Abstract
Mesoporous bioactive glasses (MBGs), which belong to the category of modern porous nanomaterials, have garnered significant attention due to their impressive biological activities, appealing physicochemical properties, and desirable morphological features. They hold immense potential for utilization in diverse fields, including adsorption, separation, catalysis, bioengineering, and medicine. Despite possessing interior porous structures, excellent morphological characteristics, and superior biocompatibility, primitive MBGs face challenges related to weak encapsulation efficiency, drug loading, and mechanical strength when applied in biomedical fields. It is important to note that the advantageous attributes of MBGs can be effectively preserved by incorporating supramolecular assemblies, miscellaneous metal species, and their conjugates into the material surfaces or intrinsic mesoporous networks. The innovative advancements in these modified colloidal inorganic nanocarriers inspire researchers to explore novel applications, such as stimuli-responsive drug delivery, with exceptional in-vivo performances. In view of the above, we outline the fabrication process of calcium-silicon-phosphorus based MBGs, followed by discussions on their significant progress in various engineered strategies involving surface functionalization, nanostructures, and network modification. Furthermore, we emphasize the recent advancements in the textural and physicochemical properties of MBGs, along with their theranostic potentials in multiple cancerous and non-cancerous diseases. Lastly, we recapitulate compelling viewpoints, with specific considerations given from bench to bedside.
Keywords: Mesoporous bioactive glasses, Engineered strategies, Nanoarchitectural morphology, Biofunctionalization, Stimuli-responsive, Disease theranostics
Graphical abstract

Highlights
-
•
MBGs show remarkable biological activities and physicochemical performances.
-
•
Significant progress in MBGs development stems from various engineered strategies.
-
•
MBGs-based stimuli-responsive drug delivery systems hold promise for disease theranostics.
1. Introduction
Ever since Gleiter's groundbreaking work in 1984, which led to the fabrication of nano-metal materials with exceptional performance, a series of cutting-edge disciplines and technologies have emerged successively, including nanomaterials, nanobiology, and nano-micrography [[1], [2], [3]]. Nanotechnology, as an encompassing system of emerging technologies, involves manipulating atoms and molecules at the nanometer scale to process materials, manufacture products with specific functions, and study their compositions or structures [4]. The internationally recognized nanoscale range falls between 0.1 and 100 nm, where physical phenomena occur at the mesoscopic level, serving as a relatively independent intermediate realm between the macroscopic and microscopic scales [5]. Nanomaterials have made unprecedented advancements in numerous fields, such as biomaterials, artificial organs, interventional therapies, diagnostics, drug delivery systems, blood purification, and biological molecular separation, with the potential to drive clinical diagnosis and therapy towards a functional and intelligent direction in the future [[6], [7], [8], [9], [10]].
Recent progress in biomaterials indicates that mesoporous materials, a type of porous nanomaterials, hold promising prospects in biomolecule adsorption and separation, enzyme immobilization, intelligent drug delivery, biosensors, biomarkers, and other applications [[11], [12], [13]]. According to the definition by the International Union of Pure and Applied Chemistry (IUPAC), porous materials with pore sizes ranging from 2 nm to 50 nm are classified as mesoporous materials [14]. Ordered mesoporous materials have garnered significant attention in interdisciplinary research over the past two decades owing to their exceptional performances [[15], [16], [17]]. Among various inorganic nanomaterials, mesoporous bioactive glasses (MBGs) stand out due to the abundant silicon hydroxyl groups on pore surfaces that act as active sites for binding with functional organic molecules, thereby enhancing structure stability and functionality [[18], [19], [20]]. The pioneering work by Zhao and colleagues in 2004 integrated the sol-gel method with surfactant supramolecular chemistry to first prepare MBGs [21]. As anticipated, MBGs have drawn considerable attention from researchers due to their highly desirable bioactivity and superior morphological features, including large specific surface area, ordered mesoporous structures, adjustable pore size and volume, easily controllable surface functionalization, and advantageous physicochemical properties [22]. Nevertheless, the standardized preparation of ordered mesoporous structures in MBGs is often affected by various factors such as components, additives, pH, solvent evaporation temperature, template properties, and concentration, given the complex nature of their multiple structural systems [23]. In recent years, the successful preparation of MBGs with diverse topographic and structural characteristics, such as particles, microspheres, fibers, 3D-printing scaffolds, and hierarchical structures, has been achieved through the utilization of mesoporous templates combined with other preparation techniques [[24], [25], [26], [27], [28]].
Although abundant studies ground on MBGs have been highlighted by researchers, the spectrum of our review covers crucial breakthroughs of MBGs over last twenty years, emphasized MBGs-based intelligent nano system for applications in cancerous/non-cancerous diseases [29,30]. Furthermore, we begin by providing an overview of the general fabrication of MBGs, elucidating the underlying roles of molecular reaction dynamics and the factors that impact their formation. Next, we discuss material modifications made to improve MBGs, focusing on three important areas: surface functionalization, tuning of nanostructures, and network modification. Subsequently, we delve into recent advances in MBGs performances, highlighting the progress made in fabricating these materials with excellent morphological, structural, and physicochemical properties, which have led to their application in disease theranostics. Finally, we conclude the review with critical views, emphasizing the next challenges that need to be addressed for MBGs to transition from the laboratory to the clinic.
2. Development of MBGs
MBGs represent a class of nanostructured bioceramic materials renowned for their exceptional bioactive characteristics. Upon interaction with biological fluids, MBGs exhibit rapid formation of a surface apatite layer within a few hours and have the capacity to uptake and release biomolecules [31]. The synthesis of MBGs was pioneered by Zhao and colleagues in 2004, who developed a multicomponent SiO2–CaO–P2O5 system. This breakthrough enabled the creation of highly ordered mesoporous structures with large surface areas, well-defined surface properties, tunable pore volumes and sizes ranging from 5 to 20 nm [21].
The fabrication process of MBGs shares similarities with that of silica-based ordered mesoporous materials, which were first reported in the early 1990s [32,33]. Typically, MBGs are synthesized using sol–gel processing, and detailed synthetic strategies for MBGs with varying characteristics have been comprehensively summarized and discussed in corresponding literature [34,35]. In essence, integrating supramolecular chemistry into the sol-gel process requires various structure-directing agents to facilitate the effective fabrication of mesoscopic materials. Under suitable conditions, surfactants such as hexadecyltrimethylammonium bromide (CTAB), polyethylene glycol (PEG), and pluronic (P123) self-organize into micelles and self-assemble to form an organized organic-inorganic mesophase [36,37]. Once surfactants are removed by calcination or extraction, the well-ordered mesoporous structure is developed, exhibiting stable texture of hexagonal symmetry, cubic pore morphology, well-ordered mesoscopic scale, high surface area and large pore volume [38]. Furthermore, due to the intricate nature of the sol solution in the preparation process, multiple factors including surfactant properties and concentrations, reactive composition proportions, self-assembly in hydrophilic or hydrophobic microenvironments, and calcination temperature can be manipulated to yield advantageous topographic and structural attributes, alongside excellent physicochemical properties [39]. Owing to these favorable performances, sol-gel derived MBGs are recognized as unique drug delivery nanoplatforms, prospective building blocks of innovative composite materials, versatile inks for 3D bio-printing, novel implant coatings and injectable biomaterials [[40], [41], [42], [43]].
3. Novel engineering strategies of MBGs: surface functionality and nanostructured modification
Advanced MBGs-based materials have been successfully fabricated through emerging nano-engineering technology in the last decade [50]. It is crucial for chemically engineered MBGs to exhibit ideal properties for intelligent drug delivery, characterized by the typical features of easily tailored mesoporous networks [51]. Surface chemistry of MBGs significantly influences the body reaction of drug delivery system. Further, nanostructured features and modified networks of MBGs exhibit improved concentration in the circulating blood and delivery efficiency as well as meliorative immune rejection reaction [35,[52], [53], [54]]. These superior attributes of chemically engineering MBGs make them to be one of remarkable inorganic nanomaterials for theranostic application in various diseases.
In this section, we mainly concentrate on the discussion based on recent progress in the above three aspects. First, we outline the utilization of miscellaneous components such as polymers, peptides, and molecules to achieve surface modification of MBGs. Further, we discuss the reengineering of nanostructure in MBGs, such as shape modification and particle-size design, as well as tailorable porosity for hetero-structures. In the end, the changes in the siliceous networks of MBGs using various strategies, including insertion of organic groups, doping of metal or metal oxide, and transformation of integral structure, are also elaborately discussed (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17).
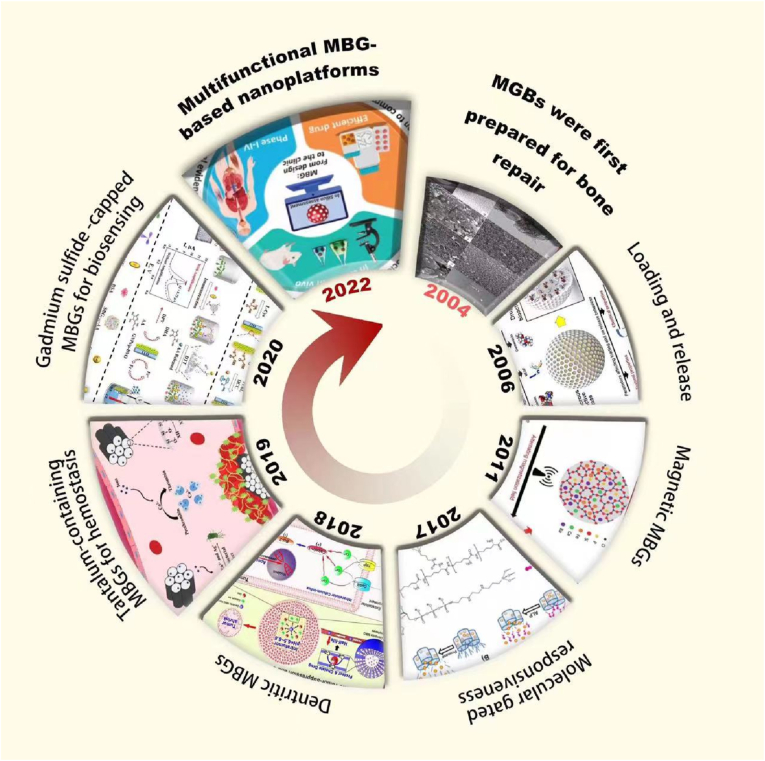
Fig. 1.
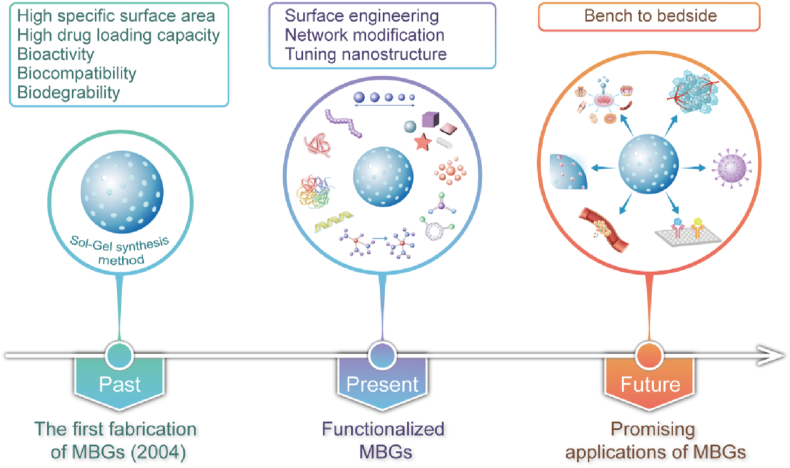
Overview of the integral development of MBGs-based nanomaterials: the timeline and representative studies of MBGs. Figures for years 1960–2022, reproduced with permission [21,35,[44], [45], [46], [47], [48], [49]].
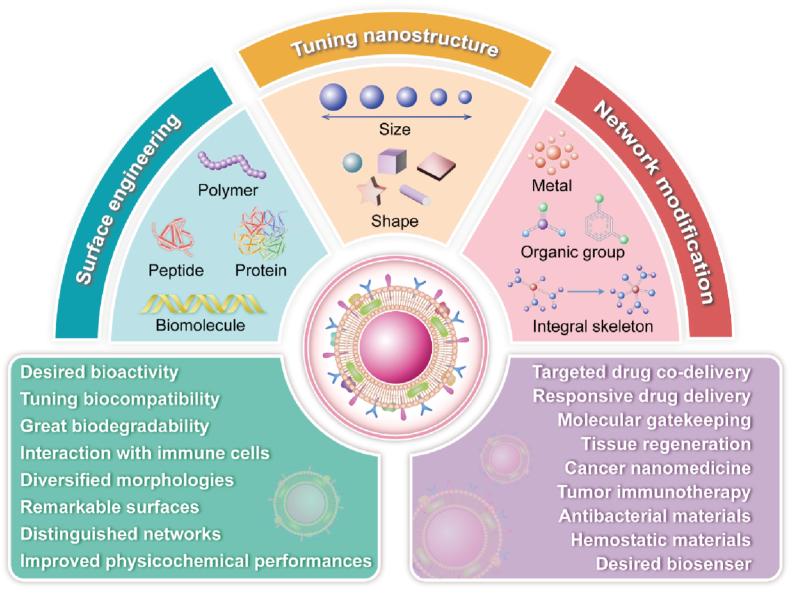
Fig. 2.
The schematic illustration shows systematically engineered strategies of advanced MBGs involved in surface functionalization, nanostructured morphology, and network modification.
Fig. 3.
The schematic illustration shows various type of stimuli-responsive MBGs-based nanoplatforms are fabricated for emerging intelligent drug delivery and theranostic applications.
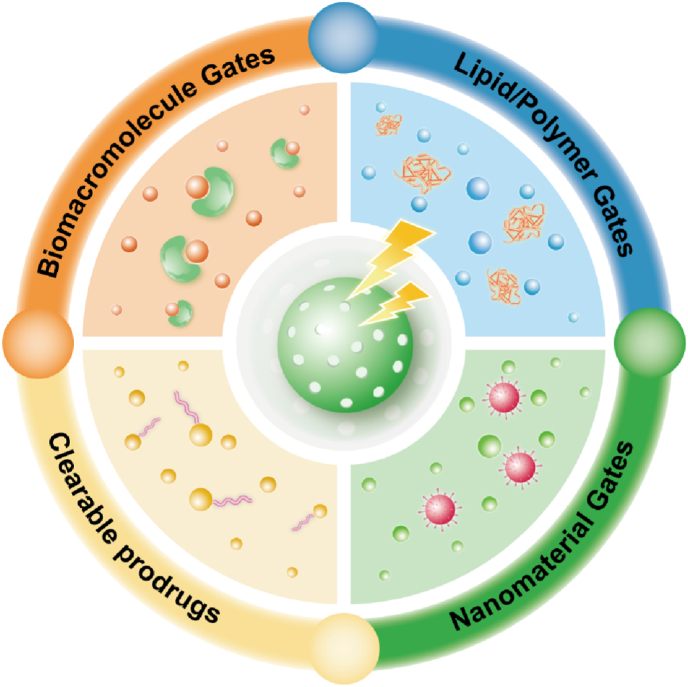
Fig. 4.
Schematic illustration of molecule gates in MBGs-based nanomaterials are prepared for stimuli-responsive and intelligent drug delivery.
Fig. 5.
Schematic showing the development and tumor suppression of pH-responsive DOX-loaded dendritic MBGs-based nanoplatforms step by step [46]. Copyright 2018, ACS publications.
Fig. 6.
(A) The synthesis of bioinspired trimodal macro/micro/nano-porous MBGs scaffolds. (B) Schematic showing the preparation of trimodal MBGs scaffolds by a modified multi-template method. (C) Micro-CT evaluation of orthotopic bone formation in vivo [116]. Copyright 2016, Elsevier Ltd.
Fig. 7.
Schematic showing smart soft-templating synthesis of hollow MBGs for controlled drug delivery and stimuli-responsive MBGs for disease theranostics in future. Reproduced with permission [119]. Copyright 2015, European Chemical Societies Publishing.
Fig. 8.
(A) The design of poly-l-glutamic acid embedded MBGs-based nanospheres for pH-stimulated chemotherapeutic drug delivery. (B) SEM images representation of (a) spherical MBGs, (b) the corresponding EDX spectrum, and (c, d, and e) elemental mapping of Si, Ca, P as green, red and yellow color, respectively in MBGs [88]. Copyright 2021, Elsevier Ltd.
Fig. 9.
Schematic showing the synthesis and characterizations of copper-containing magnetic MBGs-based nanocarriers. Reproduced with permission [173]. Copyright 2018, ACS publications.
Fig. 10.
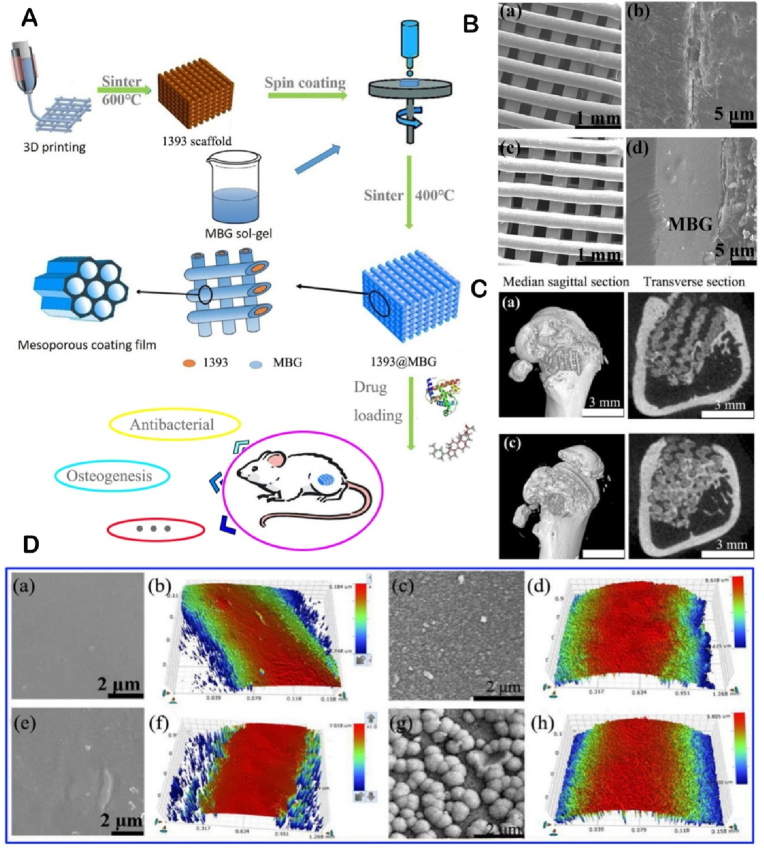
(A) The flow chart of preparing a novel vehicle-like drug delivery 3D printing MBGs-based composite scaffold for bone defect repair. (B) FESEM images of (a, c) as fabricated 1393 and 1393@MBGs scaffold; (b, d) the cross section of as fabricated 1393 and 1393@MBGs scaffold. (C) Micro-CT evaluation of bone regeneration in the rat cylindrical defects. (D) (a, b) FESEM images and the surface profile of as fabricated 1393 scaffold; (c, d) after immersed scaffold; (e, f) FESEM image and the surface profile of as fabricated 1393@MBGs scaffold; (g, h) after immersed scaffold [191]. Copyright 2020, International Publisher.
Fig. 11.
Schematic illustration showing the development of MBGs and engineered MBGs-based nanoplatforms with superior properties for theranostic applications in multiple diseases in future.
Fig. 12.
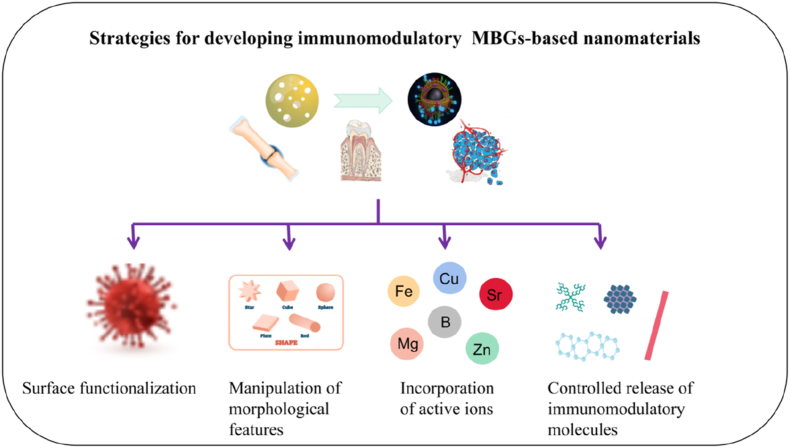
Schematic illustration of strategies for developing immunomodulatory MBGs, such as surface functionalization, manipulation of nanoarchitectures, controlled release of immunomodulatory molecules, and incorporation of bioactive ions.
Fig. 13.
Schematic illustration of advanced MBGs-based nanoplatforms for theranostic applications in diversified diseases.
Fig. 14.
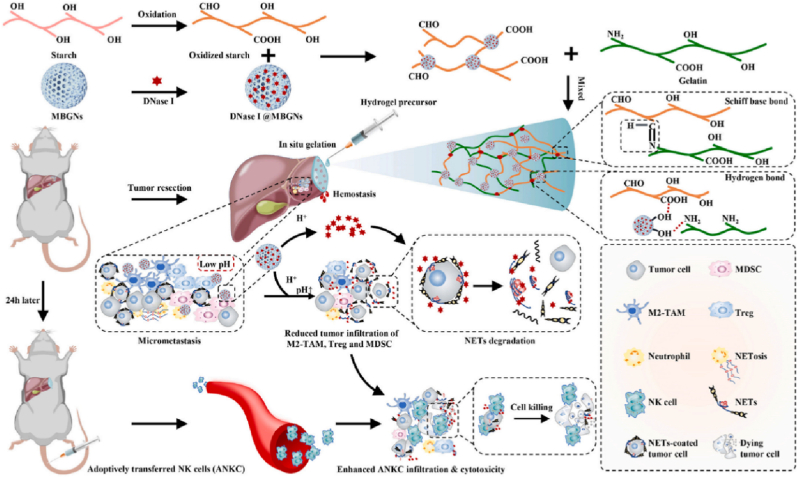
Schematic illustration of Gel-OS/DNase I@MBGNs hydrogel was prepared for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma [240]. Copyright 2022, Elsevier Ltd.
Fig. 15.
Schematic illustration showing the chemical engineering of MBGs for prospective theranostic applications in regenerative medicine.
Fig. 16.
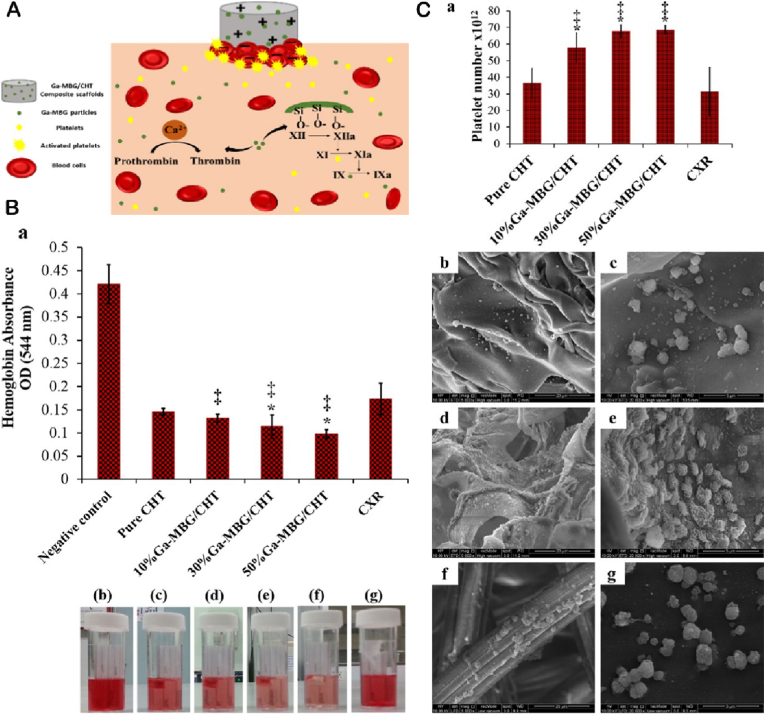
Schematic summary of (A) the hemostatic functions and antibacterial effects of MBGs-based materials with unique structure and desirable bioactivity. (B) Blood clotting rate of scaffolds and CXR (a); Photographs depicting more hemoglobin leaked from pure CHT, 10 % Ga-MBG/CHT, and CXR than that from 30 % Ga-MBG/CHT and 50 % Ga-MBG/CHT (b–g). (C) Effects of materials on platelet adhesion after 30 min of incubation with PRP (a). Low- and high-magnification FESEM images of platelets adhered to the surfaces of (b, c) pure CHT, (d, e) 50 % Ga MBG/CHT, and (f, g) CXR [276]. Copyright 2017, ACS publication.
Fig. 17.
Schematic illustration showing the fabrication of MBGs–based electrochemical biosensors from enzymatic to nonenzymatic. (A) XRD patterns of BOI NSs and p-BVO Nas. SEM images of (B) BOI NSs and (C) p-BVONAs. (D) SEM mapping images depicting the elemental distribution of Bi, V, and O in p-BVO. (E) Assembly process and immunoassay procedure of the FcAI/L-Cys/GNPs/p-BVO/ITO EC sensing interface [48]. Copyright 2021, ACS Publications.
3.1. Surface functionalization
3.1.1. Chemical group modification
It is important to note that a comprehensive understanding of the principles of modifying MBGs is crucial for developing novel surface engineering strategies [55]. In general, chemical modification in MBGs is based on the highly reactive silicon hydroxyl groups. There are three types of silicon hydroxyl groups on the surface of MBGs, namely isolated, twin, and hydrogen bonded hydroxyl groups. The isolated and twin silicon hydroxyl groups exhibit higher reactivity compared to the hydrogen bonded hydroxyl group, which transforms into a free silicon hydroxyl group when heated [56]. Effective surface modification is achieved by interacting chemically active surface silicon hydroxyl groups with various active components, introducing catalytic active sites into the pores or networks [57].
Among the different strategies, organosilane coupling agents play a crucial role in the surface engineering of MBGs. These agents are amphiphilic substances containing both organic functional groups and hydrolyzable groups [58,59]. After hydrolyzing, the alkoxy groups form silanol bonds that react with inorganic surface groups, creating strong chemical bonds and achieving functionalized surfaces with coupling agents [60]. Co-condensation and post grafting are the main methods used. Co-condensation, known as the one-step synthesis method, involves directly adding organosilane coupling agents to the sol during MBGs synthesis. However, the amount of introduced organic functional groups through co-condensation is limited as the silane coupling agent groups cannot cross-link and polymerize with other tetrahedral silicon atoms [61]. Post grafting is a relatively simple method for surface modification. It involves removing physically adsorbed water molecules from the newly synthesized mesoporous materials by heating under vacuum, followed by reacting with organosilane coupling agents in inert organic solvents like benzene, toluene, and cyclohexane to obtain surface-functionalized silicon-based mesoporous materials [62]. Mesoporous materials modified through post grafting possess a relatively high architectural order and protect the primary structures from damage [63].
Taken together, various chemical modifications can be carefully designed on the surface of MBGs with customizable nanoporous structures. These unique advantages provide an excellent opportunity for the efficient preparation of composite MBGs-based materials with uniform nano-sized pores and desirable properties. This makes MBGs an ideal platform for synthesis, self-assembly, and targeted delivery of guest molecules. As a result, the application of advanced MBGs has been expanded, allowing their use in various fields such as catalysis, separation, adsorption, biosensing, and controlled drug delivery.
3.1.2. Polymer coatings
As a novel class of organic/inorganic biomaterials, polymer-based nanocomposites ingeniously combine the advantages of organic polymers and inorganic nanoparticles. These nanoparticles act as drug delivery carriers with superior properties, improving the fate and performance of therapeutic molecules. By tailoring their delivery patterns and offering structural and functional diversities, these composites have demonstrated great potential for a wide range of applications [74,75]. Currently, silica-based mesoporous materials, known as MBGs, have been incorporated into polymers to create multifunctional composite scaffolds with improved attributes compared to matrix materials used alone. Various materials including collagen, chitosan, alginate, gelatin, polyglycerol, aliphatic polyurethane, polycaprolactone, poly lactic-co-glycolic acid, poly glycerol sebacate, poly-l-lactic acid, poly-1,8-octanediol citrate, and poly-3-hydroxybutyrate-co-3-hydroxyhexanoate have been fabricated as polymer coatings for surface functionalization of MBGs [72,[76], [77], [78], [79], [80], [81], [82], [83]].
Intriguingly, the polymer surface-coating MBGs have been designed through functional modification as novel drug delivery carriers in response to various external and biological stimuli [[84], [85], [86], [87]]. In a case, pH-responsive nanohybrids were fabricated through aminosilane functionalization of MBGs utilizing 3-aminopropyltrie thoxysilane. With daunomycin (DAN) as a model chemotherapeutic drug, the designed nanospheres enhanced drug loading and delivery rate in a pH-dependent way, where the release rate of DAN was higher at pH 5.5 compared to pH 7.4 [88]. Another example involves an injectable formulation based on polyurethane hydrogel and MBGs that can achieve sustained co-delivery of functional ions and drugs in a temperature-sensitive manner, making drug release timing and dosages controllable. The utilization of poly (ether-urethane) hydrogels with a typical LEGO-like chemical structure evokes polymer attractive properties to be finely tuned by changing their constituting building blocks. Excellent versatility opens the way to preparation of stimuli-responsive and environment-sensitive nanohybrids [89]. Whereas, coating polymeric architectures onto the surface of mesoporous silicon endows various functional attributes such as pH-responsive, molecule-responsive, ultrasound-responsive, and multielement-responsive, in contrast to the utilization observed with MBGs [[90], [91], [92], [93]]. For this reason, further design of multifunctional and stimulus-responsive MBGs/polymer nanohybrids will be the next direction to obtain a novel type of intelligent and environment-sensitive drug delivery system with remarkable bioactivity in the future [94,95].
3.1.3. Peptide modification
Proteins are unique and multifunctional biological macromolecules with abundant sources, excellent biocompatibility, and biodegradability. The fundamental structural unit of proteins is the peptide chain/poly-peptide, which consists of amino acids connected by peptide bonds. Through noncovalent bonding, the peptide chain folds into a flexible three-dimensional protein molecule. Proteins contain numerous functional groups, such as amino, carboxyl, and sulfhydryl groups, along with adjustable electrical properties, which provide binding sites for metal ions and organic compounds. Additionally, proteins possess diverse morphological structures and special biological activities, making them an excellent template for synthesizing composites with various structures [96]. In the biomedical field, protein composites are gradually emerging as promising candidates, particularly in drug targeted delivery systems. One effective strategy involves surface functionalization of nanoparticles using poly-glycerol (PG) grafting through ring-opening polymerization of glycidol, followed by poly-stage organic conversions within the PG layer, and coupling of primary polypeptides using click chemistry [97]. By employing this approach, cationic poly-arginine conjugated MBGs with poly-glycerol coating have been synthesized as potential vectors for gene delivery. The abundant silanol groups on the surface of MBGs serve as starting points for PG functionalization, resulting in a hydrophilic PG layer with versatile architecture for further modification. The cationic poly-arginine moieties play dual roles in efficiently loading plasmid DNA and facilitating cell transfection efficiency [98]. Furthermore, poly-peptide copolymers have been explored as sustained and controlled drug delivery systems due to their non-toxic, non-immunogenic, and minimal accumulation characteristics. A novel dual-drug delivery system was designed by combining MBGs with poly-peptide graft copolymers to create composites for pH-controlled drug release [99]. Among them, water-soluble gentamicin was predominantly released from MBGs in an acid environment, followed by a rapid release of fat-soluble naproxen from poly peptide nano-micelles in an alkaline environment.
In the field of intelligent drug delivery, functional protein composites enhance drug-specific selectivity by regulating their interaction with biological molecules. These composites exhibit excellent biocompatibility and cellular uptake, making them highly promising for biomedical applications. Something needs to be pointed out, although numerous examples have demonstrated the remarkable advantages of protein composites in biomedical and engineering fields, their translation from the laboratory to clinical settings still requires substantial efforts.
3.1.4. Molecule gate valve
It should be noted that surface modification using molecular gate valves is an effective strategy for achieving controlled drug delivery of MBGs in an environment-responsive manner [100]. Researchers have developed a class of artificial solid-state nanochannel materials [101]. Compared to natural ion channels, biomimetic artificial nanochannels offer higher stability, variable shapes, and surface chemical compositions, which make them highly promising for applications in biosensing, analysis, and drug delivery [102]. Currently, by combining with specific gating systems such as inorganic nanoparticles, macrocyclic compounds, biological macromolecules, and polymers, silicon-based nanoparticles have emerged as functional drug delivery systems that respond to stimuli such as pH, temperature, light, redox, magnetic fields, ultrasound, and biological molecules [103]. However, these gated systems have mainly been designed in single pure silica nanoparticles, and their functionality in bioactive glasses remains largely uncertain. Recently, Polo et al. [94] reported advanced enzymatic responsive molecular-gated nanodevices that utilize MBGs as an inorganic support for incorporating adenosine triphosphate (ATP) and polyllysine molecular gates. These stimuli-responsive MBGs-based systems exhibit excellent bioactivity, the ability to form apatite-like phases, and appropriate interactions with fluorescence probes, antibiotics, and anti-tumor drugs. Based on these concepts, tailor-made MBGs-based materials hold great potential for intelligent drug delivery and targeted disease therapy.
In conclusion, nano valves as advanced gating units play a crucial role in stimulative-responsive drug delivery systems by rhythmically blocking pores in inorganic nanoparticles. Mechanistically, molecular gating, such as the rotaxane structure, polymer, plug structure, bridge structure, and liposome, encapsulates drugs in a container and releases them upon specific stimulus response. As biomimetic nanochannels, MBGs have wide-ranging applications in biosensing analysis, gated release, and other areas due to their controllable shapes and ease of chemical modification, attracting significant attention from scientists across various fields.
3.2. Nanostructured morphology
3.2.1. Shape modification
Generally, bioactive glasses have a three-dimensional irregular network structure consisting of interconnected silicon oxygen tetrahedra with bridged oxygen at the top corner [[104], [105], [106]]. Phosphorus oxygen tetrahedra are linked to silicon oxygen tetrahedra through Si–O–P bonds, which play a crucial role in the formation of the glass network structure. Specifically, the presence of a double bond in the phosphorus oxygen tetrahedron creates a network breakpoint due to pentavalent phosphorus [107]. Metal ions existing in the shape of network modifiers destroy the integrity of silicon oxygen network texture and form a non-bridged oxygen bond of -Si-O-M+ [66]. With increasing non-bridged oxygen bonds and network breakpoints, the release rate of Na+ and Ca2+ in bioactive glasses as well as biomineralization rate are quicken. Interestingly, despite with similar network structures, MBGs prepared through different methods have varying nanostructured morphology, which is a significant aspect of material functionalization [108].
Concentrating on this novel modification strategy, variety of advanced MBGs with unique shapes have been prepared for targeted drug delivery, gene transfection, bioengineering and tumor therapy [63]. In one instance, radial mesoporous bioactive glass (rMBGs) nanoparticles were successfully synthesized using a sol gel process combined with microemulsion and gelation-induced phase separation techniques, employing CPB templates. These particles possess a distinctive design characterized by a radial and fibrous texture, customizable pore architecture, excellent dispersion, and superior mineralization ability. Osteoactivin, a model gene, was effectively protected from degradation by Deoxyribonuclease I through adsorption onto aminated rMBGs. Moreover, the aminated rMBGs were taken up by cells in a time- and dose-dependent manner, with primary localization observed in the cytoplasm. Based on these results, rMBGs show great potential as advanced non-viral carriers for targeted DNA gene transfection [109,110]. Optimized MBGs with high specific surface area and variable mesoporous channels were also fabricated by controlling calcium concentration in the silica matrix. Dendritic MBGs nanospheres effectively activated transient receptor potential channels and calcium-sensing receptors on tumor cells through calcium influx, specifically inhibiting tumor growth without affecting normal cells. Furthermore, the dendritic-shaped nanoparticles intelligently synergized with cancer drugs in an acidic microenvironment to enhance anti-tumor efficacy and reduce systemic toxicity [46,111].
Indeed, traditionally synthesized irregularly shaped MBGs often exhibit lower biological and physicochemical performances compared to novel materials that have undergone shape modification, such as regularly spherical particles or radial and dendritic nanoarchitectures. Therefore, there is great potential for bioengineering applications in the development of advanced MBGs-based biomaterials with controllable structures and morphologies through optimized synthesis technologies.
3.2.2. Particle-size design
The size and pore structure of MBGs are widely recognized as critical determinants of their biological and physicochemical properties [112,113]. When reduced to the nanometer level, the particle size of MBGs undergoes significant changes in geometric morphology and electronic structure, resulting in nanoscale characteristics such as the small size effect, surface effect, quantum size effect, and quantum tunneling effect [114]. These characteristics confer advantageous properties to MBGs, including a larger specific surface area, higher proportion of coordination unsaturated atoms and surface atoms, and high catalytic activity due to the increased proportion of surface atoms and insufficient coordination number of surface atoms [113]. Nevertheless, for biomedical applications, larger-sized MBGs are preferred for drug delivery due to their larger pores, which facilitate enhanced mass transfer, penetration, and diffusivity of proteins and genes, leading to improved drug loading rates and enhanced activity and stability of biomolecules. To achieve larger particle sizes, pore-forming templates and pore swelling agents have been developed. The combination of hydrothermal treatment and amphiphilic block copolymers as templates can result in the formation of relatively large pores (15–20 nm), while pore swelling agents such as tetramethylbenzidine (TMB) and ethyl ether have been reported to enlarge the pore size of MBGs up to 40 nm. However, excessive addition of pore swelling agents can potentially disrupt the hexagonal crystal pore structure, resulting in a heterogeneous worm-like pore structure. Therefore, achieving controllable synthesis of particle size and conducting detailed studies on the relationship between size changes and properties are crucial for optimizing the nanostructured morphology of MBGs [67].
In addition, while MBGs demonstrate better performance in gene transfection, drug delivery, and bone regeneration compared to individual silica particles, there is a need for further studies focusing on intracellular localization and long-term biosafety. Recent in vitro studies have indicated that the potential toxicity of nanomaterials is highly dependent on their particle size and shape, with spherical MBGs exhibiting higher bioactivity without obvious cytotoxicity than large-size and irregular bioactive glasses. This encourages researchers to clarify the potential size-dependent mechanisms of intracellular trafficking and cytotoxicity of MBGs. Studies have shown that smaller-size MBGs exhibit weaker cytotoxicity compared to larger particles, indicating a size-dependent mechanism that affects intracellular localization and cytotoxicity [115].
Overall, size-designed nanoparticles play a significant role in catalysis and drug delivery, with smaller-size MBGs generally demonstrating higher catalytic activity and lower cytotoxicity. Further, it is also essential to prepare nanoscale MBGs with larger pores through the selection of appropriate templates to achieve biomolecule loading and delivery, thereby expanding the range of size control under the micro level and widening the applications of MBGs with improved performances.
3.2.3. Tailorable mesoporosity
Currently, the structural functionalization of MBGs using advanced technology to adjust mesoporous pore size is a promising research focus [115]. The highly ordered MBGs display excellent biological activity, rapid hydroxyapatite-induced formation rates, and higher drug loading efficiency compared to traditional pure silicon-based mesoporous materials [117]. While small molecule drugs can be effectively loaded into MBGs with small mesoporous pore sizes, the loading of macromolecular drugs and proteins into mesoporous channels poses significant challenges, limiting their applications in drug delivery. To address this issue, long-chain surfactants are currently used as templates to prepare macro-porous biological glasses. Furthermore, 1,3,5-trimethylbenzene and N, N-dimethyl decamine are favorable pore-enlarging agents that effectively increase the mesoporous pore size of MBGs. In terms of unique structural characteristics, hollow MBG (HMBG) spheres consist of a large hollow interior with vertical mesochannels in the shell, enabling the uptake and sustained release of drugs on a larger scale [118]. Recently, Li et al. [119] reported the direct synthesis of a novel type of HMBG spheres using a dual-template system, including diblock copolymer and cationic surfactants. The study concluded that the primary contributing factors to the adsorption of large amounts of payloads were the large specific surface area and vertical mesochannels on the shells. The presence of calcium (Ca) ions also facilitated the efficiency of drug loading, as Ca ions and organic drug molecules synergistically formed chelate complexes. Structurally, the HMBG spheres exhibited two types of pores with different sizes: a hollow center with a diameter of approximately 150 nm and a mesoporous shell with a uniform thickness of 50–60 nm. These pores provide effective space for multiple therapeutic agents with different release profiles. Overall, the potential to load two drugs with different release profiles within a single particle has the capacity to overcome some clinical drug antagonism [120].
Indeed, structural optimization of MBGs-based materials are promising strategies to modulate healthy or diseased tissue microenvironments for regeneration or cancer therapy. Considering advantageous features of tailorable nanochannels, there has been significantly interested in altering microscopic nanoarchitecture such as mesopores to generate desirable properties of MBGs for their applications in multiple diseases. In this case, synthetic scaffolds with MBGs as matrix, a trimodal macro/micro/nano architecture with enhanced compressive strength was successfully designed through a multi-template method. Trimodal porous architecture and loading bone morphogenetic protein-2 (BMP2) molecules endowed advanced MBGs-based scaffolds the osteoconductive and osteoinductive activity, which may represent a novel direction for the development of orthopedic materials in future [116].
3.3. Network modification
Researchers are actively exploring novel functional strategies to enhance the mechanical and physicochemical properties of MBGs. Among these, network modification through insertion of organic groups, doping of metals and metal oxides, and transformation of integral skeleton have garnered significant attention. We will introduce above strategies in following chapters [[121], [122], [123]].
3.3.1. Insertion of organic groups
Due to the abundant presence of silicon hydroxyl groups on the surface of MBGs, which can serve as bioactive sites, MBGs-based nanohybrids with unique networks can be developed by doping organic components into these active sites using advanced methods such as co-polycondensation or grafting [40,124]. Among these methods, the co-polycondensation process aims to load organic groups onto MBGs by polymerizing organosilane and orthosilicate. For example, the organic polymer polylactic acid (PLA) was hybridized with MBGs through co-polycondensation, resulting in functional nanohybrids with superior biological induction activity, physical and mechanical properties [125]. For another, the grafting method involves combining organic functional groups with existing MBGs to enhance thermal stability and hydrolysis. Recently, a novel hybrid biomimetic formulation was developed by combining nano-sized MBGs enriched with strontium (Sr) and type I collagen. This bioactive ink was designed for 3D printing bone-like scaffolds and showed well-distributed Sr-MBGs along collagen fibrils, mimicking the organization of hydroxyapatite crystals in the bone extracellular matrix (ECM) [126]. Additionally, various active components such as biopolymers and organic acids have been incorporated into MBGs for structure modification. For instance, polycaprolactone was grafted onto the structure of MBGs using electrospinning technology, resulting in a three-dimensional skeleton with improved mechanical and physicochemical attributes, as well as excellent biodegradability [127]. Moreover, the supercritical carbon dioxide foaming technique was utilized to graft the bioactive lipid FTY720 into MBGs-PLGA composites, creating a favorable microenvironment for cellular adhesion and proliferation [128]. In summary, incorporating active compositions or organic groups into the network of MBGs enables the fabrication of advanced drug delivery carriers with enhanced structural and functional diversity, as well as stimulus responsiveness.
3.3.2. Incorporation of metal or metal oxide
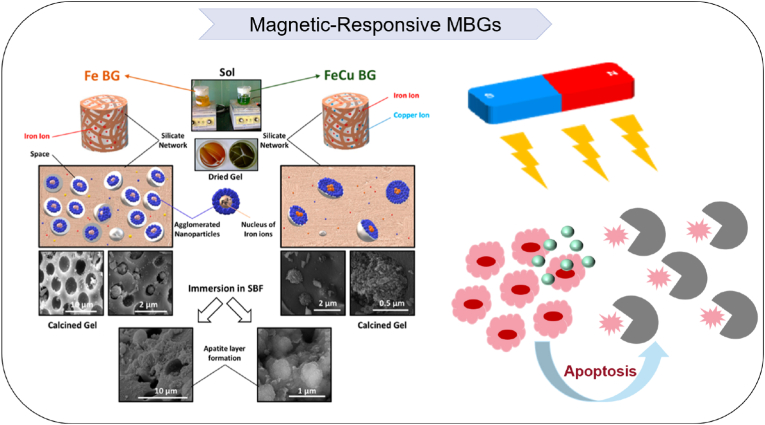
In addition to microstructure tailoring, recent efforts have focused on modifying the networks of MBGs by incorporating metal species (Cu, Zn, Sr, Co, Fe, Zn, Tb, or Eu, etc.) into the siliceous matrix. These metal dopants provide additional attributes such as osteogenesis, angiogenesis, antibacterial, and anti-tumor activities (Table 1) [[129], [130], [131], [132], [133], [134], [135], [136]]. For instance, a three-dimensional magnetic Fe3O4 nanoparticle composite containing MBGs/PCL has been successfully synthesized using rapid prototyping techniques. This composite exhibits a high porosity of 60 %, uniform macropores of 400 nm, and a notable compressive strength of 13–16 MPa. It also allows sustained drug loading and release. Importantly, the incorporation of Fe3O4 imparts superior magnetothermal effects for cancer therapy and significantly improves biological activity without affecting apatite mineralization. Another effective strategy for tissue regeneration involves pre-impregnating therapeutic cations into nanoparticles to modulate the microenvironment [137]. Zhang et al. [138] fabricated a 3D-printed nano-sized MBGs/collagen scaffold enriched with Sr ions, which effectively avoided premature release of Sr and significantly enhanced mechanical and thermal stability while maintaining high biocompatibility. Notably, when nanoscale bioactive structures mimic the natural features of bone tissue, they can reproduce the physiological environment and influence cell behavior during the regeneration process. In another case, an ordered MBGs network was modified with Ce, Ga, and Zn. The Ce and Zn played important roles in the physiological functions related to the bone metabolism environment, while Ga improved the mechanical properties of newly formed bone. However, the biocompatibility evaluation of these pore-blocking caps must be addressed, as some metal and metal oxide nanoparticles are toxic and may cause severe adverse effects and inflammatory reactions when administered [139] (see Table 2).
Table 1.
The advantages and disadvantages of surface functionalized MBGs for disease therapy.
| Representations | Ref. | |
|---|---|---|
| Advantages | Desirable biocompatibility; | [23] |
| Acceptable biodegradability; | [64] | |
| Feasible immunomodulation; | [65] | |
| Tunable particle size; | [66] | |
| Controllable pore size and distribution; | [67] | |
| High specific surface area and pore volume; | [68] | |
| Diversified surface modifications; | [69] | |
| Flexible morphologies and forms. | [70] | |
| Disadvantages | Potential toxicity; | [35] |
| Poor mechanical property; | [71] | |
| High synthesis cost; | [72] | |
| Sophisticated modification process. | [73] |
Table 2.
Incorporation of versatile metal or metal oxide into MBGs for biomedical applications.
| Metal | Function | Biomedical Applications | References |
|---|---|---|---|
| Ag | Antibacterial activity Biocompatibility with soft tissue cells |
Soft tissue regeneration Infectious diseases Dentin hypersensitivity |
[140,141] |
| B | Osteogenesis | Bone regeneration Osteoporosis |
[142,143] |
| Ce | Osteogenesis Antibacterial Antioxidant |
Bone regeneration Infectious diseases |
[144,145] |
| Co | Osteogenesis Angiogenesis |
Hypoxic diseases Tissue regeneration |
[146] |
| Cu | Osteogenesis Angiogenesis Odontogenesis Antibacterial Immune modulation Hyperthermia for tumor cells |
Bone regeneration Dental tissue regeneration Tumor photothermal therapy |
[147,148] |
| Eu | Osteogenesis Anticancer |
Bone regeneration Bone cancer therapy |
[149] |
| Ga | Antibacterial Hemostatic |
Soft tissue regeneration Wound healing |
[150,151] |
| Fe Fe3O4 |
Osteogenesis Hyperthermia for tumor cells |
Bone regeneration Cancer magnetic-heat therapy |
[152] |
| K | Osteogenesis | Bone regeneration | [153] |
| Li | Osteogenesis Cementogenesis |
Bone regeneration Periodontal tissue regeneration |
[154] |
| Mg | Osteogenesis | Bone regeneration | [155] |
| Mn | Antibacterial Osteogenesis |
Infectious diseases Bone regeneration |
[156] |
| Na | Osteogenesis | Bone regeneration | [157] |
| Rb | Osteogenesis Angiogenesis Antibacterial |
Bone regeneration | [158] |
| Se | Osteogenesis Anticancer |
Bone regeneration Bone tumor therapy |
[136] |
| Sm | Anticancer | Bone tumor therapy | [78] |
| Sr | Osteogenesis Cementogenesis |
Bone regeneration Periodontal tissue regeneration |
[[159], [160], [161]] |
| Ta | Hemostasis | Hemorrhagic disease | [162] |
| Tb | Osteogenesis | Bone regeneration | [51] |
| Te | Antibacterial Anticancer |
Bone tumor therapy | [163] |
| Zn | Osteogenesis Anti-inflammatory |
Bone regeneration Soft tissue regeneration |
[139,[164], [165], [166]] |
| Zr | Osteogenesis Radio-opacity at the implant site |
Bone regeneration Nanomolecular imaging |
[167,168] |
Furthermore, recent advances have focused on enhancing the adsorption and acid-base strength of mesoporous surfaces by doping metals or metal oxides to replace specific silicon atoms in MBGs. This takes advantage of the exchangeability of metal elements and ultimately improves the catalytic performance of MBGs [[169], [170], [171]]. Various transition metal elements with different valence states have been doped into MBGs to create redox properties. Typically, the metal or metal oxide is added during the material preparation process to ensure even dispersion within the network structure [172].
Another method involves doping metal ion-containing solutions into the prepared MBGs, followed by physical drying and a final firing step. However, metal elements obtained through this method are prone to loss due to weak van der Waals forces. Additionally, the addition of metal ion precursors, usually salts, may destabilize nanoparticles by affecting condensation and altering their surface charge. It is crucial to carefully optimize the metal concentration relative to that of silica when incorporating metals [79]. Proper control of processing parameters, such as the timing of metallic precursor addition and the molar concentration ratio between silica and the metal ion precursor, is necessary along this line.
3.3.3. Transformation of integral architecture
It is particularly significant that the beneficial and desirable properties of MBGs can be harnessed by transforming their integral network into advantageous modalities such as coating materials, composites, core-shell structures, porous spheres, fibrous constructs, and three-dimensional scaffolds [123,[174], [175], [176], [177], [178]]. For instance, MBGs have been incorporated into polymers to create multifunctional composite scaffolds with superior attributes compared to using matrix materials alone. Additionally, innovative MBGs-based nanohybrids with unique core-shell structures have been developed as nanocontainers for sequential multi-drug loading and delivery. Irregularly shaped MBGs synthesized through traditional methods often exhibit lower flow properties compared to regularly spherical particles, limiting their applications in injectable materials and drug delivery systems. Therefore, there has been a significant research interest in fabricating microspheres based on MBGs, as spherical biological glasses demonstrate improved bioactivity, hemostatic activity, excellent apatite mineralization, and high drug-loading capacity, owing to their high surface area and adjustable morphology [179,180]. Ordered microspheres can be synthesized through aerosol-assisted or emulsification-solvent evaporation methods. However, these methods have certain limitations. For instance, spray drying can result in a rapid decrease in surface area and microstructure, while the formation of mesostructure through the self-assembly of silica precursor and surfactant molecules requires a lengthy solvent evaporation process [181]. To address these challenges, Miao et al. developed a novel synthesis method for MBGs-based microspheres by combining sol-gel and water-in-oil micro-emulsion techniques, with or without the presence of ammonia as a catalyst. By adjusting the hydrolysis and polymerization rates of the precursors, they successfully synthesized acid-catalyzed MBGs with dense microsphere structures. Conversely, acid-alkali co-catalyzed MBGs with loose and porous structures were produced by incorporating ammonia during the gelation process. Furthermore, the addition of ammonia increased the mesopore size, pore volume, and surface area of the MBGs [182]. Both types of MBGs exhibited outstanding drug carrier properties with sustained release capabilities and demonstrated high in vitro apatite-forming activity. However, it was observed that acid-alkali co-catalyzed MBGs exhibited a faster rate of apatite deposition compared to acid-catalyzed MBGs [183].
Although MBGs are generally used either in powder or bulk form in most studies, fibrous frames have recently gained more attention [184,185]. For instance, Wang and coworkers reported the preparation of MBGs-based nanofibers by electrospinning β-cyclodextrin-modified MBGs nanoparticles with silk fibroin, resulting in a nanofibrous mesh that acts as a drug delivery system for hydrophobic molecules [186]. Scaffolds should mimic the structure of nanofibrous ECM, which is crucial for achieving biological functions. Pores within the scaffolds provide space for tissue in-growth and efficient mass transport of nutrients, oxygen, growth factors, and waste products. Nanofibers contribute to large surface areas that facilitate cell adhesion. Hsu et al. [123] successfully fabricated and characterized macroporous microbeads containing apatite-modified MBGs nanofibers using poly (methyl methacrylate) as the template combined with electrospray technology. The results showed that MBGs nanofibers provided a favorable environment for cell adhesion.
Developing three-dimensional hierarchical porous scaffolds with optimal features such as pore structure, mechanical strength, degradation rate, controllable drug delivery, and cell response poses a significant challenge in biomedical engineering [[187], [188], [189], [190]]. One study utilized a modified F127/PU co-templating method combined with a simple adsorption and lyophilization process to design and prepare a multiparameter-adjustable MBGs-based composite coating with poly (glycerol sebacate) (PGS) that meets various clinical application requirements. By adjusting the polymer coating amount and pore parameters, the composite scaffolds exhibited a tunable degradation rate for both the MBGs matrix and PGS coating, improved toughness, adjustable mechanical strength over a wide range, multilevel controlled release of proteins while maintaining their activity, and excellent in vitro cytocompatibility [140]. However, traditional fabrication approaches, including sol-gel, gas foaming, and fiber bonding methods, lack accurate control over scaffold architecture, pore shape, porosity, and interconnectivity. They also exhibit very low mechanical strength, making them unsuitable for clinical application. To overcome these disadvantages and challenges, advanced 3D printing technology utilizing computer-aided design (CAD) and computer-aided manufacturing (CAM) has been employed to design and fabricate three-dimensional MBGs-based scaffolds, significantly improving mechanical properties and speeding up production processes [[191], [192], [193]]. For instance, Zhang et al. prepared MBGs modified β-tricalcium phosphate (MBGs/β-TCP) scaffolds with hierarchical pore structures and functional surfaces via 3D printing and spin coating, resulting in enhanced apatite mineralization ability and compressive strength compared to pure β-TCP scaffolds [194]. Despite substantial advancements in engineering fabrication technology and the development of multifunctional MBGs-based nanocontainers, various factors ranging from initial processing and manufacturing costs to product implantation and safety must be carefully considered to achieve successful clinical transformation.
4. Superior properties of engineered MBGs
After undergoing versatile modification processes, advanced MBGs exhibit exceptional properties such as desirable bioactivity, biocompatibility, biodegradability, and interaction with immune cells. These attributes position them as potential candidates for disease theranostics. The subsequent subsection focuses on the advantageous properties of engineered MBGs-based nanomaterials and critical considerations for clinical translation.
4.1. Bioactivity
Bioactivity, in the field of biomedical materials, refers to the unique characteristics that can induce specific biological and chemical reactions at the interface between materials and biological tissues. This leads to the formation of chemical bonds between materials and living tissues [195]. MBGs are a type of bioactive material that can repair and regenerate living tissues by forming cooperative bonds between tissues and materials. The degradation products of MBGs facilitate the generation of growth factors, cell proliferation, and gene expression [196]. Engineered MBGs with uniform mesoporous channels and high specific surface area can quickly form hydroxyapatite when immersed in a simulated physiological solution. They also exhibit exceptional biological activity and strong degradability while minimally affecting the pH value of the surrounding microenvironments. Furthermore, they maintain the acid-base stability of local body fluids [197]. This makes MBGs highly valuable for tissue repair and regeneration.
4.2. Biocompatibility
Safety, based on the biocompatibility of carrier-based formulations, is a crucial consideration for biomedical applications, as it significantly influences the translation of such materials from the laboratory to clinical application [198]. MBGs, recognized as third-generation bioactive glasses, possess a nanostructured pore size distribution. These materials are designed to exhibit functional and structural compatibility with living tissues without adverse effects [199]. Furthermore, under biological conditions, MBGs can be distributed throughout the body and primarily eliminated through renal clearance. Therefore, understanding the biodistribution and elimination of MBGs is crucial in assessing long-term biocompatibility and potential toxicity concerns [200]. It is important to consider the potential risks associated with MBGs and ensure that the composition falls below safe biological limits for certain elements. Additionally, sustained release of these elements should not exhibit cytotoxic effects. Histological evaluations of kidney and hepatic structures have indicated non-toxic characteristics of all MBGs when using different surfactants. Numerous studies have demonstrated that the morphology, particle size, surface area, pore volume, pore size, and surface functionality of MBGs can influence their biocompatibility [42,169,201].
Some studies have shown that high concentrations of MBGs can lead to severe acute toxicity, highlighting the need for performance improvements. Mao et al. evaluated the acute toxicity and biodistribution of sub-micrometer MBG spheres (SMBG) when intravenously administered to mice. After 14 days of injection at doses of 20, 100, and 180 mg/kg, SMBG exhibited low in vivo toxicity based on factors such as mortality rates, organ coefficients, hematology data, and blood biochemical indexes. The lethal dose 50 (LD50) was found to be higher than 250 mg/kg. However, these nanocarriers primarily distributed in resident macrophages in the liver and spleen and could be cleared from the body within two weeks. High doses resulted in lymphocytic infiltration and granuloma formation in hepatocytes, as well as megakaryocyte hyperplasia in the spleen [202]. Therefore, when considering the use of MBGs as nanocarriers, it is crucial to consider their beneficial attributes, administration route, and dosage to ensure excellent biocompatibility, low toxicity, controlled biodegradation, and effective clearance. These factors are essential prerequisites for achieving safe and efficient nanocarriers.
4.3. Biodegradability
In addition to improved cytocompatibility and optimal surface architecture, the degradability of MBGs in biological fluids is another crucial attribute directly related to their biosafety. Unlike soft polymeric constructs, inadequate degradation of stable inorganic nanomaterials can result in long-term accumulation in the body, which poses potential risks of toxicity due to the materials' structural integrity and their chemical, thermal, and mechanical stability. It is worth noting that the degradation of MBGs in simulated body fluid occurs in two stages: an initial rapid degradation within the first 72 h, followed by a slower degradation over the subsequent 28 days [203]. Their degradation rates were generally affected by pore size and volume, chemical composition, degree of hydrolysis and condensation, morphology, surface functionality, and type of degradation medium.
Currently, researchers are actively developing bioactive porous scaffolds with time-dependent biodegradation to achieve control over the stability and release profile of guest molecules. These factors are closely associated with considerations of stability, biodistribution, excretion, and toxicity [204]. Ghamor-Amegavi et al. have successfully prepared biphasic yolk-shell granules consisting of a highly bioactive and biodegradable yolk component and a self-curing silicate shell component. They incorporated MBGs into a self-curing β-dicalcium silicate (Ca2SiO4) cement shell to form spherical granules known as MBGs@ Ca2SiO4. The incorporation of MBGs was found to enhance the bioactivity of the composites, control the degradation rate and ion release, and improved the bone repairing ability of the Ca2SiO4 shell-coated granules. Throughout the immersion stage, MBGs@Ca2SiO4 granules exhibited a rapid decrease in mass, with approximately 10 % greater decrease in mass during the first week (65.2 % vs. 75.3 %) and a cumulative decrease of nearly 15 % after 7 weeks (47.3 % vs. 62.6 %). Additionally, the MBGs@Ca2SiO4 granules displayed a slow increase in calcium concentration and a fast release of silicon ions. This confirmed that MBGs@ Ca2SiO4 granules possessed excellent compressive resistance and biodegradability [140,205]. Given these findings, MBGs present themselves as promising candidates for medical applications, as they can effectively regulate the degradation rate to mimic the microenvironments of target tissues.
4.4. Immunomodulation
The size, shape, and deformability of materials significantly influence their uptake by diverse immune cells and subsequent immune responses [206]. Given the vast potential of MBGs in various biomedical fields such as tissue engineering, cancer, and infectious processes, this study aims to explore the in vitro interaction between MBGs and immune cells [118,207]. Montes-Casado et al. [200] demonstrated the beneficial impacts of MBGs on the immune system by assessing different immune cells, including bone marrow-derived dendritic cells, subsets of spleen cells, and cell lines such as dendritic cells and lymphocytes. The findings revealed that MBGs did not disturb the balance of spleen cell subsets, the expression of T cell activation surface markers (CD25 and CD69), or the production of intracellular or secreted inflammatory cytokines. In vitro experiments indicated that T and B lymphocytes, along with dendritic cells, could efficiently and rapidly uptake MBGs by activating phosphoinositide 3-kinase, actin, and clathrin-dependent pathways. Furthermore, the appropriate expression of major histocompatibility complex and co-stimulatory molecules (CD40, CD80, and CD86) suggested that MBGs did not alter the maturation process of dendritic cells. These studies highlight that MBGs with distinct particle sizes, pore structures, and surface functionalities can influence the expression of activation surface markers and pro-inflammatory cytokines in antigen-presenting cells. Through the stimulation of co-stimulatory molecules and cytokines released by antigen-presenting cells, naive T cells can be effectively activated, subsequently influencing Th2 or Th1 cell responses.
With a highly ordered mesoporous structure, large surface area and porosity, MBGs enable high ionic exchange of Ca cation and soluble silica with surrounding media, which may stimulate the expression of several genes of osteoblastic cells and regulate immune reactions by altering the ionic microenvironment between implants and hosts. Gómez-Cerezo et al. evaluated the effects of MBGs-75S scaffold on the osteoimmune microenvironment. MBGs-75S displayed high biocompatibility in contact with Saos-2 osteoblast-like cells and allowed differentiation of macrophages into osteoclast-like cells. In vitro studies focusing on the innate immune response showed that MBGs-75S would facilitate suitable proliferation of macrophages without inducing their polarization towards the M1 pro-inflammatory phenotype. Therefore, MBGs with unique surface topography, precise control of pore size, shape, arrangement, interconnectivity, and hierarchical structure, may serve as potential osteoimmunology biomaterials in tissue engineering [200,208].
In summary, the immune system constitutes a complex network of cells and lymphoid organs that collaborate to defend the host against invading pathogens and tumor growth. Chemically engineered MBGs have the potential to interact with the immune system in various ways. Once inside the human body, MBGs can interact with and be taken up by immune cells. Enhancing our understanding of these interactions and the underlying mechanisms can facilitate the translation of MBGs-based materials from the laboratory to clinical applications.
5. Emerging theranostic applications of multifunctional MBGs
Engineering MBGs-based nanocarriers indicate promising candidates to enhance the bioavailability and safety of active molecules, which will act as a stimuli-responsive intelligent delivery platform for therapeutic applications in multiple diseases, including cancer, tissue regeneration, bacterial infections, hemorrhagic disorders and so forth [[209], [210], [211], [212], [213]].
5.1. Intelligent delivery systems
5.1.1. Drug delivery
In recent years, various organic materials such as organic micelles, liposomes, and biodegradable polymers have been studied as carriers for drug delivery systems. However, these materials often face challenges due to their susceptibility to attacks by immune system and their limited thermal and chemical stability [214]. In contrast, MBGs offer advantages in terms of accurately positioning and sustaining release of assembled drugs. This is made possible by the presence of abundant surface silicon hydroxyl groups that provide active sites and allow for specific surface modifications, enabling the packaging of a variety of drugs within the mesoporous phase (Table 3) [215]. Surface functionalization of the mesoporous channels helps maintain the high structural order of MBGs, strengthens the interaction between drug molecules and the pore surface, and slows down the rate of drug release [41]. For instance, a slower release rate of therapeutic cargo was obtained after NH2 group was modified on the pore surface of MCM-41, taking advantage of the strong ionic bond generated by acid-base reaction. Additionally, by employing self-assembly technology, stimuli-responsive components can be layered outside the hollow mesoporous sphere. These active molecules can respond to specific conditions such as pH value or ionic strength, effectively blocking or opening the mesoporous channels and acting as a switch for controlled drug release. Moreover, mesoporous drug carriers can achieve targeted drug delivery through magnetic control. By integrating iron oxide particles as the core and MBGs as the shell, highly dispersed core-shell magnetic nanocomposites have been developed. Under external magnetic fields, these composite particles can be separated, allowing for targeted transmission of drugs within the human body [216].
Table 3.
Therapeutic guest molecules incorporated into MBGs-based hierarchical scaffolds and systems.
| Cargo | Type | Delivery system | Function | Biomedical applications | Ref. |
|---|---|---|---|---|---|
| Alendronate | Drug | MBGs Aminated MBGs Acid-alkali MBGs |
Osteogenesis | Bone regeneration Osteoporosis |
[224,225] |
| Ampicillin | Drug | MBGs | Antimicrobial | Infectious diseases | [226] |
| Ciprofloxacin | Drug | MBGs | Antimicrobial | Bone or joint infections | |
| Dexamethasone | Drug | MBGs MBGs/Polycaprolactone MBGs/Alginate |
Osteogenesis | Bone regeneration | [191] |
| Dimethyloxallyl glycine | Drug | MBGs | Osteogenesis Angiogenesis |
Bone regeneration | [227] |
| Doxorubicin | Drug | MBGs/Alginate Molecular-gated MBGs Dendritic MBGs |
Anticancer | Cancer therapy | [137] |
| Fingolimod (FTY720) | Drug | MBGs | Osteogenesis Immunomodulation |
Bone regeneration | [228] |
| Gentamicin | Drug | MBGs MBGs/Gelatin |
Antibacterial | Bone regeneration | [79] |
| Ibuprofen | Drug | MBGs MBGs/PLGA/SBA-15 |
Antibacterial Anti-inflammatory |
Bone regeneration | [187,229] |
| Ievofloxacin | Drug | Molecular-gated MBGs | Antimicrobial | Bone regeneration Infectious diseases |
[94] |
| Imatinib | Drug | MBGs MBGs/Polyurethane |
Anticancer | Cancer therapy | [41,230] |
| Isoniazid | Drug | MBGs | Antitubercular | Bone regeneration Osteoarticular Tuberculosis |
[231] |
| Metformin | Drug | MBGs MBGs/PLGA/SBA-15 |
Antidiabetic Osteogenesis |
Diabetes Bone regeneration |
[229] |
| Rifampin | Drug | MBGs MBGs/Gelatin |
Antitubercular | Bone regeneration Osteoarticular Tuberculosis |
[231] |
| Vancomycin | Drug | MBGs/PLGA | Osteogenesis Angiogenesis |
Bone regeneration Infectious diseases |
[232] |
| BDNF | GF | MBGs/Cement | Osteogenesis | Bone regeneration Osteoporosis |
[233] |
| BMP-2 | GF | Trimodal MBGs MBGs/TiO2 nanorod MBGs/Chitosan MBGs/PEG |
Osteogenesis | Bone regeneration | [234,235] |
| bFGF | GF | MBGs | Osteogenesis | Bone regeneration | [220] |
| EGF | GF | MBGs | Osteogenesis Angiogenesis Antibacterial Odontogenesis |
Bone regeneration Dental regeneration |
[221,236] |
| FGF18 | GF | MBGs/Collagen | Osteogenesis | Bone regeneration | [76] |
| IGF-1 | GF | MBGs | Osteogenesis | Bone regeneration | [223] |
| VEGF | GF | MBGs/Cap cement | Angiogenesis | Bone regeneration | [237] |
| IL-8 | Chemokine | MBGs/Chitosan MBGs/PEG |
Immunomodulation Osteogenesis | Bone regeneration | [217,238] |
| Lysozyme | Protein | MBGs/Cap cement | Antimicrobial | Bone regeneration | [237] |
| Bull serum albumin | Protein | MBGs/Sodium alginate | Osteogenesis | Bone regeneration | [187] |
| CGRP | Peptide | MBGs/Gelatin | Osteogenesis | Bone regeneration | [193] |
| Osteostatin | Peptide | MBGs | Osteogenesis | Bone regeneration | [95] |
| BMP2-pDNA | Gene | MBGs | Anti-osteoclastogenesis | Bone regeneration | |
| RANK siRNA | Gene | MBGs | Anti-osteoclastogenesis | Bone regeneration | [239] |
In summary, the preparation of MBGs with superior performances, including uniform pores, large pore volume, and a chemically modifiable pore surface, holds great importance in advancing the field of bioengineering. Such advancements will pave the way for intelligent drug delivery systems.
5.1.2. Gene delivery
There has been a significant focus on utilizing hierarchical MBGs as intelligent platforms for gene delivery. These platforms facilitate specific targeted responses and the dissolution of ionic products, subsequently activating orchestrated cascade signaling in the tissue's physiological environment [40,164,217]. Gene delivery, in general, involves the transportation of nucleic acids with biological functions into target cells, their expression within cells, and the realization of specific functions. This process has been widely utilized in studying genome function and disease therapy. The target gene must overcome various obstacles to successfully enter cells, express in the nucleus, and undergo replication and expression. Each step can significantly impact the efficiency of gene delivery and ultimately determine the success of gene transfection.
Of note, MBGs exhibit favorable morphological attributes that make them desirable for gene therapy through the hosting and delivery of biological molecules such as plasmid DNA or siRNA [218,219]. For instance, Kim et al. [220] successfully prepared a gene delivery nanocarrier based on MBGs to load BMP2 plasmid DNA, resulting in high cell uptake levels in mesenchymal stem cells and subsequent promotion of the expression of bone-related genes. Additionally, amine-functionalized MBGs were fabricated as siRNA delivery systems to silence genes and suppress the expression of the receptor activator of nuclear factor kappa B (RANK) for repairing damaged bone tissue. This degradable inorganic nanoplatform exhibited strong complexation with siRNA and effectively inhibited osteoclastic activity [121].
In conclusion, engineered MBGs hold promise as gene delivery systems for disease therapy due to their superior attributes, such as biocompatibility, biodegradability, high loading capacity, and intracellular uptake rate.
5.1.3. Growth factor delivery
It is promising to incorporate growth factor into MBGs and to exert collaborative effects with bioactive materials [221,222]. MBGs, with their excellent properties such as a higher specific surface area and pore volume, have been utilized to load various functional growth factors like BMP, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), aiming to promote bone regeneration. In addition to these typical growth factors, brain-derived neurotrophic factor (BDNF) has emerged as another therapeutic molecule involved in fracture healing [83]. Kauschke et al. designed MBGs/bone cement composite scaffolds with or without BDNF to compare their effects on fracture healing. The results demonstrated that BDNF-functionalized MBGs/cement significantly enhanced bone formation ability, increased ALP activity, while reducing infiltrated leukocyte numbers and concentrations of C-reactive protein (CRP) compared to controls without BDNF. Therefore, the controlled delivery of growth factors or cytokines may prove to be an efficient approach for disease treatment [223].
5.2. Cancer diagnosis and therapy
MBGs hold great promise for cancer therapy due to their unique properties including low toxicity, high biodegradability and biocompatibility, ease of targeted and controllable anti-tumor drug delivery, and being doped with anti-tumor and magnetic ions [[240], [241], [242]]. Recently, advanced fabrication of MBGs-based nanoplatforms with optimal nanostructure and surface functionalization has sparked great interest in cancer nanomedicine (see Table 4). For example, MBGs have been proven to be valuable vehicles for anti-tumor drug delivery, producing multifunctional actions [225]. Modulation of certain parameters such as the degradation rate of MBGs, initial concentration of loaded drugs, and variation in the pH of the microenvironment could be promising strategies to achieve high-efficiency anticancer therapy. Furthermore, targeted therapy is another focus of cancer research with excellent characteristics that reduce side effects of drugs and elicit powerful lethality to tumor cells. MBGs-based targeted drug delivery has recently been proposed for anti-cancer therapy. In one case, Lin et al. fabricated functionalized MBGs with folate groups and then loaded them with camptothecin, which selectively bonded with folic acid receptors, resulting in a higher kill rate of tumor cells [243].
Table 4.
Representatively engineered MBGs-based nanoplatforms and systems for cancer diagnosis and therapy.
| Nanoplatforms | Engineering strategies | Therapeutic advantages | Ref. |
|---|---|---|---|
| Multifunctional Eu-Gd-Si-Ca MBGs | Integration of Eu-Gd-Si-Ca active irons into MBGs for tumor imaging, inhibiting recurrence and promoting impaired tissue regeneration. | Controlled biodegradation; Ultrahigh drug loading; PH responsive release; Tumor cell-targeted surface; Branched mesoporous structure; Special magneto-optical properties. |
[254] |
| Aminated MBGs | Aminated MBGs were applied for alendronate delivery. | Large surface area; Controlled drug delivery; Stimulatory bone regeneration. |
[225] |
| MBGs/polyurethane nanocomposites | Bioactive nanocomposite was fabricated by using MBGs and biodegradable polyurethane. | Controlled delivery; Enhanced mechanical property. |
[41] |
| Dendritic MBGs nanosphere | Dendritic MBGs were fabricated by adjusting calcium content in silica matrix system. | Large specific surface area; Variable mesoporous channels; Controlled anti-cancer drug delivery; Calcium influx-mediated specific tumor suppression. |
[46] |
| Europium-containing MBGs | Doping of Eu ions into MBGs for intelligent delivery of doxorubicin. | Large surface area; High pore volume; Enhanced mechanical property; Controlled drug delivery; Stimulatory bone regeneration. |
[149] |
| Sm/MBGs/Alginate microspheres | Incorporation of Sm into the network of MBGs; Surface functionalization of MBGs with alginate hydrogels. |
Acceptable biocompatibility; Controlled drug delivery; Stimulatory bone regeneration; Microenvironment modulation. |
[78] |
| Multifunctional magnetic MBGs | Incorporating Fe/Fe3O4 into the nanostructure of MBGs for tumor therapy. | Super-paramagnetic features; Improved mechanical property; Controlled drug delivery; Hyperthermia and photothermal properties. |
[[255], [256], [257]] |
| Copper-containing magnetic MBGs | Impregnation of copper ion into the nanostructure of magnetic MBGs for infectious diseases and tumor therapy. | Acceptable bioactivity; Antibacterial activity; Super-paramagnetism features; Controlled drug delivery; Stimulatory bone regeneration. |
[216] |
| Molecular gated MBGs | Surface functionalization of MBGs with adenosine triphosphate (ATP) and e-poly-llysine molecular gates. | Superior biodegradability; High surface area; Dual-drug loading system; Controlled drug delivery. |
[45] |
Immunotherapy is widely recognized as a promising targeted-tumor therapeutic strategy due to its high specificity, efficiency, and adaptability, utilizing the patient's own immune system to recognize and destroy tumor cells instead of relying on exogenous toxic substances [[244], [245], [246]]. Certain tumor immunotherapies, such as immune checkpoint blockade therapy, adoptive cell therapy, and cancer vaccines, have demonstrated significant advantages in clinical trials [[247], [248], [249], [250]]. Thanks to advancements in nanotechnology, multifunctional MBGs materials have been developed with high biocompatibility, porosity, self-adjuvanticity, and ease of surface modification, showing great promise in cancer immunotherapy and modulation of the tumor microenvironment [251,252]. It is important to note that the tumor microenvironment often creates immune-suppressive conditions and weakens immune activity. However, multifunctional MBGs can deliver various drugs specifically to tumors and release them responsively, as well as enhance the efficacy of immunotherapy by modulating the tumor microenvironment where immune cells may encounter antigens, stimulatory signals, cancerous cells, or other immunomodulatory cells [253].
Taken together, multiple unique features involving tailorable nanoscale morphology and modifiable surface chemistry, endow the advanced MBGs with excellent capability of molecule loading/release, which make it become a promising nanoplatform for targeted drug delivery.
5.3. Tissue regeneration
Numerous studies, conducted through in vivo experiments and animal models, have showcased the immense potential of engineered MBGs in the regeneration of both hard and soft tissues [[258], [259], [260]]. MBGs possess inherent properties that promote vascularization, enzyme activity, and mesenchymal stem cell differentiation. They find utility as bone graft materials and play crucial roles in coatings for dental or orthopedic implants [195,[261], [262], [263]]. For instance, Wang et al. utilized 3D-bioprinting technology to create polycaprolactone/MBGs/doxycycline scaffolds, which demonstrated dual functionality by increasing osteogenic activity and broad-spectrum bacterial inhibition. These scaffolds released BMP2 to actively stimulate osteoblast differentiation and induce ectopic bone formation [83]. Similarly, the reconstruction of osteochondral defects necessitates scaffold materials that simultaneously promote cartilage and subchondral bone morphogenesis to restore joint structural integrity and functionality. Lin et al. synthesized hierarchical PEGylated poly (glycerol sebacate) scaffolds through a spontaneous pore-forming and solvent-free urethane cross-linking procedure. By combining MBGs with viscoelastic low-crosslinked PEGS, they constructed a bifunctional MBGs/PEGS bilayer scaffold. This scaffold facilitated chondrogenic differentiation, enhanced cartilage matrix secretion, maintained chondrocyte phenotype, and successfully reconstructed integrated articular hyaline cartilage and its subchondral bone [80]. Recent studies also indicate great interest in using MBGs for dental regeneration [221]. For example, Mocquot et al. fabricated fine and large particles with fixed biological glass composition, which exhibited excellent cytocompatibility and bioactivity on human dental pulp cells [195]. Lee et al. employed 3 % propylamino triethoxysilane to aminate MBGs (MBGs-NH2), effectively reversing their charge from negative to positive. Consequently, MBGs-NH2 induced odontogenic differentiation of dental pulp stem cells [238,264,265]. MBGs-based biomaterials may serve as potential additives for dentin regeneration [266].
Chronic wounds present a significant global health issue and impose a substantial burden on patients and healthcare systems. Recent studies have demonstrated the potential of utilizing biological glass to stimulate angiogenesis and accelerate healing in soft tissue wounds, positioning MBGs as promising materials for chronic wound applications. For instance, Wang et al. developed a composite scaffold by combining copper-containing MBGs (Cu-MBGs) with nanofibrillated cellulose (NFC). Cu-MBGs exhibited high bioactivity when exposed to simulated body fluids and displayed dose-dependent cytotoxicity on fibroblasts due to the presence of copper cations. Furthermore, the incorporation of Cu-MBGs into the NFC matrix enhanced the angiogenic effects on human umbilical vein endothelial cells [267]. These findings suggest that Cu-MBGs could serve as dressing materials for chronic wound healing. In another case, researchers successfully prepared an Ag-doped MBGs/poly (1,8-octanediol citrate) elastomeric composite scaffold using the salt-leaching technique. Among these scaffolds, Ag-MBGs exhibited favorable biocompatibility with human dermal fibroblast cells, highlighting the potential of MBGs-based biomaterials as intriguing candidates for soft tissue healing [261]. In conclusion, MBGs, as captivating materials possessing excellent bioactivity, biocompatibility, and biodegradability, hold promise for a variety of soft tissue repair and organ regeneration applications in the future.
In summary, although MBGs have been applied in the repair and regeneration of hard and soft tissue defects, the next research direction lies in developing multifunctional MBGs through advanced nanoengineering techniques. This will aim to enhance their in vivo biological activity, reduce foreign body reactions, and ultimately enable theranostic applications.
5.4. Infectious diseases
The prevention and treatment of bacterial infection is one of the biggest challenges in biomedical field, which is related to the increase of bacterial resistance to antibiotics. Recent studies have highlighted the use of multifunctional MBGs as carriers for controlled release of organic or inorganic antibacterial agents [[268], [269], [270]]. Leveraging the exceptional characteristics of MBGs, such as high surface area, pore volume, and bioactive behavior, researchers have introduced platforms based on antibacterial drug-loaded and ion-doped MBGs for combating infectious diseases [241]. For example, biomimetic gelatin-coated MBGs, enhanced with 4 % zinc oxide and antibacterial agents like levofloxacin, vancomycin, gentamicin, and rifampicin, have demonstrated excellent antibacterial properties. This suggests that zinc ions released from MBGs can effectively complement lower antibiotic dosages in fighting bacterial infections [79]. Furthermore, various antibacterial metallic ions (including Ag+, Ga3+, Cu+, Cu2+, Ce3+, and Ce4+ cations) have been incorporated into the nanostructure of MBGs, yielding significant antibacterial effects [271,272]. Additionally, recent reports have highlighted the emerging applications of MBGs in tissue engineering. The development of multifunctional MBGs-based platforms, modified with antibacterial ions and loaded with carefully controlled concentrations of antibiotics, presents a promising approach for treating infectious soft or hard tissue defects.
In summary, the unique porous textures of MBGs make them desirable candidates for theranostic applications in bacterial infectious diseases, with the potential to load and deliver a variety of antibacterial metallic cations and biological molecules.
5.5. Hemostatic diseases
Efficiently controlling life-threatening bleeding and its complications through the use of hemostatic materials is crucial for saving lives [273]. In recent times, inorganic MBGs and composite materials have shown promise in accelerating hemostasis and controlling infection due to their distinctive attributes, including porous structures, blood clot-accelerating surfaces, and rapid plasma absorbency [274]. Generally, well-ordered MBGs, composed of the SiO2–CaO–P2O5 structural system, leverage calcium ions as coagulation co-factors to effectively promote the expression of coagulation factors I (fibrinogen), II (prothrombin), and III (tissue coagulation kinase). Notably, doping Ag ions into the mesoporous channels of MBGs confers hemostatic and antibacterial properties, significantly improving wound healing efficiency upon application at the wound site. Dissolution of MBGs releases calcium ions, accelerating blood coagulation, promoting migration and proliferation of epidermal cells, and thereby facilitating wound healing while preventing infection [275].
In conclusion, the surface chemistry, textural performance, morphological features, and therapeutic ion release from MBGs play a pivotal role in promoting blood clot formation and antibacterial effects, offering significant value in controlling hemorrhage and infection in both battlefield and civilian settings.
5.6. Biosensor
Research and development of high-efficiency biosensors with excellent analytical properties and precise real-time measurement feedback is desirable to improve the survival rate of severely ill patients and avoid poor prognosis at the early stages of diseases [277,278]. Biosensors can be defined as bioelectrochemical analysis systems composed of immobilized biological substances such as enzymes, proteins, antigens, antibodies, and biofilms, along with appropriate chemical signal converters. The goal is to preserve the reaction and efficiency of biological molecules to the greatest extent [[279], [280], [281]]. Recently, MBGs have gained recognition as attractive materials for biosensing devices due to their stability, multi-channel architectures, well-defined surface performance, easily functionalized mesoporous walls, and superior biocompatibility [[282], [283], [284]]. The porous structure of MBGs provides better response, recovery, and sensitivity, thanks to the active sites and interfaces that facilitate interaction between the solid network and analytes. This is highly beneficial for mass diffusion, surface reaction, adsorption, and desorption involved in the sensing process [285,286]. Electrochemical immunoassay, which enables specific recognition and detection of signal molecules, has been extensively explored in biosensing devices. For instance, Qu et al. proposed a novel split-type biosensor that integrates a controlled-release cadmium sulfide-capped spherical MBGs nanocarrier, which encapsulates ascorbic acid using electrochemical detection. To minimize nanomaterial accumulation-induced immune disturbance and steric inhibition, the immunoreaction procedure was conducted using a 96-well microplate combined with detection antibody-labeled bioconjugate, independently of the electrochemical determination process. By utilizing dithiothreitol's role in breaking disulfide bonds, ascorbic acid could be efficiently released and subsequently oxidized based on the synergetic catalysis of the electrochemical sensing platform, ultimately triggering AA-mediated cascaded signal amplification. Consequently, the MBGs-based biosensor exhibited sensitive biomarker analysis with a low detection limit of 1.08 pg/ml within a linear range of 0.001–100 ng/ml. Based on the aforementioned discussion, MBGs hold promise as biomaterials for biosensing devices due to their unique structures and fascinating performances [48].
In summary, MBGs, as a novel type of porous nanomaterials, possess high specific surface area, ordered pore structure, adjustable pore size, and easily modified surface, making them ideal candidates for adsorption, fixation, and separation of various proteins, biological enzymes, and other biological molecules. They can be applied in enzyme immobilization, biocatalysis, biomarkers, and other medical fields.
6. Conclusion and perspective
The advance of knowledge in the natural tissue microenvironment, at structural, mechanical, and compositional levels, has inspired scientific researchers to design novel materials with attractive properties for biomedical applications. MBGs-based hierarchical scaffolds and systems have been discovered and utilized in drug delivery, tissue engineering, tumor therapy, biosensing, and other fields. The synthesis of multifunctional MBGs has opened the door to numerous advantages, including great biocompatibility, biodegradability, superior physicochemical properties, controlled release of biomolecules, improved targeting properties, immune cell recruitment and activation, and more. Recently, chemically engineered strategies of MBGs have been proposed to respond to stimulated microenvironments. The capability of MBGs to deliver various therapeutic elements and bioactive agents, such as cations, chemical drugs, proteins, peptides, cytokines, growth factors, genes, and antibodies, makes the material very promising for theranostic applications in multiple diseases. Under dynamic culturing environments, an accurate combination of optimized MBGs nanoparticles with biopolymers or disease- and tissue-specific ECM scaffolding systems via rapid prototyping technology may develop an ideal bio-inspired platform for biomedicine and engineering. This platform will create a new era of precision medicine, individual disease diagnosis and treatment, targeted and efficient drug delivery, well-matched artificial tissues and organs for human health, and patient-specific cancer vaccine development in the future.
The optimal combination of the new generation of MBGs with therapeutic cancer vaccines holds tremendous potential for inducing a potent anti-tumor immune response, much like the fields of mesoporous silica chemistry and immunobiology. This approach not only generates robust tumor-specific cytotoxic T lymphocytes (CTLs), but also inhibits tumor-induced immunosuppression. Furthermore, materials-based cancer vaccines are expected to be an attractive form of immune-precision therapeutic protocol, rapidly advancing to the forefront of cancer immunotherapy and synergizing with checkpoint blockade and adoptive cell therapy. The incorporation of patient-specific neoantigens, identified through genetic profiling, can be combined with MBGs to enhance the efficacy of cancer vaccines. In addition, MBGs can serve as tumor microenvironment-sensitive nanocarriers, enabling more efficient cancer therapy and imaging.
It is important to emphasize that safety should be the primary concern when considering the use of MBGs-based materials in laboratories and clinics. Exhaustive and elaborate studies involving in vivo biodistribution, clearance, and biocompatibility of administered particles are necessary. Despite the great potential of MBGs for disease therapy, it is crucial to investigate the influence of shape, size, and surface chemistry of MBGs on the immune compartment or body organ affected, as the nano- or micro-size could potentially cause adverse reactions with surrounding tissues. Accurate toxicity evaluation is also an essential consideration factor for choosing appropriate dosage. This helps to achieve a balance between systemic toxicity and treatment efficacy, minimizing side effects while maintaining therapeutic effects. Additionally, a reliable manufacturing process for MBGs with excellent biological functions and well-matched physicochemical properties needs to be established. This is obligatory to facilitate the clinical translation of MBGs-based hierarchical systems for biomedical applications.
Ethics approval and consent to participate
The current review does not involve any experimental work on human subjects or animals, and thus does not require approval from an ethics committee. As this work solely involves the synthesis and analysis of pre-existing literature, it doesn't necessitate any form of direct involvement or consent from patients or healthy volunteers.
CRediT authorship contribution statement
Ya Cui: Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Shebin Hong: Writing – review & editing, Formal analysis, Visualization. Weidong Jiang: Formal analysis, Project administration, Writing – review & editing, Funding acquisition. Xiaojing Li: Writing – review & editing. Xingyu Zhou: Writing – review & editing. Xiaoya He: Writing – review & editing. Jiaqiang Liu: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Kaili Lin: Writing – original draft, Writing – review & editing, Visualization, Conceptualization. Lixia Mao: Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Engineering mesoporous bioactive glasses for emerging stimuli-responsive drug delivery and theranostic applications”.
Acknowledgments
This study was funded by National Natural Science Foundation of China, grant 81701020; National Natural Science Foundation of China, grant 82071081; Shanghai Municipal Health and Family Planning Commission, grant 201740035; China Postdoctoral Science Foundation, grant 2023M742318. Ya Cui, Shebin Hong and Weidong Jiang contributed equally to this work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2024.01.001.
Contributor Information
Jiaqiang Liu, Email: lklecnu@aliyun.com.
Kaili Lin, Email: liujqmj@163.com.
Lixia Mao, Email: Maolx1302@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Yang W., Luo Z.P., Bao W.K., Xie H., You Z.S., Jin H.J. Light, strong, and stable nanoporous aluminum with native oxide shell. Sci. Adv. 2021;7(28) doi: 10.1126/sciadv.abb9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz E., Sarp G., Uzcan F., Ozalp O., Soylak M. Application of magnetic nanomaterials in bioanalysis. Talanta. 2021;229 doi: 10.1016/j.talanta.2021.122285. [DOI] [PubMed] [Google Scholar]
- 3.Yadav R.S. Multifunctional nanomaterials: synthesis, properties and applications. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222112073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F.R., Van Dyck D., Kisielowski C., Hansen L.P., Barton B., Helveg S. Probing atom dynamics of excited Co-Mo-S nanocrystals in 3D. Nat. Commun. 2021;12(1):5007. doi: 10.1038/s41467-021-24857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinnear C., Moore T.L., Rodriguez-Lorenzo L., Rothen-Rutishauser B., Petri-Fink A. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 2017;117(17):11476–11521. doi: 10.1021/acs.chemrev.7b00194. [DOI] [PubMed] [Google Scholar]
- 6.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1) doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Y., Li H., Li Y., Mao L. Novel insights into nanomaterials for immunomodulatory bone regeneration. Nanoscale Adv. 2022;4(2):334–352. doi: 10.1039/d1na00741f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z., Liu Y., Fang X., Shu Z. Nanomaterials enhance the immunomodulatory effect of molecular targeted therapy. Int. J. Nanomed. 2021;16:1631–1661. doi: 10.2147/IJN.S290346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouha J., Xiong H. DNAzyme-functionalized nanomaterials: recent preparation, current applications, and future challenges. Small. 2021;17(51) doi: 10.1002/smll.202105439. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Salcedo S., García A., González-Jiménez A., Vallet-Regí M. Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 2023;155:654–666. doi: 10.1016/j.actbio.2022.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Xu C., Yang X., Pu K. Photoactivatable protherapeutic nanomedicine for cancer. Adv. Mater. 2020;32(34) doi: 10.1002/adma.202002661. [DOI] [PubMed] [Google Scholar]
- 12.Wu R., Huang L., Xia Q., Liu Z., Huang Y., Jiang Y., Wang J., Ding H., Zhu C., Song Y., Liu L., Zhang L., Feng G. Injectable mesoporous bioactive glass/sodium alginate hydrogel loaded with melatonin for intervertebral disc regeneration. Mater Today Bio. 2023;22 doi: 10.1016/j.mtbio.2023.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D., Liang Z., Su Z., Huang J., Pi Y., Ouyang Y., Luo T., Guo L. Selenium-doped mesoporous bioactive glass regulates macrophage metabolism and polarization by scavenging ROS and promotes bone regeneration in vivo. ACS Appl. Mater. Interfaces. 2023;15(29):34378–34396. doi: 10.1021/acsami.3c03446. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira M.B., Li F., Choi J., Mano J.F. Nanomaterials for biomedical applications. Biotechnol. J. 2020;15(12) doi: 10.1002/biot.202000574. [DOI] [PubMed] [Google Scholar]
- 15.Li Q., Chen C., Li C., Liu R., Bi S., Zhang P., Zhou Y., Mai Y. Ordered bicontinuous mesoporous polymeric semiconductor photocatalyst. ACS Nano. 2020;14(10):13652–13662. doi: 10.1021/acsnano.0c05797. [DOI] [PubMed] [Google Scholar]
- 16.Awoke Y., Chebude Y., Díaz I. Controlling particle morphology and pore size in the synthesis of ordered mesoporous materials. Molecules. 2020;25(21) doi: 10.3390/molecules25214909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L., Fan L., Zhang F.M., Jiang Y., Cai M., Dai C., Luo Y.A., Tu L.J., Zhou Z.N., Li X.J., Ning C.Y., Zheng K., Boccaccini A.R., Tan G.X. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021;6(3):890–904. doi: 10.1016/j.bioactmat.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukrun Farrell E., Schilt Y., Moshkovitz M.Y., Levi-Kalisman Y., Raviv U., Magdassi S. 3D printing of ordered mesoporous silica complex structures. Nano Lett. 2020;20(9):6598–6605. doi: 10.1021/acs.nanolett.0c02364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H., Wang X., Jin A., Wang M., Wang Z., Huang X., Dai J., Wang X., Lin D., Shen S.G. Reducing relapse and accelerating osteogenesis in rapid maxillary expansion using an injectable mesoporous bioactive glass/fibrin glue composite hydrogel. Bioact. Mater. 2022;18:507–525. doi: 10.1016/j.bioactmat.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu S., Li M., Wang Z., Feng Q., Gao H., Li Q., Chen X., Cao X. Bioactive glasses-based nanozymes composite macroporous cryogel with antioxidative, antibacterial, and pro-healing properties for diabetic infected wound repair. Adv. Healthcare Mater. 2023;12(29) doi: 10.1002/adhm.202302073. [DOI] [PubMed] [Google Scholar]
- 21.Yan X., Yu C., Zhou X., Tang J., Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem. Int. Ed. Engl. 2004;43(44):5980–5984. doi: 10.1002/anie.200460598. [DOI] [PubMed] [Google Scholar]
- 22.Migneco C., Fiume E., Verné E., Baino F. A guided walk through the world of mesoporous bioactive glasses (MBGs): fundamentals, processing, and applications. Nanomaterials. 2020;10(12) doi: 10.3390/nano10122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher M., Habibovic P., van Rijt S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioact. Mater. 2021;6(7):1921–1931. doi: 10.1016/j.bioactmat.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontremoli C., Pagani M., Maddalena L., Carosio F., Vitale-Brovarone C., Fiorilli S. Polyelectrolyte-coated mesoporous bioactive glasses via layer-by-layer deposition for sustained Co-delivery of therapeutic ions and drugs. Pharmaceutics. 2021;13(11) doi: 10.3390/pharmaceutics13111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guduric V., Belton N., Richter R.F., Bernhardt A., Spangenberg J., Wu C., Lode A., Gelinsky M. Tailorable zinc-substituted mesoporous bioactive glass/alginate-methylcellulose composite bioinks. Materials. 2021;14(5) doi: 10.3390/ma14051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pontremoli C., Izquierdo-Barba I., Montalbano G., Vallet-Regí M., Vitale-Brovarone C., Fiorilli S. Strontium-releasing mesoporous bioactive glasses with anti-adhesive zwitterionic surface as advanced biomaterials for bone tissue regeneration. J. Colloid Interface Sci. 2020;563:92–103. doi: 10.1016/j.jcis.2019.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Wang C., Gao G., Yin X., Pu X., Shi B., Liu Y., Huang Z., Wang J., Li J., Yin G. MBG/PGA-PCL composite scaffolds provide highly tunable degradation and osteogenic features. Bioact. Mater. 2022;15:53–67. doi: 10.1016/j.bioactmat.2021.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J., Liu L., Huang S., Zheng W., Liu H., Bai Z., Jiang K., Wang X. PCL nanofibrous incorporating unique matrix fusion protein adsorbed mesoporous bioactive glass for bone tissue engineering. Int. J. Biol. Macromol. 2022;208:136–148. doi: 10.1016/j.ijbiomac.2022.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Chang L., Liu Y., Wu C. Copper-doped mesoporous bioactive glass for photothermal enhanced chemotherapy. J. Biomed. Nanotechnol. 2018;14(4):786–794. doi: 10.1166/jbn.2018.2542. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Chen W., Liu Q., Liu L. Genistein adsorbed mesoporous bioactive glass with enhanced osteogenesis properties. Biotechnol. Lett. 2020;42(2):321–328. doi: 10.1007/s10529-019-02773-4. [DOI] [PubMed] [Google Scholar]
- 31.Wu C., Chang J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Contr. Release. 2014;193:282–295. doi: 10.1016/j.jconrel.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Antonelli D.M., Nakahira A., Ying J.Y. Ligand-assisted liquid crystal templating in mesoporous niobium oxide molecular sieves. Inorg. Chem. 1996;35(11):3126–3136. doi: 10.1021/ic951533p. [DOI] [PubMed] [Google Scholar]
- 33.Tang F., Li L., Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 2012;24(12):1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 34.Zheng K., Sui B., Ilyas K., Boccaccini A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: developments, properties and emerging biomedical applications. Mater. Horiz. 2021;8(2):300–335. doi: 10.1039/d0mh01498b. [DOI] [PubMed] [Google Scholar]
- 35.Sharifi E., Bigham A., Yousefiasl S., Trovato M., Ghomi M., Esmaeili Y., Samadi P., Zarrabi A., Ashrafizadeh M., Sharifi S., Sartorius R., Dabbagh Moghaddam F., Maleki A., Song H., Agarwal T., Maiti T.K., Nikfarjam N., Burvill C., Mattoli V., Raucci M.G., Zheng K., Boccaccini A.R., Ambrosio L., Makvandi P. Mesoporous bioactive glasses in cancer diagnosis and therapy: stimuli-responsive, toxicity, immunogenicity, and clinical translation. Adv. Sci. 2022;9(2) doi: 10.1002/advs.202102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng T.Y., Tsai P.Y., Chen M.S., Mine Y., Wu S.H., Chen C.Y., Lin D.J., Lin C.K. Mesoporous properties of bioactive glass synthesized by spray pyrolysis with various polyethylene glycol and acid additions. Polymers. 2021;13(4) doi: 10.3390/polym13040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galarraga-Vinueza M.E., Mesquita-Guimarães J., Magini R.S., Souza J.C.M., Fredel M.C., Boccaccini A.R. Mesoporous bioactive glass embedding propolis and cranberry antibiofilm compounds. J. Biomed. Mater. Res. 2018;106(6):1614–1625. doi: 10.1002/jbm.a.36352. [DOI] [PubMed] [Google Scholar]
- 38.Fernando D., Colon P., Cresswell M., Journet C., Pradelle-Plasse N., Jackson P., Grosgogeat B., Attik N. The influence of precursor addition order on the porosity of sol-gel bioactive glasses. Dent. Mater. 2018;34(9):1323–1330. doi: 10.1016/j.dental.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Ciraldo F.E., Liverani L., Gritsch L., Goldmann W.H., Boccaccini A.R. Synthesis and characterization of silver-doped mesoporous bioactive glass and its applications in conjunction with electrospinning. Materials. 2018;11(5) doi: 10.3390/ma11050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu A., Lin D., Zhao H., Chen L., Cai B., Lin K., Shen S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120718. [DOI] [PubMed] [Google Scholar]
- 41.Shoaib M., Ur Rahman M.S., Saeed A., Naseer M.M. Mesoporous bioactive glass-polyurethane nanocomposites as reservoirs for sustained drug delivery. Colloids Surf. B Biointerfaces. 2018;172:806–811. doi: 10.1016/j.colsurfb.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 42.Kang M.S., Lee N.H., Singh R.K., Mandakhbayar N., Perez R.A., Lee J.H., Kim H.W. Nanocements produced from mesoporous bioactive glass nanoparticles. Biomaterials. 2018;162:183–199. doi: 10.1016/j.biomaterials.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Yin C., Zhao Q., Li W., Zhao Z., Wang J., Deng T., Zhang P., Shen K., Li Z., Zhang Y. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020;102:416–426. doi: 10.1016/j.actbio.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive glasses entering the mainstream. Drug Discov. Today. 2018;23(10):1700–1704. doi: 10.1016/j.drudis.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Polo L., Gómez-Cerezo N., Aznar E., Vivancos J.L., Sancenón F., Arcos D., Vallet-Regí M., Martínez-Máñez R. Molecular gates in mesoporous bioactive glasses for the treatment of bone tumors and infection. Acta Biomater. 2017;50:114–126. doi: 10.1016/j.actbio.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Sui B., Liu X., Sun J. Dual-functional dendritic mesoporous bioactive glass nanospheres for calcium influx-mediated specific tumor suppression and controlled drug delivery in vivo. ACS Appl. Mater. Interfaces. 2018;10(28):23548–23559. doi: 10.1021/acsami.8b05616. [DOI] [PubMed] [Google Scholar]
- 47.Pourshahrestani S., Kadri N.A., Zeimaran E., Towler M.R. Well-ordered mesoporous silica and bioactive glasses: promise for improved hemostasis. Biomater. Sci. 2018;7(1):31–50. doi: 10.1039/c8bm01041b. [DOI] [PubMed] [Google Scholar]
- 48.Qu L., Ren X., Fan D., Kuang X., Sun X., Wang B., Wei Q., Ju H. Split-type electrochemical immunoassay system triggering ascorbic acid-mediated signal magnification based on a controlled-release strategy. ACS Appl. Mater. Interfaces. 2021;13(24):29179–29186. doi: 10.1021/acsami.1c07780. [DOI] [PubMed] [Google Scholar]
- 49.Niu W., Guo Y., Xue Y.M., Wang M., Chen M., Winston D.D., Cheng W., Lei B. Biodegradable multifunctional bioactive Eu-Gd-Si-Ca glass nanoplatform for integrative imaging-targeted tumor therapy-recurrence inhibition-tissue repair. Nano Today. 2021;38 [Google Scholar]
- 50.Hooshmand S., Mollazadeh S., Akrami N., Ghanad M., El-Fiqi A., Baino F., Nazarnezhad S., Kargozar S. Mesoporous silica nanoparticles and mesoporous bioactive glasses for wound management: from skin regeneration to cancer therapy. Materials. 2021;14(12) doi: 10.3390/ma14123337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Zhang Y., Lin C., Zhong W. Sol-gel derived terbium-containing mesoporous bioactive glasses nanospheres: in vitro hydroxyapatite formation and drug delivery. Colloids Surf. B Biointerfaces. 2017;160:406–415. doi: 10.1016/j.colsurfb.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 52.Vallet-Regi M., Salinas A.J. Mesoporous bioactive glasses for regenerative medicine. Mater Today Bio. 2021;11 doi: 10.1016/j.mtbio.2021.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye X., Leeflang S., Wu C., Chang J., Zhou J., Huan Z. Mesoporous bioactive glass functionalized 3D Ti-6Al-4V scaffolds with improved surface bioactivity. Materials. 2017;10(11) doi: 10.3390/ma10111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gisbert-Garzarán M., Vallet-Regí M. Nanoparticles for bio-medical applications. Nanomaterials. 2022;12(7) doi: 10.3390/nano12071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang S., Zhang Y., Shu Y., Wu Z., Cao W., Huang W. Amino-functionalized mesoporous bioactive glass for drug delivery. Biomed. Mater. 2017;12(2) doi: 10.1088/1748-605X/aa645d. [DOI] [PubMed] [Google Scholar]
- 56.Qian G., Zhang L., Liu X., Wu S., Peng S., Shuai C. Silver-doped bioglass modified scaffolds: a sustained antibacterial efficacy. Mater. Sci. Eng., C. 2021;129 doi: 10.1016/j.msec.2021.112425. [DOI] [PubMed] [Google Scholar]
- 57.Ji L., Gong M., Xu T., Gu J., Jiang X., Liang T., Chen Y., Liu Q. Engineering the structure of mesoporous bioactive glass microspheres by the surface effect of inverse opal templates and temperature. Small. 2019;15(52) doi: 10.1002/smll.201905451. [DOI] [PubMed] [Google Scholar]
- 58.Šinkovec R., Mušič B. Effect of organosilane coupling agents on thermal, rheological and mechanical properties of silicate-filled epoxy molding compound. Materials. 2020;13(1) doi: 10.3390/ma13010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong X., Wang Y., Guan R., Ren J., Xie Z. Silane-functionalized carbon dots and their polymerized hybrids: from optoelectronics to biotherapy. Small. 2021;17(50) doi: 10.1002/smll.202105273. [DOI] [PubMed] [Google Scholar]
- 60.Reyes-Peces M.V., Pérez-Moreno A., de-Los-Santos D.M., Mesa-Díaz M.D.M., Pinaglia-Tobaruela G., Vilches-Pérez J.I., Fernández-Montesinos R., Salido M., de la Rosa-Fox N., Piñero M. Chitosan-GPTMS-silica hybrid mesoporous aerogels for bone tissue engineering. Polymers. 2020;12(11) doi: 10.3390/polym12112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmoodi M., Behzad-Behbahani A., Sharifzadeh S., Abolmaali S.S., Tamaddon A. Co-condensation synthesis of well-defined mesoporous silica nanoparticles: effect of surface chemical modification on plasmid DNA condensation and transfection. IET Nanobiotechnol. 2017;11(8):995–1004. doi: 10.1049/iet-nbt.2017.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zucchetto N., Brühwiler D. Functionalization of arrays of silica nanochannels by post-condensation. Dalton Trans. 2016;45(36):14363–14369. doi: 10.1039/c6dt02033j. [DOI] [PubMed] [Google Scholar]
- 63.Schmitz S., Beltrán A.M., Cresswell M., Boccaccini A.R. A structural comparison of ordered and non-ordered ion doped silicate bioactive glasses. Materials. 2020;13(4) doi: 10.3390/ma13040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salètes M., Vartin M., Mocquot C., Chevalier C., Grosgogeat B., Colon P., Attik N. Mesoporous bioactive glasses cytocompatibility assessment: a review of in vitro studies. Biomimetics. 2021;6(1) doi: 10.3390/biomimetics6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai X., Liu W., Xu L., Ye Q., Zhou H., Berg C., Yuan H., Li J., Xia W. Sequential macrophage transition facilitates endogenous bone regeneration induced by Zn-doped porous microcrystalline bioactive glass. J. Mater. Chem. B. 2021;9(12):2885–2898. doi: 10.1039/d0tb02884c. [DOI] [PubMed] [Google Scholar]
- 66.Gunawidjaja P.N., Mathew R., Lo A.Y., Izquierdo-Barba I., García A., Arcos D., Vallet-Regí M., Edén M. Local structures of mesoporous bioactive glasses and their surface alterations in vitro: inferences from solid-state nuclear magnetic resonance. Philos Trans A Math Phys Eng Sci. 2012;370(1963):1376–1399. doi: 10.1098/rsta.2011.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anand A., Lalzawmliana V., Kumar V., Das P., Devi K.B., Maji A.K., Kundu B., Roy M., Nandi S.K. Preparation and in vivo biocompatibility studies of different mesoporous bioactive glasses. J. Mech. Behav. Biomed. Mater. 2019;89:89–98. doi: 10.1016/j.jmbbm.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Kumar A., Aditya A., Murugavel S. Effect of surfactant concentration on textural characteristics and biomineralization behavior of mesoporous bioactive glasses. Mater. Sci. Eng., C. 2019;96:20–29. doi: 10.1016/j.msec.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Zheng K., Torre E., Bari A., Taccardi N., Cassinelli C., Morra M., Fiorilli S., Vitale-Brovarone C., Iviglia G., Boccaccini A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater Today Bio. 2020;5 doi: 10.1016/j.mtbio.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bano S., Akhtar M., Yasir M., Salman Maqbool M., Niaz A., Wadood A., Ur Rehman M.A. Synthesis and characterization of silver-strontium (Ag-Sr)-Doped mesoporous bioactive glass nanoparticles. Gels. 2021;7(2) doi: 10.3390/gels7020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arcos D., Vila M., López-Noriega A., Rossignol F., Champion E., Oliveira F.J., Vallet-Regí M. Mesoporous bioactive glasses: mechanical reinforcement by means of a biomimetic process. Acta Biomater. 2011;7(7):2952–2959. doi: 10.1016/j.actbio.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Moses J.C., Mandal B.B. Mesoporous silk-bioactive glass nanocomposites as drug eluting multifunctional conformal coatings for improving osseointegration and bactericidal properties of metal implants. ACS Appl. Mater. Interfaces. 2022;14(13):14961–14980. doi: 10.1021/acsami.2c00093. [DOI] [PubMed] [Google Scholar]
- 73.El-Fiqi A., Allam R., Kim H.W. Antioxidant cerium ions-containing mesoporous bioactive glass ultrasmall nanoparticles: structural, physico-chemical, catalase-mimic and biological properties. Colloids Surf. B Biointerfaces. 2021;206 doi: 10.1016/j.colsurfb.2021.111932. [DOI] [PubMed] [Google Scholar]
- 74.Gómez-Cerezo N., Casarrubios L., Saiz-Pardo M., Ortega L., de Pablo D., Díaz-Güemes I., Fernández-Tomé B., Enciso S., Sánchez-Margallo F.M., Portolés M.T., Arcos D., Vallet-Regí M. Mesoporous bioactive glass/ɛ-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019;90:393–402. doi: 10.1016/j.actbio.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bae J., Son W.S., Yoo K.H., Yoon S.Y., Bae M.K., Lee D.J., Ko C.C., Choi Y.K., Kim Y.I. Effects of poly(amidoamine) dendrimer-coated mesoporous bioactive glass nanoparticles on dentin remineralization. Nanomaterials. 2019;9(4) doi: 10.3390/nano9040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahapatra C., Singh R.K., Kim J.J., Patel K.D., Perez R.A., Jang J.H., Kim H.W. Osteopromoting reservoir of stem cells: bioactive mesoporous nanocarrier/collagen gel through slow-releasing FGF18 and the activated BMP signaling. ACS Appl. Mater. Interfaces. 2016;8(41):27573–27584. doi: 10.1021/acsami.6b09769. [DOI] [PubMed] [Google Scholar]
- 77.Min Q., Wang C., Zhang Y., Tian D., Wan Y., Wu J. Strong and elastic hydrogels from dual-crosslinked composites composed of glycol chitosan and amino-functionalized bioactive glass nanoparticles. Nanomaterials. 2022;12(11) doi: 10.3390/nano12111874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Wang X., Su Y., Chen D., Zhong W. A doxorubicin delivery system: samarium/mesoporous bioactive glass/alginate composite microspheres. Mater. Sci. Eng., C. 2016;67:205–213. doi: 10.1016/j.msec.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 79.Heras C., Jiménez-Holguín J., Doadrio A.L., Vallet-Regí M., Sánchez-Salcedo S., Salinas A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020;114:395–406. doi: 10.1016/j.actbio.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin D., Cai B., Wang L., Cai L., Wang Z., Xie J., Lv Q.X., Yuan Y., Liu C., Shen S.G. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials. 2020;253 doi: 10.1016/j.biomaterials.2020.120095. [DOI] [PubMed] [Google Scholar]
- 81.Covarrubias C., Agüero A., Maureira M., Morelli E., Escobar G., Cuadra F., Peñafiel C., Von Marttens A. In situ preparation and osteogenic properties of bionanocomposite scaffolds based on aliphatic polyurethane and bioactive glass nanoparticles. Mater. Sci. Eng., C. 2019;96:642–653. doi: 10.1016/j.msec.2018.11.085. [DOI] [PubMed] [Google Scholar]
- 82.El-Fiqi A., Kim J.H., Kim H.W. Osteoinductive fibrous scaffolds of biopolymer/mesoporous bioactive glass nanocarriers with excellent bioactivity and long-term delivery of osteogenic drug. ACS Appl. Mater. Interfaces. 2015;7(2):1140–1152. doi: 10.1021/am5077759. [DOI] [PubMed] [Google Scholar]
- 83.Wang M., Li H., Yang Y., Yuan K., Zhou F., Liu H., Zhou Q., Yang S., Tang T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021;6(5):1318–1329. doi: 10.1016/j.bioactmat.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sordi M.B., Fredel M.C., da Cruz A.C.C., Sharpe P.T., de Souza Magini R. Enhanced bone tissue regeneration with hydrogel-based scaffolds by embedding parathyroid hormone in mesoporous bioactive glass. Clin. Oral Invest. 2023;27(1):125–137. doi: 10.1007/s00784-022-04696-3. [DOI] [PubMed] [Google Scholar]
- 85.Arcos D., Gómez-Cerezo N., Saiz-Pardo M., de Pablo D., Ortega L., Enciso S., Fernández-Tomé B., Díaz-Güemes I., Sánchez-Margallo F.M., Casarrubios L., Feito M.J., Portolés M.T., Vallet-Regí M. Injectable mesoporous bioactive nanoparticles regenerate bone tissue under osteoporosis conditions. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.07.067. [DOI] [PubMed] [Google Scholar]
- 86.Pant S., Thomas S., Loganathan S., Valapa R.B. 3D bioprinted poly(lactic acid)/mesoporous bioactive glass based biomimetic scaffold with rapid apatite crystallization and in-vitro Cytocompatability for bone tissue engineering. Int. J. Biol. Macromol. 2022;217:979–997. doi: 10.1016/j.ijbiomac.2022.07.202. [DOI] [PubMed] [Google Scholar]
- 87.Lu J., Liu Z., Wang K., Gu M., Peng X., Zhang Y., Chen X., Chen Y., Zhang L. Odontogenesis by endocytosis of peptide embedding bioactive glass composite. J. Dent. Res. 2022;101(9):1055–1063. doi: 10.1177/00220345221085186. [DOI] [PubMed] [Google Scholar]
- 88.Das M.P., Pandey G., Neppolian B., Das J. Design of poly-l-glutamic acid embedded mesoporous bioactive glass nanospheres for pH-stimulated chemotherapeutic drug delivery and antibacterial susceptibility. Colloids Surf. B Biointerfaces. 2021;202 doi: 10.1016/j.colsurfb.2021.111700. [DOI] [PubMed] [Google Scholar]
- 89.Boffito M., Pontremoli C., Fiorilli S., Laurano R., Ciardelli G., Vitale-Brovarone C. Injectable thermosensitive formulation based on polyurethane hydrogel/mesoporous glasses for sustained Co-delivery of functional ions and drugs. Pharmaceutics. 2019;11(10) doi: 10.3390/pharmaceutics11100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Llopis-Lorente A., García-Fernández A., Lucena-Sánchez E., Díez P., Sancenón F., Villalonga R., Wilson D.A., Martínez-Máñez R. Stimulus-responsive nanomotors based on gated enzyme-powered Janus Au-mesoporous silica nanoparticles for enhanced cargo delivery. Chem. Commun. 2019;55(87):13164–13167. doi: 10.1039/c9cc07250k. [DOI] [PubMed] [Google Scholar]
- 91.Lee J., Oh E.T., Yoon H., Kim C.W., Han Y., Song J., Jang H., Park H.J., Kim C. Mesoporous nanocarriers with a stimulus-responsive cyclodextrin gatekeeper for targeting tumor hypoxia. Nanoscale. 2017;9(20):6901–6909. doi: 10.1039/c7nr00808b. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Q., Geng H., Wang Y., Gao Y., Huang J., Wang Y., Zhang J., Wang S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces. 2014;6(22):20290–20299. doi: 10.1021/am505824d. [DOI] [PubMed] [Google Scholar]
- 93.Zhang B.B., Chen X.J., Fan X.D., Zhu J.J., Wei Y.H., Zheng H.S., Zheng H.Y., Wang B.H., Piao J.G., Li F.Z. Lipid/PAA-coated mesoporous silica nanoparticles for dual-pH-responsive codelivery of arsenic trioxide/paclitaxel against breast cancer cells. Acta Pharmacol. Sin. 2021;42(5):832–842. doi: 10.1038/s41401-021-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Polo L., Gómez-Cerezo N., García-Fernández A., Aznar E., Vivancos J.L., Arcos D., Vallet-Regí M., Martínez-Máñez R. Mesoporous bioactive glasses equipped with stimuli-responsive molecular gates for controlled delivery of levofloxacin against bacteria. Chemistry. 2018;24(71):18944–18951. doi: 10.1002/chem.201803301. [DOI] [PubMed] [Google Scholar]
- 95.Heras C., Sanchez-Salcedo S., Lozano D., Peña J., Esbrit P., Vallet-Regi M., Salinas A.J. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn(2+) ions in human mesenchymal stem cells. Acta Biomater. 2019;89:359–371. doi: 10.1016/j.actbio.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi H., Zhang C., Guo H., Zheng W., Yang J., Zhou X., Zhang L. Bioinspired multifunctional protein coating for antifogging, self-cleaning, and antimicrobial properties. ACS Appl. Mater. Interfaces. 2019;11(27):24504–24511. doi: 10.1021/acsami.9b03522. [DOI] [PubMed] [Google Scholar]
- 97.Zou Y., Ito S., Yoshino F., Suzuki Y., Zhao L., Komatsu N. Polyglycerol grafting shields nanoparticles from protein corona formation to avoid macrophage uptake. ACS Nano. 2020;14(6):7216–7226. doi: 10.1021/acsnano.0c02289. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Zhao L., Liang Q., Liang Q., Ye J., Komatsu N., Zhang Q., Gao W., Xu M., Chen X. Cationic polyarginine conjugated mesoporous bioactive glass nanoparticles with polyglycerol coating for efficient DNA delivery. J. Biomed. Nanotechnol. 2017;13(3):280–289. doi: 10.1166/jbn.2017.2350. [DOI] [PubMed] [Google Scholar]
- 99.Xia W., Chang J., Lin J., Zhu J. The pH-controlled dual-drug release from mesoporous bioactive glass/polypeptide graft copolymer nanomicelle composites. Eur. J. Pharm. Biopharm. 2008;69(2):546–552. doi: 10.1016/j.ejpb.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 100.Fiorilli S., Pagani M., Boggio E., Gigliotti C.L., Dianzani C., Gauthier R., Pontremoli C., Montalbano G., Dianzani U., Vitale-Brovarone C. Sr-containing mesoporous bioactive glasses bio-functionalized with recombinant ICOS-fc: an in vitro study. Nanomaterials. 2021;11(2) doi: 10.3390/nano11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krainov I.V., Klier J., Dmitriev A.P., Klyatskaya S., Ruben M., Wernsdorfer W., Gornyi I.V. Giant magnetoresistance in carbon nanotubes with single-molecule magnets TbPc(2) ACS Nano. 2017;11(7):6868–6880. doi: 10.1021/acsnano.7b02014. [DOI] [PubMed] [Google Scholar]
- 102.Wen J., Yang K., Liu F., Li H., Xu Y., Sun S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017;46(19):6024–6045. doi: 10.1039/c7cs00219j. [DOI] [PubMed] [Google Scholar]
- 103.Kankala R.K., Han Y.H., Na J., Lee C.H., Sun Z., Wang S.B., Kimura T., Ok Y.S., Yamauchi Y., Chen A.Z., Wu K.C. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020;32(23) doi: 10.1002/adma.201907035. [DOI] [PubMed] [Google Scholar]
- 104.Kumar A., Murugavel S., Aditya A., Boccaccini A.R. Mesoporous 45S5 bioactive glass: synthesis, in vitro dissolution and biomineralization behavior. J. Mater. Chem. B. 2017;5(44):8786–8798. doi: 10.1039/c7tb01738c. [DOI] [PubMed] [Google Scholar]
- 105.Baino F., Fiorilli S., Vitale-Brovarone C. Composite biomaterials based on sol-gel mesoporous silicate glasses: a review. Bioengineering. 2017;4(1) doi: 10.3390/bioengineering4010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanchez-Salcedo S., Malavasi G., Salinas A.J., Lusvardi G., Rigamonti L., Menabue L., Vallet-Regi M. Highly-bioreactive silica-based mesoporous bioactive glasses enriched with gallium(III) Materials. 2018;11(3) doi: 10.3390/ma11030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiménez-Holguín J., Sánchez-Salcedo S., Vallet-Regí M., Salinas A.J. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Microporous Mesoporous Mater. 2020;308 doi: 10.1016/j.micromeso.2020.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Niu H., Lin D., Tang W., Ma Y., Duan B., Yuan Y., Liu C. Surface topography regulates osteogenic differentiation of MSCs via crosstalk between FAK/MAPK and ILK/β-Catenin pathways in a hierarchically porous environment. ACS Biomater. Sci. Eng. 2017;3(12):3161–3175. doi: 10.1021/acsbiomaterials.7b00315. [DOI] [PubMed] [Google Scholar]
- 109.Li X., Chen X., Miao G., Liu H., Mao C., Yuan G., Liang Q., Shen X., Ning C., Fu X. Synthesis of radial mesoporous bioactive glass particles to deliver osteoactivin gene. J. Mater. Chem. B. 2014;2(40):7045–7054. doi: 10.1039/c4tb00883a. [DOI] [PubMed] [Google Scholar]
- 110.Galarraga-Vinueza M.E., Mesquita-Guimarães J., Magini R.S., Souza J.C., Fredel M.C., Boccaccini A.R. Anti-biofilm properties of bioactive glasses embedding organic active compounds. J. Biomed. Mater. Res. 2017;105(2):672–679. doi: 10.1002/jbm.a.35934. [DOI] [PubMed] [Google Scholar]
- 111.Kermani F., Vojdani-Saghir A., Mollazadeh Beidokhti S., Nazarnezhad S., Mollaei Z., Hamzehlou S., El-Fiqi A., Baino F., Kargozar S. Iron (Fe)-doped mesoporous 45S5 bioactive glasses: implications for cancer therapy. Transl Oncol. 2022;20 doi: 10.1016/j.tranon.2022.101397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chotchindakun K., Pekkoh J., Ruangsuriya J., Zheng K., Unalan I., Boccaccini A.R. Fabrication and characterization of cinnamaldehyde-loaded mesoporous bioactive glass nanoparticles/PHBV-based microspheres for preventing bacterial infection and promoting bone tissue regeneration. Polymers. 2021;13(11) doi: 10.3390/polym13111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung J.H., Park S.B., Yoo K.H., Yoon S.Y., Bae M.K., Lee D.J., Ko C.C., Kwon Y.H., Kim Y.I. Effect of different sizes of bioactive glass-coated mesoporous silica nanoparticles on dentinal tubule occlusion and mineralization. Clin. Oral Invest. 2019;23(5):2129–2141. doi: 10.1007/s00784-018-2658-9. [DOI] [PubMed] [Google Scholar]
- 114.Gómez-Cerezo M.N., Peña J., Ivanovski S., Arcos D., Vallet-Regí M., Vaquette C. Multiscale porosity in mesoporous bioglass 3D-printed scaffolds for bone regeneration. Mater. Sci. Eng., C. 2021;120 doi: 10.1016/j.msec.2020.111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Hu Q., Miao G., Zhang Q., Yuan B., Zhu Y., Fu X., Chen X., Mao C. Size-dependent mechanism of intracellular localization and cytotoxicity of mono-disperse spherical mesoporous nano- and micron-bioactive glass particles. J. Biomed. Nanotechnol. 2016;12(5):863–877. doi: 10.1166/jbn.2016.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang W., Lin D., Yu Y., Niu H., Guo H., Yuan Y., Liu C. Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomater. 2016;32:309–323. doi: 10.1016/j.actbio.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Choi A., Yoo K.H., Yoon S.Y., Park B.S., Kim I.R., Kim Y.I. Anti-microbial and remineralizing properties of self-adhesive orthodontic resin containing mesoporous bioactive glass. Materials. 2021;14(13) doi: 10.3390/ma14133550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polo-Montalvo A., Casarrubios L., Serrano M.C., Sanvicente A., Feito M.J., Arcos D., Portolés M.T. Effective actions of ion release from mesoporous bioactive glass and macrophage mediators on the differentiation of osteoprogenitor and endothelial progenitor cells. Pharmaceutics. 2021;13(8) doi: 10.3390/pharmaceutics13081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y., Bastakoti B.P., Yamauchi Y. Smart soft-templating synthesis of hollow mesoporous bioactive glass spheres. Chemistry. 2015;21(22):8038–8042. doi: 10.1002/chem.201406570. [DOI] [PubMed] [Google Scholar]
- 120.Mutlu N., Beltrán A.M., Nawaz Q., Michálek M., Boccaccini A.R., Zheng K. Combination of selective etching and impregnation toward hollow mesoporous bioactive glass nanoparticles. Nanomaterials. 2021;11(7) doi: 10.3390/nano11071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou J., Xiong Z., Liu M., Yang L., Yao S., Chen K., Yu K., Qu Y., Sun T., Guo X. Creation of bony microenvironment with extracellular matrix doped-bioactive ceramics to enhance osteoblast behavior and delivery of aspartic acid-modified BMP-2 peptides. Int. J. Nanomed. 2020;15:8465–8478. doi: 10.2147/IJN.S272571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sistanipour E., Meshkini A., Oveisi H. Catechin-conjugated mesoporous hydroxyapatite nanoparticle: a novel nano-antioxidant with enhanced osteogenic property. Colloids Surf. B Biointerfaces. 2018;169:329–339. doi: 10.1016/j.colsurfb.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 123.Hsu F.Y., Hsu H.W., Chang Y.H., Yu J.L., Rau L.R., Tsai S.W. Macroporous microbeads containing apatite-modified mesoporous bioactive glass nanofibres for bone tissue engineering applications. Mater. Sci. Eng., C. 2018;89:346–354. doi: 10.1016/j.msec.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 124.El-Fiqi A., Kim J.H., Kim H.W. Highly bioactive bone cement microspheres based on α-tricalcium phosphate microparticles/mesoporous bioactive glass nanoparticles: formulation, physico-chemical characterization and in vivo bone regeneration. Colloids Surf. B Biointerfaces. 2022;217 doi: 10.1016/j.colsurfb.2022.112650. [DOI] [PubMed] [Google Scholar]
- 125.Xu Y., Wu P., Feng P., Guo W., Yang W., Shuai C. Interfacial reinforcement in a poly-l-lactic acid/mesoporous bioactive glass scaffold via polydopamine. Colloids Surf. B Biointerfaces. 2018;170:45–53. doi: 10.1016/j.colsurfb.2018.05.065. [DOI] [PubMed] [Google Scholar]
- 126.Montalbano G., Borciani G., Pontremoli C., Ciapetti G., Mattioli-Belmonte M., Fiorilli S., Vitale-Brovarone C. Development and biocompatibility of collagen-based composites enriched with nanoparticles of strontium containing mesoporous glass. Materials. 2019;12(22) doi: 10.3390/ma12223719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terzopoulou Z., Baciu D., Gounari E., Steriotis T., Charalambopoulou G., Tzetzis D., Bikiaris D. Composite membranes of poly(ε-caprolactone) with bisphosphonate-loaded bioactive glasses for potential bone tissue engineering applications. Molecules. 2019;24(17) doi: 10.3390/molecules24173067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li S., Song C., Yang S., Yu W., Zhang W., Zhang G., Xi Z., Lu E. Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019;94:253–267. doi: 10.1016/j.actbio.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 129.Marovic D., Par M., Tauböck T.T., Haugen H.J., Negovetic Mandic V., Wüthrich D., Burrer P., Zheng K., Attin T., Tarle Z., Boccaccini A.R. Impact of copper-doped mesoporous bioactive glass nanospheres on the polymerisation kinetics and shrinkage stress of dental resin composites. Int. J. Mol. Sci. 2022;23(15) doi: 10.3390/ijms23158195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Batool S.A., Ahmad K., Irfan M., Ur Rehman M.A. Zn-Mn-Doped mesoporous bioactive glass nanoparticle-loaded zein coatings for bioactive and antibacterial orthopedic implants. J. Funct. Biomater. 2022;13(3) doi: 10.3390/jfb13030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atkinson I., Seciu-Grama A.M., Petrescu S., Culita D., Mocioiu O.C., Voicescu M., Mitran R.A., Lincu D., Prelipcean A.M., Craciunescu O. Cerium-containing mesoporous bioactive glasses (MBGs)-Derived scaffolds with drug delivery capability for potential tissue engineering applications. Pharmaceutics. 2022;14(6) doi: 10.3390/pharmaceutics14061169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiménez-Holguín J., Sánchez-Salcedo S., Cicuéndez M., Vallet-Regí M., Salinas A.J. Cu-doped hollow bioactive glass nanoparticles for bone infection treatment. Pharmaceutics. 2022;14(4) doi: 10.3390/pharmaceutics14040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marovic D., Haugen H.J., Negovetic Mandic V., Par M., Zheng K., Tarle Z., Boccaccini A.R. Incorporation of copper-doped mesoporous bioactive glass nanospheres in experimental dental composites: chemical and mechanical characterization. Materials. 2021;14(10) doi: 10.3390/ma14102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kurtuldu F., Mutlu N., Michálek M., Zheng K., Masar M., Liverani L., Chen S., Galusek D., Boccaccini A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng., C. 2021;124 doi: 10.1016/j.msec.2021.112050. [DOI] [PubMed] [Google Scholar]
- 135.Westhauser F., Decker S., Nawaz Q., Rehder F., Wilkesmann S., Moghaddam A., Kunisch E., Boccaccini A.R. Impact of zinc- or copper-doped mesoporous bioactive glass nanoparticles on the osteogenic differentiation and matrix formation of mesenchymal stromal cells. Materials. 2021;14(8) doi: 10.3390/ma14081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu M., Fang J., Zhang Y., Wang X., Zhong W., Zhou Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020;579:654–666. doi: 10.1016/j.jcis.2020.06.122. [DOI] [PubMed] [Google Scholar]
- 137.Zhang J., Zhao S., Zhu M., Zhu Y., Zhang Y., Liu Z., Zhang C. 3D-printed magnetic Fe(3)O(4)/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B. 2014;2(43):7583–7595. doi: 10.1039/c4tb01063a. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J., Zhao S., Zhu Y., Huang Y., Zhu M., Tao C., Zhang C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10(5):2269–2281. doi: 10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 139.Neščáková Z., Zheng K., Liverani L., Nawaz Q., Galusková D., Kaňková H., Michálek M., Galusek D., Boccaccini A.R. Multifunctional zinc ion doped sol - gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019;4:312–321. doi: 10.1016/j.bioactmat.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin D., Yang K., Tang W., Liu Y., Yuan Y., Liu C. A poly(glycerol sebacate)-coated mesoporous bioactive glass scaffold with adjustable mechanical strength, degradation rate, controlled-release and cell behavior for bone tissue engineering. Colloids Surf. B Biointerfaces. 2015;131:1–11. doi: 10.1016/j.colsurfb.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 141.Kung J.C., Wang W.H., Chiang Y.C., Yang-Wang Y.T., Wang Y.C., Chen W.C., Shih C.J. The antibacterial and remineralization effect of silver-containing mesoporous bioactive glass sealing and Er-yag laser on dentinal tubules treated in a Streptococcus mutans cultivated environment. Pharmaceuticals. 2021;14(11) doi: 10.3390/ph14111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen X., Zhao Y., Geng S., Miron R.J., Zhang Q., Wu C., Zhang Y. In vivo experimental study on bone regeneration in critical bone defects using PIB nanogels/boron-containing mesoporous bioactive glass composite scaffold. Int. J. Nanomed. 2015;10:839–846. doi: 10.2147/IJN.S69001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yin C., Jia X., Miron R.J., Long Q., Xu H., Wei Y., Wu M., Zhang Y., Li Z. Setd7 and its contribution to Boron-induced bone regeneration in Boron-mesoporous bioactive glass scaffolds. Acta Biomater. 2018;73:522–530. doi: 10.1016/j.actbio.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 144.Varini E., Sánchez-Salcedo S., Malavasi G., Lusvardi G., Vallet-Regí M., Salinas A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng., C. 2019;105 doi: 10.1016/j.msec.2019.109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie H., Sha S., Lu L., Wu G., Jiang H., Boccaccini A.R., Zheng K., Xu R. Cerium-containing bioactive glasses promote in vitro lymphangiogenesis. Pharmaceutics. 2022;14(2) doi: 10.3390/pharmaceutics14020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu C., Zhou Y., Fan W., Han P., Chang J., Yuen J., Zhang M., Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33(7):2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 147.Kaur G., Sriranganathan N., Waldrop S.G., Sharma P., Chudasama B.N. Effect of copper on the up-regulation/down-regulation of genes, cytotoxicity and ion dissolution for mesoporous bioactive glasses. Biomed. Mater. 2017;12(4) doi: 10.1088/1748-605X/aa7664. [DOI] [PubMed] [Google Scholar]
- 148.Bari A., Bloise N., Fiorilli S., Novajra G., Vallet-Regí M., Bruni G., Torres-Pardo A., González-Calbet J.M., Visai L., Vitale-Brovarone C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017;55:493–504. doi: 10.1016/j.actbio.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Y., Hu M., Wang X., Zhou Z., Liu Y. Design and evaluation of europium containing mesoporous bioactive glass nanospheres: doxorubicin release kinetics and inhibitory effect on osteosarcoma MG 63 cells. Nanomaterials. 2018;8(11) doi: 10.3390/nano8110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pourshahrestani S., Zeimaran E., Adib Kadri N., Gargiulo N., Samuel S., Naveen S.V., Kamarul T., Towler M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B. 2016;4(1):71–86. doi: 10.1039/c5tb02062j. [DOI] [PubMed] [Google Scholar]
- 151.Gómez-Cerezo N., Verron E., Montouillout V., Fayon F., Lagadec P., Bouler J.M., Bujoli B., Arcos D., Vallet-Regí M. The response of pre-osteoblasts and osteoclasts to gallium containing mesoporous bioactive glasses. Acta Biomater. 2018;76:333–343. doi: 10.1016/j.actbio.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 152.Wang D., Lin H., Jiang J., Han X., Guo W., Wu X., Jin Y., Qu F. One-pot synthesis of magnetic, macro/mesoporous bioactive glasses for bone tissue engineering. Sci. Technol. Adv. Mater. 2013;14(2) doi: 10.1088/1468-6996/14/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shoaib M., Saeed A., Akhtar J., Rahman M.S.U., Ullah A., Jurkschat K., Naseer M.M. Potassium-doped mesoporous bioactive glass: synthesis, characterization and evaluation of biomedical properties. Mater. Sci. Eng., C. 2017;75:836–844. doi: 10.1016/j.msec.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 154.Maçon A.L.B., Jacquemin M., Page S.J., Li S., Bertazzo S., Stevens M.M., Hanna J.V., Jones J.R. Lithium-silicate sol-gel bioactive glass and the effect of lithium precursor on structure-property relationships. J. Sol. Gel Sci. Technol. 2017;81(1):84–94. doi: 10.1007/s10971-016-4097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu Y., Li X., Yang J., Wang S., Gao H., Hanagata N. Composition–structure–property relationships of the CaO–MxOy–SiO2–P2O5 (M = Zr, Mg, Sr) mesoporous bioactive glass (MBG) scaffolds. J. Mater. Chem. 2011;21(25):9208–9218. [Google Scholar]
- 156.Nawaz Q., Rehman M.A.U., Burkovski A., Schmidt J., Beltrán A.M., Shahid A., Alber N.K., Peukert W., Boccaccini A.R. Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2018;29(5):64. doi: 10.1007/s10856-018-6070-4. [DOI] [PubMed] [Google Scholar]
- 157.Vaid C., Murugavel S. Alkali oxide containing mesoporous bioactive glasses: synthesis, characterization and in vitro bioactivity. Mater. Sci. Eng., C. 2013;33(2):959–968. doi: 10.1016/j.msec.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 158.He X., Liu Y., Tan Y., Grover L.M., Song J., Duan S., Zhao D., Tan X. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng., C. 2019;105 doi: 10.1016/j.msec.2019.110155. [DOI] [PubMed] [Google Scholar]
- 159.Jia X., Long Q., Miron R.J., Yin C., Wei Y., Zhang Y., Wu M. Setd2 is associated with strontium-induced bone regeneration. Acta Biomater. 2017;53:495–505. doi: 10.1016/j.actbio.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 160.Lee J.H., Mandakhbayar N., El-Fiqi A., Kim H.W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017;60:93–108. doi: 10.1016/j.actbio.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 161.Zhang Q., Chen X., Geng S., Wei L., Miron R.J., Zhao Y., Zhang Y. Nanogel-based scaffolds fabricated for bone regeneration with mesoporous bioactive glass and strontium: in vitro and in vivo characterization. J. Biomed. Mater. Res. 2017;105(4):1175–1183. doi: 10.1002/jbm.a.35980. [DOI] [PubMed] [Google Scholar]
- 162.Mendonca A., Rahman M.S., Alhalawani A., Rodriguez O., Gallant R.C., Ni H., Clarkin O.M., Towler M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon-calcium-phosphorous mesoporous bioactive glasses. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(7):2229–2237. doi: 10.1002/jbm.b.34310. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y., Hu M., Zhang W., Zhang X. Construction of tellurium-doped mesoporous bioactive glass nanoparticles for bone cancer therapy by promoting ROS-mediated apoptosis and antibacterial activity. J. Colloid Interface Sci. 2022;610:719–730. doi: 10.1016/j.jcis.2021.11.122. [DOI] [PubMed] [Google Scholar]
- 164.Garg S., Thakur S., Gupta A., Kaur G., Pandey O.P. Antibacterial and anticancerous drug loading kinetics for (10-x)CuO-xZnO-20CaO-60SiO(2)-10P(2)O(5) (2 ≤ x ≤ 8) mesoporous bioactive glasses. J. Mater. Sci. Mater. Med. 2017;28(1):11. doi: 10.1007/s10856-016-5827-x. [DOI] [PubMed] [Google Scholar]
- 165.Sun H., Zheng K., Zhou T., Boccaccini A.R. Incorporation of zinc into binary SiO(2)-CaO mesoporous bioactive glass nanoparticles enhances anti-inflammatory and osteogenic activities. Pharmaceutics. 2021;13(12) doi: 10.3390/pharmaceutics13122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choi Y., Sun W., Kim Y., Kim I.R., Gong M.K., Yoon S.Y., Bae M.K., Park B.S., Park S.B., Kim Y.I. Effects of Zn-doped mesoporous bioactive glass nanoparticles in etch-and-rinse adhesive on the microtensile bond strength. Nanomaterials. 2020;10(10) doi: 10.3390/nano10101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu Y., Zhang Y., Wu C., Fang Y., Yang J., Wang S. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous Mesoporous Mater. 2011;143(2):311–319. [Google Scholar]
- 168.Tallia F., Gallo M., Pontiroli L., Baino F., Fiorilli S., Onida B., Anselmetti G.C., Manca A., Vitale-Brovarone C. Zirconia-containing radiopaque mesoporous bioactive glasses. Mater. Lett. 2014;130:281–284. [Google Scholar]
- 169.Westhauser F., Wilkesmann S., Nawaz Q., Hohenbild F., Rehder F., Saur M., Fellenberg J., Moghaddam A., Ali M.S., Peukert W., Boccaccini A.R. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. 2021;109(8):1457–1467. doi: 10.1002/jbm.a.37136. [DOI] [PubMed] [Google Scholar]
- 170.Min D., Zhang X., He W., Zhang Y., Li P., Zhang M., Liu J., Liu S., Xu F., Du Y., Zhang Z. Direct immobilization of glucose oxidase in magnetic mesoporous bioactive glasses. J. Mater. Chem. B. 2013;1(26):3295–3303. doi: 10.1039/c3tb20480d. [DOI] [PubMed] [Google Scholar]
- 171.Hohenbild F., Arango Ospina M., Schmitz S.I., Moghaddam A., Boccaccini A.R., Westhauser F. An in vitro evaluation of the biological and osteogenic properties of magnesium-doped bioactive glasses for application in bone tissue engineering. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bains R., Sharma P., Mir R.A., Jeet S., Kaur G., Pandey O.P. Influence of CuO/MgO ratio on the gene expression, cytocompatibilty, and antibacterial/anticancerous/analgesic drug loading kinetics for (15-x) CuO-xMgO-10P(2) O(5) -60SiO(2) -10CaO-5ZnO (2.5 ≤ x ≤ 12.5) mesoporous bioactive glasses. J. Biomed. Mater. Res. 2018;106(8):2116–2130. doi: 10.1002/jbm.a.36415. [DOI] [PubMed] [Google Scholar]
- 173.Koohkan R., Hooshmand T., Mohebbi-Kalhori D., Tahriri M., Marefati M.T. Synthesis, characterization, and in vitro biological evaluation of copper-containing magnetic bioactive glasses for hyperthermia in bone defect treatment. ACS Biomater. Sci. Eng. 2018;4(5):1797–1811. doi: 10.1021/acsbiomaterials.7b01030. [DOI] [PubMed] [Google Scholar]
- 174.Mo Y., Zhao F., Lin Z., Cao X., Chen D., Chen X. Local delivery of naringin in beta-cyclodextrin modified mesoporous bioactive glass promotes bone regeneration: from anti-inflammatory to synergistic osteogenesis and osteoclastogenesis. Biomater. Sci. 2022;10(7):1697–1712. doi: 10.1039/d1bm01842f. [DOI] [PubMed] [Google Scholar]
- 175.Richter R.F., Ahlfeld T., Gelinsky M., Lode A. Composites consisting of calcium phosphate cements and mesoporous bioactive glasses as a 3D plottable drug delivery system. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.01.034. [DOI] [PubMed] [Google Scholar]
- 176.Ilyas K., Singer L., Akhtar M.A., Bourauel C.P., Boccaccini A.R. Boswellia sacra extract-loaded mesoporous bioactive glass nano particles: synthesis and biological effects. Pharmaceutics. 2022;14(1) doi: 10.3390/pharmaceutics14010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wei S.M., Pei M.Y., Pan W.L., Thissen H., Tsai S.W. Gelatin hydrogels reinforced by absorbable nanoparticles and fibrils cured in situ by visible light for tissue adhesive applications. Polymers. 2020;12(5) doi: 10.3390/polym12051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qi X., Wang H., Zhang Y., Pang L., Xiao W., Jia W., Zhao S., Wang D., Huang W., Wang Q. Mesoporous bioactive glass-coated 3D printed borosilicate bioactive glass scaffolds for improving repair of bone defects. Int. J. Biol. Sci. 2018;14(4):471–484. doi: 10.7150/ijbs.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mosqueira L., Barrioni B.R., Martins T., Ocarino N.M., Serakides R., Pereira M.M. In vitro effects of the co-release of icariin and strontium from bioactive glass submicron spheres on the reduced osteogenic potential of rat osteoporotic bone marrow mesenchymal stem cells. Biomed. Mater. 2020;15(5) doi: 10.1088/1748-605X/ab9095. [DOI] [PubMed] [Google Scholar]
- 180.Arcos D., López-Noriega A., Ruiz-Hernández E., Terasaki O., Vallet-Regí M. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009;21(6):1000–1009. [Google Scholar]
- 181.Zhu M., Shi J., He Q., Zhang L., Chen F., Chen Y. An emulsification–solvent evaporation route to mesoporous bioactive glass microspheres for bisphosphonate drug delivery. J. Mater. Sci. 2012;47(5):2256–2263. [Google Scholar]
- 182.Li S., Song C., Yang S., Yu W., Zhang W., Zhang G., Xi Z., Lu E. Supercritical CO(2) foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019;94:253–267. doi: 10.1016/j.actbio.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 183.Miao G., Chen X., Dong H., Fang L., Mao C., Li Y., Li Z., Hu Q. Investigation of emulsified, acid and acid-alkali catalyzed mesoporous bioactive glass microspheres for bone regeneration and drug delivery. Mater. Sci. Eng., C. 2013;33(7):4236–4243. doi: 10.1016/j.msec.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 184.Kuo Y.J., Chen C.H., Dash P., Lin Y.C., Hsu C.W., Shih S.J., Chung R.J. Angiogenesis, osseointegration, and antibacterial applications of polyelectrolyte multilayer coatings incorporated with silver/strontium containing mesoporous bioactive glass on 316L stainless steel. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.818137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xie P., Du J., Li Y., Wu J., He H., Jiang X., Liu C. Robust hierarchical porous MBG scaffolds with promoted biomineralization ability. Colloids Surf. B Biointerfaces. 2019;178:22–31. doi: 10.1016/j.colsurfb.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 186.Wang D., Steffi C., Wang Z., Kong C.H., Lim P.N., Shi Z., Thian E.S., Wang W. Beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers as an implantable estradiol delivery system for the potential treatment of osteoporosis. Nanoscale. 2018;10(38):18341–18353. doi: 10.1039/c8nr05268a. [DOI] [PubMed] [Google Scholar]
- 187.Fu S., Du X., Zhu M., Tian Z., Wei D., Zhu Y. 3D printing of layered mesoporous bioactive glass/sodium alginate-sodium alginate scaffolds with controllable dual-drug release behaviors. Biomed. Mater. 2019;14(6) doi: 10.1088/1748-605X/ab4166. [DOI] [PubMed] [Google Scholar]
- 188.Saberi A., Behnamghader A., Aghabarari B., Yousefi A., Majda D., Huerta M.V.M., Mozafari M. 3D direct printing of composite bone scaffolds containing polylactic acid and spray dried mesoporous bioactive glass-ceramic microparticles. Int. J. Biol. Macromol. 2022;207:9–22. doi: 10.1016/j.ijbiomac.2022.02.067. [DOI] [PubMed] [Google Scholar]
- 189.Zhu H., Monavari M., Zheng K., Distler T., Ouyang L., Heid S., Jin Z., He J., Li D., Boccaccini A.R. 3D bioprinting of multifunctional dynamic nanocomposite bioinks incorporating Cu-doped mesoporous bioactive glass nanoparticles for bone tissue engineering. Small. 2022;18(12) doi: 10.1002/smll.202104996. [DOI] [PubMed] [Google Scholar]
- 190.Bento R., Gaddam A., Oskoei P., Oliveira H., Ferreira J.M.F. 3D printing of macro porous sol-gel derived bioactive glass scaffolds and assessment of biological response. Materials. 2021;14(20) doi: 10.3390/ma14205946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang H., Deng Z., Chen J., Qi X., Pang L., Lin B., Adib Y.T.Y., Miao N., Wang D., Zhang Y., Li J., Zeng X. A novel vehicle-like drug delivery 3D printing scaffold and its applications for a rat femoral bone repairing in vitro and in vivo. Int. J. Biol. Sci. 2020;16(11):1821–1832. doi: 10.7150/ijbs.37552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gómez-Cerezo M.N., Lozano D., Arcos D., Vallet-Regí M., Vaquette C. The effect of biomimetic mineralization of 3D-printed mesoporous bioglass scaffolds on physical properties and in vitro osteogenicity. Mater. Sci. Eng., C. 2020;109 doi: 10.1016/j.msec.2019.110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu J., Miao G., Zheng Z., Li Z., Ren W., Wu C., Li Y., Huang Z., Yang L., Guo L. 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair. J. Biomater. Appl. 2019;33(6):755–765. doi: 10.1177/0885328218810269. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y., Xia L., Zhai D., Shi M., Luo Y., Feng C., Fang B., Yin J., Chang J., Wu C. Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale. 2015;7(45):19207–19221. doi: 10.1039/c5nr05421d. [DOI] [PubMed] [Google Scholar]
- 195.Mocquot C., Colon P., Fernando D., Jackson P., Pradelle-Plasse N., Grosgogeat B., Attik N. The influence of experimental bioactive glasses on pulp cells behavior in vitro. Dent. Mater. 2020;36(10):1322–1331. doi: 10.1016/j.dental.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 196.Aguilar-Rabiela A.E., Leal-Egaña A., Nawaz Q., Boccaccini A.R. Integration of mesoporous bioactive glass nanoparticles and curcumin into PHBV microspheres as biocompatible composite for drug delivery applications. Molecules. 2021;26(11) doi: 10.3390/molecules26113177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liang W., Wu X., Dong Y., Shao R., Chen X., Zhou P., Xu F. In vivo behavior of bioactive glass-based composites in animal models for bone regeneration. Biomater. Sci. 2021;9(6):1924–1944. doi: 10.1039/d0bm01663b. [DOI] [PubMed] [Google Scholar]
- 198.Wang Y.C., Lin S.H., Chien C.S., Kung J.C., Shih C.J. In vitro bioactivity and antibacterial effects of a silver-containing mesoporous bioactive glass film on the surface of titanium implants. Int. J. Mol. Sci. 2022;23(16) doi: 10.3390/ijms23169291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Batool S.A., Liaquat U., Channa I.A., Gilani S.J., Makhdoom M.A., Yasir M., Ashfaq J., Jumah M.N.B., Rehman M.A.U. Development and characterization of zein/Ag-Sr doped mesoporous bioactive glass nanoparticles coatings for biomedical applications. Bioengineering. 2022;9(8) doi: 10.3390/bioengineering9080367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Montes-Casado M., Sanvicente A., Casarrubios L., Feito M.J., Rojo J.M., Vallet-Regí M., Arcos D., Portolés P., Portolés M.T. An immunological approach to the biocompatibility of mesoporous SiO(2)-CaO nanospheres. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21218291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qin X., Cao R., Zheng J., Shi G., Ji L., Zhu A., Yao H. A new strategy for synthesizing silver doped mesoporous bioactive glass fibers and their bioactivity, antibacterial activity and drug loading performance. RSC Adv. 2020;10(73):44835–44840. doi: 10.1039/d0ra08656h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mao C., Chen X., Hu Q., Miao G., Lin C. Acute toxicity and in vivo biodistribution of monodispersed mesoporous bioactive glass spheres in intravenously exposed mice. Mater. Sci. Eng., C. 2016;58:682–691. doi: 10.1016/j.msec.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 203.Wang X., Li W. Biodegradable mesoporous bioactive glass nanospheres for drug delivery and bone tissue regeneration. Nanotechnology. 2016;27(22) doi: 10.1088/0957-4484/27/22/225102. [DOI] [PubMed] [Google Scholar]
- 204.Kenry B. Liu. Recent advances in biodegradable conducting polymers and their biomedical applications. Biomacromolecules. 2018;19(6):1783–1803. doi: 10.1021/acs.biomac.8b00275. [DOI] [PubMed] [Google Scholar]
- 205.Ghamor-Amegavi E.P., Yang X., Qiu J., Xie L., Pan Z., Wang J., Zhang X., Ke X., Zhao T., Zhang L., Gou Z. Composition control in biphasic silicate microspheres on stimulating new bone regeneration and repair of osteoporotic femoral bone defect. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(2):377–390. doi: 10.1002/jbm.b.34396. [DOI] [PubMed] [Google Scholar]
- 206.Feito M.J., Casarrubios L., Oñaderra M., Gómez-Duro M., Arribas P., Polo-Montalvo A., Vallet-Regí M., Arcos D., Portolés M.T. Response of RAW 264.7 and J774A.1 macrophages to particles and nanoparticles of a mesoporous bioactive glass: a comparative study. Colloids Surf. B Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112110. [DOI] [PubMed] [Google Scholar]
- 207.Jewell C.M., Collier J.H. Biomaterial interactions with the immune system. Biomater. Sci. 2019;7(3):713–714. doi: 10.1039/c8bm90063a. [DOI] [PubMed] [Google Scholar]
- 208.Gómez-Cerezo N., Casarrubios L., Morales I., Feito M.J., Vallet-Regí M., Arcos D., Portolés M.T. Effects of a mesoporous bioactive glass on osteoblasts, osteoclasts and macrophages. J. Colloid Interface Sci. 2018;528:309–320. doi: 10.1016/j.jcis.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 209.Gholipourmalekabadi M., Sameni M., Hashemi A., Zamani F., Rostami A., Mozafari M. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns. 2016;42(1):131–140. doi: 10.1016/j.burns.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 210.Atkinson I., Seciu-Grama A.M., Mocioiu O.C., Mocioiu A.M., Predoana L., Voicescu M., Cusu J.P., Grigorescu R.M., Ion R.M., Craciunescu O. Preparation and biocompatibility of poly methyl methacrylate (PMMA)-Mesoporous bioactive glass (MBG) composite scaffolds. Gels. 2021;7(4) doi: 10.3390/gels7040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Baino F. Copper-doped ordered mesoporous bioactive glass: a promising multifunctional platform for bone tissue engineering (†) Bioengineering. 2020;7(2) doi: 10.3390/bioengineering7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li J., Li J., Wei Y., Xu N., Li J., Pu X., Wang J., Huang Z., Liao X., Yin G. Ion release behavior of vanadium-doped mesoporous bioactive glass particles and the effect of the released ions on osteogenic differentiation of BMSCs via the FAK/MAPK signaling pathway. J. Mater. Chem. B. 2021;9(37):7848–7865. doi: 10.1039/d1tb01479j. [DOI] [PubMed] [Google Scholar]
- 213.Ur Rahman M.S., Tahir M.A., Noreen S., Yasir M., Ahmad I., Khan M.B., Ali K.W., Shoaib M., Bahadur A., Iqbal S. Magnetic mesoporous bioactive glass for synergetic use in bone regeneration, hyperthermia treatment, and controlled drug delivery. RSC Adv. 2020;10(36):21413–21419. doi: 10.1039/c9ra09349d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Szewczyk A., Skwira A., Konopacka A., Sądej R., Prokopowicz M. Mesoporous silica-bioglass composite pellets as bone drug delivery system with mineralization potential. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Casarrubios L., Gómez-Cerezo N., Feito M.J., Vallet-Regí M., Arcos D., Portolés M.T. Ipriflavone-loaded mesoporous nanospheres with potential applications for periodontal treatment. Nanomaterials. 2020;10(12) doi: 10.3390/nano10122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Koohkan R., Hooshmand T., Mohebbi-Kalhori D., Tahriri M., Marefati M.T. Synthesis, characterization, and in vitro biological evaluation of copper-containing magnetic bioactive glasses for hyperthermia in bone defect treatment. ACS Biomater. Sci. Eng. 2018;4(5):1797–1811. doi: 10.1021/acsbiomaterials.7b01030. [DOI] [PubMed] [Google Scholar]
- 217.Lin D., Chai Y., Ma Y., Duan B., Yuan Y., Liu C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials. 2019;196:122–137. doi: 10.1016/j.biomaterials.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 218.Xue Y., Guo Y., Yu M., Wang M., Ma P.X., Lei B. Monodispersed bioactive glass nanoclusters with ultralarge pores and intrinsic exceptionally high miRNA loading for efficiently enhancing bone regeneration. Adv. Healthcare Mater. 2017;6(20) doi: 10.1002/adhm.201700630. [DOI] [PubMed] [Google Scholar]
- 219.Berkmann J.C., Herrera Martin A.X., Pontremoli C., Zheng K., Bucher C.H., Ellinghaus A., Boccaccini A.R., Fiorilli S., Vitale Brovarone C., Duda G.N., Schmidt-Bleek K. In vivo validation of spray-dried mesoporous bioactive glass microspheres acting as prolonged local release systems for BMP-2 to support bone regeneration. Pharmaceutics. 2020;12(9) doi: 10.3390/pharmaceutics12090823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Perez R.A., El-Fiqi A., Park J.H., Kim T.H., Kim J.H., Kim H.W. Therapeutic bioactive microcarriers: co-delivery of growth factors and stem cells for bone tissue engineering. Acta Biomater. 2014;10(1):520–530. doi: 10.1016/j.actbio.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 221.El-Fiqi A., Mandakhbayar N., Jo S.B., Knowles J.C., Lee J.H., Kim H.W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2021;6(1):123–136. doi: 10.1016/j.bioactmat.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yin C., Jia X., Zhao Q., Zhao Z., Wang J., Zhang Y., Li Z., Sun H., Li Z. Transcription factor 7-like 2 promotes osteogenic differentiation and boron-induced bone repair via lipocalin 2. Mater. Sci. Eng., C. 2020;110 doi: 10.1016/j.msec.2020.110671. [DOI] [PubMed] [Google Scholar]
- 223.Lalzawmliana V., Anand A., Kumar V., Das P., Devi K.B., Mukherjee J., Maji A.K., Kundu B., Roy M., Nandi S.K. Potential of growth factor incorporated mesoporous bioactive glass for in vivo bone regeneration. J. Mech. Behav. Biomed. Mater. 2019;91:182–192. doi: 10.1016/j.jmbbm.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 224.Wang X., Zeng D., Weng W., Huang Q., Zhang X., Wen J., Wu J., Jiang X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells, Nanomed. Biotechnol. 2018;46(sup2):171–181. doi: 10.1080/21691401.2018.1453825. [DOI] [PubMed] [Google Scholar]
- 225.Ravanbakhsh M., Labbaf S., Karimzadeh F., Pinna A., Houreh A.B., Nasr-Esfahani M.H. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater. Sci. Eng., C. 2019;104 doi: 10.1016/j.msec.2019.109994. [DOI] [PubMed] [Google Scholar]
- 226.El-Fiqi A., Kim T.H., Kim M., Eltohamy M., Won J.E., Lee E.J., Kim H.W. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale. 2012;4(23):7475–7488. doi: 10.1039/c2nr31775c. [DOI] [PubMed] [Google Scholar]
- 227.Min Z., Shichang Z., Chen X., Yufang Z., Changqing Z. 3D-printed dimethyloxallyl glycine delivery scaffolds to improve angiogenesis and osteogenesis. Biomater. Sci. 2015;3(8):1236–1244. doi: 10.1039/c5bm00132c. [DOI] [PubMed] [Google Scholar]
- 228.Liu L., Zhao F., Chen X., Luo M., Yang Z., Cao X., Miao G., Chen D., Chen X. Local delivery of FTY720 in mesoporous bioactive glass improves bone regeneration by synergistically immunomodulating osteogenesis and osteoclastogenesis. J. Mater. Chem. B. 2020;8(28):6148–6158. doi: 10.1039/d0tb00982b. [DOI] [PubMed] [Google Scholar]
- 229.Ma J., Lin H., Li X., Bian C., Xiang D., Han X., Wu X., Qu F. Hierarchical porous bioactive glasses/PLGA-magnetic SBA-15 for dual-drug release. Mater. Sci. Eng., C. 2014;39:21–28. doi: 10.1016/j.msec.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 230.Shoaib M., Saeed A., Rahman M.S.U., Naseer M.M. Mesoporous nano-bioglass designed for the release of imatinib and in vitro inhibitory effects on cancer cells. Mater. Sci. Eng., C. 2017;77:725–730. doi: 10.1016/j.msec.2017.03.288. [DOI] [PubMed] [Google Scholar]
- 231.Zhu M., Li K., Zhu Y., Zhang J., Ye X. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015;16:145–155. doi: 10.1016/j.actbio.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 232.Cheng T., Qu H., Zhang G., Zhang X. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif. Cells, Nanomed. Biotechnol. 2018;46(8):1935–1947. doi: 10.1080/21691401.2017.1396997. [DOI] [PubMed] [Google Scholar]
- 233.Kauschke V., Schneider M., Jauch A., Schumacher M., Kampschulte M., Rohnke M., Henss A., Bamberg C., Trinkaus K., Gelinsky M., Heiss C., Lips K.S. Effects of a pasty bone cement containing brain-derived neurotrophic factor-functionalized mesoporous bioactive glass particles on metaphyseal healing in a new murine osteoporotic fracture model. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ge F., Yu M., Yu C., Lin J., Weng W., Cheng K., Wang H. Improved rhBMP-2 function on MBG incorporated TiO(2) nanorod films. Colloids Surf. B Biointerfaces. 2017;150:153–158. doi: 10.1016/j.colsurfb.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 235.Chai Y., Lin D., Ma Y., Yuan Y., Liu C. RhBMP-2 loaded MBG/PEGylated poly(glycerol sebacate) composite scaffolds for rapid bone regeneration. J. Mater. Chem. B. 2017;5(24):4633–4647. doi: 10.1039/c7tb00505a. [DOI] [PubMed] [Google Scholar]
- 236.Wang X., Chen W., Liu Q., Gao K., Wang G., Gao L., Liu L. Function and mechanism of mesoporous bioactive glass adsorbed epidermal growth factor for accelerating bone tissue regeneration. Biomed. Mater. 2017;12(2) doi: 10.1088/1748-605X/aa65d8. [DOI] [PubMed] [Google Scholar]
- 237.Schumacher M., Reither L., Thomas J., Kampschulte M., Gbureck U., Lode A., Gelinsky M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017;5(3):578–588. doi: 10.1039/c6bm00903d. [DOI] [PubMed] [Google Scholar]
- 238.Cai L., Lin D., Chai Y., Yuan Y., Liu C. MBG scaffolds containing chitosan microspheres for binary delivery of IL-8 and BMP-2 for bone regeneration. J. Mater. Chem. B. 2018;6(27):4453–4465. doi: 10.1039/c8tb00875b. [DOI] [PubMed] [Google Scholar]
- 239.Kim T.H., Singh R.K., Kang M.S., Kim J.H., Kim H.W. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2016;29:352–364. doi: 10.1016/j.actbio.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 240.Cheng Y., Gong Y., Chen X., Zhang Q., Zhang X., He Y., Pan L., Ni B., Yang F., Xu Y., Zhou L., Yang Y., Chen W. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121506. [DOI] [PubMed] [Google Scholar]
- 241.Ciraldo F.E., Schnepf K., Goldmann W.H., Boccaccini A.R. Development and characterization of bioactive glass containing composite coatings with ion releasing function for antibiotic-free antibacterial surgical sutures. Materials. 2019;12(3) doi: 10.3390/ma12030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu T., Chen Y., Lai D., Zhang L., Pan X., Chen J., Weng H. Biomimetic fabrication of new bioceramics-introduced fibrous scaffolds: from physicochemical characteristics to in vitro biological properties. Mater. Sci. Eng., C. 2019;94:547–557. doi: 10.1016/j.msec.2018.09.063. [DOI] [PubMed] [Google Scholar]
- 243.Lin H.M., Lin H.Y., Chan M.H. Preparation, characterization, and in vitro evaluation of folate-modified mesoporous bioactive glass for targeted anticancer drug carriers. J. Mater. Chem. B. 2013;1(44):6147–6156. doi: 10.1039/c3tb20867b. [DOI] [PubMed] [Google Scholar]
- 244.Miola M., Pakzad Y., Banijamali S., Kargozar S., Vitale-Brovarone C., Yazdanpanah A., Bretcanu O., Ramedani A., Vernè E., Mozafari M. Glass-ceramics for cancer treatment: so close, or yet so far? Acta Biomater. 2019;83:55–70. doi: 10.1016/j.actbio.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 245.Shi Y., Lammers T. Combining nanomedicine and immunotherapy. Acc. Chem. Res. 2019;52(6):1543–1554. doi: 10.1021/acs.accounts.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 248.Zimmermannova O., Caiado I., Ferreira A.G., Pereira C.F. Cell fate reprogramming in the era of cancer immunotherapy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.714822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sharma P., Jang N.Y., Lee J.W., Park B.C., Kim Y.K., Cho N.H. Application of ZnO-based nanocomposites for vaccines and cancer immunotherapy. Pharmaceutics. 2019;11(10) doi: 10.3390/pharmaceutics11100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang D., Wang T., Yu H., Feng B., Zhou L., Zhou F., Hou B., Zhang H., Luo M., Li Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci Immunol. 2019;4(37) doi: 10.1126/sciimmunol.aau6584. [DOI] [PubMed] [Google Scholar]
- 251.Vallet-Regí M., Ruiz-Hernández E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv. Mater. 2011;23(44):5177–5218. doi: 10.1002/adma.201101586. [DOI] [PubMed] [Google Scholar]
- 252.Richter R.F., Ahlfeld T., Gelinsky M., Lode A. Development and characterization of composites consisting of calcium phosphate cements and mesoporous bioactive glass for extrusion-based fabrication. Materials. 2019;12(12) doi: 10.3390/ma12122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sanz-Ortega L., Rojas J.M., Portilla Y., Pérez-Yagüe S., Barber D.F. Magnetic nanoparticles attached to the NK cell surface for tumor targeting in adoptive transfer therapies does not affect cellular effector functions. Front. Immunol. 2019;10:2073. doi: 10.3389/fimmu.2019.02073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yang Q., Yin H., Xu T., Zhu D., Yin J., Chen Y., Yu X., Gao J., Zhang C., Chen Y., Gao Y. Engineering 2D mesoporous Silica@MXene-integrated 3D-printing scaffolds for combinatory osteosarcoma therapy and NO-augmented bone regeneration. Small. 2020;16(14) doi: 10.1002/smll.201906814. [DOI] [PubMed] [Google Scholar]
- 255.Wu C., Fan W., Zhu Y., Gelinsky M., Chang J., Cuniberti G., Albrecht V., Friis T., Xiao Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011;7(10):3563–3572. doi: 10.1016/j.actbio.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 256.Zhu Y., Shang F., Li B., Dong Y., Liu Y., Lohe M.R., Hanagata N., Kaskel S. Magnetic mesoporous bioactive glass scaffolds: preparation, physicochemistry and biological properties. J. Mater. Chem. B. 2013;1(9):1279–1288. doi: 10.1039/c2tb00262k. [DOI] [PubMed] [Google Scholar]
- 257.Liu Y.Z., Li Y., Yu X.B., Liu L.N., Zhu Z.A., Guo Y.P. Drug delivery property, bactericidal property and cytocompatibility of magnetic mesoporous bioactive glass. Mater. Sci. Eng., C. 2014;41:196–205. doi: 10.1016/j.msec.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 258.Kermani F., Mollazadeh Beidokhti S., Baino F., Gholamzadeh-Virany Z., Mozafari M., Kargozar S. Strontium- and cobalt-doped multicomponent mesoporous bioactive glasses (MBGs) for potential use in bone tissue engineering applications. Materials. 2020;13(6) doi: 10.3390/ma13061348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Du J., Xie P., Lin S., Wu Y., Zeng D., Li Y., Jiang X. Time-phase sequential utilization of adipose-derived mesenchymal stem cells on mesoporous bioactive glass for restoration of critical size bone defects. ACS Appl. Mater. Interfaces. 2018;10(34):28340–28350. doi: 10.1021/acsami.8b08563. [DOI] [PubMed] [Google Scholar]
- 260.Shen X., Yu P., Chen H., Wang J., Lu B., Cai X., Gu C., Liang G., Hao D., Ma Q., Li Y. Icariin controlled release on a silk fibroin/mesoporous bioactive glass nanoparticles scaffold for promoting stem cell osteogenic differentiation. RSC Adv. 2020;10(20):12105–12112. doi: 10.1039/d0ra00637h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pourshahrestani S., Zeimaran E., Kadri N.A., Gargiulo N., Jindal H.M., Hasikin K., Naveen S.V., Sekaran S.D., Kamarul T. Elastomeric biocomposite of silver-containing mesoporous bioactive glass and poly(1,8-octanediol citrate): physiochemistry and in vitro antibacterial capacity in tissue engineering applications. Mater. Sci. Eng., C. 2019;98:1022–1033. doi: 10.1016/j.msec.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 262.Montalbano G., Borciani G., Cerqueni G., Licini C., Banche-Niclot F., Janner D., Sola S., Fiorilli S., Mattioli-Belmonte M., Ciapetti G., Vitale-Brovarone C. Collagen hybrid formulations for the 3D printing of nanostructured bone scaffolds: an optimized genipin-crosslinking strategy. Nanomaterials. 2020;10(9) doi: 10.3390/nano10091681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Son S.A., Kim D.H., Yoo K.H., Yoon S.Y., Kim Y.I. Mesoporous bioactive glass combined with graphene oxide quantum dot as a new material for a new treatment option for dentin hypersensitivity. Nanomaterials. 2020;10(4) doi: 10.3390/nano10040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee J.H., Kang M.S., Mahapatra C., Kim H.W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pérez R., Sanchez-Salcedo S., Lozano D., Heras C., Esbrit P., Vallet-Regí M., Salinas A.J. Osteogenic effect of ZnO-mesoporous glasses loaded with osteostatin. Nanomaterials. 2018;8(8) doi: 10.3390/nano8080592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ahn J.H., Kim I.R., Kim Y., Kim D.H., Park S.B., Park B.S., Bae M.K., Kim Y.I. The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials. 2020;10(4) doi: 10.3390/nano10040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang X., Cheng F., Liu J., Smått J.H., Gepperth D., Lastusaari M., Xu C., Hupa L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016;46:286–298. doi: 10.1016/j.actbio.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 268.Park S.Y., Yoo K.H., Yoon S.Y., Son W.S., Kim Y.I. Synergetic effect of 2-methacryloyloxyethyl phosphorylcholine and mesoporous bioactive glass nanoparticles on antibacterial and anti-demineralisation properties in orthodontic bonding agents. Nanomaterials. 2020;10(7) doi: 10.3390/nano10071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Choi A., Yoo K.H., Yoon S.Y., Park S.B., Choi Y.K., Kim Y.I. Enhanced antimicrobial and remineralizing properties of self-adhesive orthodontic resin containing mesoporous bioactive glass and zwitterionic material. J. Dent. Sci. 2022;17(2):848–855. doi: 10.1016/j.jds.2021.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Huang Y.C., Yang T.Y., Chen B.X., Kung J.C., Shih C.J. Evaluation of antibacterial effects of matrix-induced silver ions against antibiotic-resistant ESKAPE pathogens. Pharmaceuticals. 2021;14(11) doi: 10.3390/ph14111094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jung J.H., Kim D.H., Yoo K.H., Yoon S.Y., Kim Y., Bae M.K., Chung J., Ko C.C., Kwon Y.H., Kim Y.I. Dentin sealing and antibacterial effects of silver-doped bioactive glass/mesoporous silica nanocomposite: an in vitro study. Clin. Oral Invest. 2019;23(1):253–266. doi: 10.1007/s00784-018-2432-z. [DOI] [PubMed] [Google Scholar]
- 272.Liu X., Chen H.H., Lin Y.C., Nabilla S.C., Liu W.C., Wang W.C., Shih S.J., Li Y., Lin C.P., Zhao G., Chung R.J. Composite polyelectrolyte multilayer and mesoporous bioactive glass nanoparticle coating on 316L stainless steel for controlled antibiotic release and biocompatibility. J. Biomed. Nanotechnol. 2018;14(4):725–735. doi: 10.1166/jbn.2018.2531. [DOI] [PubMed] [Google Scholar]
- 273.Sung Y.K., Lee D.R., Chung D.J. Advances in the development of hemostatic biomaterials for medical application. Biomater. Res. 2021;25(1):37. doi: 10.1186/s40824-021-00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen J., Ai J., Chen S., Xu Z., Lin J., Liu H., Chen Q. Synergistic enhancement of hemostatic performance of mesoporous silica by hydrocaffeic acid and chitosan. Int. J. Biol. Macromol. 2019;139:1203–1211. doi: 10.1016/j.ijbiomac.2019.08.091. [DOI] [PubMed] [Google Scholar]
- 275.Nagrath M., Gallant R., Yazdi A.R., Mendonca A., Rahman S., Chiu L., Waldman S.D., Ni H., Towler M.R. Tantalum-containing mesoporous bioactive glass powder for hemostasis. J. Biomater. Appl. 2021;35(8):924–932. doi: 10.1177/0885328220965150. [DOI] [PubMed] [Google Scholar]
- 276.Pourshahrestani S., Zeimaran E., Kadri N.A., Gargiulo N., Jindal H.M., Naveen S.V., Sekaran S.D., Kamarul T., Towler M.R. Potency and cytotoxicity of a novel gallium-containing mesoporous bioactive glass/chitosan composite scaffold as hemostatic agents. ACS Appl. Mater. Interfaces. 2017;9(37):31381–31392. doi: 10.1021/acsami.7b07769. [DOI] [PubMed] [Google Scholar]
- 277.Shaw W.M., Zhang Y., Lu X., Khalil A.S., Ladds G., Luo X., Ellis T. Screening microbially produced Δ(9)-tetrahydrocannabinol using a yeast biosensor workflow. Nat. Commun. 2022;13(1):5509. doi: 10.1038/s41467-022-33207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sondhi P., Maruf M.H.U., Stine K.J. Nanomaterials for biosensing lipopolysaccharide. Biosensors. 2019;10(1) doi: 10.3390/bios10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dong Q., Ding Q., Yuan R., Yuan Y. AuNPs/CdS QDs/CeO(2) ternary nanocomposite coupled with scrollable three-dimensional DNA walker mediated cycling amplification for sensitive photoelectrochemical miRNA assay. Anal. Chim. Acta. 2022;1228 doi: 10.1016/j.aca.2022.340344. [DOI] [PubMed] [Google Scholar]
- 280.Liu S.T., Chen J.S., Liu X.P., Mao C.J., Jin B.K. A photoelectrochemical biosensor based on b-TiO(2)/CdS:Eu/Ti(3)C(2) heterojunction for the ultrasensitive detection of miRNA-21. Talanta. 2022;253 doi: 10.1016/j.talanta.2022.123601. [DOI] [PubMed] [Google Scholar]
- 281.Nam K.H., Abdulhafez M., Castagnola E., Tomaraei G.N., Cui X.T., Bedewy M. Laser direct write of heteroatom-doped graphene on molecularly controlled polyimides for electrochemical biosensors with nanomolar sensitivity. Carbon N Y. 2022;188:209–219. doi: 10.1016/j.carbon.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Patel K.D., Buitrago J.O., Parthiban S.P., Lee J.H., Singh R.K., Knowles J.C., Kim H.W. Combined effects of nanoroughness and ions produced by electrodeposition of mesoporous bioglass nanoparticle for bone regeneration. ACS Appl. Bio Mater. 2019;2(11):5190–5203. doi: 10.1021/acsabm.9b00859. [DOI] [PubMed] [Google Scholar]
- 283.Mubina M.S.K., Shailajha S., Sankaranarayanan R., Saranya L. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO(2)-CaO-Na(2)O-P(2)O(5) nano bioactive glass ceramics. J. Mech. Behav. Biomed. Mater. 2019;100 doi: 10.1016/j.jmbbm.2019.103379. [DOI] [PubMed] [Google Scholar]
- 284.Chitra S., Bargavi P., Balakumar S. Effect of microwave and probe sonication processes on sol-gel-derived bioactive glass and its structural and biocompatible investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(1):143–155. doi: 10.1002/jbm.b.34373. [DOI] [PubMed] [Google Scholar]
- 285.He Y., Zeng Z., Cao Y., Zhang X., Wu C., Luo X. Ultrasenstive SERS biosensor based on Zn(2+) from ZnO nanoparticle assisted DNA enzyme amplification for detection of miRNA. Anal. Chim. Acta. 2022;1228 doi: 10.1016/j.aca.2022.340340. [DOI] [PubMed] [Google Scholar]
- 286.Lara S., Perez-Potti A. Applications of nanomaterials for immunosensing. Biosensors. 2018;8(4) doi: 10.3390/bios8040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.