Introduction
Stable-treated patients with pulmonary vascular disease (PVD) defined as pulmonary arterial or distal chronic thromboembolic pulmonary hypertension (PAH/CTEPH) wish to participate in daily activities including travel to high altitude or by air, but may be at increased risk of adverse events at high altitude (AEHA).1,2 Thus, pulmonary hypertension (PH) guidelines recommend that symptomatic PVD patients should not travel >1500 m or fly without supplemental oxygen therapy (SOT),3 but evidence is scarce and ambiguous.4
Methods
Pulmonary vascular disease patients were investigated at 470 m (Zurich, low altitude, LA) and 2500 m (high altitude, HA) during a 30 h overnight stay at Mount Säntis according to a randomized-sequence, cross-over design (LA–HA vs. HA–LA) including >2-week washout between study sites. Patients were transported within 3 h by car and ropeway from home to 2500 m. The trial was performed between October 2021 and April 2022, ethically approved and registered (ClinicalTrials.gov: NCT05107700).
Patients were adults of all genders diagnosed with pre-capillary PH, classified as PAH or distal CTEPH,3 stable on medical therapy and providing written informed consent. Chronic thromboembolic pulmonary hypertension patients were inoperable or had persistent PH after interventional therapy. Patients with baseline PaO2 <8 kPa, FEV1 or FVC <70%, significant concomitant diseases, pregnancy or breast feeding were excluded.
The main outcomes were AEHA, pre-defined as (i) severe hypoxaemia [oxygen saturation by pulse oximetry (SpO2) < 80% > 30 min], or (ii) acute mountain sickness (AMS) by Lake Louise score ≥4 with headache or AMSc score ≥0.7, or (iii) any new illness including symptomatic cardiac arrhythmia, severe rise in systolic/diastolic blood pressure (>200/100 mmHg) or angina, and the therapeutic effect of SOT to restore baseline values in AEHA.
Participants’ SpO2 and condition were frequently monitored during daytime and continuously overnight at 2500 m. Patients with severe hypoxaemia were treated with SOT 3 L/min via nasal cannula (EverFlow, Philips Respironics) and had further HA assessments using SOT.
Other outcomes the second day at 2500 vs. 470 m were change in resting SpO2, systolic pulmonary artery pressure (sPAP) calculated from tricuspid regurgitation pressure velocity by echocardiography and 6 min walk distance (6MWD) including SpO2, heart rate, and Borg dyspnoea scale (Borg) at end-walk. Statistical analysis was performed with Stata (StataCorp, College Station, TX, USA) using mixed-linear regression models, and variables are presented as means ± standard deviation, numbers or mean differences (95% confidence intervals). A two-sided P-value <.05 was considered statistically significant.
Results
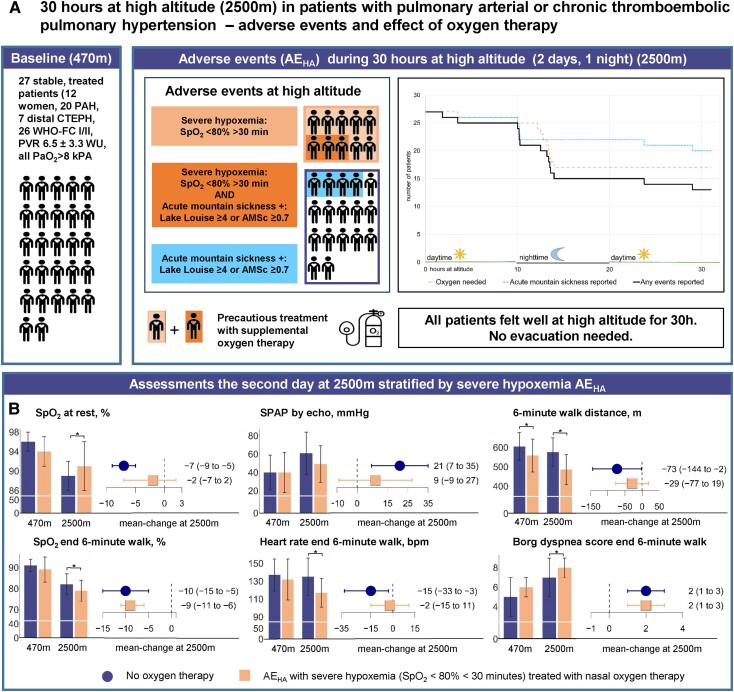
Of 65 assessed, 27 patients (12 women, 20 PAH, 7 distal CTEPH, age 62 ± 14 years) were included (Figure 1). All felt well during the study including 3 months thereafter. Pre-defined AEHA occurred in 14/27: 10/27 (37%) had severe hypoxaemia (9/10 nocturnal), thereafter treated with SOT, and 7/27 scored positive for AMS, of which 3/7 combined with severe hypoxaemia.
Figure 1.
(A) Baseline characteristics of 27 patients with pulmonary vascular disease defined as pulmonary arterial hypertension or distal chronic thromboembolic pulmonary hypertension are shown (left sector, WHO-FC, WHO dyspnoea functional class; PVR, pulmonary vascular resistance; PaO2, partial pressure of oxygen in arterial blood). Adverse events at high altitude (AEHA) including severe hypoxaemia and acute mountain sickness are graphically illustrated (middle sector). The sector on the right reveals the time to AEHA overall (solid black line) and stratified for patients with severe hypoxaemia treated with supplemental oxygen therapy (long dashed orange line) and those with acute mountain sickness without severe hypoxaemia (dotted blue line), with colours corresponding to the illustration in the middle sector. (B) Assessments at 470 and 2500 m are shown stratified for patients without supplemental oxygen therapy at 2500 m (left dark blue bars and whiskers) and patients with severe hypoxaemia AEHA at 2500 m (right orange bars and whiskers). Bars at the left of each of the six subpanels represent means and standard deviations, whiskers represent mean difference and 95% confidence interval. *Significant difference (P < .05) as calculated by a mixed-linear regression model by intervention (2500 vs. 470 m) corrected for the intervention sequence as a fixed effect and subjects as a random effect. SPAP, systolic pulmonary artery pressure assessed from tricuspid regurgitation velocity without adding right atrial pressure by echocardiography
In patients without severe hypoxaemia at 2500 vs. 470 m, the SpO2 was lower (89 ± 3 vs. 96 ± 2%, P < .001), the sPAP was higher (61 ± 23 vs. 40 ± 19 mmHg, P = .004), and 6MWD was equal (576 ± 74 vs. 605 ± 72 m, P = .240) with lower SpO2 (82 ± 5 vs. 91 ± 3%, P < .001), higher Borg (7 ± 2 vs. 7 ± 2, P < .001), but unchanged heart rate (135 ± 21 vs. 137 ± 18 b.p.m., P = .741) at end-walk. In patients with severe hypoxaemia needing SOT, SpO2 and sPAP were restored to baseline (91 ± 5 vs. 94 ± 3%, P = .250 and 49 ± 20 vs. 40 ± 22 mmHg, P = .322), whereas the 6MWD was reduced (485 ± 78 vs. 558 ± 85 m, P = .045) with a lower SpO2 (79 ± 5 vs. 89 ± 6%, P < .001), higher Borg (8 ± 1 vs. 6 ± 1, P < .001) but unchanged heart rate (117 ± 16 vs. 132 ± 23 b.p.m., P = .095).
Discussion
This randomized controlled trial in stable PVD patients, mostly in functional Class I/II, showed that during 30 h at 2500 m in accordance with a weekend getaway, pre-defined AEHA occurred in 14/27 patients, mostly as severe hypoxaemia during the night, which was effectively treated with subsequent SOT.5 All patients felt subjectively well at 2500 m. The threshold of SpO2 desaturation which forces physicians to provide SOT at HA is debated, but strongly depends on whether healthy or patients are concerned. Whilst anaesthetists maintain SpO2 >92% by routinely administering high-dose SOT, many tourists and mountaineers feel well for prolonged times at very HA with much lower SpO2 even under strenuous exercise.6 Healthy report SpO2 around 85% at 4500 m that decreases to 45% at 8848 m without experiencing long-term consequences. However, observing PVD patients with SpO2 <80% at HA would be considered hazardous by physicians and ethical board in fear of serious AEHA and guidelines recommend to treat PVD patients with PaO2 <8 kPa with SOT.3 We thus administered SOT if SpO2 dropped <80% >30 min at HA, a threshold proven confident in >300 chronic obstructive pulmonary disease (COPD) patients, preliminary data in PVD and by the present PVD cohort.1,5,7
Data about PVD patients systematically investigated in a hypoxic environment are scarce and restricted to very short time, mostly to normobaric hypoxia.1,2,8 During a daytrip to 2500 m, 3/30 PVD patients needed SOT according to similarly defined AEHA.1 Presently, severe hypoxaemia occurred almost exclusively during nights, which is in accordance with COPD patients at 2590 m and a pilot of 9 PVD patients at 2048 m.8
Of the 7/27 PVD patients who scored positive for AMS due to mild headache, only 3 needed SOT, none wanted painkillers and all stayed at HA without subjective complaints. Thus, current AMS questionnaires may not define clinically relevant altitude illness in PVD patients, who may indicate occasional headache even at low altitude, potentially associated with vasodilator treatment. Albeit PVD patients with AEHA were less fit, logistic regression models did not identify low-altitude measures that significantly predict AEHA.
At 2500 m, SOT restored SpO2 and sPAP to low-altitude levels, whereas patients not needing SOT revealed lower SpO2 and higher sPAP, but revealed similar 6MWD, suggesting unchanged pressure flow.9 Contrarily, patients needing SOT at 2500 m revealed a lower 6MWD along with a reduced SpO2 and higher Borg at end-walk. Thus, in contrast to high-flow SOT,10 3 L/min via nasal cannula was not sufficient to reverse 6MWD and exercise-induced hypoxaemia at HA, potentially due to increased mouth breathing or insufficient flow. The main limitations are the relatively fit PVD collective with a mean baseline 6MWD of 580 m, the short HA exposure, and studying only central European PVD patients.
To conclude, stable, low-risk PVD patients tolerated a weekend getaway to 2500 m for up to 30 h generally well, none needed evacuation. In case of severe hypoxaemia, SOT restores resting but not exercise low-altitude physiology. The results of this field study help to counsel PVD patients for HA sojourns and call for future longer term studies.
Acknowledgements
S.U. is the guarantor and takes responsibility for the content of the manuscript, including the data and analysis.
Contributor Information
Simon R Schneider, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Julian Müller, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Meret Bauer, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Laura Mayer, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Lea Lüönd, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Tanja Ulrich, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Michael Furian, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Aglaia Forrer, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Arcangelo Carta, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Esther I Schwarz, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Konrad E Bloch, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Mona Lichtblau, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Silvia Ulrich, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, CH-8091 Zürich, Switzerland.
Declarations
Disclosure of Interest
S.U. reports grants from Johnson and Johnson SA, Switzerland, during the conduct of the study; and grants from the Swiss National Science Foundation and Zurich Lung, grants and personal fees from Orpha Swiss, and personal fees from Actelion SA and MSD SA, outside the submitted work. M.L. reports travel grants from Johnson and Johnson SA, Switzerland, and personal fees from MSD, Switzerland, during the conduct of the study, grants from the Swiss Lung League, all outside the submitted work. All other authors have nothing to disclose.
Data Availability
The data presented in this study are available on reasonable request from the corresponding author. Data will be uploaded to an online repository after publication including substudies.
Funding
The trial is funded by the Swiss National Science Foundation (SNSF, grant number 32003B_197706).
Ethical Approval
The trial was approved by the ethics committee Zurich (KEK Project-ID 2021-00243).
Pre-registered Clinical Trial Number
The pre-registered clinical trial numbers are (ClinicalTrials.gov: NCT05107700 and NCT05112172).
References
- 1. Schneider SR, Mayer LC, Lichtblau M, Berlier C, Schwarz EI, Saxer S, et al. . Effect of a day-trip to altitude (2500 m) on exercise performance in pulmonary hypertension: randomised crossover trial. ERJ Open Res 2021;7:00314-2021. 10.1183/23120541.00314-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider SR, Mayer LC, Lichtblau M, Berlier C, Schwarz EI, Saxer S, et al. . Effect of normobaric hypoxia on exercise performance in pulmonary hypertension: randomized trial. Chest 2021;159:757–71. 10.1016/j.chest.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 3. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 4. Carta AF, Lichtblau M, Berlier C, Saxer S, Schneider SR, Schwarz EI, et al. . The impact of breathing hypoxic gas and oxygen on pulmonary hemodynamics in patients with pulmonary hypertension. Front Med (Lausanne) 2022;9:791423. 10.3389/fmed.2022.791423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lichtblau M, Furian M, Aeschbacher SS, Bisang M, Ulrich S, Saxer S, et al. . Dexamethasone improves pulmonary hemodynamics in COPD-patients going to altitude: a randomized trial. Int J Cardiol 2019;283:159–64. 10.1016/j.ijcard.2018.12.052 [DOI] [PubMed] [Google Scholar]
- 6. Luks AM, Hackett PH. Medical conditions and high-altitude travel. N Engl J Med 2022;386:364–73. 10.1056/NEJMra2104829 [DOI] [PubMed] [Google Scholar]
- 7. Furian M, Mademilov M, Buergin A, Mayer L, Schneider S, Emilov B, et al. . Acetazolamide to prevent adverse altitude effects in COPD and healthy adults. NEJM Evidence 2022;1:EVIDoa2100006. 10.1056/EVIDoa2100006 [DOI] [PubMed] [Google Scholar]
- 8. Lichtblau M, Saxer S, Latshang TD, Aeschbacher SS, Huber F, Scheiwiller PM, et al. . Altitude travel in patients with pulmonary hypertension: randomized pilot-trial evaluating nocturnal oxygen therapy. Front Med (Lausanne) 2020;7:502. 10.3389/fmed.2020.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joseph P, Oliveira RKF, Eslam RB, Agarwal M, Waxman AB, Systrom DM. Fick principle and exercise pulmonary hemodynamic determinants of the six-minute walk distance in pulmonary hypertension. Pulm Circ 2020;10:2045894020957576. 10.1177/2045894020957576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulrich S, Hasler ED, Saxer S, Furian M, Müller-Mottet S, Keusch S, et al. . Effect of breathing oxygen-enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham-controlled cross-over trial. Eur Heart J 2017;38:1159–68. 10.1093/eurheartj/ehx099 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. Data will be uploaded to an online repository after publication including substudies.