Abstract
We have compared the in vitro responses of peripheral blood T cells from malaria-unexposed donors to live Plasmodium falciparum schizonts, freeze-thawed schizont extracts (P. falciparum schizont extracts [PfSE]), and parasite culture supernatants. We show that the cells responding to PfSE and parasite culture supernatants are predominantly CD4+ TCRαβ+ while in the presence of live schizonts there is an additional activation of TCRγδ+ cells. Activation of TCRγδ+ cells in response to PfSE was seen only when irradiated autologous feeder cells or recombinant interleukin-2 (IL-2) was added to the cultures. Live schizonts but not PfSE induced significant IL-2 production in vitro in the first 5 days after stimulation, suggesting that induction of early IL-2 by live parasites may contribute to the marked activation of the TCRγδ+ population.
Plasmodium falciparum schizonts induce proliferation of and cytokine production by peripheral blood mononuclear cells (PBMC) from humans with no prior exposure to malaria (1, 12, 16, 28, 37). The production by these cells of proinflammatory cytokines, such as gamma interferon (IFN-γ) (6, 9, 15), has led to the hypothesis that they may contribute to the pathology of the disease (5, 6, 9), which is characterized by release of macrophage-derived cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 (23), and the consequent induction of fever. On the other hand, rapid activation of inflammatory responses may act to limit parasite growth and so be beneficial for the host (9). Identifying the parasite products responsible for polyclonal lymphocyte activation would represent a significant step forward in understanding the pathogenesis of malaria and in the development of a malaria vaccine.
Cells from unprimed donors which respond to malaria antigens are almost exclusively CD3+ T lymphocytes (1, 9, 14, 20, 37), but they have been variously described as predominantly CD4− CD8− TCRγδ+ (2, 13, 14, 31) or predominantly CD4+ TCRαβ+ (5, 6, 9, 30). The kinetics of the response and the requirement for antigen processing and presentation via HLA class II suggest that the effect is mediated by a classical antigen rather than a mitogen or superantigen (5, 9, 20), although some workers have proposed that a superantigen may be responsible for the activation of large numbers of Vγ9Vδ2+ T cells (2, 13, 25).
The in vivo expansion of the TCRγδ+ subset of peripheral blood T cells during primary P. falciparum or Plasmodium vivax infections has been reported (4, 17, 18, 27, 31), and in vitro, preferential activation of TCRγδ+ cells has been reported when PBMC from naive donors were incubated with live, intact P. falciparum-parasitized erythrocytes (14) or freeze-thawed merozoites and/or schizonts (2, 13, 31). In contrast, several groups (including our own) have reported that in vitro stimulation of naive PBMC with freeze-thawed schizonts leads almost exclusively to expansion of CD4+ TCRαβ+ cells (5, 6, 9, 30). Interpretation of the existing data is complicated by differences in methodology between studies (e.g., the presence or absence of exogenous IL-2 or irradiated feeder cells) and made more difficult by the recent realization that many isolates of P. falciparum maintained in long-term culture are contaminated by mycoplasma and that this can lead to artifactual results with respect to cytokine induction (35).
In order to clarify the requirements for activation of naive human T cells by malaria antigens, we have compared the effects of (i) freeze-thawed P. falciparum schizont extract (PfSE), (ii) live (intact) schizont-infected erythrocytes, and (iii) supernatants from overnight cultures of mature P. falciparum schizonts (containing the products of rupturing schizonts) on the lymphoblastic response of naive PBMC and on the surface phenotype of the responding cells. In order to determine the effect of variations in experimental protocols, which might explain contradictory conclusions in the literature, cells were grown in the presence or absence of irradiated autologous feeder cells or recombinant IL-2 (rIL-2).
P. falciparum clone 3D7 (36) was maintained in continuous culture and periodically synchronized by sorbitol treatment (24). Cultures were used when parasitemia reached ∼6 to 8% mature schizonts. Mature schizonts were separated on a 60% Percoll gradient (Pharmacia, Uppsala, Sweden) and adjusted to a concentration of ∼108 schizont-infected cells per ml. PfSE was prepared by two cycles of freeze-thawing in liquid nitrogen and was stored for up to 4 weeks at −70°C. Supernatant was collected from overnight cultures of rupturing schizonts and centrifuged at 10,000 × g for 60 min to remove cellular debris. Freeze-thaw preparations of uninfected erythrocytes (uRBC) (108/ml) and supernatants from uRBC cultures were used as controls. Parasite and uRBC preparations were screened for mycoplasma contamination by using a commercial PCR kit (Stratagene, Cambridge, United Kingdom). PBMC were obtained from malaria-unexposed European blood donors by density centrifugation (Lymphoprep; Nycomed, Oslo, Norway) and resuspended at a concentration of 106 cells/ml in complete medium (9). Autologous feeder cells were prepared by irradiating PBMC in a cesium source (7,500 rads).
PBMC (105 cells/100 μl of complete medium) were cultured, in triplicate, with malaria antigens (104 live schizont-infected erythrocytes or 104 uRBC, or the equivalent mass of PfSE or culture supernatants diluted 1:2) or phytohemagglutinin (PHA) (2 μg/ml; Sigma) for 6 days and pulsed with tritiated thymidine (1 μCi/well; Amersham Life Sciences, Little Chalfont, United Kingdom) for 18 h; incorporation of [3H]thymidine was assessed by liquid scintillation counting. The change in counts per minute was calculated as the geometric mean (GM) counts per minute for triplicate antigen-stimulated wells minus the GM counts per minute of control wells.
Lymphocyte phenotyping was performed by two-color flow cytometry as described previously (9). PBMC (106 cells/ml) were cultured for 7 days with or without antigen (105 live schizonts or uRBC or an equivalent mass of PfSE) and with or without 5 × 105 irradiated feeder cells or exogenous rIL-2 (20 U/ml; Genzyme Diagnostics, Cambridge, Mass.). Viable cells were counted by trypan blue exclusion. Fluorescein isothiocyanate-conjugated antibodies to CD20/H147 (Caltag, San Francisco, Calif.), CD4/S3.5 (Caltag), TCRαβ/BW242/412 (T Cell Diagnostics, Woburn, Mass.), and TCRγδ/5A6.E9 (T Cell Diagnostics) were used. Phycoerythrin-conjugated antibodies to CD3/S4.1(7D6) (Caltag), CD45RO/UCHL1 (Caltag), and CD8/3B5 (Caltag) were used.
The IL-2 concentration was measured by a two-site enzyme-linked immunosorbent assay (Human IL-2 Duoset; Genzyme Diagnostics).
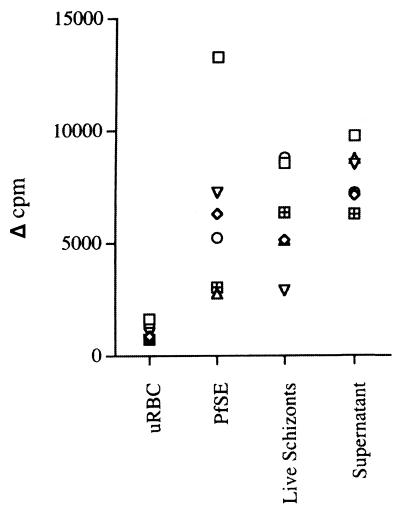
Lymphoproliferative response to P. falciparum.
Cells from all donors responded to live schizonts, parasite culture supernatant, and PfSE, with changes in counts per minute ranging from ∼2,000 to 13,600 (Fig. 1). There was no significant difference in the magnitudes of the proliferative responses to the different malaria antigen preparations. There was no significant proliferative response to either uRBC or control culture supernatant (not shown).
FIG. 1.
Lymphoproliferative responses of naive human PBMC to P. falciparum. Each symbol represents data from a single donor (7-day cultures). Cells from all donors proliferated in response to PHA, showing that they were viable (data not shown).
The proportions of resting (R1) and blasting (R2) lymphocytes were determined, on the basis of forward and side scatter, by flow cytometry (Table 1). The proportions of lymphoblasts in both PfSE- and live-schizont-stimulated cultures were similar and were significantly increased compared to those of uRBC cultures.
TABLE 1.
Proportions and phenotypes of resting lymphocytes and lymphoblasts after 7 days of in vitro culture (n = 7)
| Antigen | Mean % (SEM) of cells in R2 | Mean % (SEM) of CD3+ cells expressing:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4+
|
CD8+
|
TCRαβ
|
TCRγδ
|
||||||
| R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 | ||
| uRBC | 2.8 (0.5)a,b | 67.8 (2.27) | 57.9 (2.64) | 33.4 (1.81) | 34.3 (2.41) | 91.7 (4.37) | 92.2 (1.7) | 5.46 (0.75) | 7.8 (1.7) |
| PfSE | 11.7 (1.2)a,c | 67.2 (2.32) | 74.7 (3.7) | 34.7 (1.00) | 18.7 (2.6) | 91.4 (3.43) | 93.4 (1.3) | 5.04 (0.98) | 6.6 (1.3) |
| Live P. falciparum | 17.1 (5.5)b,c | 58.9 (6.04) | 29.2 (4.6) | 41.3 (2.99) | 22.2 (2.2) | 86.6 (4.07) | 51.4 (4.0) | 12.3 (2.35) | 48.6 (4.0) |
For uRBC versus PfSE, paired t = 10.38, df = 6, and P < 0.001.
For uRBC versus live schizonts, paired t = 2.59, df = 6, and P < 0.05.
For PfSE versus live schizonts, paired t = 0.90, df = 6, and P > 0.2.
Comparison of phenotypes of cells responding to live parasites and PfSE.
Although the percentages of lymphoblasts induced by PfSE and live schizonts were not significantly different, the phenotypes of the responding cells were markedly and significantly different for the two stimuli (Table 1). In PfSE-stimulated cultures, 93% of the CD3+ blasts expressed the αβ T-cell receptor (TCR) and 7% expressed the γδ TCR; similar proportions of TCRαβ+ and TCRγδ+ cells were seen in the R1 population. This is in full agreement with our previous data (9) and that of others (5, 6, 30). However, in cultures stimulated with live schizonts, 49% of CD3+ blasts expressed the γδ TCR and 51% were TCRαβ+; the percentage of TCRγδ+ cells in the resting lymphocyte population was also slightly increased.
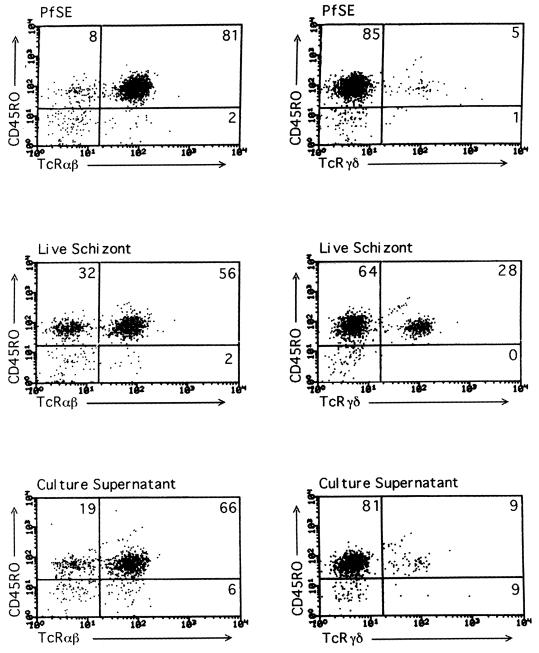
Differences in the proportion of γδ+-T-cell blasts between PfSE and live parasites were highly significant (paired t = 9.17; df = 4; P < 0.001). Within the TCRαβ+ population, CD4+ blasts were less prevalent in cultures stimulated with live parasites than in PfSE-stimulated cultures (paired t = 9.58; df = 4; P < 0.001), but there was no significant difference in the proportion of CD8+ blasts (paired t = 1.78; df = 4; P > 0.1). For both stimuli, 80 to 97% of lymphoblasts expressed the activation-memory marker CD45RO (Fig. 2). In cultures stimulated with parasite supernatant, the percentages of TCRγδ+ cells were low and similar to the percentages in PfSE cultures (Fig. 2). Subsequent experiments therefore concentrated on comparing the effects of live schizonts and PfSE.
FIG. 2.
Fluorescence-activated cell sorting plots showing proportions of TCRαβ+ and TCRγδ+ CD45RO+ lymphoblasts in cultures stimulated for 7 days with PfSE, live schizonts, or parasite culture supernatant for a single, representative donor. Only blasting cells were included in the gate. The numbers in each quadrant are the percentage of gated cells in that quadrant.
When the absolute numbers of cells were compared between PfSE and live schizonts, the GM number of TCRγδ+ blasts was almost sevenfold higher in live-schizont-stimulated cultures than in PfSE-stimulated cultures, but the numbers of CD4+, CD8+, and TCRαβ+ blasts were not significantly different (Table 2). Thus, the expansion of the TCRγδ+ population in live-schizont cultures is in addition to the response of TCRαβ+ cells. The absolute numbers of R1 γδ+ T cells are not significantly different between PfSE-stimulated and live-parasite-stimulated cultures.
TABLE 2.
Resting lymphocytes and lymphoblasts present in 7-day cultures stimulated with PfSE or live P. falciparum schizontsa
| Cell phenotype | R1 stimulated with:
|
R2 stimulated with:
|
tb | P | ||
|---|---|---|---|---|---|---|
| PfSE | Live schizonts | PfSE | Live schizonts | |||
| CD3+ | 50.1 (35.7, 98.7) | 24.3 (17.3, 34.0) | 13.4 (9.2, 19.5) | 11.3 (1.6, 79.9) | 1.07 | >0.2 |
| CD3+ CD4+ | 32.0 (22.1, 46.4) | 14.5 (7.6, 27.5) | 9.9 (6.4, 15.3) | 3.1 (0.5, 18.9) | 1.74 | >0.1 |
| CD3+ CD8+ | 16.8 (12.3, 23.1) | 9.5 (7.6, 11.7) | 2.4 (1.5, 3.9) | 2.4 (0.3, 21.8) | 0.01 | >0.5 |
| CD3+ TCRαβ | 44.1 (27.9, 69.6) | 21.3 (13.8, 33.0) | 12.4 (8.4, 18.4) | 5.7 (0.8, 40.5) | 1.09 | >0.2 |
| CD3+ TCRγδ | 2.2 (0.7, 6.5) | 2.02 (0.5, 8.3) | 0.8 (0.4, 1.6) | 5.4 (0.8, 37.3) | 2.60 | 0.03 |
Results are given as absolute numbers (104) (GM [n = 5]); values in parentheses are lower and upper values, respectively, for the 95% confidence interval.
t test on log-transformed data (log10) for lymphoblast numbers in PfSE cultures versus live-schizont cultures.
Effect of irradiated feeder cells or exogenous IL-2 on activation of naive T cells by P. falciparum.
In previous studies of naive-T-cell responses to P. falciparum, exogenous rIL-2 had been added or cells had been cultured in the presence of autologous, irradiated feeder cells. Irradiated cells are frequently used, in vitro, as a source of antigen-presenting cells and as a nonspecific support mechanism, but there is little recent data on their precise function (26) and little information on the cytokines they secrete. However, their ability to support proliferating cells suggests that they are a useful source of cell growth factors. We therefore compared the effect of rIL-2 or irradiated feeder cells on the T-cell response to PfSE and live schizonts.
In the presence of irradiated feeder cells, the proportion of cells undergoing blastogenesis in response to PfSE was significantly increased, the proportion of TCRαβ+, CD4+, and CD8+ blasts was significantly decreased, and the proportion of TCRγδ+ blasts significantly increased (Table 3). The proportion of γδ+ T cells in the resting lymphocyte population was not affected by the addition of feeder cells (for PfSE with or without feeder cells, t = 1.7; df = 8 [not significant]).
TABLE 3.
Proportions and phenotypes of blast cells after culture with and without irradiated feeder cells (n = 5)
| Culture | % of cells in R2 | % of CD3+ blasts (mean ± SEM) expressing:
|
|||
|---|---|---|---|---|---|
| CD4 | CD8 | TCRαβ | TCRγδ | ||
| uRBC | 2.8 ± 0.58 | 56.49 ± 1.97 | 35.51 ± 1.82 | 92.00 ± 1.77 | 8.00 ± 1.77 |
| uRBC with feeder cells | 2.8 ± 0.58 | 45.87 ± 3.50 | 25.72 ± 4.09 | 71.20 ± 5.61 | 28.8 ± 5.61 |
| Paired t (P [df = 4]) | 3.55 (<0.05) | 2.29 (>0.05) | 4.47 (<0.02) | 4.47 (<0.02) | |
| PfSE | 9.00 ± 1.31 | 66.68 ± 4.25 | 24.32 ± 2.17 | 91.00 ± 2.12 | 9.00 ± 2.12 |
| PfSE with feeder cells | 14.4 ± 2.51 | 45.56 ± 7.42 | 16.24 ± 2.77 | 61.80 ± 6.28 | 36.00 ± 7.80 |
| Paired t (P [df = 4]) | 4.32 (<0.02) | 3.19 (<0.05) | 8.76 (<0.001) | 4.61 (<0.01) | 3.63 (<0.003) |
When rIL-2 (20 U/ml) was added to cultures containing PfSE, there was a significant decrease in the proportion of CD4+ TCRαβ+ blasts and a sixfold increase in the percentage of TCRγδ+ lymphoblasts (Table 4). This is consistent with previous data (13). Again, the percentage γδ+ T cells in the resting lymphocyte population was not affected by the addition of IL-2 (for PfSE with or without IL-2, t = 1.04; df = 8 [not significant]).
TABLE 4.
Proportions and phenotypes of blast cells following in vitro culture for 7 days with or without rIL-2 (n = 5)
| Culture | % of cells in R2 | % of CD3+ blasts (mean ± SEM) expressing:
|
|||
|---|---|---|---|---|---|
| CD4 | CD8 | TCRαβ | TCRγδ | ||
| uRBC | 3.0 ± 0.7 | 57.9 ± 2.6 | 34.3 ± 2.4 | 92.2 ± 1.7 | 7.8 ± 1.7 |
| uRBC with IL-2 | 29.2 ± 11.9 | 37.9 ± 9.0 | 32.7 ± 5.3 | 70.6 ± 6.1 | 29.4 ± 6.1 |
| Paired t (P [df = 4]) | 2.15 (0.1) | 2.11 (>0.1) | 0.24 (>0.5) | 3.99 (<0.02) | 3.99 (<0.02) |
| PfSE | 12.0 ± 1.52 | 74.6 ± 3.69 | 18.8 ± 2.62 | 93.4 ± 1.29 | 6.6 ± 1.29 |
| PfSE with IL-2 | 42.0 ± 10.9 | 38.1 ± 10.8 | 21.3 ± 2.60 | 59.4 ± 10.85 | 40.6 ± 10.85 |
| Paired t (P [df = 4]) | 2.66 (>0.05) | 3.76 (<0.02) | 1.36 (>0.2) | 3.41 (<0.03) | 3.41 (<0.03) |
| Live P. falciparum | 20.0 ± 7.40 | 29.3 ± 4.57 | 22.1 ± 2.23 | 51.4 ± 4.02 | 48.6 ± 4.02 |
| Live P. falciparum with IL-2 | 27.0 ± 5.52 | 40.7 ± 6.70 | 20.7 ± 1.58 | 61.4 ± 5.65 | 38.6 ± 5.65 |
| Paired t (P [df = 8]) | 1.50 (>0.1) | 1.80 (>0.1) | 0.61 (>0.5) | 1.58 (>0.1) | 1.58 (>0.1) |
The effects of feeder cells and rIL-2 are not antigen specific, as similar but less marked effects on blast cell phenotype were observed in uRBC-stimulated cultures. Activation of resting human TCRγδ+ cells upon stimulation with exogenous IL-2 has been reported previously (22) and may reflect the presence of recently activated IL-2 receptor (IL-2R)-positive cells in the peripheral circulation.
Interestingly, there were no significant changes in response when rIL-2 was added to live schizont cultures. Also, there was no significant difference, in terms of the percentage or the phenotype of lymphoblasts, between PfSE with rIL-2 and live schizonts without rIL-2 (P > 0.5 in all cases). These data indicate that the lymphoblastic response to live schizonts can be mimicked by adding rIL-2 or irradiated feeder cells to PfSE-stimulated cultures and support the findings of Elloso et al. (10) that proliferation of γδ+ T cells in this system is dependent on cytokines binding to IL-2R. The effect of IL-2 may be to facilitate activation of TCRγδ+ cells once they have encountered parasite antigens.
Is endogenous IL-2 present in cultures stimulated with live schizonts?
As many human pathogens have acquired the ability to interact directly with the immune system by producing homologs of human cytokines or cytokine receptors (reviewed by Yao et al. [38]) and as extremely high levels of IL-2R in the plasma of malaria patients have been reported (8, 19, 21, 29), we investigated whether IL-2 or IL-2-like substances were secreted by live P. falciparum schizonts. We tested fresh P. falciparum culture supernatants (from cultures with 10 to 12% parasitemia that had undergone schizogony in the previous 12 h) for the presence of IL-2 by bioassay (33) but found no evidence of any IL-2-like activity (data not shown). This is consistent with the failure of culture supernatant alone to activate large numbers of γδ T cells (Fig. 2).
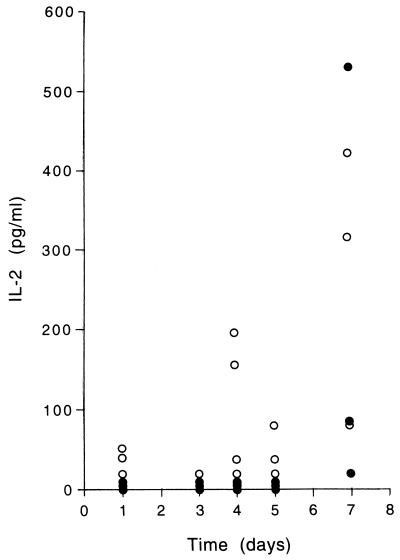
We then hypothesized that live schizonts induced more IL-2 production by naive PBMC than did PfSE (leading to activation of TCRγδ+ cells in the presence of live schizonts). By enzyme-linked immunosorbent assay, we tested culture supernatants from PBMC cultured with PHA, uRBC, PfSE, or live schizonts for IL-2 (Fig. 3). IL-2 was not found at any time in cultures stimulated with uRBC but was at a high level (>650 pg/ml) in all PHA-stimulated cultures. After 24 h, significant levels of IL-2 were detectable in two of three cultures with live schizonts but in none of the cultures with PfSE. There was no detectable IL-2 in cultures after 3 days, but at 4 and 5 days there was detectable IL-2 in 3 of 4 and 2 of 3 live-parasite cultures, respectively; again, there was no detectable IL-2 in PfSE cultures. After 7 days, high levels of IL-2 were found in both PfSE and live-parasite cultures. These data strongly suggest that live P. falciparum schizonts (but not killed parasites) are able to induce an early burst of IL-2 production which may facilitate activation of TCRγδ+ cells. Experiments are under way to determine the cellular source of this early IL-2.
FIG. 3.
IL-2 in culture supernatants of cells stimulated with PfSE (•) or live schizonts (○). Control cultures (stimulated with uRBC) all contained <20 pg of IL-2 per ml at each time point. PHA-stimulated cultures all contained >650 pg of IL-2 per ml after 24 h of stimulation.
These experiments have demonstrated that there are two distinct patterns of activation of naive human T cells by malaria parasites. CD4+ TCRαβ+ cells are activated by components of freeze-thawed schizonts; we have previously shown this to be due to an insoluble, partially heat-stable membrane-associated component of schizont-infected erythrocytes (9) which may contain antigens which cross-react with common commensal or environmental organisms (5, 6). In contrast, TCRγδ+ cells are preferentially activated by components of live schizonts, a response which seems to be at least partly dependent on rapid induction of IL-2 from PBMC. This may explain the observation of Tsuji et al. (34), who found that γδ+-T-cell clones induced by immunization of mice with live Plasmodium yoelii did not respond to extracts of dead parasites.
Culture supernatants from freshly ruptured schizonts did not contain noticeable γδ+-T-cell-activating components, indicating that the antigens released by live schizonts are either present at a low concentration or are unstable. An alternative explanation might be that direct contact is required between the parasitized erythrocyte and the lymphocyte. We propose, as a working hypothesis, that activation of naive TCRγδ+ cells by malaria schizonts requires the presence of an extremely labile parasite product, which may either induce or mimic the effects of IL-2. In support of this hypothesis, we show that live schizonts induce an early burst of IL-2 production from PBMC, which is not seen when PBMC are cultured with PfSE. The presence of a very labile TCRγδ ligand may explain discrepancies with respect to activation of γδ+ T cells by freeze-thaw parasite extracts; immediate use of rapidly freeze-thawed parasites may retain γδ cell activation, but activity may be lost in extracts which are stored for any length of time.
This scenario is similar to that described for the TCRγδ response to mycobacteria, in which it is proposed that the Vγ9Vδ2 T-cell ligands are phosphorylated metabolites of living bacteria and have short half-lives (7). Accordingly, a P. falciparum-derived antigenic stimulus for γδ+ T cells has recently been ascribed to two phosphorylated molecules, similar to those previously described for Mycobacterium tuberculosis, present at extremely low levels in supernatants of freshly lysed schizonts (3). High-performance liquid chromatography fractions containing these molecules were able to induce proliferation of naive PBMC and activation of the TCRγδ+ subset without exogenous IL-2, but the requirement for CD4+ TCRαβ+ cells as a potential source of IL-2 was not analyzed. The data presented here are consistent with the in vivo finding of very high proportions of TCRγδ+ cells in the peripheral circulation of patients with acute malaria (4, 17, 18, 27, 31) and may explain the lack of such γδ+-T-cell expansion in patients with very low levels of peripheral parasitemia (32). Acute malaria is also characterized by extremely high levels of circulating soluble IL-2R (8, 19, 21, 29), indicating significant secretion of IL-2 somewhere in the body. Our data suggest that products of live parasites induce this IL-2 response and that both parasite antigens and IL-2 contribute to γδ+-T-cell activation in vivo.
In addition to identifying the ligands of the αβ+ and γδ+ T cells, it is important to characterize their function. Both P. falciparum-stimulated TCRγδ+ cells and PfSE-stimulated PBMC have been reported to synthesize proinflammatory cytokines (9, 15). Some studies claim that TCRγδ+ cells are the major source of IFN-γ during the response to schizont antigens (15) and are able to inhibit the growth of P. falciparum in vitro (11)—although there is no direct evidence that these two effects are causally associated—while others report high levels of IFN-γ secretion in the absence of significant γδ+-T-cell activation (6, 9, 39). In order to determine the relative importance of these two T-cell populations in mediating the pathogenesis of malaria or controlling the infection, it is necessary to determine the sequence of events leading to and the cells and cytokines involved in the activation of each cell population.
Acknowledgments
We thank Susan Haley for technical assistance and David McGuinness for statistical advice.
This work was supported by grants from the Wellcome Trust.
REFERENCES
- 1.Ballet J, Druilhe P, Querleux M, Schmitt C, Agrapart M. Parasite-derived mitogenic activity for human T cells in Plasmodium falciparum continuous cultures. Infect Immun. 1981;33:758–762. doi: 10.1128/iai.33.3.758-762.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr C, Dubois P. Preferential expansion of Vγ9 Vδ2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol. 1992;4:361–366. doi: 10.1093/intimm/4.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Behr C, Poupot R, Peyrat M, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie J. Plasmodium falciparum stimuli for human γδ T cells are related to the phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–2896. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang W, van der Heyde H, Maki D G, Malkovsky M, Weidanz W P. Subset heterogeneity among γδ T cells found in peripheral blood during Plasmodium falciparum malaria. Immunol Lett. 1992;32:273–274. doi: 10.1016/0165-2478(92)90061-r. [DOI] [PubMed] [Google Scholar]
- 5.Currier J, Beck H, Currie B, Good M F. Antigens released at schizont burst stimulate Plasmodium falciparum-specific CD4+ T cells from nonexposed donors: potential for cross-reactive memory T cells to cause disease. Int Immunol. 1995;7:821–833. doi: 10.1093/intimm/7.5.821. [DOI] [PubMed] [Google Scholar]
- 6.Currier J, Sattabongkot J, Rosenberg R, Good M F. Natural T cells responsive to malaria: evidence implicating immunological cross reactivity in the maintenance of TCRαβ+ malaria specific responses from non-exposed donors. Int Immunol. 1992;4:985–994. doi: 10.1093/intimm/4.9.985. [DOI] [PubMed] [Google Scholar]
- 7.De Libero G. Sentinel function of broadly reactive human γδ T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 8.Deloron P, Lepers J P, Coulanges P. Evolution of levels of soluble interleukin-2 receptors during Plasmodium falciparum and Plasmodium vivax malaria. J Clin Microbiol. 1989;27:1887–1889. doi: 10.1128/jcm.27.8.1887-1889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick S, Waterfall M, Currie J, Maddy A, Riley E. Naive human αβ T cells respond to membrane-associated components of malaria-infected erythrocytes by proliferation and production of IFNγ. Immunology. 1996;88:412–420. doi: 10.1046/j.1365-2567.1996.d01-661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elloso M M, van der Heyde H C, Troutt A, Manning D D, Weidanz W P. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol. 1996;157:2096–2102. [PubMed] [Google Scholar]
- 11.Elloso M M, van der Heyde H C, Vande Waa J A, Manning D D, Weidanz W P. Inhibition of Plasmodium falciparum in vitro by human γδ T cells. J Immunol. 1994;153:1187–1194. [PubMed] [Google Scholar]
- 12.Gabrielsen A A, Jensen J B. Mitogenic activity of extracts from continuous cultures of Plasmodium falciparum. Am J Trop Med Hyg. 1982;31:441–448. doi: 10.4269/ajtmh.1982.31.441. [DOI] [PubMed] [Google Scholar]
- 13.Goerlich R, Häcker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparum merozoites primarily stimulate the Vγ9 subset of human γδ T cells. Eur J Immunol. 1991;21:2613–2616. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 14.Goodier M, Fey P, Eichmann K, Langhorne J. Human peripheral blood γδ T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992;4:33–41. doi: 10.1093/intimm/4.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Goodier M, Lundqvist C, Hammarstrom M, Troye-Blomberg M, Langhorne J. Cytokine profiles for human Vγ9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol. 1995;17:413–423. doi: 10.1111/j.1365-3024.1995.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood B M, Vick R M. Evidence for a malaria mitogen in human malaria. Nature. 1975;257:592–594. doi: 10.1038/257592a0. [DOI] [PubMed] [Google Scholar]
- 17.Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, Shafiq J, Suntharsamai P, Looareesuwan S, Webster H K, Elliott J F. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparum malaria. Infect Immun. 1994;62:855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho M, Webster H K, Tongtawe P, Pattanapanyasat K, Weidanz W P. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen P H, Morris-Jones S, Theander T G, Hviid L, Hansen M B, Bendtzen K, Ridley R G, Greenwood B M. Increased plasma levels of soluble Il-2R are associated with severe Plasmodium falciparum malaria. Clin Exp Immunol. 1994;96:98–103. doi: 10.1111/j.1365-2249.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones K R, Hickling J K, Targett G A T, Playfair J H L. Polyclonal in vitro proliferative responses from non-immune donors to Plasmodium falciparum malaria antigens require UCHL1+ (memory) T cells. Eur J Immunol. 1990;20:307–315. doi: 10.1002/eji.1830200212. [DOI] [PubMed] [Google Scholar]
- 21.Josimovic-Alasevic O, Feldmeier H, Zwingenberger K, Harms G, Hahn H, Shrisuphanunt M, Diamantstein T. Interleukin-2 receptor in patients with localised and systemic parasitic diseases. Clin Exp Immunol. 1988;72:249–254. [PMC free article] [PubMed] [Google Scholar]
- 22.Kjeldsen-Kragh J, Quayle A J, Skålhegg B S, Sioud M, Forre O. Selective activation of resting human γδ T lymphocytes by interleukin-2. Eur J Immunol. 1993;23:2092–2099. doi: 10.1002/eji.1830230908. [DOI] [PubMed] [Google Scholar]
- 23.Kwiatkowski D, Hill A V S, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D, Greenwood B M. TNF concentration in fatal, non-fatal cerebral and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;ii:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 24.Lambros J, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 25.Langhorne J, Goodier M, Behr C, Dubois P. Is there a role for γδ T cells in malaria? Immunol Today. 1992;13:298–300. doi: 10.1016/0167-5699(92)90041-5. [DOI] [PubMed] [Google Scholar]
- 26.Miller R A. Quantitation of functional T cells by limiting dilution. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 13.12.1–13.12.11. [DOI] [PubMed] [Google Scholar]
- 27.Perera M K, Carter R, Goonewardene R, Mendis K N. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley E M, Jepsen S, Andersson G, Otoo L N, Greenwood B M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988;71:377–382. [PMC free article] [PubMed] [Google Scholar]
- 29.Riley E M, Rowe P, Allen S J, Greenwood B M. Soluble plasma IL-2 receptors and malaria. Clin Exp Immunol. 1993;91:495–499. doi: 10.1111/j.1365-2249.1993.tb05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roussilhon C, Agrapart M, Behr C, Dubois P, Ballet J-J. Interactions of CD4+ and CD8+ human T lymphocytes from malaria-unprimed donors with Plasmodium falciparum schizont stage. J Clin Microbiol. 1989;27:2544–2551. doi: 10.1128/jcm.27.11.2544-2551.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet J J. Human TcRγδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–97. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzepczyk C, Stamiatou S, Anderson K, Stowers A, Cheng Q, Saul A, Allworth A, McCormack J, Whitby M, Olive C, Lawrence G. Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to γδ T cells. Scand J Immunol. 1996;43:219–227. doi: 10.1046/j.1365-3083.1996.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 33.Swain S L, Dennert G, Warner J F, Dutton R W. Culture supernatants of a stimulated T-cell line have helper activity that acts synergistically with interleukin 2 in the response of B cells to antigen. Proc Natl Acad Sci USA. 1981;78:2517–2521. doi: 10.1073/pnas.78.4.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji M, Eyster C L, O’Brien R L, Born W K, Bapna M, Reichel M, Nussenzweig R S, Zavala F. Phenotypic and functional properties of murine γδ T cell clones derived from malaria immunized, αβ T cell-deficient mice. Int Immunol. 1996;8:359–366. doi: 10.1093/intimm/8.3.359. [DOI] [PubMed] [Google Scholar]
- 35.Turrini F, Giribaldi G, Valente E, Arese P. Mycoplasma contamination of Plasmodium cultures—a case of parasite parasitism. Parasitol Today. 1997;13:367–368. doi: 10.1016/s0169-4758(97)01088-0. [DOI] [PubMed] [Google Scholar]
- 36.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T R, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 37.Wyler D J, Herrod H G, Weinbaum F I. Response of sensitized and unsensitized human lymphocyte subpopulations to Plasmodium falciparum antigens. Infect Immun. 1979;24:106–110. doi: 10.1128/iai.24.1.106-110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Fanslow W C, Seldin M F, Rousseau A, Painter S L, Comeau M R, Cohen J I, Spriggs M K. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 39.Zevering Y, Amante F, Smillie A, Currier J, Smith G, Houghten R A, Good M F. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992;22:689–696. doi: 10.1002/eji.1830220311. [DOI] [PubMed] [Google Scholar]