Abstract
The lethal factor (LF) protein of Bacillus anthracis lethal toxin contains the thermolysin-like active-site and zinc-binding consensus motif HEXXH (K. R. Klimpel, N. Arora, and S. H. Leppla, Mol. Microbiol. 13:1093–1100, 1994). LF is hypothesized to act as a Zn2+ metalloprotease in the cytoplasm of macrophages, but no proteolytic activities have been previously shown on any target substrate. Here, synthetic peptides are hydrolyzed by LF in vitro. Mass spectroscopy and peptide sequencing of isolated cleavage products separated by reverse-phase high-pressure liquid chromatography indicate that LF seems to prefer proline-containing substrates. Substitution mutations within the consensus active-site residues completely abolish all in vitro catalytic functions, as does addition of 1,10-phenanthroline, EDTA, and certain amino acid hydroxamates, including the novel zinc metalloprotease inhibitor ZINCOV. In contrast, the protease inhibitors bestatin and lysine CMK, previously shown to block LF activity on macrophages, did not block LF activity in vitro. These data provide the first direct evidence that LF may act as an endopeptidase.
Lethal toxin (LeTx) is a vital virulence factor of Bacillus anthracis and has been postulated to act as a Zn2+ protease mediating the fatal symptoms observed during anthrax infections by hyperstimulation of host macrophage inflammatory pathways (5, 6, 8, 10). LeTx is an A-B toxin comprised of two distinct proteins. Protective antigen (PA; 735 residues, 82.6 kDa) serves as the B moiety, directing binding to cellular membrane receptors and translocation of its catalytic partners into the cytoplasm (5, 11). Lethal factor (LF; 776 residues, 90.2 kDa) acts as the A moiety (5, 11). Evidence presented by Klimpel et al. demonstrates that LF is a zinc-binding protein which contains the HEXXH motif in its carboxy-terminal (activity) region at residues 686 to 690 (LF686–690) (10). They hypothesized that LF requires zinc for activity and perhaps functions as a Zn2+-dependent protease, thus having functions similar to those of, and having an active-site motif in common with, the botulinum and tetanus neurotoxins, albeit with differing cell tropisms, target substrates, and disease sequellae (16). Here, we demonstrate LF-specific, Zn2+-dependent cleavage of synthetic peptides in vitro. These data, as well as those from protease inhibitor profiles, metal ion substitution studies, and mutational analysis of residues within LF686–690 that arrest activity, strongly support LF as demonstrating the activities of a Zn2+-dependent neutral endoprotease.
Anthrax toxin purification.
LF, PA, and mutants were purified either from B. anthracis Sterne or as recombinant proteins from Escherichia coli (6–8, 14). B. anthracis cultures were grown in defined RM toxin production medium (13). Culture supernatants were sterilized by passage through a 0.22-μm-pore-size filter (Millipore, Bedford, Mass.) and concentrated to 500 ml with the Minitan ultrafiltration system (Millipore). Ammonium sulfate was added to 75%, and the protein pellet was collected and suspended in 20 mM Tris-HCl (pH 8.0) and dialyzed extensively against the same buffer. Very efficient purification was performed by MonoQ anion-exchange fast-performance liquid chromatography (FPLC) (Pharmacia Biotech, Piscataway, N.J.). PA eluted at 130 to 140 mM NaCl, and LF eluted at 250 to 270 mM NaCl. The recombinant proteins expressed in pET15b are produced with an amino-terminal hexa-histidine tag, allowing purification by FPLC affinity chromatography on a HiTrap (Pharmacia Biotech) chelating column. Cultures of E. coli BLR(DE3)/pET15b-LF (or indicated LF mutants) were grown in Luria broth containing ampicillin (100 μg ml−1) to an optical density at 600 nm of 0.7 to 1.0, and expression was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) for 12 h at 18°C. Cell lysates were prepared by French press, cleared by centrifugation, and injected via FPLC (Pharmacia Biotech) onto a HiTrap column charged with Ni2+. Recombinant histidine-tagged LF (wild type [wt] or mutant) eluted at approximately 100 mM imidazole. Eluted protein was further purified by gel filtration on a 320-ml Sephacryl-200 FPLC column. By these methods, PA and LF were each determined to be 95 to 99% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. PA and LF proteins were assayed for cytoxicity activities by a standard 51Cr release assay of a sensitive macrophage cell line (RAW 264.7 ATCC TIB-71) (11, 13). Mutant proteins LFE687C, LFH686A, LFH690A, and LFH686A+H690A were obtained from Kurt Klimpel (10) as direct mutants of wt anthrax LF. LFE687D was created as a recombinant mutant in E. coli BLR(DE3) by using the pET15b plasmid and purified as described above.
Protease assay conditions.
Oligopeptides were obtained from Sigma. The reaction buffer used was 25 mM potassium phosphate (pH 7.0) containing 20 μM ZnSO4 and 20 μM CaCl2. Each reaction mixture contained as indicated from 250 μM to 1 mM substrate and 0.25 to 2.8 μM enzyme (23 to 250 μg/ml) in a final volume of 100 μl. After incubation at 37°C for 3 to 24 h (as indicated), the reactions were quenched with 1 μl of 10 M HCl and the mixtures were injected onto a C18 HP Hypersil-octyldecyl silane column (100 by 4.6 mm, 120-Å pore size, 5-μm particle size) or a C8 Rainin Microsorb-MV column (250 by 4.6 mm, 300-Å pore size, 5-μm particle size) with a Hewlett-Packard 1050 high-performance liquid chromatography (HPLC) system. The aqueous phase used with the C18 column was 25 mM phosphate buffer (pH 7.5) with 2% (vol/vol) methanol (MeOH) and 2% (vol/vol) tetrahydrofuran, and the mobile phase was 100% MeOH. The aqueous phase used with the C8 column was 0.1% (vol/vol) trifluoroacetic acid (TFA) in water, and the mobile phase was 0.1% (vol/vol) TFA in acetonitrile. Peptide peaks were detected by UV absorption at 215 and 274 nm. For further analysis by mass spectrometry or protein sequencing, peaks were collected as they eluted. For kinetic studies, reaction mixtures of 100 μM to 1 mM substrate with 250 nM LF were run at 25°C. Samples were injected into the HPLC at 3-h intervals. Velocities were then calculated by measuring the reduction of the starting substrate peak over time or by measuring the formation of product peaks over time. In inhibitor studies, enzyme and inhibitors were coincubated at room temperature for 1 h before addition of substrate. For studies with chelating inhibitors, there was no addition of exogenous metals. Otherwise, the conditions were the same as those described above. For pH studies, 250 ng of LF was added to 500 μM substrate in a buffer of 25 mM phosphate with pHs ranging from 5.8 to 8.2. Phosphate buffers were made from appropriate amounts of mono- and dibasic sodium phosphate. Reactions were analyzed after 4 h at 37°C as described above. Results indicated a pH optimum of approximately 6.75, with less than 5% activity at pH 5.5 (results not shown). Thus, the addition of HCl to reaction mixtures was determined to be the best way to quench reactions before HPLC analysis. To create apo-enzyme (no bound metals), purified LF was dialyzed against 10 mM EDTA–1 mM 1,10-phenanthroline for 24 h at 4°C. The proteins were then dialyzed against 20 mM Tris (pH 7.5) in ultrapure water (≥15 MΩ), for 24 h at 4°C, with four buffer changes.
Peptide fragment analysis.
Peptides purified by reverse-phase HPLC (RP-HPLC) were analyzed by time-of-flight mass spectrometry. The mass spectrum was recorded by using nitrocellulose targets (9) in an Applied Biosystems Bio-Ion plasma-desorption instrument. The spectrum was accumulated for 106 fission events corresponding to approximately 10 min. Further details of the instrumentation and spectral analysis have been described elsewhere (19). Peptide analysis was kindly performed by the Duke Comprehensive Cancer Center Facility, directed by Jan J. Enghild.
Hydrolysis of synthetic peptides.
To directly assess whether LF is capable of endopeptidase activity, synthetic peptides (or p-nitroanilide-derivatized peptides) as well as a variety of purified proteins were obtained as test substrates in an arbitrary manner. However, the lengths of the synthetic peptides ranged from 2 to 39 residues with efforts to vary both amino acid composition and primary sequence. The overwhelming majority of these substrates were not affected by LF in any discernible way, although they were cleaved by appropriate control proteases (e.g., trypsin, pronase, thermolysin) (data not shown). A complete list of substrates tested in this study is available upon request.
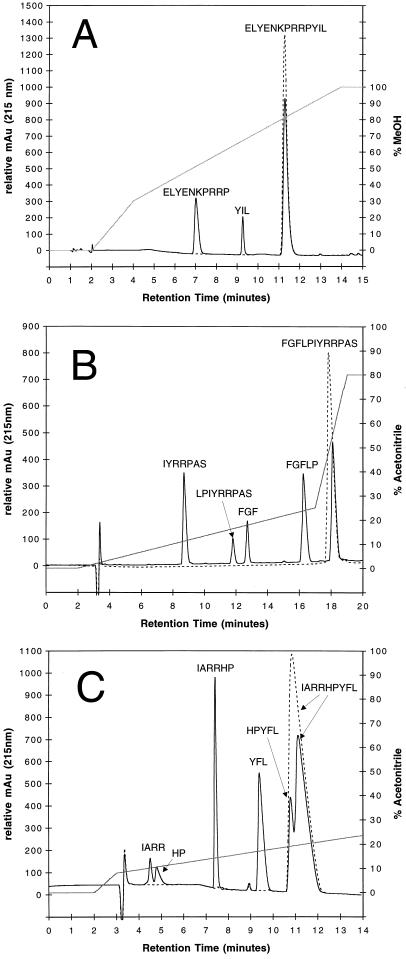
Sixteen oligopeptides 6 to 21 residues in length containing a large variety of amino acids were then obtained and assayed as LF substrates (Table 1). Results presented in Fig. 1 indicate LF cleavage of three peptides. Data illustrated in Fig. 1A show significant hydrolysis of peptide 1 (with ELYENKPRRPYIL hydrolyzed into ELYENKPRRP and YIL) after incubation with LF. The kcat/Km for this reaction was calculated to be 8.9 s−1 M−1. Peptide 2, FGFLPIYRRPAS, was hydrolyzed by LF into the major products FGFLP and IYRRPAS, as well as minor products FGF and LPIYRRPAS (Fig. 1B). The kcat/Km for this reaction was calculated to be 29.4 s−1 M−1. It is important to note that this value reflects the consumption of substrate by both major and minor reactions. Peptide 3, IARRHPYFL, was hydrolyzed by LF into the major products IARRHP and YFL and the minor products IARR and HPYFL (Fig. 1C). Kinetics were not determined for peptide 3; however, the cleavage of peptide 3 occurs faster than that of either peptide 1 or peptide 2 and could have a kcat/Km value greater than 50 s−1 M−1. Unlike peptide 2, in which FGFLP is not split into FGF and LP, the peptide 3 major product IARRHP is hydrolyzed to IARR and HP. These three proteins have some sequence homology at their major cleavage sites, the most notable of which is proline at the new C terminus. The requirement for additional amino acids at key positions is clear from peptides that contain proline but show no signs of cleavage. Additionally, tyrosine is consistently present at or adjacent to the new N-terminal side of the cleavage site. Whether LF requires a tyrosine or simply a bulky uncharged residue is still under investigation. Cleavage of peptide 4 (YGGFLRRI) into YGGFLR and RI is similar to the secondary cleavage of peptide 3, that is, IARRHP to IARR and HP, and occurs at approximately the same rate. Cleavage of peptide 5 (DRVYIHPFHL) occurs extremely slowly into the three products DRV, YIHP, and FHL. No evidence of possible intermediates DRVYIHP and YIHPFDL could be found. Due to the very poor nature of this substrate, drawing similarities between this reaction and the others, while possible, may lead to incorrect assumptions.
TABLE 1.
Peptide substrates used to measure protease activity of anthrax LF
| Cleaved peptidesa | kcat/Km (s−1 M−1)b |
|---|---|
| IARR/HP/YFL | 50* |
| FGF/LP/IYRRPAS | 29.4 |
| ELYENKPRRP/YIL | 8.9 |
| YGGFLR/RI | 5* |
| DRV/YIHP/FHL | 0.3* |
| ELAGAPPEPA | NR |
| YFLFRPRN | NR |
| EGLPPRPKIPP | NR |
| LRRASLG | NR |
| SYSMEHFRWG | NR |
| IVPFLGPLLGLLT | NR |
| GWTLQSAGYLLGPNNFFGLM | NR |
| ESPLIAKVLTTEPPIITPVRR | NR |
| HDMNKVLDL | NR |
| HLGLAR | NR |
A slash indicates a known cleavage site as determined by mass spectrometry or peptide sequencing. Peptides were initially chosen at random until the peptide FGFLPIYRRPAS was found to cleave. From then on, peptides were selected for their potential to react with LF.
An asterisk indicates that the kcat/Km value is estimated. NR, no reaction detected. Kinetics were determined at 25°C with 500 μM substrate and 23 ng of LF/ml (250 nM), and values are averages of two experiments.
FIG. 1.
HPLC elution profiles of synthetic peptides cleaved by anthrax LF. The dashed line represents 500 μM substrate alone without LF, and the solid black line represents substrate plus LF. The gray line indicates percent mobile phase. All reactions occurred at pH 7.0 in 25 mM phosphate buffer. (A) RP-HPLC elution profile of the reaction with peptide 1 (ELYENKPRRPYIL) and 23 ng of LF per ml after 18 h at 37°C. The HPLC running buffer contained 25 mM potassium phosphate (pH 7.5), 2% MeOH, and 2% tetrahydrofuran. (B) Elution profile of the reaction with peptide 2 (FGFLPIYRRPAS) and 250 ng of LF per ml after 4 h at 37°C. The HPLC running buffer was 0.1% TFA in water. The mobile phase was 0.1% TFA in acetonitrile. (C) Elution profile of the reaction with peptide 3 (IARRHPYFL) and 250 ng of LF per ml after 90 min at 37°C. The HPLC running buffer was 0.1% TFA in water. The mobile phase was 0.1% TFA in acetonitrile. All reactions were monitored by UV absorption at 215 nm (see text for more details). The HPLC flow rate was 1.0 ml/min. These results are typical examples of experiments repeated many times.
The calculated reaction rates are considerably lower than what is normally seen in general metalloproteases such as thermolysin. This may not be unusual considering that tetanus and botulinum neurotoxins, zinc metalloproteases with which LF has active-site homology, are unable to cleave small peptides corresponding to the cleavage sites of their targets. It has been hypothesized that three-dimensional structure plays a more important a role in target specificity than linear amino acid sequence with these neurotoxins (16). It is possible that LF may recognize important structural elements of its target rather than primary sequence, and the relatively slow cleavage of these peptides may demonstrate LF’s restriction of its active site to its pertinent cellular target(s). Alternatively, in vivo conditions found within the macrophage may somehow modify LF to a more active form (e.g., phosphorylation and nicking, etc.).
Inhibitor profiles.
LF cleavage of peptides was completely inhibited by the addition of either 1 mM 1,10-phenanthroline or 10 mM EDTA, both of which chelate zinc (Table 2). Activity was partially inhibited by 5 μM EGTA. This indicates that certain metal ions are essential for LF activity in vitro. LF that was cleared of metals through dialysis with EDTA and phenanthroline (see Materials and Methods) showed no propensity to cleave peptides. As individual metals are added back to LF, it is clear that both zinc and calcium are essential for full protease activity (Fig. 2). Additionally, specific amino acid hydroxamates, selective inhibitors of zinc metalloproteases, have been shown to inhibit LF both in vitro and in vivo. Amino acid hydroxamates are reversible inhibitors that fit the enzyme’s active site while chelating the zinc ion (3). Tyrosine and leucine hydroxamate showed the best in vitro inhibition with 50% inhibitory concentrations of approximately 300 and 350 μM, respectively. Additionally, ZINCOV, a novel hydroxamate, inhibits LF in vitro protease activity at 350 μM and completely protects macrophages in vivo at a concentration of 500 μM (in vivo data not shown). The ability of hydroxamates to inhibit LF supports LF having zinc metalloprotease activity. Further, the specific ability of tyrosine and leucine hydroxamates to inhibit LF may implicate these amino acids as important for active site binding. Previously, relatively high concentrations (200 μM) of the protease inhibitors bestatin and lysine CMK were shown to protect cultured macrophages from lysis by anthrax LeTx (10). In contrast, bestatin and lysine CMK did not inhibit in vitro LF proteolysis of peptides 1 and 2 at concentrations ranging from 50 μM to 1 mM (Table 2), suggesting that the protective effect of these inhibitors observed with LF-challenged cultured cells might not be due to direct inhibition of the toxin’s enzymatic activity but rather to some other event in the cytolytic cascade. As an example of this phenomenon, in lipopolysaccharide-stimulated monocytes, inhibitors of metalloproteases were observed to block maturation and release of shock-inducing cytokines (15). It is possible that bestatin and lysine CMK inhibit an event downstream from initial target cleavage by LF. Other classes of protease inhibitors, such as phenylmethylsulfonyl fluoride, N-succinyl-l-proline, tosyl lysine CMK, tosyl phenylalanine CMK, and nitrobestatin, did not inhibit LF activity in vitro (Table 2).
TABLE 2.
Chelator and protease inhibitor profile of anthrax LF
| Metal chelator or protease inhibitor | Concn | % Initial activitya |
|---|---|---|
| Metal chelators | ||
| EDTA | 10 mM | 0 |
| EDTA | 5 mM | 6.9 |
| EGTA | 5 mM | 19.7 |
| 1,10-Phenanthroline | 1 mM | 0 |
| Protease inhibitors | ||
| Alanine hydroxamate | 500 μM | >99 |
| Arginine hydroxamate | 1 mM | >99 |
| Glutamate γ-hydroxamate | 10–500 μM | >99 |
| Glycine hydroxamate | 500 μM | >99 |
| Isoleucine hydroxamate | 500 μM | >99 |
| Leucine hydroxamate | 350 μM | 50 |
| Phenylalanine hydroxamate | 300 μM | 50 |
| Tyrosine hydroxamate | 650 μM | 50 |
| ZINCOV | 350 μM | 50 |
| Amastatin | 100 μM | >99 |
| Aprotinin | 0.05 U | >99 |
| l-Arginine | 500 μM | >99 |
| Bestatin | 50 μM–1 mM | >99 |
| Calpeptin | 75 μM | >99 |
| Captopril | 1 mM | >99 |
| Lysine CMK | 0.1–1 mM | >99 |
| Nitrobestatin | 10 μM | >99 |
| N-Succinyl-l-Proline | 100 μM | >99 |
| PMSFb | 100 μM | >99 |
| Phosphoramidon | 500 μM | >99 |
| Tosyl lysine CMK | 100 μM | >99 |
| Tosyl phenylalanine CMK | 10 μM | >99 |
| Trifluoracetyl LysPro | 100 μM | >99 |
| Sodium dodecyl sulfate | 1% | 0 |
A 500 μM concentration of peptide 1 (ELYENKPRRPYIL) was mixed with 250 nM of LF mutant and allowed to react for 18 h at 37°C. Samples were then analyzed on an HPLC as described in Materials and Methods. The areas of the substrate and product peaks were measured and compared to that of substrate controls. The results are averages of duplicate experiments.
PMSF, phenylmethylsulfonyl fluoride.
FIG. 2.
In vitro protease activity of metal-reconstituted anthrax LF. Comparison of the areas of the major product peaks (see Fig. 1A, peak labeled ELYENKPRRP) created from the reaction with peptide 1 (ELYENKPRRPYIL) and LF. Reactions of 50 ng of LF per ml with 1 mM ELYENKPRRPYIL occurred at pH 7.0 in 25 mM potassium phosphate buffer containing a 100 μM concentration of the indicated metal(s). Reactions were monitored as described in the text. All metals were chloride salts, except for zinc and nickel which were sulfate salts. After 9 h at 37°C, reaction mixtures were injected into the HPLC. It is important to note that the first three columns indicate the need for both zinc and calcium ions for full catalysis. No reaction was ever observed in the absence of metals, or with calcium alone, cobalt alone, magnesium alone, manganese alone, or nickel alone. The fastest reaction consistently was that in which only zinc and calcium were added. These results are an average of two experiments.
Active-site mutations.
Amino acid substitutions in LF686–690 (the HEXXH motif) were previously shown to be incapable of killing cultured macrophages, suggesting that this region is important for cytotoxicity and perhaps is involved in catalytic function (10). To determine whether this consensus thermolysin-like active-site motif may be directly involved with cleavage of peptides, and to ensure that all hydrolytic activities observed in our assays are specific to LF, amino acid substitutions in LF686–690 were tested for the ability to cleave peptides 1 and 2. Mutant toxin molecules assayed were LFE687C, LFE687D, LFH686A, LFH690A, and LFH686A+H690A. The recombinant mutants proteins were purified as stable, full-length molecules as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis with high-titer polyclonal antitoxin. All histidine mutants were found to have decreased zinc binding as compared to that of wt LF and were unable to kill macrophages in LeTx assays (10; also data not shown). All mutants were mixed individually with peptide 1, 2, or 3 to determine hydrolytic activity. No significant reduction in substrate concentration or generation of cleavage product was observed by RP-HPLC even after 18 h at 37°C (Table 3). This indicates that LFH686 and LFH690 are important residues for both Zn2+ binding and catalysis as well as cytotoxicity. The LFE687C mutant was found to bind zinc at a level equal to that of wt LF yet was also unable to kill cultured cells in LeTx assays (10; also data not shown). However, as with the histidine mutants, LFE687C showed no observable cleavage of peptide 1 even after incubation for 18 h at 37°C (Table 3). Additionally, work with our mutant LFE687D showed no ability to cleave peptides in vitro, even at a very high concentration of enzyme. This mutant was also unable to kill macrophages in vivo (data not shown). This indicates that LFE687 is important for cytotoxicity and catalysis but not for Zn2+ binding. Importantly, the loss of hydrolytic activities associated with point mutations in the active-site region acts as a control for assignment of hydrolytic functions to LF and not to some undefined contaminating protease.
TABLE 3.
Proteolytic activity of anthrax LF mutants
| Enzyme | % Cleavage of peptide 1a |
|---|---|
| LF (wt) | 85.9 |
| LF (recombinant) | 82.1 |
| LFH686A | 3.4 |
| LFH690A | 3.2 |
| LFH686A+H690A | 3.0 |
| LFE687C | 2.4 |
| LFE687D | 0 |
| BSAb | 0 |
| Trypsin | 100 |
| Pronase | 100 |
A 500 μM concentration of peptide 1 (ELYENKPRRPYIL) was mixed with 23 ng (250 nM) of enzyme and allowed to react for 18 h at 37°C. Samples were analyzed on an HPLC as described in Materials and Methods. The areas of the substrate and products peaks were measured and compared to that of substrate controls (no enzyme). The results are averages of duplicate experiments.
BSA, bovine serum albumin.
There were other supporting data for all activities being specific to LF, namely, that LF purified from the supernatant of B. anthracis and recombinant LF purified from E. coli-soluble extract maintain identical substrate specificities and kinetics (data not shown). For these reasons, it is clear that the observed proteolytic activity is exclusively a property of LF, not of contaminating proteases.
Near the center of the primary amino acid sequence of LF (residues 315 to 416) are five homologous repeats, each 19 amino acids long (Fig. 3). These repeats were first investigated by Quinn et al. (18), who discovered that dipeptide insertions into this region resulted in unstable gene products. Recently, these repeats were proposed to form an EF-hand calcium binding motif (17a; motif reviewed in reference 17) through alignments with sequences of other EF-hand-containing proteins, such as parvalbumin, and an EF-hand consensus sequence (reviewed in reference 17). The results of current metal ion reconstitution experiments are in agreement with this EF-hand hypothesis and clearly indicate the need for calcium as well as the catalytic zinc to achieve maximum catalysis. EF-hand motifs are the most common calcium binding motifs which are involved mainly in regulation (e.g., calmodulin) and calcium buffering (e.g., parvalbumin) (2). However, only a few EF-hand motifs have been found in prokaryotes, calerythrin being the only well-documented example (1). For this reason, gene transfer has been suggested for the existence of EF-hand motifs in prokaryotes (2). Since LF is likely to contain an EF-hand motif and since gene transfer of bacterial toxins has been a popular hypothesis, especially of ADP-ribosylation toxins (18), it is inviting to suggest that gene transfer is involved with LF. A curious coincidence is that edema factor, an adenylate cyclase that serves as another A domain in anthrax toxin, requires, as a cofactor, calmodulin, an EF-hand-containing protein (12).
FIG. 3.
Known LF domains. The first 255 amino acids are involved in binding to PA, and this region has been termed LFn. This region has a high degree of homology to the first 255 amino acids of anthrax edema factor, which also binds PA. Residues 315 to 416 contain five repeat regions that follow the consensus sequence for two EF-hand calcium-binding motifs (e.g., calmodulin). Residues 686 to 690 contain a thermolysin-like zinc metalloprotease motif HEXXH. Additionally, residues 745 to 749 (HSTDH), which are similar to the inverted zinc metalloprotease motif HXXEH (e.g., insulin-degrading enzyme), could potentially act as another zinc site, either structural or enzymatic.
B. anthracis LeTx is the major virulence factor responsible for symptoms associated with systemic anthrax (reviewed in references 5 and 11). Although LF, through its association with PA, can bind to and enter the cytoplasm of most cells tested, only macrophages seem to be affected (4). In macrophages, LF induces hyperstimulation of the oxidative burst, expression of proinflammatory cytokines tumor necrosis factor alpha and interleukin 1β, and cytolysis (6, 8). The release of these potent host mediators are responsible for the dramatic hypotension, shock, and death of the victim (6, 8, 11). It is interesting to speculate that the proteolytic activities associated with LF cleave some cytoplasmic protein responsible for regulation of macrophage inflammatory processes. The exact nature of pertinent cellular LF targets and whether proline specificity is maintained within these targets remain to be determined.
Acknowledgments
We are grateful to Sylvia Hill for providing expert technical assistance and to Carlo Petosa, Terry Dixon, and John Ireland for useful discussions.
This study was supported in part by National Institutes of Health grants AI08649 and AI40644, American Cancer Society grant ACS-IRG158K, and funds from the Duke University Medical Center.
REFERENCES
- 1.Bylsma N, Drakenberg T, Anderson I, Leadlay P F, Försen S. Prokaryotic calcium-binding protein of the calmodulin superfamily. Calcium binding to a Saccharopolyspora erythraea 20kDa protein. FEBS Lett. 1992;299:44–47. doi: 10.1016/0014-5793(92)80096-y. [DOI] [PubMed] [Google Scholar]
- 2.Celio M R. Guidebook to the calcium-binding proteins. New York, N.Y: Oxford University Press; 1996. [Google Scholar]
- 3.Chan W W C, Dennis P, Demmer W, Brand K. Inhibition of leucine aminopeptidase by amino acid hydroxamates. J Biol Chem. 1982;257:7955–7957. [PubMed] [Google Scholar]
- 4.Friedlander A M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 5.Hanna P C. Anthrax pathogenesis and host response. Curr Top Microbiol Immunol. 1998;225:13–35. doi: 10.1007/978-3-642-80451-9_2. [DOI] [PubMed] [Google Scholar]
- 6.Hanna P C, Acosta D, Collier R J. On the role of macrophages in anthrax. Proc Natl Acad Sci USA. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna P C, Kochi S, Collier R J. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol Biol Cell. 1992;3:1269–1277. doi: 10.1091/mbc.3.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna P C, Kruskal B A, Allen R, Ezekowitz B, Bloom B R, Collier R J. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol Med. 1994;1:7–18. [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson G P, Hodin A B, Häkansson P L, Sundqvist B V R, Säve B G S, Nielson P F, Roepstorff P, Johansson K E, Kamensky I, Lindborg M S L. Plasma desorption mass spectrometry of peptides and proteins adsorbed on nitrocellulose. Anal Chem. 1986;58:1084. [Google Scholar]
- 10.Klimpel K R, Arora N, Leppla S H. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol Microbiol. 1994;13:1093–1100. doi: 10.1111/j.1365-2958.1994.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 11.Leppla S H. Anthrax toxins: bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. [Google Scholar]
- 12.Leppla S H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eukaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- 13.Leppla S H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- 14.Milne J C, Blanke S R, Hanna P C, Collier R J. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol Microbiol. 1995;15:661–666. doi: 10.1111/j.1365-2958.1995.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohler K M, Sleath P R, Fitzner J N, Cerretti D P, Alderson M, Kerwar S S, Torrance D S, Otten-Evans C, Greenstreet T, Weerawarna K, Kronheim S R, Petersen M, Gerhart M, Kozlosky C J, March C J, Black R A. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 16.Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama S, Kretsinger R H. Evolution of EF-hand proteins. Annu Rev Biophys Biomol Struct. 1994;23:473–507. doi: 10.1146/annurev.bb.23.060194.002353. [DOI] [PubMed] [Google Scholar]
- 17a.Petosa, C. Personal communication.
- 18.Quinn C P, Singh Y, Klimpel K R, Leppla S H. Functional mapping of anthrax toxin lethal factor by in-frame insertion mutagenesis. J Biol Chem. 1991;266:20124–20130. [PubMed] [Google Scholar]
- 19.Sundqvist B, Kamensky I, Hakansson P, Kjellberg J, Salehpour M, Widdiyasekera S, Fohlman J, Peterson P A, Roepstorff P. Californium-252 plasma desorption time of flight mass spectroscopy of proteins. Biomed Mass Spectrom. 1984;11:242–257. doi: 10.1002/bms.1200110509. [DOI] [PubMed] [Google Scholar]