdominal surgery. The MC were isolated according to techniques modified from the work of Nicholson et al. (10) and Wu et al. (22) and cultured as described previously (23). The identity of MC was demonstrated by the absence of von Willebrand factor staining (13) and the presence of intracellular cytokeratins by immunofluorescence with monoclonal antibodies (Dakopatts, Glostrup, Denmark) (5).
A C. trachomatis clinical urogenital isolate was used for all experiments. Strains were propagated on a buffalo green monkey (BGM) cell line with minimal essential medium with Earle’s salts (Life Technologies Ltd., Paisley, Scotland) supplemented with 10% fetal calf serum. Confirmation of strain identity was performed with a monoclonal antibody (De Beer Medicals, Diessen, The Netherlands).
MC monolayers (second passage) grown to confluence in 48-well tissue culture plates were used for infection experiments. Heat-inactivated (30 min at 56°C) and ultracentrifuged (2 h at 100,000 × g) C. trachomatis suspensions were also investigated. MC monolayers incubated with 500 and 100 μl of sucrose-phosphate-glutamic acid (SPG) served in all experiments as the control medium. After 24 h the conditioned medium was collected and centrifuged (800 × g). The supernatants were stored at −70°C for immunoassessment. Fresh supplemented M-199 was added to the MC monolayers at 24 and 48 h after initial infection. For quantification of infection, cells were fixed for 30 min in absolute methanol-acetone, air dried, and stained by fluorescein-conjugated murine monoclonal antibodies and examined microscopically for inclusions. The number of inclusion-forming units was counted in 10 random fields at a ×200 magnification. MC toxicity was assessed by examining the monolayers under phase-contrast microscopy and by measurement of lactate dehydrogenase activity (2, 4). Interleukin 1β (IL-1β) and IL-8 were detected by an enzyme-linked immunosorbent assay (Pelikine-compact; Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands). Quantification of PAI-1 activity was also determined in the same supernatants by means of an enzyme-linked immunosorbent assay (Spectrolyse; Biopool, Umea, Sweden). Determinations were done in duplicate, while all experiments were performed in triplicate. Procoagulant studies were performed in 48-well tissue culture plates at 37°C and assayed as described previously (21). Tissue factor (TF) expression was quantified as the amount of pM factor Xa formed per minute by 105 MC compared with the amount formed in the control medium (100%).
Results are expressed as means and standard deviations (SD). The data were subjected to statistical analysis by the paired two-tailed Student t test, where groups were compared with the control.
Susceptibility and cell damage of MC monolayers.
MC were highly susceptible to infection with C. trachomatis. Significant cell injury occurred 72 h after initial infection for nondiluted (1:1) and diluted (1:5) chlamydia suspensions (data not shown). Therefore, a 1:10 dilution of the chlamydia stock solution was used in all subsequent experiments. The actual infection percentage of MC monolayers after 48 h of incubation with a 1:10-diluted stock solution of C. trachomatis was comparable to that of the standard BGM cell line, i.e., approximately 17% (30 inclusion bodies per 500 MC at a ×200 magnification).
IL-1β and -8 production by human MC.
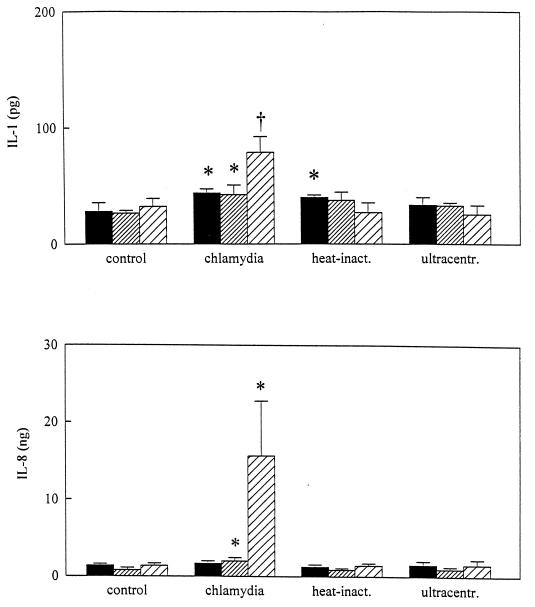
Unstimulated cultured MC monolayers released small amounts of IL-1β (28.2 ± 7.4 pg) and IL-8 (1.38 ± 0.2 ng) during incubation with control medium (M-199 with 0.2% SPG). When MC were cultured with viable chlamydiae, IL-1β concentrations in the supernatants reached maximal levels 72 h after initiation of cultures (78.9 ± 13.5 pg) (Fig. 1). The release of IL-8 in response to infection with a 1:10-diluted stock of C. trachomatis rose significantly above background levels after 24 to 48 h (2.0 ± 0.4 ng) and rapidly increased after 48 to 72 h (11.9 ± 3.9 ng) of incubation. Heat-killed chlamydiae were ingested by MC, and no intracellular growth was observed (Fig. 2). Figure 1 also indicates that heat-killed C. trachomatis significantly enhanced the amount of IL-1β 0 to 24 h after initial infection and failed to induce the amount of IL-8 during the 72-h period.
FIG. 1.
IL-1 and -8 concentrations in the supernatants of MC monolayers infected with C. trachomatis. Levels are expressed as mean IL-1 concentrations (±SD) and IL-8 concentrations (±SD) per 105 MC based on results from four separate experiments performed in duplicate. Patterns in bars reflect lengths of incubation, as follows: ▪, 0 to 24 h; ▨, 24 to 48 h; and ▨, 48 to 72 h. ∗, P < 0.05; †, P < 0.01. Statistically significant differences reflect comparisons with MC monolayers exposed to control medium (supplemented M-199 containing 0.2% [vol/vol] SPG medium). inact., inactivated; ultracentr., ultracentrifuged.
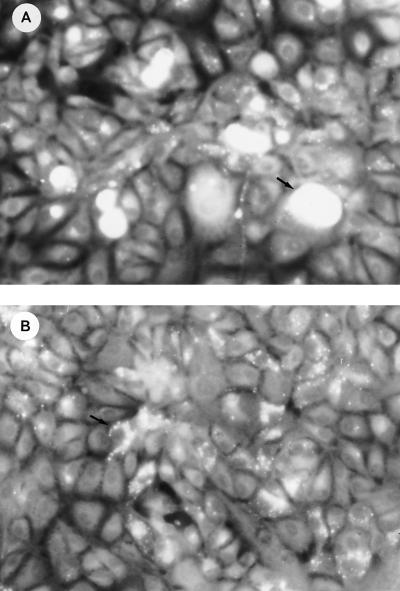
FIG. 2.
Phase-contrast microscopy of chlamydia incorporation during 48 h of incubation with a 1:10-diluted stock suspension of viable C. trachomatis (A) and a suspension of heat-killed chlamydiae (B). Magnification, ×188. Arrows indicate intracellular fluorescent chamydiae.
Procoagulant and antifibrinolytic activities of human MC.
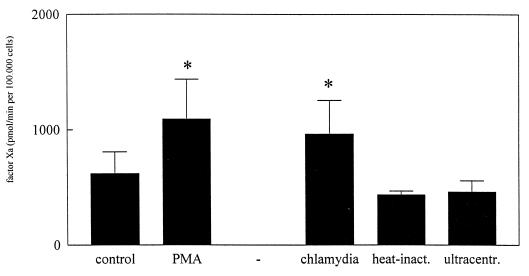
Without cells or without one of the clotting factors (VII or X), no Xa was formed. As a positive control we used phorbol myristate acetate (10 ng/ml). Forty-eight hours after initial chlamydial infection, TF expression was significantly induced. Compared to the level of TF expression in the negative control, a 1.5-fold increase was observed (Fig. 3).
FIG. 3.
TF activity of MC monolayers 72 h after initial infection with C. trachomatis. Results are depicted as mean amounts of factor Xa generated (± standard errors) and are based on values from four separate experiments performed in triplicate. ∗, P < 0.05. Statistically significant differences reflect comparisons with MC monolayers exposed to control medium (supplemented M-199 containing 0.2% [vol/vol] SPG medium). inact., inactivated; ultracentr., ultracentrifuged.
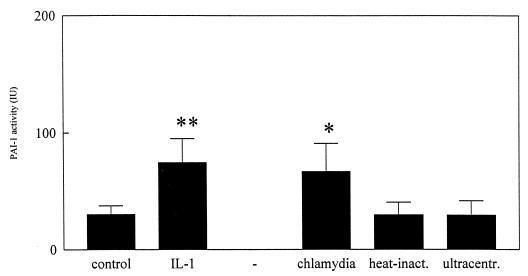
The supernatants from unstimulated, noninfected, and infected MC monolayers were assessed for fibrinolytic activity by enzyme-linked immunosorbent assay. In response to IL-1β, plasminogen activator inhibitor 1 (PAI-1) activity of MC monolayers was altered directly after stimulation. This response continued 48 and 72 h after initial stimulation. Kinetics of chlamydia-induced PAI-1 activity were different from those of the IL-1β-induced response and were significantly enhanced only in supernatants obtained 72 h after initial infection (Fig. 4).
FIG. 4.
PAI-1 activity of MC monolayers 72 h after initial infection with C. trachomatis. Results are depicted as mean levels of PAI-1 activity (in international units per 105 cells ± SD) and are based on values from four separate experiments performed in triplicate. ∗, P < 0.05; ∗∗, P < 0.01. Statistically significant differences reflect comparisons with MC monolayers exposed to control medium (supplemented M-199 containing 0.2% [vol/vol] SPG medium). inact., inactivated; ultracentr., ultracentrifuged.
Local immune system activation by C. trachomatis, and not the infection per se, may be responsible for the tissue damage and fibrosis seen in chlamydia-associated infections. C. trachomatis has a very limited host range in vivo and appears to be exclusively a parasite of squamocolumnar epithelial cells. In this study, MC, obtained from human omental species, showed themselves to be very sensitive host cells in vitro. Their inflammatory response after primary inoculation with Chlamydia spp. in vivo is characterized by infiltration with neutrophils and cytokine secretion in the acute phase (3, 12, 14). During inflammatory events, MC are activated either directly by bacterial products or by cytokines secreted by peritoneal cells and they release inflammatory mediators, such as IL-1 (7). The mesothelium is also involved in the process of transmesothelial migration of neutrophils, via synthesis of IL-8 (20, 23). The results of this study show induction of IL-8 production by MC at the onset of infection with C. trachomatis. We suggest that the chemoattractant IL-8, produced at serosal surfaces, is responsible for the neutrophil influx that occurs during invasive chlamydial infection. Epithelial cells may be an important source of IL-8 during the initial urogenital infection (1). Recently, Rasmussen and coworkers showed increased production of proinflammatory cytokines by epithelial cells in response to chlamydial infection (16). It can be hypothesized that chlamydial inclusion results in endogenous production of IL-1β by MC, which might activate neighboring cells and thus spread the peritoneal inflammatory response. Heat-killed chlamydiae induced IL-1β production after 0 to 24 h only slightly, and this may be explained by activation in response to the outer membrane of C. trachomatis or to ingestion of the heat-killed chlamydiae, which we observed after staining with monoclonal antibodies. In contrast, Rothermel et al. (17) observed no uptake of heat-killed chlamydiae by monocytes and found that inactivated chlamydiae induce IL-1 production of monocytes as effectively as viable chlamydiae. In our experiments heat-killed chlamydiae failed to initiate prolonged production of IL-1, a prerequisite for initiation of the inflammatory cascade.
Local cytokine production during infection may play an important role in modulating host defenses against C. trachomatis. The formation of fibrin is mediated via local coagulation and fibrinolytic pathways. If coagulation is activated, TF expression on the cell membrane initiates activation of the extrinsic pathway (directly activating factor VII, which subsequently activates factor X) (15). The fibrin clot formed is susceptible to lysis by tissue plasminogen activator. The process is inhibited when tissue plasminogen activator is blocked by PAI-1. This reduction in peritoneal fibrinolytic activity, which occurs after injury or inflammation, is associated with adhesions (19) and may facilitate, e.g., postinfectious tubal infertility. We demonstrated induction of TF expression by MC monolayers in the early stage of infection with C. trachomatis. High levels of TF were detectable after 48 to 72 h of incubation. Upregulation of TF expression can be due to both activated inflammatory responses and interaction of the intracellular parasite with MC cytoplasmatic systems. Infection of MC monolayers with C. trachomatis resulted also in enhanced activity of the inhibitor of fibrinolysis, PAI-1.
In conclusion, this study demonstrates that, after initial chlamydial infection, local proinflammatory responses and procoagulant activity are enhanced but that fibrinolytic activity is inhibited. Heat-killed chlamydiae failed to induce a prolonged IL-1 response, probably due to ingestion by MC. These data further stress the pivotal role of local immune system activation in the pathogenesis of intraabdominal complications after chlamydial infection.
Acknowledgments
We thank Edwin Boel for preparing the C. trachomatis stock and Peter K. von dem Borne for isolation of factors VII and X.
REFERENCES
- 1.Agace W W, Hedges S R, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmeyer H U, Bernt E, Hess B. Lactate dehydrogenase (II 2a) In: Bergmeyer H U, editor. Methods of enzymatic analysis—1965. New York, N.Y: Academic Press; 1965. pp. 736–743. [Google Scholar]
- 3.Bobo L, Novak N, Mkocha H, Vitale S, West S, Quinn T C. Evidence for a predominant proinflammatory conjuctival cytokine response in individuals with trachoma. Infect Immun. 1996;64:3273–3279. doi: 10.1128/iai.64.8.3273-3279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breborowiczs A, Rodela H, Oreopouos D G. Toxicity of osmotic solutions on human mesothelial cells in vitro. Kidney Int. 1992;41:1280–1285. doi: 10.1038/ki.1992.190. [DOI] [PubMed] [Google Scholar]
- 5.Connell N D, Rheinwald J G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983;34:245. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- 6.Dörr P J, Brommer E J P, Dooijewaard G, Vemer H M. Peritoneal fluid and plasma fibrinolytic activity in women with pelvic inflammatory disease. Thromb Haemostasis. 1992;68:102–105. [PubMed] [Google Scholar]
- 7.Douvdevani A, Rapoport J, Konforty A, Argov S, Ovnat A, Chaimovitz C. Human peritoneal mesothelial cells synthesize IL-1α and β. Kidney Int. 1994;46:993–1001. doi: 10.1038/ki.1994.359. [DOI] [PubMed] [Google Scholar]
- 8.Heinonen P K, Teisela K, Punnonen R, Miettenen A, Lehtinen M, Pavoonen J. Anatomic sites of upper genital tract infection. Obstet Gynecol. 1985;66:348–390. [PubMed] [Google Scholar]
- 9.Malinverni R. The role of cytokines in chlamydial infections. Curr Opin Infect Dis. 1996;9:150–155. [Google Scholar]
- 10.Nicholson L J, Clarke J M F, Pittilo R M, Machin S J, Woolf N. The mesothelial cell as a non-thrombogenic surface. Thromb Haemostasis. 1984;52:102–104. [PubMed] [Google Scholar]
- 11.Patton D L. Immunopathology and histopathology of experimental salpingitis. Rev Infect Dis. 1985;7:746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- 12.Patton D L, Kuo C C. Histopathology of Chlamydial trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 13.Pronk A, de Groot P G, Hoynck van Papendrecht A A G M, Verbrugh H A, Leguit P, van Vroonhoven T J M V, Sixma J J. Thrombogenicity and procoagulant activity of human mesothelial cells. Arterioscler Thromb. 1992;12:1428–1436. doi: 10.1161/01.atv.12.12.1428. [DOI] [PubMed] [Google Scholar]
- 14.Rank R G, Sanders M M, Kidd A T. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am J Pathol. 1993;142:1291–1296. [PMC free article] [PubMed] [Google Scholar]
- 15.Rapaport S I. Regulation of the tissue factor pathway. Ann N Y Acad Sci. 1991;614:51–62. doi: 10.1111/j.1749-6632.1991.tb43691.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y-X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothermel C D, Schachter J, Lavrich P, Lipsitz E C, Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989;57:2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor H R, Johnson S L, Schachter J, Caldwell H D, Prendergast R A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987;138:3023–3027. [PubMed] [Google Scholar]
- 19.Thompson J N, Paterson-Brown S, Harbourne T, Whawell S A, Kalodiki E, Dudley H A F. Reduced human peritoneal plasminogen activity: possible mechanism of adhesion formation. Br J Surg. 1989;76:382–384. doi: 10.1002/bjs.1800760422. [DOI] [PubMed] [Google Scholar]
- 20.Topley N, Brown Z, Jörres A, Westwick J, Davies M, Coles G A, Williams J D. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by IL-1β and TNF-α. Am J Pathol. 1993;142:1876–1886. [PMC free article] [PubMed] [Google Scholar]
- 21.Verhagen H J M, Heijnen-Snyder G J, Vink T, Pronk A, van Vroonhoven T J M V, Eikelboom B C, Sixma J J, de Groot P G. Tissue factor expression on mesothelial cells is induced during in vitro culture—manipulation of culture conditions creates perspectives for mesothelial cells as a source for cell seeding procedures on vascular grafts. Thromb Haemostasis. 1995;74:1096–1102. [PubMed] [Google Scholar]
- 22.Wu Y-J, O’Connel T M, Rheinwald J G. Human mesothelial cell culture. Cell. 1983;31:693–703. [Google Scholar]
- 23.Zeillemaker A M, Mul F J P, Hoynck van Papendrecht A A G M, Kuijpers T W, Roos D, Leguit P, Verbrugh H A. Neutrophil adherence to and migration across monolayers of human peritoneal cells. J Lab Clin Med. 1996;127:279–286. doi: 10.1016/s0022-2143(96)90096-7. [DOI] [PubMed] [Google Scholar]