Abstract
The protozoan parasite Cryptosporidium parvum invades intestinal epithelial cells and can cause life-threatening diarrhea in immunocompromised individuals. Despite the clinical importance of this organism, much remains to be learned about the pathogenesis of C. parvum-induced diarrhea. To explore the role of the intestinal inflammatory response in C. parvum disease, using C. parvum oocysts we infected human intestinal xenografts in severe combined immunodeficient (SCID) mice. Seven days after infection, we found levels of human tumor necrosis factor alpha and interleukin-8 in C. parvum-infected human intestinal xenografts that were significantly higher than those seen in uninfected control xenografts. These results demonstrate that human intestinal cells produce proinflammatory cytokines in response to C. parvum infection and establish SCID-HU-INT mice as a model system to study the interactions of C. parvum with the human intestine.
Cryptosporidium parvum is a coccidian protozoan that has been recognized as an important cause of diarrhea worldwide. Waterborne outbreaks of C. parvum are a major threat to public health in the United States (6, 12, 15). C. parvum causes self-limited diarrhea in immunocompetent humans and ruminants, but infection may persist in immunodeficient or immunosuppressed hosts. This can result in severe and protracted diarrhea, with malabsorption and fatal wasting. There is an urgent need for a better understanding of how C. parvum causes diarrhea and for effective therapeutic and palliative agents to treat cryptosporidiosis. Studies in this area have been hampered by a number of factors, including a lack of access to parasites, difficulties in assessing parasite virulence, the lack of inexpensive animal models that mimic human disease, and a paucity of immunologic and pathologic data from human infections.
One possible mechanism by which C. parvum could cause diarrhea is by inducing gut inflammation. C. parvum infection reportedly causes human intestinally derived epithelial cell lines to produce interleukin-8 (IL-8) and another inflammatory cell chemoattractant, GRO-α (7, 11). The production of proinflammatory cytokines by intestinal epithelial cells in response to C. parvum infection could result in the influx of inflammatory cells such as neutrophils, monocytes, and immune cells to the gut, with resultant tissue damage and diarrhea (1, 9, 13, 14, 16). Independent support for this contention comes from both experimental and human observational studies (reviewed in reference 5). Intestinal samples from C. parvum-infected piglets possess tumor necrosis factor alpha (TNF-α) activity (8). Intestinal biopsy samples from individuals with AIDS and cryptosporidiosis indicate that some individuals with severe disease have a localized inflammatory reaction, including a pronounced neutrophilic infiltrate in the gut tissue (2, 4). An autopsy study of intestines from individuals with cryptosporidiosis revealed interstitial edema with a mixed inflammatory cell infiltrate (3). A heightened inflammatory state in individuals with cryptosporidiosis is suggested by the finding that among 250 children with cryptosporidiosis (77% of whom had profuse diarrhea) studied in Cote D’Ivoire, 58% had fever (10).
The purpose of this study was to determine whether C. parvum infection of human intestinal xenografts induces the production of the cytokines IL-8, IL-1β, and/or TNF-α from human intestinal epithelial cells in vivo. We chose these three cytokines because of the reported findings that C. parvum induces IL-8 in human intestinal epithelial cell lines (7), our previous findings that infection of human intestinal epithelial cells with the extracellular intestinal parasite Entamoeba histolytica induces IL-1β and IL-8 production in vivo (18), and reports that TNF-α activity is present in intestinal tissue from C. parvum-infected piglets (8). SCID-HU-INT mice were produced by our previously described protocol (18). In brief, SCID mice were anesthetized and a small incision was made in both rear flanks and both suprascapular regions. A subcutaneous tunnel was then produced between the two ipsilateral incisions by blunt dissection. Sections (5-cm) of 100- to 120-day gestational age fetal human intestine (obtained from tissues destined for discard following prostaglandin-saline-induced therapeutic abortions from the Central Laboratory for Human Embryology at the University of Washington Health Sciences Center) were threaded through the tunnel by using a blunt forceps. The tissue was trimmed as necessary, and the incision was closed with a 7-mm Michel clip. Grafts were allowed to develop for 8 to 10 weeks. These intestinal grafts become extensively vascularized, secrete mucus, and develop into morphologically normal-appearing small intestine, with elongated villi and paneth cells, or large intestine, with blunter villi, goblet cells, and crypts, depending on the tissue of origin (18).
When mature, human intestinal xenografts were infected by direct intraluminal inoculation of C. parvum oocysts. Oocysts, purified from the feces of C. parvum-infected calves, were stored in 2.5% potassium dichromate at 4°C prior to use and then were rinsed and treated with hypochlorite according to a standard protocol (17). Treated oocysts (5 × 106), suspended in 0.1 ml of phosphate-buffered saline (PBS), were then inoculated directly into the lumen of one of the intestinal xenografts in each of eight SCID-HU-INT mice. This dosage of oocysts and the time course for studying infection (7 days) were chosen based on a previous report of successful infection of rabbit fetal intestinal xenografts with C. parvum (20). The contralateral graft of each SCID-HU-INT mouse was inoculated with PBS alone to serve as a control. Infection was allowed to proceed for 7 days, and then SCID-HU-INT mice were sacrificed and sections of the human intestinal xenograft were analyzed for histologic evidence of infection and were processed for reverse transcription (RT)-PCR and enzyme-linked immunosorbent assays (ELISAs) exactly as previously described (18). For RT-PCR, 100 mg of human intestinal tissue was suspended in 1 ml of Trizol reagent (GibcoBRL, Gaithersburg, Md.) and then homogenized for 15 s with a Polytron. Samples underwent phase separation using chloroform, the aqueous layer was removed, and the RNA was precipitated with isopropyl alcohol. Following a 15-min 12,000 × g centrifugation, the RNA pellet was washed in 70% ethanol, dried, resuspended in diethyl pyrocarbonate-water, and quantified by measuring absorbance at 260 nm. cDNA was synthesized from 2 μg of total RNA by using 0.5 μg of oligo(dT) primer, 50 mM dithiothreitol, 10 μM (each) deoxynucleoside triphosphate, and 200 U of RNase H− Moloney murine leukemia virus reverse transcriptase (GibcoBRL) in a final volume of 20 μl at 37°C for 1 h. PCR (using cDNA equivalent to 0.2 μg of starting RNA) was performed in a 100-μl volume containing 0.5 μM concentrations of the appropriate sense and antisense oligonucleotide primers, 200 mM (each) deoxynucleoside triphosphate, 5% dimethyl sulfoxide, 1 U of Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.), and the supplied 10× buffer. PCR was performed with a program of 35 cycles of denaturing at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min. A 20-μl aliquot of the PCR product was subjected to electrophoresis in a 1.5% agarose gel and stained with ethidium bromide for visualization. Primers that specifically amplify transcripts for human actin, human IL-1β, and IL-8 in RT-PCRs have been previously described (18); primers that specifically amplify human and not murine TNF-α were 5′AAGAAGACAGGGGGGCCCCAGGG (sense) and 3′AGACTCGGCAAAGTCGAGATAGTC (antisense). Protein samples for ELISA were prepared by homogenizing human intestinal tissue at a concentration of 50 mg/ml in a solution of PBS with aprotinin, leupeptin, and pepstatin A (1 μg/ml each). Samples were then spun for 5 min at 500 × g to remove particulate matter, and the supernatants were assayed. ELISA kits specific for human IL-1β (Endogen, Cambridge, Mass.), human TNF-α (Endogen), and IL-8 (R&D Systems, Minneapolis, Minn.) were used according to the manufacturer’s instructions.
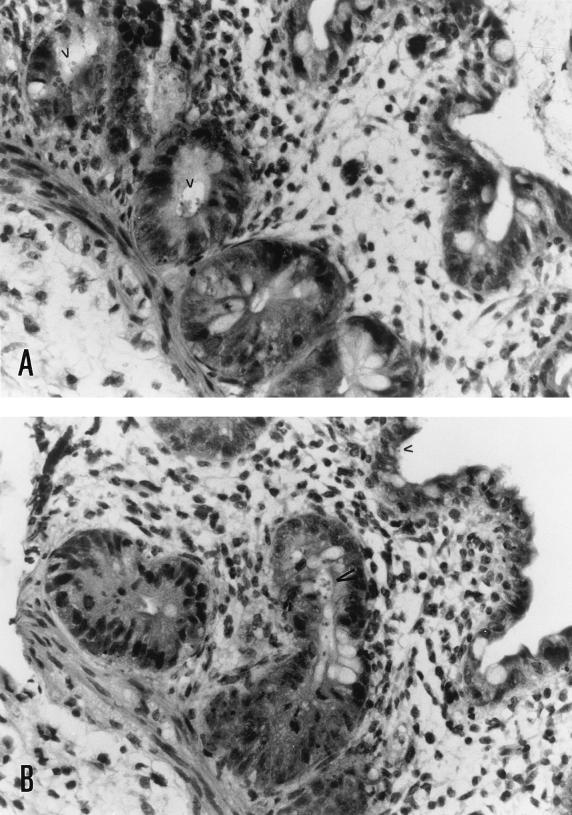
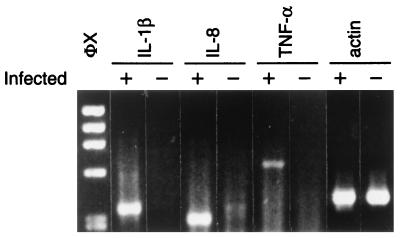
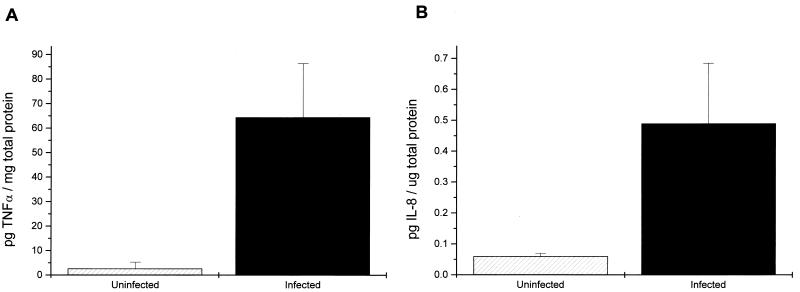
Human intestinal xenografts infected with C. parvum did not appear different from the control xenografts on gross examination. Microscopic examination of hematoxylin-eosin-stained sections showed C. parvum parasites in villi and crypts of infected xenografts (Fig. 1). Some sections of C. parvum-infected human intestinal xenografts showed scattered foci of villous blunting, enterocyte degeneration, and some influx of inflammatory cells, but in most areas the intestine appeared normal. No C. parvum parasites were seen in uninfected xenografts. To determine whether C. parvum infection induces cytokine production by human intestinal epithelial cells, we assayed sections from human intestinal xenografts for human IL-1β, human TNF-α, and IL-8. As shown in Fig. 2, 7 days following infection C. parvum-infected human intestinal xenografts had mRNA transcripts for human IL-1β, human TNF-α, and IL-8. In contrast, no mRNA transcripts for human IL-1β, human TNF-α, or IL-8 could be seen in the control uninfected xenografts. The intensity of the actin control mRNA was equivalent between C. parvum-infected and uninfected human intestinal xenografts, indicating that equivalent quantities of cDNA were present in the samples. We attempted to confirm the results of the RT-PCR assay and quantify cytokine production by assaying C. parvum-infected and control human intestinal xenografts for human IL-1β, human TNF-α, and IL-8 by ELISA. As shown in Fig. 3A, the mean level of TNF-α was significantly higher in C. parvum-infected human intestinal xenografts (n = 8) than in control xenografts (n = 8) at the 7-day time point (P < 0.002). Interestingly, we have not seen TNF-α production in either E. histolytica-infected (18) or Shigella flexneri-infected (19) human xenografts, indicating that the elevated human intestinal production of TNF-α is not a stereotypic response to intracellular or extracellular intestinal infection. The mean level of IL-8 in C. parvum-infected human intestinal xenografts was also significantly higher than the level of IL-8 in uninfected control xenografts (P < 0.002) (Fig. 3B). While IL-8 was detectable in C. parvum-infected human intestinal xenografts, the quantity of IL-8 found was approximately 10-fold lower than that seen with E. histolytica infection of human intestinal xenografts (18). Consistent with this finding, we saw a more pronounced inflammatory response, with many more neutrophils in E. histolytica-infected human intestinal xenografts than in the C. parvum infected xenografts described in this study. Despite the finding of mRNA transcripts for human IL-1β by RT-PCR, we were unable to detect IL-1β in ELISAs (data not shown), indicating that the quantity of IL-1β produced in response to C. parvum infection was below the level of detection of the assay (<1 pg/ml). Our finding that IL-8 is produced by intestinal epithelial cells in response to C. parvum infection confirms the recent findings of Laurent et al., who documented IL-8 and GRO-α production by intestinal epithelial cell lines in vitro and found increased mRNA for both cytokines in human xenografts in SCID-HU-INT mice (11). Our results extend those findings by demonstrating that TNF-α and IL-1β are also produced in vivo in response to C. parvum infection and establish that increased levels of both TNF-α and IL-8 are found in intestinal tissue.
FIG. 1.
C. parvum infects human intestinal xenografts. These photomicrographs from sections from two different C. parvum-infected intestinal xenografts 7 days following infection show C. parvum parasites (arrowheads) present in crypt cells (A) and in crypt and villous cells (B). Magnification, ×290.
FIG. 2.
mRNA transcripts for human IL-1β, IL-8, and human TNF-α are induced when human intestinal xenografts are infected with C. parvum. The results of an RT-PCR assay for mRNA transcripts for human IL-1β, IL-8, human TNF-α, and actin from tissue samples from an intestinal xenograft infected 7 days previously with C. parvum (lanes +) and the control uninfected contralateral graft (lanes −) are shown. Transcripts of the predicted sizes for human IL-1β (339 bp), IL-8 (281 bp), and human TNF-α (610 bp) are amplified in the C. parvum-infected graft but are not detectable in control xenografts. The equivalent density of the actin control suggests that equivalent quantities of cDNA were present in the samples. Lane φX contains the φX174RF/HaeIII DNA size standards.
FIG. 3.
Human intestinal xenografts produce human TNF-α and IL-8 in response to C. parvum infection. (A) Seven days following infection, the mean level of human TNF-α in C. parvum-infected human intestinal xenografts (n = 8) is significantly higher (P < 0.002) than the level of human TNF-α in uninfected grafts (n = 8). (B) At the same time point, the mean level of IL-8 in C. parvum-infected human intestinal xenografts (n = 8) is significantly higher (P < 0.002) than the level of IL-8 in uninfected grafts (n = 8). Error bars, standard errors of the means.
The SCID-HU-INT model of C. parvum infection enables one to analyze the initial interactions between C. parvum sporozoites or oocysts and a mucin-secreting human intestinal tissue. This may be especially valuable in studies of C. parvum adherence and pathogenesis (20). Importantly, the chimeric nature of SCID-HU-INT mice allows one to dissect the role of intestinally derived (human) versus inflammatory-cell-derived (murine) cytokines in the pathogenesis of C. parvum infection and, potentially, to perform interventions that directly target human intestinal tissue in the mouse. These interventional studies will be critical in determining whether the cytokine production induced in this system has physiologic relevance to the disease process. This is especially relevant to our finding that C. parvum infection induces elevated levels of TNF-α in human intestine, since in addition to its role in mediating inflammatory responses, TNF-α potentially plays a role in C. parvum induced diarrhea by stimulating prostaglandins which in turn can increase intestinal secretion of chloride (8). The important question of whether interventions that reduce gut-induced inflammation, especially TNF-α production by human intestine, can affect the course of experimental C. parvum infection, is under investigation.
Acknowledgments
This work was supported by NIH grant AI-30084 and Research Career Development Award AI-01231 to S.L.S. and NIAID grant AI-33384 to J.K.G. and S.T. K.B.S. was supported by NIH Training Grant 5T32AI-07172. The Central Laboratory for Human Embryology is supported by NIH grant HD 00836 to Alan G. Fantel.
REFERENCES
- 1.Evans C W, Taylor J E, Walker J D, Simmons N L. Transepithelial chemotaxis of rat peritoneal exudate cells. Br J Exp Pathol. 1983;64:644–654. [PMC free article] [PubMed] [Google Scholar]
- 2.Genta R M, Chappell C L, White A C, Jr, Kimball K T, Goodgame R W. Duodenal morphology and intensity of infection in AIDS-related intestinal cryptosporidiosis. Gastroenterology. 1993;105:1769–1775. doi: 10.1016/0016-5085(93)91075-s. [DOI] [PubMed] [Google Scholar]
- 3.Godwin T A. Cryptosporidiosis in the acquired immunodeficiency syndrome: a study of 15 autopsy cases. Hum Pathol. 1991;22:1215–1224. doi: 10.1016/0046-8177(91)90103-v. [DOI] [PubMed] [Google Scholar]
- 4.Goodgame R W, Kimball K, Ou C N, White A C, Jr, Genta R M, Lifschitz C H, Chappell C L. Intestinal function and injury in acquired immunodeficiency syndrome-related cryptosporidiosis. Gastroenterology. 1995;108:1075–1082. doi: 10.1016/0016-5085(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths, J. K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv. Parasitol., in press. [DOI] [PubMed]
- 6.Hayes E B, Matte T D, O’Brien T R, McKinley T W, Logsdon G S, Rose J B, Ungar B L, Word D M, Pinsky P F, Cummings M L, et al. Large community outbreak of cryptosporidiosis due to contamination of a filtered public water supply. N Engl J Med. 1989;320:1372–1376. doi: 10.1056/NEJM198905253202103. [DOI] [PubMed] [Google Scholar]
- 7.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandil H M, Berschneider H M, Argenzio R A. Tumour necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut. 1994;35:934–940. doi: 10.1136/gut.35.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H S, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol. 1992;27:529–537. doi: 10.3109/00365529209000116. [DOI] [PubMed] [Google Scholar]
- 10.Kone M, Penali L K, Enoh S, Gershy-Damet G M, Anderson M. La cryptosporidiose chez les enfants ivoiriens de Yopougon. Bull Soc Pathol Exot. 1992;85:167–169. [PubMed] [Google Scholar]
- 11.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Naciri M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKenzie W R, Hoxie N J, Proctor M E, Gradus M S, Blair K A, Peterson D E, Kazmierczak J J, Addiss D G, Fox K R, Rose J B, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 13.Madara J L, Parkos C A, Colgan S P, MacLeod R J, Nash S, Matthews J, Delp C, Lencer W. Cl− secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madara J L, Patapoff T W, Gillece-Castro B, Colgan S P, Parkos C A, Delp C, Mrsny R J. 5′-Adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash S, Stafford J, Madara J L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riggs M W, Perryman L E. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987;55:2081–2087. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanley, S. L., Jr., and K. B. Seydel. 1997. Unpublished observations.
- 20.Thulin J D, Kuhlenschmidt M S, Rolsma M D, Current W L, Gelberg H B. An intestinal xenograft model for Cryptosporidium parvum infection. Infect Immun. 1994;62:329–331. doi: 10.1128/iai.62.1.329-331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]