Abstract
Many RNA delivery strategies require efficient endosomal uptake and release. To monitor this process, we developed a 2′-OMe RNA-based ratiometric pH probe with a pH-invariant 3ʹ-Cy5 and 5ʹ-FAM whose pH sensitivity is enhanced by proximal guanines. The probe, in duplex with a DNA complement exhibits a 48.9-fold FAM fluorescence enhancement going from pH 4.5 to pH 8.0, and reports on both endosomal entrapment and release when delivered to HeLa cells. In complex with an antisense RNA complement, the probe constitutes an siRNA mimic capable of protein knockdown in HEK293T cells. This illustrates a general approach for measuring the localization and pH microenvironment of any oligonucleotide.
Graphical Abstract

The development of RNA vaccines and therapeutics has focused attention on several remaining challenges in RNA delivery. Endosomal release into the cytoplasm has been a persistent barrier to successful delivery.1,2 Because the endosome and cytoplasm are acidic and slightly basic, respectively, real-time measurements of intracellular pH can directly and quantitatively monitor endosomal escape.3 Split protein complementation is sensitive and robust, but very complex.4–6 The classic calcein assay is simpler but entirely qualitative.7–10 6-carboxyfluorescein (FAM) is a well-established and ubiquitous dye in biological research.11 In aqueous solution, the equilibrium between the mono- and dianion (Scheme S1) gives rise to FAM’s characteristic pH sensitivity. Many pH-sensitive fluorophores have been investigated in the context of nucleic acid delivery9,12–20 but FAM conjugates, in addition to possessing favorable spectral properties21–23 and sequence-dependent fluorescence intensity,24–28 are inexpensive and can often be synthesized in a single step. Conjugation of pH-sensitive dyes provides a strategy for directly monitoring the oligonucleotide payload. In a pioneering example, Modi et al. reported a three-component DNA nanomachine relying on an i-motif to modulate the FRET efficiency of a dye pair with 5-fold signal enhancement from pH 5 to 7 in vitro and in vivo.29,30 While effective, this FRET-based approach restricts the sequence scope and must employ further bioconjugation to monitor any cargo of interest. Other methods often rely on inefficient or nonspecific labeling chemistry along with time-consuming synthesis.31–34 The uptake of siRNA is commonly inferred using reporter genes that are susceptible to artifacts and blind to the spatiotemporal details of cell delivery. Due to the significance of endosomes for siRNA delivery,35,36 we developed a simple yet robust ratiometric pH probe to enable the direct visualization and quantitation of cell uptake.
In our design (Scheme 1), the 5′- and 3′-termini of an RNA oligonucleotide are labeled with FAM and Cyanine 5 (Cy5); the latter is sufficiently pH-stable37,38 for ratiometric purposes and acts as an internal standard.20,31 Following delivery to cells, intracellular pH can be quantified from the FAM / Cy5 fluorescence intensity ratio to assess the extent of endosomal release at every voxel and time point.
Scheme 1.

Ratiometric probe design enables oligonucleotide visualization and local pH measurement
Under physiologic conditions, the pH sensitivity of FAM conjugated to many biomolecules is muted compared to the free dye.20,39,40 To ascertain the extent of this effect in a nuclease-resistant41,42 2′-OMe RNA probe, we compared the fluorescence dynamic range (FAM signal at pH 8.0 relative to pH 4.5) for both free fluorescein and a 5′-FAM-U5-3′ conjugate in PBesque, a buffer series of constant ionic strength (Table 1).21
Table 1.
Dynamic Range (DR) and Relative Quantum Yield (QY) for FAM-Labeled Oligos.a
| Construct | DR (fold) | QYa/QYa0 | QYb/QYb0 |
|---|---|---|---|
| 5′-FAM-U5-3′ | 13.0 | 0.55 | 0.94 |
| 5′-FAM-U4G-3′ | 15.6 | 0.38 | 0.77 |
| 5′-FAM-GU4-3′ | 18.1 | 0.13 | 0.33 |
| 5′-FAM-G2U3-3′ | 30.7 | 0.06 | 0.28 |
| Fluorescein | 15.4 | 1.00 | 1.00 |
| Probe | 55.9 | N/A | N/A |
| Probe-DNA | 48.9 | N/A | N/A |
| Probe-RNA | 30.3 | N/A | N/A |
Underlined bases signify 2′-OMe RNA. QYa and QYa0 refer to sample and free dye quantum yields, respectively, at pH 4.9. QYb and QYb0 are measured at pH 8.0.
As reported,43–45 modest pH sensitivity was observed for free fluorescein in buffer, with a 15.4-fold fluorescence dynamic range (Figures 1 and S1, Table 1). The FAM-U5 construct achieved an emission enhancement of only 13.0-fold (Figure 1, Table 1) with a slight reduction in quantum yield compared to the free dye (Tables 1, S1, and Figure S1). To enhance the dynamic range of the FAM-oligonucleotide conjugate, we investigated guanine (G) near the dye. When FAM and G are adjacent on a DNA strand, the fluorescence quantum yield can decrease by as much as 40% via photoinduced electron transfer.25,27 It is unknown, however, whether G-quenching is pH-sensitive and capable of increasing pH sensitivity in FAM-oligo conjugates. Our G-containing sequences, while quenched relative to FAM-U5 (Table 1), achieved superior dynamic ranges with each U-to-G substitution (Table 1, Figure 1): FAM-U4G (15.6-fold), FAM-GU4 (18.1-fold) and FAM-G2U3 (30.7-fold). These data highlight the potential for using multiple proximal G nucleobases to increase pH sensitivity in FAM-oligo conjugates.
Figure 1.

Proximal guanine increases n-fold integrated fluorescence change of FAM (λex = 488 nm) attached to 5ʹ end of 2ʹ-OMe RNA, as a function of pH (1.0 μM, n = 4). Error bars represent ± 1 S. D.
To investigate the role of guanine in this system, we analyzed the fluorescence quantum yields of the free dye and all test constructs at 1.0 μM at pH 4.9 and pH 8.0 PBesque as described by the Sawyer group (Tables 1 and S1).22 As expected, in acidic solution a lower quantum yield was observed for the free fluorophore (QYa0) and all FAM conjugates (QYa). Conjugation to U5 decreased the ratio QYa/QYa0 to 0.55. Single distal, single proximal and double proximal G-substitution decreased QYa/QYa0 to 0.38, 0.13, and 0.06, highlighting the efficient, distance-dependent G quenching in acidic solution (Table 1). Conjugation decreased QYb/QYb0 to 0.94 in basic (pH 8.0) conditions while the same G-substitutions resulted in QYb/QYb0 values of 0.77, 0.33, and 0.28, respectively (Table 1). These findings, along with literature describing the quenching of FAM by G,46–49 suggest a pH dependence of the G-quenching effect that is positively correlated to the observed dynamic ranges. At pH 8, more G-bases are anionic than at pH 4.9 and less likely to be oxidized.
To validate G-quenched FAM as a pH sensor for biological cargo, we synthesized (Figure S2) the sequence 5ʹ-FAM-GGA CAA AUU UAA UCU UAC UdTdT-Cy5-3ʹ (Probe), which is based on the sense strand of a known siRNA against human CSNK2β and offers two G nucleobases proximal to FAM.50 We recovered 60 nmol ( 31% yield), ESI-MS confirmed the correct mass (Figure S3), and analytical HPLC indicated a purity of 98% (Figure S4). The dTdT overhang was included to protect the construct from 3′ exonucleases. In the presence of RNase A at 37 °C, the single-stranded Probe remained intact for 24 h (Figure S5). CSNK2β plays roles in proliferation and cell cycle regulation in mammalian cells and is implicated in apoptosis in triple negative breast cancer.50 In line with data for the 5-mers (Figures 1, S1 and Table 1), Probe demonstrated 55.9-fold fluorescence dynamic range. However, much FAM emission was lost to FRET with Cy5, which showed the expected pH invariance (Figure S6).
To abrogate FRET, we duplexed Probe with its antisense DNA complement, 5ʹ-AGT AAG ATT AAA TTT GTC CTG-3ʹ, to form a stable duplex (Tm = 48 °C, Figure S7). Between pH 4.5 and 8.0, the FAM signal increased 48.9-fold (Figures 2, S8, S9, and Table 1), while the Cy5 signal remained constant. We then evaluated Probe-DNA for its pH measurement performance in live HeLa cells, which do not express CSNK2β,50 via flow cytometry. The antisense DNA complement prevents the construct from triggering off-target RNAi, and HeLa cells are amenable to quantitative live cell imaging and lack CSNK2β mRNA transcripts that could engage the Probe. We focused initially on a slow uptake process that can be monitored over several hours, to facilitate visualization by microscopy and quantitation by flow cytometry.
Figure 2.

Dynamic ranges of 5ʹ-FAM and 3ʹ-Cy5 attached to Probe-DNA duplex in PBesque buffer, expressed as relative fold change in integrated fluorescence intensity as a function of pH (1.0 μM, n = 4). Error bars represent ± 1 S.D.
Using Poly-l-lysine (PLL) ± 100 μM chloroquine, we delivered Probe-DNA to HeLa cells, incubated them at 37 °C and 5% CO2 for 2 and 4 h, and measured the average intracellular pH at each time point using flow cytometry (Figures 3 and S10). In PBesque, Probe-DNA in complex with PLL behaves similarly to Probe-DNA in solution. The FAM and Cy5 emission intensities increased 50.9-fold and 1.2-fold, respectively, between pH 4.4 and 8.1 (Figure S11). Calibration curves from pH 5.0 to 7.5 demonstrated dynamic ranges of up to 3.4-fold in cells (Figure S10). In the absence of chloroquine, we measured pH = 6.7 ± 0.2 at 2 h and pH = 5.3 ± 0.3 at 4 h. With chloroquine, we measured pH = 5.9 ± 0.4 at 2 h and pH = 5.8 ± 0.2 at 4 h.
Figure 3.
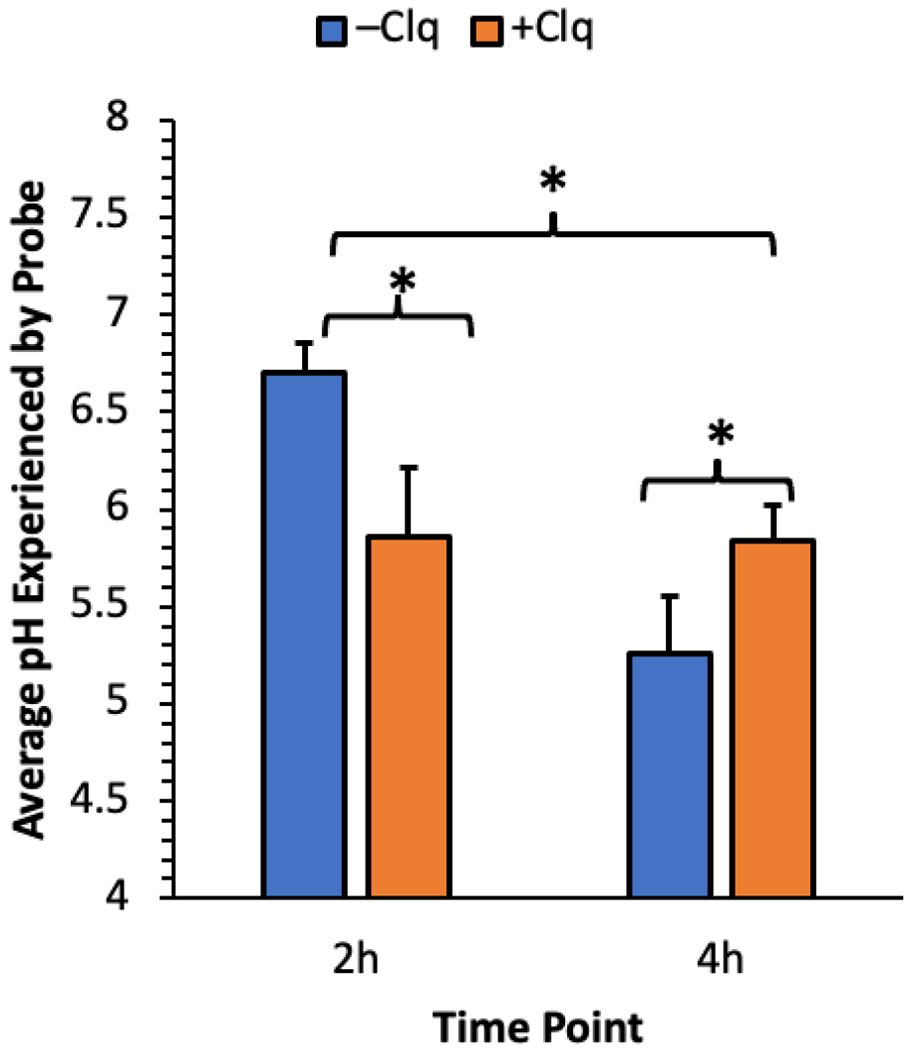
pH in the microenvironment of Probe-DNA for PLL polyplexes ± 100 μM chloroquine in HeLa cells by flow cytometry as a function of time (n = 3, * = p < 0.05, two sample t-test within time points and two-way ANOVA between time points). Error bars represent ± 1 S.D.
Live cell confocal laser scanning microscopy provided greater insight into the fate of the Probe-DNA duplex following uptake. HeLa cells were transfected with polyplexes of Probe-DNA and PLL ± 20 μM chloroquine, after which confocal micrographs were acquired at 2 and 4 h post transfection (see SI for method and all micrographs). From representative Sum-of-Slices (SoS) projections in the PLL conditions (Figure 4, left), we observed mostly green puncta at 2 h, suggesting along with the measured pH of 6.7 (Figure 3) that Probe is largely confined to endocytic vesicles and early endosomes. By 4 h, we observed perinuclear accumulation of red signal with remaining green signal restricted to puncta; the measured pH of 5.3 (Figure 3) indicates that, in line with previous reports, Probe is trapped in late endosomes.20,31,51–53 With chloroquine present (Figure 4, right), we observed punctate yellow signal at 2 h and 4 h, with more yellow puncta at the latter time point. The measured pH values of 5.9 and 5.8 at these time points (Figure 3) indicate that Probe was trafficked to early endosomes faster in the presence of chloroquine, but endosomal acidification was inhibited.20,31,51–54
Figure 4.
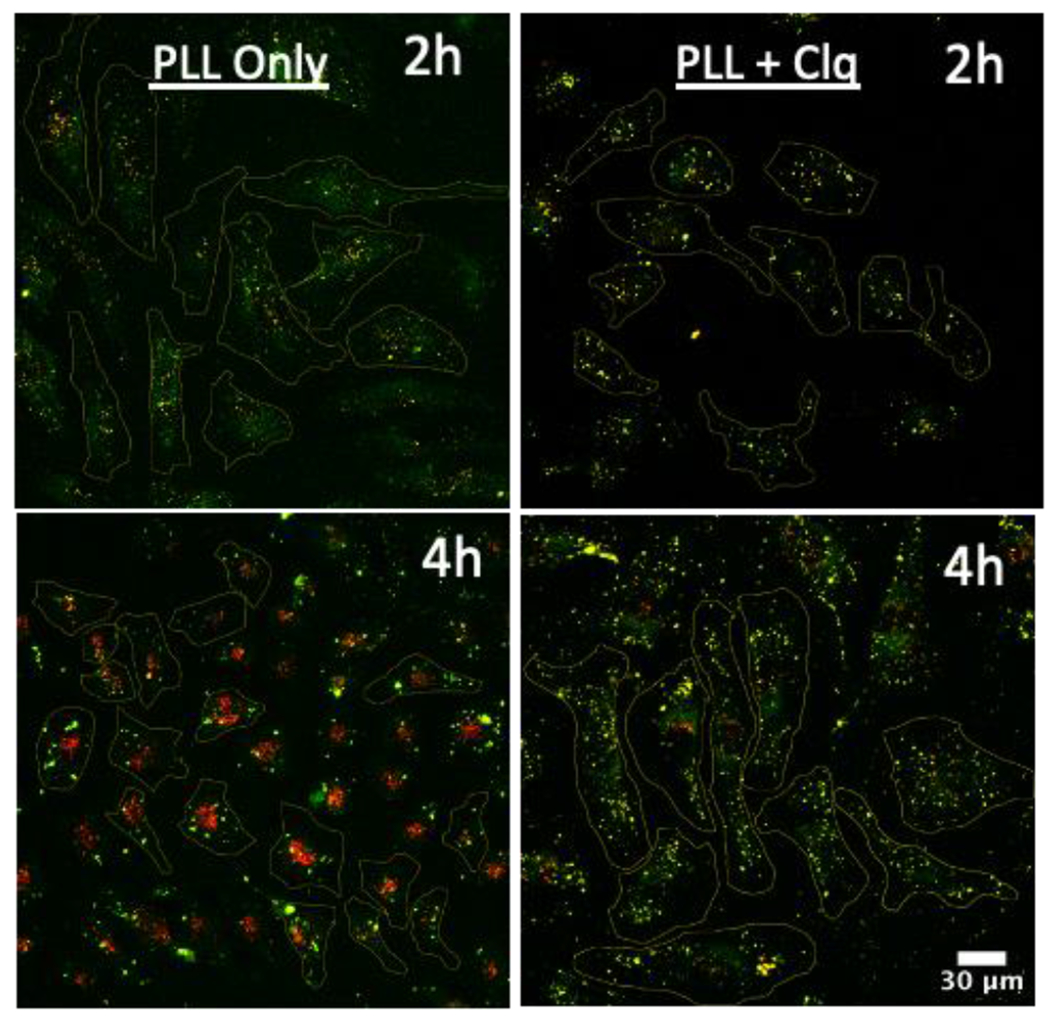
Fluorescence micrographs of live HeLa cells transfected with Probe-DNA in the absence and presence of 20 μM chloroquine. FAM (green) and Cy5 (red) channels are shown for each time point as an overlay. Micrographs are shown as SoS projections.
Chloroquine has been shown in other contexts to raise endosomal pH without triggering escape,55,56 so we verified that Probe-DNA can measure the intracellular pH in a system exhibiting endosomal release. After testing a panel of non-viral vectors for compatibility (Figure S12), we transfected HeLa cells with Probe-DNA using jetPEI (Polyplus-transfection) and quantitatively imaged them after 2 h incubation. Using our quantitative SoS methodology (see SI), we found that the probe was reporting an average pH value of 7.1 (Figures 5 and S13), consistent with endosomal release.
Figure 5.

Fluorescence micrographs of live HeLa cells transfected with Probe-DNA using JetPEI and incubated for 2 h at 37 °C and 5% CO2. FAM (green) and Cy5 (red) channels are shown for each replicate as an overlay. Micrographs are shown as SoS projections.
The placement of fluorescent dyes on both termini of an siRNA sense strand may abrogate gene silencing,57 but there are reports in which RISC interaction and silencing have been preserved.58,59 To ascertain the ability of Probe to report on the delivery of functional antisense RNA to the cytoplasm, we annealed it to its natural RNA antisense complement,50 5ʹ-AGU AAG AUU AAA UUU GUC CdTdG-3ʹ. The resultant duplex (Probe-RNA) was thermally stable (Tm = 64.5 °C, Figure S14) and minimized the FAM-Cy5 FRET interaction (Figure S15), with Cy5 remaining pH stable (Figure S16). The thermal stability was greater than in the Probe-DNA duplex, likely because 2ʹ-OMe RNA and natural RNA prefer the same anti-nucleosidic conformation. Probe-RNA displayed a 30.3-fold fluorescence dynamic range at pH 4.5-8.0 (Table 1). After transfecting HEK293T cells with Probe-RNA/JetPEI polyplexes, a Western blot (Figure S17) revealed substantial knockdown of CSNK2β in the experimental and positive control conditions, which demonstrated that a functional payload was delivered to the cytoplasm. Flow cytometric analysis of HEK293T cells transfected with Probe-RNA revealed an average intracellular pH of 7.4 after 4 h incubation, consistent with endosomal escape (Figure S18).
In summary, we demonstrated that Probe, readily synthesized from 2ʹ-OMe RNA phosphoramidites and incorporating both FAM and Cy5, has a 48.9-fold FAM signal enhancement over the physiologic pH range in buffer when complexed with DNA. Probe-DNA duplexes allowed mutually complementary intracellular visualization and pH quantitation by live cell imaging and flow cytometry during uptake and endosomal trafficking. Probe-RNA was found to cause gene knockdown in HEK293T cells, which confirmed RNA delivery to the cytoplasm. These examples highlight that the Probe pH reporter is compatible with both RNA and DNA cargo. This work validates the use of G-quenched FAM-oligo constructs as specific and sensitive reporters of intracellular pH and demonstrates their potential for monitoring oligo cargo in real time.
Supplementary Material
ACKNOWLEDGMENT
Ryan Kubanoff maintained cell culture facilities. Dora von Trentini and Dr. Linlin Yang provided guidance in solid-phase oligonucleotide synthesis and RP-HPLC purification. The Penn Cytomics and Cell Sorting Resource Laboratory provided training and access to flow cytometry instrumentation. Dr. Megan Matthews provided access to a ChemiDoc gel imaging system. We thank the National Institutes of Health (grant R35-GM-131907 to IJD) for funding.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website. Supporting_Information provides additional figures, tables, schemes, materials, and experimental methods. All confocal micrographs acquired for the experiment described in Figure 4 are included as an appendix.
Underlined nucleobases indicate ribose 2′-O-methylation.
REFERENCES
- (1).Kole R; Krainer AR; Altman S RNA Therapeutics: Beyond RNA Interference and Antisense Oligonucleotides. Nat. Rev. Drug Discovery 2012, 11 (2), 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Shin H; Park S-J; Yim Y; Kim J; Choi C; Won C; Min D-H Recent Advances in RNA Therapeutics and RNA Delivery Systems Based on Nanoparticles. Adv. Ther 2018, 1 (7), 1800065. [Google Scholar]
- (3).Smith SA; Selby LI; Johnston APR; Such GK The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019, 30 (2), 263–272. [DOI] [PubMed] [Google Scholar]
- (4).Milech N; Longville BA; Cunningham PT; Scobie MN; Bogdawa HM; Winslow S; Anastasas M; Connor T; Ong F; Stone SR; Kerfoot M; Heinrich T; Kroeger KM; Tan Y-F; Hoffmann K; Thomas WR; Watt PM; Hopkins RM GFP-Complementation Assay to Detect Functional CPP and Protein Delivery into Living Cells. Sci. Rep 2015, 5 (1), 18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Schmidt S; Adjobo-Hermans MJW; Wallbrecher R; Verdurmen WPR; Bovée-Geurts PHM; van Oostrum J; Milletti F; Enderle T; Brock R Detecting Cytosolic Peptide Delivery with the GFP Complementation Assay in the Low Micromolar Range. Angew. Chem., Int. Ed 2015, 54 (50), 15105–15108. [DOI] [PubMed] [Google Scholar]
- (6).Schmidt S; Adjobo-Hermans MJW; Kohze R; Enderle T; Brock R; Milletti F Identification of Short Hydrophobic Cell-Penetrating Peptides for Cytosolic Peptide Delivery by Rational Design. Bioconjugate Chem. 2017, 28 (2), 382–389. [DOI] [PubMed] [Google Scholar]
- (7).Hu Y; Litwin T; Nagaraja AR; Kwong B; Katz J; Watson N; Irvine DJ Cytosolic Delivery of Membrane-Impermeable Molecules in Dendritic Cells Using pH-Responsive Core–Shell Nanoparticles. Nano Lett. 2007, 7 (10), 3056–3064. [DOI] [PubMed] [Google Scholar]
- (8).Convertine AJ; Diab C; Prieve M; Paschal A; Hoffman AS; Johnson PH; Stayton PS pH-Responsive Polymeric Micelle Carriers for siRNA Drugs. Biomacromolecules 2010, 11 (11), 2904–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kongkatigumjorn N; Cortez-Jugo C; Czuba E; Wong ASM; Hodgetts RY; Johnston APR; Such GK Probing Endosomal Escape Using pHlexi Nanoparticles. Macromol. Biosci 2017, 17 (4), 1600248. [DOI] [PubMed] [Google Scholar]
- (10).Wong ASM; Mann SK; Czuba E; Sahut A; Liu H; Suekama TC; Bickerton T; Johnston APR; Such GK Self-Assembling Dual Component Nanoparticles with Endosomal Escape Capability. Soft Matter 2015, 11 (15), 2993–3002. [DOI] [PubMed] [Google Scholar]
- (11).Baeyer A. Ueber Eine Neue Klasse von Farbstoffen. Ber. Dtsch. Chem. Ges 1871, 4 (2), 555–558. [Google Scholar]
- (12).Kim SY; Podder A; Lee H; Cho Y-J; Han EH; Khatun S; Sessler JL; Hong KS; Bhuniya S Self-Assembled Amphiphilic Fluorescent Probe: Detecting pH-Fluctuations within Cancer Cells and Tumour Tissues. Chem. Sci 2020, 11 (36), 9875–9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang P; Meng J; Li Y; Wang Z; Hou Y pH-Sensitive Ratiometric Fluorescent Probe for Evaluation of Tumor Treatments. Materials 2019, 12 (10), 1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li G; Zhang B; Song X; Xia Y; Yu H; Zhang X; Xiao Y; Song Y Ratiometric Imaging of Mitochondrial pH in Living Cells with a Colorimetric Fluorescent Probe Based on Fluorescein Derivative. Sens. Actuators, B 2017, 253, 58–68. [Google Scholar]
- (15).Wang X; Feng Y; Liu J; Cheng K; Liu Y; Yang W; Zhang H; Peng H Fluorescein Isothiocyanate-Doped Conjugated Polymer Nanoparticles for Two-Photon Ratiometric Fluorescent Imaging of Intracellular pH Fluctuations. Spectrochim. Acta, Part A 2022, 267 (Pt 1), 120477. [DOI] [PubMed] [Google Scholar]
- (16).Anees P; Sudheesh KV; Jayamurthy P; Chandrika AR; Omkumar RV; Ajayaghosh A A Protein–Dye Hybrid System as a Narrow Range Tunable Intracellular pH Sensor. Chem. Sci 2016, 7 (11), 6808–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wilson JT; Keller S; Manganiello MJ; Cheng C; Lee C-C; Opara C; Convertine A; Stayton PS pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano 2013, 7 (5), 3912–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hilderbrand SA; Kelly KA; Niedre M; Weissleder R Near Infrared Fluorescence-Based Bacteriophage Particles for Ratiometric pH Imaging. Bioconjugate Chem. 2008, 19 (8), 1635–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).He L; Duan Y; Feng J; Wan Z; Liu X; Wang Y; Yu F; Wang Y; Xiong Y; Wu Y Apoferritin-Based Tunable Nano-Indicator for Intracellular pH Sensing: Regulating Response Performances and Minimizing Effects of System Fluctuations. Sens. Actuators, B 2020, 323, 128661. [Google Scholar]
- (20).Deng ZJ; Morton SW; Bonner DK; Gu L; Ow H; Hammond PT A Plug-and-Play Ratiometric pH-Sensing Nanoprobe for High-Throughput Investigation of Endosomal Escape. Biomaterials 2015, 51, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lavis LD; Rutkoski TJ; Raines RT Tuning the pKa of Fluorescein to Optimize Binding Assays. Anal. Chem 2007, 79 (17), 6775–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Klonis N; Sawyer WH Spectral Properties of the Prototropic Forms of Fluorescein in Aqueous Solution. J. Fluoresc 1996, 6 (3), 147–157. [DOI] [PubMed] [Google Scholar]
- (23).Martin MM; Lindqvist L The pH Dependence of Fluorescein Fluorescence. J. Lumin 1975, 10 (6), 381–390. [Google Scholar]
- (24).Sjöback R; Nygren J; Kubista M Characterization of Fluorescein-Oligonucleotide Conjugates and Measurement of Local Electrostatic Potential. Biopolymers 1998, 46 (7), 445–453. [DOI] [PubMed] [Google Scholar]
- (25).Lietard J; Ameur D; Somoza MM Sequence-Dependent Quenching of Fluorescein Fluorescence on Single-Stranded and Double-Stranded DNA. RSC Adv. 2022, 12 (9), 5629–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Torimura M; Kurata S; Yamada K; Yokomaku T; Kamagata Y; Kanagawa T; Kurane R Fluorescence-Quenching Phenomenon by Photoinduced Electron Transfer between a Fluorescent Dye and a Nucleotide Base. Anal. Sci 2001, 17 (1), 155–160. [DOI] [PubMed] [Google Scholar]
- (27).Nazarenko I; Pires R; Lowe B; Obaidy M; Rashtchian A Effect of Primary and Secondary Structure of Oligodeoxyribonucleotides on the Fluorescent Properties of Conjugated Dyes. Nucleic Acids Res. 2002, 30 (9), 2089–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang L; Gaigalas AK; Blasic J; Holden MJ Spectroscopic Characterization of Fluorescein- and Tetramethylrhodamine-Labeled Oligonucleotides and Their Complexes with a DNA Template. Spectrochim. Acta, Part A 2004, 60 (12), 2741–2750. [DOI] [PubMed] [Google Scholar]
- (29).Modi S; M. G. S; Goswami D; Gupta GD; Mayor S; Krishnan Y. A DNA Nanomachine That Maps Spatial and Temporal pH Changes inside Living Cells. Nat. Nanotechnol 2009, 4 (5), 325–330. [DOI] [PubMed] [Google Scholar]
- (30).Surana S; Bhat JM; Koushika SP; Krishnan Y An Autonomous DNA Nanomachine Maps Spatiotemporal pH Changes in a Multicellular Living Organism. Nat. Commun 2011, 2 (1), 340. [DOI] [PubMed] [Google Scholar]
- (31).Akinc A; Langer R Measuring the pH Environment of DNA Delivered Using Nonviral Vectors: Implications for Lysosomal Trafficking. Biotechnol. Bioeng 2002, 78 (5), 503–508. [DOI] [PubMed] [Google Scholar]
- (32).Wilson DR; Routkevitch D; Rui Y; Mosenia A; Wahlin KJ; Quinones-Hinojosa A; Zack DJ; Green JJ A Triple-Fluorophore-Labeled Nucleic Acid pH Nanosensor to Investigate Non-Viral Gene Delivery. Mol. Ther 2017, 25 (7), 1697–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhang Y; Guo S; Cheng S; Ji X; He Z Label-Free Silicon Nanodots Featured Ratiometric Fluorescent Aptasensor for Lysosomal Imaging and pH Measurement. Biosens. Bioelectron 2017, 94, 478–484. [DOI] [PubMed] [Google Scholar]
- (34).Itaka K; Harada A; Yamasaki Y; Nakamura K; Kawaguchi H; Kataoka K In Situ Single Cell Observation by Fluorescence Resonance Energy Transfer Reveals Fast Intra-Cytoplasmic Delivery and Easy Release of Plasmid DNA Complexed with Linear Polyethylenimine. J. Gene Med 2004, 6 (1), 76–84. [DOI] [PubMed] [Google Scholar]
- (35).Haraszti RA; Miller R; Didiot M-C; Biscans A; Alterman JF; Hassler MR; Roux L; Echeverria D; Sapp E; DiFiglia M; Aronin N; Khvorova A Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles. Mol. Ther 2018, 26 (8), 1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sharma VK; Osborn MF; Hassler MR; Echeverria D; Ly S; Ulashchik EA; Martynenko-Makaev YV; Shmanai VV; Zatsepin TS; Khvorova A; Watts JK Novel Cluster and Monomer-Based GalNAc Structures Induce Effective Uptake of siRNAs in Vitro and in Vivo. Bioconjugate Chem. 2018, 29 (7), 2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Moreira BG; You Y; Owczarzy R Cy3 and Cy5 Dyes Attached to Oligonucleotide Terminus Stabilize DNA Duplexes: Predictive Thermodynamic Model. Biophys. Chem 2015, 198, 36–44. [DOI] [PubMed] [Google Scholar]
- (38).Kroutil O; Romancová I; Šíp M; Chval Z Cy3 and Cy5 Dyes Terminally Attached to 5′C End of DNA: Structure, Dynamics, and Energetics. J. Phys. Chem. B 2014, 118 (47), 13564–13572. [DOI] [PubMed] [Google Scholar]
- (39).Kim HC; Park WH Fluorescent Property of Glycol Chitosan-Fluorescein Isothiocyanate Conjugate for Bio-Imaging Material. Int. J. Biol. Macromol 2019, 135, 1217–1221. [DOI] [PubMed] [Google Scholar]
- (40).Yao Q; Lu S; Lin F; Zhao T; Zhao L; Chen X A Co-Precipitation Strategy for Making a Ratiometric pH Nanosensor for Intracellular Imaging. Sens. Actuators, B 2017, 250, 484–490. [Google Scholar]
- (41).Yeldell SB; Yang L; Lee J; Eberwine JH; Dmochowski IJ Oligonucleotide Probe for Transcriptome in Vivo Analysis (TIVA) of Single Neurons with Minimal Background. ACS Chem. Biol 2020, 15 (10), 2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yeldell SB; Ruble BK; Dmochowski IJ Oligonucleotide Modifications Enhance Probe Stability for Single Cell Transcriptome in Vivo Analysis (TIVA). Org. Biomol. Chem 2017, 15 (47), 10001–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Doughty MJ pH Dependent Spectral Properties of Sodium Fluorescein Ophthalmic Solutions Revisited. Ophthal. Physiol. Opt 2010, 30 (2), 167–174. [DOI] [PubMed] [Google Scholar]
- (44).Bidmanova S; Hlavacek A; Damborsky J; Prokop Z Conjugation of 5(6)-Carboxyfluorescein and 5(6)-Carboxynaphthofluorescein with Bovine Serum Albumin and Their Immobilization for Optical pH Sensing. Sens. Actuators, B 2012, 161 (1), 93–99. [Google Scholar]
- (45).Munkholm C; Parkinson DR; Walt DR Intramolecular Fluorescence Self-Quenching of Fluoresceinamine. J. Am. Chem. Soc 1990, 112 (7), 2608–2612. [Google Scholar]
- (46).Mao H; Luo G; Zhan Y; Zhang J; Yao S; Yu Y The Mechanism and Regularity of Quenching the Effect of Bases on Fluorophores: The Base-Quenched Probe Method. Analyst (London, U. K.) 2018, 143 (14), 3292–3301. [DOI] [PubMed] [Google Scholar]
- (47).Xiang D; Li F; Wu C; Shi B; Zhai K The G-BHQ Synergistic Effect: Improved Double Quenching Molecular Beacons Based on Guanine and Black Hole Quencher for Sensitive Simultaneous Detection of Two DNAs. Talanta 2017, 174, 289–294. [DOI] [PubMed] [Google Scholar]
- (48).Song C; Zhang C; Zhao M Singly Labeled Smart Probes for Real-Time Monitoring of the Kinetics of dNTP Misincorporation and Single Nucleotide Extension in DNA Intra-Molecular Polymerization. Biosens. Bioelectron 2009, 25 (2), 301–305. [DOI] [PubMed] [Google Scholar]
- (49).Hwang GT Single-Labeled Oligonucleotides Showing Fluorescence Changes upon Hybridization with Target Nucleic Acids. Molecules 2018, 23 (1), 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Karna SKL; Lone BA; Ahmad F; Shahi N; Pokharel YR Knockdown of CSNK2ß Suppresses MDA-MB231 Cell Growth, Induces Apoptosis, Inhibits Migration and Invasion. EXCLI J. 2020, 19, 1211–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wattiaux R; Laurent N; Coninck SW-D; Jadot M Endosomes, Lysosomes: Their Implication in Gene Transfer. Adv. Drug Delivery Rev 2000, 41 (2), 201–208. [DOI] [PubMed] [Google Scholar]
- (52).Wagner E Effects of Membrane-Active Agents in Gene Delivery. J. Controlled Release 1998, 53 (1), 155–158. [DOI] [PubMed] [Google Scholar]
- (53).Kargaard A; Sluijter JPG; Klumperman B Polymeric siRNA Gene Delivery – Transfection Efficiency versus Cytotoxicity. J. Controlled Release 2019, 316, 263–291. [DOI] [PubMed] [Google Scholar]
- (54).Xia M-C; Cai L; Yang Y; Zhang S; Zhang X Tuning the pKa of Carboxyfluorescein with Arginine-Rich Cell-Penetrating Peptides for Intracellular pH Imaging. Anal. Chem 2019, 91 (14), 9168–9173. [DOI] [PubMed] [Google Scholar]
- (55).Hwang HS; Hu J; Na K; Bae YH Role of Polymeric Endosomolytic Agents in Gene Transfection: A Comparative Study of Poly(L-lysine) Grafted with Monomeric L-histidine Analogue and Poly(L-histidine). Biomacromolecules 2014, 15 (10), 3577–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Forrest ML; Pack DW On the Kinetics of Polyplex Endocytic Trafficking: Implications for Gene Delivery Vector Design. Mol. Ther 2002, 6 (1), 57–66. [DOI] [PubMed] [Google Scholar]
- (57).Raemdonck K; Remaut K; Lucas B; Sanders NN; Demeester J; Smedt SCD In Situ Analysis of Single-Stranded and Duplex siRNA Integrity in Living Cells. Biochemistry 2006, 45 (35), 10614–10623. [DOI] [PubMed] [Google Scholar]
- (58).Shin S; Kim YS; Kim J; Kwon H-M; Kim D-E; Hah SS Sniffing for Gene-Silencing Efficiency of siRNAs in HeLa Cells in Comparison with That in HEK293T Cells: Correlation between Knockdown Efficiency and Sustainability of siRNAs Revealed by FRET-Based Probing. Nucleic Acid Ther. 2013, 23 (2), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Kraynack BA; Baker BF Small Interfering RNAs Containing Full 2’-O-Methylribonucleotide-Modified Sense Strands Display Argonaute2/EIF2C2-Dependent Activity. RNA 2006, 12 (1), 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


