Abstract
Cancer patients have increased thrombosis and bleeding compared with the general population. Cancer is associated with activation of both platelets and coagulation. Mouse models have been used to study the dysregulation of platelets and coagulation in cancer. We established a mouse model of pancreatic cancer in which tissue factor-expressing human pancreatic tumors (BxPC-3) are grown in nude mice. Tumor-bearing mice have an activated coagulation system and increased venous thrombosis compared to control mice. We also showed that tumor-derived, tissue factor-positive extracellular vesicles activated platelets ex vivo and in vivo. In this study, we determined the effect of tumors on a platelet-dependent arterial thrombosis model. Unexpectedly, we observed significantly reduced carotid artery thrombosis in tumor-bearing mice compared to controls. In addition, we observed significantly increased tail bleeding in tumor-bearing mice compared to controls. These results suggested that the presence of the tumor affected platelets. Indeed, tumor-bearing mice exhibited a significant decrease in platelet count and an increase in mean platelet volume and percentage of reticulated platelets, findings that are consistent with increased platelet turnover. Levels of the platelet activation marker platelet factor 4 were also increased in tumor-bearing mice. We also observed decreased platelet receptor expression in tumor-bearing mice and reduced levels of active αIIb/β3 integrin in response to PAR4 agonist peptide and convulxin in platelets from tumor-bearing mice compared with platelets from control mice. In summary, our study suggests that in tumor-bearing mice there is chronic platelet activation, leading to thrombocytopenia, decreased receptor expression, and impaired platelet adhesive function.
Keywords: bleeding, cancer, extracellular vesicles, platelet, thrombosis
Graphical Abstract:
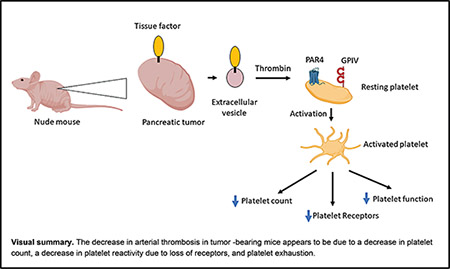
Introduction
Cancer patients have a dysregulated hemostatic system and experience both increased thrombosis and bleeding compared with the general population.1–3 A recent study determined the rates of thrombocytopenia (<100,000/μL) and severe thrombocytopenia (<50,000/μL) in >3,500 patients with cancer-associated thrombosis.4 Rates of thrombocytopenia for solid tumors and hematologic malignancies were 22 and 47%, respectively, and rates of severe thrombocytopenia for solid tumors and hematologic malignancies were 7 and 30%.4 In addition, cancer patients with venous thromboembolism (VTE) receiving anticoagulant therapy have increased rates of VTE and major bleeding compared to a general population of VTE patients.5,6
Many studies have measured biomarkers of platelet activation including soluble P-selectin, soluble CD40 ligand, platelet factor 4 (PF4), and thrombospondin-1, in a variety of cancer patients.7 One study analyzed these biomarkers in the same cohort of cancer patients and found an increase in thrombospondin-1 but not the other biomarkers compared to healthy controls.8 However, levels of soluble P-selectin were higher in cancer patients with VTE compared with healthy controls, which is consistent with findings from an earlier study.8,9 Interestingly, a study with pancreatic cancer patients found that the level of PF4 was associated with an increased risk for VTE and poor prognosis.10 Another study analyzed the responsiveness of platelets from cancer patients to ex vivo stimulation with a variety of agonists and found that patients with decreased platelet reactivity had a poor prognosis.11 Taken together, these studies indicate that platelets are activated in cancer patients.
Activation of platelets and the coagulation system in cancer patients can lead to disseminated intravascular coagulation (DIC).12 One study found that 6.8% of cancer patients with solid tumors had DIC.13 Some of the most common coagulation abnormalities observed in cancer patients with DIC were thrombocytopenia and elevated D-dimer.13 DIC observed in cancer patients is typically less intense compared with DIC in other clinical settings, such as sepsis, and proceeds more slowly and can remain asymptomatic.12
Cancer cells release extracellular vesicles (EVs) that contain podoplanin and tissue factor (TF) that can directly and indirectly activate platelets.14–17 We found that TF+ EVs derived from the human pancreatic cancer cell line BxPC-3 activated platelets ex vivo in a thrombin-dependent manner.18
Tumor-bearing mice have been used to study mechanisms of thrombosis in cancer.19 We and others found that mice bearing either murine TF-expressing pancreatic tumors (Panc02) or human TF-expressing pancreatic tumors (HPAF-II and BxPC-3) have increased levels of circulating TF+ EVs, an activated coagulation system, and increased venous thrombosis compared to control mice.20–24 Further-more, we found that injection of BxPC-3-derived TF+ EVs increased PF4 in mice, which indicated platelet activation, and other studies found that injected TF+ EVs enhanced venous thrombosis.18,21,24–26 One study found that the platelet inhibitor clopidogrel reduced thrombosis in mice bearing Panc02 tumors.27 We also found that administration of the platelet inhibitor clopidogrel or a deficiency of the thrombin receptor protease-activated receptor 4 (PAR4) reduced exogenous BxPC-3-derived TF+ EV-dependent enhancement of venous thrombosis.18 These data indicate that platelets are activated in mice bearing human and murine pancreatic tumors.
In this study, we analyzed carotid arterial thrombosis in mice bearing BxPC-3 tumors. Based on our previous studies, we hypothesized that tumor-bearing mice would have increased arterial thrombosis compared with controls. Unexpectedly, however, we observed that tumor-bearing mice had decreased arterial thrombosis. In addition, tumor-bearing mice had increased tail bleeding, increased levels of the platelet activation marker PF4, a decrease in platelet count, and a decrease in receptor expression on platelets.
Methods
Mouse Tumor Model
We used a human pancreatic cancer cell line called BxPC-3 that expresses a high level of TF and a low level of podoplanin (J. Geddings and N. Mackman, 2016, unpublished data).18 Cells were stably transfected with a vector expressing the firefly luciferase reporter to allow noninvasive monitoring of tumor growth in the pancreas.22 We injected 2.0 × 106 BxPC-3 cells and Matrigel into the pancreas of Crl:NU-Foxn1nu male mice (nude mice) and tumor growth was monitored by measuring luciferase expression using the IVIS spectrum imaging system (Caliper Life Sciences, Hopkinton, Massachusetts, United States).22 The median time from injection of cancer cells to experiment is 84 days (interquartile range [IQR]: 64–106). All mice were injected with the same number of cancer cells and the tumors allowed to grow until they were ≥2.0 to ≤3.2 g. Phosphate-buffered saline (PBS) and Matrigel were injected into control mice. We used male mice because we have established this model with male mice and wanted to compare the data from the current study with our previous studies.18,22,28 All animal studies were approved by the University of North Carolina at Chapel Hill Animal Care and Use Committee and complied with National Institutes of Health guidelines.
Arterial Thrombosis Model
A ferric chloride (FeCl3)-induced common carotid artery occlusion model was performed as previously described.29 Briefly, mice were anesthetized with 2% isoflurane and placed in a supine position. After isolation of the right common carotid artery, a 2 mm2 piece of filter paper soaked in FeCl3 (2.5%) was applied to the ventral surface of the exposed carotid artery for 5 minutes. We also examined injury induced with 10% FeCl3 for 2 minutes. A microvascular ultrasonic flow probe (MA0.5PSB, Transonic Systems Inc, Ithaca, New York, United States) was used to detect blood flow for 30 minutes after removal of the filter paper. Occlusion time was defined as a cessation of blood flow (0 mL/minutes) for ≥2 minutes.
Tail Bleeding
We used the tail tip amputation model as previously described.30 Mice were anesthetized with isoflurane and placed in a prone position. The distal 5 mm segment of the tail was amputated with a scalpel. The tail was immediately immersed in 50 mL falcon tube containing isotonic saline prewarmed at 37°C. The bleeding time within a 20-minute period was recorded.
Measurement of Platelet Number
Platelet number and mean platelet volume in whole blood were measured using an Element HT5 analyzer (Heska, Loveland, Colorado, United States). Platelet count was also determined by flow cytometry using an Alexa Fluor 488–labeled antibody to GPIX (clone Xia.B4, Emfret Analytics, Eibelstadt, Germany). Samples were diluted with PBS, and the number of GPIX-positive events per volume was determined. Forward scatter (FSC) was used to quantify platelet size.
Measurement of Reticulated Platelets
Reticulated platelets were measured in heparinized blood. Blood was incubated with 50 ng/mL of thiazole orange (Sigma-Aldrich, St. Louis, Missouri, United States) diluted in PBS + 2 mM EDTA for 30 minutes at room temperature followed by labeling with Alexa Fluor 647-conjugated anti-GPIX antibody. GPIX-positive events were measured by flow cytometry. To account for differences in platelet size, we analyzed our samples using FlowJo and used the derived parameter of FL1-H/FSC-H (thiazole orange signal/platelet size) to set our gates for determining the percent of platelets that are reticulated.
Blood Collection
For measurement of platelet count and plasma proteins, sodium citrate was injected into the inferior vena cava and blood was collected into syringes as described.31 Mouse platelet-poor plasma was prepared by centrifuging whole blood at 4,500 × g for 15 minutes. Plasma was frozen and stored at −80°C.
Measurement of Platelet and Endothelial Activation Markers
We measured plasma levels of different proteins by ELISA: PF4 (Mouse CXCL4/PF4 DuoSet ELISA, Cat. #DY595, R&D Systems, Minneapolis, Minnesota, United States), soluble P-selectin (Mouse P-selectin/CD62P DuoSet ELISA, Cat. #DY737, R&D Systems), soluble VCAM-1 (Mouse VCAM-1/CD106 Quantikine ELISA kit, Cat. #MVC00, R&D Systems), and soluble ICAM-1 (Mouse ICAM-1/CD54 Quantikine ELISA kit, Cat. #MIC100, R&D Systems).
Measurement of Platelet Receptors and Activation
Blood (50 μL) was collected with heparin-coated capillaries (VWR, Radnor, Pennsylvania, United States) from the retro-orbital plexus. Platelet adhesion receptors were quantified by incubating diluted whole blood in a saturating concentration of PE-labeled anti-GPIbα (glycoprotein Ibα) antibody (clone Xia.G5, Emfret Analytics), Alexa Fluor 488-labeled antibody anti-GPIX antibody (clone Xia.B4, Emfret Analytics), PE-labeled anti-αIIb/β3 antibody (clone MWReg30, BD Biosciences) or Alexa Fluor 647-labeled anti-GPVI (glycoprotein VI) antibody (clone JAQ1, Emfret Analytics).
Diluted heparinized blood (modified Tyrode’s buffer containing 1 mM CaCl2) was activated or not with adenosine diphosphate (ADP; 5 and 50 μM) (Sigma-Aldrich), convulxin (25 and 50 ng/mL) (purchased from Kenneth Clemetson, Theodor Kocher Institute, University of Berne, Bern, Switzerland), or PAR4-agonist peptide (125 and 250 μM) (PAR4p; GL Biochem Inc. Shanghai, China). Levels of the activated form of murine αIIbβ3 and P-selectin were measured using either JON/A-PE (Emfret Analytics) or an Alexa Fluor-647 conjugated anti-P-selectin antibody (clone RB40.34, BD Biosciences, Franklin Lakes, New Jersey, United States). Following 15 minutes of incubation, samples were diluted with PBS and analyzed by flow cytometry. All flow cytometric analyses were performed using a BD C6 flow cytometer (BD Biosciences) as described.32
Aggregometry
Washed platelets were diluted to a concentration of 3 × 108 platelets/mL in modified Tyrode’s buffer containing 1 mM CaCl2. Experiments were performed at 37 °C under stirring conditions (1,200 rpm). Platelets were stimulated with PAR4-agonist peptide (150 μM), convulxin (25 ng/mL), and ADP (10 μM). Light transmission was recorded on a 4-chanel optical aggregation system (Chrono-log).
Statistics
Statistical analyses were performed by using GraphPad Prism 9.1.1 (GraphPad Software Inc., La Jolla, California, United States). All data were presented as mean±standard deviation (SD), or median±IQR. The Shapiro–Wilk test was used to determine normality. The two-tailed Student’s t-test was used for normally distributed data, and the Mann–Whitney test for nonnormally distributed data. Spearman’s rank correlation test was used to assess the association between different biomarkers. We used two-way analysis of variance (ANOVA) with Bonferroni’s post-hoc comparisons in multiple analysis. p-Values <0.05 were regarded as significant.
Results
Tumor-Bearing Mice Have Decreased Arterial Thrombosis
Based on our studies that showed an increase in venous thrombosis in tumor-bearing mice,22 we anticipated that tumor-bearing mice would have increased arterial thrombosis. We used the FeCl3-induced carotid artery occlusion model because it triggers robust formation of platelet-rich thrombi and is a commonly used model of arterial thrombosis.33 We chose conditions that would allow us to observe increased thrombosis (2.5% FeCl3 for 5 minutes). Surprisingly, we observed a significant increase in the occlusion time in tumor-bearing mice compared with control mice (Fig. 1A). There was no difference in occlusion times between tumor-bearing mice and controls when the injury was increased (10% FeCl3 for 2 minutes) (data not shown).
Fig. 1.
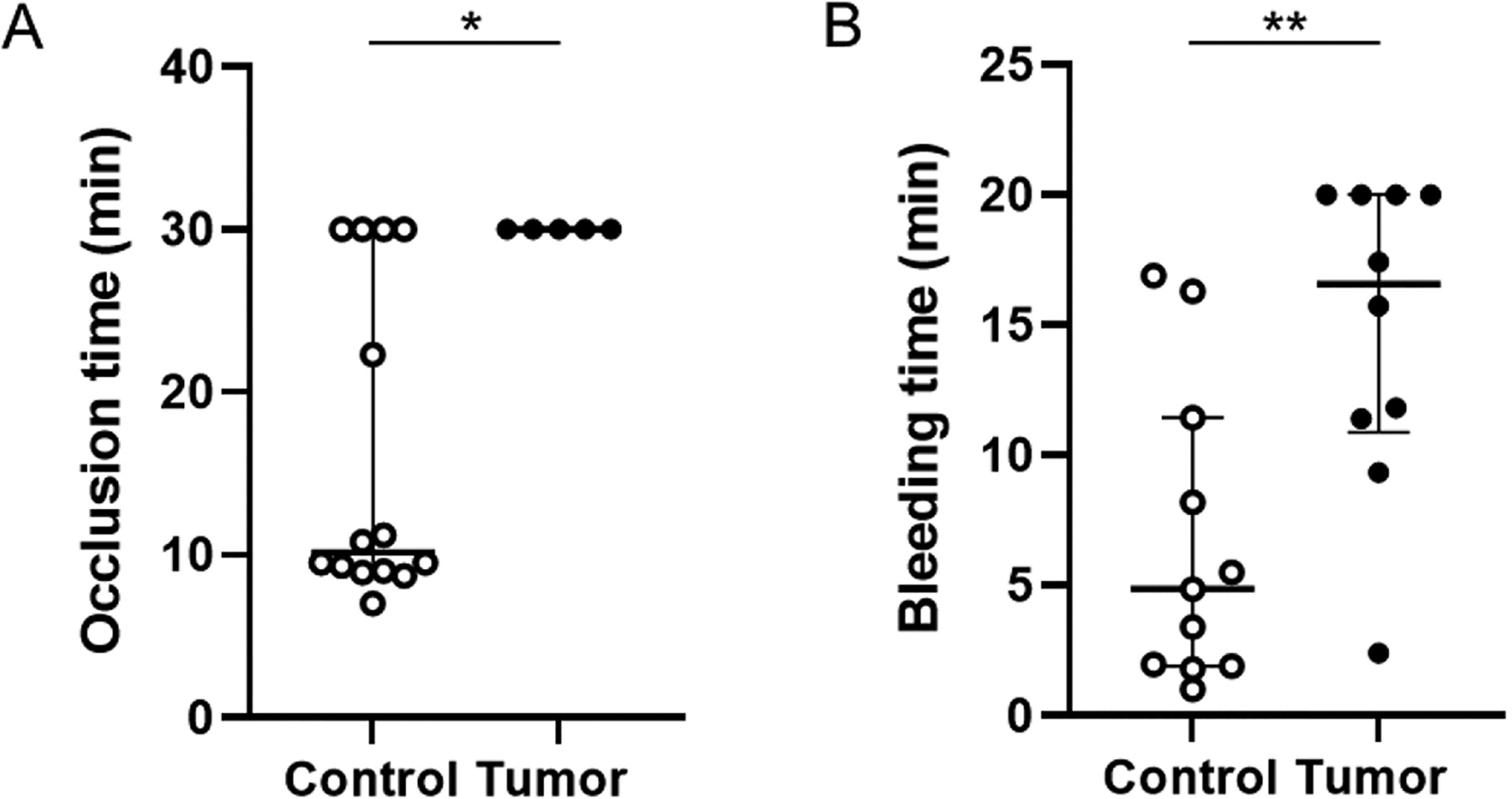
Carotid artery occlusion time and tail bleeding in control mice and tumor-bearing mice. (A) Carotid artery occlusion times in control mice (n = 14) and tumor-bearing mice (n = 5). (B) Tail bleeding times in control mice (n = 11) and tumor-bearing mice (n = 10). All data are shown as median (IQR) and analyzed by the Mann–Whitney test. *p < 0.05, **p < 0.01. IQR, interquartile range.
Tumor-Bearing Mice Have Increased Tail bleeding
We measured tail bleeding in tumor-bearing and control mice. We observed a significant increase in the bleeding time in tumor-bearing mice compared with control mice (Fig. 1B). This is consistent with a decrease in arterial thrombosis.
Tumor-Bearing Mice Have a Decrease in Platelet Count
We determined the effect of human pancreatic tumors in mice on the platelet count. Mice bearing tumors had a significant decrease in the platelet count compared with control mice (Fig. 2A). In addition, platelets from tumor-bearing mice were significantly larger compared with platelets from control mice (Fig. 2B). Similar results were observed when platelet count and size were measured by flow cytometry (data not shown). We measured platelet count in mice bearing smaller BxPC-3 tumors (0.51 to <2.0 g) and also observed a significant decrease in platelet count (mean±SD, 1,047±209 103/μL, n = 15; p < 0.001).
Fig. 2.
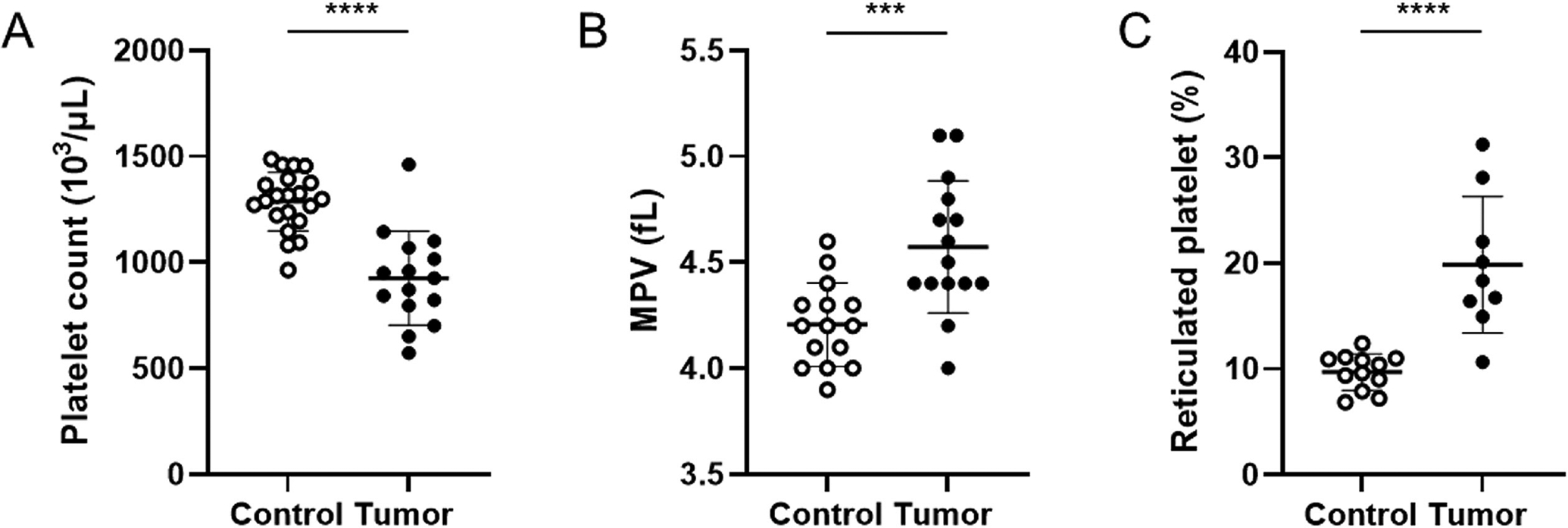
Platelet count, platelet size, and percentage of reticulated platelet in control mice and tumor-bearing mice. Platelet count and size were measured using an Element HT5 analyzer. (A) Platelet count in control mice (n = 21) and tumor-bearing mice (n = 15). (B) Platelet size in control mice (n = 15) and tumor-bearing mice (n = 15). (C) Percentage of reticulated platelets in control mice (n = 15) and tumor-bearing mice (n = 9). All data are shown as mean ± SD and analyzed by the two-tailed Student’s t-test. ***p < 0.001, ****p < 0.0001. MPV, mean platelet volume; SD, standard deviation.
We speculated that the observed thrombocytopenia in tumor-bearing mice is the result of increased platelet turnover triggered by TF+ EV-dependent thrombin generation and platelet activation rather than a defect in platelet production. Consistent with increased platelet turnover, we found that mice bearing tumors had a significantly higher percentage of reticulated platelets compared with control mice (Fig. 2C).
Circulating Platelet and Endothelial Activation Markers in Tumor-Bearing Mice
We measured plasma levels of markers of platelet and endothelial activation in tumor-bearing mice and control mice. Tumor-bearing mice had significantly higher levels of PF4 and soluble P-selectin compared with control mice (Fig. 3A, B). Tumor-bearing mice also had significantly higher levels of soluble VCAM-1 and soluble ICAM-1 compared with control mice (Fig. 3C, D). These data suggest that tumor-bearing mice have activated platelets and endothelium.
Fig. 3.
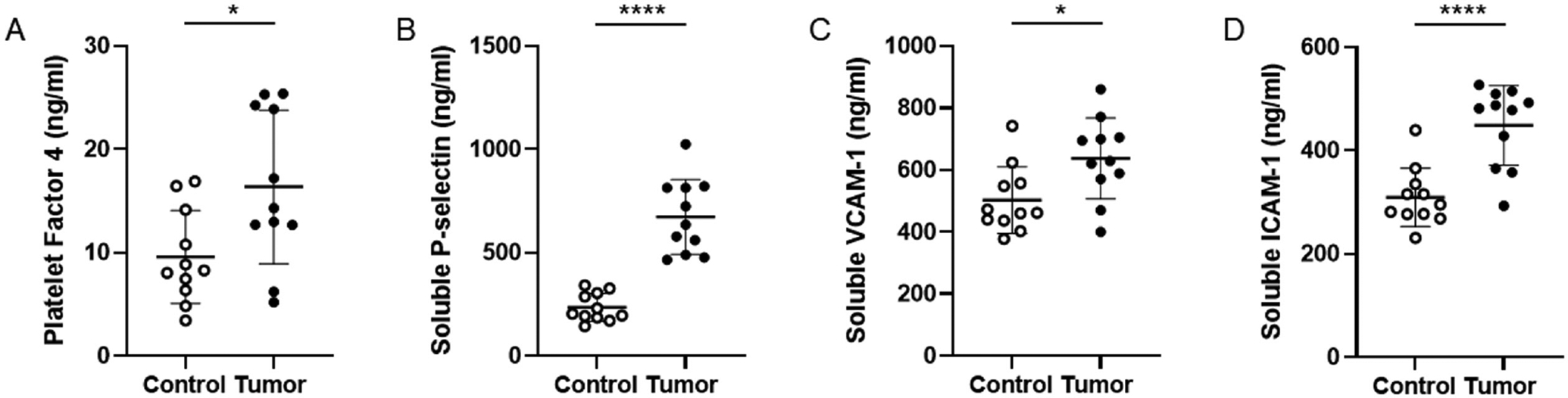
Levels of platelet and endothelial activation markers in control mice and tumor-bearing mice. (A) Plasma levels of platelet factor 4 in control mice (n = 11) and tumor-bearing mice (n = 11). (B) Plasma levels of soluble P-selectin in control mice (n = 11) and tumor-bearing mice (n = 11). (C) Plasma levels of soluble VCAM-1 in control mice (n = 11) and tumor-bearing mice (n = 11). (D) Plasma levels of soluble ICAM-1 in control mice (n = 11) and tumor-bearing mice (n = 11). All data are shown as mean ± SD and analyzed by the two-tailed Student’s t-test. *p < 0.05, ****p < 0.0001. SD, standard deviation.
Levels of PF4 significantly correlated with soluble P-selectin (Spearman’s r = 0.467, p = 0.028) and soluble ICAM-1 (Spearman’s r = 0.474, p = 0.026). Levels of soluble P-selectin significantly correlated with soluble VCAM-1 (Spearman’s r = 0.449, p = 0.036) and soluble ICAM-1 (Spearman’s r = 0.753, p < 0.0001), and levels of soluble VCAM-1 significantly correlated with soluble ICAM-1 (Spearman’s r = 0.798, p < 0.001).
Platelets from Tumor-Bearing Mice Have Reduced Receptor Expression
We analyzed if platelets in mice bearing tumors showed signs of in vivo activation. Compared to controls, platelets isolated from tumor-bearing mice had a significant decrease in surface expression of GPIX, GPVI, αIIbβ3, and GPIbα (Fig. 4).
Fig. 4.
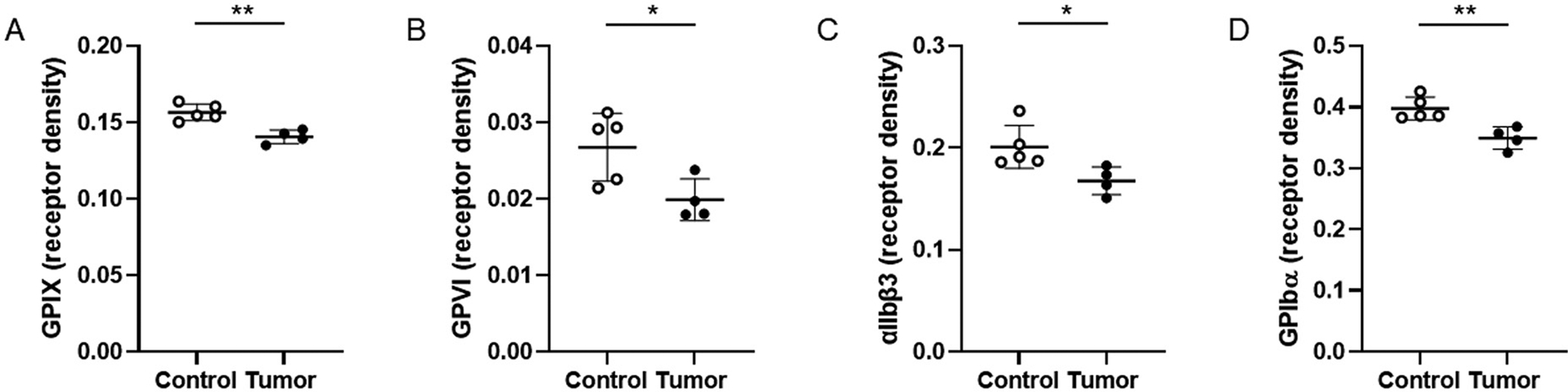
Receptor expression on platelets from control and tumor-bearing mice. Levels of GPIX (A), GPVI (B), αIIbβ3 (C), and GPIbα (D) were determined by flow cytometry. Levels of the different receptors were normalized to mean platelet size for each mouse. We analyzed platelets from control mice (n = 5) and tumor-bearing mice (n = 4). Data are shown as mean ± SD and analyzed by the two-tailed Student’s t-test. *p < 0.05, **p < 0.01. GPVI, glycoprotein VI, GPIX, glycoprotein IX; SD, standard deviation.
Platelets from Tumor-Bearing Mice Have Reduced Activation Ex Vivo
We investigated if platelets from tumor-bearing mice have reduced ability to activate αIIbβ3 and to secrete granules after agonist stimulation ex vivo. Platelets were stimulated with various agonists (PAR4 agonist peptide, convulxin, and ADP) and levels of activated αIIbβ3 and surface P-selectin measured by flow cytometry. We did not observe any differences in levels of activated αIIbβ3 and surface P-selectin in resting platelets from tumor-bearing mice (Fig. 5). Platelets from tumor-bearing mice had reduced activated αIIbβ3 in response to PAR4 agonist peptide and convulxin-induced but not in response to ADP (Fig. 5A–C). There was no difference in surface expression of P-selectin on platelets activated with PAR agonist peptide, convulxin, or ADP compared with platelets from control mice (Fig. 5D–F). These data indicate that platelets from tumor-bearing mice are impaired in their ability to activate integrin receptors.
Fig. 5.
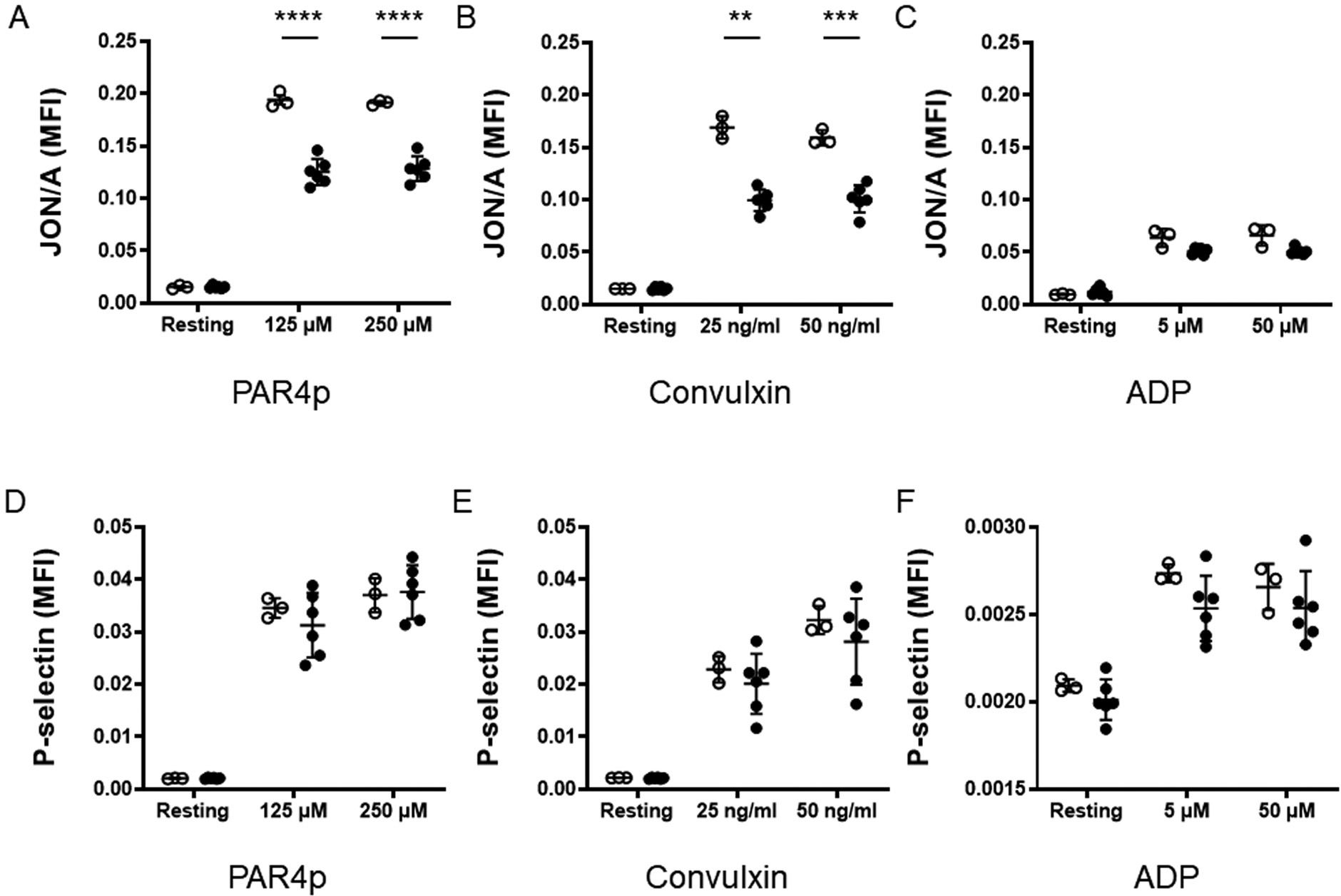
Analysis of ex vivo activation of platelets from control and tumor-bearing mice. Levels of activated αIIbβ3 (A–C) and P-selectin (D–F) on the cell surface of platelets from control mice (white circles) and tumor-bearing mice (black circles) were determined by flow cytometry in response to a PAR4 agonist peptide (PAR4p) (A, D), convulxin (B, E), and ADP (C, F). We analyzed the expression of activated αIIbβ3 and P-selectin on resting and activated platelets from control mice (n = 3) and tumor-bearing mice (n = 4). Results were normalized to platelet count. Data are shown as mean ± SD and analyzed by two-way analysis of variance, followed by the Bonferroni multiple comparison tests. **p < 0.01, ***p < 0.001, ****p < 0.0001. ADP, adenosine diphosphate; SD, standard deviation.
Measurement of Platelet Aggregation
We measured platelet aggregation using light transmission aggregometry. Washed platelets from tumor-bearing mice and controls were activated with PAR4 agonist peptide 150 μM, convulxin 25 ng/mL, or ADP 10 μM. We did not observe differences in maximal aggregation (%) and aggregation area under the curve between platelets from tumor-bearing mice and controls (Fig. 6).
Fig. 6.
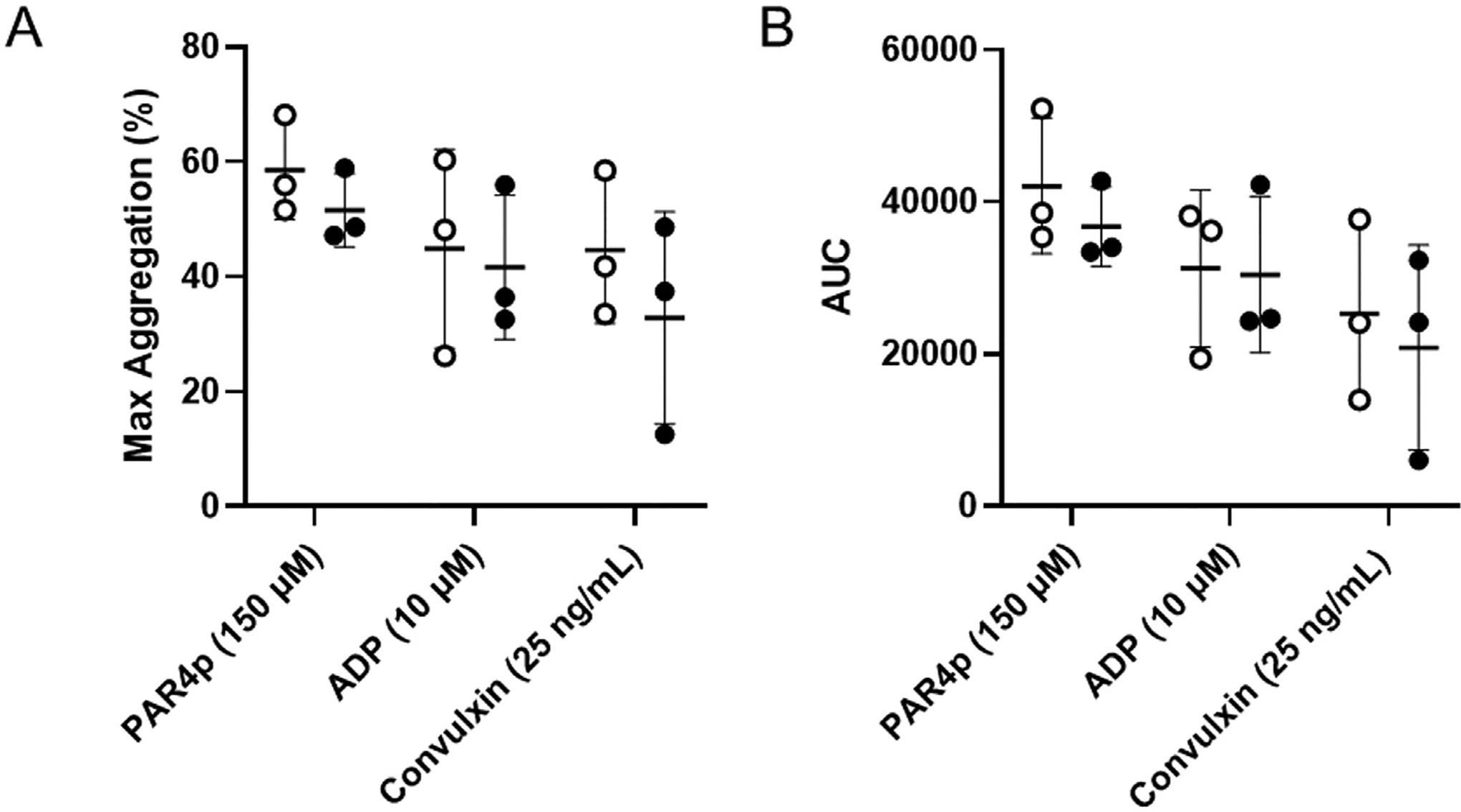
Aggregation of platelets from control and tumor-bearing mice. Washed platelets from control mice (white circles, n = 3) and tumor-bearing mice (black circles, n = 3) were activated with PAR4p (150 μM), convulxin (25 ng/mL), or ADP (10 μM). Maximum aggregation (A) and area under the curve (AUC) (B) were measured using a light transmission aggregometry. Data are shown as mean ± SD and analyzed by the two-tailed Student’s t-test. ADP, adenosine diphosphate; SD, standard dseviation.
Discussion
The major finding of this study is that the presence of large human BxPC-3 pancreatic tumors in mice is associated with a decrease in carotid artery thrombosis. This result was unexpected because the coagulation cascade is activated in this tumor model and mice have increased venous thrombosis.18,22 Tumor-bearing mice also had a decrease in platelet count and an increase in tail bleeding compared with control mice. A recent study found that growth of murine Panc02 pancreatic tumors for 25 days in mice was associated with a decrease in platelet count and decreased thrombosis in a cremaster vessel laser injury model.34 This result is consistent with our observations with mice bearing BxPC-3 tumors. In contrast, growth of Panc02 tumors for 10 days was associated with an increase in platelet count and an increase in thrombosis.34 At present, the mechanism by which Panc02 tumors increase count in mice has not been determined.
A recent study found that severe thrombocytopenia (approximately 80% decrease in platelet count) was required to affect tail bleeding and carotid artery occlusion time.35 Therefore, the observed reduction in platelet count in mice bearing BxPC-3 tumors is likely to be a contributing factor but cannot completely explain the reduced carotid artery thrombosis and prolongation in tail bleeding observed in these mice. In contrast, protection from FeCl3-induced arterial thrombosis and/or impaired hemostasis are frequently reported for mice with partially impaired platelet adhesive function.33,36 Indeed, we observed a significant decrease in receptor expression in platelets from tumor-bearing mice, including GPIbα, GPIX, GPVI, and αIIbβ3. The reduction in GPIbα is consistent with shedding of the extracellular domain of the GPIbα subunit.37,38 In addition, we observed a significant increase in the platelet activation marker PF4 in tumor-bearing mice. These results suggest that platelets are activated in tumor-bearing mice and have decreased function.
We hypothesized that the decrease in platelet count is due to an increase in platelet turnover caused by elevated levels of tumor-derived TF+ EVs that leads to chronic thrombin stimulation of platelets. Indeed, we have previously shown that BxPC-3-derived TF+ EVs activate platelets in a thrombin-dependent manner.18 Consistent with this hypothesis, tumor-bearing mice also had an increased percentage of reticulated platelets compared to control mice.
Levels of soluble ICAM-1 and soluble VCAM-1 were increased in tumor-bearing mice compared with control mice. This suggests that the presence of tumors leads to endothelial activation. It is possible that endothelial activation might contribute to the decreased arterial thrombosis in tumor-bearing mice.
Levels of activated αIIbβ3 and surface P-selectin in resting platelets from tumor-bearing mice were not increased compared with platelets from control mice. We did observe significantly lower levels of active αIIbβ3 in stimulated platelets from tumor-bearing mice compared to stimulated platelets from control mice. However, analysis of levels of active αIIbβ3 in platelets from tumor-bearing mice as a marker of platelet activation in this study is complicated by the reduced levels of this receptor. We did not observe a difference in platelet aggregation between platelets from tumor-bearing mice and controls. It should be noted that platelets can have defects that lead to reduced function in vivo and ex vivo that do not cause defective aggregation in in vitro studies. For instance, Pld1 −/− mice have a significantly increased occlusion time in the FeCl3 artery thrombosis model and activated Pld1 −/− platelets have reduced binding to fibrinogen but the platelets from these mice exhibit normal aggregation ex vivo.39 This indicates that under conditions of submaximal αIIbβ3 integrin activation, robust aggregation can occur.
A recent study examined the reactivity of platelets obtained from cancer patients before the initiation of therapy.11 Platelet counts did not differ between the cancer patients and healthy controls. Platelets were stimulated with PAR1 or PAR4 agonist peptides or a GPVI agonist and levels of activated αIIbβ3 integrin and surface P-selectin were measured. Cancer patients that died within the first year after diagnosis had significantly decreased platelet reactivity compared with platelets from cancer patients that were alive after 1 year.11 This indicated that decreased platelet reactivity was associated with poor prognosis. Similarly, we observed that platelets from tumor-bearing mice had decreased platelet reactivity ex vivo compared with platelets from control mice.
In summary, most mouse studies have focused on mechanisms of thrombosis.19,40–42 In our previous studies with mice bearing BxPC-3 tumors, we found increased levels of tumor-derived TF+ EVs, an activated coagulation system, increased neutrophils and neutrophil extracellular traps, and increased venous thrombosis.18,22,28 In the current study, we found a decrease in arterial thrombosis and an increase in bleeding. It was surprising to us that mice bearing BxPC-3 tumors of the same size could have increased venous thrombosis and decreased arterial thrombosis. To explain these observations, we propose that the increase in venous thrombosis in mice bearing BxPC-3 tumors is primarily driven by TF-dependent activation of coagulation, neutrophils, and neutrophil extracellular traps and not by platelets. In contrast, the decrease in arterial thrombosis in tumor-bearing mice is due to a decrease in platelet count, a decrease in platelet reactivity due to loss of receptor, and platelet exhaustion. It should be noted that we have recently shown that platelets do contribute to venous thrombosis in the inferior vena cava stenosis model in mice without tumors.43 Together, these data demonstrate that this mouse model is a powerful tool to study how tumors affect platelet count and function, coagulation, hemostasis, and thrombosis. A better understanding of the molecular changes underlying the throm-bocytopathy induced by tumors may help to prevent thrombocytopenia, platelet dysfunction, and bleeding in cancer patients.
What is known about this topic?
Cancer patients have a dysregulated hemostatic system and activated platelets.
In mouse models of pancreatic cancer, platelets have been shown to contribute to thrombosis, tumor growth, and metastasis.
What does this paper add?
Most studies using mouse models of pancreatic cancer have focused on mechanisms of venous thrombosis. In this study, we analyzed arterial thrombosis in tumor-bearing mice.
Surprisingly, we found that mice bearing human pancreatic tumors had decreased carotid artery thrombosis. Tumor-bearing mice also had decreased platelet count, increased levels of a platelet activation marker and decreased receptor expression on platelets suggesting platelet activation.
In addition, platelets from tumor-bearing mice had reduced αIIbβ3 activation ex vivo. Our study may help to explain the increased bleeding observed in some cancer patients.
Acknowledgment
The authors would like to thank Dr. Julia Riedl (Medical University of Vienna) for helpful comments.
Funding
The presented study was supported by grant from the NIH (N.M.: 1R35HL155657; W.B.: R35HL144976) and SEN-SHIN Medical Foundation (T.K.).
Footnotes
Conflict of Interest
None declared.
References
- 1.Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119(03):648–655 [DOI] [PubMed] [Google Scholar]
- 2.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017;70(08): 926–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122(10): 1712–1723 [DOI] [PubMed] [Google Scholar]
- 4.Hsu CPatell R, Zwicker JI. The prevalence of thrombocytopenia in patients with acute cancer-associated thrombosis. Blood Adv 2022. (e-pub ahead of print). Doi: 10.1182/bloodadvances.2022008644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100(10):3484–3488 [DOI] [PubMed] [Google Scholar]
- 6.Weitz JI, Haas S, Ageno W, et al. ; GARFIELD-VTE investigators. Cancer associated thrombosis in everyday practice: perspectives from GARFIELD-VTE. J Thromb Thrombolysis 2020;50(02):267–277 [DOI] [PubMed] [Google Scholar]
- 7.Riedl J, Pabinger I, Ay C. Platelets in cancer and thrombosis. Hamostaseologie 2014;34(01):54–62 [DOI] [PubMed] [Google Scholar]
- 8.Riedl J, Hell L, Kaider A, et al. Association of platelet activation markers with cancer-associated venous thromboembolism. Platelets 2016;27(01):80–85 [DOI] [PubMed] [Google Scholar]
- 9.Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008;112(07):2703–2708 [DOI] [PubMed] [Google Scholar]
- 10.Poruk KE, Firpo MA, Huerter LM, et al. Serum platelet factor 4 is an independent predictor of survival and venous thromboembolism in patients with pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2010;19(10):2605–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedl J, Kaider A, Marosi C, et al. Decreased platelet reactivity in patients with cancer is associated with high risk of venous thromboembolism and poor prognosis. ThrombHaemost 2017;117(01):90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi M Disseminated intravascular coagulation in cancer: an update. Semin Thromb Hemost 2019;45(04):342–347 [DOI] [PubMed] [Google Scholar]
- 13.Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost 2001;86(03):828–833 [PubMed] [Google Scholar]
- 14.Dvorak HF, Quay SC, Orenstein NS, et al. Tumor shedding and coagulation. Science 1981;212(4497):923–924 [DOI] [PubMed] [Google Scholar]
- 15.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost 2004;2(11):2065–2067 [DOI] [PubMed] [Google Scholar]
- 16.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 2013;122(11):1873–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mir Seyed Nazari P, Riedl J, Pabinger I, Ay C. The role of podoplanin in cancer-associated thrombosis. Thromb Res 2018;164(Suppl 1): S34–S39 [DOI] [PubMed] [Google Scholar]
- 18.Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost 2016;14(01):153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost 2015;13(08):1372–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davila M, Amirkhosravi A, Coll E, et al. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 2008;6(09):1517–1524 [DOI] [PubMed] [Google Scholar]
- 21.Wang JG, Geddings JE, Aleman MM, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012;119 (23):5543–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisada Y, Ay C, Auriemma AC, Cooley BC, Mackman N. Human pancreatic tumors grown in mice release tissue factor-positive microvesicles that increase venous clot size. J Thromb Haemost 2017;15(11):2208–2217 [DOI] [PubMed] [Google Scholar]
- 23.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lom-bardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med 2009;206(09):1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas GM, Brill A, Mezouar S, et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost 2015;13(07):1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasano T, Cho MS, Rodriguez-Aguayo C, et al. Role of tissue-factor bearing extracellular vesicles released from ovarian cancer cells in platelet aggregation in vitro and venous thrombosis in mice. Thrombosis Update 2021;2:100020 [Google Scholar]
- 26.Stark K, Schubert I, Joshi U, et al. Distinct pathogenesis of pancreatic cancer microvesicle-associated venous thrombosis identifies new antithrombotic targets in vivo. Arterioscler Thromb Vasc Biol 2018;38(04):772–786 [DOI] [PubMed] [Google Scholar]
- 27.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer 2015;136(02):462–475 [DOI] [PubMed] [Google Scholar]
- 28.Hisada Y, Grover SP, Maqsood A, et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica 2020;105(01):218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens AP III, Lu Y, Whinna HC, Gachet C, Fay WP, Mackman N. Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J Thromb Haemost 2011;9(09):1862–1863 [DOI] [PubMed] [Google Scholar]
- 30.Schiviz A, Magirr D, Leidenmühler P, Schuster M, Muchitsch EM, Höllriegl W Subcommittee on Animal Models of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Influence of genetic background on bleeding phenotype in the tail-tip bleeding model and recommendations for standardization: communication from the SSC of the ISTH. J Thromb Haemost 2014;12(11):1940–1942 [DOI] [PubMed] [Google Scholar]
- 31.Sommeijer DW, van Oerle R, Reitsma PH, et al. Analysis of blood coagulation in mice: pre-analytical conditions and evaluation of a home-made assay for thrombin-antithrombin complexes. Thromb J 2005;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanini L, Paul DS, Robledo RF, et al. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest 2015;125 (04):1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grover SP, Mackman N. How useful are ferric chloride models of arterial thrombosis? Platelets 2020;31(04):432–438 [DOI] [PubMed] [Google Scholar]
- 34.Palacios-Acedo AL, Mezouar S, Mège D, Crescence L, Dubois C, Panicot-Dubois L. P2RY12-inhibitors reduce cancer-associated thrombosis and tumor growth in pancreatic cancers. Front Oncol 2021;11:704945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morowski M, Vögtle T, Kraft P, Kleinschnitz C, Stoll G, Nieswandt B. Only severe thrombocytopenia results in bleeding and defective thrombus formation in mice. Blood 2013;121(24):4938–4947 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Jennings NL, Dart AM, Du XJ. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J Exp Med 2012;2(02):30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergmeier W, Rackebrandt K, Schröder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood 2000;95(03):886–893 [PubMed] [Google Scholar]
- 38.Montague SJ, Andrews RK, Gardiner EE. Mechanisms of receptor shedding in platelets. Blood 2018;132(24):2535–2545 [DOI] [PubMed] [Google Scholar]
- 39.Elvers M, Stegner D, Hagedorn I, et al. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci Signal 2010;3(103):ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hisada Y, Mackman N. Mouse models of cancer-associated thrombosis. Thromb Res 2018;164(Suppl 1):S48–S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mege D, Mezouar S, Dignat-George F, Panicot-Dubois L, Dubois C. Microparticles and cancer thrombosis in animal models. Thromb Res 2016;140(Suppl 1):S21–S26 [DOI] [PubMed] [Google Scholar]
- 42.Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res 2016;139:65–76 [DOI] [PubMed] [Google Scholar]
- 43.Mwiza JMN, Lee RH, Paul DS, et al. Both G protein-coupled and immunoreceptor tyrosine-based activation motif receptors mediate venous thrombosis in mice. Blood 2022;139(21):3194–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]


