Abstract
Lipoproteins can bind lipopolysaccharide (LPS) and decrease LPS-stimulated cytokine production. Lipoprotein(a) [Lp(a)] was as potent as low-density lipoproteins (LDL) in inhibiting LPS-stimulated tumor necrosis factor synthesis by human mononuclear cells. The kinetics of LPS inhibition by Lp(a) was similar to that of LDL. This suggests that circulating Lp(a) may be an important factor determining the amplitude of the response to LPS in humans.
The systemic toxicity of gram-negative sepsis is largely due to endotoxin, a lipopolysaccharide (LPS) component of the outer membrane of gram-negative bacteria. LPS stimulates the production of proinflammatory cytokines such as tumor necrosis factor alpha (TNF) and interleukin-1, which in turn may induce disseminated intravascular coagulation, hypotension, and renal, hepatic, and cerebral damage (15). Once LPS enters the circulation, initial interactions of LPS with LPS-binding protein and lipoproteins are essential for stimulation of cytokine production. While LPS-binding protein mediates binding of LPS to CD14, the main LPS receptor, leading to stimulation of cytokine synthesis (23), hydrophobic interaction between the lipid A component of LPS and lipoproteins neutralizes LPS and inhibits LPS-induced cytokine release (14). Preincubation of LPS with low-density lipoproteins (LDL) (24), high-density lipoproteins (HDL) (8), or very-low-density lipoproteins (10) reduces LPS-induced cytokine production in vitro, whereas hyperlipoproteinemia protects animals against lethal endotoxemia (11, 13) and gram-negative sepsis (17, 21) in vivo. In humans, reconstituted HDL inhibits LPS-induced cytokine release in whole blood (20), and infusion of reconstituted HDL has potent anti-inflammatory effects during human endotoxemia (19). These data are strong arguments for the important role played by lipoproteins in the neutralization of LPS during endotoxemia and gram-negative infections. All the studies to date have focused on the interaction of LPS with the major lipoprotein subfractions LDL, HDL, and very-low-density lipoproteins, while no studies investigated the LPS-neutralizing properties of the relatively recently described lipoprotein(a) [Lp(a)]. Lp(a) is a lipoprotein particle having apolipoprotein B-100 (ApoB-100) as a protein moiety, linked by disulfide bridges to one or two molecules of Apo(a) (22). It is now accepted that Lp(a) is an important and independent risk factor for the development of atherosclerosis (for a review see reference 22). Lp(a) levels in plasma are genetically determined and vary widely, with differences as high as 100-fold being found among individuals, ranging from 20 to 2,000 mg/liter (22). If Lp(a) as an LDL-like particle had LPS-neutralizing properties, the large variation in concentrations of Lp(a) in plasma among individuals could play an important role in the amplitude of the response to LPS. Moreover, since Lp(a) reacts as an acute-phase reactant (12, 16, 18), this could have an additional impact on the capacity of an individual to neutralize LPS. The aims of the present study were to investigate whether Lp(a) is able to inhibit LPS-induced cytokine production and to compare the LPS-neutralizing capacity of Lp(a) with that of LDL, on the basis of their cholesterol content.
LPS (Escherichia coli O55:B5) was obtained from Sigma (St. Louis, Mo.). LPS-free LDL, Lp(a), and lipoprotein-deficient plasma (LPDP) were isolated by single-spin density gradient centrifugation from EDTA-fresh human plasma, as previously described (3), with minor modifications to obtain Lp(a). For the density gradient ultracentrifugation, 0.77 g of KBr was dissolved in 2 ml of plasma from donors with high Lp(a) concentrations (>1,000 mg/liter) and overlaid by solutions with subsequent densities of 1.10 g/ml (2 ml), 1.065 g/ml (3 ml), 1.040 g/ml (3 ml), and 1.006 g/ml (2 ml). After ultracentrifugation for 22 h at 285,000 × g (L7-55; Beckman, Palo Alto, Calif.), Lp(a) was isolated on the basis of the banding pattern. Lp(a) was clearly visible between the LDL and HDL fractions. Two distinct bands were separately aspirated from this density region and were analyzed by agarose gel electrophoresis (Paragon; Beckman, Brea, Calif.). Because the lightest fraction was contaminated with LDL, only the heavier fraction was used. This fraction showed pure pre-beta mobility typical for Lp(a), slightly contaminated with small amounts of HDL and LDL. The purity of the Lp(a) fractions was 90 to 98% in all assays. After isolation, the LDL and Lp(a) fractions were dialyzed against 0.05 M phosphate buffer, pH 7.4, for at least 16 h before being used in the experiments.
Separation and stimulation of peripheral blood mononuclear cells (PBMC) were performed as described elsewhere (6). The cells were counted on a Coulter counter (Coulter Electronics, Mijdrecht, The Netherlands), and the number was adjusted to 5 × 106 cells/ml. A total of 5 × 105 cells/well were incubated in 96-well plates (Greiner B. V., Alphen a/d Rijn, The Netherlands), with or without LPS, for 24 h at 37°C. Unless otherwise indicated, the lipoprotein concentrations used were 10% of the normal LDL concentration present in the circulation, expressed as total (free plus esterified) cholesterol, and the final LPS concentration used for stimulation was 1 ng/ml, representing the same lipoprotein:LPS ratio as in patients with severe gram-negative sepsis, in which LPS levels reach 10 to 100 ng/ml (2). After collection, the samples were stored at −70°C until assayed.
To assess the capacity of Lp(a) and LDL to inhibit LPS-induced cytokine production, the lipoproteins were preincubated with various LPS concentrations (1, 5, and 10 ng/ml) before the mixture was added to PBMC, and cells were stimulated for 24 h. Secondly, the effect of various Lp(a) and LDL concentrations on LPS (1 ng/ml)-induced TNF production was investigated. The kinetics of LPS binding and neutralization by Lp(a) and LDL was assessed by preincubation of lipoproteins with LPS for various intervals of time (1, 2, 4, 8, and 24 h), before the mixtures were added to PBMC for stimulation. Data are given as percentage of the production stimulated by the mixture of LPS plus LPDP, after the corresponding preincubation interval. TNF concentrations were determined by radioimmunoassay (4). Comparison between the groups was performed by using the Mann-Whitney U test.
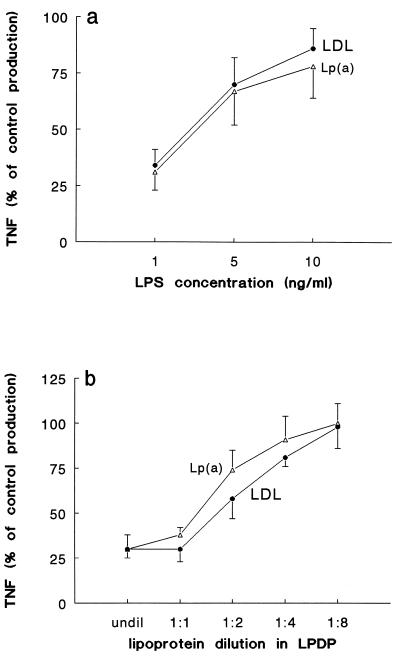
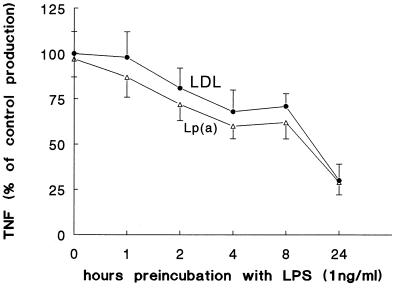
Incubation of PBMC with the various lipoprotein preparations in the absence of LPS did not induce TNF synthesis (not shown), indicating the absence of LPS contamination. When LPS in various concentrations (1, 5, and 10 ng/ml) was preincubated with Lp(a) or LDL for 24 h before being added to the cells, a significant reduction of TNF production relative to levels for the control (24-h preincubation of LPS with LPDP) was apparent, which was inversely correlated with the LPS concentration (Fig. 1a). A dose-response curve for the neutralization effect was obtained when LPS (1 ng/ml) was preincubated with various dilutions of Lp(a) and LDL in LPDP (Fig. 1b). Lp(a) and LDL had similar capacities to neutralize LPS (Fig. 1a and b). The kinetics of LPS neutralization by Lp(a) and LDL is shown in Fig. 2. The lipoproteins needed more than 8 h to neutralize half of the TNF induced by LPS, while the neutralization was more than 70% of control production when LPS was preincubated with Lp(a) or LDL for 24 h. No differences in the kinetics of LPS neutralization by Lp(a) and LDL could be observed (Fig. 2).
FIG. 1.
Lp(a) inhibits LPS-induced TNF production by human PBMC. (a) LPS in various concentrations was preincubated with Lp(a) or LDL for 24 h before being added to the cells. Lp(a) and LDL determined a significant inhibition of LPS-induced TNF production (data are given as percentage of control production in the presence of LPDP. (b) A dose response curve for the neutralization effect was obtained when LPS (1 ng/ml) was preincubated with various dilutions of Lp(a) and LDL in LPDP. The data represent means plus or minus the standard deviations of four experiments with similar results (n = 8).
FIG. 2.
The kinetics of LPS neutralization by Lp(a) and LDL. The kinetics of neutralization was investigated by preincubation of LPS with lipoproteins for various time intervals. The lipoproteins needed more than 8 h to neutralize half of the TNF production induced by LPS, while the neutralization was more than 70% when LPS was preincubated with Lp(a) or LDL for 24 h. No differences in the kinetics of LPS neutralization by Lp(a) and LDL could be observed. The data represent means plus or minus the standard deviations of three experiments with similar results (n = 6).
To our knowledge, this is the first study to show that Lp(a) is able to neutralize the biological effects of LPS, by reducing the endotoxin-stimulated TNF production by human PBMC. The capacity of Lp(a) to neutralize LPS is similar to that of LDL. The relatively slow kinetics of LPS neutralization by Lp(a), requiring at least 8 h for 50% neutralization, is similar to that reported for LDL and HDL at these high LPS levels (8). LPS binds to CD14 on the cells much faster under these conditions (9), and this may explain why endogenous lipoproteins are not able to protect against LPS toxicity in gram-negative sepsis. However, when the lipoprotein-to-LPS ratio increases, such as in hyperlipoproteinemia, the kinetics of neutralization is much faster (16a) and the host is protected against endotoxemia (17, 21). Similarly, endogenous lipoproteins are protective against lower concentrations of LPS, as demonstrated by Feingold and colleagues, who have shown increased susceptibility to LPS in hypolipoproteinemic mice (7).
The importance of LPS neutralization by Lp(a) is strengthened by the fact that Lp(a) levels in plasma vary greatly among individuals, with differences as great as 100-fold being not uncommon (22). The lipid content of Lp(a) can reach half of the lipid content of circulating LDL, and therefore, circulating Lp(a) concentration may represent an important factor for the amount of LPS neutralized by endogenous lipoproteins. The capacity of Lp(a) to neutralize LPS is not surprising, since Lp(a) is an LDL-like molecule in which only the apolipoprotein components differ, with Apo(a) being present in Lp(a) but not in LDL. The data published in the literature are controversial regarding whether the apolipoproteins or the lipid components are the most important for LPS neutralization. While some studies have suggested that ApoAI and ApoB are able to inactivate endotoxin by themselves (1, 5), others insisted on the importance of lipid components (10, 24). The similar capacities of Lp(a) and LDL to neutralize LPS and the virtually identical kinetics of LPS neutralization by the two lipoprotein subfractions strongly suggest that Apo(a) does not play an important role in the Lp(a)-LPS interaction.
An additional argument for the importance of LPS neutralization by Lp(a) is represented by the studies reporting that Lp(a) reacts as an acute-phase reactant (12, 16, 18). This cytokine-mediated response of the organism suggests that feedback modulation of Lp(a) concentration may have a role in host defense. The acute hyperlipidemic response as a component of the host defense during endotoxemia and infections is well documented (14).
REFERENCES
- 1.Berger D, Schleich S, Seidelmann M, Berger H G. Correlation between endotoxin-neutralizing capacity of plasma as tested by the limulus-amebocyte-lysate and plasma protein levels. FEBS Lett. 1990;277:33–36. doi: 10.1016/0014-5793(90)80803-q. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun J N, Halvorsen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Demacker P N M, Vos-Jansen H E, Jansen A P, van’t Laar A. Evaluation of the dual precipitation method by comparison with the ultracentrifugation method for the measurement of lipoproteins in serum. Clin Chem. 1977;23:1238–1244. [PubMed] [Google Scholar]
- 4.Drenth J P H, van Uum S H M, van Deuren M, Pesman G J, van der Ven-Jongekrijg J, van der Meer J W M. Endurance run increases circulating IL-6 and IL-1ra, but downregulates ex vivo TNF-alpha and IL-1beta production. J Appl Physiol. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- 5.Emancipator K, Csako G, Elin R J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endres S, Ghorbani R, Lonnemann G, van der Meer J W M, Dinarello C A. Measurement of immunoreactive interleukin-1beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988;49:424–438. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- 7.Feingold K R, Funk J L, Moser A H, Shigenaga J K, Rapp J H, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegel W A, Baumstark M W, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallay P, Jongeneel C V, Barras C, Burnier M, Baumgartner J-D, Glauser M P, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–5093. [PubMed] [Google Scholar]
- 10.Harris H W, Grunfeld C, Feingold K R, Rapp J H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990;86:696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubsch A P, Powell F S, Lerch P G, Doran J E. A reconstituted, apolipoprotein A-I containing lipoprotein reduces tumor necrosis factor release and attenuates shock in endotoxemic rabbits. Circ Shock. 1993;40:14–23. [PubMed] [Google Scholar]
- 12.Kawade M, Maeda S, Abe A, Yamashiro M. Alterations in plasma Lp(a) lipoprotein (Lp(a)) and acute phase proteins after surgical operations. Clin Chem. 1984;30:941–947. [Google Scholar]
- 13.Levine D M, Parker T S, Donelly T M, Walsh A, Rubin A L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao W, Claes-Henrik F. Hyperlipidemic response to endotoxin-a part of the host-defence mechanism. Scand J Infect Dis. 1993;25:675–682. doi: 10.3109/00365549309008562. [DOI] [PubMed] [Google Scholar]
- 15.Lynn W A, Cohen J. Adjunctive therapy for septic shock: a review of experimental approaches. Clin Infect Dis. 1995;20:143–158. doi: 10.1093/clinids/20.1.143. [DOI] [PubMed] [Google Scholar]
- 16.Magnani B, Massone P P B, Meriggi F, di Jeso F. The variation of serum lipoprotein (a) during surgical operations. Clin Chim Acta. 1992;212:149–151. doi: 10.1016/0009-8981(92)90182-p. [DOI] [PubMed] [Google Scholar]
- 16a.Netea, M. G., P. N. M. Demacker, B. J. Kullberg, L. E. H. Jacobs, T. J. G. Verver-Jansen, O. C. Boerman, A. F. H. Stalenhoef, and J. W. M. Van der Meer. Bacterial lipopolysaccharide binds and stimulates cytokine-producing cells before neutralization by endogenous lipoproteins can occur. Cytokine, in press. [DOI] [PubMed]
- 17.Netea M G, Demacker P N M, Kullberg B J, Boerman O C, Verschueren I, Stalenhoef A F H, Van der Meer J W M. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe Gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noma A, Abe A, Maeda S, Seishima M, Makino K, Yano Y, Shimokawa K. Lp(a): an acute phase reactant? Chem Phys Lipids. 1994;67–68:411–417. doi: 10.1016/0009-3084(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 19.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, van der Poll T, ten Cate J W, van Deventer S J H. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker T S, Levine D M, Chang J C C, Laxer J, Coffin C C, Rubin A L. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun. 1995;63:253–258. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read T E, Grunfeld C, Kumwenda Z L, Calhoun M C, Kane J P, Feingold K R, Rapp J H. Triglyceride-rich lipoproteins prevent septic death in rats. J Exp Med. 1995;182:267–272. doi: 10.1084/jem.182.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanu A M. Lipoprotein (a). A genetic risk for premature coronary heart disease. JAMA. 1992;267:3326–3329. doi: 10.1001/jama.267.24.3326. [DOI] [PubMed] [Google Scholar]
- 23.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 24.van Lenten B J, Fogelman A M, Haberland M E, Edwards P A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1986;83:2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]