Abstract
Thrombosis is a leading cause of morbidity and mortality. Detection of a prothrombotic state using biomarkers would be of great benefit to identify patients at risk of thrombosis that would benefit from thromboprophylaxis. The addition of biomarkers to these scores could improve risk assessment accuracy. Tissue factor (TF) is a highly procoagulant protein that under normal conditions is not present in the blood. However, increased levels of TF in the blood in the form of microparticles (MPs) (also called extracellular vesicles) are observed under various pathological conditions. In this review, we will discuss studies that have measured MP-TF activity using two similar FXa generation assays in a variety of diseases. One of the most robust signals for MP-TF activity (16-26 fold higher than healthy controls) is observed in pancreatic cancer patients with venous thromboembolism. In this case, the TF+ MPs appear to be derived from the cancer cells. Surprisingly, cirrhosis and acute liver injury are associated with 17-fold and 38-fold increases in MP-TF activity, respectively. Based on mouse models, we speculate that the TF+ MPs are derived from hepatocytes. More modest increases are observed in patients with urinary tract infections (6-fold) and in a human endotoxemia model (9-fold) where monocytes are the likely source of the TF+ MPs. Finally, there is no increase in MP-TF activity in the majority of cardiovascular disease patients. These studies indicate that MP-TF activity may be a useful biomarker to identify patients with particular diseases that have an increased risk of thrombosis.
Keywords: cancer, microparticles, thrombosis, tissue factor
Introduction
Thrombosis is a common pathology associated with a variety of diseases, particularly cardiovascular diseases [1]. Risk assessment scores use clinical information to try to identify patients at risk of thrombosis that would benefit from thromboprophylaxis. The addition of biomarkers to these scores could improve the accuracy of these scores. There are a variety of biomarkers that can be used to monitor the activation of the coagulation cascade. These include initiators, such as tissue factor, proteases that can be detected in complexes, such as thrombin-antithrombin complexes, and finally degradation products of fibrin, such as D-dimer [2]. In addition, global assays that measure levels of thrombin generation have been used to try to identify patients with a prothrombotic state [3]. However, the thrombin generation assay can be quite variable and usually employs an exogenous activator, such as TF, that may mask low levels of endogenous TF. D-dimer can be measured using commercial ELISAs and in our opinion is the most reliable biomarker of a prothombotic state. There are limitations with D-dimer that include an increase with age [4, 5].
In this review, we will focus on studies reporting the measurement of functional TF activity in plasma samples from a variety of different types of patient populations. TF is a transmembrane glycoprotein that functions as the primary initiator of the coagulation cascade [6]. TF can be present in two major forms: a cryptic form that is inactive and a decrypted form that is active [7]. Due to its high procoagulant activity, TF likely triggers many forms of thrombosis, such as formation of occlusive clots after rupture of atherosclerotic plaques and venous thromboembolism (VTE) [8–10]. Microparticles (MPs) (also known as microvesicles or extracellular vesicles) are submicron vesicles that are shed from apoptotic cells, activated cells, and cancer cells [11]. Levels of TF+ MPs in the blood can be measured using activity- and antigen-based assays [12]. We believe that activity assays are more sensitive and reliable than antigen-based assays and the two assays often do not correlate with each other [13, 14]. In addition, antigen-based assays will detect both cryptic and decrypted TF.
In healthy individuals, levels of TF+ MPs are very low or undetectable [12]. However, increased levels of MP-TF activity may contribute to thrombosis associated with certain disease states. This has led to the notion that TF+MP could be used as a biomarker to identify patients who have an increased risk for thrombosis. Table 1 summarizes all of the published MP-TF activity studies. In addition, it contains 5 unpublished studies measuring levels of MP-TF activity in multiple myeloma patients, in cardiovascular disease patients with or without statins and in healthy volunteers receiving endotoxin.
Table 1.
Clinical studies measuring MP-TF activity.
| Disease | Conclusion | Ref |
|---|---|---|
| 1. Cancer & VTE | MP-TF activity is increased in metastatic breast and pancreatic cancer patients | 15 |
| 2. Cancer & VTE | Increased MP-TF activity precedes VTE in pancreatic cancer patients | 16 |
| 3. Cancer & DIC | MP-TF activity is associated with DIC in cancer patients | 18 |
| 4. Cancer & VTE | Cancer patients with VTE have higher levels of MP-TF activity than those without VTE | 19 |
| 5. Cancer & VTE | Cancer patients with VTE have higher levels of MP-TF activity than those without VTE | 36 |
| 6. PE | MP-TF activity is not associated with PE | 25 |
| 7. Breast cancer | MP-TF activity is not increased after chemotherapy | 43 |
| 8. MM & chemo | MP-TF activity is increased in MM patients and decreased after chemotherapy | 27 |
| 9. FH | Familial hypercholesterolemia patients have elevated MP-TF activity | 24 |
| 10. Cancer & VTE | MP-TF activity is associated with mortality in pancreatic cancer patients | 17 |
| 11. Cancer & VTE | MP-TF activity is associated with VTE in cancer patients | 42 |
| 12. PNH | MP-TF activity is not increased in PNH patients | 56 |
| 13. Endotoxemia | MP-TF activity is increased in human endotoxemia model | 50 |
| 14. Cancer & chemo | Chemotherapy does not increase MP-TF activity in testicular cancer patients | 26 |
| 15. Cancer & VTE | MP-TF activity is associated with VTE and mortality in pancreaticobiliary cancer | 41 |
| 16. Cancer | MP-TF activity is associated with stage and grade of pancreatic cancer | 20 |
| 17. Liver injury | MP-TF activity is increased in acute liver injury patients | 47 |
| 18. HIV | Antiretroviral therapy is not associated with decreased MP-TF activity in HIV patients | 51 |
| 19. Leukemia & DIC | Acute myelocytic leukemia patients with DIC have elevated MP-TF activity | 21 |
| 20. Cirrhosis | MP-TF activity is increased in cirrhosis patients | 28 |
| 21. APS | MP-TF activity is not increased in antiphospholipid syndrome patients | 29 |
| 22. UTI | MP-TF activity is increased in urinary tract infection patients | 33 |
| 23. HCV/HIV | HCV patients have higher levels of MP-TF activity than HIV patients | 30 |
| 24. LA | MP-TF activity is not increased in LA patients | 55 |
| 25. Obesity | Bariatric surgery reduces MP-TF activity in morbidly obese patients | 52 |
| 26. Influenza A | MP-TF activity is increased in influenza A patients | 22 |
| 27. MM | MP-TF activity is not increased in MM patients | NP |
| 28. CVD & atorvastatin | Atorvastatin does not reduce MP-TF activity in CVD patients | NP |
| 29. CVD & atorvastatin | Atorvastatin does not reduce MP-TF activity in CVD patients | NP |
| 30. CVD & Statin | Statins do not alter MP-TF activity in paired patients | NP |
| 31. Endotoxemia | LPS increases MP-TF activity in human volunteers | NP |
APS: antiphospholipid syndrome, FH: familial hypercholesterolemia, HCV: hepatitis C virus, HIV: human immunodeficiency virus, LA: lupus anticoagulant, MM: multiple myeloma, NP: not published, PE: pulmonary embolism, PNH: paroxysmal nocturnal hemoglobinuria, UTI: urinary tract infection
Measurement of MP-TF activity in plasma
There are two slightly different “in-house” assays that have been reported for the measurement of MP-TF activity in plasma using factor Xa (FXa) generation that we will refer to as “kinetic” and “endpoint” assays [15, 16]. The units of the kinetic assay are fM Xa min−1 whereas the units for the endpoint assay are pg/mL of TF. Both assays uses commercially available Innovin™ as a standard. Dr. Pabinger’s group in Vienna has used both assays and they have adopted the endpoint assay because it is simpler to perform. In one study, both “in-house” assays were used to measure MP-TF activity in 54 pancreatic cancer patients and a robust correlation was found (Spearman correlation coefficient r =0.61, P < 0.001) [17]. We used these data to create an equation that allows a conversion between the assays (y = 0.0009x + 0.0831 where y = pg/mL, x = fM Xa min−1). In addition, a commercial assay has been developed for the measurement of MP-TF activity that captures MPs using a non-inhibitory anti-TF antibody; however, there are no reports of this assay being used for clinical samples [13]. Finally, one study isolated MPs from plasma and measured their procoagulant activity in a one-stage clotting assay with or without an inhibitory anti-TF antibody [18].
Effects of pre-analytical and analytical variables on measurement of MP-TF activity
The level of MP-TF activity can be affected by many pre-analytical variables, but the most important is plasma preparation. This has been discussed extensively in a recent review [12]. For example, there are several centrifugation conditions reported for preparing platelet-poor-plasma (PPP) [15, 16, 19–26]. Other studies used various conditions to prepare platelet-free-plasma (PFP) [17, 27–30]. This makes comparison between studies difficult. Levels of MP-TF activity are significantly lower in PFP compared with PPP since some MPs are lost with the additional centrifugation involved in preparing PFP. For instance, two separate studies reported 52 - 80% reductions in the number of MPs obtained from PFP compared with PPP [14, 31]. We recommend that PFP should be prepared by centrifugation at 2500 x g for 15 minutes without a brake and then repeated as described by the Scientific Collaborative Workshop of the International Society of Thrombosis and Hemostasis [32]. There is a need to standardize the measurement of MP-TF activity in human plasma sample. Indeed, this has been discussed at the Scientific Standardization Committees of the International Society of Thrombosis and Hemostasis. However, in general, people prefer to use their own “in-house” assay and until a reliable commercial assay is developed it will be difficult to compare levels of MP-TF activity in different studies.
For the kinetic assay, healthy controls were reported to have median values of 79 and 127 fM Xa min−1 for PPP [19, 33] compared with a median value of 4.1 fM Xa min−1 for PFP [27] (Tables 2 and 4). For the endpoint assay, healthy controls were reported to have mean values of 0.21 and 0.25 pg/mL for PPP [16] (unpublished data) compared with 0.01 pg/mL for PFP [28] (Tables 3 and 4). Tables 2–4 indicate the use of either PPP or PFP in the different studies. The source and batch of recombinant TF used to create standard curves also varies between studies and will affect the reported amount of MP-TF activity. For the endpoint assay, we propose four response categories of MP-TF activity for PPP: zero (0.0 – <0.5 pg/ml); weak (0.5 - <1.0 pg/mL), moderate (1.0 – <2.0 pg/mL), and strong (>2.0 pg/mL).
Table 2.
MP-TF activity in cancer – kinetic assay.
| Group | MP-TF activity (fM Xa min−1) | PPP/PFP | Ref | |
|---|---|---|---|---|
| Mean±SD (range) | Median (range) | |||
| Healthy controls (n=37) | 132±47 | PPP | 15 | |
| VTE, non-cancer (n=6) | 148 (135–331) | |||
| Cancer no VTE (n=33) | 209 | 170 (59–665) | ||
| Cancer VTE (n=7) | 3643 | 2620 (410–14180)* | ||
|
| ||||
| Healthy controls (n=37) | 132 | 127 (44–247) | PPP | 19 |
| Cancer no VTE (n=49) | 162 | 150 (23–535) | ||
| Cancer VTE (n=51) | 1125 | 355 (19–12333)* | ||
| Colorectal no VTE (n=14) | 119 (23–535) | |||
| Colorectal VTE (n=14) | 246 (55–1578) | |||
| Pancreatic no VTE (n=10) | 138 (80–228) | |||
| Pancreatic VTE (n=10) | 2080 (510–12333) | |||
|
| ||||
| Healthy controls (n=20) | 4.1 (2.3–6.6)‡ | PFP | 27 | |
| Multiple myeloma (n=122) | 17.6 (8.6–33.2)*‡ | |||
| Before chemo (n=75) | 17.4 (10.2–32.8)‡ | |||
| After chemo (n=75) | 12.0 (7.0–18.5)*‡ | |||
| No VTE (n=107) | 17.8 (8.1–32.5)‡ | |||
| VTE (n=15) | 16.8 (11.4–36.1)‡ | |||
|
| ||||
| Pancreatic cancer (n=72) | 45.9 (12.3–193.9)‡ | PFP | 17 | |
| Brain cancer (n=148) | 16.4 (5.5–30.1)‡ | |||
|
| ||||
| Healthy controls (n=19) | (8–113) | PPP | 26 | |
| Testicular cancer (n=13) | 16±8 (10–30) | |||
significant difference compared with controls
25th–75th percentile
Table 4.
MP-TF activity in different diseases.
| Disease | Group | MP-TF activity | PPP/PFP | Ref | ||
|---|---|---|---|---|---|---|
| Endpoint | Kinetics | |||||
| Mean±SD (range) | Median (range) | Median±SD (range) | ||||
| Hypercholesterolemia | Healthy controls (n=17) | 0.01 | PPP | 24 | ||
| FH patients (n=25) | 0.66 | |||||
|
| ||||||
| Endotoxemia | Healthy controls (n=7) | 47±41 | 50 | |||
| After LPS treatment (n=7) | 309±267 | |||||
|
| ||||||
| Acute liver injury | Healthy controls (n=13) | 0.24±0.14 | PPP | 47 | ||
| ALI patients (n=34) | 9.05±8.82* | |||||
|
| ||||||
| HIV | Untreated (n=54) | 0.35 (0.24–0.54)‡ | PPP | 51 | ||
| Antiretroviral (n=109) | 0.22 (0.14–0.34)*‡ | |||||
|
| ||||||
| Cirrhosis | Healthy controls (n=9) | 0.01±0.01 | PFP | 28 | ||
| Child–Pugh A (n=10) | 0.05±0.05 | |||||
| Child–Pugh B (n=9) | 0.10±0.07 | |||||
| Child–Pugh C (n=14) | 0.22±0.15* | |||||
|
| ||||||
| APS | Asymptomatic (n=72) | 0.09 (0.05–0.14)‡ | PFP | 29 | ||
| Symptomatic (n=30) | 0.13 (0.10–0.17)‡ | |||||
|
| ||||||
| UTI | Healthy controls (n=19) | 79 (57–126)‡ | PPP | 33 | ||
| UTI (n=215) | 197 (113–398)*‡ | |||||
| Non bacteremic (n=167) | 186 (110–324)‡ | |||||
| Bacteremic (n=48) | 325 (166–641)‡ | |||||
| APACHE II score 0–4 (n=78) | 152 (98–280)‡ | |||||
| APACHE II score 5–9 (n=93) | 227 (123–501)‡ | |||||
| APACHE II score 10–14 (n=34) | 259 (144–599)‡ | |||||
| APACHE II score 15–17 (n=10) | 471 (180–848)‡ | |||||
|
| ||||||
| HIV/HCV | HIV (n=15) | 0.31±0.32 | PFP | 30 | ||
| HCV (n=15) | 0.15±0.21 | |||||
| HIV/HCV (n=15) | 0.14±0.05 | |||||
|
| ||||||
| Lupus anticoagulant | Healthy controls (n=30) | 0.06 (0.00–0.10)‡ | PPP | 55 | ||
| Patients (n=113) | 0.04 (0.00–0.11)‡ | |||||
|
| ||||||
| Obesity | Before surgery (n=74) | 0.32±0.43 | PFP | 52 | ||
| After surgery (n=74) | 0.18±0.41* | |||||
|
| ||||||
| Influenza A | Healthy controls (n=27) | 0.05±0.02 | PPP | 22 | ||
| Influenza A patients | 3.80±0.90* | |||||
|
| ||||||
| CVD | Baseline (n=19) | 0.26±0.35 | PPP | NP | ||
| Placebo (n=19) | 0.22±0.26 | |||||
| Atorvastatin (n=19) | 0.20±0.18 | |||||
|
| ||||||
| CVD | Before high dose atorvastatin (n=19) | 0.23±0.64 | PPP | NP | ||
| After high dose atorvastatin (n=19) | 0.21±0.76 | |||||
| Before low dose atorvastatin plus ezetimibe (n=14) | 0.09±0.22 | |||||
| After low dose atorvastatin plus ezetimibe (n=14) | 0.25±0.53 | |||||
|
| ||||||
| Endotoxemia | Endotoxemia 0 hour (n=13) | 0.06±0.05 | PFP | NP | ||
| Endotoxemia 3 hours (n=13) | 0.51±0.18* | |||||
| Endotoxemia 6 hours (n=13) | 0.47±0.23* | |||||
APS: antiphospholipid syndrome, CVD: cardiovascular disease, HCV: hepatitis C virus, HIV: human immunodeficiency virus, NP: not published, UTI: urinary tract infection
significant difference compared with controls
25th–75th percentile
Table 3.
MP-TF activity in cancer – endpoint assay.
| Group | MP-TF activity (pg/mL) | PPP/PFP | Ref | |
|---|---|---|---|---|
| Mean±SD (range) | Median (range) | |||
| Healthy controls (n=15) | 0.21±0.11 | PPP | 16 | |
| Pancreatic cancer (n=10) | 0.95 (0–3.1) | |||
| No VTE (n=8) | 0.46±1.26* | |||
| VTE patient No.5 | 4.4 | |||
| VTE patient No.10 | 5.5 | |||
|
| ||||
| Cancer no VTE (n=13) | 0.5±0.5 | PPP | 36 | |
| Cancer VTE (n=53) | 1.7±3.8* | |||
| Pancreatic (n=5) | 6.6±10.8 | |||
| Lung (n=10) | 2.4±2.5 | |||
| Colorectal (n=14) | 0.7±0.8 | |||
|
| ||||
| Pancreatic (n=60) | 0.10 (0.04–0.19)‡ | PFP | 17 | |
| Stomach (n=43) | 0.07 (0.0–0.17)‡ | |||
| Colorectal (n=126) | 0.05 (0.01–0.15)‡ | |||
| Brain (n=119) | 0.04 (0.0–0.08)‡ | |||
|
| ||||
| Healthy controls (n=22) | 0.26 (0.06–0.41)‡ | PPP | 42 | |
| Cancer no VTE (n=38) | 0.21 (0.04–0.35)‡ | |||
| Cancer VTE (n=5)† | 0.82 (0.25–6.9)‡ | |||
|
| ||||
| Pancreaticobiliary (n=117) | 2.15±3.8 | 1.20 (0.17–31.01) | PPP | 41 |
| No VTE (n=65) | 1.40±1.5 | 1.13 (0.23–10.65) | ||
| VTE (n=36) | 3.08±5.3 | 1.20 (0.17–31.01)* | ||
|
| ||||
| Healthy controls (n=22) | 0.05 (0.00–0.76) | PPP | 20 | |
| Pancreatic cancer (n=73) | 0.37 (0.00–11.91) | |||
| Localized resected (n=18) | 0.18 (0.00–1.39) | |||
| Localized unresected (Stage II–III) (n=13) | 0.29 (0.00–0.88) | |||
| Metastatic recurrent (Stage IV) (n=13) | 0.28 (0.04–0.74) | |||
| Metastatic non-resectable (Stage IV) (n=29) | 0.88 (0.19–11.91)* | |||
|
| ||||
| AML patient (n=7) | 1.26±1.77 | PPP | 21 | |
| No VTE, No DIC (n=2) | 0.17±0.08 | |||
| VTE (n=3) | 0.30±0.14 | |||
| DIC patient No.1 | 4.43 | |||
| DIC patient No.2 | 3.16 | |||
|
| ||||
| Healthy controls (n=5) | 0.25±0.08 | PPP | NP | |
| Multiple myeloma (n=11) | 0.25±0.20 | |||
| Before chemo (n=9) | 0.24±0.19 | |||
| After chemo (n=9) | 0.28±0.29 | |||
AML: acute myelocytic leukemia, NP: not published
significant difference compared with controls
including 3 pancreatic cancer patients
25th – 75th percentile
1. MP-TF activity in cancer patients
Levels of MP-TF in different types of cancer with or without VTE
The incidence of VTE varies between different cancers and can be broadly divided into high risk, such as pancreatic and brain cancer, moderate risk, such as lung, colorectal and hematologic cancers, and low risk, such as breast and prostate cancers [34, 35]. In general, higher levels of MP-TF activity are observed in cancers associated with the highest rates of VTE. For instance, Tesselaar and colleagues found that pancreatic cancer patients with VTE had much higher levels of MP-TF activity than colorectal cancer patients with VTE (Table 2) [19]. Higher levels of MP-TF activity were also observed in pancreatic cancer patients with VTE compared with lung and colorectal cancer patients with VTE (Table 3) [36]. In non-VTE cancer patients, MP-TF activity was highest in those with pancreatic cancer followed by stomach, colorectal and brain cancer (Table 3) [17].
One study reported 4-fold higher levels of MP-TF activity in PFP from 122 multiple myeloma patients compared with healthy controls [27]. In a separate study, we collected serial samples (1-6 samples per patient) from 16 multiple myeloma patients during the course of chemotherapy (cycle 1 and 2) (Supplementary Table 1).. We did not observed an increase in MP-TF activity in multiple myeloma patients and there was no increase in MP-TF activity after chemotherapy 1 and 4 days after treatment (Supplementary Table 2). The reason for these discrepant results between the two studies is unclear but is unlikely to be due to the use of PFP versus PPP as a starting material.
A small study measured MP-TF activity in acute myeloid leukemia (AML), although no healthy controls were included [21]. However, it appears that the 5 AML patients without disseminated intravascular coagulation (DIC) in this study did not have increased MP-TF activity when compared to healthy controls from other studies.
Levels of MP-TF activity with stage of cancer in pancreatic cancer
Previous studies have found that TF expression correlates with histological grade in cancer [37–39]. Circulating TF+ MPs present in cancer patients appear to be derived from the tumor since levels are dramatically reduced after resection [40]. Therefore, one would expect levels of MP-TF activity to increase with cancer stage. Thaler and colleagues measured MP-TF activity in pancreatic cancer patients with localized (stage II-III) and metastatic tumors (stage IV) [20]. MP-TF activity was significantly higher in the cancer patients compared to healthy controls irrespective of the stage. However, levels of MP-TF activity were highest in those patients with metastatic, non-resectable disease. In a separate study [41], MP-TF activity also increased with stage of pancreatic cancer (Figure 1). In summary, levels of MP-TF activity increase with stage of cancer, which is most likely due to increased TF expression in the tumor. Accordingly, the highest MP-TF activity levels reported to date are in patients with stage IV pancreatic cancer. We would anticipate a similar relationship between levels of MP-TF activity and stage for other types of cancer.
Figure 1. Levels of MP-TF activity in pancreatic cancer patients.
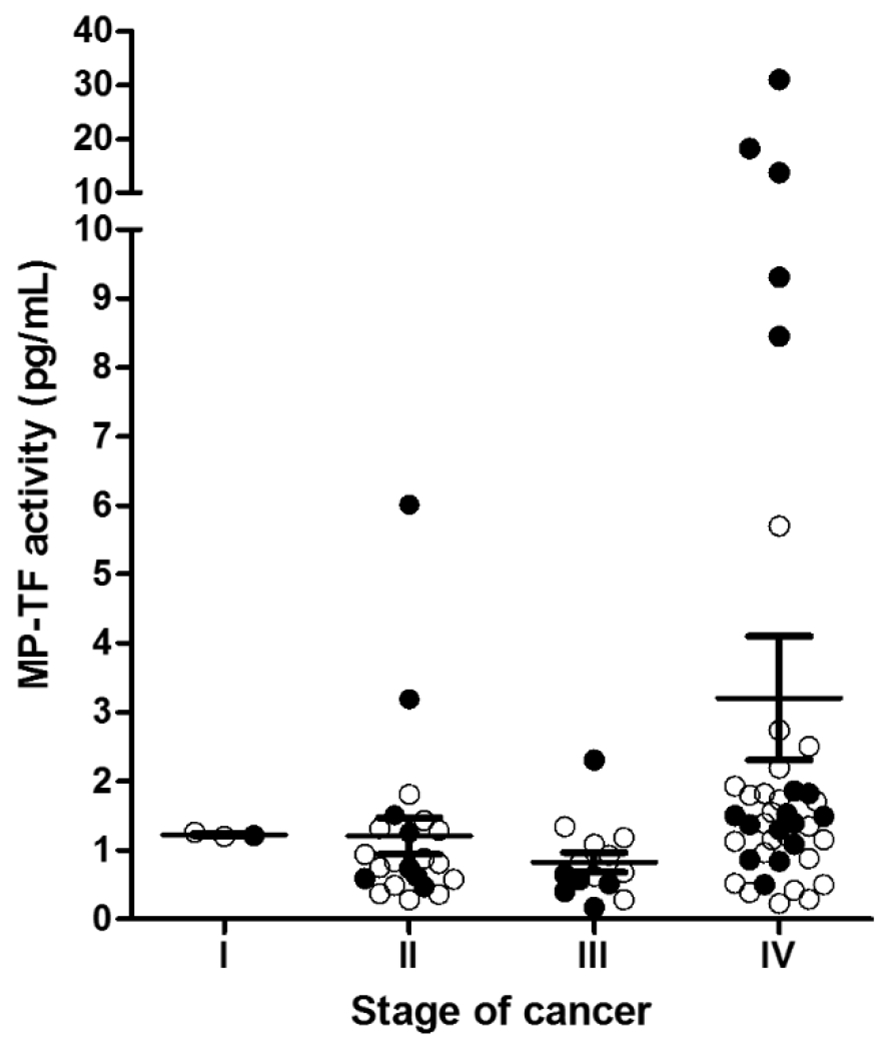
MP-TF activity in patients with stage I (n=3), II (n=22), III (n=14), and IV (n=41) pancreatic cancer. Data are shown by mean ± SEM. The filled circles represent patients with VTE.
Association of MP-TF activity with VTE in cancer patients
A number of retrospective studies have shown that cancer patients with VTE have higher levels of MP-TF activity than cancer patients without VTE (Table 2 and 3) [15, 19, 36]. One study found that levels of MP-TF activity were higher in 51 cancer patients with VTE compared to 49 cancer patients without VTE [19]. The two groups were matched for age, sex, type of cancer, stage of disease and mode of treatment. The study analyzed many different types of cancer, including gastro-intestinal, genito-urinary, breast and ovarian. Importantly, pancreatic cancer patients with VTE showed 15-fold higher levels of MP-TF activity than those without VTE, whereas colorectal cancer patients with VTE showed only a 2-fold increase in MP-TF activity compared with those without VTE. Of note, 10 out of 32 patients with elevated MP-TF activity were pancreatic cancer patients. However, a potential problem with retrospective studies is that MP-TF activity may be increased as a consequence of the VTE rather than MP-TF activity contributing to thrombosis.
Prospective studies are more informative because they measure MP-TF activity as patients are being monitored for VTE over a defined follow-up period. Three prospective studies have shown a correlation between elevated levels of MP-TF activity and VTE in cancer patients (Table 3) [16, 41, 42]. Khorana and colleagues measured MP-TF activity in serial samples from 10 pancreatic cancer patients and found that MP-TF activity increased in the two patients that developed VTE [16]. A second study found a correlation between MP-TF activity and VTE in 43 cancer patients, 5 of which developed VTE in the 6 month follow-up period [42]. Of note, 3 of the 5 patients that developed VTE had pancreatic cancer. MP-TF activity was also correlated with VTE in 117 pancreaticobiliary cancer patients that were followed for 6 months [41]. Thaler and colleagues found “borderline non-significant associations between MP-TF activity and increased risk of VTE in Cox proportional hazard models” in pancreatic cancer patients using the endpoint assay [17]. No associations were observed between MP-TF activity and VTE in brain, stomach or colorectal cancer patients [17]. This study had a 2 year follow-up period that may explain the weak association between MP-TF activity and VTE. Finally, there was no correlation between MP-TF activity and VTE in multiple myeloma patients [27]. In summary, several studies have found the highest levels of MP-TF activity in pancreatic cancer patients and a relationship between MP-TF activity and VTE in these patients but not in other types of cancer. At present, it is unclear why this association seems to be confined to pancreatic cancer.
MP-TF activity and survival of cancer patients
Four studies have reported a relationship between the level of MP-TF activity and survival in cancer patients [15, 17, 19, 41]. In one study, the median survival of 32 cancer patients (10 of whom had pancreatic cancer) with elevated MP-TF activity was 3.5 months compared to 13 months for 68 cancer patients with low MP-TF activity [19]. Thaler and colleagues also found an association between elevated levels of MP-TF activity and reduced survival in pancreatic cancer patients [17]. Finally, Bharthuar and colleagues found that median survival of pancreaticobiliary cancer patients with elevated levels of MP-TF activity was 98.5 days compared with 231 days for those with low levels of MP-TF activity [41]. These studies indicate that MP-TF activity is a biomarker for survival in pancreatic cancer patients.
MP-TF and chemotherapy
Chemotherapy is designed to induce cytoreduction of cancer cells and therefore would be expected to increase levels of MP-TF activity in plasma. A number of studies have measured MP-TF activity in cancer patients before and after chemotherapy [26, 27, 43]. Mukherjee and colleagues found that chemotherapy did not increase MP-TF activity in patients with early stage breast cancer [43]. A second study reported no change in the levels of MP-TF activity in patients with metastatic testicular cancer following two courses of chemotherapy [26]. As described above, we did not see an increase in the levels of MP-TF activity after chemotherapy in patients with multiple myeloma (Supplementary Table 2). Only one study reported an association between MP-TF activity and chemotherapy; it was shown that chemotherapy significantly decreased the level of MP-TF activity in patients with multiple myeloma [27]. These studies suggest that chemotherapy does not increase MP-TF activity in cancer patients.
Levels of MP-TF activity in malignancy induced DIC
DIC is a condition characterized by systemic activation of coagulation. One study found that 5 patients with DIC and a variety of cancers had elevated levels of TF+ MPs measured using a one-stage clotting assay [18]. A second study observed very high levels of MP-TF activity in 2 AML patients with DIC compared with 5 AML patients without DIC [21]. Importantly, the levels of MP-TF activity fell dramatically after the DIC was resolved. These studies suggest that TF+MPs may contribute to DIC associated with malignancy but larger studies are needed to confirm these results. Additional prospective studies are needed to show that MP-TF activity is increased prior to the DIC and is not caused by the DIC.
2. MP-TF activity in non-cancer patients
Levels of MP-TF activity in patients with cardiovascular disease
Hypercholesterolemia is associated with atherosclerosis and atherothrombosis [10]. Various studies have reported elevated levels of TF antigen in the circulation of cardiovascular disease patients [44]. Levels of MP-TF activity were elevated in familial hypercholesterolemia patients compared with controls [24]. We measured MP-TF activity in 3 different studies with cardiovascular disease patients treated with statins. In the first study, we measured MP-TF activity in 19 patients with peripheral arterial disease who received high-dose atorvastatin (80 mg/day) or placebo for 8 weeks in a cross-over fashion (Supplementary Table 3) [45]. There was no difference in the mean values of MP-TF activity at baseline, during atorvastatin or during placebo (Figure 2A). Analysis of the levels in individual patients did not reveal any consistent changes. Atorvastatin treatment was associated with decreased MP-TF activity in the three patients with the highest basal levels of MP-TF activity, but was also associated with increases in MP-TF activity in 4 other patients (Figure 2A). In a second study, coronary artery disease patients were given either a moderately high-dose of atorvastatin (40 mg/day) (n=19) or a low-dose of atorvastatin (10 mg/day) plus ezetimibe (10 mg/day) (n=14) for 4 weeks [46]. There was no difference in the mean value of MP-TF activity in either group before or after treatment (Figure 2B). In the atorvastatin alone group, MP-TF activity was decreased in 2 patients and increased in another patient, whereas in the atorvastatin and ezetimibe group, MP-TF activity was decreased in 2 patients and increased in 3 other patients (Figure 2B). Finally, we compared levels of MP-TF activity in a small study consisting of 11 matched pairs of patients with or without statins (Supplementary Table 4). Surprisingly, the mean level of MP-TF activity in the statin group was higher than the level observed in the non-statin group (Supplementary Figure 1). Five patients in the statin group and 3 patients in the non-statin group had levels of MP-TF activity that were above the cut-off of healthy individuals (Supplementary Figure 1). It is unclear why some of the patients in this study had elevated levels of MP-TF activity. Combining baseline data from the 3 studies indicates that 10/63 (16%) of cardiovascular disease patients had moderately elevated levels of MP-TF activity and that statin treatment did not consistently reduce these levels.
Figure 2. Effect of statins on MP-TF activity.
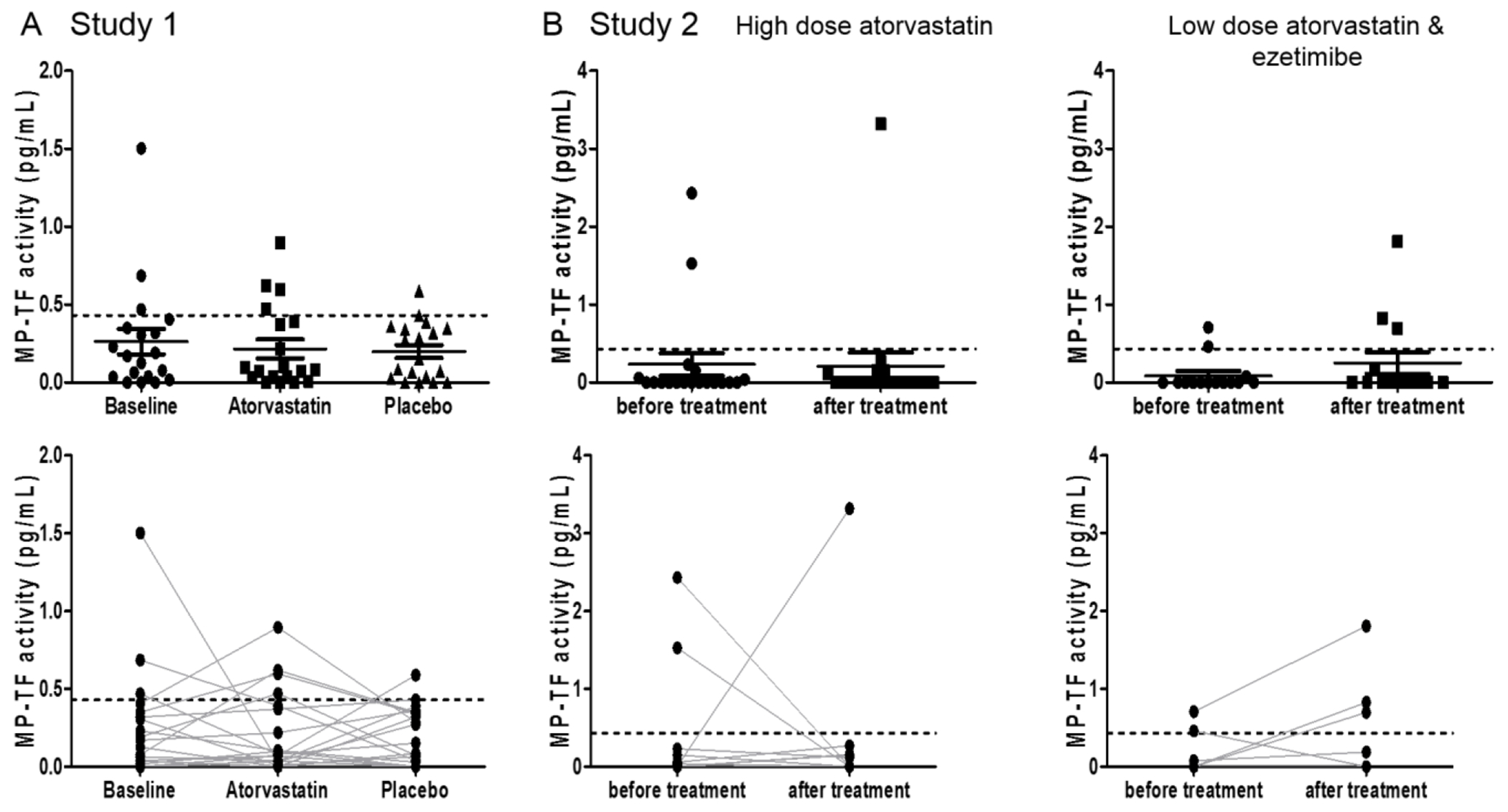
(A) Effect of atorvastatin on MP-TF activity in patients with peripheral arterial occlusive disease randomized to 8 weeks of treatment with atorvastatin or placebo in a cross-over study. Each sample was collected after 8 weeks of treatment with atorvastatin or after 8 weeks of treatment with placebo. (Top panel) MP-TF activity (mean ± SEM). (Bottom panel) Changes in the levels of MP-TF activity in each patient during atorvastatin treatment. (B) Effect of atorvastatin on MP-TF activity in patients with coronary artery disease randomized to either high dose atorvastatin (40 mg/day) only (left panels) or low dose atorvastatin (10 mg/day) plus ezetimibe (10 mg/day) (right panels). (Top panel) MP-TF activity (mean ± SEM). (Bottom panel) Changes in the levels of MP-TF activity in each patient during treatment. Data are shown by mean ± SEM. The dotted line represents the upper limit of the normal MP-TF activity range (0.43 pg/mL, mean plus 2 standard deviations).
Levels of MP-TF activity in patients with cirrhosis and liver injury
Cirrhosis is associated with various forms of thrombosis, particularly portal vein thrombosis. A recent study measured levels of MP-TF activity in patients with varying degrees of cirrhosis as determined by the Child-Pugh score [28]. MP-TF activity increased in a step-wise manner with disease severity with the 14 patients with the most severe cirrhosis having 17-fold higher levels of MP-TF activity compared with healthy controls (Table 4). Similarly, Stravitz and colleagues reported a 38-fold increase in MP-TF activity in 34 subjects with acute liver injury (ALI) compared with healthy controls [47]. In a separate study, patients with hepatitis C virus had detectable levels of MP-TF activity, although no healthy controls were included in the study [30]. Although the liver expresses low levels of TF, it is likely that TF+ MPs are released from the liver after acute and chronic injury. Indeed, in a mouse model of chronic liver injury we found that deletion of the TF gene in hepatocytes but not myeloid cells abolished the activation of coagulation [48].
Levels of MP-TF activity in a human endotoxemia model and infection
An early study reported increased levels of MP-TF activity in human volunteers injected with Escherichia coli lipopolysaccharide (LPS) using a functional assay that involved capture of the TF+ MPs [49]. A more recent study demonstrated a 6.6-fold increase in the mean level of MP-TF activity using the kinetic assay in 7 volunteers receiving LPS [50]. Similarly, we found that the mean level of MP-TF activity was increased 8.5-fold 3 hours after LPS administration using the endpoint assay (Table 4) (Mooberry M, manuscript in revision).
MP-TF activity was also measured in patients with urinary tract infections where the APACHE II score was used to measure disease severity [33]. Infections induce TF expression and activation of coagulation. Again, MP-TF activity increased in a step-wise manner with severity in urinary tract infection with a 6-fold increase in patients with the highest APACHE II score [33]. Similarly, patients with severe influenza A infection had increased levels of MP-TF activity compared with healthy controls (Table 4), and the level of MP-TF activity correlated with mortality [22].
Levels of MP-TF activity in patients with HIV
Baker and colleagues measured levels of MP-TF activity in HIV patients with or without antiretroviral therapy and found a 39% decrease in the treated patients (Table 4) [51]. In the untreated patients, those with hepatitis B/C coinfection (n=14) had higher levels of MP-TF activity compared to those without hepatitis (n=40). In a separate study, higher levels of MP-TF activity were found in patients with hepatitis C virus compared to HIV patients [30]. However, neither of these studies included healthy controls which makes it difficult to conclude if the MP-TF activity is really increased in HIV patients.
Levels of MP-TF activity in patients with other diseases
A study comparing levels of MP-TF activity in 74 morbidly obese patients before and after bariatric surgery reported significantly decreased MP-TF activity following surgery [52]. This finding is consistent with the fact that obesity is associated with activation of coagulation [53, 54]. In contrast, several studies have reported no increases in MP-TF activity. A study of antiphospholipid syndrome (APS) revealed APS patients expressed MP-TF activity levels below assay sensitivity limits [29]. Similarly, MP-TF activity was not increased in patients with lupus anticoagulant with a history of thrombosis compared with healthy controls [55]. Another study reported no difference in MP-TF activity in paroxysmal nocturnal hemoglobinuria patients before and after treatment with eculizumab, a monoclonal antibody to complement protein C5 [56].
Summary
The majority of the studies of MP-TF activity have focused on cancer patients because cancer cells express high levels of TF and because certain types of cancer are associated with high rates of VTE. Indeed, MP-TF activity was increased 16 to 26-fold over basal levels for pancreatic cancer patients with VTE. However, the mechanism by which TF+ MPs contribute to VTE is not well understood [57]. In contrast, lower levels of MP-TF activity are observed with other types of cancer. Liver injury is also associated with a robust increase in MP-TF activity. As expected, MP-TF activity increases in a human model of endotoxemia (7 to 9-fold) and in patients with urinary tract infection (6-fold) but these increases are more modest compared with pancreatic cancer. Surprisingly, significant increases in MP-TF activity were not observed in the majority of cardiovascular disease patients and statins did not generally decrease the signal. Efforts should be made to standardize pre-analytic variables in plasma preparation and the assay itself so that different studies can be directly compared. In addition, it would be helpful to identify the cellular origin of the TF+ MPs in different diseases and to increase the sensitivity of the detection of TF+ MPs in plasma.
Supplementary Material
Acknowledgements:
We would like to acknowledge technical support from Rebecca Lee and helpful discussions from Dr. Jianguo Wang (Harvard Medical School) and Dr. Johannes Thaler (Medical University of Vienna).
Footnotes
Disclosure of Conflict of Interests: The authors do not declare any conflict of interest.
Reference
- [1].Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. [DOI] [PubMed] [Google Scholar]
- [2].Spronk HM, Cannegieter S, Morange P, Hackeng T, Huisman M, Nagler M, et al. Theme 2: Epidemiology, Biomarkers, and Imaging of Venous Thromboembolism (and postthrombotic syndrome). Thromb Res. 2015;136 Suppl 1:S8–S12. [DOI] [PubMed] [Google Scholar]
- [3].Lance MD. A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J. 2015;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Linkins LA, Bates SM, Lang E, Kahn SR, Douketis JD, Julian J, et al. Selective D-dimer testing for diagnosis of a first suspected episode of deep venous thrombosis: a randomized trial. Ann Intern Med. 2013;158:93–100. [DOI] [PubMed] [Google Scholar]
- [5].Righini M, Kamphuisen PW, Le Gal G. Age-adjusted D-dimer cutoff levels and pulmonary embolism--reply. JAMA. 2014;312:557–8. [DOI] [PubMed] [Google Scholar]
- [6].Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. [DOI] [PubMed] [Google Scholar]
- [7].Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 2014;111:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Owens AP 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rautou PE, Mackman N. Microvesicles as risk markers for venous thrombosis. Expert Rev Hematol. 2013;6:91–101. [DOI] [PubMed] [Google Scholar]
- [10].Owens AP 3rd, Mackman N. Role of tissue factor in atherothrombosis. Curr Atheroscler Rep. 2012;14:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. [DOI] [PubMed] [Google Scholar]
- [12].Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–75. [DOI] [PubMed] [Google Scholar]
- [13].Tatsumi K, Antoniak S, Monroe DM 3rd, Khorana AA, Mackman N, Subcommittee on H, et al. Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1932–4. [DOI] [PubMed] [Google Scholar]
- [14].Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. [DOI] [PubMed] [Google Scholar]
- [16].Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. [DOI] [PubMed] [Google Scholar]
- [18].Langer F, Spath B, Haubold K, Holstein K, Marx G, Wierecky J, et al. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann Hematol. 2008;87:451–7. [DOI] [PubMed] [Google Scholar]
- [19].Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–3. [DOI] [PubMed] [Google Scholar]
- [20].Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, et al. Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Invest. 2013;43:277–85. [DOI] [PubMed] [Google Scholar]
- [21].Thaler J, Pabinger I, Sperr WR, Ay C. Clinical evidence for a link between microparticle-associated tissue factor activity and overt disseminated intravascular coagulation in patients with acute myelocytic leukemia. Thromb Res. 2014;133:303–5. [DOI] [PubMed] [Google Scholar]
- [22].Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle Tissue Factor Activity and IL-8 Levels are Associated with Mortalitiy in Patients with Influenza A/H1N1 Infection. Crit Care Med. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thaler J, Koppensteiner R, Pabinger I, Ay C, Gremmel T. Microparticle-associated tissue factor activity in patients with acute unprovoked deep vein thrombosis and during the course of one year. Thromb Res. 2014;134:1093–6. [DOI] [PubMed] [Google Scholar]
- [24].Owens AP 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garcia Rodriguez P, Eikenboom HC, Tesselaar ME, Huisman MV, Nijkeuter M, Osanto S, et al. Plasma levels of microparticle-associated tissue factor activity in patients with clinically suspected pulmonary embolism. Thromb Res. 2010;126:345–9. [DOI] [PubMed] [Google Scholar]
- [26].van den Hengel LG, van Steijn-van Tol AQ, Bertina RM, Versteeg HH, Osanto S. Microparticle-associated tissue factor activity in plasma is unaffected by cytolytic chemotherapy treatment in metastatic testicular cancer patients. Thromb Res. 2013;131:187–9. [DOI] [PubMed] [Google Scholar]
- [27].Auwerda JJ, Yuana Y, Osanto S, de Maat MP, Sonneveld P, Bertina RM, et al. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105:14–20. [DOI] [PubMed] [Google Scholar]
- [28].Rautou PE, Vion AC, Luyendyk JP, Mackman N. Circulating microparticle tissue factor activity is increased in patients with cirrhosis. Hepatology. 2014;60:1793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Willemze R, Bradford RL, Mooberry MJ, Roubey RA, Key NS. Plasma microparticle tissue factor activity in patients with antiphospholipid antibodies with and without clinical complications. Thromb Res. 2014;133:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hodowanec AC, Lee RD, Brady KE, Gao W, Kincaid S, Plants J, et al. A matched cross-sectional study of the association between circulating tissue factor activity, immune activation and advanced liver fibrosis in hepatitis C infection. BMC Infect Dis. 2015;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lechner D, Weltermann A. Circulating tissue factor-exposing microparticles. Thromb Res. 2008;122 Suppl 1:S47–54. [DOI] [PubMed] [Google Scholar]
- [32].Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F, et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Woei AJFJ, van der Starre WE, Tesselaar ME, Garcia Rodriguez P, van Nieuwkoop C, Bertina RM, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133:799–803. [DOI] [PubMed] [Google Scholar]
- [34].Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–8. [DOI] [PubMed] [Google Scholar]
- [35].Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- [36].Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, et al. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125:511–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82:1101–4. [DOI] [PubMed] [Google Scholar]
- [38].Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. [DOI] [PubMed] [Google Scholar]
- [39].Goldin-Lang P, Tran QV, Fichtner I, Eisenreich A, Antoniak S, Schulze K, et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep. 2008;20:123–8. [PubMed] [Google Scholar]
- [40].Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, et al. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–4. [DOI] [PubMed] [Google Scholar]
- [42].van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, et al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–5. [DOI] [PubMed] [Google Scholar]
- [43].Mukherjee SD, Swystun LL, Mackman N, Wang JG, Pond G, Levine MN, et al. Impact of chemotherapy on thrombin generation and on the protein C pathway in breast cancer patients. Pathophysiol Haemost Thromb. 2010;37:88–97. [DOI] [PubMed] [Google Scholar]
- [44].Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mobarrez F, He S, Broijersen A, Wiklund B, Antovic A, Antovic J, et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thromb Haemost. 2011;106:344–52. [DOI] [PubMed] [Google Scholar]
- [46].Piorkowski M, Fischer S, Stellbaum C, Jaster M, Martus P, Morguet AJ, et al. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets? J Am Coll Cardiol. 2007;49:1035–42. [DOI] [PubMed] [Google Scholar]
- [47].Stravitz RT, Bowling R, Bradford RL, Key NS, Glover S, Thacker LR, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rautou PE, Tatsumi K, Antoniak S, Owens AP 3rd, Sparkenbaugh E, Holle LA, et al. Hepatocyte Tissue Factor Contributes to the Hypercoagulable State in a Mouse Model of Chronic Liver Injury. J Hepatol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. [DOI] [PubMed] [Google Scholar]
- [50].Woei AJFJ De Kruif MD, Garcia Rodriguez P, Osanto S, Bertina RM. Microparticles expressing tissue factor are concurrently released with markers of inflammation and coagulation during human endotoxemia. J Thromb Haemost. 2012;10:1185–8. [DOI] [PubMed] [Google Scholar]
- [51].Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating levels of tissue factor microparticle procoagulant activity are reduced with antiretroviral therapy and are associated with persistent inflammation and coagulation activation among HIV-positive patients. J Acquir Immune Defic Syndr. 2013;63:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ay L, Thaler J, Brix JM, Schernthaner G, Ay C, Pabinger I, et al. Decrease in microvesicle-associated tissue factor activity in morbidly obese patients after bariatric surgery. Int J Obes. in press. [DOI] [PubMed] [Google Scholar]
- [53].Allman-Farinelli MA. Obesity and venous thrombosis: a review. Semin Thromb Hemost. 2011;37:903–7. [DOI] [PubMed] [Google Scholar]
- [54].Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89:493–8. [PubMed] [Google Scholar]
- [55].Hell L, Ay C, Posch F, Koder S, Gebhart J, Pabinger I, et al. Low microparticle-associated tissue factor activity in patients with persistent lupus anticoagulant and a history of thrombosis. J Thromb Haemost. 2015;13 (Suppl. 2):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Weitz IC, Razavi P, Rochanda L, Zwicker J, Furie B, Manly D, et al. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res. 2012;130:361–8. [DOI] [PubMed] [Google Scholar]
- [57].Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


